Abstract
Intranasal oxytocin affects a suite of human social behaviors, including trust, eye contact, and emotion recognition. However, it is unclear where oxytocin receptors (OXTR) and the structurally related vasopressin 1a receptors (AVPR1a) are expressed in the human brain. We have previously described a reliable, pharmacologically informed receptor autoradiography protocol for visualizing these receptors in postmortem primate brain tissue. We used this technique in human brainstem tissue to identify the neural targets of oxytocin and vasopressin. To determine binding selectivity of the OXTR radioligand and AVPR1a radioligand, sections were incubated in four conditions: radioligand alone, radioligand with the selective AVPR1a competitor SR49059, and radioligand with a low or high concentration of the selective OXTR competitor ALS-II-69. We found selective OXTR binding in the spinal trigeminal nucleus, a conserved region of OXTR expression in all primate species investigated to date. We found selective AVPR1a binding in the nucleus prepositus, an area implicated in eye gaze stabilization. The tissue's postmortem interval was not correlated with either the specific or nonspecific binding of either radioligand, indicating that it will not likely be a factor in similar postmortem studies. This study provides critical data for future studies of OXTR and AVPR1a in human brain tissue.
Keywords: brainstem, receptor autoradiography, neuropeptides, spinal trigeminal nucleus, nucleus prepositus
Introduction
Decades of research have shown that oxytocin (OT) can act as a potent neuromodulator in a variety of species to influence complex social behaviors, including social bonding (A. S. Smith, Ågmo, Birnie, & French, 2010; Young & Wang, 2004), social memory (Dantzer, Bluthé, Koob, & Le Moal, 1987; Ferguson, Aldag, Insel, & Young, 2001; Ferguson et al., 2000), affiliation (Rosenblum et al., 2002; Winslow, Noble, Lyons, & Sterk, 2003), and recently, social reward (Dölen, Darvishzadeh, Huang, & Malenka, 2013). In a growing number of studies (Young & Flanagan-Cato, 2012), administering intranasal OT to healthy humans can affect a suite of social behaviors, such as trust (Kosfeld, Heinrichs, Zak, Fischbacher, & Fehr, 2005), eye contact (Guastella, Mitchell, & Dadds, 2008), emotion recognition (Domes, Heinrichs, Michel, Berger, & Herpertz, 2007), socially-reinforced learning (Hurlemann et al., 2010), and pair-bonding-related behaviors (Scheele et al., 2012; 2013). Due to the ability of OT to modulate social function in animals as well as humans, the OT system has been highly implicated in the neurobiology and treatment of autism spectrum disorder (Modi & Young, 2012), a condition that is characterized in part by deficits in sociality. However, it is still unclear where oxytocin receptors (OXTR) and the structurally related vasopressin 1a receptors (AVPR1a) are expressed in the human brain. Thus, the plethora of intranasal OT studies has been conducted without knowledge of the neural targets of OT in the human brain or the mechanism by which intranasal OT enhances social function (Insel, 2016).
Mapping the locations of OXTR expression in human brain tissue has been a difficult endeavor due to the pharmacological cross reactivity between the OT and vasopressin (AVP) receptor systems. There is a high degree of structural homology between OT and AVP and between OXTR and the vasopressin 1a receptor (AVPR1a), which causes both neuropeptides to have a high affinity for each other's receptors. This mixed affinity is particularly high in humans and nonhuman primates in comparison to rodents (Manning et al., 2012). Similar to the neuropeptides themselves, most of the pharmacological tools that are currently available to study these receptors also have a high affinity for both OXTR and AVPR1a in humans (Freeman, Inoue, Smith, Goodman, & Young, 2014a; Manning et al., 2012). As a result, research on the basic physiology of OXTR and AVPR1a in primates has been markedly hindered, especially the fundamental neuroanatomical research to localize the distributions of these receptors in brain tissue.
Despite two early attempts to visualize OXTR and AVPR1a in human brain tissue using receptor autoradiography (Loup, Tribollet, Dubois-Dauphin, & Dreifuss, 1991; Loup, Tribollet, Dubois-Dauphin, Pizzolato, & Dreifuss, 1989), the problematic issue of pharmacological crosstalk in the OT and AVP systems has prevented adequate localization of these receptor subtypes in the human brain. Receptor autoradiography is the most common and reliable way to localize OXTR in rodent brain tissue, but the OXTR radioligand is now known to bind to both human OXTR and AVPR1a with a high affinity (Freeman, Inoue, Smith, Goodman, & Young, 2014a). At the time of publication of the human OXTR mapping paper over 20 years ago, the high cross-reactivity between OXTR and AVPR1a was not yet fully appreciated. As a result, the authors used concentrations of the radioligands and competitors that are by today's standards extremely high and should result in mixed binding to both OXTR and AVPR1a in human brain tissue. Thus, it is critical to revisit the results of this publication and revise the available localization techniques to disentangle the OXTR and AVPR1a distributions in human brain tissue.
Furthermore, there are currently no reliable, commercially available OXTR antibodies for immunohistochemistry (Yoshida et al., 2009). In a previous study, six different commercially available OXTR antibodies were used in brain tissue from wildtype mice and OXTR knock out mice. The pattern of staining produced by each of the antibodies was the same for both genotypes, which demonstrates that immunohistochemistry is not a reliable method for localizing OXTR in brain tissue (Yoshida et al., 2009). However, one study in human brain tissue used a novel, custom antibody to localize OXTR, but the authors did not conduct control experiments in brain tissue (Boccia, Petrusz, Suzuki, Marson, & Pedersen, 2013). A very recent publication using another novel antibody in mouse cortical tissue reported reliable OXTR staining that is not present in OXTR knock out mice (Marlin, Mitre, D'amour, Chao, & Froemke, 2015). While the results of these studies are promising, immunohistochemistry will not become a real option for localizing OXTR or AVPR1a until validated antibodies are widely available. In addition, despite recent efforts, there are currently no in vivo neuroimaging agents for OXTR for PET imaging, a technique that would be invaluable in research using humans and nonhuman primates (A. L. Smith et al., 2012; A. L. Smith, Freeman, Voll, Young, & Goodman, 2013a; 2013b).
