Abstract
Pituitary adenomas represent the third most common primary intracranial tumor in neurosurgical practice. To understand the biological behaviour of the pituitary adenomas previous studies have determined the tumor proliferation rate using monoclonal antibodies targeted against the Ki-67 antigen. The aim of this study was to correlate the Ki-67 index with hormonal profiles of pituitary adenomas. The study included 50 pituitary adenomas. For histopathologic evaluation, the sections were stained with routine hematoxylin and eosin method. Additional paraffin sections from each tumor were immunostained using primary antibodies against the following pituitary hormones: somatotropin (STH), prolactin (PRL), adrenocorticotrophic hormone (ACTH), thyroid-stimulating hormone (TSH), follicle-stimulating hormone (FSH), and luteinizing hormone (LH). To detect the expression of Ki-67 we used a mouse anti-human monoclonal antibody (clone K2). The percentage of Ki-67 positive nuclei (Ki-67 labeling index) was assessed by counting approximately 1000 nuclei of the tumor cells at ×400 magnification. Out of the 50 tumor samples, 31 (62%) pituitary adenomas showed proliferative activity, and the proliferation rate was variable in this group. The overall mean Ki-67 labeling index was 1.59 ± 1.47, ranging from 0.3% to 6.6%. In 5 cases, the Ki-67 index was >3%, all of them being prolactinomas. The Ki-67 index was higher in PRL-secreting adenomas (mean ± SD was 3.37 ± 1.80, range 0.9 - 6.6%). Our study provides the evidence that a higher Ki-67 value is associated with pituitary adenomas that secrete PRL (prolactinomas and mixed STH/PRL-secreting adenomas).
KEY WORDS: Pituitary adenomas, Ki-67 labeling index, prognostic factor, prolactin, PRL, PRL-secreting adenoma
INTRODUCTION
Pituitary adenomas are the most common type of benign neoplasm arising in the sellar region, and account for approximately 10% of all intracranial tumors in adults. A high incidence is observed in patients aged between 30 to 60 years. Pituitary adenomas occur earlier in women (between 20 and 45 years) compared with men (between 35 and 60 years), and this is mainly due to a higher frequency of prolactinoma in younger women [1]. These tumors have a variable proliferation rate, invasion, and recurrence potential. Some of them are fast-growing, others remain dormant for a long period of time [2].
In earlier classification systems, pituitary adenomas were classified according to the tumor size (microadenomas <10 mm and macroadenomas >10 mm), cellular morphology, and cell affinity for basic or acidic dyes (acidophilic, basophilic, or chromophobe pituitary adenomas). Today, several subtypes of pituitary adenomas can be differentiated based on their hormonal profiles, using immunohistochemistry (IHC) [3]. For prognostic purposes, the diagnostic criteria for the group of atypical pituitary adenomas (APAs) are revised in the World Health Organization (WHO) classification of endocrine organ tumors from 2004. Moreover, according to the WHO classification of central nervous system tumors, APAs are characterized as tumors with the Ki-67 index of proliferation >3% and overexpression of tumor protein p53. In addition, APAs were considered as precursor lesions of rare pituitary carcinomas, representing the only malignant primary sellar tumor entity (0.2%) able to produce systemic or cerebrospinal metastases [4]. Pituitary carcinomas have been reported to metastasize to the cerebral cortex, cerebellum, spinal cord, cervical lymph nodes, liver, and ovaries. In most of the cases, pituitary carcinomas secrete adrenocorticotrophic hormone (ACTH) and prolactin (PRL) [5].
Several studies in this field have been focused on the identification of new markers that may predict the tumor behavior.
In pituitary adenomas, increased Ki-67 index has been observed in patients with high recurrence rates [2]. The Ki-67 antigen is a protein responsible for cell proliferation throughout the entire cell cycle [6]. The ability to predict the proliferative potential of pituitary adenomas may have major implications for clinical management. Currently, there is lack of accurate information about the association between the hormonal profile of pituitary adenomas and their proliferative index. The purpose of the present study was to investigate the relation between pituitary hormones and the rate of proliferation in pituitary adenomas.
MATERIALS AND METHODS
The study included 50 pituitary adenomas. The tissue specimens were fixed in 10% buffered formalin for 48 hours and paraffin embedded. Five micrometers thick serial sections were obtained from each paraffin block and the sections were mounted on silanized slides. The sections were stained with routine hematoxylin and eosin (H&E) method for histopathologic evaluation.
