Abstract
Background
Breast cancer (BC) disparities may widen with genomic advances. We compared non-Hispanic white (NHW), Black, and Hispanic BC survivors for: 1) cancer risk management practices (CRM) among BRCA carriers; and 2) provider discussion and receipt of genetic testing.
Methods
A population-based sample of NHW, Black, and Hispanic women diagnosed with invasive BC ≤ age 50 in 2009–2012 were recruited through the state cancer registry. Using multiple logistic regression we compared CRM in BRCA carriers and association of demographic and clinical variables with provider discussion and receipt of testing.
Results
Of the 1622 participants, 36.1% (159/440) Blacks, 64.5% (579/897) NHW, 49.6% (58/117) Spanish-speaking Hispanics, and 69.0% (116/168) English-speaking Hispanics had BRCA testing, of whom 90 had a pathogenic BRCA mutation. Among BRCA carriers, RRM and RRSO rates were significantly lower among Blacks compared to Hispanics and NHW after controlling clinical and demographic variables (p=0.025 and 0.008, respectively). Compared to NHW, discussion of genetic testing with a provider was 16 times less likely among Blacks (p<0.0001) and nearly two times less likely among Spanish-speaking Hispanics (p=0.04) after controlling clinical and sociodemographic factors.
Conclusions
Our results suggest lower rates of RRSO among Black compared to Hispanic and NHW BRCA carriers, which is concerning as benefits from genetic testing arise from CRM options. Furthermore, lower BRCA testing rates among Blacks may partially be due to lower likelihood of provider discussion. Future studies are needed to improve cancer risk identification and management practices across all populations to prevent the widening of disparities.
Introduction
Breast cancer (BC) is the most common cancer among women in the United States, with 5–10% due to inherited gene mutations most commonly in the BRCA1 and BRCA2 (BRCA) genes.1, 2 BRCA mutation carriers have a 60–70% lifetime risk of BC and up to a 44% risk of ovarian cancer,3–6 compared to 12% and <2% for women in the general population. Furthermore, the risk of a second primary BC among BRCA mutation carriers may be over 50%, particularly among those who develop their first BC at an early age.7, 8 These risks may be reduced by 90% or more (i.e., to below that of the general population) through preventive options such as risk-reducing mastectomy (RRM) and risk-reducing salpingo-oophorectomy (RRSO).9, 10 Once an individual is tested for and identified to have inherited cancer predisposition, they will only reap health benefits from acting on this information. Consequently, clinical practice guidelines in the United States (US) for BRCA carriers have been developed through the National Comprehensive Cancer Network (NCCN) including: 1) annual BC surveillance (through mammogram and breast MRI) or RRM for BC risk management; and 2) RRSO for ovarian cancer risk management.11
Prior efforts to explore cancer risk management practices among BRCA carriers have primarily been based on non-Hispanic White (NHW) populations at academic institutions12–15 or integrated health systems.16 Studies among US-based women consistently suggest higher rates of RRSO (~70%) than RRM (~40%).12–16 Yet no prior efforts have compared cancer risk management across ethnically and racially diverse populations with BRCA mutations, treated across varied settings.
Identification of a BRCA mutation has potential to empower women with options to detect cancers early or prevent them altogether.17–19 Yet only ~10% of those with BRCA mutations in the US are aware they carry a mutation.20 Furthermore, there are substantial disparities across populations in awareness and utilization of genetic testing for inherited BC, with considerably lower rates reported among Blacks and Hispanics compared to NHW.21–24 Per NCCN guidelines, all women diagnosed with BC ≤50 should be offered cancer genetic risk assessment (which includes genetic counseling and consideration for testing),11 yet few discuss testing with their healthcare provider.25–28
Through a population-based sample of young Black, Hispanic, and NHW women with BC, we sought to compare: 1) cancer risk management practices among BRCA carriers; and 2) provider discussion and receipt of genetic testing.
Methods
Participants
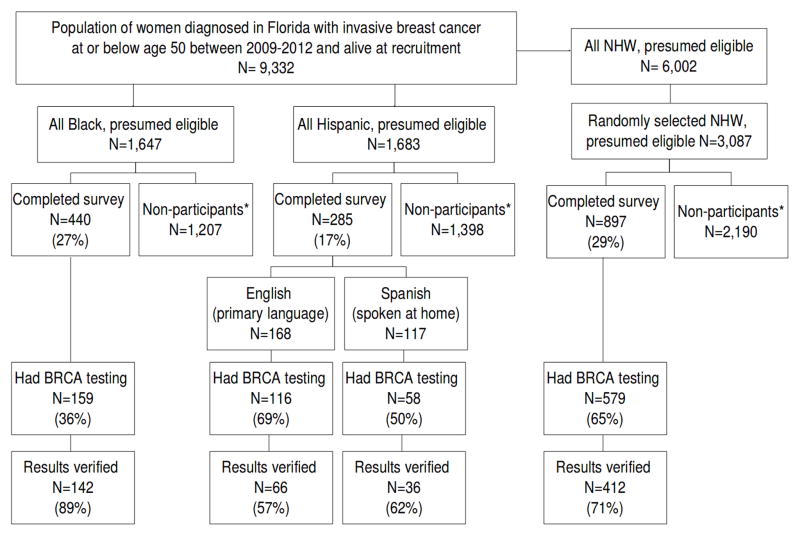
Eligible participants were women diagnosed with invasive BC ≤50 between the years 2009–2012 living in Florida at the time of diagnosis, and alive at the time of recruitment. Through protocols approved through the Institutional Review Boards at the University of South Florida and the Florida Department of Health, recruitment of Black women was initiated in 2012 as previously described, 29, 30 and that of White and Hispanic women was initiated in 2014. Using information on all eligible participants released by the Florida State Cancer Registry, contact was attempted among all Black and Hispanic women in the sampling frame and in a random sample of White women (Figure 1).