In order to overcome these limitations on reliably detecting OXTR in primate tissue, we have previously designed a reliable, pharmacologically informed protocol for visualizing OXTR in postmortem primate brain tissue by determining the precise concentrations at which these ligands can be used to selectively occupy one receptor over the other (Freeman, Inoue, Smith, Goodman, & Young, 2014a). This pharmacologically optimized modification for OXTR receptor autoradiography is the first reliable technique for identifying OXTR (and AVPR1a) in human and nonhuman primate brain tissue.
In the current study, we use this optimized technique in human brainstem tissue in order to determine whether this approach is selective for detecting OXTR in the human brain. We chose to use blocks of brainstem tissue because it contains the spinal trigeminal nucleus, which is a region that should serve as a positive control based on comparative work across other primates. The spinal trigeminal nucleus in the brainstem has been shown to express OXTR across all primate species that have had OXTR distributions mapped to date (Freeman, Inoue, Smith, Goodman, & Young, 2014a; Freeman, Walum, Inoue, Smith, Goodman, Bales, et al., 2014b; Schorscher-Petcu, Dupré, & Tribollet, 2009), and the early autoradiographic studies in human tissue also detected a signal in this regions of the brainstem (Loup et al., 1989). Thus, the current study sought to use brainstem tissue containing this region in order to validate our technique for use in human brain tissue more broadly and to establish where OXTR and AVPR1a are expressed in adjacent regions in the human brainstem.
Methods
Specimens
Unfixed, frozen blocks (n=7) of de-identified human brainstem at the level of the medulla were provided by the University of Maryland Brain and Tissue Bank, which is a Brain and Tissue Repository of the NIH Neurobiobank. All specimens came from male donors aged 15.3 to 45.9 years (with a mean±SEM of 29.2±4.6 years). These specimens varied in postmortem interval (PMI) in order to assess the potential effect of PMI on receptor binding (PMI ranged from 5h to 33h with a mean±SEM of 15±4.2h and median of 9h). The details of this proposal were reviewed by the Institutional Review Board (IRB) at the University of California-Davis and were found not to meet criteria for human subjects research. The UC-Davis Institutional Biosafety Committee has approved this research under the Biological Use Authorization #R1840.
Tissue preparation
Detailed information about the postmortem tissue processing protocol is provided online by the NICHD Brain Bank. Briefly, brains were chilled in wet ice prior to sectioning. The medulla was removed and sectioned separately. The entire brain was sectioned into left and right hemispheres. The right hemisphere was fixed in 10% formalin. The midbrain/pons/cerebellum were removed from the left hemisphere. Then, the cerebrum was blocked coronally at 1 cm intervals. The midbrain/pons was sectioned at 0.03 cm sections. All tissue blocks were frozen in isopentane/dry ice or liquid nitrogen, placed in sealed, labeled bags, and then stored at -80°C. Upon receipt from the brain bank, the frozen blocks of brainstem tissue were kept at -80°C until sectioning. Brain blocks were brought up to -20°C then sectioned at 20 μm on a cryostat and mounted on Fisher Frost-plus slides. Slides were stored in a sealed slide box with desiccant and kept at -80°C until use for receptor autoradiography.
Competitive binding receptor autoradiography
Sections were allowed to thaw in the sealed slide box containing a desiccant packet for 1 hour at 4°C followed by 1 hour at RT. The slides were processed for OXTR receptor autoradiography as described previously (Freeman, Inoue, Smith, Goodman, & Young, 2014a). Specifically, sections were incubated with either 90pM of the OXTR radioligand, 125I-ornithine vasotocin analog (125I-OVTA) or 30 pM of the AVPR1a radioligand, 125I-linearized vasopressin antagonist (125I-LVA). These concentrations were determined from previous pharmacological studies to be at the radioligand's binding affinity (Kd) for the appropriate human receptor (Freeman, Inoue, Smith, Goodman, & Young, 2014a). Adjacent sections for each of the two radioligands were incubated in the following four conditions: 1) radioligand alone, 2) radioligand plus 10 nM of the AVPR1a selective antagonist SR49059 (Gal, Wagnon, & Garcia, 1993), 3) radioligand plus 100 nM of the OXTR selective antagonist ALS-II-69, or 4) radioligand plus 1 μM of ALS-II-69. SR49059 is available from Tocris (Minneapolis, MN) and has been previously shown in ligand-binding assays to selectively occupy human AVPR1a over human OXTR at 10 nM (Freeman, Inoue, Smith, Goodman, & Young, 2014a). ALS-II-69 is a novel, human-selective OXTR antagonist synthesized and provided by Dr. Aaron Smith at Emory University (A. L. Smith, Freeman, Voll, Young, & Goodman, 2013b). ALS-II-69 has been shown to be selective for human OXTR in previous ligand-binding studies (Freeman, Inoue, Smith, Goodman, & Young, 2014a). In the previous studies in nonhuman primates, only one concentration of the OXTR competitor ligand (ALS-II-69) was used (Freeman, Inoue, Smith, Goodman, & Young, 2014a; Freeman, Walum, Inoue, Smith, Goodman, Bales, et al., 2014b). For the current study, we decided to add an additional, higher concentration (1 μM) to confirm selective binding to OXTR. The slides were exposed to BioMax MR film (Kodak, Rochester, NY) for 8 days, then developed and analyzed.
Acetylcholinesterase (AChE) counterstain and neuroanatomical analysis
After film development, slides were stained for AChE, a common neuroanatomical counterstain to aid in the specific identification of anatomical regions. Slides were processed as described previously (Freeman, Inoue, Smith, Goodman, & Young, 2014a; Freeman, Walum, Inoue, Smith, Goodman, Bales, et al., 2014b; Lim, Hammock, & Young, 2004) and compared to an atlas of the human brainstem (Paxinos & Huang, 2013).
