Abstract
Background
Ewing sarcoma survivors (ESS) are at increased risk for treatment-related complications. The incidence of treatment-related morbidity and late mortality with aging is unknown.
Patients and Methods
This study reports survival probabilities, estimated by the Kaplan-Meier method, and cumulative incidence of cause-specific mortality and chronic conditions among ESS in the Childhood Cancer Survivor Study who were treated between 1970 and 1986. Piecewise exponential models were used to estimate relative rates (RR) and 95% confidence intervals (CI) for these outcomes. Chronic conditions were graded using CTCAE version 4.03
Results
Among 404 5-year ESS (median age at last follow-up 34.8 years, range 9.1-54.8 years), 35-year survival was 70% (95% CI 66%-74%). Late recurrence (cumulative incidence 15.1% at 35 years) was the most common cause of death followed by treatment-related causes (11.2%). There were 53 patients with subsequent neoplasms (cumulative incidence 24.0% at 35 years), 38 were malignant (14.3% at 35 years). Standardized incidence ratios were 377.1 (95% CI 172.1-715.9) for osteosarcoma, 28.9 (95% CI 3.2-104.2) for acute myeloid leukemia, 14.9 (95% CI 7.9-25.5) for breast cancer, and 13.1 (95% CI 4.8-28.5) for thyroid cancer. Rate of chronic conditions were highest for musculoskeletal [RR 18.1, 95% CI 12.8-25.7] and cardiac complications [RR 1.8, 95% CI 1.4-2.3]. At 35 years from diagnosis, the cumulative incidence of any and 2 or more chronic conditions were 84.6% (95% CI 80.4-88.8%) and 73.8% (95% CI 67.8-79.9%).
Conclusions
With extended follow-up, ESS' risk for late mortality and subsequent neoplasms do not plateau. Treatment-related chronic conditions develop years after therapy, supporting the need for life-long follow-up.
Keywords: Ewing sarcoma, childhood cancer survivors, treatment-related complications, late mortality, chronic health conditions
Introduction
The use of multimodality therapy for children and adolescents with Ewing sarcoma (ES) has incrementally improved the 5-year event-free survival to 60-70%.1-5 However, treatment requires the use of high-doses of chemotherapy including alkylating agents and anthracyclines, and aggressive local control with surgery and/or high-dose radiotherapy. Though effective, these strategies place survivors at risk for long-term medical complications including anthracycline-induced cardiomyopathy6, 7 and subsequent neoplasms (SNs).8-11 As a result, survivors are at increased risk for late (>5 years from diagnosis) mortality,12 but also to organ toxicities.13 and chronic conditions14 from exposure to chemotherapy and radiation early in life.
Initial estimates of late mortality, SNs and chronic conditions among Ewing sarcoma survivors in the CCSS were previously reported using data from the baseline questionnaire administered 1994 through 1996 when survivors were a mean age of 26.3 years (9-45).15 Since then, CCSS has followed this population through serial questionnaires. Given that the risk for SNs (particularly second solid tumors)8, 11, 16, 17 and other chronic conditions increases over time among survivors,14 it is important to identify health complications specific to aging ESS to inform survivorship care.
This report updates cumulative incidence rates for SNs, late mortality and chronic conditions in 5-year survivors of childhood Ewing sarcoma. We further evaluated musculoskeletal complications by including those related to surgical resection as functional outcomes are influenced by surgical procedure.18 We hypothesized that rates of adverse outcomes would increase over time, be greater among survivors compared to siblings/population norms, and be associated with greater intensity of original therapy.
Patients and Methods
Patient Population
The CCSS is a retrospective cohort with longitudinal follow-up of children (<21 years of age) diagnosed with cancer at one of 26 participating institutions between January 1, 1970 and December 31, 1986, and who survived at least five years.19, 20 The study protocol was reviewed and approved by institutional review boards at each institution. Informed consent was obtained from participants. Participants completed a baseline questionnaire, and two subsequent questionnaires that captured major health events (www.stjude.org/ccss). To provide a comparison population, from a random sample of 50% of survivors, one full sibling closest in age was recruited. Siblings received questionnaires identical to survivors, excluding questions specific to cancer treatment.
For this report, ESS eligible for participation in CCSS (N=566) were included in mortality analyses and 404 completed the baseline questionnaire (1994-96) and were included in the chronic conditions and SN analyses. Among the 404 Ewing survivors who completed the baseline questionnaire, 364 completed follow up questionnaire in 2000, 238 completed the follow-up 2003 questionnaire and 202 completed the follow-up 2007 questionnaire. We report information up until the last questionnaire for each survivor. A flow diagram detailing these numbers is provided as Supplemental Figure 1. Only those consenting to medical record abstraction were included in analyses that utilized treatment data.
Cancer Treatment Information
Information about cancer diagnosis and treatment were abstracted and included chemotherapy cumulative dose, radiation and surgery data.21 Radiation records were centrally coded by the CCSS Radiation Physics Center for estimation of tissue-specific dosimetry.22 For these analyses we included age at diagnosis, local control modality with surgery (amputation, limb-sparing, thoracotomy, abdominal surgery), radiotherapy (limb, abdomen, chest), and chemotherapy exposures (anthracyclines, alkylating agents, epipodophyllotoxins and their doses) as independent variables in multivariable models.