Figure 1.
Recruitment from the Florida cancer registry and participants with prior BRCA testing and results verification
Participants were recruited using previously described state-mandated recruitment methods,29, 30 which consisted of 2 mailings, 3 weeks apart, including a ‘telephone response card’ to give potential participants the option to either decline (i.e., indicating they did not wish to be contacted by phone) or express interest in participation with follow-up by a study team member. If no response was received within 3 weeks of the second mailing, a member of the study team attempted to contact the potential participant by telephone to explain the study and determine interest in participation. For those willing to participate, written informed consent was obtained and a baseline study questionnaire was completed.
Measures
Clinical (i.e., age at diagnosis, stage of diagnosis, histologic subtype, tumor receptor status) and demographic (i.e., primary payer at diagnosis, race/ethnicity) data were obtained from the cancer registry for all potential participants meeting inclusion criteria. Tumor receptor status was coded as triple negative (TN) if registry data indicated the tumor was negative for all three receptors (ER, PR, and HER2) and non-triple negative (non-TN) if at least one of these receptors was present. Tumors that were missing data for one or more receptors, but were negative for the other receptors were categorized as undetermined. For all participants in the undetermined group, clarification was attempted through medical record verification and patient self-report. Data obtained through the baseline questionnaire included healthcare provider discussion of genetic testing for inherited cancer risk, and receipt of BRCA testing. Medical record verification was attempted in all participants who indicated receipt of BRCA testing in whom a signed a medical release was available. Participants were categorized through self-reported race/ethnicity into NHW, Black, and Hispanic groups. Hispanics were further categorized as Spanish-speaking (if they spoke Spanish at home) or English-speaking (all others). Additional information obtained through the baseline questionnaire included: partner status, biological children, income, family cancer history, education, insurance status and cancer risk management (including receipt of an RRSO, RRM; and high-risk BC screening (mammograms and breast MRIs).
Data Analysis
Demographic and clinical characteristics available through the cancer registry for all eligible participants were summarized for each racial/ethnic strata using descriptive statistics. Consented participants in each racial/ethnic strata were compared to all other presumed eligible women from the cancer registry using Pearson’s chi-square tests. For participants in each of the four racial/ethnic groups, demographic and clinical characteristics were summarized and compared using Pearson’s chi-square tests for categorical variables and Mann-Whitney test for continuous variables.
Among those with a known BRCA mutation at the time of the baseline questionnaire, proportions with RRSO, RRM and breast surveillance were calculated based on self-report. Comparisons between Blacks, Hispanics and NHW were made using multiple logistic regression to control for age at enrollment, time since diagnosis, income, family history of breast and ovarian cancer, and private insurance at diagnosis. Analyses were conducted using SAS version 9.4. The goodness-of-fit for all regression models was evaluated by the Hosmer-Lemeshow statistic. For all analyses, a two-sided p-value of <0.05 was considered statistically significant.
Proportion who discussed genetic testing with a healthcare provider and proportion who underwent genetic testing were calculated for each racial/ethnic strata. Two multiple logistic regression models were then conducted using the 1,325 cases for whom all data were available. The first regression model evaluated racial/ethnic differences in genetic testing discussion and the second model evaluated receipt of genetic testing. To simultaneously control for key variables and evaluate the relative strength of relationship between the two outcomes (i.e., discussed testing and receipt of testing), a path model was conducted using Mplus version 6.12. Variables with a p-value <0.15 from the two logistic regression models were included in the model as follows. Race/ethnicity, having children, diagnosed at or below age 45, annual income over $25,000, college educated, family history of breast cancer, and having private insurance were included as predictors of having testing. Simultaneously, race/ethnicity, having children, triple negative tumor, diagnosed at or below age 45, annual income over $25,000, college educated, family history of breast cancer, family history of ovarian cancer, having private insurance, and years from diagnosis to survey were included as predictors for receipt of testing. A direct path was included to evaluate the strength of relationship between discussed testing and receipt of testing while controlling for all other variables specified in the path model.
Results
Participants included a total of 1622 BC survivors, consisting of 440 Blacks, 168 English-speaking Hispanics, 117 Spanish-speaking Hispanics, and 897 NHW (Figure 1). Comparisons between participants and all others within each respective racial/ethnic strata revealed no statistically significant differences with regard to median age, stage, histologic subtype, marital status, or employment at diagnosis (results not shown). Among those reporting genetic testing, medical record verification was obtained in 72% overall, and 78% of BRCA carriers. Participants differed across racial/ethnic strata on several clinical and demographic variables (Table 1).
Table 1.
