Abstract
The frequent appearance of non-lamellar membrane arrangements such as cubic membranes (CMs) in cells under stressed or pathological conditions points to an intrinsic cellular response mechanism. CM represents highly curved, three-dimensional nano-periodic structures that correspond to mathematically well-defined triply periodic minimal surfaces. Specifically, cellular membrane may transform into CM organization in response to pathological, inflammatory and oxidative stress conditions. CM organization, thus, may provide an advantage to cope with various types of stress. The identification of inducible membrane systems, such as in the mitochondrial inner membranes to cubic morphology upon starvation, opens new avenues for understanding the molecular mechanisms of cellular responses to oxidative stress. In this study, we compared the cellular responses of starved and fed amoeba Chaos carolinense to oxidative stress. Food deprivation from C. carolinense induces a significant increase in prooxidants such as superoxide and hydrogen peroxide. Surprisingly, we observed a significant lower rate of biomolecular damage in starved cells (with higher free radicals generation) when compared with fed cells. Specifically, lipid and RNA damages were significantly less in starved cells compared with fed cells. This observation was not due to the upregulation of intracellular antioxidants, as starved amoeba show reduced antioxidant enzymatic activities; however, it could be attributed to CM formation. CM could uptake and retain short segments of nucleic acids (resembles cellular RNA) in vivo and in vitro. Previous results showed that nucleic acids retained within CM sustain a minimal oxidative damage in vitro upon exposure to high level of superoxide. We thus propose that CM may act as a ‘protective’ shelter to minimize the oxidation of biologically essential macromolecules such as RNA. In summary, we examined enzymatic antioxidant activities as well as oxidative damage biomarkers in starved amoeba C. carolinense in correlation with the potential role of CM as an optimal intracellular membrane organization for the protection of biological macromolecules against oxidative damage.
Keywords: cubic membrane, mitochondria, starvation, oxidative stress, antioxidant, cell survival
1. Introduction
Oxidative stress is the resultant damage that arises due to redox imbalances, more specifically an increase in destructive free radicals and reduction in protection from antioxidants and antioxidant defence pathways. In living organisms, antioxidant enzymes form the first line of defence against reactive oxygen species (ROS) in the cellular environments [1]. The key enzymes involved in the protection against oxidative stress include superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX). These enzymes work in tandem to decrease the damaging effects of ROS in cells. However, living organisms developed several other defence mechanisms to cope with oxidative stress. For example, in the unicellular organism Escherichia coli, level of fumarase C (a key enzyme in Krebs cycle catalysing the reversible hydration/dehydration of fumarate to malate) which is insensitive to superoxide anions, increases during oxidative stress, probably to replace fumarases A and B which are susceptible to damage by superoxide anions [2]. In mammals, there exist ‘sacrificial agents’, for example albumin, which are oxidized preferentially under oxidative stress conditions to protect important biomolecules [3]. These observations suggest that living organisms may use a wide range of biomolecules and mechanisms other than antioxidant enzymes to ameliorate the damaging effects of ROS. Interestingly, virus-infected host cells can undergo membrane transitions, which are generally referred to as ‘cytomembraneous inclusions’. The basis for this interesting observation has yet to be established although there are numerous reports that the virus-induced convoluted membranes are essential for viral replication and assembly [4]. It has been shown that virus-induced ‘cytomembraneous inclusions’ are indeed cubic membranes (CMs) [5]. It was further speculated that these membrane complexes could provide partial protection against host immune response [4]. Possibly, the formation of convoluted CMs in the host upon viral infection serves to protect the viral genome, especially RNA viruses [4], from oxidative stress generated by defence mechanisms.
It is well known that total removal of the food organism (Paramecium multimicronucleatum) from amoeba Chaos carolinense culture (starvation condition) induces greater levels of free radicals than continuous fed cells [6]. Meanwhile, starvation induces CM formation within C. carolinense mitochondria. CMs have been observed in numerous cell types from all kingdoms of life and in virtually any membrane-bound subcellular organelles. This induced membrane transition is frequently accompanied by alterations in cellular oxidative stress response which led to speculation that CM formation may be associated with oxidative stress [6]. In addition, CM mitochondrial particles are able to uptake and retain short segments of nucleic acids in vitro; we have, therefore, proposed that CM may act as a ‘protective’ shelter to minimize or prevent the oxidation of biologically essential macromolecules such as micro RNAs (miRNA) [7].
In this study, we investigated the enzymatic antioxidant activities as well as oxidative damage biomarkers in starved amoeba C. carolinense in correlation with the potential role of CM as an optimal intracellular membrane organization for macromolecule protection against oxidative damage.
2. Material and methods
2.1. Amoeba culture
Amoebae (C. carolinense) were cultured in flat-bottomed glass dishes at room temperature in an appropriate inorganic medium both in the presence of the amoeba food (fed condition) and in its absence (starved condition) as described earlier [8].
2.2. Measurement of reactive oxygen species production via electron paramagnetic resonance
Fed and starved amoebae C. carolinense were homogenized in 1 ml of 1 µM α-phenyl-N-tert-butylnitrone (PBN), then the same amounts of proteins in the homogenized samples were immediately frozen by immersion in liquid nitrogen. For specific electron paramagnetic resonance (EPR) analysis, samples were thawed on ice, mixed with 1.5 ml of spectroscopic-grade toluene and centrifuged at 2000g for 5 min at 4°C. The upper phase was collected for spectral analysis using a Bruker (Bruker BioSpin GmbH, Rheinstetten/Karlsruhe, Germany) Elexsys Series E500 CW-EPR X-band (9–10 GHz) spectrometer with these settings: 50 mW microwave power, 9.83 GHz microwave frequency, 1 G modulation amplitude, 100 kHz modulation frequency, 3448 G centre field, 110 G field sweep, 40.96 ms sampling time, 60 dB receiver gain, 163.84 ms receiver time constant and 20 averaged scans.