Outcome Variables
Cause of Death
Patients eligible for participation were included in a National Death Index (NDI) search through 2013.23 For deaths that predated the NDI (i.e., those in 1975-1978), death certificates from states where deaths occurred were requested. Deaths were grouped into three mutually exclusive categories using ICD-9 and ICD-10 coding: 1) recurrence/progression of primary cancer; 2) external causes (accidents, suicides, poisonings, and other external causes; ICD 9: 800-999, ICD 10: V00-V99, Y00-Y89, X00-X99, W00-W99); and, 3) non-recurrence, non-external causes including SNs (ICD 9: 140-239, ICD 10: C00- C97, D10-D36), cardiac (ICD 9: 390-398, 402, 404, 410-429, ICD 10: I00- I02, I05-I09, I11, I13, I14, I20-I28, I30-I52), pulmonary (ICD 9: 460-519, ICD 10: J00-J99), and all other causes.
SNs
SNs, occurring five or more years after initial diagnosis,17 included new neoplasms (malignant and benign), not including recurrence of the primary childhood malignancy. Cases were reported by participants and confirmed by pathology report, or when not available, confirmed by death certificate or other medical records. Second malignant neoplasms (SMN) were defined as those diagnoses included in the U.S. Surveillance, Epidemiology and End Results (SEER) registry, and do not include non-melanoma skin cancers and benign meningiomas.
Chronic Conditions
Chronic conditions were determined using previous methods,14 with grades assigned to each event according to the Common Terminology Criteria for Adverse Events, version 4.03: grade 1 (mild), grade 2 (moderate), grade 3 (severe), grade 4 (life threatening or disabling) or grade 5 (fatal). In addition to condition severity, the presence of multiple conditions, specific type of conditions and interval between cancer diagnosis and condition onset were assessed.
Statistical Analysis
Descriptive statistics were used to characterize the study population. Survival probabilities were estimated by the Kaplan-Meier product-limit method and cause-specific mortality by the cumulative incidence method, treating other causes of deaths as competing risks. Follow-up for death started at cohort entry (5 years post-diagnosis) and ended at date of death or censoring (December 31, 2007) whichever was earlier. Standardized mortality ratios (SMRs) for overall and cause-specific mortality were computed by dividing observed deaths among survivors by expected deaths from age-, sex-, and calendar-year-specific U.S. mortality data.24 For SMRs, 95% confidence intervals (CI) assumed a Poisson distribution for observed number of deaths. For survivors with treatment information, the effects of exposures on mortality were assessed using piecewise exponential models, adjusting for sex, race/ethnicity, age at diagnosis, treatment era, and attained age.
Cumulative incidence of SNs was estimated using death as a competing risk.24 Neoplasms that occurred before the cohort entry were considered prevalent at cohort entry in cumulative incidence curves. Standardized Incidence Ratios (SIRs) of overall and specific types of SMNs were calculated in the same manner as SMRs, using age-, sex-, calendar-year specific incidence rates from SEER for reference.
Cumulative incidence of chronic conditions was estimated using death, late recurrence and SMN after 5 years as competing risks, stratified by radiation exposure. Rates of chronic conditions with onset after cohort entry were estimated among survivors and compared with siblings using piecewise exponential models adjusting for age, sex, and race/ethnicity. We also evaluated treatment exposures associated with chronic conditions among survivors.
Results
The characteristics of the 404 ESS and 4,022 siblings who completed the baseline questionnaire are described in Table 1. Compared to siblings, survivors were more commonly male and Caucasian. The mean age at last follow-up among survivors and siblings was 33.8 (interquartile range [IQR]: 26.1-41.7) and 34.3 (IQR: 26.7-41.7) years respectively. The most common primary tumor site was the lower extremity followed by the upper extremity and pelvis. Most survivors received chemotherapy and radiotherapy (67.8%) with or without surgery. The most common radiation site was extremity, followed by the chest and abdomen. As expected, 96.9% of patients received alkylating agents and 85.2% anthracyclines.
Table 1. Demographic and treatment characteristics of the study population.
| Characteristic | Survivors (N=404) N(%) |
Siblings (N=4,022) N(%) |
|---|---|---|
| Sex | ||
| Male | 213 (52.7) | 1937 (48.2) |
| Female | 191 (47.3) | 2085 (51.8) |