All procedures were done according to the principles embodied in the Declaration of Helsinki and approved by the Institutional Review Board of „Victor Babeş” University of Medicine and Pharmacy Timişoara.
Based on histopathological examination, sections from each sample were selected to determine their hormonal profiles and immunostained using the specific antibodies against pituitary hormones, including: somatotropin (STH), PRL, ACTH, thyroid-stimulating hormone (TSH), follicle-stimulating hormone (FSH), and luteinizing hormone (LH) [DAKO, Carpinteria, California, USA]. IHC workflow was completely automated, performed according to the existing protocols selected from the list of BondMax Autostainer (Leica Biosystems, Newcastle upon Tyne, UK), using a biotin-free system specific for this device. Briefly, according to the selected program, incubation with primary antibodies was followed by the use of Bond Polymer Refine Detection System with 3,3’-diaminobenzidine dihydrochloride applied as chromogen for 10 minutes, and hematoxylin applied for 5 minutes, as counterstain. The immunostaining results for each patient were graded as 0 (0-10% of positive cells), 1+ (11-30% of positive cells), 2+ (31-50% of positive cells), or 3+ (over 50% of positive cells). If more than 10% of hormone-positive cells was observed, the tumors were classified as secretory.
Heat-induced epitope retrieval with a ready-to-use Novocastra Bond Epitope Retrieval Solution 2, pH 9.0 solution (Leica Biosystems, Newcastle Ltd, Newcastle upon Tyne NE 12 8EW, UK) was performed for 20 minutes to detect the expression of Ki-67. Endogenous peroxidase blocking was achieved with 3% hydrogen peroxide for 5 minutes. Then, incubation with the primary antibody Ki67 (Leica Biosystems, Newcastle Ltd, Newcastle upon Tyne NE 12 8EW, UK) monoclonal mouse anti-human (clone K2, RTU) was performed for 20 minutes. The results were visualized with the Bond Polymer Refine Detection System using 3,3’-diaminobenzidine dihydrochloride as chromogen for 10 minutes and hematoxylin as counterstain for 5 minutes. The entire immunohistochemical procedure was performed on a Leica Bond-Max (Leica Biosystems, Newcastle upon Tyne, UK) autostainer.
Immunoreactivity was estimated as positive in the cells with nuclear expression. The percentage of Ki-67 positive nuclei (the Ki-67 labeling index) was assessed by counting approximately 1000 nuclei of the tumor cells at ×400 magnification. Microscopic evaluation was performed using Nikon Eclipse E600 Microscope (Nikon CEE GmbH Microscopes/Instruments Division, Austria, Europe). Image acquisition was performed with Coolpix 950 Digital Camera (Nikon CEE GmbH Microscopes/Instruments Division, Austria, Europe) followed by image analysis using Version 13 Lucia G software.
Statistical analysis
Statistical Package for the Social Sciences (SPSS) Statistics for Windows, Version 17.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Correlation analysis was done using Pearson’s chi-squared, Kendall rank correlation coefficient, and Spearman’s rank correlation coefficient tests. A value of p < 0.05 was considered statistically significant.
RESULTS
The results of histopathological evaluation of pituitary adenoma samples showed that solid pattern was most frequently observed (31/50; 62%), followed by trabecular (8/50; 16%), papillary (3/50; 6%), and mixed pattern (8/50; 3.2%) (Figure 1).
FIGURE 1.
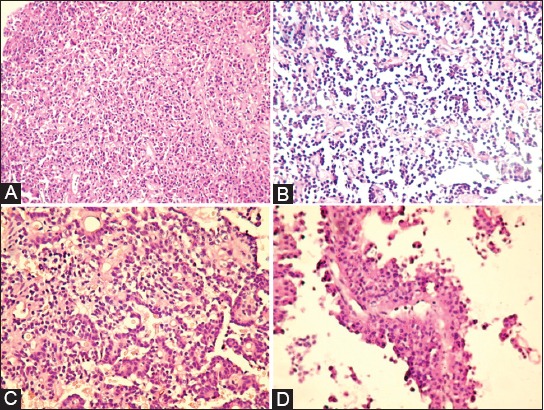
Histopathological features of pituitary adenomas: (A) solid pattern (B) trabecular pattern (C) mixed pattern (D) papillary pattern (Hematoxylin and eosin staining, magnification ×20).
