Abstract
Pair bonding leads to increases in dopamine D1 receptor (D1R) binding in the nucleus accumbens of monogamous prairie voles. In the current study, we hypothesized that there is similar upregulation of D1R in a monogamous primate, the titi monkey (Callicebus cupreus). Receptor binding of the D1R antagonist [11C]-SCH23390 was measured in male titi monkeys using PET scans before and after pairing with a female. We found that within-subject analyses of pairing show significant increases in D1R binding in the lateral septum, but not the nucleus accumbens, caudate, putamen, or ventral pallidum. The lateral septum is involved in a number of processes that may contribute to social behavior, including motivation, affect, reward, and reinforcement. This region also plays a role in pair bonding and paternal behavior in voles. Our observations of changes in D1R in the lateral septum, but not the nucleus accumbens, suggest that there may be broadly similar dopaminergic mechanisms underlying pair bonding across mammalian species, but that the specific changes to neural circuitry differ. This study is the first research to demonstrate neuroplasticity of the dopamine system following pair bonding in a non-human primate; however, substantial variability in the response to pairing suggests the utility of further research on the topic.
Keywords: Dopamine receptor, lateral septum, attachment, titi monkey, monogamy
Introduction
The dopamine (DA) system plays a key role in both the formation [Hostetler et al., 2011; Wang et al., 1999] and maintenance [Aragona et al., 2006] of pair bonds in monogamous prairie voles. The prairie vole model has been invaluable for identifying neuroendocrine systems underlying social attachment in mammals [Gobrogge and Wang, 2015; Young et al., 2010]. However, the specific neural mechanisms that regulate prairie vole pair bonding do not always generalize to other monogamous species [Fink et al., 2006; Turner et al., 2010]. Therefore it is important not to rely on a single animal model, but to maintain a comparative approach across many taxa in order to truly understand the biology and evolution of social attachment.
Titi monkeys (Callicebus cupreus) are monogamous New World primates that form selective pair bonds between mates and raise offspring biparentally [Mendoza and Mason, 1986b; Mendoza and Mason, 1997]. Pair bonding is characterized as a strong motivation to maintain proximity to a partner, distress upon separation from the partner, and selective aggression toward novel opposite-sex conspecifics. The neural substrates of pair bonding in titi monkeys appear to be generally similar to those identified in prairie voles. A previous brain imaging study found differences in glucose utilization in a number of brain regions between pair bonded versus lone-housed male titi monkeys [Bales et al., 2007]. Specifically, the regions affected by pair bonding were brain areas involved in social memory, reward, and limbic processes that have also been shown to play a role in prairie vole pair bonding. These included dopaminergic regions such as the nucleus accumbens (NAcc), lateral septum (LS), and ventral pallidum (VP).
While there appear to be similarities to rodent pair bonding, we have already identified a number of ways in which the neural substrates of titi monkey pair bonding may differ from that of voles. Titi monkeys have oxytocin receptor (OTR) and vasopressin receptor (V1aR) binding in quite different areas than do prairie voles [Freeman et al., 2014; Freeman and Young, 2016]. In particular, titi monkeys do not have OTR in the NAcc, an area strongly implicated in OT-DA interactions involved in prairie vole pair bonding. In voles, pair bonding leads to increases in D1 receptor binding in the NAcc shell, and this change is associated with direct effects on aggression towards intruders that might threaten the bond [Aragona et al., 2006], as well as indirect protective effects on drug-related behaviors [Liu et al., 2011]. In contrast to voles, titi monkeys have V1aR rather than OTR in the NAcc, and no OTR or V1aR at all in the ventral pallidum (VP) [Freeman et al., 2014]. However, titi monkeys do have OTR in the lateral septum [Freeman et al., 2014], an area also innervated by the dopamine system in primates [Sheehan et al., 2004].
In the current study, we hypothesized that there would be an upregulation of D1 receptors in pair bonded titi monkeys, particularly in areas that contain OTR or V1aR – the LS and NAcc. We investigated this hypothesis by measuring receptor binding of the D1 receptor antagonist [11C]-SCH23390 in male titi monkeys using PET scans before and after forming pair bonds. We focused on dopaminergic regions, predicting that we would be more likely to see changes in areas in which titi monkeys also have OTR or V1aR (LS and NAcc); rather than regions void of these receptors (VP, caudate, and putamen).
We also measured blood plasma levels of the hormones oxytocin (OT), arginine vasopressin (AVP), and cortisol. There is some evidence that plasma levels of neurohypophyseal peptides, including OT and AVP, may reflect central activity [Kenkel et al., 2012; Landgraf and Neumann, 2004]; at the least, they will represent the output of the pituitary. We measured OT and AVP to examine basal levels of these hormones as an indirect measure of potential central changes in these neuropeptide systems following pairing. Plasma cortisol was measured to assess the subjects' physiological status at the time of the scan.
Methods
Subjects
Thirteen captive-born and reproductively naïve adult male titi monkeys (Callicebus cupreus) housed at the California National Primate Research Center (CNPRC) in Davis, CA were used in this study. Subjects were removed from their natal group and housed alone for greater than one month prior to the beginning of the study. Animals were fed twice daily (0830 and 1330 h) a commercial primate diet (Mazuri) supplemented with rice cereal, banana, apples, raisins, and baby carrots at each feeding. Water was available ad libitum. Animals were housed in cages (2.13 m high × 1.27 m wide × 1.27 m deep) throughout the study [Jarcho et al., 2011; Mendoza, 1999; Tardif et al., 2006].
