Abstract
Background
Household air pollution is a major contributor to death and disability worldwide. Over 95% of rural Guatemalan households use woodstoves for cooking or heating. Woodsmoke contains carcinogenic or fetotoxic polycyclic aromatic hydrocarbons (PAHs) and volatile organic compounds (VOCs). Increased PAHs and VOCs have been shown to increase levels of oxidative stress.
Objective
We examined PAH and VOC exposures among recently pregnant rural Guatemalan women exposed to woodsmoke and compared exposures to levels seen occupationally or among smokers.
Methods
Urine was collected from 23 women who were 3 months post-partum 3 times over 72-hours: morning (fasting), after lunch, and following dinner or use of wood-fired traditional sauna baths (samples=68). Creatinine-adjusted urinary concentrations of metabolites of 4 PAHs and 8 VOCs were analyzed by liquid chromatography—mass spectrometry. Creatinine-adjusted urinary biomarkers of oxidative stress, 8-isoprostane and 8-OHdG, were analyzed using enzyme-linked immunosorbent assays (ELISA). Long-term (pregnancy through 3 months prenatal) exposure to particulate matter and airborne PAHs were measured.
Results
Women using wood-fueled chimney stoves are exposed to high levels of particulate matter (median 48-hour PM2.5 105.7 μg/m3; inter-quartile range (IQR): 77.6–130.4). Urinary PAH and VOC metabolites were significantly associated with woodsmoke exposures: 2-naphthol (median (IQR) in ng/mg creatinine: 295.9 (74.4–430.9) after sauna versus 23.9 (17.1–49.5) fasting; and acrolein: 571.7 (429.3–1040.7) after sauna versus 268.0 (178.3–398.6) fasting. Urinary PAH (total PAH: ρ = 0.89, p < 0.001) and VOC metabolites of benzene (ρ=0.80, p < 0.001) and acrylonitrile (ρ=0.59, p < 0.05) were strongly correlated with long-term exposure to particulate matter. However urinary biomarkers of oxidative stress were not correlated with particulate matter (ρ = 0.01 to 0.05, p > 0.85) or PAH and VOC biomarkers (ρ =−0.20 to 0.38, p > 0.07). Urinary metabolite concentrations were significantly greater than those of heavy smokers (mean cigarettes/day = 18) across all PAHs. In 15 (65%) women, maximum 1-hydroxypyrene concentrations exceeded the occupational exposure limit of coke-oven workers.
Conclusions
The high concentrations of urinary PAH and VOC metabolites among recently pregnant women is alarming given the detrimental fetal and neonatal effects of prenatal PAH exposure. As most women used chimney woodstoves, cleaner fuels are critically needed to reduce smoke exposure.
Keywords: solid fuel use, household air pollution, urinary biomarkers, polycyclic aromatic hydrocarbons, volatile organic compounds
1. Introduction
Household air pollution (HAP) from solid fuels is a significant risk factor for death and disability worldwide. In 2013, it was the seventh leading cause of Disability Adjusted Life years (DALYs) and remains one of the leading causes of acute lower respiratory infections, chronic obstructive pulmonary disease, lung cancer, cerebrovascular and ischemic heart disease (Collaborators et al., 2015). The disease burden is highest among the very young (under 5 years old) and women (IHME, 2015), and would be even higher if evidence of the effect of HAP on preterm birth and low birthweight were included in global estimates (Patelarou and Kelly, 2014). Reducing HAP exposures is an important mission of a recent funding opportunity supported by the National Institutes of Health, the Gates Foundation and the Global Alliance for Clean Cookstoves (NIH, 2015).
In Guatemala, 64% of all households and 95% of rural households use wood fuel for cooking (WHO, 2013). HAP ranks as the fifth leading cause of death and is responsible for 4% of all DALYs for children under 5 (IHME, 2015). Annually, over 5,000 deaths are attributable to HAP with lower respiratory infections and ischemic heart disease causing the most deaths (McCracken et al., 2015). It will continue to contribute to the epidemiologic transition within Guatemala as the predominant health burden shifts from communicable diseases, such as lower respiratory infections, to non-communicable diseases, such as ischemic heart disease, to which HAP is a major contributing factor.