Clinical and demographic comparisons between racial/ethnic groups
| Characteristics | Black N=440 |
English- speaking Hispanic N=168 |
Spanish- speaking Hispanic N=117 |
Non-Hispanic white N=897 |
P | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Previous genetic testing | <.0001 | ||||||||
| No | 281 | 63.9% | 50 | 29.8% | 59 | 50.4% | 317 | 35.3% | |
| Yes | 159 | 36.1% | 118 | 70.2% | 58 | 49.6% | 580 | 64.7% | |
| Referred for genetic testing | <.0001 | ||||||||
| No | 276 | 62.7% | 24 | 14.3% | 35 | 29.9% | 126 | 14.1% | |
| Yes | 164 | 37.3% | 144 | 85.7% | 82 | 70.1% | 769 | 85.7% | |
| Unknown | - | - | - | - | - | - | 2 | 0.2% | |
| Has children | <.0001 | ||||||||
| No | 54 | 12.3% | 54 | 32.1% | 50 | 42.7% | 263 | 29.3% | |
| Yes | 386 | 87.7% | 110 | 65.5% | 64 | 54.7% | 611 | 68.1% | |
| Unknown | - | - | 4 | 2.4% | 3 | 2.6% | 23 | 2.6% | |
| Married or cohabiting | <.0001 | ||||||||
| No | 262 | 59.55% | 56 | 33.3% | 37 | 31.6% | 253 | 28.2% | |
| Yes | 178 | 40.45% | 112 | 66.7% | 80 | 68.4% | 644 | 71.8% | |
| Triple Negative | <.0001 | ||||||||
| No | 300 | 68.2% | 128 | 76.2% | 88 | 75.2% | 696 | 77.6% | |
| Yes | 101 | 22.9% | 21 | 12.5% | 9 | 7.7% | 119 | 13.3% | |
| Unknown | 39 | 8.9% | 19 | 11.3% | 20 | 17.1% | 82 | 9.1% | |
| Diagnosed ≤ age 45 | <.0001 | ||||||||
| No | 154 | 35.0% | 76 | 45.2% | 65 | 55.6% | 465 | 51.8% | |
| Yes | 286 | 65.0% | 92 | 54.8% | 52 | 44.4% | 432 | 48.2% | |
| Income ≥ 25k | <.0001 | ||||||||
| No | 154 | 35.0% | 33 | 19.6% | 48 | 41.0% | 80 | 8.9% | |
| Yes | 256 | 58.2% | 126 | 75.0% | 60 | 51.3% | 754 | 84.1% | |
| Unknown | 30 | 6.8% | 9 | 5.4% | 9 | 7.7% | 63 | 7.0% | |
| College education | <.0001 | ||||||||
| No | 263 | 59.8% | 72 | 42.9% | 65 | 55.6% | 391 | 43.6% | |
| Yes | 175 | 39.8% | 95 | 56.5% | 52 | 44.4% | 504 | 56.2% | |
| Unknown | 2 | 0.4% | 1 | 0.6% | - | - | 2 | 0.2% | |
| Family history of breast cancer | 0.175 | ||||||||
| No | 193 | 43.9% | 85 | 50.6% | 61 | 52.1% | 396 | 44.15% | |
| Yes | 247 | 56.1% | 83 | 49.4% | 56 | 47.9% | 501 | 55.85% | |
| Family history of ovarian cancer | 0.453 | ||||||||
| No | 381 | 86.6% | 150 | 89.3% | 107 | 91.45% | 795 | 88.6% | |
| Yes | 59 | 13.4% | 18 | 10.7% | 10 | 8.55% | 102 | 11.4% | |
| Private insurance | <.0001 | ||||||||
| No | 172 | 39.1% | 38 | 22.6% | 59 | 50.4% | 142 | 15.8% | |
| Yes | 255 | 57.95% | 130 | 77.4% | 58 | 49.6% | 754 | 84.1% | |
| Unknown | 13 | 2.95% | - | - | - | - | 1 | 0.1% | |
| Median years from diagnosis to survey (range) | 1 (0 – 4) | 4 (0 – 6) | 4 (2 – 6) | 4 (0 – 6) | <.0001 | ||||
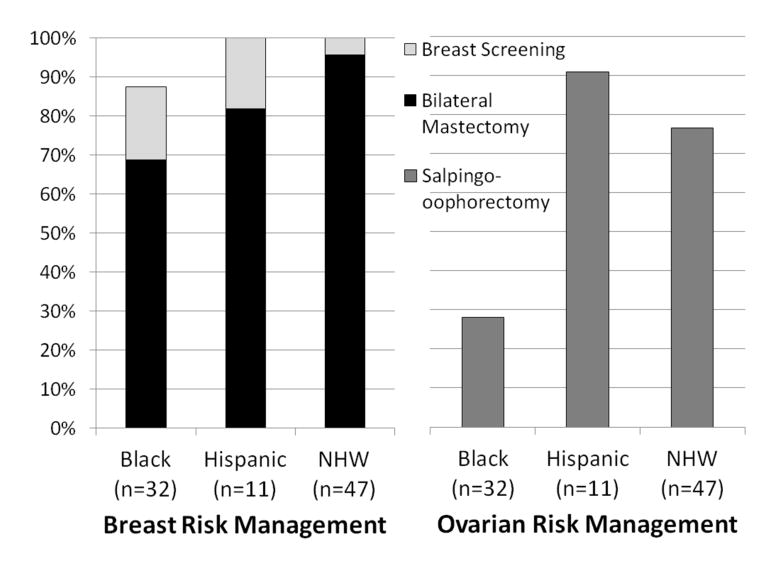
Among NHW, Black and Hispanic BRCA carriers, uptake of: 1) RRSO was 76.6%, 28.1%, and 90.9%, respectively; 2) RRM was 95.7%, 68.8%, and 81.8%, respectively; and 3) guideline-based BC screening or RRM was 100%, 85.7%, and 100%, respectively (Figure 2). Among BRCA carriers with remaining breast tissue who reported no breast screening, 2 had not yet completed their BC treatment, both of whom were Black. With Blacks as the referent group, even after controlling for possible confounders, Hispanics and NHW remained significantly more likely to have RRSO (p=0.025) and RRM (p=0.008). Hosmer-Lemeshow tests provided evidence of adequate model fit for all logistic regression models (all p>0.05).