| Race | ||
| White, non-Hispanic | 377 (93.3) | 3508 (87.2) |
| Black, non-Hispanic | 6 (1.5) | 112 (2.8) |
| Hispanic/Latino | 12 (3.0) | 148 (3.7) |
| Other | 9 (2.2) | 254 (6.3) |
| Age at diagnosis | ||
| <5 years | 29 (7.2) | |
| 5-9 years | 108 (26.7) | |
| 10-14 years | 148 (36.6) | |
| ≥15 years | 119 (29.5) | |
| Year of diagnosis | ||
| 1970-1974 | 57 (14.1) | |
| 1975-1979 | 146 (36.1) | |
| 1980-1986 | 201 (49.8) | |
| Length of Follow-up* | ||
| 5-9 years | 57 (14.1) | |
| 10-14 years | 35 (8.7) | |
| 15-19 years | 31 (7.7) | |
| 20-24 years | 99 (24.5) | |
| ≥25 years | 182 (45.0) | |
| Primary tumor site | ||
| Upper extremity | 71 (17.6) | |
| Lower extremity | 144 (35.6) | |
| Chest wall | 53 (13.1) | |
| Pelvis | 65 (16.1) | |
| Other** | 55 (13.6) | |
| Missing | 16 (4.0) | |
| Treatment Exposure | ||
| Chemotherapy only | 8 (2.2) | |
| Chemotherapy and radiation | 140 (38.31) | |
| Chemotherapy and surgery | 62 (16.9) | |
| Chemotherapy and radiation and surgery | 134 (36.5) | |
| Other*** | 23 (6.3) | |
| Chest Radiotherapy | ||
| Yes | 101 (28.5) | |
| No | 253 (71.5) | |
| Median dose (range), Gy | 34.5 (5.5 - 66.0) | |
| Abdomen radiotherapy | ||
| Yes | 47 (13.3) | |
| No | 307 (86.7) | |
| Median dose (range) , Gy | 40.0 (5.5 - 69.0) | |
| Brain radiotherapy | ||
| Yes | 23 (6.5) | |
| No | 331 (93.5) | |
| Median dose (range) , Gy | 45.0 (2.0 - 60.0) | |
| Other head radiotherapy | ||
| Yes | 15 (4.2) | |
| No | 339 (95.8) | |
| Median dose (range) , Gy | 45.0 (5.0 - 60.0) | |
| Extremity radiotherapy | ||
| Yes | 139 (39.3) | |
| No | 215 (60.7) | |
| Median dose (range) , Gy | 55.2 (10.0 - 112.0) | |
| Total body irradiation | ||
| Yes | 3 (0.8) | |
| No | 351 (99.2) | |
| Median dose (range) , Gy | 5.5 (5.0 - 8.0) | |
| Alkylating agents | ||
| Yes | 347 (96.9) | |
| No | 11 (3.1) | |
| Median dose (range), mg/m2 | 14771.4 (11.3 - 51267.3) | |
| Anthracycline | ||
| Yes | 305 (85.2) | |
| No | 53 (14.8) | |
| Median dose (range), mg/m2 | 382.8 (1.6 - 1070.0) |
To the latest questionnaire or death, whichever is earlier
Diagnosis site code (C40.9, C41.9, C76.7, C80.9)
Twenty three survivors missing one or two of chemotherapy, RT, and surgery, and 1 patient had no chemotherapy or radiation only surgery
Survival and Late Mortality
Among 566 eligible participants with Ewing sarcoma there were 169 deaths. Survival 35 years from diagnosis, conditioned on 5-year survival, for all eligible ESS was 70.4% (Supplemental Figure 2). The cumulative incidence of death due to recurrent disease was 15.2% at 35 years since diagnosis, followed by non-recurrent/non-external (11.2%) and external (2.0%) causes (Supplemental Figure 3). Corresponding SMRs were: all 8.5 (95% CI 7.3-9.9); external 1.1 (95% CI 0.5-2.1); and non-recurrent/non-external 5.8 (95%CI 4.5-7.4) including: SN 7.1 (95% CI 4.5-10.7); cardiac 6.4 (95% CI 3.6-10.6); and pulmonary 3.3 (95% CI 0.4 -12.0) causes. Host and treatment-related risk factors for mortality among survivors are shown in Table 2. Older age at diagnosis and female sex were associated with higher mortality. The only treatment-related risk factors associated with mortality were exposure to radiation or anthracyclines.
Table 2. Rate ratios (RR) and 95% confidence intervals (CI) for late mortality by demographic and treatment-related factors*.
| Risk factors | RR (95% CI) |
|---|---|
| Age at Diagnosis (years) | |
| <5 | 1.0 |
| 5-9 | 2.8 (0.8-9.8) |
| 10-14 | 3.8 (1.1-3.6) |
| ≥15 | 4.3 (1.2-15.9) |
| Sex | |
| Male | 2.0 (1.3-3.1) |
| Female | 1.0 |
| Race | |
| White, non-Hispanic | 1.3 (0.5-3.3) |
| Other | 1.0 |
| Year of Diagnosis | |
| 1970-1974 | 1.7 (0.9-3.3) |
| 1975-1979 | 1.0 (0.6-1.6) |
| 1980-1986 | 1.0 |
| Radiation | |
| Yes | 3.2 (1.5-6.9) |
| No | 1.0 |
| Surgery (excluding biopsy) | |
| Yes | 1.4 (0.9-2.1) |
| No | 1.0 |
| Alkylating agents | |
| Yes | 0.7 (0.2-3.0) |
| No | 1.0 |
| Anthracycline | |
| Yes | 2.9 (1.3-6.3) |
| No | 1.0 |
Attainted age as was adjusted using natural cubic splines
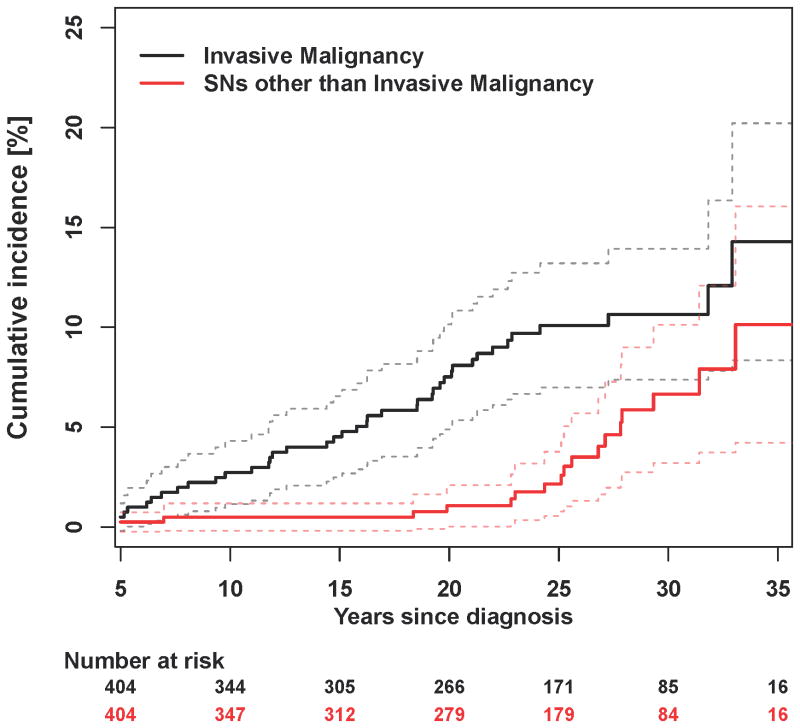
Subsequent Neoplasms
Among the 53 ESS with SNs, 38 were malignancies (SMN). The cumulative incidence of SMN was 14.3% at age 35 years (Figure 1) with an overall SIR of 7.8 (95% CI 5.6-10.6). As seen in Table 3, breast cancer was the most frequent SMN and SIRs were highest for osteosarcoma, acute myeloid leukemia, breast cancer, and thyroid cancer. Nearly all survivors who developed SMNs were previously exposed to alkylating agents, anthracyclines and radiotherapy. The only treatment exposure associated with the risk of SMN was chest radiotherapy.