Based on the IHC analysis, the following hormonal profiles were observed in the pituitary adenomas: 8 PRL-secreting adenomas (16%) (Figure 2), 13 STH-secreting adenomas (26%), 5 mixed STH/PRL-secreting adenomas (10%), 7 gonadotropin-secreting (LH-FSH) adenomas (14%), 13 null cell adenomas (non-secreting) (26%), and 4 plurihormonal adenomas (8%) which were represented by 2 subtypes (STH/PRL/ACTH and STH/PRL/LH).
FIGURE 2.
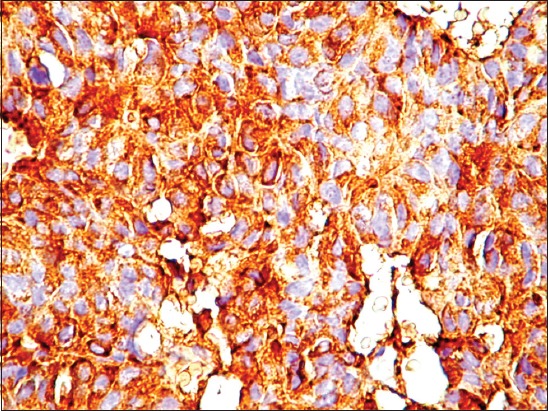
Cytoplasmic immunoreactivity for prolactin in pituitary adenoma samples (magnification ×40).
Out of the 50 tumor samples, 31 (62%) pituitary adenomas showed proliferative activity, and the proliferation rate was variable in this group. The overall mean Ki-67 labeling index was 1.59 ± 1.47, ranging from 0.3% to 6.6%. In 5 samples (all of them being prolactinomas), the Ki-67 index was >3%.
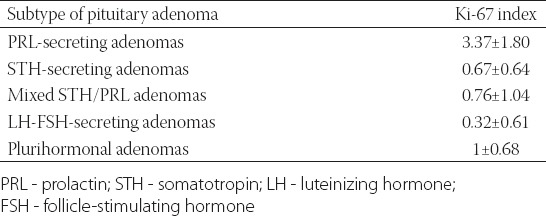
The Ki-67 index was higher in PRL-secreting adenomas (mean ± SD = 3.37 ± 1.80, range 0.9 - 6.6%). In STH-secreting adenomas, the Ki-67 index ranged from 0 to 2.1% (0.67 ± 0.64), for mixed STH/PRL-secreting adenomas the index ranged from 0 to 2.1% (0.76 ± 1.04). For LH-FSH-secreting adenomas the Ki-67 index ranged from 0 to 1.7% (0.32 ± 0.61), and five cases were negative. Plurihormonal adenomas showed a low proliferative index that ranged from 0 to 1.5 % (1 ± 0.68). Out of 13 null cell adenomas, 8 were negative; all samples ranged from 0 to 1.1% [0.28 ± 0.40] (Figure 3). The results of Ki-67 index in the pituitary adenoma samples are shown in Table 1.
FIGURE 3.
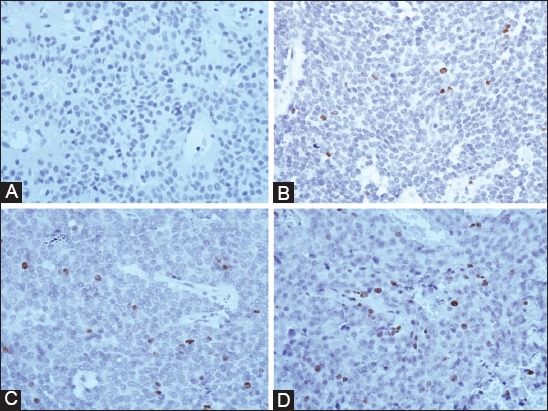
Immunostaining for Ki-67 antigen in pituitary adenoma samples. (A) Null cell adenoma (0% Ki-67 expression); (B) Prolactin (PRL)-secreting adenoma with 4.3% of Ki-67 expression; (C) PRL-secreting adenoma with 4.5% expression of Ki-67; (D) PRL-secreting adenoma with 6.6% expression of Ki-67.
TABLE 1.
Ki-67 labeling index (mean±SD) in pituitary adenoma samples according to their hormonal profiles
Finally, we correlated the Ki-67 index with hormone expression of the pituitary adenomas (0 to 3+). A significant direct correlation was observed between the Ki-67 index and tissue expression of prolactin (r = 0.048, p < 0.001). No other hormones showed significant correlation with the Ki-67 proliferation index (STH, p = 0.268; ACTH, p = 0.847; FSH, p = 0.479; LH, p = 0.170).