2.3. Antioxidant enzyme activity quantification
2.3.1. Catalase level measurement
Fed versus starved C. carolinense cells were collected, homogenized separately in cold buffer (50 mM potassium phosphate, pH 7, containing 1 mM EDTA), and same amounts of proteins in the homogenized samples were centrifuged at 9300g for 15 min at 4°C. Twenty millilitres of supernatant collected was then incubated in 100 µl of assay buffer, 30 µl of methanol and 20 µl H2O2 was added to initiate CAT peroxidatic activity (at room temperature with shaking). The reaction was terminated with 30 µl KOH after 20 min; 30 µl of Purpald (chromogen) followed by 10 µl KIO4 was added for 10 min and 5 min, respectively. The light absorbance was then measured at 540 nm with an optical density (OD) reader (Biotek µQuant Microplate Scanning Spectrometer; Biotek Instruments, Inc.).
2.3.2. Glutathione peroxidase level measurement
Fed and starved C. carolinense cells were homogenized in cold buffer (i.e. 50 mM Tris–HCl, pH 7.5, 5 mM EDTA and 1 mM DTT) separately, then same amounts of proteins in the homogenized samples were spun at 9300g for 15 min at 4°C. Supernatant was collected subsequently. An assay buffer and a mixture (of NADPH, glutathione and glutathione reductase) were added in quantities of 120 µl and 50 µl, respectively, for background control while fed and starved amoeba lysate supernatants (20 µl each) were each separately mixed with 100 µl assay buffer and 50 µl mixture. Cumene hydroperoxide (20 µl) added as the initiator is reduced to oxidized glutathione by GPX, which is then reduced back to the reduced glutathione by glutathione reductase and NADPH. The NADPH oxidation to NADP+ was accompanied by reduction in the absorbance at 340 nm which indicates GPX activity.
2.3.3. Superoxide dismutase level measurement
SOD levels in the samples were determined with the SOD Assay Kit purchased from (Cayman Chemicals, catalogue no. 706002) following the manufacturer's protocol. Mitochondria were isolated from starved and fed amoeba [9]. Same amounts of mitochondrial proteins were resuspended in HEPES buffer (in accordance with Cayman Chemicals protocol) and the superoxide reaction was initiated by the addition of 10 µl xanthine oxidase to the samples. After 20 min of incubation at room temperature the absorbance (OD) was read at 450 nm for SOD activity measurement.
2.4. Oxidative damage assessment
2.4.1. Thiobarbituric acid reactive substances assay
Malondialdehyde (MDA) concentrations were determined in fed and starved amoeba C. carolinense cells using thiobarbituric acid reactive substances (TBARS) Assay Kit (Cayman Chemicals). Both fed and starved samples were homogenized in 250 µl of 1× phosphate buffer saline using a 5 ml glass homogenizer (Teflon Potter Elvehjem). A measure of 105 µl of sodium dodecylsulfate (SDS) was then added to same amount of proteins in the homogenized samples in glass test tubes (Kimble, Division of Owens-Illinois) and mixed by swirling 4 ml of the colour reagent containing thiobarbituric acid added to the samples and incubated in a water bath over a stir plate (Sybron Thermolyne Nuova II) at 90°C for 1 h. The samples were subsequently incubated on ice for 10 min to stop the reaction and spun for 10 min at 1600g at 4°C. The absorbance (OD) was measured at 533 nm.
2.4.2. Protein carbonyl concentration measurement
Fed and starved amoeba samples were lysed and the cell lysates were each transferred separately to a 2 ml microtube (Axygen) and spun at 186g for 6 min at 4°C. The supernatants from the same amount of proteins in the homogenized samples were transferred to another 2 ml microtube (Axygen) and they were spun again at 1015g for 12 min at 4°C. Mixtures of 100 µl supernatants from fed and starved samples (which resulted from the second spin) were each mixed with 100 µl of SDS in separate tubes and further separated into two microtubes (Axygen)—100 µl each. A measure of 400 µl of 2,4-dinitrophenylhydrazine was added to the sample tube while 400 µl of 2.5 M HCl was added to the control tube. Both tubes were incubated in the dark at room temperature for 1 h, and subsequently mixed with 500 µl of 20% trichloroacetic acid (TCA). Tubes were incubated on ice for 30 min and spun at 10 961g for 10 min at 4°C. The supernatants were discarded and the pellets were resuspended in 1 ml of 1% TCA and incubated on ice for 5 min and spun at 10 961g for 10 min at 4°C. The pellets were then washed in 1 ml of (1 : 1) ethanol/ethyl acetate mixture and spun at 10 961g for 10 min at 4°C. Following that, they were resuspended in 220 µl of guanidine hydrochloride. The supernatant from each tube was then transferred to a 96-well plate and OD was measured at a wavelength of 375 nm to determine the protein carbonyl concentrations.
