Abstract
Biominerals are typically indispensable structures for their host organism in which they serve varying functions, such as mechanical support and protection, mineral storage, detoxification site, or as a sensor or optical guide. In this perspective article, we highlight the occurrence of both structural diversity and uniformity within these biogenic ceramics. For the first time, we demonstrate that the universality–diversity paradigm, which was initially introduced for proteins by Buehler et al. (Cranford & Buehler 2012 Biomateriomics; Cranford et al. 2013 Adv. Mater. 25, 802–824 (doi:10.1002/adma.201202553); Ackbarow & Buehler 2008 J. Comput. Theor. Nanosci. 5, 1193–1204 (doi:10.1166/jctn.2008.001); Buehler & Yung 2009 Nat. Mater. 8, 175–188 (doi:10.1038/nmat2387)), is also valid in the realm of biomineralization. A nanogranular composite structure is shared by most biominerals which rests on a common, non-classical crystal growth mechanism. The nanogranular composite structure affects various properties of the macroscale biogenic ceramic, a phenomenon we attribute to emergence. Emergence, in turn, is typical for hierarchically organized materials. This is a clear call to renew comparative studies of even distantly related biomineralizing organisms to identify further universal design motifs and their associated emergent properties. Such universal motifs with emergent macro-scale properties may represent an unparalleled toolbox for the efficient design of bioinspired functional materials.
Keywords: biomineralization, hierarchy, emergence, bioinspired materials
1. Introduction
In recent years, considerable scientific endeavour has been devoted to understanding and imitating the processes, structures and features of biological systems. The research field of biomimetic and bioinspired material design and synthesis has consequently prospered, as more and more material design concepts have been identified [1–6]. In nature, such concepts arise from the process of evolutionary optimization. This describes the inheritance of genotypes caused by fortuitous genetic variations combined with selection of those individuals which are better adapted to an external threat or to performing a vital task. In that sense, biological diversity can be regarded as a library of solutions each of which addresses the challenge of an external selective pressure. Despite being a standard optimization process, evolution does not result in convergence on a predetermined objective or set target. Evolution is a non-teleological process of adaptation and selection; the maximal degree of optimization is not (necessarily) reached, as the evolutionary driving force ceases as soon as the material's performance reaches a level where it sufficiently abates selection pressure. For biological materials, it is not only performance that is essential—the robustness and resilience of the material is of similar importance during evolutionary selection [7]. The level of complexity which is reached by such a non-teleological, unguided and unsighted sampling of the solution space is astounding.
Peptides and proteins represent an impressive and unparalleled example of a versatile, evolutionarily developed materials class. Despite the conspicuous diversity of their structure and functions, they feature on certain hierarchical levels a noticeable universality such as reoccurring motifs present in most proteins. This universality–diversity paradigm, as proposed by Buehler et al. for the case of proteins [8–11], is at first sight perplexing. By considering the hierarchical organization of proteins, the coexistence of diversity and universality within one class of biopolymers becomes comprehensible. In the next section, we will further clarify this concept using proteins as our archetype. After this explanation, we will examine whether a similar principle is found for biominerals, another important evolutionarily developed materials class. Are there fundamental, universally repetitive building blocks or design motifs which can ideally be found in all if not most biominerals? We will show that biominerals also obey the universality–diversity paradigm introduced by Buehler et al. even though biominerals—as inorganic solid-state components of the organism—are structurally disparate from proteins and show a fundamentally different hierarchical architecture.
2. Proteins teach us the universality–diversity paradigm
The simplest way to define a protein is to describe its primary structure; a polypeptide, or more simply a polymer, constituted of amino acids as monomers in a given sequence. Most proteins are constructed from a given set of 23 proteinogenic amino acids. This set can be extended by chemical modification of the amino acids, such as post-translational modification, or by addition of non-proteinogenic amino acids. From this diverse set of fundamental building blocks, certain folding motifs emerge on the next level of hierarchy, the level of secondary structures, such as the well-known α-helices or β-sheets. These architectures form by minimization of the conformational and interfacial energy of the protein, resulting from optimization of favourable torsion angles of the peptide backbone brought about by the steric demands of the amino acid side chains or structure-stabilizing hydrogen bonding. The secondary structure elements are a common and fundamental building block on this special level of hierarchy. When the diverse array of primary sequences from which these two architectures are formed, the ubiquity of these folding motifs becomes truly remarkable, this phenomenon demonstrates that diversity can merge into universality in a hierarchical system.
Buehler and co-workers [8,10] established and demonstrated the principle of the universality–diversity paradigm using three different intermediate proteinaceous filaments, vimentin, keratin and lamin as examples. These three filaments serve different functions at different loci within the organism, as outlined in figure 1. The primary sequence of the three filaments differs significantly, yet each sequence leads to the formation of an α-helix on the secondary level. Remarkably, this diversity in the primary sequence hardly affects the formation of the α-helical secondary structure, but it does impact on a higher-level, the quaternary structure, i.e. the dimer level. Vimentin, keratin and lamin, each form a so-called coiled-coil structure, where two α-helical strands are coiled together like the cords in a rope. On this level, we observe partial uncoiling as a function of the primary sequence, an effect that is often paraphrased as a ‘stutter’. These subtle structural differences translate to pronounced changes in the patterns of self-assembly; affecting and matching the properties of the filament network on the super-structural level, foremost the mechanical functions. Across the length-scales, we observe multiple changeovers from diverse to universal structural features and vice versa (figure 1 on the left). The three fibrous proteins are diverse and uniform at the same time and are excellent examples of materials obeying the universality–diversity paradigm.
Figure 1.