Figure 2.
Uptake of Risk Management Options among BRCA Mutation Carriers
All participants met national guidelines for genetic risk assessment and counseling;31 however, among Blacks, Spanish-speaking Hispanics, English-speaking Hispanics and NHW, the proportion who reported: 1) having discussed genetic testing with a provider was 37.3%, 70.1%, 85.7% and 85.7%, respectively; and 2) receipt of genetic testing was 36.1%, 49.6%, 69.05%, and 64.55%, respectively. Compared to NHW, Blacks were 16.6 times less likely to have discussed genetic testing with a healthcare provider (p<0.0001) and Spanish-speaking Hispanics were nearly two times less likely to have discussed testing (p=0.04) after controlling for other variables (Table 2). Rates of genetic testing discussion were similar among NHW and primarily English-speaking Hispanics.
Table 2.
Results of multiple logistic regression showing associations with healthcare provider discussion of genetic testing and receipt of genetic testing
| Discussed Genetic Testing
|
P | Underwent Genetic Testing
|
P | |||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |||
| Race group | ||||||
| Black vs. NHW | 0.06 | 0.04 – 0.10 | <.0001 | 0.18 | 0.11 – 0.29 | <.0001 |
| English-speaking Hispanic vs. NHW | 1.08 | 0.60 – 1.94 | .80 | 1.52 | 0.96 – 2.39 | .07 |
| Spanish-speaking Hispanic vs. NHW | 0.54 | 0.30 – 0.96 | .04 | 1.07 | 0.62 – 1.83 | .82 |
| Has children | ||||||
| Yes vs. No | 1.38 | 0.96 – 1.98 | .08 | 1.51 | 1.11 – 2.05 | .01 |
| Married or cohabiting | ||||||
| Yes vs. No | 0.87 | 0.63 – 1.20 | .39 | 0.88 | 0.66 – 1.17 | .37 |
| Triple negative | ||||||
| Yes vs. No | 1.19 | 0.81 – 1.76 | .37 | 1.74 | 1.22 – 2.48 | .002 |
| Diagnosed ≤ age 45 | ||||||
| Yes vs. No | 3.16 | 2.28 – 4.38 | <.0001 | 5.17 | 3.89 – 6.87 | <.0001 |
| Income ≥ 25k | ||||||
| Yes vs. No | 1.52 | 1.00 – 2.30 | .05 | 2.27 | 1.54 – 3.37 | <.0001 |
| College graduate | ||||||
| Yes vs. No | 1.63 | 1.19 – 2.23 | .002 | 1.71 | 1.31 – 2.24 | <.0001 |
| Family history of breast cancer | ||||||
| Yes vs. No | 1.76 | 1.31 – 2.38 | .0002 | 1.98 | 1.53 – 2.57 | <.0001 |
| Family history of ovarian cancer | ||||||
| Yes vs. No | 1.35 | 0.85 – 2.15 | .20 | 1.87 | 1.24 – 2.81 | .003 |
| Private insurance | ||||||
| Yes vs. No | 1.79 | 1.23 – 2.59 | .002 | 2.88 | 2.04 – 4.07 | <.0001 |
| Years from diagnosis to survey (per 1 year increase) | 0.97 | 0.84 – 1.11 | .65 | 0.91 | 0.81 – 1.02 | .10 |
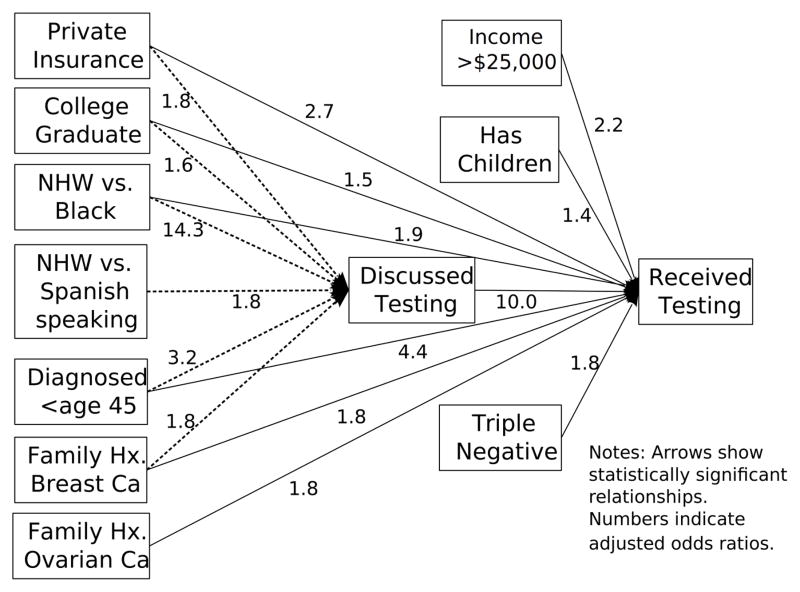
Blacks were 5.6 times less likely to have had genetic testing than NHW when controlling for other variables (Table 2), but differences between NHW and Spanish-speaking Hispanics were no longer significant (p=0.82) after controlling for clinical and socioeconomic differences. The path model reveals the strongest association with receipt of testing is having a healthcare provider discuss testing (Figure 3).