Experimental Design
All 13 males (mean±SE age: 4.09±0.35, range 2.2-5.5 years) underwent a baseline “unpaired” positron emission tomography (PET) scan. Males had been separate from their natal group and housed alone for at least one month to minimize the impact of separation from their attachment figure (the father). After the unpaired baseline scan (“Timepoint 1”), seven males (“Experimental group”) were paired with females and six males served as their unpaired age-matched controls (“Control group”: see experimental design in Figure 1). It is however, important to note that at all times during the study all males had visual, olfactory, and auditory access to other titi monkeys. “Unpaired” housing was thus not isolation housing. There is also evidence from field studies that males travel alone for a “bachelor” period after leaving their natal group, thus lending ethological validity to this control group (Bossuyt, unpublished data). Age-matched controls were included in order to be sure that the inherent change in age with a longitudinal design does not lead to confusion of changes associated with aging, with changes induced by pair-bonding.
Figure 1.
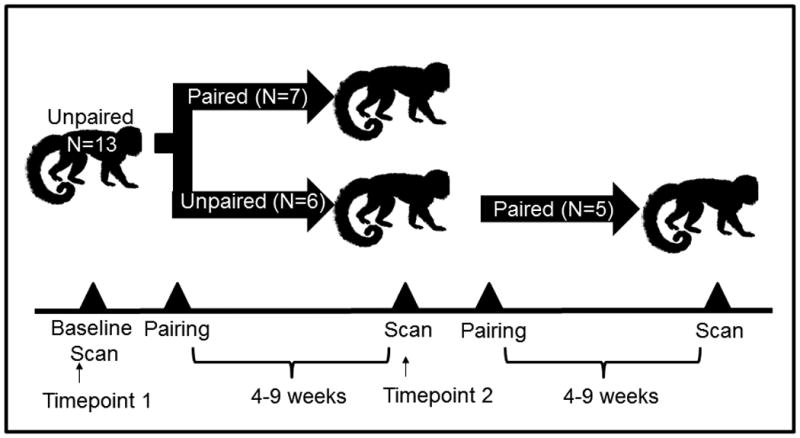
Study Design Schema. All subjects (n=13) were scanned in the “unpaired” condition prior to pairing (“Timepoint 1”). Six of these subjects (“Experimental group”) were paired while age-matched controls remained in the unpaired condition, and all underwent a second scan 4-9 weeks later (“Timepoint 2”). Finally, the Control group was paired and PET scanned 4-9 weeks post-pairing.
The newly-paired subjects and age-matched controls underwent another PET scan 4-8 weeks after pair formation (“Timepoint 2”; 6.0±0.45weeks post-pairing). After serving as age-matched controls, five of the six control males were paired with females and scanned 4-9 weeks after pairing (6.2±0.76 weeks post-pairing). All experimental procedures were approved by the Animal Care and Use Committee of the University of California, Davis; adhered to the legal requirements of the United States to the American Society of Primatologists Principles for the Ethical Treatment of Non Human Primates; and complied with National Institutes of Health ethical guidelines as set forth in the Guide for Lab Animal Care.
Preparation of [11C]SCH23390
[11C]CO2 was produced with a Siemens RDS111 cyclotron via the 14N(p,α)11C reaction. Typical yields for a 45 minute bombardment with a 40 μA beam were on the order of 1.5 Ci. [11C]CO2 was converted to [11C]CH3I in the gas phase, using a GE Tracerlab FX-C Pro synthesis unit. [11C]CH3I was bubbled into a freshly prepared slurry of precursor SCH24518 (ABX; 0.5 mg) and NaHCO3 (0.25 mg) in DMF (0.5 mL) at -20 °C. The reaction solution was heated to 50 °C and incubated for 7 minutes. [11C]SCH23390 was then purified by HPLC, using a Phenomenex C18 (5 μ) 250 × 10 mm column eluted in 65:35 (v/v) 0.1 M ammonium acetate (aq), pH 6.5/acetonitrile at 5 mL/min. In this system, the tR of precursor, major radioactive side product, and [11C]SCH23390, were 5.1, 7.7, and 9.4 minutes, respectively. The product peak was diluted in water (25 mL), applied to a Sep-Pak Plus C18 solid phase elution cartridge (Waters), washed in water (5 mL), and eluted in ethanol (2.5 mL). [11C]SCH23390 was dried at 80 °C under a gentle stream of helium, formulated in PBS, and filtered (0.22 μm; Millipore). Identity and purity were evaluated by analytical HPLC of [11C]SCH23390 coinjected with authentic 12C standard (ABX). The total synthesis time, including formulation, was 65 minutes. Typical decay-corrected yields from [11C]CH3I were 40 to 60%. The radiopurity of all doses was >95%, and the mean specific activity EOS was 2300 Ci/mmol.
Positron Emission Tomography (PET)
Males were fasted 6-12 h prior to the scan, with water available throughout the pre-scan period. On the day of the PET scan, the subject was caught and manually restrained for intramuscular administration of sedative [ketamine (25 mg/kg IM) and medetomidine (0.05 mg/kg IM)]. Anesthesia for the PET imaging was induced and maintained with isoflurane (1–2%) and the animal positioned in the microPET P4 scanner at the CNPRC. Image acquisition began immediately following administration of [11C]SCH23390 and scanned for 90 minutes to generate the dynamic binding activity of [11C]SCH23390. As a precautionary measure, the subject's EtCO2, oxygen saturation, heart rate and blood pressure were monitored throughout the scanning procedure [Bales et al., 2007].
Following imaging, subjects were maintained in metabolism cages for 24 h after scanning, at which time radiation decayed to background levels and animals returned to home cage. The 90 minute PET listmode data was binned (full 3D binning, span 3 and ring difference 31) into 24 frames (10 frames at 60s, 5 frames at 120s, 4 frames at 5 minutes and 5 frames at 10 minutes). Sinogram data was further reconstructed into PET images with a 3DRP reconstruction (vendor-supplied algorithm) resulting in images with an isotropic spatial resolution of approximately 1.8 mm [Qi et al., 1998]. Image voxels size was 1.8973 × 1.8973 × 1.2115 mm3. Because the head is small and similar across all subjects, and comparisons are made across the same structure in different animals, the PET data were not corrected for photon attenuation or scatter.