2.4.3. Determination of DNA and RNA oxidative damage
Trizol reagent (Gibco Life Technologies) was used to extract DNA and RNA from fed and starved amoeba samples according to manufacturer's instructions. 8-Hydroxydeoxyguanosine (8-OHdG) and 8-hydroxyguanosine (8-OHG) concentrations were determined.
Following OHdG EIA Assay Kit (Cayman Chemicals), DNA extracted from the same amount of proteins in the homogenized fed and starved amoeba C. carolinense cells was incubated with four units of DNase I for 1 h at 37°C and one unit of alkaline phosphatase for 1 h at 37°C. The samples were boiled for 10 min and 50 µl of the sample, 5 µl of acetylcholinesterase linked to 8-OHdG and 50 µl of monoclonal antibody to 8-OHdG were added into each well in the plate coated with goat anti-mouse immunoglobin G. The plate was then incubated for 18 h at 4°C before the wells were rinsed with wash buffer. A measure of 200 µl of Ellman's reagent was added to each well and the OD was read at a wavelength of 412 nm after 60 min. 8-OHdG concentrations obtained were adjusted according to DNA concentrations measured previously.
Following Oxiselect Oxidative RNA Damage Enzyme-linked Immunoassay Kit (CellBioLabs), RNA extracted from fed and 7-day-starved amoeba C. carolinense samples was incubated with 5–20 units of RNase A for 1 h at 37°C and 5–10 units of alkaline phosphatase for 1 h at 37°C. A measure of 100 µl of 8-OHG conjugate was added to each well of the protein binding plate, which was then incubated overnight at 4°C. The wells were subsequently blocked with 200 µl of assay diluent for 1 h at room temperature before the plate was transferred to 4°C. The assay diluent was removed from each well and 50 µl of each sample was then added to each well and incubated at room temperature for 10 min on an orbital shaker. A measure of 50 µl of the diluted anti-8-OHG antibody was then added to each well and incubated at room temperature for 1 h. The wells were washed with 250 µl of 1× wash buffer per well before 100 µl of the diluted secondary antibody-enzyme conjugate was added to all wells and incubated at room temperature for 1 h. Finally, 100 µl of substrate solution was added to all wells and the plate was incubated at room temperature. After 2–30 min, the enzyme reaction was stopped by adding 100 µl of stop solution into each well. The plate was read at a wavelength of 450 nm using an OD reader. OHG concentrations obtained were adjusted according to RNA concentrations measured previously.
2.5. Assessment of antioxidant properties of cubic membrane in vitro
Isolated mitochondria from starved amoeba C. carolinense and mouse liver mitochondria (50.4 µg protein) were mixed with 17.8 µg of oligonucleotides (ODNs) (5′-TCGGGGAGCGTGCGCCAT-3′, 1st Base Holdings) separately. The mixtures were incubated for 1 h at room temperature. To start the Fenton reaction, 0.03% H2O2 and 0.026 mM FeSO4 were added to the mixtures and incubated at 37°C for 1 h° [10]. Starved amoeba C. carolinense mitochondria and mouse liver mitochondria were subsequently pelleted from the mixture by spinning at 1015g for 12 min at 4°C and 6713 g for 10 min at 4°C, respectively; 25 µl of 5 M sodium chloride (NaCl) and 375 µl of ice-cold ethanol were added to the supernatant followed by overnight incubation at −20°C. The mixture was then spun at 10 961g for 30 min and the ODN pellets recovered were dissolved in 50 µl of 10 mM NaOH. The concentration of ODNs in each solution was determined using a Nanodrop spectrophotometer (Biofrontier, ND-1000). The isolated ODNs were then assessed for damage using the 8-OHdG enzyme immunoassay kit as described above.
2.6. Statistics
All data are expressed as means ± s.d. (n as indicated in the figure legends). Paired Student's t-test and ANOVA test were performed in this study, and the statistical significance was set at p < 0.05.
3. Results
3.1. Starved amoeba Chaos carolinense generates higher levels of superoxide
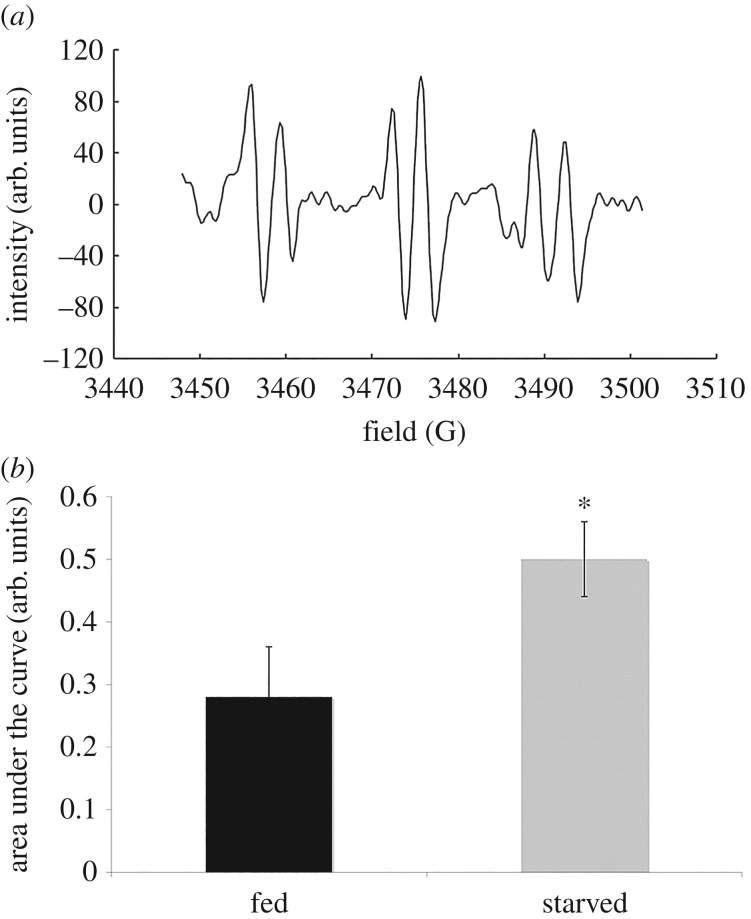
The production of superoxide by both fed and starved cells was measured using EPR technique. EPR signal of PBN was specific for superoxide anion (figure 1). The amplitude of EPR signal was lower in fed cells than in starved cells indicating that the superoxide production is at a higher level in starved cells than in fed cells. The result obtained is consistent with the previous experimental work that was conducted earlier [6].