Figure 1. Cumulative incidence of second neoplasms (malignant and non-malignant).
Table 3. Characteristics of Ewing Sarcoma Survivors with Subsequent Malignant Neoplasms and Standardized Incidence Ratios (SIR).
| Characteristic | N (%) | SIR |
|---|---|---|
| Gender | ||
| Male | 12 (31.6) | 5.3 (2.7-9.2) |
| Female | 26 (68.4) | 9.8 (6.6-14.1) |
| Age at diagnosis of primary malignancy: Mean (Range) | 13.3 (4.2-20.5) | |
| Age at diagnosis of subsequent malignancy: Mean (Range)* | 30.5 (14.1-46.2) | |
| Interval between primary malignancy and subsequent malignancy* | ||
| 0-4 years | ||
| 5-9 years | 9 (22.0) | |
| 10-14 years | 7 (17.1) | |
| 15-19 years | 11 (26.8) | |
| ≥20 years | 14 (34.1) | |
| Subsequent Malignant Neoplasm** | ||
| Breast | 13 (31.7) | 14.9 (7.9-25.5) |
| Osteosarcoma | 9 (22.0) | 377.1 (172.1–715.9) |
| Other cancers | 7 (17.1) | 3.7 (1.5-7.6) |
| Thyroid | 6 (14.6) | 13.1 (4.8-28.5) |
| Melanoma | 1 (2.4) | 1.8 (0.0-9.8) |
| Acute Myeloid Leukemia | 2 (4.9) | 28.9 (3.2-104.2) |
| Lymphoid Leukemia | 1 (2.4) | 15.5 (0.2-86.3) |
| Soft Tissue Sarcomas | 1 (2.4) | 6.2 (0.7-22.3) |
| Alkylating agents | ||
| Yes | 33 (94.3) | 7.6 (5.3-10.6) |
| No | 2 (5.7) | 17.8 (3.6-52.0) |
| Anthracyclines | ||
| Yes | 28 (80.0) | 7.9 (5.3-11.3) |
| No | 7 (20.0) | 8.3 (3.8-15.7) |
| Topoisomerase II inhibitors | ||
| Yes | 1 (2.9) | 12.4 (0.2-68.8) |
| No | 34 (97.1) | 7.8 (5.5-10.8) |
| Radiation | ||
| Yes | 31 (88.6) | 8.6 (5.9-12.0) |
| No | 4 (11.4) | 4.2 (1.1-10.8) |
3 patients with 2 SMNs contributed two records in these calculations.
total adds to 41 since it includes 3 patients with two different SMN
Chronic Conditions
At 25 years from diagnosis, the cumulative incidence of any grade 1-5 and 2 or more grade 1-5 chronic conditions were 80.0% (95% CI 75.8-84.1) and 61.8% (95% CI 56.7-66.8), respectively (Supplemental figures 4a and 4b). At 35 years from diagnosis, the corresponding cumulative incidence is 84.6% (95% CI 80.4-88.8) and 73.8% (95% CI 67.8-79.9), respectively. The frequency of events and relative rates (compared to siblings) were highest for musculoskeletal and cardiac complications (Table 4). Survivors were significantly more likely than siblings to have an amputation, leg lengthening procedure, joint replacement or scoliosis surgery, five or more years from diagnosis. Similarly, they were at increased risk for congestive heart failure, serious arrhythmias and myocardial infarction. The musculoskeletal events occurring before 5 years were counted as prevalence in supplemental Figure 5.