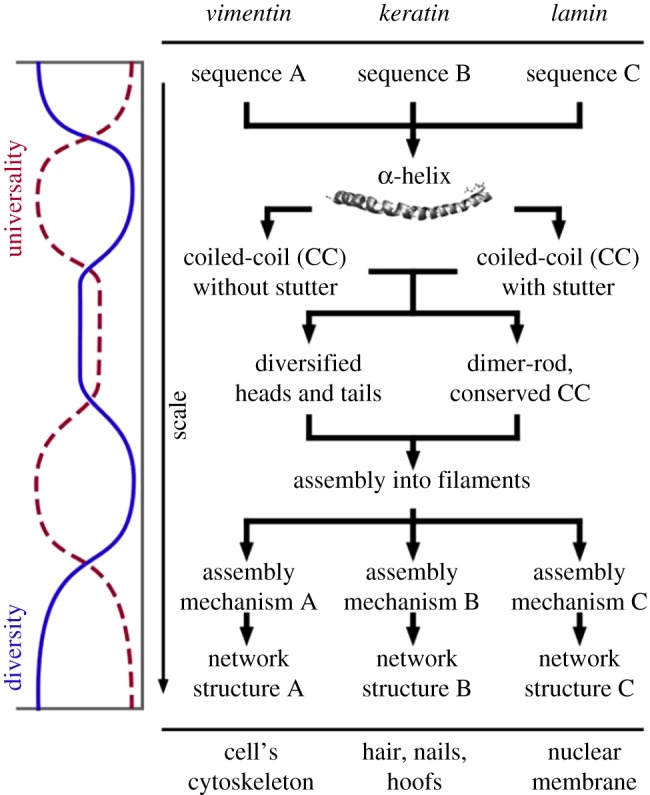
Hierarchical biological materials, here exemplified for the example of three types of intermediate filaments, are governed through the interplay of universal and diverse patterns, which combined with silencing and activation, are unified over multiple hierarchical scales. This enables to forward information that is completely coded at the lowest scale (amino acid sequence), safely by means of silencing through intermediate scales (α-helix, coiled-coil) up to higher scales, where they are activated in order to fulfil specific requirements. There is a balancing between universal features (e.g. α-helical structures, filament assembly) and diverse functionality. The plot on the left shows the balancing exchange between universality and diversity across scales. Reproduced with permission from Cranford & Buehler [8] (Copyright 2012 Springer); caption reproduced as stipulated by the licence agreement.
3. Coexistence of universality and diversity in biominerals?
In this section, we look to see if it is possible to extend the universality–diversity paradigm to the realm of biominerals. The ability to do so would aid biomimesis, as identification of universal structures would allow for targeting of methods for their synthesis. First, we examine biominerals to exemplify and document their extensive structural diversity. Second, we will search for a common, conserved element which demonstrates clear coexistence of universality and diversity in biominerals.
Biominerals are solid-state composite structures composed of an inorganic component and organic intra- and pericrystalline matrix, typically of proteinaceous origin. The fundamental, inorganic component of biominerals ranges from organism to organism, but is typically calcium carbonate, calcium phosphate, calcium sulfate, calcium oxalate, iron oxide, silica or silicate [12–15]. In addition to this degree of variety, the inorganic component is far from a homogeneous solid-state material. Instead, it characteristically incorporates a multitude of various dopant ions or consists of multiple different mineral phases, this variation is observed within a single species or a single microstructure [16,17]. The degree of inorganic diversity is shown clearly for the case of bone, the mineral phase of which is often named under the umbrella term hydroxyapatite. A closer look reveals that this is, however, an imprecise description of the inorganic component of bone. Besides minor ionic substitutions (such as 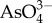 ,
, 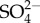 ,
, 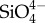 , K+, Na+, Mn2+, Ni2+, Cu2+, Co2+, Zn2+, Sr2+, Ba2+, Pb2+, Cd2+, etc.), replacement of phosphate anions by carbonate leading to B-type substitution is prevalent (4–8% by mass). Magnesium substitution into the calcium site also occurs, increasing the lattice strain and solubility of the bone apatite [18–22]. The manifold substitutions change the crystal lattice of bone apatite markedly, to the extent that X-ray diffraction patterns and infrared absorption spectra are distinct from those of pure geological apatite [23]. These indicate, among other details, a nearly complete lack of OH− in the crystal lattice, i.e. to call bone apatite, hydroxyl apatite has been criticized to be imprecise [18].
, K+, Na+, Mn2+, Ni2+, Cu2+, Co2+, Zn2+, Sr2+, Ba2+, Pb2+, Cd2+, etc.), replacement of phosphate anions by carbonate leading to B-type substitution is prevalent (4–8% by mass). Magnesium substitution into the calcium site also occurs, increasing the lattice strain and solubility of the bone apatite [18–22]. The manifold substitutions change the crystal lattice of bone apatite markedly, to the extent that X-ray diffraction patterns and infrared absorption spectra are distinct from those of pure geological apatite [23]. These indicate, among other details, a nearly complete lack of OH− in the crystal lattice, i.e. to call bone apatite, hydroxyl apatite has been criticized to be imprecise [18].
In addition to the diversity and complexity associated with the inorganic component, the intracrystalline and pericrystalline organic matrix of biominerals also exhibits diversity [12–15]. This material normally consists of proteins, polysaccharides or low-molecular weight molecules such as citrate or individual amino acids. The composition of the mineral-associated organic matrix changes from species to species; even between biominerals from related species with the same microstructure. This was demonstrated for the shell matrix proteins of Pinctada margaritifera by SDS/PAGE and mass spectroscopy analysis [24]. The shell of P. margaritifera is composed of two mineral layers, an outer calcitic prismatic layer adjacent to an inner aragonitic nacreous layer; both layers are surrounded by an insoluble organic matrix. These organic matrices were individually analysed to allow for a comparison of the protein content of both microstructures, with 48 proteins found in the prismatic layer and 33 in the nacreous layer. Of these, only three of the proteins identified were found in both microstructures. This demonstrates the diversity of proteins present in different mineral architectures. Furthermore, the protein content of a closely related oyster Pinctada maxima was studied in the same work. It was found to have 28 different proteins in the prismatic layer and 17 in the nacreous layer. Of these proteins, only two were identified in both microstructures. Moreover, 24 proteins were found to be common between the two prismatic layers and 15 between the two nacreous layers. Of the 80 different proteins identified in total in these closely related species, only three were present in all of the different organic matrices. This exemplary study clearly illustrates the proteomic diversity which makes efficient understanding and mimesis difficult. Diversity in the proteome is even more extensive when more distantly related species are analysed. In fact, the mineral-related proteome in calcareous shells (the ‘shellome’) exhibits no authoritative conservation of specific proteins or peptide motifs [24–31]. Moreover, it seems relatively typically for proteins belonging to the mineral-related proteome to have terminal sequences with an unfolded, labile and dynamic conformation [32], a trait that seems to be inherently linked to their unusual acidicity [33]. This lack of a defined and fixed secondary structure element enables these proteins to undergo an adaptive refolding upon interaction with a given target.
This overwhelming diversity on the level of fundamental constituents, both the organic and inorganic components, raises the question as to whether we can identify a commonality among biominerals which may pave the way to an efficient bioinspired material design. On the microstructural level, several different architectures, each possessing various subclasses of microstructure, are found in the class of bivalves (Mollusca) alone [34]. When the search is expanded beyond Mollusca, an even greater selection of microstructures is found, a handful of which are presented in figure 2a–c. This mass of diversified structures, spanning various length scales, presents a clear challenge for the identification of a structurally universal trait. The absence of such a structure, which would be auspicious for biomimesis, is prohibitive for the development of an overarching understanding of bioceramic materials.