Quantifiction and statistical analysis
Digital images were obtained from the films using an Epson Perfection V500 Photo scanner. Quantification of the optical binding density (OBD) was conducted on these resulting digitized autoradiogram images in the following manner using ImageJ 64 software (NIH, Bethesda, MD). OBD values from a set of autoradiography standards (American Radiolabeled Chemicals, Inc., St. Louis, MO) were loaded into the software and used to generate a standard curve, from which OBD values for the regions of interest (ROI) can be extrapolated. For each specimen, OBD values were calculated for each ROI, as well as one background area where no binding is detected. Three measurements were taken for each ROI and for the background area. These three background OBD values were subtracted from the three measurements for each ROI in order to account for any individual differences in nonspecific binding and to yield normalized OBDs across specimens. We examined four ROIs: the spinal trigeminal nucleus (Sp5), the nucleus prepositus (NP), the inferior olivary nucleus (IO), and the reticular formation (RF). Due to anatomical variance across specimens, some ROIs were not present in all specimens, so the sample size for each ROI varied from 5 to 7. In order to take into account this missing data, we analyzed each ROI using mixed models. In addition to analyzing radioligand binding selectivity, we also performed correlations in order to determine the potential associations between PMI and OBD. The correlations between PMI and OBD were performed for both non-specific background binding and specific radioligand binding for both radioligands.
Results
Association between PMI and OBD
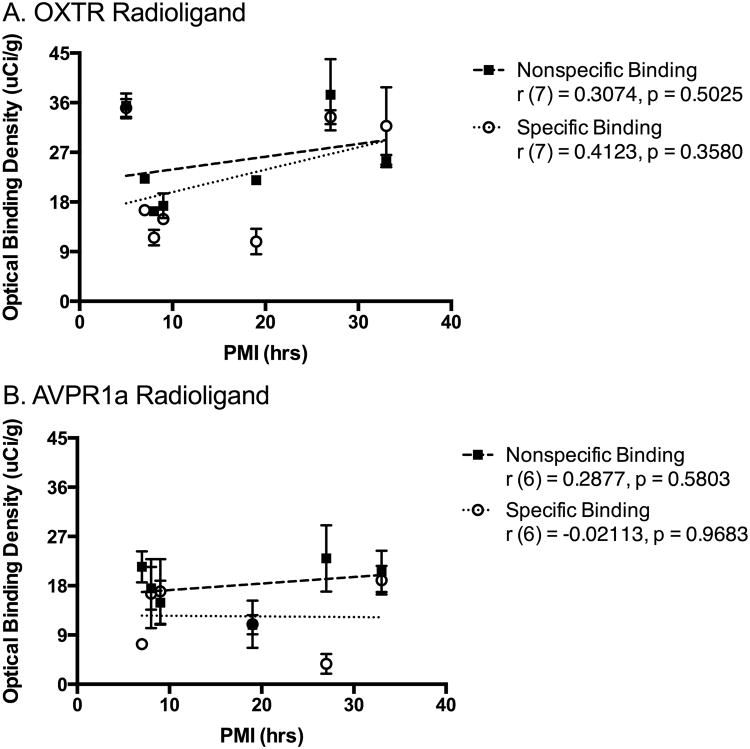
We found no significant association between PMI and OBD for either radioligand for either specific or nonspecific background binding (Figure 1). This indicates that PMI does not significantly correlate with specific OXTR or AVPR1a binding or background binding in human brain tissue and should therefore not be a consideration in future tissue selection if properly acquired prior to 33 hours.
Fig 1. No association between postmortem interval (PMI) and optical binding density.
We found no significant correlations between the PMI of each specimen and the nonspecific or specific binding of either the OXTR radioligand (A) or the AVPR1a radioligand (B).
Areas of specific OXTR binding
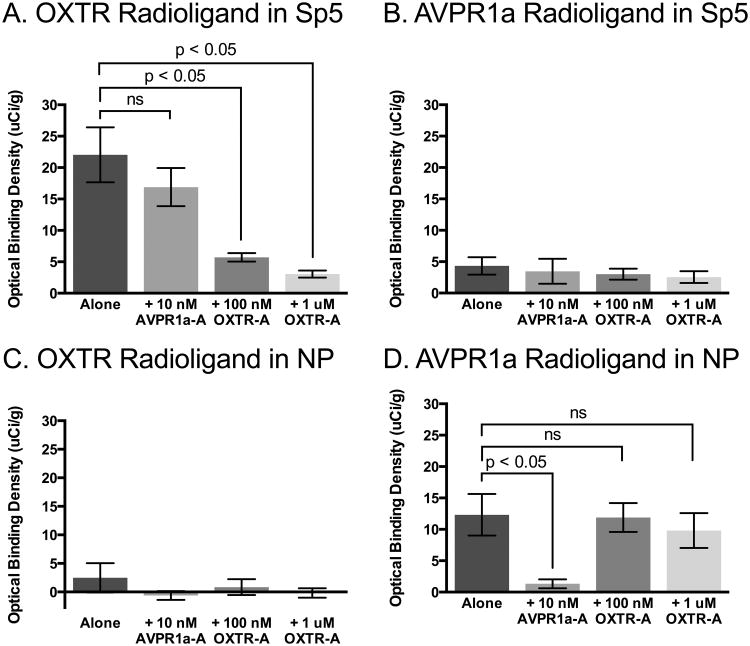
We found a main effect of competitor in the Sp5 (Figure 2 and 3) for the OXTR radioligand, F (3, 18) = 18.39; MSE = 31.00, p < 0.01, but not for the AVPR1a radioligand, F (3, 13) = 0.22; MSE = 11.36, p = 0.88. Post-hoc tests for the OXTR radioligand in the Sp5 indicate a significant reduction in binding in the presence of both the low and high concentrations of the OXTR competitor compared to radioligand alone, but in the presence of the AVPR1a competitor, OXTR radioligand binding was not significantly different from radioligand alone (Figure 4A). These results, and the lack of selective binding of the AVPR1a radioligand (Figure 4B), indicate selective OXTR expression in the Sp5.