Figure 1.
EPR spectra study in amoeba Chaos carolinense cells. Sample of the EPR spectra of signature of spin-trap (PBN) signals detected in amoeba C. carolinense (a) and the bar graph depicting calculated area under the curve of EPR spectra of PBN signal for superoxide anion (b) show the PBN signal detected was statistically higher in starved amoeba when compared with fed cells. *p < 0.05.
3.2. Antioxidant enzyme activity is lower in starved amoeba Chaos carolinense when compared with fed cells
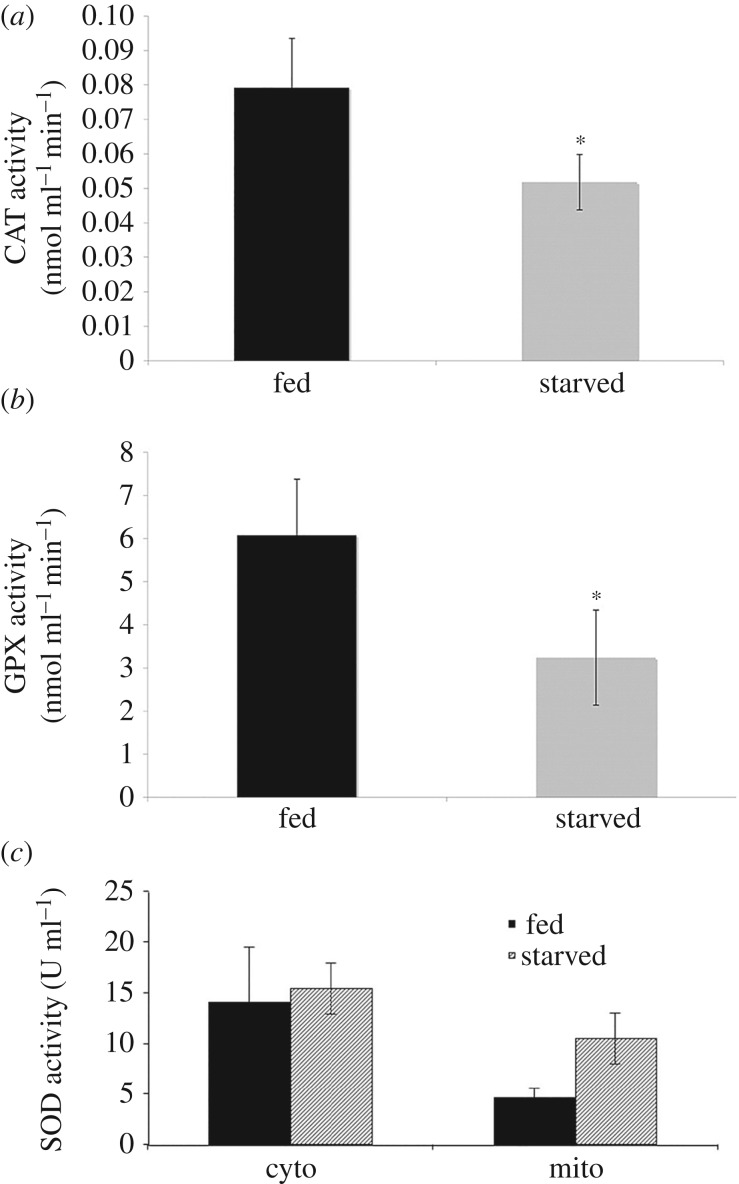
The CAT activity in fed amoeba C. carolinense was relatively higher than that of the starved amoeba cells (figure 2). Similarly, the enzyme GPX showed a higher activity in fed cells than in starved cells (figure 2). This kinetic study implies that fed amoeba cell has a higher activity of GPX than starved one. The mitochondrial SOD enzyme activity was found to be higher in starved amoeba samples compared with fed amoeba; however, the cytoplasmic SOD activity in 7-day-starved cells showed similar activity in comparison with fed cells (figure 2).
Figure 2.
Bar graphs depict the antioxidant enzyme activities in amoeba C. carolinense cells. Catalase (a) and glutathione peroxidase (b) activities are higher in fed amoeba C. carolinense cells when compared with starved cells. (c) Mitochondrial SOD activity was significantly higher in starved amoeba C. carolinense cells when compared with well-fed cells, while cytosolic SOD activity was the same in starved and fed amoeba C. carolinense. Data presented as means (±s.d.) of five independent experiments. *p-value < 0.05.