Figure 3.
Path model to demonstrate factors associated with healthcare provider discussion and subsequent receipt of BRCA testing
Discussion
To our knowledge, this is the first study to compare differences in cancer risk management practices across an ethnically and racially diverse sample of BRCA mutation carriers tested and treated across multiple settings. Our findings suggest lower rates of RRSO and RRM among Blacks. Furthermore, our results demonstrate lower genetic testing rates among Blacks compared to NHW, most strongly associated with lower genetic testing discussions by healthcare providers.
When considering BRCA testing, it is important to recognize that benefits do not arise from BRCA testing itself, but rather acting on test results to detect cancers early or prevent them altogether. A number of studies have evaluated cancer risk management practices among BRCA carriers; however, these primarily encompass NHW populations mainly based at academic institutions12–15 or integrated health systems.16 A recent retrospective cohort study of primarily NHW BRCA carriers from a community healthcare system in Northern California reported uptake of RRSO and RRM among BRCA carriers of 74% and 44%, respectively,16 which is slightly higher than that recently reported from US-based academic centers.12, 15 Similarly, uptake of preventive options reported through an international study of BRCA carriers reported RRSO and RRM rates of 71.1% and 36.3%, respectively, among US-based women.13 Taken together, these RRSO rates are similar to those found in our study among NHW and Hispanics, yet substantially higher than the RRSO rate of 28% observed among Blacks in our study. Consistent with the low RRSO rates we observed among Blacks are results from African American BRCA carriers in which breast and ovarian cancer surveillance was preferred over risk-reducing surgery, however this earlier study was based on a single African American kindred.32 Consequently, our study represents the first to evaluate and compare follow-up care among unselected BRCA carriers across minority populations, with results suggesting substantial racial differences in cancer risk management practices.
Although RRSO remains the only reliable option for ovarian cancer risk management among BRCA carriers, RRM or heightened screening through annual MRI and mammograms are both considered appropriate options for BC risk management.11 However, adherence to screening over time among unaffected BRCA carriers in an integrated healthcare system identified low compliance with annual MRI (35%) and mammograms (43%) at baseline among those without RRM with compliance at 5 years dropping to only 3% and 7%, respectively.16 More recently, a follow-up study of primarily NHW BRCA carriers who received genetic counseling and BRCA testing through an academic center indicated 51% had RRM and 72% had RRSO.33 Interestingly, despite the limited number of minorities in this study (~11%; n=11), study authors reported both white race and higher BC genetics knowledge to be significantly associated with adherence to recommended management, highlighting the potential for genomic testing to widen existing disparities among minority populations. Ultimately, our study is the sole population-based effort to compare differences in cancer risk management practices across minority BRCA carriers treated across diverse settings, underscoring the need for further studies to confirm and address observed disparities in follow-up care.
The majority of BRCA testing has occurred in Caucasian populations,24, 34–37 with disproportionately lower rates among Blacks and Hispanics,23, 24, 37 consistent with our results among Blacks and Spanish-speaking Hispanics. However, English-speaking Hispanics and NHW had similar testing rates which may reflect acculturation of Hispanics over generations. Black women in our study were not recent immigrants and did not have a language barrier, yet their testing rates were the lowest demonstrating a concerning health disparity that requires focused attention. This is particularly alarming given that limited studies among high risk Hispanics38–41 and Blacks42 suggest high interest in these services once it is explained to them.
Reasons for lower testing rates among Blacks and Hispanics include both lower awareness about genetic testing21 and access to cancer genetics experts,43 geographic barriers,44 language barriers,45 and socioeconomic factors such as insurance, education and income.46 In fact, the presence of private insurance had a direct impact on both genetic testing discussion and receipt of testing in the path model (Figure 3). Based on our own clinical experience this is not surprising because private insurers tend to be more likely to cover genetic testing than Medicaid. Additionally, a genetic test discussion may not even occur if testing is not perceived to be feasible by patients or providers, as might be the case if the patient is uninsured or on Medicaid. In our study, even after controlling for socioeconomic factors, Blacks were less likely to be tested but the single strongest predictor was provider discussion of genetic testing. Consequently, in addition to patient-level factors, provider and system level factors may contribute to suboptimal testing rates among minorities. In particular, multiple studies demonstrate the importance of healthcare provider recommendations in receipt of genetic testing, with lack of physician referral amongst the most highly cited barriers to testing among BC survivors. 23, 35, 47–50 Our findings that healthcare provider discussion of testing was the strongest predictor for receipt of BRCA testing with lowest rates of both testing discussion and testing receipt among Blacks, is consistent with prior studies. Although not explored through our study, other potential explanations for observed differences include provider characteristics and distribution, as well as variability in clinical practice situations, which should be explored further through future efforts. Ultimately, our findings are concerning and suggest the need for the development of multi-level interventions targeted at both the patient and provider level in order to successfully address the widening disparities due to genomic advances.