Table 4. Frequency, rate and rate ratios (RR) for chronic conditions comparing Ewing sarcoma survivors to siblings.
| Condition | Survivors | Siblings | RR (95% CI)* | ||
|---|---|---|---|---|---|
|
| |||||
| # of events | Rate per 10,000 person years | # of events | Rate per 10,000 person years | ||
| Any grade 1-5 | 105 | 555.3 | 1818 | 232.4 | 2.2 (1.8-2.7) |
|
| |||||
| A 2nd grade 1-5 | 57 | 412.6 | 930 | 197.2 | 1.7 (1.3-2.2) |
|
| |||||
| Cardiac | 71 | 134.4 | 586 | 52.8 | 1.8 (1.4-2.3) |
|
| |||||
| Arrhythmias | 31 | 53.2 | 199 | 17.3 | 2.3 (1.6-3.4) |
| Myocardial infarction | 8 | 12.8 | 24 | 2.0 | 5.0 (2.2-11.5) |
| Congestive heart failure | 26 | 43.5 | 18 | 1.5 | 21.5 (11.5-40.1) |
| Hypertension | 40 | 68.2 | 392 | 34.3 | 1.3 (1.0-1.8) |
| Stiff or leaky valve | 13 | 21.1 | 72 | 6.2 | 2.8 (1.6-5.2) |
| Heart complications requiring transplant | 2 | 3.2 | 0 | 0.0 | - |
|
| |||||
| Pulmonary | 57 | 110.8 | 607 | 58.3 | 2.3 (1.7-3.0) |
|
| |||||
| Chronic cough | 29 | 49.2 | 162 | 14.1 | 3.1 (2.0-4.6) |
| Lung fibrosis | 9 | 15.1 | 30 | 2.6 | 5.0 (2.3-10.8) |
| Emphysema | 23 | 38.6 | 438 | 41.1 | 1.4 (0.9-2.2) |
| Other respiratory conditions | 6 | 9.7 | 24 | 2.0 | 5.2 (2.0-13.4) |
| Blood clot in head, lung, arm, leg or pelvis | 12 | 19.4 | 60 | 5.1 | 2.8 (1.5-5.2) |
| Lung complications requiring transplant | 0 | 0.0 | 0 | 0.0 | - |
|
| |||||
| Musculoskeletal | 58 | 125.4 | 90 | 7.7 | 18.1 (12.8-25.7) |
|
| |||||
| Amputation | 22 | 39.2 | 18 | 1.5 | 29.7 (14.9-59.2) |
| Joint replacement | 13 | 21.1 | 14 | 1.2 | 14.1 (6.5-30.7) |
| Osteoporosis | 16 | 27.2 | 38 | 3.2 | 10.4 (5.6-19.3) |
| Scoliosis surgery | 6 | 9.7 | 17 | 1.4 | 8.7 (3.3-22.9) |
| Leg lengthening | 19 | 31.7 | 6 | 0.5 | 126.2 (46.5-342.8) |
|
| |||||
| Neurological | 44 | 102.0 | 399 | 35.5 | 2.2 (1.6-3.0) |
|
| |||||
| Pain or weakness | 17 | 31.5 | 104 | 9.0 | 2.8 (1.7-4.7) |
| Paralysis | 7 | 11.6 | 40 | 3.4 | 2.6 (1.1-5.9) |
| Abnormal sensation | 41 | 89.1 | 357 | 31.5 | 2.1 (1.5-2.8) |
Adjusted for age (using natural cubic splines), sex, and race/ethnicity.
Rate Ratios were not provided because of the zero or one number of events.
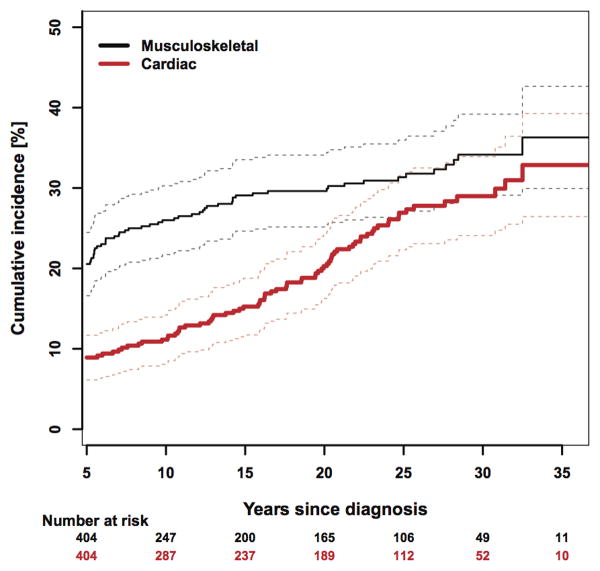
Figure 2 illustrates the cumulative incidence of musculoskeletal and cardiac complications and demonstrates that while most musculoskeletal complications occur in the first 10 years of follow-up, new onset musculoskeletal complications are possible decades after treatment has ended. In contrast, cardiac complications were relatively uncommon in the first ten years, began to increase and did not appear to plateau even at 30 years of follow-up. Radiation exposure was associated with both musculoskeletal and cardiac complication (Table 5). We evaluated the impact of radiation therapy on each musculoskeletal condition using separate regression analyses. Rate ratios (RR) and 95% confidence intervals (CI) for each of Musculoskeletal Chronic Conditions by radiation exposure are shown in supplemental Table 1. While statistical significance cannot be achieved due to the small number of events in each condition, radiation exposure showed a positive association with all the musculoskeletal conditions.
Figure 2. Cumulative incidence of musculoskeletal and cardiac complications.