DISCUSSION
The first report about Ki-67 expression in pituitary adenomas and its potential role in these tumors was published in 1986, where the percentage of Ki-67 positive tumor cells varied between 0.2% and 1.5% [7]. Another study, conducted by Mastronardi et al. [8], has shown that the overall mean (SD) Ki-67 labeling index was 2.64 (3.69). Our Ki-67 labeling index was 1.59% and this is in agreement with data from the literature.
Recent series have reported that in some cases the Ki-67 index can be higher, 15.48% [9] or 23% [10]. It seems that the increased proliferation rate is associated with a more aggressive behavior in pituitary adenomas compared with the low proliferation rate, observed in noninvasive tumors [11]. Moreover, a significantly higher Ki-67 index was reported in younger patients versus older patients [12]. Mastronardi et al. [8] used the Ki-67 index to determine the invasive potential in pituitary adenomas by establishing two different thresholds: 3.5% for invasive adenomas and 5% for adenomas with invasion of the cavernous sinus. Recently, Miermeister et al. [13] suggested a new cut-off value for the Ki-67 index (>4%), as the best marker in diagnosis of atypical pituitary adenomas [13].
In most of the published studies, the Ki-67 proliferation index has been correlated with hormonal profile, size, and degree of tumor invasion. Variable expression of Ki-67 antigen, observed in different types of pituitary adenomas, suggests the implication of pituitary hormones in the proliferation potential of those tumors.
Thapar et al. [14] reported that hormone-secreting adenomas had a significantly higher mean Ki-67 labeling index (3.25%) than non-functioning adenomas (2.06%). The highest Ki-67 labeling index was found in prolactinomas [14]. In addition, other series, in which prolactinomas were studied, have demonstrated that a higher Ki-67 index is related to higher prolactin levels and larger macroprolactinomas [15-17]. In 2010, Pawlikowski et al. [18] suggested that plurihormonal adenomas, especially ACTH-secreting tumors, have a higher Ki-67 index, as compared to monohormonal tumors. A high Ki-67 index in ACTH-secreting adenomas was found in another study as well [9]. In the case of gonadotropin-secreting and null cell adenomas, other studies showed low levels of the Ki-67 proliferation marker [19,20]. A study performed on a relatively low number of pituitary carcinoma samples [15] concluded that the mean Ki-67 index was 2.6% for primary tumors and 11% for metastatic tumors [21].
In this study, prolactin appeared to be an important indicator of proliferative potential in pituitary adenomas compared with the other hormones, based on the highest value of the Ki-67 index observed in the PRL-secreting tumors.
In addition to the pituitary gland, the PRL gene expression has been confirmed in various regions of the brain, decidua, myometrium, thymus, spleen, circulating lymphocytes, lymphoid cells of bone marrow, and mammary epithelial cells and tumors [22].
PRL is involved in both, mammary gland development and lactation, and it may play a role in the malignant changes of the breast [22-24]. Also, prolactin and its receptors affect the immune system by inducing lymphocyte proliferation [25], and are involved in tumorigenesis of prostate cancer [26]. PRL receptors (PRLRs) are highly expressed in breast cancer, suggesting that the PRL pathway may be a potential therapeutic target in these tumors [24]. The relation between increased secretion of PRL and high Ki-67 proliferation index supports the role of Ki-67 proliferation index as a predictive marker for invasive tumors.
CONCLUSION
Our study provides the evidence that a higher Ki-67 value is associated with PRL-secreting pituitary adenomas, mainly prolactinomas and mixed GH/PRL-secreting adenomas. The high Ki-67 labeling index and PRL expression in tissue samples suggest the need for careful clinical and radiological follow-up. Variable expression of Ki-67 antigen in different immunophenotypes of pituitary adenomas suggests that pituitary hormones affect the tumor proliferation rate in a specific manner, despite controversial data reported in the literature regarding its utility as a predictive marker of recurrence in pituitary adenomas.