The current study has several strengths including the first population-based design to systematically compare cancer risk management practices among BRCA carriers drawn from an ethnically and racially diverse sample of BC survivors treated across multiple settings, enhancing the generalizability of our findings. Furthermore, our estimates of provider discussion and genetic testing across diverse populations provides updated and novel data, compared to prior efforts with limited minority representation, non-population based sampling, or sampling frame of women diagnosed before 2008. 23, 35, 47–50 Furthermore, BRCA testing confirmation in over 72% of all cases further strengthens the accuracy and validity of our observations.
Despite these strengths, there remain some limitations including our inability to fully determine reasons for the observed differences in uptake of cancer risk management options and testing rates across populations. Furthermore, although participants were diagnosed within the same 4 years and eligibility criteria were the same, the Blacks and non-Blacks were recruited under separate protocols. However, time since diagnosis and age at diagnosis (or age at the time of the survey) were included in the models in order to minimize bias. Furthermore, our study is cross-sectional and represents a single snapshot in time, thus longitudinal follow-up is critical to determine whether these disparities persist or widen. As well, given the time between diagnosis and recruitment, there is potential for recall bias. Moreover, our sample size of carriers was limited, given that they represented a subset drawn from a much larger unselected population of BC survivors. Nevertheless, we observed clear differences in uptake of RRSO and RRM among Black carriers, which requires confirmation and additional longitudinal follow-up. Additionally, survey completion rates across racial subgroups was below 30% which may lead to selection bias, although the study population was comparable to the source population based on available clinical and demographic variables. As well, the sample was confined to Florida, thus may not be generalizable to other parts of the country where clinical practices may vary. Finally, all participants were diagnosed prior to a number of practice changing events that occurred around 2013 and beyond, including: plummeting sequencing costs due to technological advances in conjunction with the fall of the BRCA patent, implementation of the Affordable Care Act, and celebrity disclosures.51, 52 To determine if these changes impacted populations with existing health disparities, more recent studies across ethnically and racially diverse populations of high risk patients are needed, as was recently identified as a research gap by the US Preventive Services Task Force (USPSTF).53
Ultimately, it is critical to better understand the reasons for the lower uptake of cancer risk management options among Black BRCA carriers, in order to develop interventions and assure access to preventive care. In this regard, coverage for genetic testing does not equate to coverage for preventive care, which is essential to improve health outcomes.54 Consequently, variations in preventive services coverage may exacerbate health disparities without policies to ensure equitable access to these services. Given that BRCA testing and cancer risk management are choices, it remains imperative to identify and discuss genetic testing with high risk patients across all populations, communicate the information in a culturally congruent and understandable way, and ensure access to testing and follow-up care regardless of socioeconomic factors.
In summary, our study is the first to demonstrate differences in cancer risk management across Blacks, Hispanics and NHW recruited through population-based efforts. The lower RRSO rates observed among Black BRCA carriers are particularly concerning given that most ovarian cancers are diagnosed at a later stage without reliable means for early detection. Furthermore, our findings demonstrate that healthcare provider discussion was the strongest predictor of testing. Taken together, the underlying etiology of differences observed in testing rates and follow-up care require further study to identify facilitators and barriers such as psychological, cultural and geographic factors. In addition to patient-specific factors, provider and system-level factors must be examined to develop solutions to narrow existing health disparities in gene-based care. Ultimately, multi-level interventions are needed to reduce the growing healthcare disparities in clinical cancer genetics.
Acknowledgments
Research Support: This work was supported by grants through the Bankhead Coley Granting agency (4BB15 and IBG10-34199), the American Cancer Society (RSG-11-268-01-CPPB). Support for Deborah Cragun’s time was provided in part by a NCI R25T training grant awarded to Moffitt Cancer Center (5R25CA147832-04). This work has been supported in part by the Biostatistics Core (5P30CA076292-16) and Survey Core at the Moffitt Cancer Center, a National Cancer Institute Comprehensive Cancer Center (P30-CA076292).
Footnotes
Conflict of Interest: The authors declare that no conflict of interest exists.
Author Contributions: Drs. Cragun and Pal had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Cragun, Pal
Acquisition, analysis, or interpretation of data: Cragun, Weidner, Lewis, Bonner, Kim, Vadaparampil, Pal
Drafting of the manuscript: Cragun, Weidner, Lewis, Bonner, Kim, Vadaparampil, Pal
Statistical Analysis: Cragun, Kim, Pal
Obtained funding: Pal, Vadaparampil
Administrative, technical or material support: Weidner, Lewis, Bonner
Study Supervision: Pal, Vadaparampil
LITERATURE CITED
- 1.Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 2.Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- 3.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Litton JK, Ready K, Chen H, et al. Earlier age of onset of BRCA mutation-related cancers in subsequent generations. Cancer. doi: 10.1002/cncr.26284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25:1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 7.Graeser MK, Engel C, Rhiem K, et al. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2009;27:5887–5892. doi: 10.1200/JCO.2008.19.9430. [DOI] [PubMed] [Google Scholar]
- 8.Malone KE, Begg CB, Haile RW, et al. Population-based study of the risk of second primary contralateral breast cancer associated with carrying a mutation in BRCA1 or BRCA2. J Clin Oncol. 28:2404–2410. doi: 10.1200/JCO.2009.24.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. Jama. 304:967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finch AP, Lubinski J, Moller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547–1553. doi: 10.1200/JCO.2013.53.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. [accessed Sept 19, 2016];Genetic/Familial High-risk Assessment: Breast and Ovarian. Available from URL: http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf.