Polycyclic aromatic hydrocarbons (PAHs) and volatile organic compounds (VOCs), two groups of chemicals created during the incomplete combustion of organic substances, are systemically absorbed. Many are known carcinogens, causes of pulmonary and cardiovascular disease, immune impairment and/or adverse birth outcomes. Multiple PAHs and VOCs, such as benzene, 1,3-butadiene, and acrylamide, are classified as carcinogenic (IARC, 2015). In addition, adult exposure to PAHs and VOCs is associated with cardiovascular disease (Alshaarawy et al., 2016; Haussmann, 2012). Prenatal exposure to ambient levels of PAHs is associated with adverse birth outcomes such as neural tube defects (Ren et al., 2011), small for gestational age and preterm birth (Choi et al., 2008; Padula et al., 2014). Similarly, residential exposures to VOCs have been shown to be associated with small for gestational age in newborns (Sorensen et al., 2010).
A common source of PAHs is dietary intake (WHO and IARC, 2010). In addition, elevated urinary concentrations of PAH and VOC metabolites have been found in the urine of cigarette smokers (Alwis et al., 2012; Benowitz et al., 2015), those exposed to secondhand smoke (St Helen et al., 2014; Suwan-ampai et al., 2009), and from occupational exposures, such as coal processing or aluminum production (Jongeneelen, 2001). Smoke from burning solid fuels typically contains high levels of PAHs (Titcombe and Simcik, 2011), VOCs (Vanker et al., 2015), and airborne fine particulate matter (PM2.5) (Li et al., 2011; Titcombe and Simcik, 2011) and exposure to wood smoke from cooking is associated with high urinary levels of PAH metabolites (Pruneda-Alvarez et al., 2012). These reported levels are higher than those found in studies within high-income countries (Alshaarawy et al., 2016) and many are higher than the occupational exposure limit set by Jongeneelen (Jongeneelen, 2001).
The biological mechanisms by which PAH and VOC metabolites exert effects on health outcomes are not well established but they have been shown to induce oxidative stress (Li et al., 2015; Wang et al., 2015). Two urinary markers of oxidative stress are 8-isoprostane, a measure of lipid peroxidation (Milne et al., 2005), and 8-hydroxy-2′-deoxyguanosine (8-OHdG), a measure of DNA oxidation (Evans et al., 2010; Poulsen et al., 2014). Levels of both have been found to be elevated in the urine of those exposed to ambient air pollution (Svecova et al., 2009), household air pollution (Commodore et al., 2013) and welding fumes (Nuernberg et al., 2008).
Previous studies have shown that women in Guatemala are exposed to high concentrations of PM2.5 and carbon monoxide (CO), two major constituents produced during the incomplete combustion of solid fuel (Smith et al., 2010; Smith et al., 2011; Thompson et al., 2011a). PAHs and VOCs are also significant by-products of incomplete combustion. Thus, this study aimed to measure the urinary concentrations of PAHs, VOCs and oxidative stress metabolites in recently pregnant Guatemalan women, to compare these concentrations to long-term personal exposures to airborne PM2.5 and PAHs, and to compare PAH and VOC urinary metabolite concentrations in this study to levels found with other known high exposures, namely cigarette smoking or the industrial processing of coal products.
2. Methods
2.1. Study population and sampling strategy
This study was nested within a larger cohort study, the NACER (Neurodevelopment and anthropometric growth of infants exposed to household air pollution in rural Guatemala) study, which explored the effect of household woodsmoke on birth outcomes and child development among rural Mam-speaking Mayan or Spanish-speaking ladino women in the Western Highlands of Guatemala between April 2012 and December 2013. Study participants were pregnant women between 18 and 45 years of age who met the following criteria: non-smoking; used wood fuel for cooking; had no plans to migrate in the next 1 ½ years; and attended the Ministry of Health clinic for prenatal care. Thirty-six pregnant women were recruited consecutively from the clinic if their gestational age was <20 weeks based on ultrasound examination and met the inclusion criteria. Sociodemographic characteristics were collected for all participants.
Airborne kitchen concentrations of particulate matter (PM2.5) were measured at three prenatal measurements (< 20 weeks, 24–28 weeks (second trimester) and 32–36 weeks (third trimester) of gestation), two neonatal measurements (< 48 hours since birth and one month after birth) and at 3 months of infant life (to correspond with health outcomes from the parent study) (Figure 1A). Personal exposure to airborne PAHs was measured at three times during the course of the study: at 32–36 weeks gestational age and at one and three months after birth (Figure 1A).
Figure 1.