Blood sampling and hormone analysis
When animals were sedated for PET scanning blood samples were collected via femoral venipuncture. Immediately after collection blood samples were placed on ice. Blood samples were centrifuged at 3000 RPM for 15 minutes at 4° C. Plasma was extracted and stored at -70° C until assay. Plasma samples were assayed for OT, AVP, and cortisol. AVP and OT concentrations were estimated in duplicate using commercial enzyme immunoassay kits (Enzo Life Sciences, Farmingdale, NY) previously validated for titi monkeys [Bales et al., 2005]. Assay sensitivity was determined to be 2.34 pg/ml for AVP and 15.55 pg/ml for OT. Intra- and inter-assay coefficients of variation (CV) were 3.36% and 14.34% respectively for AVP, and 10.62% and 12.78%, respectively for OT. Plasma cortisol concentrations were estimated in duplicate using commercial radioimmunoassay kits (Siemens Healthcare, Malvern, PA). Prior to assay, samples were diluted 1:4 in PBS gel buffer. Assay procedures were modified with the addition of 0.5 and 2.35 μg/dl concentrations of standards along with the provided range of 1.0-49 μg/dl. Assay sensitivity has been determined to be 0.261 μg/dl. Intra- and inter-assay CV were 3.20% and 6.26%, respectively.
Magnetic Resonance Imaging (MRI)
Structural magnetic resonance imaging (MRI) scans were performed to define regions of interest for each subject. MRI scans were conducted in a GE Signa LX 9.1 scanner (General Electric Corporation, Milwaukee, WI) with a 1.5 T field strength and a 3” surface coil. Each male was fasted 8-12 h before the procedure. At the start of the procedure, the male was sedated with ketamine (10 mg/kg IM) and medazolam (0.1 mg/kg IM), and an endotracheal tube was placed. A catheter was also placed in the saphenous vein in order to administer fluids as necessary. Anesthesia was maintained with isoflurane (1-2%) while the male was positioned in the MRI scanner. Each scan lasted approximately 20 min and consisted of a 3D SPGR pulse sequence in a coronal plane. Images of the entire brain were collected using the following parameters: echo time TE=7.9 ms, repetition time TR=22.0 ms, flip angle=30.0°, field of view=8 cm, number of excitations=3, matrix=256×256, and slice thickness=1 mm. Similar to PET scanning, as a precautionary measure, the male's EtCO2, oxygen saturation, heart rate and blood pressure were monitored throughout.
Co-Registration of MRI and PET Scans and Calculation of Binding Potentials
The images were reconstructed using the 3DRP algorithm with no projection filter. There was no additional post-smoothing applied on the reconstructed images. The volume of the septum is about 2-3 PET voxels. Note that quantitative values will be in error due to the partial volume effect, but they typically are in PET imaging of the brain as most structures are small or similar in size to the resolution of the scanner. The current work is focused on group comparison using ANOVA. Given there is no size difference expected between groups and any systematic bias in binding potential estimates can be cancelled out in the group difference, the results on the statistical test are less affected than that of absolute quantification [Martinez et al., 2001].
Regions of interest (listed in Table 1) were drawn on structural MRI images using Siemen's Inveon Research Workplace software (IRW, Siemens Healthcare, USA) (Figure 2). These regions were examined as each has been implicated in either prairie vole or titi monkey pair bonding. MRI images were co-registered with PET scan images using automatic rigid registration in IRW. Binding potentials of [11C]SCH23390 to dopamine D1 receptors in the brain were calculated using a simplified reference tissue model (SRTM) and nonlinear least square fitting with the basis function method [Gunn et al., 1997]. The cerebellum was chosen as the reference region [Chan et al., 1998].
Table 1.
Demographic characteristics and hormone values of male titi monkeys at Timepoints (TP) 1 and 2. At Timepoint 1, all animals are unpaired. At Timepoint 2, Control animals remain unpaired while Experimental animals were 4-9 weeks post-pairing.
| Control (n=6) | Experimental (n=7) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (TP1) | Unpaired (TP2) | Baseline (TP1) | Paired (TP2) | |||||||||
| Age at Scan (years) | 4.11 | ± | 0.53 | 4.38 | ± | 0.57 | 4.08 | ± | 0.47 | 4.44 | ± | 0.48 |
| Cortisol (ug/dL) | 76.6 | ± | 11.2 | 64.9 | ± | 5.9 | 61.1 | ± | 4.7 | 42.0 | ± | 5.8* |
| AVP (pg/mL) | 389 | ± | 125 | 428 | ± | 147 | 342.0 | ± | 39.7 | 419.6 | ± | 81.9 |
| OT (pg/mL) | 802 | ± | 97 | 683 | ± | 114 | 783.8 | ± | 75.2 | 821.6 | ± | 57.7 |
indicates significant difference between groups, p<0.05.
Figure 2.
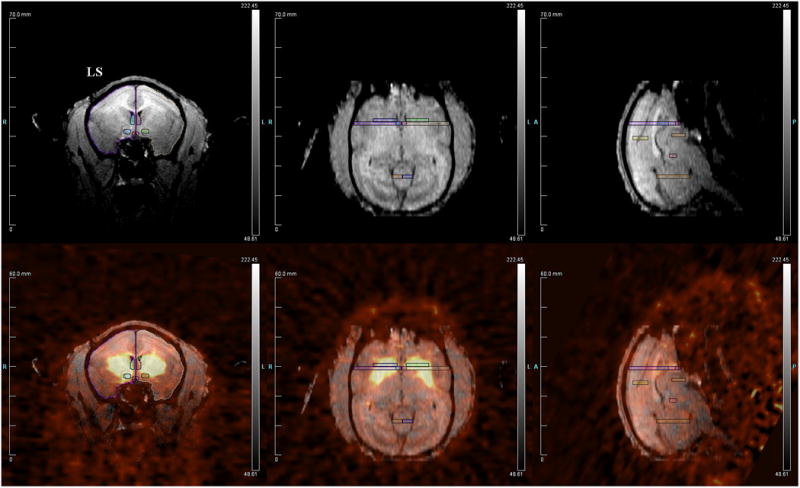
Representative figure showing: (top) titi monkey MRI with several regions of interest drawn; (bottom) MRI overlaid with PET data. LS = lateral septum.