3.3. Starved amoeba Chaos carolinense has less lipid peroxidation and RNA damage, but similar protein and DNA damage when compared with fed cells
As superoxide and hydrogen peroxide production were higher in starved amoeba cells and the activities of antioxidant enzymes were lower than in fed cells, we further investigated the effect of the extent of oxidative stress on cellular biomolecules. Multiple oxidative damage biomarker levels in fed amoeba C. carolinense were compared with that in 7-day-starved cells.
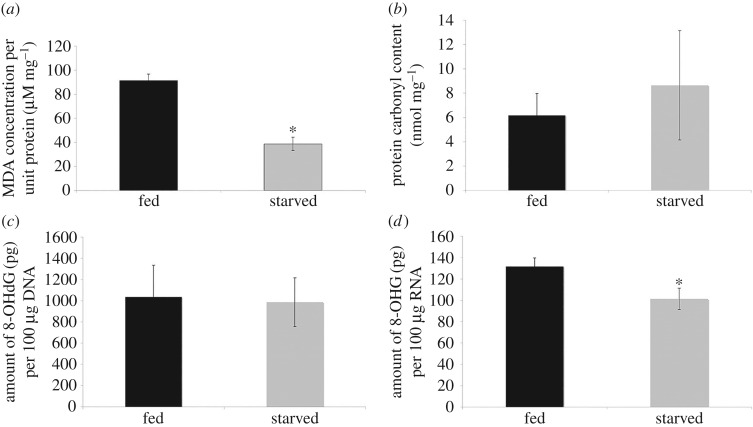
TBARS assay was used to assess lipid peroxidation [11,12]. The data showed that the levels of MDA formed in peroxidizing lipid systems [13] in fed amoeba were approximately threefold greater than in starved cells indicating higher lipid peroxidation in fed amoeba (figure 3a). On the contrary, protein carbonyl content in cytosolic lysates of fed amoeba C. carolinense was an average of 6.17 nmol mg−1 protein while that of starved amoeba C. carolinense with cubic mitochondria was an average of 8.64 nmol mg−1 protein (figure 3b).
Figure 3.
Bar graphs depict the oxidative damage biomarkers in amoeba C. carolinense cells. Lipid peroxidation (a) and RNA oxidation (d) were significantly lower in starved amoeba C. carolinense cells when compared with fed cells, while protein (b) and DNA (c) oxidation were not significantly different. Data presented as means ± s.d. of five independent experiments. *p-value < 0.05.
Furthermore, the quantification of 8-OHdG and 8-OHG, products of DNA and RNA damage, respectively, showed significantly low RNA oxidative damage (figure 3d) but not DNA oxidative damage (figure 3c) in starved amoeba when compared with fed cells.
3.4. Isolated mitochondria with cubic membrane organization protect short segment of nucleic acids against oxidative damage in vitro
The results from the RNA oxidative damage assays suggest that a protective mechanism was in place to protect RNA strands in starved amoeba C. carolinense from oxidative damage; thus, a subsidiary experiment was performed to test if CMs play a direct role in this antioxidant effect.
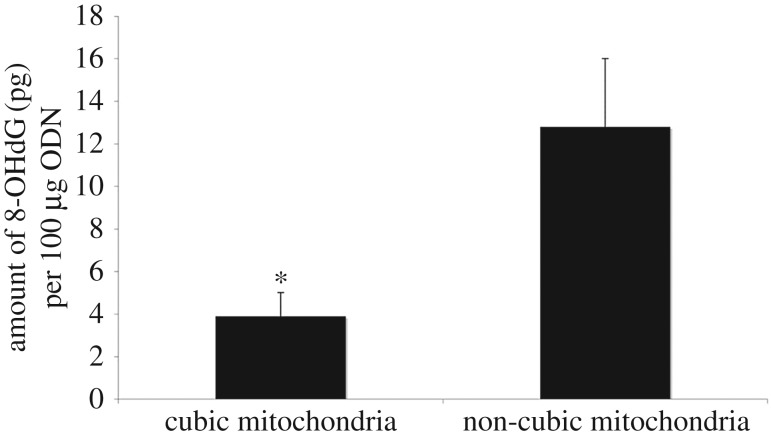
In this experiment, mitochondria with CM organization were isolated from 7-day-starved amoeba C. carolinense and the mitochondria without CM were isolated from mouse liver. Same amounts of mitochondrial proteins from 7-day-starved amoeba C. carolinense and mouse liver were incubated with ODNs in separate tubes before the mixture was exposed to superoxide anions from the Fenton reaction. After exposure to the Fenton reaction, the ODNs were isolated and assessed for oxidative damage. As shown in figure 4, the amount of 8-OHdG in the mixture with cubic mitochondria was approximately four times less than the amount of 8-OHdG incubated with non-cubic mitochondria. Similar findings were obtained when mitochondria from fed amoeba with tubular membrane organization were used as a control.
Figure 4.
Bar graph describes the difference in the amount of 8-OHdG (picograms) per 100 µg of ODN in the mixture containing cubic mitochondria and that containing non-cubic mitochondria. The mixture containing non-cubic mitochondria has approximately four times as much 8-OHdG as the mixture containing cubic mitochondria. Data presented as means (±s.d.) of five independent experiments. *p-value < 0.05.