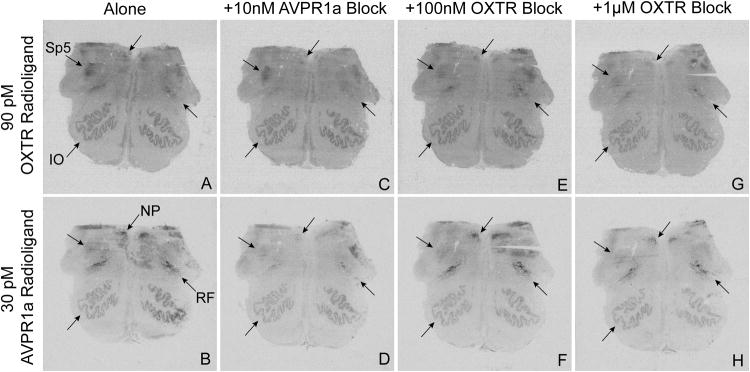
Fig 2. Selectivity of radioligand binding in human brainstem.
The OXTR radioligand (A, C, E, G) and AVPR1a radioligand (B, D, F, H) were used to identify the locations of OXTR and AVPR1a in human brainstem. To determine selectivity of binding, these radioligands were incubated alone (A, B) and also co-incubated with unlabeled competitors: 10 nM of an AVPR1a block (C, D), 100 nM of an OXTR block (E, F), and 1 μM of the same OXTR block (G, H). Here we show selective binding of the OXTR radioligand to the spinal trigeminal nucleus (Sp5) and inferior olivary nucleus (IO) and of the AVPR1a radioligand to the nucleus prepositus (NP). We also found that both competitors were capable of reducing radioligand binding in the reticular formation (RF), indicating mixed binding in this region.
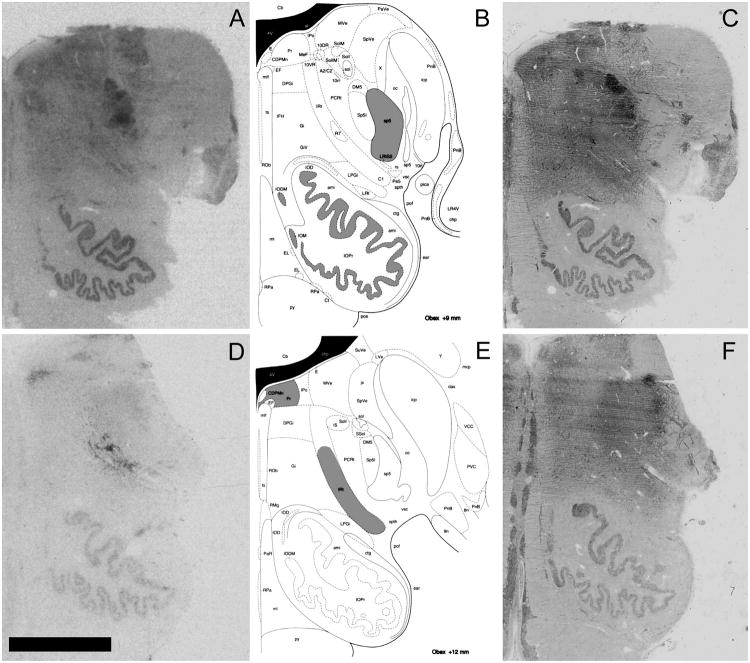
Fig 3. Anatomical determination.
A, D. Representative OXTR binding (A) and AVPR1a binding (D) in human brainstem. B, E. Anatomical locations (highlighted in gray) of OXTR binding (B) and AVPR1a binding (E) (reprinted with permission from Paxinos & Huang, 2013). C, F. Acetylcholinesterase counterstained sections from A and D, respectively, which were used to anatomically align sections with the published atlas. Scale bar = 5 mm. Relevant abbreviations from published atlas images: sp5, spinal trigeminal nucleus; IOPr, inferior olive, principal nucleus; IRt, intermediate reticular zone (also called the reticular formation in the atlas's introduction; the adjacent PCRt is the parvicellular reticular nucleus, a portion of the reticular formation that likely also contains AVPR1a binding); Pr, nucleus prepositus.
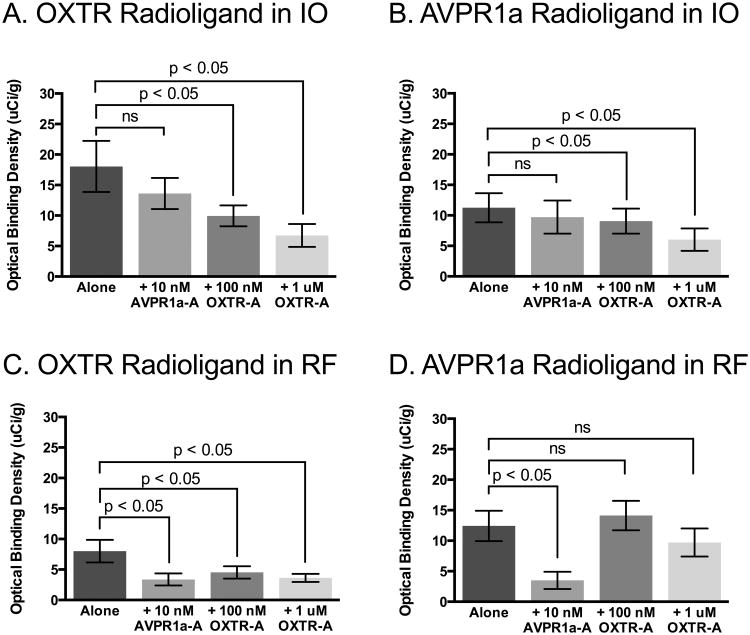
Fig 4. Quantification of competitive binding results, showing binding specificity of radioligands.