- 12.Beattie MS, Crawford B, Lin F, Vittinghoff E, Ziegler J. Uptake, time course, and predictors of risk-reducing surgeries in BRCA carriers. Genet Test Mol Biomarkers. 2009;13:51–56. doi: 10.1089/gtmb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metcalfe KA, Birenbaum-Carmeli D, Lubinski J, et al. International variation in rates of uptake of preventive options in BRCA1 and BRCA2 mutation carriers. Int J Cancer. 2008;122:2017–2022. doi: 10.1002/ijc.23340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metcalfe KA, Lubinski J, Ghadirian P, et al. Predictors of contralateral prophylactic mastectomy in women with a BRCA1 or BRCA2 mutation: the Hereditary Breast Cancer Clinical Study Group. J Clin Oncol. 2008;26:1093–1097. doi: 10.1200/JCO.2007.12.6078. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz MD, Isaacs C, Graves KD, et al. Long-term outcomes of BRCA1/BRCA2 testing: risk reduction and surveillance. Cancer. 2012;118:510–517. doi: 10.1002/cncr.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia C, Wendt J, Lyon L, et al. Risk management options elected by women after testing positive for a BRCA mutation. Gynecol Oncol. 2014;132:428–433. doi: 10.1016/j.ygyno.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Watson M, Kash KM, Homewood J, Ebbs S, Murday V, Eeles R. Does genetic counseling have any impact on management of breast cancer risk? Genet Test. 2005;9:167–174. doi: 10.1089/gte.2005.9.167. [DOI] [PubMed] [Google Scholar]
- 18.Roukos DH, Briasoulis E. Individualized preventive and therapeutic management of hereditary breast ovarian cancer syndrome. Nat Clin Pract Oncol. 2007;4:578–590. doi: 10.1038/ncponc0930. [DOI] [PubMed] [Google Scholar]
- 19.Narod SA, Offit K. Prevention and management of hereditary breast cancer. J Clin Oncol. 2005;23:1656–1663. doi: 10.1200/JCO.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 20.Drohan B, Roche CA, Cusack JC, Jr, Hughes KS. Hereditary breast and ovarian cancer and other hereditary syndromes: using technology to identify carriers. Ann Surg Oncol. 2012;19:1732–1737. doi: 10.1245/s10434-012-2257-y. [DOI] [PubMed] [Google Scholar]
- 21.Mai PL, Vadaparampil ST, Breen N, McNeel TS, Wideroff L, Graubard BI. Awareness of cancer susceptibility genetic testing: the 2000, 2005, and 2010 National Health Interview Surveys. Am J Prev Med. 2014;46:440–448. doi: 10.1016/j.amepre.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pagan JA, Su D, Li L, Armstrong K, Asch DA. Racial and ethnic disparities in awareness of genetic testing for cancer risk. Am J Prev Med. 2009;37:524–530. doi: 10.1016/j.amepre.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 23.Jones T, Lockhart JS, Mendelsohn-Victor KE, et al. Use of Cancer Genetics Services in African-American Young Breast Cancer Survivors. Am J Prev Med. 2016 doi: 10.1016/j.amepre.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Hall MJ, Reid JE, Burbidge LA, et al. BRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast-ovarian cancer. Cancer. 2009;115:2222–2233. doi: 10.1002/cncr.24200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellcross CA, Kolor K, Goddard KA, Coates RJ, Reyes M, Khoury MJ. Awareness and utilization of BRCA1/2 testing among U.S. primary care physicians. Am J Prev Med. 40:61–66. doi: 10.1016/j.amepre.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 26.Bellcross CA, Leadbetter S, Alford SH, Peipins LA. Prevalence and Healthcare Actions of Women in a Large Health System with a Family History Meeting the 2005 USPSTF Recommendation for BRCA Genetic Counseling Referral. Cancer Epidemiol Biomarkers Prev. 2013;22:728–735. doi: 10.1158/1055-9965.EPI-12-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trivers KF, Baldwin LM, Miller JW, et al. Reported referral for genetic counseling or BRCA 1/2 testing among United States physicians: a vignette-based study. Cancer. 2011;117:5334–5343. doi: 10.1002/cncr.26166. [DOI] [PubMed] [Google Scholar]
- 28.Wood ME, Kadlubek P, Pham TH, et al. Quality of cancer family history and referral for genetic counseling and testing among oncology practices: a pilot test of quality measures as part of the American Society of Clinical Oncology Quality Oncology Practice Initiative. J Clin Oncol. 2014;32:824–829. doi: 10.1200/JCO.2013.51.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pal T, Rocchio E, Garcia A, Rivers D, Vadaparampil S. Recruitment of black women for a study of inherited breast cancer using a cancer registry-based approach. Genet Test Mol Biomarkers. 2011;15:69–77. doi: 10.1089/gtmb.2010.0098. [DOI] [PubMed] [Google Scholar]
- 30.Bonner D, Pal T, Tallo C, Vadaparampil ST. The utility of a state-wide cancer registry in recruiting a clinically representative population-based sample of young Black women diagnosed with early-onset breast cancer. Fifth Conference on the Science of Cancer Health Disparities in Racial/Ethnic Minorities; San Diego, CA. 2012. [Google Scholar]
- 31. [accessed March 9, 2016];Genetic/Familial High-risk Assessment: Breast and Ovarian. Available from URL: http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf.