4. Discussion
It has been established that the inner mitochondrial membrane of starved amoeba C. carolinense exhibits cubic morphology [14]. The appearance of this CM morphology is not restricted to amoeba C. carolinense cells, rather, it has been frequently reported in different cell types and tissues in association with an increase in cellular ROS levels [15]. Likewise, C. carolinense cells under starvation stress condition generated higher level of superoxide (figure 1b) and hydrogen peroxide [6]. As the free radical generation was observed to be greater in the starved C. carolinense, the antioxidant enzyme system was assumed to be upregulated in the starved cells, in order to diminish the oxidative damage caused by free radicals. Interestingly, the major cytoplasmic antioxidant enzyme system was found to be more active in fed cells compared with that in starved cells. With the exception of mitochondrial SOD, the activities of other antioxidants such as CAT and GPX were higher in fed cells (figure 2). SOD is an enzyme catalysing the dismutation of superoxide into hydrogen peroxide and oxygen. The presence of SOD enzyme activity in high levels in starved amoeba C. carolinense mitochondria may explain the tremendous generation of hydrogen peroxide in the starved amoeba C. carolinense [6].
Despite the lower antioxidant activities for a greater amount of free radical production, the starved C. carolinense shows lower lipid peroxidation and RNA damage.
It is likely that the lower levels of lipid peroxidation observed after starvation is attributed to the concurrent increase in the levels of plasmalogen lipids. It was reported that the levels of plasmalogen phosphatidylcholine (PC) were highly elevated after 7-day starvation [16]. Several studies have shown that plasmalogens have protective roles in lipid peroxidation [17,18]. In an investigation by Sindelar et al. [18], brain phospholipids with and without plasmalogens in separate liposomal systems were subjected to oxidative stress. The results revealed that in the presence of plasmalogens, biomarkers for lipid peroxidation were significantly decreased. This implies that plasmalogens protect polyunsaturated fatty acids from oxidative damage. Although the mechanism for this phenomenon has not been elucidated, it probably involves vinyl ether bonds in plasmalogens, which are more susceptible to oxidative attack than ester bonds in phospholipids [19]. The radical species formed during the peroxidation of vinyl ether bond may either be more stable or be less efficient to abstract hydrogen than alkyl radicals produced during the peroxidation of polyunsaturated fatty acids [18]. Also, it is likely that the oxygenated vinyl ether radical is broken down into water-soluble radical compounds, which are unable to further propagate the lipid peroxidation [18].
On the other hand, it has been observed in this study that there is no significant difference between the protein carbonyl content in the cytosolic lysates of fed and starved amoeba C. carolinense. It is possible that degradation and repair mechanisms are in play to eliminate oxidized proteins, masking the differences in protein oxidation in fed and starved amoeba C. carolinense as a result.
The assessment of DNA and RNA damage in fed and starved C. carolinense cells has revealed interesting results. It can be observed in figure 3 that 8-OHG levels are significantly lower in starved amoeba although there is no significant difference between 8-OHdG levels in fed and starved amoeba C. carolinense. 8-OHG and 8-OHdG measurements are indicative of RNA and DNA damage, respectively.
It has been shown previously that cubic mitochondria isolated from starved amoeba C. carolinense interact significantly with phosphorothioate ODNs. It is important to note that the ODNs used in this study are short and single stranded. Hence, they are similar to miRNA in the cell. As miRNA strands are localized in the cytosol in the same compartment as mitochondria in the cell [20], it is possible that they possess the ability to accumulate within the CMs of starved amoeba C. carolinense. On the other hand, such interaction is not possible for nuclear DNA, which accumulate within the nucleus.
It is likely also that alterations in lipid content during the starvation period play a protective role against ROS. As shown earlier, CM transition of amoeba C. carolinense mitochondria upon food deprivation is accompanied by major changes in fatty acid composition [16]. In particular, docosapentaenoic acid (DPA) content increased by 1.6-fold during the fasting process and is the most distinguished fatty acid species in C. carolinense lipids under starvation conditions. Recent investigations show that very long chain polyunsaturated fatty acids (VLC-PUFAs) such as DPA may have antioxidant abilities. In one study [21], it was noted that fatty acid micelles containing LC-PUFAs were able to scavenge greater amounts of superoxide anions than those containing saturated fatty acids. In another investigation [22], it was observed that E. coli cells with elevated levels of LC-PUFAs were resistant to hydrogen peroxide. These cells exhibit lower intracellular concentrations of hydrogen peroxide than the control strain when treated with exogenous hydrogen peroxide [22]. It was postulated that the membrane-shielding effects observed in cells with elevated levels of LC-PUFAs may be attributed to the inherent structure of phospholipids that contain these fatty acids. It is probable that phospholipids containing both LC-PUFAs and saturated fatty acids form more hydrophobic interfaces between the phospholipid bilayers. This hydrophobic interface could prevent the entry of hydrogen peroxide, thus protecting biomolecules contained within from damage.
Apart from the increased levels of mSOD and DPA, it is important to reiterate that the mitochondrial inner membranes of starved amoeba C. carolinense are rich in plasmalogen PC. This phospholipid can be preferentially oxidized to produce a relatively stable radical, thus inhibiting the chain reaction initiated by ROS [17]. Hence, the increased plasmalogen PC content in mitochondrial membranes is possibly another factor that makes cubic mitochondrial particles in starved amoeba C. carolinense a good shield against ROS.