A. The OXTR radioligand binding in the spinal trigeminal nucleus (Sp5) was not competed off by co-incubation with the selective AVPR1a antagonist, SR49059 (AVPR1a-A), but was successfully competed off by co-incubation with the selective OXTR antagonist, ALS-II-69 (OXTR-A). B. The AVPR1a radioligand did not show significant binding to the Sp5, where specific OXTR binding was detected, indicating that the signal detected in this area is due to OXTR expression. C. The OXTR radioligand did not show significant binding to the nucleus prepositus (NP), where specific AVPR1a binding was detected, indicating that the signal detected in this area is due to AVPR1a expression. D. The AVPR1a radioligand binding in the NP was successfully competed off by co-incubation with the AVPR1a-A, but was not competed off by co-incubation with the OXTR-A.
In the IO (Figure 2 and 3), we found a main effect of competitor for both the OXTR radioligand F (3, 16) = 6.95; MSE = 18.20, p < 0.01, and the AVPR1a radioligand, F (3, 16) = 5.02; MSE = 1.814, p < 0.02. Interestingly, for both radioligands, post-hoc tests indicate a significant reduction in binding compared to radioligand alone in the presence of the low and high concentrations of the OXTR block, but not in the presence of the AVPR1a block (Figure 5A,B). This result indicates some nonselective binding of the AVPR1a radioligand to OXTR in this region. However, given the high levels of background binding that remain in the presence of the OXTR competitor for both radioligands, it is likely that much of this signal is nonspecific binding in the IO.
Fig 5. Quantification of competitive binding results, showing nonselective binding of radioligands.
A. The OXTR radioligand binding in the inferior olivary nucleus (IO) was not competed off by co-incubation with AVPR1a-A, but was successfully competed off by co-incubation with OXTR-A, indicating specific OXTR binding in this area, however there are very high levels of background binding remaining in the presence of the OXTR competitor. B. The AVPR1a radioligand was not competed off by co-incubation with AVPR1a-A, but was successfully competed off by co-incubation with OXTR-A, indicating off-target binding of the AVPR1a radioligand to OXTR in the IO. C. The OXTR radioligand binding in the reticular formation (RF) was significantly reduced in the presence of all three competitor conditions, indicating that the OXTR radioligand may be binding to both OXTR and AVPR1a that are present in this region. D. The AVPR1a radioligand binding in the RF was successfully competed off by co-incubation with the AVPR1a-A, but was not competed off by co-incubation with the OXTR-A, indicating specific binding of the AVPR1a radioligand to AVPR1a in the RF.
Areas of specific AVPR1a binding
We found a main effect of competitor in the NP (Figure 2 and 3) for the AVPR1a radioligand, F (3, 13) = 8.13; MSE = 19.12, p < 0.01, but not for the OXTR radioligand, F (3, 12) = 0.94; MSE = 10.10, p = 0.45. Post-hoc tests for the AVPR1a radioligand in the NP indicate a significant reduction in binding in the presence of the AVPR1a competitor compared to radioligand alone, but not in the presence of either the low or high concentrations of the OXTR competitor (Figure 4D). These results, and the lack of selective binding of the OXTR radioligand (Figure 4C), indicate selective AVPR1a expression in the NP.
In the RF (Figure 2 and 3), we found a main effect of competitor for both the OXTR radioligand, F (3, 15) = 4.08; MSE = 6.738, p < 0.05, and the AVPR1a radioligand, F (3, 13) = 11.20; MSE = 11.62, p < 0.01. Post-hoc tests for the AVPR1a radioligand in the RF indicate a significant reduction in binding in the presence of presence of the AVPR1a competitor compared to radioligand alone, but not in the presence of either the low or high concentrations of the OXTR competitor (Figure 5D). These results indicate selective binding of the AVPR1a radioligand to AVPR1a in the RF. However, the binding of the OXTR radioligand in the RF was significantly reduced in all of the competitor conditions (Figure 5C), which suggests that the OXTR radioligand may be binding to both AVPR1a and OXTR present in the RF. This interpretation would then imply that OXTR expressed in this region are not being bound by the AVPR1a radioligand, indicating some preferential binding of the AVPR1a radioligand to AVPR1a in brain regions that express both receptor subtypes.
Individual differences
We found a main effect of specimen ID for the OXTR radioligand binding in the Sp5, F (6, 18) = 3.58; MSE = 31.00, p < 0.02, and in the IO, F (6, 16) = 8.37; MSE = 18.20, p < 0.01. We found a main effect of specimen ID for the AVPR1a radioligand binding in the NP, F (5, 13) = 3.63; MSE = 19.12, p < 0.05, in the IO, F (6, 16) = 70.14; MSE = 1.814, p < 0.01, and in the RF, F (5, 13) = 5.70; MSE = 11.62, p < 0.01. These main effects of ID for both radioligands were found in all of the regions that also showed a main effect of competitor (except for the OXTR radioligand in RF), which indicates that there is significant individual variation in binding density in regions that exhibit specific receptor binding.
Discussion
This study has demonstrated conclusively for the first time that it is possible to reliably and specifically identify the neural targets of OT and AVP in humans. Our competitive binding approach has shown that the commercially available radioligands can be used in combination with selective competitors to reveal specific distributions of both OXTR and AVPR1a in postmortem human brain tissue. Although this study was limited to the brainstem, our approach should also yield reliable results in tissue specimens throughout the human brain, which makes a variety of research questions concerning OXTR and AVPR1a neurophysiology in humans now possible.
Comparison to previous studies
Although the current study is the first to show conclusive and reliable receptor binding results in human brain tissue, it is not the first attempt to visualize OXTR and AVPR1a in the human nervous system using receptor autoradiography. Thus, it is important to discuss the results of this study in the context of the two previously published studies that sought to localize OXTR and AVPR1a in postmortem human brain tissue. Both of these studies were authored by Loup and colleagues and examined OXTR binding in the brainstem and upper spinal cord (Loup et al., 1989) as well as OXTR and AVPR1a binding in a variety of other regions throughout the brain (Loup et al., 1991). These two studies both utilized the same basic experimental approach as the current study—receptor autoradiography. The paper examining OXTR binding in the brainstem (Loup et al., 1989) found mostly overlapping areas of OXTR binding as our study, such as Sp5 and IO. However, there are a few key differences that merit discussion. First, the Loup papers did not quantify binding densities, and therefore, we cannot directly compare our results with theirs.