Table 5. Rate ratios (RR) and 95% confidence intervals (CI) for chronic conditions by demographic and treatment-related factors among survivors of Ewing sarcoma.
| Any grade 1-5 condition | RR (95% CI) |
|---|---|
| Radiation Exposure | |
| Yes | 2.1 (1.0-4.4) |
| No | 1.0 |
| Surgery Exposure | |
| Yes | 1.0 (0.6-1.7) |
| No | 1.0 |
|
| |
| Two or more grade 1-5 conditions | |
|
| |
| Radiation Exposure | |
| Yes | 0.8 (0.3-1.9) |
| No | 1.0 |
| Surgery Exposure | |
| Yes | 1.0 (0.5-2.0) |
| No | 1.0 |
|
| |
| Cardiac condition | RR (95% CI) |
|
| |
| Anthracycline Dose | |
| None | 1.0 |
| <300 mg/m2 | 1.0 (0.4-2.6) |
| ≥300 mg/m2 | 1.0 (0.5-2.1) |
| Chest Radiation | |
| No | 1.0 |
| Yes | 1.9 (1.0-3.5) |
|
| |
| Pulmonary condition | |
|
| |
| Chest Radiation | |
| Yes | 1.2 (0.6-2.4) |
| No | 1.0 |
|
| |
| Musculoskeletal complication | |
|
| |
| Radiation | |
| Yes | 6.8 (1.6-29.1) |
| No | 1.0 |
| Surgery | |
| Yes | 1.0 (0.5-1.9) |
| No | 1.0 |
|
| |
| Neurological condition | |
|
| |
| Surgery | |
| Yes | 0.8 (0.4-1.7) |
| No | 1.0 |
The results for each outcome are from regression analysis adjusting for age (using natural cubic splines), smoking, and BMI as time-dependent variables, sex, race, year of diagnosis, and age at diagnosis.
Discussion
This study provides longitudinal follow-up on health outcomes among aging survivors of ES, nearly 80% of whom were twenty or more years from diagnosis. Our findings indicate that increases in both late mortality and SMN do not plateau and that chronic conditions continue to develop years after therapy, particularly among those exposed to radiation therapy. This report also includes new information on the significant risk for late onset musculoskeletal complications. This is important as these complications have previously been shown to negatively affect health status.18
Even at 35 years from diagnosis, recurrent disease remains the most common cause of late mortality in ESS. This is surprising as in the overall CCSS population, cumulative incidence of non-recurrence, non-external cause late mortality eclipses that of recurrence by 30 years from diagnosis.12 This result could be related to the fact that the number and types of effective chemotherapy agents available for ESS treated between 1970-1986 was limited, resulting in fewer durable remissions. The introduction of ifosfamide and etoposide in the 1990s has improved the outcome for patients with localized ES.3 Thus, it is possible that the proportion of patients with late recurrence of disease will decrease in more recently treated patients.3, 5
The incidence of SMNs also continued to rise in ESS (cumulative incidence of 13.8% at 35 years since diagnosis). We previously reported a rate of 9% at 25 years from diagnosis.15 The most frequent SMN was breast cancer among females. The Children's Oncology Group (COG) late effect guidelines suggest early screening with mammography and MRI for high-risk females25 and given the results presented here, this strategy may be warranted in this population. Although we were only able to identify chest radiotherapy as a treatment-related risk factor for SMNs, it will be important in the future to evaluate the impact of higher doses of alkylating agents9, 26 and epipodophyllotoxins27 on the cumulative incidence of SMNs, as these agents are reported to increase risk of subsequent neoplasms26, 28 and are used in more recent protocols for children with ES.
Based on standard ES therapy, it is not unexpected that cardiac outcomes are a significant long-term complication, related to the use of high-doses of anthracyclines and radiotherapy. Cardiac complications result in significant morbidity29, 30 and can impact the health of survivors and influence their activity level (an important factor in maintaining health). Our data show a significant dose response association between cardiac outcomes and treatment with radiotherapy. This result is similar to previous CCSS reports for patients with Hodgkin lymphoma treated with mantle radiotherapy31 and for the overall CCSS cohort.32 Since cardiac complications appear to increase from 15-30 years of follow-up without an apparent plateau, it will be important to continue following these patients as cardiac complications will increase as the population ages. In the current analysis, anthracyclines were not significantly associated with cardiac outcomes, but given the small number of patients reported to have cardiac events (n=22) and the size of the anthracycline treated Ewing cohort (n=306) this is likely related to low statistical power. Dexrazoxane administration decreases early risk for subclinical disease in recently treated patients,33, 34 thus long-term follow-up will be important to evaluate the impact of this strategy on long-term cardiac health.
This is one of the first reports documenting the frequency of major surgical procedures to manage late onset musculoskeletal complications (>5 years from diagnosis) in long-term ESS. Even after accounting for original local control surgical procedures, previous exposure to extremity radiation therapy is an important risk factor for future musculoskeletal complications. While most complications occurred 5-15 years from diagnosis; new events continued to appear even 30 years following diagnosis. These findings expand on the results recently published by Laack in a study of 79 patients treated for Ewing sarcoma at a single institution.35 These investigators reported that although many Ewing patients report excellent functional and quality-of-life outcomes, a significant number report long-term disability and impairment. Older age, female gender and pelvic tumor location identified their patients at greatest risk of long-term disability and impairment. One speculates that the recent evolution of surgical techniques and improved internal prosthetic devices will result in a decrease in the number of patients needing amputations following initial surgery and/or radiation. However, since limb sparing surgery has become a more frequent therapeutic modality,3-5 often combined with radiotherapy for microscopically positive margins, it will be important to compare our cohort's outcomes with more recently treated patients to determine the impact of combined surgery and radiation on long-term orthopedic sequelae among ESS.