ACKNOWLEDGMENTS
This work was supported by project PII-C4- TC-2016 16441-07-ANGIOHIPOFACT of the “Victor Babes” University of Medicine and Pharmacy Timisoara, Romania.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- 1.Buurman H, Saeger W. Subclinical adenomas in postmortem pituitaries: Classification and correlations to clinical data. Eur J Endocrinol. 2006;154(5):753–8. doi: 10.1530/eje.1.02107. https://doi.org/10.1530/eje.1.02107. [DOI] [PubMed] [Google Scholar]
- 2.Kontogeorgos G. Predictive markers of pituitary adenoma behavior. Neuroendocrinology. 2006;83(3-4):179–88. doi: 10.1159/000095526. https://doi.org/10.1159/000095526. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd RV, Kovacs K, Young WF, Jr, Farell WE, Asa SL, Trouillas J, et al. Tumors of the pituitary. In: DeLellis RA, Lloyd RV, Heitz PU, Eng C, editors. Pathology and genetics of tumours of endocrine organs. France, Lyon: IARC Press; 2004. pp. 159–61. [Google Scholar]
- 4.Al-Shraim M, Asa SL. The 2004 World Health Organization classification of pituitary tumors: What is new? Acta Neuropathol. 2006;111(1):1–7. doi: 10.1007/s00401-005-1093-6. https://doi.org/10.1007/s00401-005-1093-6. [DOI] [PubMed] [Google Scholar]
- 5.Ragel BT, Couldwell WT. Pituitary carcinoma: A review of the literature. Neurosurg Focus. 2004;16(4):E7. doi: 10.3171/foc.2004.16.4.8. https://doi.org/10.3171/foc.2004.16.4.8. [DOI] [PubMed] [Google Scholar]
- 6.Scholzen T, Gerdes J. The Ki-67 protein: From the known and the unknown. J Cell Physiol. 2000;182(3):311–22. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. https://doi.org/10.1002/(SICI)1097-4652(200003)182: 3<311: AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Burger PC, Shibata T, Kleihues P. The use of the monoclonal antibody Ki-67 in the identification of proliferating cells: Application to surgical neuropathology. Am J Surg Pathol. 1986;10(9):611–7. doi: 10.1097/00000478-198609000-00003. https://doi.org/10.1097/00000478-198609000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Mastronardi L, Guiducci A, Spera C, Puzzilli F, Liberati F, Maira G. Ki-67 labelling index and invasiveness among anterior pituitary adenomas: Analysis of 103 cases using the MIB-1 monoclonal antibody. J Clin Pathol. 1999;52(2):107–11. doi: 10.1136/jcp.52.2.107. https://doi.org/10.1136/jcp.52.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pizarro CB, Oliveira MC, Coutinho LB, Ferreira NP. Measurement of Ki-67 antigen in 159 pituitary adenomas using the MIB-1 monoclonal antibody. Braz J Med Biol Res. 2004;37(2):235–43. doi: 10.1590/s0100-879x2004000200011. https://doi.org/10.1590/S0100-879X2004000200011. [DOI] [PubMed] [Google Scholar]
- 10.Losa M, Franzin A, Mangili F, Terreni MR, Barzaghi R, Veglia F, et al. Proliferation index of nonfunctioning pituitary adenomas: Correlations with clinical characteristics and long-term follow-up results. Neurosurgery. 2000;47(6):1313–9. https://doi.org/10.1097/00006123-200012000-00009. [PubMed] [Google Scholar]
- 11.Hentschel SJ, McCutcheon IE, Moore W, Durity FA. P53 and MIB-1 immunohistochemistry as predictors of the clinical behavior of nonfunctioning pituitary adenomas. Can J Neurol Sci. 2003;30(3):215–9. doi: 10.1017/s0317167100002614. https://doi.org/10.1017/S0317167100002614. [DOI] [PubMed] [Google Scholar]
- 12.Yonezawa K, Tamaki N, Kokunai T. Clinical features and growth fractions of pituitary adenomas. Surg Neurol. 1997;48(5):494–500. doi: 10.1016/s0090-3019(97)00102-x. https://doi.org/10.1016/S0090-3019(97)00102-X. [DOI] [PubMed] [Google Scholar]