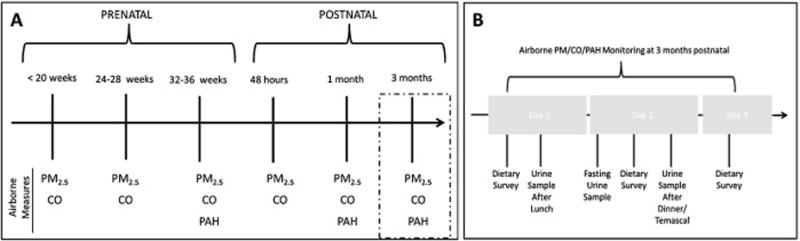
Diagram of timeline for collection of: (A) airborne particulate matter, CO, and PAHs; and (B) urine and dietary PAH survey.
2.2. Urine collection
Twenty-three NACER study participants who were 3 months post-partum participated in the present study. The remaining 13 women were past the 3rd month post-partum and were, therefore, not included in this sub-study. Since use of wood-fired sauna baths have been shown to greatly increase levels of CO exposure, one by-product of incomplete combustion of wood fuel (Lam et al., 2011; Thompson et al., 2011b), we measured urinary metabolite concentrations after multiple types of smoke exposure such as cooking and sauna baths. Urine samples (n=68) were collected three times over a 72-hour period: at first morning urine (fasting), after lunch and following dinner or sauna bath use (Figure 1B). Women were instructed to use the clean-catch method and collected their urine in sterile polypropylene cups which were stored on ice until picked up by study personnel. Samples were processed at the field laboratory and were then shipped on dry ice to the United States and stored at −20°C until laboratory analysis.
2.3. Dietary survey
Dietary surveys were constructed based on foods that are known to be high in polycyclic aromatic hydrocarbons (WHO and IARC, 2010) and that are commonly eaten in this region of rural Guatemala, such as charred, smoked or fried food, white bread, tomatoes, oranges, eggs, tortillas and tamales. Each morning during the study period, participants were asked about their consumption of these high-PAH foods over the previous 24 hours (Figure 1B).
2.4. Airborne PAH measurements
Passive samplers were used to measure airborne concentrations of four PAHs (naphthalene, pyrene, fluorene and phenanthrene). Based on pilot sampling, it was determined that monitors worn over a 72-hour period would sufficiently reduce within-subject variability over the three time periods. The PAH passive sampler is constructed from a modified 37 mm diameter polystyrene air sampling cassette by the Hammond laboratory (University of California, Berkeley) as previously described (Hammond and Leaderer, 1987). Women wore the filter affixed to their non-dominant shoulder and vapor-phase PAHs adsorbed to a finely ground XAD-4 resin impregnated Teflon coated glass fiber filter within the monitor. Ten percent of the women wore two filters to assess difference between filters worn at the same time. The correlation between these filters was 0.17–0.40 depending on the PAH. Filters were stored at 3°C until analysis when vapor-phase PAHs were desorbed in dichloromethane and analyzed by gas chromatography—mass spectrometry (GC-MS). The concentrations of desorbed PAHs were adjusted by “blank” filters worn by 10% of the women to account for background levels. Blank filters were placed in sealed cups and worn for the same time period as the passive diffusion filters. The analytical limit of detection was 1 ng per filter, the field blanks from this study indicate field limits of detection between 9 and 19 ng and the recoveries for these compounds on spiked filters ranged from 82% to 102%.
2.5. PM2.5 measurements
Kitchen PM2.5 concentrations were measured over a 48-hour period by trained field staff to reduce within-person variability as we have done with other studies in this area (Smith et al., 2010). Concentrations were measured continuously every minute using the UCB-PATS, a lightweight device that measures PM2.5 using a photoelectric (light-scattering) detector (Berkeley Air, Berkeley, CA). To correct for instrument accuracy, gravimetric measurements of particulate matter were taken concurrently with 10% of continuous measurements. This was performed by drawing air through a BGI metal cyclone separator (model SSC1.06 triplex), with a 50% cutpoint at 2.5 μm, onto 37 mm filters with 2 μm pores using battery-operated constant flow SKC air sample pumps at a rate of 1.5 liters/minute. All pumps were calibrated before and after use in the field using a Gillian Gillibrator soap bubble meter. Filters were changed after 24 hours; thus, each woman had two filters representing each 48-hour monitoring period. Filters were pre- and post-weighed using gravimetric analysis on a Mettler MT-5 balance at the University of California, Berkeley. The room was maintained at 71° F and 44 % relative humidity. All filters were conditioned for 24 hours by exposure to these conditions before weighing. Kitchen PM2.5 measurements — both continuous and gravimetric — were taken at a height of 1.45 meters and 1.10 meters from the primary cooking stove.