Our study has several limitations including the self-reported nature of our outcomes. Additionally, the cohort includes patients treated between 1970 and 1986 and thus, the outcomes reported here may not be entirely applicable to current survivors since about 68% of patients received radiotherapy and current treatment strategies attempt to limit the use of radiotherapy if feasible. The retrospective self-reported nature of the surgical outcomes make it challenging to draw concrete conclusions regarding surgical complications. Prospective data collection would more accurately help group the various surgeries and the subsequent complications especially since surgical techniques have evolved since this cohort study started. Further, since joint replacements have limited lifespans, revision of a prosthesis after it has reached its lifespan is very different from revision due to an early complication (infection or mechanical failure). Time to failure and the type or extent of management required following failure are important details that should be included in any future analysis. That information will influence the conclusions regarding these outcomes. Furthermore, the evolution of surgical interventions and newer procedures may result in better functional outcomes even for patients who require surgery for treatment-related complications. Our report however, should serve as a baseline against which future studies should compare outcomes for patients treated in more modern eras.
In conclusion, evaluation of long-term outcomes in the ES cohort followed by CCSS after treatment between 1970 and 1986 confirms recurrent disease to be the most common cause of mortality. This cohort also had an increasing cumulative incidence of SMNs. ESS are at risk of severe, disabling chronic health conditions which increase over time and are related to treatment exposures. The continued need for aggressive multimodality therapy will continue to result in significant sequelae in these patients and the continued need for long-term follow-up. Development and assessment of interventions designed to improve long-term health of ESS should be a priority.
Supplementary Material
Acknowledgments
Funding: National Cancer Institute (CA55727, CA21765) and American Lebanese-Syrian Associated Charities (ALSAC).
Footnotes
Conflict of interest disclosures: Dr. Marina consultant Nekktar and Dr. Randall consultant Zimmer Biomet
Author contributions: Conceptualization/Project Administration/Writing: Marina, Ness
Data Curation/Formal Analysis/Methodology/Software: Liu, Yasui, Leisenring, Oeffinger, Neglia, Stovall, Armstrong, Ness, Robison
Funding acquisition/Investigation: Armstrong, Robison
Supervision: Ness
Writing-Review and Editing: Marina, Liu, Donaldson, Sklar, Armstrong, Oeffinger, Ginsberg, Henderson, Neglia, Stovall, Yasui, Randall, Geller, Robison, Ness
Contributor Information
Neyssa M. Marina, Stanford University & Lucile Packard Children's Hospital, Palo Alto CA.
Qi Liu, School of Public Health, University of Alberta, Edmonton, AB Canada.
Sarah S. Donaldson, Stanford University, Stanford CA.
Charles A. Sklar, Memorial Sloan Kettering Cancer Center, New York, NY.
Gregory T. Armstrong, St Jude Children's Research Hospital, Memphis TN.
Kevin C. Oeffinger, Memorial Sloan Kettering Cancer Center, New York, NY.
Wendy M. Leisenring, Fred Hutchison Cancer Center, Seattle WA.
Jill P. Ginsberg, Children's Hospital of Philadelphia, Philadelphia, PA.
Tara O. Henderson, University of Chicago, Chicago, IL.
Joseph P. Neglia, University of Minnesota Medical Center-Fairview, Minnesota, MN.
Marilyn A. Stovall, University of Texas MD Anderson Cancer Center, Houston TX.
Yutaka Yasui, St Jude Children's Research Hospital, Memphis TN.
R. Lor Randall, Primary Children's Hospital & Huntsman Cancer Institute, University of Utah, Salt Lake City UT.
David S. Geller, Montefiore Medical Center and the Children's Hospital at Montefiore, Bronx NT.
Leslie L. Robison, St Jude Children's Research Hospital, Memphis TN.
Kirsten K. Ness, St Jude Children's Research Hospital, Memphis TN.
References
- 1.Jurgens H, Bier V, Dunst J, et al. [The German Society of Pediatric Oncology Cooperative Ewing Sarcoma Studies CESS 81/86: report after 6 1/2 years] Klin Padiatr. 1988;200:243–252. doi: 10.1055/s-2008-1033716. [DOI] [PubMed] [Google Scholar]
- 2.Bacci G, Ferrari S, Bertoni F, et al. Prognostic factors in nonmetastatic Ewing's sarcoma of bone treated with adjuvant chemotherapy: analysis of 359 patients at the Istituto Ortopedico Rizzoli. J Clin Oncol. 2000;18:4–11. doi: 10.1200/JCO.2000.18.1.4. [DOI] [PubMed] [Google Scholar]
- 3.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 4.Granowetter L, Womer R, Devidas M, et al. Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: a Children's Oncology Group Study. J Clin Oncol. 2009;27:2536–2541. doi: 10.1200/JCO.2008.19.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Womer RB, West DC, Krailo MD, et al. Randomized Controlled Trial of Interval-Compressed Chemotherapy for the Treatment of Localized Ewing Sarcoma: A Report From the Children's Oncology Group. Journal of Clinical Oncology. 2012;30:4148–4154. doi: 10.1200/JCO.2011.41.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kremer LC, van Dalen EC, Offringa M, Ottenkamp J, Voute PA. Anthracycline-induced clinical heart failure in a cohort of 607 children: long-term follow-up study. J Clin Oncol. 2001;19:191–196. doi: 10.1200/JCO.2001.19.1.191. [DOI] [PubMed] [Google Scholar]
- 7.Krischer JP, Epstein S, Cuthbertson DD, Goorin AM, Epstein ML, Lipshultz SE. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. J Clin Oncol. 1997;15:1544–1552. doi: 10.1200/JCO.1997.15.4.1544. [DOI] [PubMed] [Google Scholar]