Second, the Loup papers used two different types of radioligands: tritiated versions of the endogenous peptides themselves (3H-OT and 3H-AVP) as well as the then-novel iodinated OXTR radioligand (Elands, Barberis, Jard, Tribollet, et al., 1988b) that was used in our current study (125I-OVTA). Interestingly, the iodinated AVPR1a radioligand (125I-LVA) (Elands, Barberis, Jard, Lammek, et al., 1988a) used in our study had been developed by the time of publication of the Loup papers but was not used in either of them. Thus, the current study is the first to use 125I-LVA in postmortem human brain tissue to localize AVPR1a binding patterns. In the Loup papers, 125I-OVTA was used for the stated purpose of identifying the high-affinity binding sites, while 3H-OT was used to localize the lower affinity binding sites, possibly including AVPR1a. Thus, there was some understanding at the time of publication of the Loup papers that OT is capable of binding to AVPR1a, and therefore, the authors included binding results using the purportedly “high affinity” iodinated OXTR radioligand in order to compare the results to tritiated OT itself. However, it has recently been shown that the iodinated OXTR radioligand binds with a high affinity to AVPR1a in humans and nonhuman primates (Freeman, Inoue, Smith, Goodman, & Young, 2014a), which suggests that the presumed high affinity OXTR binding capacity of 125I-OVTA in the Loup papers likely also includes off-target binding to AVPR1a. With that said, the authors did use a concentration of 125I-OVTA (40 pM) in the same range as the current study (90 pM), which should be low enough to promote radioligand binding to OXTR over AVPR1a. However, when their binding results are compared to the results of the current study, it is clear that 125I-OVTA in the Loup papers is binding to the areas of the human brainstem that we have shown conclusively to be AVPR1a binding sites, specifically the NP and RF (Note: the area which we have labeled as RF is labeled in Loup et al., 1989 as the nucleus ambiguous, which is considered to be a part of the intermediate reticular zone in the RF (Paxinos & Huang, 2013) and is at the same neuroanatomical level as the region where we detected AVPR1a binding in the current study).
The last major difference between the Loup papers and the current study is the concentrations of the competitors that were used to demonstrate selectivity of radioligand binding. In the Loup papers, non-specific binding of both OXTR radioligands was determined by adding an excess of unlabelled OT: 5 or 10 μM for 3H-OT; 1-2 μM for 125I-OVTA. These micromolar concentrations of OT are extremely high and will result in OT binding to AVPR1a in addition to OXTR. Therefore, the reported “selective binding” is not conclusively OXTR binding and certainly represents binding to both OXTR and AVPR1a in the human brain.
Comparative expression of OXTR and AVPR1a in the brainstem of other primate species
As mentioned above, previous attempts to localize in the human brainstem with receptor autoradiography also found binding in the areas where the current study has found OXTR: Sp5 and IO, although binding in IO likely represents nonspecific background binding. In marmosets, OXTR binding was also found in Sp5 (Schorscher-Petcu et al., 2009). In titi monkeys, OXTR binding was found in the Sp5 as well, but interestingly, OXTR was also found in NP, where we detected AVPR1a binding in human brainstem. AVPR1a binding in titi monkey brainstem was found in the IO, where we detected OXTR in human tissue (Freeman, Walum, Inoue, Smith, Goodman, Bales, et al., 2014b). And lastly, in rhesus macaques, OXTR binding was detected in the Sp5 but not discussed, due to edge artifacts in the autoradiograms and a lack of OXTR mRNA detected in the same area (Freeman, Inoue, Smith, Goodman, & Young, 2014a). AVPR1a binding in rhesus macaque hindbrain was detected in the IO, as well as an area that the authors report as the locus coeruleus (Young, Toloczko, & Insel, 1999), but is actually the nucleus of the solitary tract (NTS) (Paxinos & Huang, 2013). AVPR1a binding in the NTS of the human brainstem was not found in the current study.
Future directions
With the increasing number of studies involving the administration of intranasal OT to humans, it is critical to identify where in human brain tissue OXTR are expressed. Locating the neural substrates that are sensitive to OT will enable us to better understand the mechanisms by which OT can influence human behavior. Furthermore, the results of this study shed light on the possible mechanism of action of intranasal OT, which is still unknown. Interestingly, it has recently been proposed that one potential pathway by which intranasally administered OT could reach the brain and affect behavior and physiology is via afferent nerve fibers originating in the nasal cavity and terminating in trigeminal nerve nuclei in the brainstem (Quintana, Alvares, Hickie, & Guastella, 2014). While it is possible that these nerves to take up OT and transport it to the brainstem, it is also possible that OT could bind to OXTR along this pathway. To our knowledge, it has yet to be determined whether the trigeminal nerve afferents in the nasal cavity express OXTR, but the results of the current study show dense OXTR binding in the spinal trigeminal nucleus, where these sensory nerves terminate. Future studies are needed to establish the function of OXTR in Sp5 and their potential involvement in the mechanism of action of intranasal OT.
Using the methods described in the current study, we are now capable of fully mapping OXTR expression throughout the human brain. Comparative studies mapping neural OXTR expression across mammalian species have consistently found species differences. In the primate studies conducted to date, there are several notable species differences, including dense OXTR in the hippocampus of monogamous titi monkeys (Freeman, Walum, Inoue, Smith, Goodman, Bales, et al., 2014b) that is not found in non-monogamous rhesus macaques (Freeman, Inoue, Smith, Goodman, & Young, 2014a) or marmosets (Schorscher-Petcu et al., 2009). Similarly, cooperatively breeding marmosets exhibit dense OXTR binding in the nucleus accumbens (Schorscher-Petcu et al., 2009), which is not found in rhesus macaques or titi monkeys. Establishing a map of OXTR expression in human brain tissue can be used in comparison to the existing maps in other primate species to shed light on how the similarities and differences in OXTR expression may relate to species differences in social behavior.