Statistics
Two separate analyses were performed to investigate changes in [11C]SCH23390 receptor binding following pair bonding. One male (Animal 35892) from the Paired group showed a consistently aggressive dynamic with his pairmate. In fact, this pair was dissolved subsequent to the current study. We performed all analyses with and without this male, and his removal did not change the significance of any results. Therefore, all results are presented with him included.
We did not have any a priori reason to suspect that we would find differences between hemispheres. For this reason and to avoid potential Type I error, we combined the hemispheres for each ROI and examined only the five ROIs: LS, VP, NAcc, C, and P.
In the first analysis, we used repeated measures analysis to perform between-subjects comparison between males that had been paired with a female at the second timepoint (“Experimental,” n=7) and males that were unpaired at both timepoints (“Control”, n=6). This allowed us to assess whether group (“Control” versus “Paired”) differences existed in D1 receptor binding potential in the regions of interest. Within-subjects effects were also assessed at this point.
We then paired Control males, which allowed a within-subjects approach to investigate changes in D1 receptor binding potential following pairing. One male was not paired or given a final scan (due to a removal unrelated to the study), leading to a final sample size of 12 males. A mixed model ANOVA was performed with male ID as the random effect, using Baseline and Post-Pairing scans for all subjects. All tests were two-tailed and significance was set at p < 0.05.
Results
Analysis I (Between-subjects)
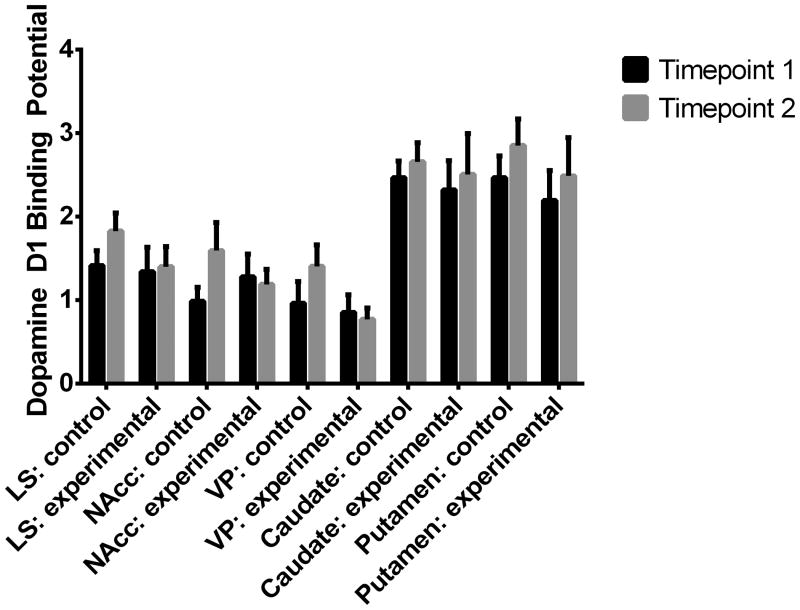
Control and Experimental animals did not differ in dopamine D1 receptor binding at either Timepoint 1 (“Baseline”), or Timepoint 2 (“Paired”) (p>0.05 for all measures). Means and standard errors (SE) for the between-subjects analysis are presented in Figure 3. For the LS, there was a significant effect of the repeated measure “male ID” (F = 6.62, p = 0.026). All other brain regions showed no difference in D1R BP between groups (Figure 3). Final sample size was n = 6 for control males and n =7 for experimental males.
Figure 3.
D1 receptor binding did not significantly differ in the Control or Experimental groups between Timepoint 1 (baseline) and Timepoint 2 (when the Experimental group had been paired), in any region of interest.
Animals housed with a pair-mate at Timepoint 2 had significantly lower plasma cortisol levels (n = 13, F=7.14, p=0.023) compared to those than remained unpaired. Neither plasma OT nor AVP differed between groups (p≥0.05).
Analysis II (Within-subjects)
All but one control animal was subsequently paired with a female and scanned for [11C]SCH23390 BP a third and final time. This allowed us to perform an additional within-subjects analysis between baseline and post-pairing [11C]SCH23390 binding in a total of 12 males. For the second PET scan, the average time since initial pairing with a female was 6.07 weeks (range: 4.3-9.1 weeks). Means and SE are presented in Table 2.
Table 2.
Demographic characteristics and hormone values of male titi monkeys in a within-subjects analysis.
| Baseline (n=12) | Post-Pairing (n = 12) | |||||
|---|---|---|---|---|---|---|
| Age at Scan (years) | 4.18 | ± | 0.4 | 4.63 | ± | 0.4 |
| Cortisol (ug/dL) | 64.4 | ± | 4.9 | 55.5 | ± | 7.2* |
| AVP (pg/mL) | 376.7 | ± | 62.4 | 370.9 | ± | 51.8 |
| OT (pg/mL) | 814.7 | ± | 57.7 | 790.7 | ± | 42.7 |
indicates significant difference between Timepoints, p<0.05; this difference was significant following removal of one male whose cortisol tripled (see Results section).