Taken together, there is a strong possibility that the CMs in the starved amoeba C. carolinense cells play a protective role against ROS attack. When oxidative stress increases during starvation, the inner mitochondria membranes in amoeba C. carolinense transit into cubic conformation [6]. Possibly, this leads to the accumulation of miRNA and alike molecule strands within the convoluted spaces of the CMs, which contain high levels of plasmalogen PC and DPA [16]. These lipid components might be involved in the shielding mechanism. Furthermore, mSOD in starved amoeba C. carolinense makes the internal compartments of the cubic mitochondria a safe environment against ROS.
The proposed mechanism for the role of CMs in macromolecule protection such as miRNA and possibly other forms of RNA (figure 5) might be supported by results from the in vitro experiment. It can be observed in figure 4 that in the absence of cubic mitochondria, 8-OHdG levels were approximately four times as much as that in the presence of cubic mitochondria. This implies that ODNs in the mixture without cubic mitochondria suffer higher levels of oxidative damage by superoxide anions generated from the Fenton reaction than in the mixture with cubic mitochondria. Possibly, the protection of ODNs observed in this experiment is due to their accumulation within the convoluted channels of the CMs (figure 5). This is in line with the proposed mechanism as these ODNs were meant to represent short and single-stranded miRNA in the cell.
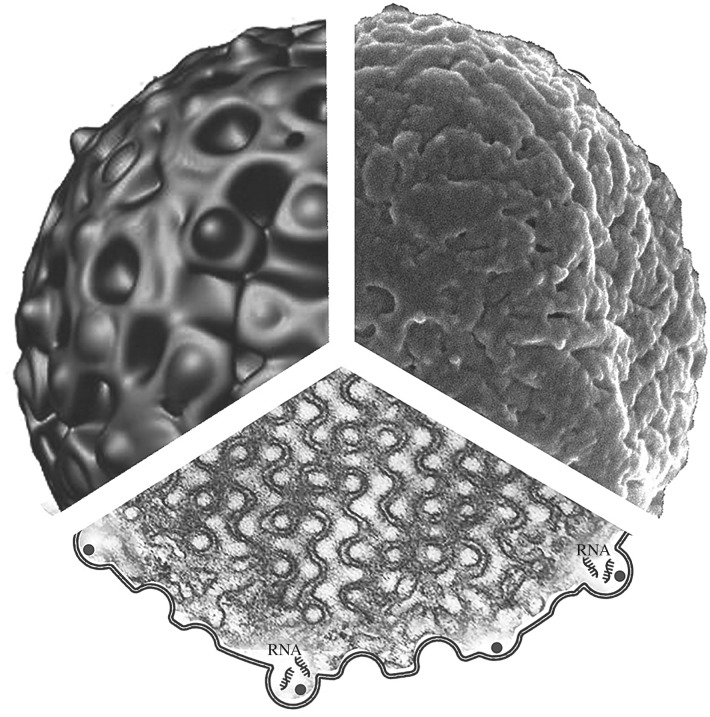
Figure 5.
The combined diagram of CM in a mitochondrion of starved amoeba C. carolinense. Upper left: a computer simulation of the outer surface of cubic mitochondrion particle matching (upper right) an original scanning electron micrograph of the same mitochondrion. Lower diagram illustrates the inner membrane organization of a mitochondrion with cubic architecture as seen in a transmission electron micrograph. The unique membrane organization may facilitate RNA retention within the convoluted channels of CMs and protect it from oxidative damage. Refer to the text for more details.
In conclusion, significantly low antioxidant enzymatic activities and intracellular lipid and RNA damage for a greater amount of free radical production were observed in starved amoeba C. carolinense when compared with the well-fed control. Thus, it seems unlikely that the antioxidant enzymes are the only protective mechanism involved in antioxidant defence system in amoeba C. carolinense. Possibly, some ‘unknown’ anti-ROS scavenger(s) induced in the amoeba C. carolinense system during starvation period is/are yet to be discovered. Moreover, the structural transition in the mitochondrial inner membrane to cubic organization seems to play an essential part in the cellular protective response to oxidative stress.
Acknowledgements
We thank Aik Kia Khaw for his artwork presented in figure 5. We also extend our gratitude to Ms Mei Yin Soong for her technical support in amoeba Chaos carolinense mass culture in this study.
Authors' contributions
E.L.-H.L. and K.C. were involved in the experimental protocols and design and applications to cubic membrane model system (amoeba Chaos carolinense). Y.D. and Z.A.A. conceived the research programme. Y.D. and Z.A.A. directed the investigations and composed the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by grants from BMRC, Singapore (R-185-000-197-305) and National Natural Science Foundation, China to Y.D. (grant no. 31670841).