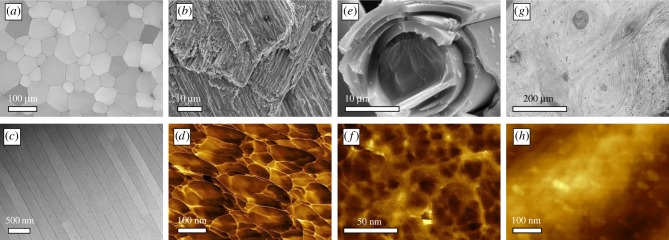
Figure 2.
Diverse microstructures and universal nanostructures in biominerals. (a) Optical micrographs of the prismatic layer of P. nobilis. (b) Electron microscopical image of crossed-lamellae in G. glycymeris. (c) STEM images of a nacreous layer in P. nobilis. (d) Atomic force microscopy reveals, here in the phase image, the nanogranules of which the prisms of P. nobilis consist. (e) The glass sponge Euplectella sp. also consists of similar granules; the phase image in (f) reveals that the silica grains are of smaller diameter as in its calcareous counterparts. (g) Bone also consists of nanoscale particles; here, the bone apatite nanoparticles are also distinctly smaller, as revealed by the height image (h).
Therefore, the question arises as to whether there is any conserved motif, shared structural feature, which unites biominerals? Is there structural universality at any length scale? Moreover, the ideal answer to this question should not only encompass the inorganic or organic constituents individually, the common structural feature should also reflect the composite design of biominerals and therefore embrace both of inorganic and organic components.
The advent of high-resolution imaging has allowed for the identification of such a structural commonality. Nanogranularity is an almost universal phenomenon in biominerals. It has been evidenced in both the nacreous plates and calcitic prisms of molluscs [35], the fibres of the siliceous sea sponge Euplectella [36], human bone and kidney stones [37,38] and other biogenic crystal structures formed across various taxa and phyla [39]. Numerous biogenic crystals, previously thought to be the lowest level of hierarchical organization of their respective microstructure, have been shown to in fact be space-filling bodies with nanogranular texture [39,40]. The size of the nanogranules varies from species to species and between the microstructures they comprise; nevertheless, they are typically in the range of 20–150 nm in diameter. Owing to the requirement for the nanogranules to tessellate such that they form a space-filling structure on the next hierarchical level, they are typically shaped like distorted spheres. The structure and composition of the granules themselves has been investigated by varying means. Atomic force microscopy imaging of polished, etched or fractured sections of biogenic crystals composed of nanogranules show a phase difference at the granule edge indicating the mineral granule comprises a mineral core enwrapped in a material of differing mechanical properties (figure 2d). TEM studies have shown that this core is of higher electron density than the sheath material [41,42]. These inferences, in combination with SS-NMR measurements, which have shown the presence of buried inorganic–organic interfaces in biominerals [43–45], have led to a consensus that the nanogranules are composed of a mineral core enwrapped in a sheath of organic molecules. The presence of organic molecules at the interface of the granules renders the microstructure component a composite structure, rather than a continuous object. The presence of this almost universal structure raises the question as to where it originates from. A possible model of formation, resting on classical models of crystallization, could describe the formation of this nanoscale composite material by ion-driven growth within a porous proteinaceous scaffold [42]. Biomimetic mineralization studies have, however, fundamentally changed our view of crystallization from supersaturated solutions in the presence of additives [46–52] and suggested additional crystallization pathways which could potentially give rise to the observed nanogranular fine structure. Initial studies of biomineralization and biomimetic crystallization were reliant on the concepts of classical crystallization theory. This theory describes mineral formation as a two-step process, initial nucleation and subsequent ion-by-ion growth [53]. These physical models were developed for crystals forming from a pure melt or from a supersaturated but passive solvent and they rest on several assumptions and an idealized model that ignores chemical processes which might interfere with the process of crystallization. This model has allowed for the derivation of a quantitative, descriptive physical model for idealized systems. Biomineralization, however, proceeds in confinement and in the presence of biopolymers, which often act as crystallization inhibitors or modifiers. Early biomimetic crystallization studies successfully demonstrated that theoretical models describing crystallization do not suffice in the case of non-ideal conditions. Subsequent experiments, especially those conducted in the presence of agents that act as crystallization inhibitors, have shown that this is not the case. The presence of crystallization inhibitors in solution opens pathways which, although they may lead to the formation of a single crystal, proceed via stabilized colloidal precursors [35,46,47,54–56]. These colloidal precursors then drive mineralization by accretion and assembly [39,49,57], and ion-by-ion growth becomes marginal. These pathways, which are still neither properly described in physical models nor sufficiently mechanistically understood, are condensed today under the notion of non-classical crystallization [49,52,58]. Importantly, such processes are known to result in the formation of crystals bearing a nanogranular texture [57]. With our increasing awareness of non-classical pathways, speculation has been raised that these processes may also take place in vivo [45,59,60]. Direct evidence of in vivo crystallization occurring via colloidal attachment processes has, however, only been shown recently for the case of nacre of the Mediterranean fan mussel Pinna nobilis [61]. By means of a high-resolution scanning tunnelling electron microscopy investigation on the nacroprismatic transition zone, Hovden et al. [61] showed that the first and still immature layers of nacre provide clear evidence for a particle-driven mode of crystal growth. During stages of incipient nacre growth, nanoparticles, which measure 50–80 nm in diameter, form relatively loose branched fibre-like extensions oriented in the growth direction of the shell. The coverage and number density of the nanoparticles progressively increases until a critical and sufficient particle number-density is reached, so that inter-particle fusion takes place resulting in dense, space-filling nacre platelets in continuum with aggregated nanoparticles. This study demonstrates that biomineral formation can occur by particle assembly and aggregation rather than via ion-by-ion growth. Moreover, the typical particle size observed in this study is identical to the mean particle sizes reported from atomic force microscopy studies [35,44,62] and allow the inference that nanogranular organization is a result of the non-classical, particle-mediated growth mode. Commensurate with this, some of the platelets formed have a nanogranular texture on their surface, a remnant of their formation process.
4. Biominerals join the universality–diversity paradigm
With nanogranularity as a universal structural motif, we propose the coexistence of structural diversity and unity in biominerals (figure 3). This allows biominerals to join the universality–diversity paradigm which Buehler and co-workers [8,10] proposed for proteins. Both the protein system and the biomineral system are diverse at their lowest hierarchical levels (differing primary sequences and inorganic ionic constituents). On the next hierarchical level, both systems converge towards universality (secondary structures and nanogranules) before again diverging on the above hierarchical levels (differing quaternary structures and various biomineral architectures). In the case of biominerals, this universality seems to originate from a fundamental mechanism of crystallization which occurs under the influence of additives which stabilize mineral particles at an early, colloidal stage. The presence of process-directing agents such as impurities and inhibitors shift the crystallization process from a classical to a non-classical route. As a consequence, colloidal interactions, aggregation, ripening processes and intermediate mineral precursor stages become key mechanisms of the mineral growth process. The generic involvement of an amorphous precursor allows us to unite the disparate bulk materials, regardless of their ionic composition, impurity content or level of biogenic additives. This gives us a universal structural motif on the meso- to nanoscale level. This fundamental building block encompasses both the organic and inorganic constituents of biominerals as the individual granules are covered by an organic ensheathment which dramatically affects the properties of the macroscopic biomineral. Moreover, for efficient biomimesis, it seems pertinent to target methods that result in the formation of this universal structural trait, as it is present in a majority of nature's functional architectures.