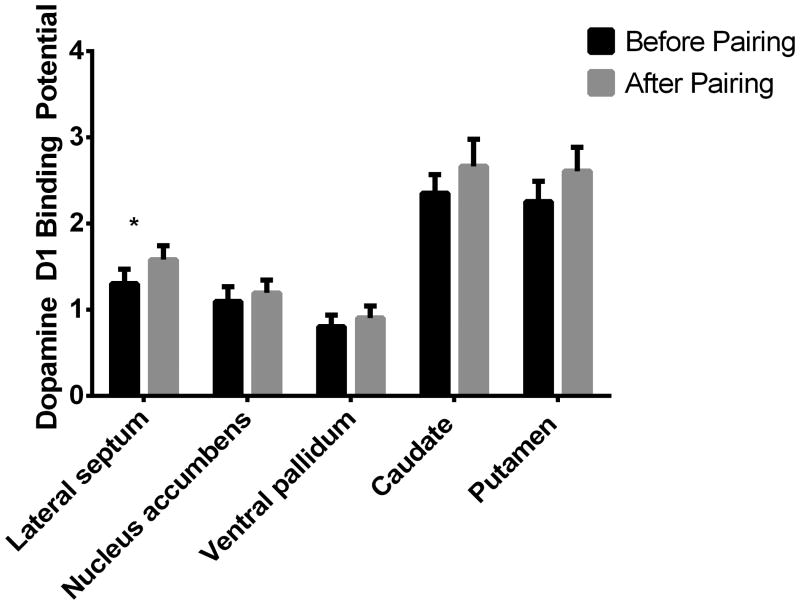
Dopamine D1 receptor binding was increased following pairing in the lateral septum (F = 4.97, p = 0.047; Figure 4). Effect size was Cohen's d = 0.4897, suggesting a medium effect size. Changes in D1 binding were characterized by significant variability between males: 4 of the 11 males experienced a decrease with pairing. No other brain regions showed a change in D1R BP.
Figure 4.
D1 receptor binding ([11C]-SCH23390) is significantly increased (*p<0.05) in the lateral septum after pair bonding, when analyzed in a within-subjects analysis.
Although plasma cortisol was reduced from Baseline to Post-Pairing (Table 2), this difference was not significant (F = 1.08, p=0.320). Upon closer examination of the data, the lack of significance appeared to be driven by a single individual whose cortisol nearly tripled from a baseline of 42.7 to 113.5 ug/dL following pairing. Removal of this individual from analysis yielded a highly significant (F = 10.41, p = 0.009) decrease in plasma cortisol following pairing (Baseline: 66.4±4.9 ug/dL; Post-Pairing: 50.2±5.4 ug/dL). Neither plasma OT nor AVP changed over time (p≥0.05).
Discussion
In this study we present findings that suggest that heterosexual pairing leads to site-specific increases in D1 receptor binding in a monogamous primate, the titi monkey. These findings are similar to observations of upregulation of D1 receptor binding in the NAcc shell of the monogamous prairie vole [Aragona et al., 2006]. In our data, we do see a suggestion of increased D1 receptor binding across most of the regions studied (Figure 4). However, in contrast to prairie voles, we observed statistically significant changes in D1 receptor binding in the lateral septum (LS), not the NAcc. This suggests that there may be broadly similar dopaminergic mechanisms underlying pair bonding across mammalian species, but that the specific changes to neural circuitry differ.
The LS is important for assessing the affective salience of sensory input and sending this information to limbic and reward-related regions. As such, this region is involved in a number of processes that may contribute to social behavior, including motivation, affect [Sheehan et al., 2004], reward and reinforcement [Olds and Milner, 1954]. The LS has been shown to play a role in social memory [Dantzer et al., 1988] and aggression [Clarke and File, 1982], both of which are required for pair bonding. We have also previously found that glucose utilization in the LS is affected by long-term pair bonding in the titi monkey [Bales et al., 2007].
Previous research has identified the LS as a key region for formation of pair bonds and parental behavior in prairie voles. Specifically, injection of AVP directly into the LS facilitates partner preference formation [Liu et al., 2001]. Partner preference is also inhibited by either V1a or OT receptor antagonists of in this region. Similarly, intraseptal AVP promotes, whereas V1a receptor blockade inhibits, parental behavior in sexually naïve male prairie voles [Wang et al., 1994]. Male voles also have changes in LS AVP-immunoreactivity following pair bonding that are dynamic across the female partner's reproductive status [Bamshad et al., 1993; Bamshad et al., 1994]. However, the specific role of the DA system in the LS remains unexplored in voles. The current findings suggest this may be an intriguing area for future research.
We did not observe an upregulation of D1R binding in the NAcc, as has been previously described in prairie voles [Aragona et al., 2006]. Dopamine's effects on pair bonding in voles are largely due to interactions with neuropeptide systems [Liu and Wang, 2003; Pitkow et al., 2001]. There are significant differences in OT and V1a receptor distribution between prairie voles [Insel and Shapiro, 1992; Insel et al., 1994] and titi monkeys [Freeman et al., 2014]. Anatomical differences in OT and AVP V1a receptor systems between voles and titi monkeys suggest that the specific role of neuropeptides in pair bonding would differ between these species [Freeman et al., 2014]. For voles, the dominant neuropeptide receptor in the NAcc is the OT receptor [Lim et al., 2004], whereas titi monkeys have no OT receptors but high V1a receptor binding in this region [Freeman et al., 2014]. There are OT receptors present in the LS of titi monkeys, which may make this region an alternate site for dopamine-oxytocin interactions that are associated with pair bonding [Freeman et al., 2014]. Therefore, underlying species differences in these neuropeptide systems may influence the specific (i.e. regional) role of dopamine on similar attachment behavior.
Given the role the LS plays in social memory and aggression, the observed increase in D1R may promote mate guarding behaviors in response to unfamiliar animals. We have observed increases in cerebral glucose metabolism in the LS when male titi monkey males view their female pair-mates next to a strange male [Maninger and Bales, unpublished data], which supports the hypothesis that upregulation of D1R in the LS facilitates mate guarding behavior. In prairie voles, the post-pairing increase in D1R is associated with aggression towards opposite-sex strangers, and is viewed as a mechanism for maintenance of the pair-bond [Aragona et al., 2006]. Although we did not include behavioral observations in the current study, such an approach is warranted in future research. Future studies should include measures of aggression and other mate guarding behaviors to see which may be correlated with changes in LS D1 receptor binding. We have now validated a method for measuring mate-guarding in titi monkeys [Fisher-Phelps et al., 2016].