We removed any continuous measures where the monitoring period was <43 hours (19% of measures). We applied a filter-based adjustment factor to continuous PM measures using filters that were placed concurrently with the UCB-PATS for > 21.5 hours and a monitor adjustment factor based on UCB-PATs colocation data to account for differences in monitors.
2.6. Laboratory procedures for urinary biomarker analysis
Urinary metabolites of several PAHs and VOCs (Table 1) were analyzed by LC-MS/MS by the Clinical Pharmacology laboratory at UCSF as previously described (Alwis et al., 2012; Jacob et al., 2007). Briefly, urine was incubated with β-glucoronidase and sulfatase to catalyze hydrolysis of hydroxylated PAHs. PAH metabolites were then extracted, converted to pentafluorobenzyl ether derivatives and analyzed by LC-MS/MS with internal standards. For VOC metabolites, urine with added internal standard was extracted, converted to pentafluorobenzyl ester derivatives and analyzed by LC-MS/MS. As reported elsewhere, quantifying urinary metabolites via LC-MS/MS has been shown to be accurate, sensitive and precise (Alwis et al., 2012; Jacob et al., 2007)
Table 1.
Airborne exposures and the associated urinary metabolite measured by LC-MS/MS.
| Exposure | Metabolite (acronym) |
|---|---|
| Polycyclic aromatic hydrocarbons | |
|
| |
| Naphthalene | 2-naphthol |
| Fluorene | 1-hydoxyfluorene |
| 2-hydroxyfluorene | |
| 3-hydroxyfluorene | |
| Phenanthrene | 1-hydroxyphenanthrene |
| 2-hydroxyphenanthrene | |
| 3,4-hydroxyphenanthrene | |
| Pyrene | 1-hydroxypyrene |
|
| |
| Volatile Organic Compounds | |
|
| |
| Benzene | phenylmercapturic acid (PMA) |
| 1,3- butadiene | 4-hydroxy-2-buten-1-yl-mercapturic acid (MHBMA-3) |
| Ethylene oxide, acrylonitrile, vinyl chloride | 2-hydroxyethylmercapturic acid (HEMA) |
| Methylating agents | methylmercapturic acid (MMA) |
| Acrylonitrile | 2-cyanoethylmercapturic acid (CNEMA) |
| Acrolein | 3-hydroxypropylmercapturic acid (3HPMA) |
| Propylene oxide | 2-hydroxypropylmercapturic acid (2HPMA) |
| Acrylamide | 2-carbamoylethylmercapturic acid (AAMA) |
| Crotonaldehyde | 3-hydroxy-1-methyl-propylmercapturic acid (HPMMA) |
Urinary markers of oxidative stress, 8-isoprostane and 8-OHdG, were analyzed by the laboratory of Joel Meyer at Duke University. Urinary 8-isoprostane levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit from Oxford Biomedical Research (Rochester Hills, MI, Cat# EA85). The lower limit of detection for this assay is 0.08 ng/ml, the upper limit is 3.5 ng/mL, and the inter- and intra-assay variation is <10%, according to the manufacturer’s specifications. Before assaying, samples were pretreated with β-glucuronidase to allow for analysis of total isoprostanes. Treated samples were then diluted 1:4 with the kit’s Enhanced Dilution Buffer, and the assay was performed according to the manufacturer’s instructions. Oxidized guanines (8-OHdG) in urine were measured using the DNA/RNA Oxidative Damage ELISA kit from Cayman Chemical (Ann Arbor, MI, Cat# 589320). According to the manufacturer’s specifications, this assay has a range of 10.3 to 3,000 pg/mL and a sensitivity of 30 pg/mL. Urine samples were diluted 1:750 in the ELISA buffer and the assay was carried out according to the manufacturer’s instructions.
Concentrations of all urinary metabolites were normalized to creatinine concentrations.