Figure 3.
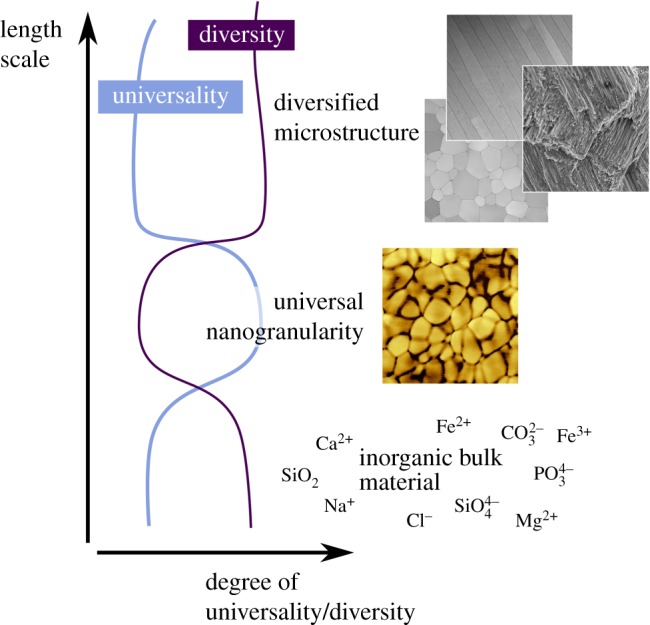
Universality–diversity coexistence in hierarchically organized biominerals. Despite the variability of the constituents, a universal nanogranularity is found across the species which builds up a diversity of microstructures. Inspired from [8].
5. Emergence from universal motifs: a guide for both structural analyses and biomimesis
Emergence is a phenomenon inherently linked to hierarchical materials. Pointedly stated in the well-known Aristotelian paraphrase that ‘the totality is not, as it were, a mere heap, but the whole is something besides the parts' (Metaphysics, Book VIII, 1045a.8–10). The emergent behaviour of a hierarchical system originates from the interplay between the individual elements and the result of this interplay cannot be attributed to individual parts. Detailed analysis of a system on only a single length scale may result in a failure to anticipate the impact of seemingly simple structures on non-adjacent hierarchical levels.
A perfect example for such behaviour may be the emergence of a ‘stutter’ in a coiled-coil structure [8,10,11], a phenomenon we introduced earlier to explain the dichotomy of universality and diversity in proteinaceous materials. The stutter is the local uncoiling of the superhelical structure which originates in peculiarities in the primary structure of the protein. This is then passed through the levels of hierarchy and emerges as a local dissociation of helical strands as a result of a lack of cooperativeness of the H-bridging patterns or steric demands of the individual strands. A localized steric incompatibility is not predictable on the basis of the analysis of an individual α-strand, nor is the genesis of such a stutter trivial to predict from the primary sequence of both strands. The genesis of this structural feature, which affects the mechanical properties of the macroscale material, arises by passing information through the hierarchy and length scales until it emerges as an important local structural variation.
When we examine biominerals, the question naturally arises as to whether emergent behaviour can also contribute to the macroscale material properties of biogenic solid-state materials. For the case of our universal motif, nanogranularity, extensive research efforts have shown that this is case. For instance, biominerals are constantly faced with the problem of dissolution due to the relatively low ionic supersaturation of the surrounding media. The use of nanograins as the building blocks at the lowest hierarchical level helps protect the host material against dissolution by invoking the effect of capillarity. Particles with a size <100 nm (i.e. within the capillary regime) show marked deviation from ideal dissolution behaviour because of the Gibbs–Thomson effect. However, the presence of a continuous organic matrix surrounding the nanograins protects against dissolution and can entrap granules of greater Gibbs free energy than those of the host material, such as amorphous grains [44,63–65]. The organic and intergranular matrix also impacts upon the mechanical properties of the nanogranular material. When cracks propagate through biominerals, the crack path is observed to be conchoidal, rather than following cleavage planes as observed for geological and synthetically produced crystals. This conchoidal fracture occurs even though biominerals are typically mesoscopically ordered such that they refract X-rays as a single crystal. The cracking behaviour arises from the nanogranular organization; cracks propagate between granules and through the organic matrix, thus taking a tortuous path, increasing the path length and concurrently the energy dissipation [39,66–71]. The proteinaceous intergranular matrix can, therefore, perform as a glue adhering granules together and increasing the work of fracture. Moreover, granule size can also impinge upon the mechanical behaviour of the host biomineral, the Griffith criterion states that below a certain granule size, a ceramic material approaches its maximal strength as the grains are too small to act as a stress concentrator, which would allow crack growth and would cause catastrophic failure. For biominerals, this has been shown to be roughly in the range of 20 nm, which is approximately the same size as a nanogranule, but above of the size of the components on the next hierarchical level [72,73]. Therefore, by tailoring the component granule size to within this regime, the mechanical properties of the host material are optimized [74].
The non-classical formation route, thought to underlie nanogranular materials, ensures the adaptivity and robustness of the biological mineralization system is increased. Changes in the chemical environment due to catastrophes, seasons or variable nutrition supply do not impinge on mineralization as would be the case in classical crystallization. Generation of a single crystal via classical ion by ion growth is drastically influenced by only a minor change in solution composition [53,75,76]. This may result in immense changes in reaction rates, morphology or phase selectivity. Relying on non-classical processes and exploiting a transient solid amorphous phase, which transforms later to a crystalline state in a solid-to-solid fashion (quasi a glass-ceramic process), renders biomineralization a resilient process for the fabrication of crystalline solid-state materials. Furthermore, formation of crystalline materials via amorphous phases allows for diversity of the inorganic and organic dopants contained in the final crystalline material. This diversity occurs because precipitation of amorphous phases in the presence of additives results in incorporation of the additive in the amorphous phase. Subsequent solid-state transformation of the amorphous to the crystalline phase entraps the dopants in the crystalline material.