Despite our discussion above, we are reluctant to overstate our findings regarding D1 binding in the LS. There were no differences in LS D1R when using a between-subjects analysis. This may be due to being statistically under-powered for the between-subjects analysis, given the level of variability. We did observe significant variability in the responses of individual males in the between subjects analysis, which argues for examining this variable again in a future, larger study and with attention to behavioral differences in the pair bonds.
Other caveats include the choice of control condition and sequence of treatments. Specifically, we chose to complete our initial scan while males were in an “unpaired” state rather than housed with another titi monkey, either their parents or another male. This choice was made because while housed in the natal group, titi monkeys show an attachment to their father [Hoffman et al., 1995; Mendoza and Mason, 1986b]. We were specifically seeking a control group that was not currently in an attachment relationship. It was also not possible to house the males with an unrelated male, as unrelated adult males are aggressive towards one another [Tardif et al., 2006]. However, the lack of an additional social control means that there is a possibility that the change we saw was due to a general change in social setting rather than the specific effect of pair-bond formation.
Finally, we used sequential rather than counter-balanced treatments (i.e., the unpaired condition always preceded the paired condition). This was chosen because we did not know whether or not changes in D1 binding induced by pairing would persist after a pair bond is ended, thus introducing the possibility of a carry-over effect post-pairing. It also introduces the possibility of grief at the loss of the pair-mate [Sun et al., 2014].
If the increases that we saw in D1 binding were caused by research design rather than pairing, the two most reasonable alternative hypotheses would be changes due to aging or to stress. The time between baseline to post-pairing scans was less than 6 months (Table 2); moreover, aging is normally associated with a decline in D1 binding, not an increase [MacDonald et al., 2012]. Stress is also a possibility, though not a particularly compelling explanation. One study in California mice found no effect of social defeat stress on D1 receptors in the only area examined, the NAcc [Campi et al., 2014]. Dopamine neurons, and specifically D1 receptors [Taylor et al., 2013], are involved in the arousal from anesthesia [Zhou et al., 2015]; and chronic stress can cause a reduction in dopamine transmission [Mizoguchi et al., 2000]. It is thus theoretically possible that reduced dopamine transmission caused by the stress of anesthesia could result in an up-regulation of D1 neurons. While we believe that this is not probable based on the less than chronic nature of our stressor, it provides testable predictions for future research.
In both analyses we also found that plasma cortisol decreased following pairing. This is consistent with previous findings in this species that heterosexual pairing decreases cortisol [Mendoza et al., 2000; Muth et al., 2015]. Conversely, separation from an attachment figure (such as the father or a mate) leads to increased cortisol which is reversed upon reunion [Mendoza et al., 2000; Mendoza and Mason, 1986a]. If the separation from the attachment figure is maintained, even in the presence of other familiar animals, elevations in cortisol are sustained. Moreover, other aspects of the stress response (e.g. acute activation of the HPA system) are similar in lone and paired animals, leading to the suggestion that the elevations in cortisol attendant to long-term separation are shifts in the regulation of basal cortisol [Mendoza et al., 2000]. Similar to these past studies, in the current study provision of an appropriate mate leads to a regulatory shift similar to return to the attachment [Mendoza et al., 2000; Mendoza and Mason, 1986a]. It is also worth noting that the conditions necessarily employed in this study were not ideal for examining changes in basal HPA activity. Exposure to novel environment activates the acute HPA stress response and masks social buffering effect to acute stress. Fasting likewise can lead to elevations in HPA activity [Dallman et al., 2004]. Thus, the down-regulation of HPA activity in paired animals across both analyses in the current study underscores the robust impact social bonding can have on physiological processes.
We do not believe that the differences in cortisol between groups account for D1R binding. This is based on research in prairie voles. In contrast to titi monkeys, chronic isolation in adult voles does not affect basal corticosterone [Grippo et al., 2007]. Therefore in voles, changes in D1R are independent of glucocorticoids, and it is likely that these measures are similarly dissociated in titi monkeys, but this should be clarified in future studies.
We did not observe any differences in plasma OT or AVP in either analysis. This suggests that there may be no major alterations in basal OT or AVP following pairing. This is not entirely surprising given that data from prairie voles indicate that these peptides are important for forming, whereas D1R play a role in maintenance of, pair bonds. Therefore we would not necessarily expect to see changes in OT or AVP under the current experimental conditions. It is also possible that our plasma measures do not reflect central activity, as the correlation between plasma and hypothalamic levels of neurohypophyseal peptides appears to be stimulus-specific [Kenkel et al., 2012; Landgraf and Neumann, 2004]. However, it is also pertinent to note that these hormones were assessed under extreme conditions, including fasting and a novel environment, and group differences may exist under undisturbed, basal conditions. Acute measurements of these neuropeptides in response to a social stimulus may prove more interesting [Kenkel et al., 2012]. Therefore, the present findings should not preclude further study of the central role of OT and AVP in pair bond maintenance in the titi monkey.
This study is the first research to demonstrate neuroplasticity of the dopamine system following pair bonding in a non-human primate. These findings also highlight the necessity of primate models for understanding the neuroscience of social behavior [Phillips et al., 2014]. Rodent models are useful for broadly identifying neural substrates of attachment and enabling nuanced experimental manipulation. Our findings in part replicate rodent research; however species differences exist for many molecular mechanisms of monogamy. Therefore examining these processes in non-human primates importantly extends our understanding of the evolution and biology of social behavior more systematically across mammals, with implications for the primate with which we are all most familiar: humans.