2.7. Statistical analysis
To determine correlations between metabolite concentrations and dietary factors, a Dietary Index was created from survey data on consumption of foods high in PAHs. Different types of foods were dichotomized (0/1) based on whether or not they had been consumed within a 24-hour period, irrespective of the quantity, and then summed to generate the index. Due to the higher daily consumption of tortillas and tamales, these were treated as continuous variables and compared independently of the index. Since dietary surveys asked about food consumed in the preceding 24 hours, dietary indices were adjusted to account for the marked (58–79%) reduction in urinary concentrations within 12 hours of ingestion (Li et al., 2012).
Urinary biomarker concentrations were right skewed and thus median differences were determined using non-parametric tests. Differences in concentration were compared by collection time using the Skillings-Mack test, an adaptation of the Friedman test (Chatfield and Mander, 2009). Associations between median urinary metabolite concentration and household characteristics, such as stove or fuel type, were made using Mann-Whitney and Kruskal-Wallis tests. Spearman rank correlation was used to determine correlations between urinary concentrations and concentrations PM2.5. Long-term exposure PM2.5 was calculated as the mean concentration of all six measurements taken from pregnancy through 3 months postnatal. Because of the different molecular weights of each PAH metabolite, total PAH concentration was calculated on a molar basis for statistical comparisons. Comparisons between the median of measured concentrations and literature values were made using the Wilcoxon signed-rank test and those between means were made using the t test. Due to the longitudinal nature of urinary data collection, generalized estimating equations were used for multivariate analyses.
2.8. Ethical approval
We received ethical approval from the Committee for Human Research at UCSF and Universidad del Valle in Guatemala. Participants were informed of the study by trained field workers fluent in both Spanish and Mam. Written informed consent was obtained.
3. Results
3.1. Socio-demographic characteristics of participants
The median age of study participants was 22.5 years (inter-quartile range (IQR), 20–27). The majority of participants was Mam-Mayan indigenous (n=17, 74%) and had only an elementary school education (n=16, 70%). All used wood as a primary cooking fuel and most used chimney stoves (n=21, 91%) in a separate structure (n=20, 87%). Most women also burned garbage (n=21, 91%) or plastic (n=13, 57%) in either an outdoor or indoor fire. None of the women were smokers and only one woman stated that a family member smoked 1 cigarette a day outside of the home. The median kitchen 48 hour PM2.5 average concentration was 105.7 μg/m3 (IQR: 77.6–130.4) (Table 2). There was little variation in kitchen PM2.5 concentrations measured at different time points on each woman; 75% and 99% of the measurements were within one and two standard deviations of the 48 hour PM2.5 average of all time points, respectively (data not shown).
Table 2.
Demographic characteristics of study participants (n=23)
| Age years, median (IQR) | 22.5 (20–27) |
| Education, n (%) | |
| None | 2 (9) |
| Elementary school | 16 (70) |
| Middle school | 5 (22) |
| Ethnicity, n (%) | |
| Indigenous Mam | 17 (74) |
| Spanish-speaking Ladina | 6 (26) |
| Exposed to second-hand smoke, n (%) | 1 (4) |
| Kitchen in separate structure, n (%) | 20 (87) |
| Primary stove, n (%) | |
| Stove with chimney | 21 (91) |
| Stove without chimney | 2 (9) |
| Secondary stove, n (%) | |
| None | 17 (74) |
| Gas stove | 4 (17) |
| 3 stone open fire | 2 (9) |
| Fuel use for cooking, n (%) | |
| Wood | 23 (100) |
| Food scraps | 17 (74) |
| Plastic | 13(57) |
| Propane | 4 (17) |
| Charcoal | 1 (4) |
| Kerosene | 1 (4) |
| Burns garbage, n (%) | 21 (91) |
| Burns away from the house, n (%) | 19 (90) |
| 24-hour dietary index, median (range) | 5 (1.3–10.3) |
| Tortillas consumed, median (range) | 1.25 (0–6.8) |
| Tamales consumed, median (range) | 2.63 (0–5.5) |
| Airborne pollutant concentrations 48-houra | |
| Particulate Matter2.5 (μg/m3), median (IQR) 72 hourb | 105.7 (77.6–130.4) |
| Naphthalene (ng/m3), median (IQR) | 18.55 (6.49–18.69) |
| Fluorene (ng/m3), median (IQR) | 162.74 (88.96–357.65) |
| Phenanthrene (ng/m3), median (IQR) | 511.04 (207.02–1345.19) |
| Pyrene (ng/m3), median (IQR) | 59.23 (29.97–96.83) |
Indicates average of measurements taken on six separate visits from pregnancy to three months prenatal
Indicates measurement taken concurrently with urinary samples.