- 13.Miermeister CP, Petersenn S, Buchfelder M, Fahlbusch R, Lüdecke DK, Hölsken A, et al. Histological criteria for atypical pituitary adenomas –data from the German pituitary adenoma registry suggests modifications. Acta Neuropathol Commun. 2015;3:50. doi: 10.1186/s40478-015-0229-8. https://doi.org/10.1186/s40478-015-0229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thapar K, Kovacs K, Scheithauer BW, Stefaneanu L, Horvath E, Pernicone PJ, et al. Proliferative activity and invasiveness among pituitary adenomas and carcinomas: An analysis using the MIB-1 antibody. Neurosurgery. 1996;38(1):99–107. doi: 10.1097/00006123-199601000-00024. https://doi.org/10.1097/00006123-199601000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Delgrange E, Trouillas J, Maiter D, Donckier J, Tourniaire J. Sex-related difference in the growth of prolactinomas: A clinical and proliferation marker study. J Clin Endocrinol Metab. 1997;82(7):2102–7. doi: 10.1210/jcem.82.7.4088. https://doi.org/10.1210/jc.82.7.2102. [DOI] [PubMed] [Google Scholar]
- 16.Ma W, Ikeda H, Yoshimoto T. Clinicopathologic study of 123 cases of prolactin-secreting pituitary adenomas with special reference to multihormone production and clonality of the adenomas. Cancer. 2002;95(2):258–66. doi: 10.1002/cncr.10676. https://doi.org/10.1002/cncr.10676. [DOI] [PubMed] [Google Scholar]
- 17.Paek K-II, Kim SH, Song SH, Choi SW, Koh HS, Youm JY, et al. Clinical significance of Ki-67 labeling index in pituitary macroadenoma. J Korean Med Sci. 2005;20(3):489–94. doi: 10.3346/jkms.2005.20.3.489. https://doi.org/10.3346/jkms.2005.20.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pawlikowski M, Kunert-Radek J, Radek M. Plurihormonality of pituitary adenomas in light of immunohistochemical studies. Endokrynol Pol. 2010;61(1):63–6. [PubMed] [Google Scholar]
- 19.Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, et al. The prevalence of pituitary adenomas: A systematic review. Cancer. 2004;101(3):613–9. doi: 10.1002/cncr.20412. https://doi.org/10.1002/cncr.20412. [DOI] [PubMed] [Google Scholar]
- 20.Prevedello DM, Jagannathan J, Jane JA, Jr, Lopes MB, Laws ER., Jr Relevance of high Ki-67 in pituitary adenomas. Case report and review of the literature. Neurosurg Focus. 2005;19(5):E11. doi: 10.3171/foc.2005.19.5.12. https://doi.org/10.3171/foc.2005.19.5.12. [DOI] [PubMed] [Google Scholar]
- 21.Pernicone PJ, Scheithauer BW, Sebo TJ, Kovacs KT, Horvath E, Young WF, Jr, et al. Pituitary carcinoma: A clinicopathologic study of 15 cases. Cancer. 1997;79(4):804–12. doi: 10.1002/(sici)1097-0142(19970215)79:4<804::aid-cncr18>3.0.co;2-3. https://doi.org/10.1002/(SICI)1097-0142(19970215)79: 4<804: AID-CNCR18>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Jonathan N, Mershon JL, Allen DL, Steinmetz RW. Extrapituitary prolactin: Distribution, regulation, functions, and clinical aspects. Endocr Rev. 1996;17(6):639–69. doi: 10.1210/edrv-17-6-639. https://doi.org/10.1210/edrv-17-6-639. [DOI] [PubMed] [Google Scholar]
- 23.Clevenger CV, Furth PA, Hankinson SE, Schuler LA. The role of prolactin in mammary carcinoma. Endocr Rev. 2003;24(1):1–27. doi: 10.1210/er.2001-0036. https://doi.org/10.1210/er.2001-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vonderhaar BK. Prolactin in human breast cancer development. In: Ethier SP, editor. Endocrine oncology. Totowa, NJ: Humana Press; 2000. pp. 101–20. https://doi.org/10.1007/978-1-59259-223-4_7. [Google Scholar]
- 25.Ignacak A, Kasztelnik M, Sliwa T, Korbut RA, Rajda K, Guzik TJ. Prolactin-not only lactotrophin. A “new” view of the “old” hormone. J Physiol Pharmacol. 2012;63(5):435–43. [PubMed] [Google Scholar]
- 26.Sackmann-Sala L, Chiche A, Mosquera-Garrote N, Boutillon F, Cordier C, Pourmir I, et al. Prolactin-induced prostate tumorigenesis links sustained Stat5 signaling with the amplification of basal/stem cells and emergence of putative luminal progenitors. Am J Pathol. 2014;184(11):3105–19. doi: 10.1016/j.ajpath.2014.07.020. https://doi.org/10.1016/j.ajpath.2014.07.020. [DOI] [PubMed] [Google Scholar]