Acknowledgments
This work was supported by the National Institutes of Health under Grants HD053555 and OD011107; and the Good Nature Institute. We thank Jaleh Janatpour, Kevin Theis, Deborah Kent, Dr. Kari Christe, Dr. Angela Colagross-Schouten, and the fantastic staff in CNPRC Husbandry, Research Services, Division of Primate Medicine, and Jennifer Fung and Michelle Connell at the CMGI for contributions to this research project.
References
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang ZX. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nature Neuroscience. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Bales KL, Kramer KM, Hostetler CM, Capitanio JP, Mendoza SP. Validation of oxytocin and vasopressin blood assay for primates: what can blood tell us? American Journal of Primatology. 2005;66:73–73. [Google Scholar]
- Bales KL, Mason WA, Catana C, Cherry SR, Mendoza SP. Neural correlates of pair-bonding in a monogamous primate. Brain Research. 2007;1184:245–253. doi: 10.1016/j.brainres.2007.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamshad M, Novak MA, De Vries GJ. Sex and species differences in the vasopressin innervation of sexually naive and parental prairie voles, Microtus ochrogaster, and meadow voles, Microtus pennsylvanicus. Journal of Neuroendocrinology. 1993;5:247–255. doi: 10.1111/j.1365-2826.1993.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Novak MA, De Vries GJ. Cohabitation alters AVP innervation and paternal behavior in prairie voles (Microtus ochrogaster) Physiol Behav. 1994;56(4):751–758. doi: 10.1016/0031-9384(94)90238-0. [DOI] [PubMed] [Google Scholar]
- Campi KL, Greenberg GD, Kapoor A, Ziegler TE, Trainor BC. Sex differences in effects of dopamine D1 receptors on social withdrawal. Neuropharmacology. 2014;77:208–216. doi: 10.1016/j.neuropharm.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan GL, Holden JE, Stoessl AJ, Doudet DJ, Wang Y, Dobko T, Morrison KS, Huser JM, English C, Legg B, et al. Reproducibility of the distribution of carbon-11-SCH 23390, a dopamine D1 receptor tracer, in normal subjects. Journal of Nuclear Medicine. 1998;39:792–797. [PubMed] [Google Scholar]
- Clarke A, File SE. Selective neurotoxin lesions of the lateral septum: changes in social and aggressive behaviours. PharmacolBiochemBehav. 1982;17(4):623–628. doi: 10.1016/0091-3057(82)90334-3. [DOI] [PubMed] [Google Scholar]
- Dallman MF, la Fleur SE, Pecoraro NC, Gomez F, Houshyar H, Akana SF. Minireview: glucocorticoids - food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology. 2004;145:2633–2638. doi: 10.1210/en.2004-0037. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Koob GF, Bluthe RM, Le MM. Septal vasopressin modulates social memory in male rats. Brain Research. 1988;457:143–147. doi: 10.1016/0006-8993(88)90066-2. [DOI] [PubMed] [Google Scholar]
- Fink S, Excoffier L, Heckel G. Mammalian monogamy is not controlled by a single gene. Proceedings of the National Academy of Sciences. 2006;103:10956–10960. doi: 10.1073/pnas.0602380103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher-Phelps ML, Mendoza SP, Serna S, Griffin LL, Schaefer TJ, Jarcho MR, Ragen BJ, Goetze LR, Bales KL. Laboratory simulations of mate-guarding as a component of the pair-bond in male titi monkeys, Callicebus cupreus. American Journal of Primatology. 2016;78:573–582. doi: 10.1002/ajp.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Walum H, Inoue K, Smith AL, Goodman MM, Bales KL, Young LJ. Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus) Neuroscience. 2014;273:12–23. doi: 10.1016/j.neuroscience.2014.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Young LJ. Comparative perspectives on oxytocin and vasopressin receptor research in rodents and primates: Translational implications. Journal of Neuroendocrinology. 2016 doi: 10.1111/jne.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobrogge K, Wang Z. Neuropeptidergic regulation of pair-bonding and stress buffering: Lessons from voles. Hormones and Behavior. 2015;76 doi: 10.1016/j.yhbeh.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Cushing BS, Carter CS. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosomatic Medicine. 2007;69:149–157. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–287. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- Hoffman KA, Mendoza SP, Hennessy MB, Mason WA. Responses of infant titi monkeys, Callicebus moloch, to removal of one or both parents: Evidence for paternal attachment. Developmental Psychobiology. 1995;28:399–407. doi: 10.1002/dev.420280705. [DOI] [PubMed] [Google Scholar]
- Hostetler CM, Harkey SL, Krzywosinski T, Aragona BJ, Bales KL. Neonatal exposure to the D1 agonist SKF38393 inhibits pair bonding in the adult prairie vole. Behavioral Pharmacology. 2011;22:703–710. doi: 10.1097/FBP.0b013e32834affd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proceedings of the National Academy of Sciences. 1992;89(13):5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Wang ZX, Ferris CF. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. Journal of Neuroscience. 1994;14(9):5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho MR, Mendoza SP, Mason WA, Yang X, Bales KL. Intranasal vasopressin affects pair bonding and peripheral gene expression in male Callicebus cupreus. Genes, Brain, and Behavior. 2011;10:375–383. doi: 10.1111/j.1601-183X.2010.00677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel WM, Paredes J, Yee JR, Pournajafi-Nazarloo H, Bales KL, Carter CS. Neuroendocrine and behavioural responses to exposure to an infant in male prairie voles. Journal of Neuroendocrinology. 2012;24:874–886. doi: 10.1111/j.1365-2826.2012.02301.x. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Frontiers in Neuroendocrinology. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lim MM, Murphy AZ, Young LJ. Ventral striatopallidal oxytocin and vasopressin V1a receptors in the monogamous prairie vole (Microtus ochrogaster) JComp Neurol. 2004;468(4):555–570. doi: 10.1002/cne.10973. [DOI] [PubMed] [Google Scholar]