3.2. Variation in urinary metabolite concentration by collection time
The concentration of PAH urinary metabolites differed significantly by collection time (Table 3) and, thus, were dependent on exposure prior to sample collection. For example, the median (IQR) concentrations for 2-naphthol are as follows: 295.9 ng/mg creatinine (74.4–430.9) following sauna; 37.0 ng/mg creatinine (24.9–62.0) following lunch; 31.7 ng/mg creatinine (20.8–40.1) following dinner; and 23.9 ng/mg creatinine (17.1–49.5) fasting. Excluding sauna bath measurements from the analysis, there were significant differences for 2-hydroxyfluorene and 3,4-hydroxyphenanthrene with higher levels measured after lunch, the largest meal of the day, compared to those at fasting and after dinner. As an example, the median 2-hydroxyfluorene (IQR) concentrations are as follows: 4.7 ng/mg creatinine (2.9–11.6) following lunch; 3.9 ng/mg creatinine (2.3–5.7) following dinner; and 3.5 ng/mg creatinine (2.4–8.7) fasting.
Table 3.
Urinary concentrations of urinary biomarkers of PAHs, VOCs and oxidative stress
| Biomarker | OH-PAH (ng/mg creatinine) | ||||
|---|---|---|---|---|---|
| Total (N=68) | Fasting (N=23) | Lunch (N=23) | Dinner (N=15) | Sauna (N=7) | |
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |
| 2-naphthol***,# | 36.2 (21.2–61.3) | 23.9 (17.1–49.5) | 37.0 (24.9–62.0) | 31.7 (20.8–40.1) | 295.9 (74.4–430.9) |
| 1-hydroxyfluorene** | 2.1 (1.1–3.4) | 1.8 (0.9–3.1) | 1.8 (1.1–2.9) | 2.1 (1.6–3.4) | 11.4 (2.3–17.6) |
| 2-hydroxyfluorene***,# | 4.7 (2.9–11.0) | 3.5 (2.4–8.7) | 4.7 (2.9–11.6) | 3.9 (2.3–5.7) | 40.4 (7.1–66.2) |
| 3-hydroxyfluorene** | 2.1 (1.1–5.0) | 2.0 (0.9–4.7) | 2.1 (1.1–5.1) | 1.8 (0.9–4.3) | 11.1 (3.3–14.5) |
| 1-hydroxyphenanthrene** | 1.9 (0.9–3.0) | 1.6 (0.7–2.7) | 1.8 (0.9–3.7) | 1.9 (0.9–2.7) | 8.9 (1.6–15.4) |
| 2-hydroxyphenanthrene* | 5.4 (2.9–8.8) | 5.1 2.5–8.8) | 5.3 (3.0–8.4) | 4.0 (2.6–6.9) | 19.8 (6.1–35.5) |
| 3,4-hydroxyphenanthrene***,# | 4.9 (2.2–8.0) | 4.7 (2.0–7.1) | 5.0 (2.2–8.3) | 3.7 (2.0–5.3) | 24.6 (5.8–56.8) |
| 1-hydroxypyrene** | 4.4 (2.6–8.7) | 3.5 (2.6–7.8) | 4.5 (2.5–10.1) | 4.2 (2.4–7.2) | 20.3 (6.0–36.2) |
| Biomarker | VOC (ng/mg creatinine) | ||||
| Total (N=68) | Fasting (N=23) | Lunch (N=23) | Dinner (N=15) | Sauna (N=7) | |
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |
| PMA***,### (Benzene) | 1.4 (0.7–2.6) | 1.3 (0.5–2.3) | 1.6 (0.8–3.8) | 1.3 (0.6–2.1) | 6.1 (1.7–10.9) |
| MHBMA-3 (1,3- butadiene) | 0.5 (0.4–0.8) | 0.6 (0.3–0.8) | 0.5 (0.4–0.8) | 0.5 (0.2–0.8) | 0.6 (0.2–1.1) |
| HEMA (ethylene oxide, acrylonitrile, vinyl chloride) | 4.5 (3.7–6.7) | 4.4 (3.3–7.0) | 4.2 (3.4–6.6) | 4.5 (3.7–6.4) | 5.8 (4.9–8.8) |
| MMA# (methylating agents) | 94.3 (49.6–177.8) | 108.3 (49.0–229.0) | 79.7 (40.7–152.9) | 111.8 (60.3–203.7) | 93.4 (77.6–189.4) |
| CNEMA*** (acrylonitrile) | 7.8 (5.5–14.7) | 6.9 (4.4–13.9) | 8.6 (5.6–14.7) | 7.0 (5.2–11.1) | 48.1 (28.2–81.2) |
| 3HPMA** (acrolein) | 339.8 (215.3–499.3) | 268.0 (178.3–398.6) | 376.8 (219.4–523.5) | 313.0 (214.6–574.3) | 571.7 (429.3–1040.7) |
| 2HPMA (propylene oxide) | 24.2 20.5–31.1) | 24.4 (21.7–31.4) | 22.5 (17.5–30.4) | 24.3 (18.6–33.6) | 27.9 (21.7–39.0) |