References
- 1.Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, Reiter RJ. 2004. Regulation of antioxidant enzymes: a significant role for melatonin. J. Pineal Res. 36, 1–9. ( 10.1046/j.1600-079X.2003.00092.x) [DOI] [PubMed] [Google Scholar]
- 2.Payá M, Halliwell B, Hoult JR. 1992. Peroxyl radical scavenging by a series of coumarins. Free Radic. Res. Commun. 17, 293–298. ( 10.3109/10715769209079522) [DOI] [PubMed] [Google Scholar]
- 3.Halliwell B, Gutteridge J. 1990. The antioxidants of human extracellular fluids. Arch. Biochem. Biophys. 280, 1–8. ( 10.1016/0003-9861(90)90510-6) [DOI] [PubMed] [Google Scholar]
- 4.Deng Y, Almsherqi ZA, Ng MM, Kohlwein SD. 2010. Do viruses subvert cholesterol homeostasis to induce host cubic membranes? Trends Cell Biol. 20, 371–379. ( 10.1016/j.tcb.2010.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almsherqi ZA, McLachlan CS, Mossop P, Knoops K, Deng Y. 2005. Direct template matching reveals a host subcellular membrane gyroid cubic structure that is associated with SARS virus. Redox Rep. 10, 167–171. ( 10.1179/135100005X57373) [DOI] [PubMed] [Google Scholar]
- 6.Deng Y, Kohlwein SD, Mannella CA. 2002. Fasting induces cyanide-resistant respiration and oxidative stress in the amoeba Chaos carolinensis: implications for the cubic structural transition in mitochondrial membranes. Protoplasma 219, 160–167. ( 10.1007/s007090200017) [DOI] [PubMed] [Google Scholar]
- 7.Deng Y, Almsherqi ZA.. 2015. Evolution of cubic membranes as antioxidant defence system. Interface Focus 5, 20150012 ( 10.1098/rsfs.2015.0012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan OLL, Almsherqi ZA, Deng Y. 2005. A simple mass culture of the amoeba Chaos carolinense: revisit. Protistology 4, 185–190. [Google Scholar]
- 9.Chong K, Tan LL, Almsherqi Z, Lin Q, Kohlwein SD, Deng Y. 2015. Isolation of mitochondria with cubic membrane morphology reveals specific ionic requirements for the preservation of membrane structure. Protoplasma 252, 689–696. ( 10.1007/s00709-014-0698-9) [DOI] [PubMed] [Google Scholar]
- 10.Wei H, Cai Q, Rahn RO. 1996. Inhibition of UV light- and Fenton reaction-induced oxidative DNA damage by the soybean isoflavone genistein. Carcinogenesis 17, 73–77. ( 10.1093/carcin/17.1.73) [DOI] [PubMed] [Google Scholar]
- 11.Matos HR, Di Mascio P, Medeiros MH. 2000. Protective effect of lycopene on lipid peroxidation and oxidative DNA damage in cell culture. Arch. Biochem. Biophys. 383, 56–59. ( 10.1006/abbi.2000.2035) [DOI] [PubMed] [Google Scholar]
- 12.Linden A, Gülden M, Martin HJ, Maser E, Seibert H. 2008. Peroxide-induced cell death and lipid peroxidation in C6 glioma cells. Toxicol. In Vitro 22, 1371–1376. ( 10.1016/j.tiv.2008.02.003) [DOI] [PubMed] [Google Scholar]
- 13.Halliwell B, Chirico S.. 1993. Lipid peroxidation: its mechanism, measurement, and significance. Am. J. Clin. Nutr. 57, 715S–724S; discussion 724S-725S. [DOI] [PubMed] [Google Scholar]
- 14.Deng Y, Marko M, Buttle KF, Leith A, Mieczkowski M, Mannella CA. 1999. Cubic membrane structure in amoeba (Chaos carolinensis) mitochondria determined by electron microscopic tomography. J. Struct. Biol. 127, 231–239. ( 10.1006/jsbi.1999.4147) [DOI] [PubMed] [Google Scholar]
- 15.Almsherqi ZA, Landh T, Kohlwein SD, Deng Y. 2009. Cubic membranes: the missing dimension of cell membrane organization. Int. Rev. Cell Mol. Biol. 274, 275–342. ( 10.1016/S1937-6448(08)02006-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng Y, Almsherqi ZA, Shui G, Wenk MR, Kohlwein SD. 2009. Docosapentaenoic acid (DPA) is a critical determinant of cubic membrane formation in amoeba Chaos mitochondria. FASEB J. 23, 2866–2871. ( 10.1096/fj.09-130435) [DOI] [PubMed] [Google Scholar]
- 17.Zoeller RA, Lake AC, Nagan N, Gaposchkin DP, Legner MA, Lieberthal W. 1999. Plasmalogens as endogenous antioxidants: somatic cell mutants reveal the importance of the vinyl ether. Biochem. J. 338, 769–776. ( 10.1042/0264-6021:3380769) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sindelar PJ, Guan Z, Dallner G, Ernster L. 1999. The protective role of plasmalogens in iron-induced lipid peroxidation. Free Radic. Biol. Med. 26, 318–324. ( 10.1016/S0891-5849(98)00221-4) [DOI] [PubMed] [Google Scholar]
- 19.Brites P, Waterham HR, Wanders RJA. 2004. Functions and biosynthesis of plasmalogens in health and disease. Biochim. Biophys. Acta 1636, 219–231. ( 10.1016/j.bbalip.2003.12.010) [DOI] [PubMed] [Google Scholar]
- 20.Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD.. 1989. Molecular biology of the cell. New York, NY: Garland Publishing. [Google Scholar]
- 21.Richard D, Kefi K, Barbe U, Bausero P, Visioli F. 2008. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 57, 451–455. ( 10.1016/j.phrs.2008.05.002) [DOI] [PubMed] [Google Scholar]
- 22.Okuyama H, Orikasa Y, Nishida T. 2008. Significance of antioxidative functions of eicosapentaenoic and docosahexaenoic acids in marine microorganisms. Appl. Environ. Microbiol. 74, 570–574. ( 10.1128/AEM.02256-07) [DOI] [PMC free article] [PubMed] [Google Scholar]