All the enhanced properties described above demonstrate that macroscopic properties of a material can be affected by emergent behaviour of building blocks on a lower hierarchical level. In our special case of a universal building block, a number of the consequent emergent effects are relevant for all nanogranular biomineral systems regardless of the structural diversity at greater hierarchical levels. Thus, such universal structure elements may help us to efficiently direct our studies on biominerals and their specific properties. For the field of biomimetic material design, the benefit may be even larger. By exploiting universally occurring structures (such as nanogranules) as design elements in bioinspired material synthesis, the macroscale properties, which have been above shown to be affected by emergence in vivo, can be bestowed upon or at least enhanced in the final man-made solid-state material.
6. Conclusion and outlook
In this perspective article, we have established that biominerals feature a universality–diversity coexistence similar to other biogenic materials such as proteins. The dichotomy of universality and diversity in these biogenic inorganic solid-state materials is unquestionably facilitated by the hierarchical organization of biominerals. We suggest that universal nanogranular features of biominerals are rooted in a non-classical crystallization process exploited by organisms for formation of their solid inorganic constituents. These processes bestow the mineralized tissues not only with functionality but also provide a robust mineralization mechanism resilient against, for instance, externally triggered compositional changes.
We have additionally expounded that the identification of such universal structural elements is remarkably advantageous if they can give rise to emergent phenomena—another material concept immanent for hierarchical systems. The identification of such relationships between universal structures and their associated emergent properties may not only point at hidden and unidentified property-affecting structural features present in mineralized tissues of other species. Moreover, the mimesis of such a structure may allow us to bestow the emergent properties upon a synthetic material, enhancing the performance and features of the man-made material and guiding us to efficient bioinspired material design.
Future comparative studies will reveal further common and overarching design motifs and process–structure–property relationships in biominerals. Some may be readily identifiable, such as layered composite structures (lamellar bone versus nacre or even wood) which can be related/mimicked with a layer-wise deposition of the material with changing composition. The origin of others may be non-trivial to evidence, such as the source of interwoven structures in lobster and insect cuticles, bone and nacre which bear a remarkable resemblance to liquid crystalline structures [77–79]. Studies of this kind should also span the length scales in the search for generic motifs across hierarchical levels. Such studies will disclose stunning and concealed concepts for biominerals, for instance, the repetition of an ‘eternal’ structural motif across all scales. Biominerals generate their building blocks (e.g. nacre tablets, lamellae) from building blocks (e.g. grains), thus they build a composite material from a composite material of smaller scale. The eternal structural motif (the Lego™ blocks in figure 4) is simple: a highly anisotropic inorganic building block coated with an organic, polymeric material. The building blocks—near to one- or two-dimensional objects such as grains, plates or rods—tessellate and feature nanoscopic dimensions and/or a remarkable aspect ratio. The resulting high surface-to-volume ratio of the building blocks increases the organic–inorganic interface and, by this, augments the composite material behaviour. The building blocks are assembled into a larger element (e.g. grains to nacre tablets; or grains to laths and laths to lamellae in crossed-lamellar shells; or grains, platelets, and collagen fibres building up lamellar bone) which in turn is a highly anisotropic inorganic building block with an organic coating. By this approach, the biomineral maximizes the interaction between the inorganic majority component and the organic minority component. Moreover, this multi-scale composite material is able to counter an external threat (e.g. a fissure or crack due to a predator's attack) with an adequate functional structure of the very same length scale and maximizes by this the efficiency of its response.
Figure 4.
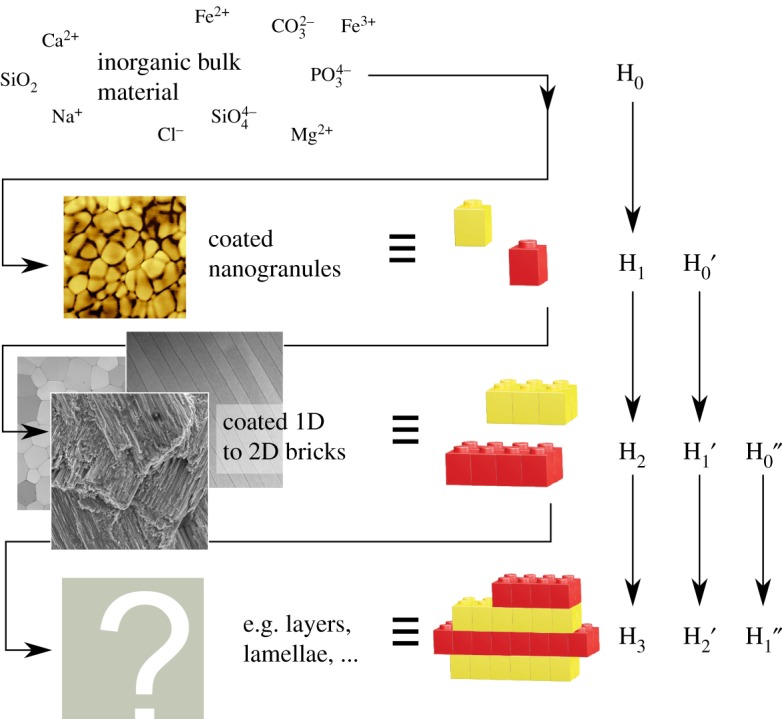
In analogy to proteins, biominerals use the same concept of creating building blocks from building blocks. In the protein world, the building blocks of the different hierarchy levels (H0 to HN) are distinct in their nature: amino acids form secondary structure elements (α-helices, β-sheets, etc.) which then form a super structure (e.g. a coiled-coil) and so forth. In biominerals, it seems that a reoccurring, eternal design motif (represented by using Lego™ blocks) is used throughout the hierarchy levels: inorganic building block with a high surface-to-volume ratio, coated with an energy-dissipative interelemental glue of organic nature. Depending on the requirement (e.g. the external threat, damage, or the material property under investigation), the material's response may be fully understood by neglecting subjacent levels, e.g. starting from H0′, taking this as the irreducible and thus fundamental element. Inspired from [8].
In consequence, we perceive that it is only with a holistic stance on biominerals that we will be able to decipher the inherent complexity of these biogenic inorganic solid-state materials. This can only happen if we realize that structure and performance may originate from various contributions across the length scales. The universality–diversity paradigm may be helpful in this venture. Only then we will be able to tap the full potential of biomimetic and bioinspired mineralization and material design and allow for emergent discoveries from our understanding of biominerals.
Authors' contributions
J.H. and S.E.W. drafted and wrote the manuscript, S.E.W. conceived, designed and coordinated the study. J.H. coordinated and carried out laboratory work; C.F.B. contributed to the laboratory work and participated in data analysis. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
S.E.W. is beholden to the German Research Foundation for generous financial support in the framework of an Emmy Noether starting grant DFG (No. WO1712/3-1). S.E.W. and J.H. acknowledge additional financial support from Cluster of Excellence 315 ‘Engineering of Advanced Materials—Hierarchical Structure Formation for Functional Devices' funded by the German Research Foundation.