- 8.Goldsby R, Burke C, Nagarajan R, et al. Second solid malignancies among children, adolescents, and young adults diagnosed with malignant bone tumors after 1976: follow-up of a Children's Oncology Group cohort. Cancer. 2008;113:2597–2604. doi: 10.1002/cncr.23860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunst J, Ahrens S, Paulussen M, et al. Second malignancies after treatment for Ewing's sarcoma: a report of the CESS-studies. Int J Radiat Oncol Biol Phys. 1998;42:379–384. doi: 10.1016/s0360-3016(98)00228-4. [DOI] [PubMed] [Google Scholar]
- 10.Kuttesch J, Wexler L, Marcus R, et al. Second malignancies after Ewing's sarcoma: radiation dose-dependency of secondary sarcomas. Journal of Clinical Oncology. 1996;14:2818–2825. doi: 10.1200/JCO.1996.14.10.2818. [DOI] [PubMed] [Google Scholar]
- 11.Strong L, Herson J, Osborne B, Sutow W. Risk of radiation-related subsequent malignant tumors in survivors of Ewing's sarcoma. Journal of the National Cancer Institute. 1979;62:1401–1406. [PubMed] [Google Scholar]
- 12.Armstrong GT, Liu Q, Yasui Y, et al. Late Mortality Among 5-Year Survivors of Childhood Cancer: A Summary From the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2328–2338. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nysom K, Holm K, Lipsitz SR, et al. Relationship between cumulative anthracycline dose and late cardiotoxicity in childhood acute lymphoblastic leukemia. J Clin Oncol. 1998;16:545–550. doi: 10.1200/JCO.1998.16.2.545. [DOI] [PubMed] [Google Scholar]
- 14.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 15.Ginsberg JP, Goodman P, Leisenring W, et al. Long-term survivors of childhood Ewing sarcoma: report from the childhood cancer survivor study. Journal of the National Cancer Institute. 2010;102:1272–1283. doi: 10.1093/jnci/djq278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meadows AT, Friedman DL, Neglia JP, et al. Second Neoplasms in Survivors of Childhood Cancer: Findings From the Childhood Cancer Survivor Study Cohort. J Clin Oncol. 2009;27:2356–2362. doi: 10.1200/JCO.2008.21.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman DL, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102:1083–1095. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marina N, Hudson MM, Jones KE, et al. Changes in health status among aging survivors of pediatric upper and lower extremity sarcoma: a report from the childhood cancer survivor study. Arch Phys Med Rehabil. 2013;94:1062–1073. doi: 10.1016/j.apmr.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the childhood cancer survivor study: A multi-institutional collaborative project. Medical and Pediatric Oncology. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 20.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 23.Armstrong GT, Chen Y, Yasui Y, et al. Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer. N Engl J Med. 2016;374:833–842. doi: 10.1056/NEJMoa1510795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 25.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: the Children's Oncology Group Long-Term Follow-Up Guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22:4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 26.Sandoval C, Pui CH, Bowman LC, et al. Secondary acute myeloid leukemia in children previously treated with alkylating agents, intercalating topoisomerase II inhibitors, and irradiation. Journal of Clinical Oncology. 1993;11:1039–1045. doi: 10.1200/JCO.1993.11.6.1039. [DOI] [PubMed] [Google Scholar]
- 27.Pui CH, Ribeiro RC, Hancock ML, et al. Acute myeloid leukemia in children treated with epipodophyllotoxins for acute lymphoblastic leukemia. New England Journal of Medicine. 1991;325:1682–1687. doi: 10.1056/NEJM199112123252402. [DOI] [PubMed] [Google Scholar]
- 28.Tucker MA, D'Angio GJ, Boice JD, Jr, et al. Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med. 1987;317:588–593. doi: 10.1056/NEJM198709033171002. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong GT, Joshi VM, Ness KK, et al. Comprehensive Echocardiographic Detection of Treatment-Related Cardiac Dysfunction in Adult Survivors of Childhood Cancer: Results From the St. Jude Lifetime Cohort Study. J Am Coll Cardiol. 2015;65:2511–2522. doi: 10.1016/j.jacc.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipshultz SE, Cochran TR, Franco VI, Miller TL. Treatment-related cardiotoxicity in survivors of childhood cancer. Nat Rev Clin Oncol. 2013;10:697–710. doi: 10.1038/nrclinonc.2013.195. [DOI] [PubMed] [Google Scholar]
- 31.Castellino SM, Geiger AM, Mertens AC, et al. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood. 2011;117:1806–1816. doi: 10.1182/blood-2010-04-278796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. Bmj. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz CL, Wexler LH, Krailo MD, et al. Intensified Chemotherapy With Dexrazoxane Cardioprotection in Newly Diagnosed Nonmetastatic Osteosarcoma: A Report From the Children's Oncology Group. Pediatr Blood Cancer. 2016;63:54–61. doi: 10.1002/pbc.25753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wexler LH, Andrich MP, Venzon D, et al. Randomized trial of the cardioprotective agent ICRF-187 in pediatric sarcoma patients treated with doxorubicin. J Clin Oncol. 1996;14:362–372. doi: 10.1200/JCO.1996.14.2.362. [DOI] [PubMed] [Google Scholar]
- 35.Stish BJ, Ahmed SK, Rose PS, Arndt CA, Laack NN. Patient-Reported Functional and Quality of Life Outcomes in a Large Cohort of Long-Term Survivors of Ewing Sarcoma. Pediatr Blood Cancer. 2015;62:2189–2196. doi: 10.1002/pbc.25710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.