- Liu Y, Curtis JT, Wang ZX. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behavioral Neuroscience. 2001;115(4):910–919. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121(3):537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Liu Y, Young KA, Curtis JT, Aragona BJ, Wang Z. Social bonding decreases the rewarding properties of amphetamine through a dopamine D1 receptor-mediated mechanism. Journal of Neuroscience. 2011;31:7960–7966. doi: 10.1523/JNEUROSCI.1006-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SWS, Karlsson S, Rieckmann A, Nyberg L, Backman L. Aging-related increases in behavioral variability: relations to losses of dopamine D1 receptors. The Journal of Neuroscience. 2012;32:8186–8191. doi: 10.1523/JNEUROSCI.5474-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Hwang D-R, Mawlawi O, Slifstein M, Kent J, Simpson N, Parsey RV, Hashimoto T, Huang Y, Shinn A, et al. Differential occupancy of somatodendritic and postsynaptic 5HT1a receptors by pindolol: a dose-occupancy study with [11C]WAY 100635 and positron emission tomography in humans. Neuropsychopharmacology. 2001;24:209–229. doi: 10.1016/S0893-133X(00)00187-1. [DOI] [PubMed] [Google Scholar]
- Mendoza SP. Squirrel Monkeys. In: Poole T, editor. The UFAW Handbook on the Care and Management of Laboratory Animals. Seventh. Vol. 1. Oxford: Blackwell Science Ltd; 1999. pp. 591–600. [Google Scholar]
- Mendoza SP, Capitanio JP, Mason WA. Chronic social stress: studies in non-human primates. In: Moberg GP, Mench JA, editors. Biology of Animal Stress: Basic Principles and Implications for Animal Welfare. New York: CABI Publishing; 2000. pp. 227–247. [Google Scholar]
- Mendoza SP, Mason WA. Contrasting responses to intruders and to involuntary separation by monogamous and polygynous New World monkeys. Physiology & Behavior. 1986a;38:795–801. doi: 10.1016/0031-9384(86)90045-4. [DOI] [PubMed] [Google Scholar]
- Mendoza SP, Mason WA. Parental division of labour and differentiation of attachments in a monogamous primate (Callicebus cupreus) Animal Behaviour. 1986b;34:1336–1347. [Google Scholar]
- Mendoza SP, Mason WA. Attachment relationships in New World primates. Annals of the New York Academy of Sciences. 1997;807:203–209. doi: 10.1111/j.1749-6632.1997.tb51921.x. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chiu D-H, Tabira T. Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. The Journal of Neuroscience. 2000;20:1568–1574. doi: 10.1523/JNEUROSCI.20-04-01568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muth C, Bales KL, Hinde K, Maninger N, Mendoza SP, Ferrer E. Alternative models for small samples in psychological research: applying linear mixed effects models and generalized estimating equations to repeated measures data. Educational and Psychological Measurement. 2015;76:64–87. doi: 10.1177/0013164415580432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. Journal of Comparative and Physiological Psychology. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, 't Hart BA, Hopkins WD, Hu SL, Miller LA, Nader MA, et al. Why primate models matter. American Journal of Primatology. 2014;76:801–827. doi: 10.1002/ajp.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkow LJ, Sharer CA, Ren XL, Insel TR, Terwilliger EF, Young LJ. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. Journal of Neuroscience. 2001;21(18):7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Leahy RM, Cherry SR, Chatziioannou A, Farquhar TH. High resolution 3D Bayesian image reconstruction using the microPET small animal scanner. Physics in Medicine and Biology. 1998;43:1001–1013. doi: 10.1088/0031-9155/43/4/027. [DOI] [PubMed] [Google Scholar]
- Sheehan TP, Chambers RA, Russell DS. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Research Brain Research Reviews. 2004;46:71–117. doi: 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Sun P, Smith AS, Lei K, Liu Y, Wang Z. Breaking bonds in male prairie voles: long-term effects on emotional and social behavior, physiology, and neurochemistry. Behavioural Brain Research. 2014;265:22–31. doi: 10.1016/j.bbr.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif S, Bales K, Williams L, Moeller E, Abbott D, Schultz-Darken N, Mendoza S, Mason W, Bourgeois S, Ruiz J. Preparing New World monkeys for laboratory research. ILARJournal. 2006;47:307–315. doi: 10.1093/ilar.47.4.307. [DOI] [PubMed] [Google Scholar]
- Taylor NE, Chemali JJ, Brown EN, Solt K. Activation of D1 dopamine receptors induces emergence from isoflurane general anesthesia. Anesthesiology. 2013;118:30–39. doi: 10.1097/ALN.0b013e318278c896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner LM, Young AR, Rompler H, Schoneberg T, Phelps SM, Hoekstra H. Monogamy evolves through multiple mechanisms: evidence from V1aR in deer mice. Molecular Biology and Evolution. 2010;27:1269–1278. doi: 10.1093/molbev/msq013. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yu G, Cascio C, Lui Y, Gingrich B, Insel TR. Dopamine D2 receptor-mediated regulation of partner preferences in female prairie voles (Microtus ochrogaster): a mechanism for pair bonding? BehavNeurosci. 1999;113:602–611. doi: 10.1037//0735-7044.113.3.602. [DOI] [PubMed] [Google Scholar]
- Wang ZX, Ferris CF, Devries GJ. Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster) Proceedings of the National Academy of Sciences. 1994;91(1):400–404. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, Wang ZX. The neurobiology of pair-bonding: insights from a socially monogamous rodent. Frontiers in Neuroendocrinology. 2010 doi: 10.1016/j.yfrne.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Wang Y, Zhang C, Wang M, Zhang M, Yu L, Yan M. The role of dopaminergic VTA neurons in general anesthesia. PLoS One. 2015;10:e0138187. doi: 10.1371/journal.pone.0138187. [DOI] [PMC free article] [PubMed] [Google Scholar]