| AAMA (acrylamide) | 141.5 (97.5–201.1) | 144.8 (104.7–203.0) | 132.0 (91.3–193.6) | 127.0 (92.3–166.9) | 207.4 (171.9–277.2) |
| HPMMA***,### (crotonaldehyde) | 211.2 (173.1–301.1) | 187.4 57.2–235.3) | 219.0 (166.6–308.3) | 209.4 (177.6–340.5) | 293.9 (263.4–371.8) |
| Biomarker | Oxidative stress (ng/mg creatinine) | ||||
| Total (N=68) | Fasting (N=23) | Lunch (N=23) | Dinner (N=15) | Sauna (N=7) | |
| Median (IQR): | Median (IQR): | Median (IQR): | Median (IQR): | Median (IQR): | |
| isoprostane* | 2.3 (1.8–3.1) | 2.7 (1.9–3.1) | 2.4 (1.9–1.2) | 1.8 (1.4–2.5) | 2.3 (2.2–3.5) |
| 8-OHdG | 85.2 (60.5–109.9) | 73.7 (47.2–109.9) | 98.7 (71.3–115.2) | 85.2 (62.5–98.5) | 57.1 (37.5–121.2) |
indicates a significant difference among fasting, lunch, dinner and sauna by the Skillings-Mack Test and # indicates a significant difference by the Skillings-Mack Test excluding the sauna samples. (*, #p < 0.05; **, ##p < 0.01; ***, ###p < 0.005)
There was a weaker association between biomarkers of oxidative stress or exposure to VOCs and collection period (Table 3). While neither biomarker of oxidative stress differed significantly among the different collection times, there was a time dependence on concentration for several VOC metabolites. Comparing across all collection times, the only significant differences were for PMA (benzene): median (IQR) of 6.1 (1.7–10.9) following sauna compared with 1.3 (0.5–2.3) fasting, ng/mg creatinine; 3HPMA (acrolein): median (IQR) of 571.7 (429.3–1040.7) following sauna compared with 268.0 (178.3–398.6) fasting, ng/mg creatinine; and HPMMA (crotonaldehyde): median (IQR) of 293.9 (263.4–371.8) following sauna compared with 187.4 (157.2–235.3) fasting, ng/mg creatinine. After excluding measurements taken after the sauna bath, the only significant differences between collection times were for MMA (methylating agents): median (IQR) of 111.8 (60.3–203.7) following dinner compared with 79.7 (40.7–152.9) following lunch, ng/mg creatinine and HPMMA (crotonaldehyde): median (IQR) of 219.0 (166.6–308.3) following lunch compared with 187.4 (157.2–235.3) fasting, ng/mg creatinine.
3.3. Correlation of urinary biomarker concentrations to personal exposure levels
Women who used cook stoves with chimneys had significantly lower urinary PAH biomarkers than those who used cook stoves without chimneys (median of total PAH (IQR): 353.8 (287.5–493.4) pmol/mg creatinine versus 1892.0 (1833.7–1950.4) pmol/mg creatinine; p = 0.03. This difference is particularly striking given only two households used a stove without a chimney. The difference in measured airborne PAHs, however, was not statistically significant. There was no significant difference in total urinary PAH metabolite concentration among those using different secondary (gas) stoves, based on the type of fuel used or whether the participant burned garbage. Additionally, there were no significant correlations between urinary PAH biomarker concentrations and the adjusted dietary index, tamales or tortillas consumed in univariate or multivariate models (data not shown; ρ < 0.17; p > 0.17) so data were not adjusted for dietary intake in the final analysis.