- 32.Kinney AY, Simonsen SE, Baty BJ, et al. Risk reduction behaviors and provider communication following genetic counseling and BRCA1 mutation testing in an African American kindred. J Genet Couns. 2006;15:293–305. doi: 10.1007/s10897-006-9026-7. [DOI] [PubMed] [Google Scholar]
- 33.Buchanan AH, Voils CI, Schildkraut JM, et al. Adherence to Recommended Risk Management among Unaffected Women with a BRCA Mutation. J Genet Couns. 2016 doi: 10.1007/s10897-016-9981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frank TS, Deffenbaugh AM, Reid JE, et al. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol. 2002;20:1480–1490. doi: 10.1200/JCO.2002.20.6.1480. [DOI] [PubMed] [Google Scholar]
- 35.McCarthy AM, Bristol M, Domchek SM, et al. Health Care Segregation, Physician Recommendation, and Racial Disparities in BRCA1/2 Testing Among Women With Breast Cancer. J Clin Oncol. 2016 doi: 10.1200/JCO.2015.66.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall MJ, Olopade OI. Disparities in genetic testing: thinking outside the BRCA box. J Clin Oncol. 2006;24:2197–2203. doi: 10.1200/JCO.2006.05.5889. [DOI] [PubMed] [Google Scholar]
- 37.Levy DE, Byfield SD, Comstock CB, et al. Underutilization of BRCA1/2 testing to guide breast cancer treatment: black and Hispanic women particularly at risk. Genet Med. 2011;13:349–355. doi: 10.1097/GIM.0b013e3182091ba4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sussner KM, Jandorf L, Thompson HS, Valdimarsdottir HB. Interest and beliefs about BRCA genetic counseling among at-risk Latinas in New York City. J Genet Couns. 2010;19:255–268. doi: 10.1007/s10897-010-9282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sussner KM, Jandorf L, Thompson HS, Valdimarsdottir HB. Barriers and facilitators to BRCA genetic counseling among at-risk Latinas in New York City. Psychooncology. 2013;22:1594–1604. doi: 10.1002/pon.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sussner KM, Edwards T, Villagra C, et al. BRCA Genetic Counseling Among At-Risk Latinas in New York City: New Beliefs Shape New Generation. J Genet Couns. 2014 doi: 10.1007/s10897-014-9746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gammon AD, Rothwell E, Simmons R, et al. Awareness and preferences regarding BRCA1/2 genetic counseling and testing among Latinas and non-Latina white women at increased risk for hereditary breast and ovarian cancer. J Genet Couns. 2011;20:625–638. doi: 10.1007/s10897-011-9376-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams I, Christopher J, Williams KP, Sheppard VB. What Black Women Know and Want to Know About Counseling and Testing for BRCA1/2. J Cancer Educ. 2014 doi: 10.1007/s13187-014-0740-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kolb B, Wallace AM, Hill D, Royce M. Disparities in cancer care among racial and ethnic minorities. Oncology (Williston Park) 2006;20:1256–1261. discussion 1261, 1265, 1268–1270. [PubMed] [Google Scholar]
- 44.Pal T, Vadaparampil ST. Genetic risk assessments in individuals at high risk for inherited breast cancer in the breast oncology care setting. Cancer Control. 19:255–266. doi: 10.1177/107327481201900402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.U.S. Census Bureau. America Speaks: A Demographic Profile of Foreign-Language Speakers for the United States: 2000 (PHC-T-42) 2010 [Google Scholar]
- 46.Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. Jama. 2005;293:1729–1736. doi: 10.1001/jama.293.14.1729. [DOI] [PubMed] [Google Scholar]
- 47.Anderson B, McLosky J, Wasilevich E, Lyon-Callo S, Duquette D, Copeland G. Barriers and facilitators for utilization of genetic counseling and risk assessment services in young female breast cancer survivors. J Cancer Epidemiol. 2012;2012:298745. doi: 10.1155/2012/298745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenberg SM, Ruddy KJ, Tamimi RM, et al. BRCA1 and BRCA2 Mutation Testing in Young Women With Breast Cancer. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2015.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jagsi R, Griffith KA, Kurian AW, et al. Concerns about cancer risk and experiences with genetic testing in a diverse population of patients with breast cancer. J Clin Oncol. 2015;33:1584–1591. doi: 10.1200/JCO.2014.58.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCarthy AM, Bristol M, Fredricks T, et al. Are physician recommendations for BRCA1/2 testing in patients with breast cancer appropriate? A population-based study. Cancer. 2013 doi: 10.1002/cncr.28268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jolie A. [accessed July 18, 2013];My Medical Choice. Available from URL: http://www.nytimes.com/2013/05/14/opinion/my-medical-choice.html?_r=0.
- 52.Cho MK, Sankar P, Wolpe PR, Godmilow L. Commercialization of BRCA1/2 testing: practitioner awareness and use of a new genetic test. Am J Med Genet. 1999;83:157–163. doi: 10.1002/(sici)1096-8628(19990319)83:3<157::aid-ajmg4>3.0.co;2-g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nelson HD, Fu R, Goddard K, et al. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer: Systematic Review to Update the U.S. Preventive Services Task Force Recommendation. Rockville MD: 2013. [PubMed] [Google Scholar]
- 54.Prince AE. Prevention for those who can pay: insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci. 2015;2:365–395. doi: 10.1093/jlb/lsv008. [DOI] [PMC free article] [PubMed] [Google Scholar]