References
- 1.Barthelat FF. 2007. Biomimetics for next generation materials. Phil. Trans. R. Soc. A 365, 2907–2919. ( 10.1098/rsta.2007.0006) [DOI] [PubMed] [Google Scholar]
- 2.Mann S. 1997. Biomineralization: the form(id)able part of bioinorganic chemistry! Dalt. Trans. 3953–3962. ( 10.1039/a704004k) [DOI] [Google Scholar]
- 3.Espinosa HD, Rim JE, Barthelat FF, Buehler MJ. 2009. Merger of structure and material in nacre and bone—perspectives on de novo biomimetic materials. Prog. Mater. Sci. 54, 1059–1100. ( 10.1016/j.pmatsci.2009.05.001) [DOI] [Google Scholar]
- 4.Chen P-Y, McKittrick J, Meyers MA. 2012. Biological materials: functional adaptations and bioinspired designs. Prog. Mater. Sci. 57, 1492–1704. ( 10.1016/j.pmatsci.2012.03.001) [DOI] [Google Scholar]
- 5.Meyers MA, Chen P-Y, Lin AY-M, Seki Y. 2008. Biological materials: structure and mechanical properties. Prog. Mater. Sci. 53, 1–206. ( 10.1016/j.pmatsci.2007.05.002) [DOI] [PubMed] [Google Scholar]
- 6.Meyers MA, Lin AYM, Seki Y, Chen P-Y, Kad BK, Bodde S. 2006. Structural biological composites: an overview. JOM 58, 35–41. ( 10.1007/s11837-006-0138-1) [DOI] [Google Scholar]
- 7.Csete ME, Doyle JC. 2002. Reverse engineering of biological complexity. Science 295, 1664–1669. ( 10.1126/science.1069981) [DOI] [PubMed] [Google Scholar]
- 8.Cranford SW, Buehler MJ. 2012. Biomateriomics. Berlin, Germany: Springer. [Google Scholar]
- 9.Cranford SW, De Boer J., Blitterswijk C, Buehler MJ, de Boer J, van Blitterswijk C, Buehler MJ. 2013. Materiomics: an -omics approach to biomaterials research. Adv. Mater. 25, 802–824. ( 10.1002/adma.201202553) [DOI] [PubMed] [Google Scholar]
- 10.Ackbarow T, Buehler MJ. 2008. Hierarchical coexistence of universality and diversity controls robustness and multi-functionality in protein materials. J. Comput. Theor. Nanosci. 5, 1193–1204. ( 10.1166/jctn.2008.001) [DOI] [Google Scholar]
- 11.Buehler MJ, Yung YC. 2009. Deformation and failure of protein materials in physiologically extreme conditions and disease. Nat. Mater. 8, 175–188. ( 10.1038/nmat2387) [DOI] [PubMed] [Google Scholar]
- 12.Westbroek P, Tanke-Visser J, De Vrind JP. M., Spuy R, van der Pol W, De Jong EW. 1983. Biomineralization and biological metal accumulation: biological and geological perspective, 4th edn Renesse, The Netherlands: D. Reidel Publishing Company. [Google Scholar]
- 13.Bäuerlein E. 2008. Handbook of biomineralization: biological aspects and structure formation. Weinheim, Germany: Wiley.
- 14.Mann S. 2001. Biomineralization: principles and concepts in bioinorganic materials chemistry. Oxford, UK: Oxford University Press. [Google Scholar]
- 15.Lowenstam HA, Weiner S. 1989. On biomineralization. Oxford, UK: Oxford University Press. [Google Scholar]
- 16.Weaver JC, et al. 2012. The stomatopod dactyl club: a formidable damage-tolerant biological hammer. Science 336, 1275–1280. ( 10.1126/science.1218764) [DOI] [PubMed] [Google Scholar]
- 17.Amini S, Masic A, Bertinetti L, Teguh JS, Herrin JS, Zhu X, Su H, Miserez A. 2014. Textured fluorapatite bonded to calcium sulphate strengthen stomatopod raptorial appendages. Nat. Commun. 5, 3187 ( 10.1038/ncomms4187) [DOI] [PubMed] [Google Scholar]
- 18.Wopenka B, Pasteris JD. 2005. A mineralogical perspective on the apatite in bone. Mater. Sci. Eng. C 25, 131–143. ( 10.1016/j.msec.2005.01.008) [DOI] [Google Scholar]
- 19.Dorozhkin SV, Epple M. 2002. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. Engl. 41, 3130–3146. ( 10.1002/1521-3773(20020902)41:17%3C3130::AID-ANIE3130%3E3.0.CO;2-1) [DOI] [PubMed] [Google Scholar]
- 20.Trueman CN, Tuross N. 2002. Trace elements in recent and fossil bone apatite. Rev. Miner. Geochem. 48, 489–521. ( 10.2138/rmg.2002.48.13) [DOI] [Google Scholar]
- 21.Pan Y, Fleet ME. 2002. Compositions of the apatite-group minerals: substitution mechanisms and controlling factors. Rev. Mineral. Geochem. 48, 13–49. ( 10.2138/rmg.2002.48.2) [DOI] [Google Scholar]
- 22.Gross KA, Berndt CC. 2002. Biomedical application of apatites. Rev. Mineral. Geochem. 48, 631–672. ( 10.2138/rmg.2002.48.17) [DOI] [Google Scholar]
- 23.LeGeros RZ, Trautz OR, Klein E, LeGeros JP. 1969. Two types of carbonate substitution in the apatite structure. Experientia 25, 5–7. ( 10.1007/BF01903856) [DOI] [PubMed] [Google Scholar]
- 24.Marie B, et al. 2012. Different secretory repertoires control the biomineralization processes of prism and nacre deposition of the pearl oyster shell. Proc. Natl Acad. Sci. USA 109, 20 986–20 991. ( 10.1073/pnas.1210552109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marin F, Roy N, Marie B, Ramos-Silva P, Wolf SE, Benhamada S, Guichard N, Immel F, Le Roy N. 2014. Synthesis of calcium carbonate biological materials: how many proteins are needed? Key Eng. Mater. 614, 52–61. [Google Scholar]
- 26.Marin F, et al. 2013. ‘Shellome’: proteins involved in mollusc shell biomineralization—diversity, functions. In Recent advances in pearl research (eds Watabe S, Maeyama K, Nagasawa H), pp. 149–166. Terrapub. [Google Scholar]
- 27.Marin F, Luquet G, Marie B, Medakovic D. 2008. Molluscan shell proteins: primary structure, origin, and evolution. Curr. Top. Dev. Biol. 80, 209–276. ( 10.1016/S0070-2153(07)80006-8) [DOI] [PubMed] [Google Scholar]