The current study establishes several additional directions for future research. First, it would be important to determine whether the densities and patterns of OXTR and AVPR1a expression change during human development, as there is a precedent for receptor expression to change over the lifetime of several species of rodents (voles: Wang & Young, 1997; Wang, Liu, Young, & Insel, 1997a; Wang, Young, Liu, & Insel, 1997b; mice: Hammock & Levitt, 2013; rats: Snijdewint, Van Leeuwen, & Boer, 1989; Tribollet, Goumaz, Raggenbass, & Dreifuss, 1991; Yoshimura, Kimura, Watanabe, & Kiyama, 1996). Second, several studies have now suggested a role for the OT system in the etiology of autism spectrum disorder and other diagnosable human conditions characterized by social deficits, and the methodology described here provides a novel avenue for investigating in postmortem tissue whether there are underlying neurobiological differences in OXTR expression across clinical populations compared to matched control samples. Finally, we found significant individual differences in specific binding for both OXTR and AVPR1a across brain areas, and this result provides a conceptual foundation for investigations of possible genetic influences on OXTR expression in the brain that could account for variation across individuals. These future studies, among many others, will provide critical information on the mechanisms by which OT influences human behavior.
Acknowledgments
Human tissue was obtained from University of Maryland Brain and Tissue Bank, which is a Brain and Tissue Repository of the NIH Neurobiobank. This work was supported by the NIH under Grant P51OD011107 to the California National Primate Research Center. All authors report no conflicts of interest.
Footnotes
Disclosure: All authors report no conflicts of interest.
References
- Boccia ML, Petrusz P, Suzuki K, Marson L, Pedersen CA. Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience. 2013;253:155–164. doi: 10.1016/j.neuroscience.2013.08.048. http://doi.org/10.1016/j.neuroscience.2013.08.048. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Bluthé RM, Koob GF, Le Moal M. Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology. 1987;91(3):363–368. doi: 10.1007/BF00518192. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biological Psychiatry. 2007;61(6):731–733. doi: 10.1016/j.biopsych.2006.07.015. http://doi.org/10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501(7466):179–184. doi: 10.1038/nature12518. http://doi.org/10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elands J, Barberis C, Jard S, Lammek B, Manning M, Sawyer WH, de Kloet ER. 125I-d(CH2)5[Tyr(Me)2, Tyr(NH2)9]AVP: iodination and binding characteristics of a vasopressin receptor ligand. FEBS Letters. 1988a;229(2):251–255. doi: 10.1016/0014-5793(88)81135-9. [DOI] [PubMed] [Google Scholar]
- Elands J, Barberis C, Jard S, Tribollet E, Dreifuss JJ, Bankowski K, et al. 125I-labelled d(CH2)5[Tyr(Me)2,Thr4,Tyr-NH2(9)]OVT: a selective oxytocin receptor ligand. European Journal of Pharmacology. 1988b;147(2):197–207. doi: 10.1016/0014-2999(88)90778-9. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience. 2001;21(20):8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nature Genetics. 2000;25(3):284–288. doi: 10.1038/77040. http://doi.org/10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Freeman SM, Inoue K, Smith AL, Goodman MM, Young LJ. The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta) Psychoneuroendocrinology. 2014a;45:128–141. doi: 10.1016/j.psyneuen.2014.03.023. http://doi.org/10.1016/j.psyneuen.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Walum H, Inoue K, Smith AL, Goodman MM, Bales KL, Young LJ. Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus) Neuroscience. 2014b doi: 10.1016/j.neuroscience.2014.04.055. http://doi.org/10.1016/j.neuroscience.2014.04.055. [DOI] [PMC free article] [PubMed]
- Gal CSL, Wagnon J, Garcia C. Biochemical and pharmacological properties of SR 49059, a new, potent, nonpeptide antagonist of rat and human vasopressin V1a receptors. Journal of Clinical Investigation. 1993;92(1):224–231. doi: 10.1172/JCI116554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biological Psychiatry. 2008;63(1):3–5. doi: 10.1016/j.biopsych.2007.06.026. http://doi.org/10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Hammock EAD, Levitt P. Oxytocin receptor ligand binding in embryonic tissue and postnatal brain development of the C57BL/6J mouse. Frontiers in Behavioral Neuroscience. 2013;7:195. doi: 10.3389/fnbeh.2013.00195. http://doi.org/10.3389/fnbeh.2013.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience. 2010;30(14):4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. http://doi.org/10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. Translating oxytocin neuroscience to the clinic: A National Institute of Mental Health perspective. Biological Psychiatry. 2016;79(3):153–154. doi: 10.1016/j.biopsych.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–676. doi: 10.1038/nature03701. http://doi.org/10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Lim MM, Hammock EAD, Young LJ. A method for acetylcholinesterase staining of brain sections previously processed for receptor autoradiography. Biotechnic & Histochemistry : Official Publication of the Biological Stain Commission. 2004;79(1):11–16. doi: 10.1080/10520290410001671344. http://doi.org/10.1080/10520290410001671344. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Research. 1991;555(2):220–232. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Pizzolato G, Dreifuss JJ. Localization of oxytocin binding sites in the human brainstem and upper spinal cord: an autoradiographic study. Brain Research. 1989;500(1-2):223–230. doi: 10.1016/0006-8993(89)90317-x. [DOI] [PubMed] [Google Scholar]
- Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, et al. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. Journal of Neuroendocrinology. 2012;24(4):609–628. doi: 10.1111/j.1365-2826.2012.02303.x. http://doi.org/10.1111/j.1365-2826.2012.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin BJ, Mitre M, D'amour JA, Chao MV, Froemke RC. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature. 2015;520(7548):499–504. doi: 10.1038/nature14402. http://doi.org/10.1038/nature14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi ME, Young LJ. The oxytocin system in drug discovery for autism: animal models and novel therapeutic strategies. Hormones and Behavior. 2012;61(3):340–350. doi: 10.1016/j.yhbeh.2011.12.010. http://doi.org/10.1016/j.yhbeh.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Huang XF. Atlas of the Human Brainstem. Elsevier; 2013. [Google Scholar]
- Quintana DS, Alvares GA, Hickie IB, Guastella AJ. Do delivery routes of intranasally administered oxytocin account for observed effects on social cognition and behavior? A two-level model. Neuroscience and Biobehavioral Reviews. 2014;49C:182–192. doi: 10.1016/j.neubiorev.2014.12.011. http://doi.org/10.1016/j.neubiorev.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Rosenblum LA, Smith ELP, Altemus M, Scharf BA, Owens MJ, Nemeroff CB, et al. Differing concentrations of corticotropin-releasing factor and oxytocin in the cerebrospinal fluid of bonnet and pigtail macaques. Psychoneuroendocrinology. 2002;27(6):651–660. doi: 10.1016/s0306-4530(01)00056-7. [DOI] [PubMed] [Google Scholar]
- Scheele D, Striepens N, Güntürkün O, Deutschländer S, Maier W, Kendrick KM, Hurlemann R. Oxytocin modulates social distance between males and females. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience. 2012;32(46):16074–16079. doi: 10.1523/JNEUROSCI.2755-12.2012. http://doi.org/10.1523/JNEUROSCI.2755-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D, Wille A, Kendrick KM, Stoffel-Wagner B, Becker B, Güntürkün O, et al. Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proceedings of the National Academy of Sciences. 2013;110(50):20308–20313. doi: 10.1073/pnas.1314190110. http://doi.org/10.1073/pnas.1314190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Dupré A, Tribollet E. Distribution of vasopressin and oxytocin binding sites in the brain and upper spinal cord of the common marmoset. Neuroscience Letters. 2009;461(3):217–222. doi: 10.1016/j.neulet.2009.06.016. http://doi.org/10.1016/j.neulet.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Smith AL, Freeman SM, Stehouwer JS, Inoue K, Voll RJ, Young LJ, Goodman MM. Synthesis and evaluation of C-11, F-18 and I-125 small molecule radioligands for detecting oxytocin receptors. Bioorganic & Medicinal Chemistry. 2012;20(8):2721–2738. doi: 10.1016/j.bmc.2012.02.019. http://doi.org/10.1016/j.bmc.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AL, Freeman SM, Voll RJ, Young LJ, Goodman MM. Carbon-11 N-methyl alkylation of L-368,899 and in vivo PET imaging investigations for neural oxytocin receptors. Bioorganic & Medicinal Chemistry Letters. 2013a;23(3):902–906. doi: 10.1016/j.bmcl.2012.10.116. http://doi.org/10.1016/j.bmcl.2012.10.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AL, Freeman SM, Voll RJ, Young LJ, Goodman MM. Investigation of an F-18 oxytocin receptor selective ligand via PET imaging. Bioorganic & Medicinal Chemistry Letters. 2013b;23(19):5415–5420. doi: 10.1016/j.bmcl.2013.07.045. http://doi.org/10.1016/j.bmcl.2013.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Ågmo A, Birnie AK, French JA. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Hormones and Behavior. 2010;57(2):255–262. doi: 10.1016/j.yhbeh.2009.12.004. http://doi.org/10.1016/j.yhbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijdewint FG, Van Leeuwen FW, Boer GJ. Ontogeny of vasopressin and oxytocin binding sites in the brain of Wistar and Brattleboro rats as demonstrated by lightmicroscopical autoradiography. Journal of Chemical Neuroanatomy. 1989;2(1):3–17. [PubMed] [Google Scholar]
- Tribollet E, Goumaz M, Raggenbass M, Dreifuss JJ. Appearance and transient expression of vasopressin and oxytocin receptors in the rat brain. Journal of Receptor Research. 1991;11(1-4):333–346. doi: 10.3109/10799899109066412. [DOI] [PubMed] [Google Scholar]
- Wang Z, Young LJ. Ontogeny of oxytocin and vasopressin receptor binding in the lateral septum in prairie and montane voles. Brain Research Developmental Brain Research. 1997;104(1-2):191–195. doi: 10.1016/s0165-3806(97)00138-7. [DOI] [PubMed] [Google Scholar]
- Wang Z, Liu Y, Young LJ, Insel TR. Developmental changes in forebrain vasopressin receptor binding in prairie voles (Microtus ochrogaster) and montane voles (Microtus montanus) Annals of the New York Academy of Sciences. 1997a;807:510–513. doi: 10.1111/j.1749-6632.1997.tb51954.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Young LJ, Liu Y, Insel TR. Species differences in vasopressin receptor binding are evident early in development: comparative anatomic studies in prairie and montane voles. The Journal of Comparative Neurology. 1997b;378(4):535–546. [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28(5):910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, Nishimori K. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience. 2009;29(7):2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009. http://doi.org/10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura R, Kimura T, Watanabe D, Kiyama H. Differential expression of oxytocin receptor mRNA in the developing rat brain. Neuroscience Research. 1996;24(3):291–304. doi: 10.1016/0168-0102(95)01003-3. [DOI] [PubMed] [Google Scholar]
- Young LJ, Flanagan-Cato LM. Editorial comment: oxytocin, vasopressin and social behavior. Hormones and Behavior. 2012;61(3):227–229. doi: 10.1016/j.yhbeh.2012.02.019. http://doi.org/10.1016/j.yhbeh.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nature Neuroscience. 2004;7(10):1048–1054. doi: 10.1038/nn1327. http://doi.org/10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Young LJ, Toloczko D, Insel TR. Localization of vasopressin (V1a) receptor binding and mRNA in the rhesus monkey brain. Journal of Neuroendocrinology. 1999;11(4):291–297. doi: 10.1046/j.1365-2826.1999.00332.x. [DOI] [PubMed] [Google Scholar]