- 28.Kocot KM, Aguilera F, McDougall C, Jackson DJ, Degnan BM. 2016. Sea shell diversity and rapidly evolving secretomes: insights into the evolution of biomineralization. Front. Zool. 13, 23 ( 10.1186/s12983-016-0155-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson DJ, et al. 2010. Parallel evolution of nacre building gene sets in molluscs. Mol. Biol. Evol. 27, 591–608. ( 10.1093/molbev/msp278) [DOI] [PubMed] [Google Scholar]
- 30.Evans JS. 2012. Aragonite-associated biomineralization proteins are disordered and contain interactive motifs. Bioinformatics 28, 3182–3185. ( 10.1093/bioinformatics/bts604) [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto H., et al. 2013. The diversity of shell matrix proteins: genome-wide investigation of the pearl oyster, Pinctada fucata. Zool. Sci. 30, 801–816. ( 10.2108/zsj.30.801) [DOI] [PubMed] [Google Scholar]
- 32.Evans JS. 2008. ‘Tuning in’ to mollusk shell nacre- and prismatic-associated protein terminal sequences. Implications for biomineralization and the construction of high performance inorganic-organic composites. Chem. Rev. 108, 4455–4462. ( 10.1021/cr078251e) [DOI] [PubMed] [Google Scholar]
- 33.Marin F, Luquet G. 2007. Unusually acidic proteins in biomineralization. In Handbook of biomineralization: biological aspects and structure formation. Weinheim, Germany: Wiley-VCH Verlag GmbH. [Google Scholar]
- 34.Bøggild OB. 1930. The shell structure of the mollusks. København: A.F. Høst & søn. [Google Scholar]
- 35.Wolf SE, Lieberwirth I, Natalio F, Bardeau J-F, Delorme N, Emmerling F, Barrea R, Kappl M, Marin F. 2012. Merging models of biomineralisation with concepts of nonclassical crystallisation: is a liquid amorphous precursor involved in the formation of the prismatic layer of the Mediterranean fan mussel Pinna nobilis? Farad. Discov. 159, 433 ( 10.1039/b000000x) [DOI] [Google Scholar]
- 36.Aizenberg J, Weaver JC, Thanawala MS, Sundar VC, Morse DE, Fratzl P. 2005. Skeleton of Euplectella sp.: structural hierarchy from the nanoscale to the macroscale. Science 309, 275–278. ( 10.1126/science.1112255) [DOI] [PubMed] [Google Scholar]
- 37.Tai K, Ulm FJ, Ortiz C. 2006. Nanogranular origins of the strength of bone. Nano Lett. 6, 2520–2525. ( 10.1021/nl061877k) [DOI] [PubMed] [Google Scholar]
- 38.Sandersius S, Rez P. 2007. Morphology of crystals in calcium oxalate monohydrate kidney stones. Urol. Res. 35, 287–293. ( 10.1007/s00240-007-0115-3) [DOI] [PubMed] [Google Scholar]
- 39.Wolf SE, et al. 2016. Nonclassical crystallization in vivo et in vitro (I): process–structure–property relationships of nanogranular biominerals. J. Struct. Biol. 196, 260–287. ( 10.1016/j.jsb.2016.07.016) [DOI] [PubMed] [Google Scholar]
- 40.Yang L, Killian CE, Kunz M, Tamura N, Gilbert PU. 2011. Biomineral nanoparticles are space-filling. Nanoscale 3, 603–609. ( 10.1039/c0nr00697a) [DOI] [PubMed] [Google Scholar]
- 41.Suzuki M, Okumura T, Nagasawa H, Kogure T. 2011. Localization of intracrystalline organic macromolecules in mollusk shells. J. Cryst. Growth 337, 24–29. ( 10.1016/j.jcrysgro.2011.10.013) [DOI] [Google Scholar]
- 42.Jacob DE, Soldati A, Wirth R, Huth J, Wehrmeister U, Hofmeister W. 2008. Nanostructure, composition and mechanisms of bivalve shell growth. Geochim. Cosmochim. Acta 72, 5401–5415. ( 10.1016/j.gca.2008.08.019) [DOI] [Google Scholar]
- 43.Shir I Ben, Kababya S, Katz I, Pokroy B, Schmidt A, Ben Shir I., Katz I, Pokroy B, Schmidt A. 2013. Exposed and buried biomineral interfaces in the aragonitic shell of Perna canaliculus revealed by solid-state NMR. Chem. Mater. 25, 4595–4602. ( 10.1021/cm4028226) [DOI] [Google Scholar]
- 44.Wolf SE, Böhm CF, Harris J, Hajir M, Mondeshki M, Marin F. 2015. Single nanogranules preserve intracrystalline amorphicity in biominerals. Key Eng. Mater. 672, 47–59. [Google Scholar]
- 45.Ma Y, et al. 2012. Structure–property relationships of a biological mesocrystal in the adult sea urchin spine. Proc. Natl Acad. Sci. USA 109, 1–6. ( 10.1073/pnas.1109243109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gower LB, Tirrell DA. 1998. Calcium carbonate films and helices grown in solutions of poly(aspartate). J. Cryst. Growth 191, 153–160. ( 10.1016/S0022-0248(98)00002-5) [DOI] [Google Scholar]
- 47.Gower LB, Odom D. 2000. Deposition of calcium carbonate films by a polymer-induced liquid-precursor (PILP) process. J. Cryst. Growth 210, 719–734. ( 10.1016/S0022-0248(99)00749-6) [DOI] [Google Scholar]
- 48.Gower LB. 2008. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem. Rev. 108, 4551–4627. ( 10.1021/cr800443h) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cölfen H, Antonietti M. 2008. Mesocrystals and nonclassical crystallization. Chichester, UK: John Wiley & Sons Ltd. [Google Scholar]
- 50.Wolf SE, Gower LB. 2016. Challenges and perspectives of the polymer-induced liquid-precursor process: the pathway from liquid-condensed mineral precursors to mesocrystalline products. In New perspectives on mineral nucleation and growth (eds Kellermeier M, van Driessche A, Benning L, Gebauer D). Berlin, Germany: Springer. [Google Scholar]
- 51.De Yoreo JJJ. 2013. Crystal nucleation: more than one pathway. Nat. Mater. 12, 284–285. ( 10.1038/nmat3604) [DOI] [PubMed] [Google Scholar]
- 52.De Yoreo JJJ, et al. 2015. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 349, aaa6760 ( 10.1126/science.aaa6760) [DOI] [PubMed] [Google Scholar]
- 53.DeYoreo JJ, Vekilov PG, De Yoreo JJJ. 2003. Principles of crystal nucleation and growth. Rev. Miner. Geochem. 54, 57–93. ( 10.2113/0540057) [DOI] [Google Scholar]
- 54.Wolf SE, Leiterer J, Pipich V, Barrea R, Emmerling F, Tremel W. 2011. Strong stabilization of amorphous calcium carbonate emulsion by ovalbumin: gaining insight into the mechanism of ‘polymer-induced liquid precursor’ processes. J. Am. Chem. Soc. 133, 12 642–12 649. ( 10.1021/ja202622g) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gal A, Weiner S, Addadi L. 2015. A perspective on underlying crystal growth mechanisms in biomineralization: solution mediated growth versus nanosphere particle accretion. CrystEngComm 17, 2606–2615. ( 10.1039/C4CE01474J) [DOI] [Google Scholar]
- 56.Gal A, Habraken W, Gur D, Fratzl P, Weiner S, Addadi L. 2013. Calcite crystal growth by a solid-state transformation of stabilized amorphous calcium carbonate nanospheres in a hydrogel. Angew. Chem. Int. Ed. Engl. 125, 4967–4970. ( 10.1002/ange.201210329) [DOI] [PubMed] [Google Scholar]
- 57.Rodriguez-Navarro C, Ruiz-Agudo E, Harris J, Wolf SE. 2016. Nonclassical crystallization in vivo et in vitro (II): nanogranular features in biomimetic minerals disclose a general colloid-mediated crystal growth mechanism. J. Struct. Biol. 196, 260–287. ( 10.1016/j.jsb.2016.09.005) [DOI] [PubMed] [Google Scholar]
- 58.Wang T, Cölfen H, Antonietti M. 2005. Nonclassical crystallization: mesocrystals and morphology change of CaCO3 crystals in the presence of a polyelectrolyte additive. J. Am. Chem. Soc. 127, 3246–3247. ( 10.1021/ja045331g) [DOI] [PubMed] [Google Scholar]
- 59.Oaki Y, Kotachi A, Miura T, Imai H. 2006. Bridged nanocrystals in biominerals and their biomimetics: classical yet modern crystal growth on the nanoscale. Adv. Funct. Mater. 16, 1633–1639. ( 10.1002/adfm.200600262) [DOI] [Google Scholar]
- 60.Oaki Y, Imai H. 2006. Nanoengineering in echinoderms: the emergence of morphology from nanobricks. Small 2, 66–70. ( 10.1002/smll.200500246) [DOI] [PubMed] [Google Scholar]
- 61.Hovden R, Wolf SE, Holtz ME, Marin F, Muller DA, Estroff LA. 2015. Nanoscale assembly processes revealed in the nacroprismatic transition zone of Pinna nobilis mollusc shells. Nat. Commun. 6, 10097 ( 10.1038/ncomms10097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dauphin Y. 2003. Soluble organic matrices of the calcitic prismatic shell layers of two pteriomorphid bivalves. Pinna nobilis and Pinctada margaritifera. J. Biol. Chem. 278, 15 168–15 177. ( 10.1074/jbc.M204375200) [DOI] [PubMed] [Google Scholar]
- 63.Politi Y, Metzler R, Abrecht M, Gilbert B, Wilt FH, Sagi I, Addadi L, Weiner S, Gilbert PU. 2008. Transformation mechanism of amorphous calcium carbonate into calcite in the sea urchin larval spicule. Proc. Natl Acad. Sci. USA 105, 17 362–17 366. ( 10.1073/pnas.0806604105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Killian CE, et al. 2009. Mechanism of calcite co-orientation in the sea urchin tooth. J. Am. Chem. Soc. 131, 18 404–18 409. ( 10.1021/ja907063z) [DOI] [PubMed] [Google Scholar]
- 65.Olson IC, Blonsky AZ, Tamura N, Kunz M, Pokroy B, Romao CP, White MA, Gilbert PU.. 2013. Crystal nucleation and near-epitaxial growth in nacre. J. Struct. Biol. 184, 454–463. ( 10.1016/j.jsb.2013.10.002) [DOI] [PubMed] [Google Scholar]
- 66.Fantner GE, et al. 2005. Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nat. Mater. 4, 612–616. ( 10.1038/nmat1428) [DOI] [PubMed] [Google Scholar]
- 67.Huang Z, Li X. 2013. Origin of flaw-tolerance in nacre. Sci. Rep. 3, 1693 ( 10.1038/srep01693) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li X, Xu Z-H, Wang R. 2006. In situ observation of nanograin rotation and deformation in nacre. Nano Lett. 6, 2301–2304. ( 10.1021/nl061775u) [DOI] [PubMed] [Google Scholar]
- 69.Smith BL., et al. 1999. Molecular mechanistic origin of the toughness of natural adhesives, fibres and composites. Nature 399, 761–763. ( 10.1038/21607) [DOI] [Google Scholar]
- 70.Currey J. 2001. Sacrificial bonds heal bone. Nature 414, 699 ( 10.1038/414699a) [DOI] [PubMed] [Google Scholar]
- 71.Thompson JB, Kindt JH, Drake B, Hansma HG, Morse DE, Hansma PK. 2001. Bone indentation recovery time correlates with bond reforming time. Nature 414, 773–776. ( 10.1038/414773a) [DOI] [PubMed] [Google Scholar]
- 72.Griffith AA. 1921. The phenomena of rupture and flow in solids. Phil. Trans. R. Soc. Lond. A 221, 163–198. [Google Scholar]
- 73.Margolin L. 1984. A generalized Griffith criterion for crack propagation. Eng. Fract. Mech. 19, 539–543. ( 10.1016/0013-7944(84)90010-9) [DOI] [Google Scholar]
- 74.Gao H, Ji B, Jager IL, Arzt E, Fratzl P. 2003. Materials become insensitive to flaws at nanoscale: lessons from nature. Proc. Natl Acad. Sci. USA 100, 5597–5600. ( 10.1073/pnas.0631609100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Place T, Mullin JW. 2002. Crystallization and precipitation. Ullmann’s Encycl. Ind. Chem. 43, 25.1–25.40. ( 10.1002/14356007.b02) [DOI] [Google Scholar]
- 76.Vekilov PG. 2004. Solid phases from solution. Cryst. Growth Des. 4, 671–685. [Google Scholar]
- 77.Al-Sawalmih A, Li C, Siegel S, Fabritius H, Yi S, Raabe D, Fratzl P, Paris O. 2008. Microtexture and chitin/calcite orientation relationship in the mineralized exoskeleton of the American lobster. Adv. Funct. Mater. 18, 3307–3314. ( 10.1002/adfm.200800520) [DOI] [Google Scholar]
- 78.Palmer LC, Newcomb CJ, Kaltz SR, Spoerke ED, Stupp SI. 2008. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 108, 4754–4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cartwright JH. E, Checa AG. 2007. The dynamics of nacre self-assembly. J. R. Soc. Interface 4, 491–504. ( 10.1098/rsif.2006.0188) [DOI] [PMC free article] [PubMed] [Google Scholar]