In general, urinary PAH biomarker concentrations were correlated with personal exposure levels to airborne PAHs. The personal mean urinary hydroxyfluorenes (ρ = 0.43 to 0.51, p < 0.05), hydroxyphenanthrenes (ρ = 0.63 to 0.72, p < 0.005) and 1-hydroxypyrene (ρ = 0.52, p < 0.05) correlated with the concurrent airborne concentration of the parent PAH (e.g. phenanthrene) (Table 4). Urinary PAH metabolites also correlated with long-term kitchen particulate matter concentration; there is a strong positive correlation between all urinary PAH biomarkers and the mean 48-hr PM2.5 level (Total PAH: ρ = 0.89, p < 0.001) (Table 5).
Table 4.
Correlation of urinary PAH metabolites to concurrent airborne PAH levels
| Biomarker | Airborne PAH | ρ (p)a |
|---|---|---|
| 2-naphthol | Naphthalene | 0.00 (0.98) |
| 1-hydroxyfluorene | Fluorene | 0.51 (0.02) |
| 2-hydroxyfluorene | Fluorene | 0.51 (0.02) |
| 3-hydroxyfluorene | Fluorene | 0.43 (0.04) |
| 1-hydroxyphenanthrene | Phenanthrene | 0.72 (<0.001) |
| 2-hydroxyphenanthrene | Phenanthrene | 0.63 (0.002) |
| 3,4-hydroxyphenanthrene | Phenanthrene | 0.64 (0.001) |
| 1-hydroxypyrene | Pyrene | 0.51 (0.01) |
Determined by Spearman rank correlation.
Table 5.
Correlation between mean urinary biomarker concentration and mean measured PM2.5 levels
| Biomarker | Mean 48 hr PM2.5a ρ (p)b |
|---|---|
| 2-naphthol | 0.82 (<0.001) |
| 1-hydroxyfluorene | 0.76 (<0.001) |
| 2-hydroxyfluorene | 0.93 (<0.001) |
| 3-hydroxyfluorene | 0.76 (<0.001) |
| 1-hydroxyphenanthrene | 0.86 (<0.001) |
| 2-hydroxyphenanthrene | 0.78 (<0.001) |
| 3,4-hydroxyphenanthrene | 0.84 (<0.001) |
| 1-hydroxypyrene | 0.84 (<0.001) |
| Total PAH | 0.89 (<0.001) |
| PMA | 0.80 (<0.001) |
| MHBMA-3 | 0.49 (0.06) |
| HEMA | 0.24 (0.39) |
| MMA | 0.13 (0.66) |
| CNEMA | 0.59 (0.02) |
| 3HPMA | 0.35 (0.20) |
| 2HPMA | 0.18 (0.52) |
| AAMA | 0.29 (0.30) |
| HPMMA | −0.09 (0.76) |
| isoprostane | 0.14 (0.66) |
| 8-OHdG | 0.22 (0.49) |
Indicates average of measurements taken on six separate visits from pregnancy to three months postnatal.
Determined by Spearman rank correlation.
The correlation between VOC metabolites and long-term exposure levels are not as robust. Several VOC biomarkers did significantly correlate with PM2.5, however, there were fewer (Table 5). Only PMA (benzene; ρ=0.80, p < 0.001) and CNEMA (acrylonitrile; ρ=0.59, p < 0.05) were correlated with the long-term mean PM2.5 kitchen concentration.
Neither 8-OHdG nor 8-isoprostane were correlated with long-term PM2.5 exposure (Table 5). Additionally, the concentrations of urinary 8-OHdG and 8-isoprostane were not correlated with the concentration of any PAH or VOC metabolite (ρ =-0.20 to 0.38, p > 0.07). Urinary biomarkers of PAHs, VOCs and oxidative stress were not significantly correlated with 48-hour PM2.5 measures taken concurrently with urine sample collection (data not shown).
3.4. Comparison of biomarker concentrations to smokers and industrial workers exposed to coal processing
We compared elevated urinary metabolite concentrations found in our study to other studies with groups known to have high exposure, such as smokers and workers in industrial coal processing. We compared our findings to published values that used the same lab methodology (Alwis et al., 2012; Benowitz et al., 2015; Campos et al., 2011; St Helen et al., 2014). The median individual mean urinary PAH metabolite concentrations among women exposed to wood smoke in this study were significantly higher than those of Chinese smokers (mean cigarettes per day = 18.0) across all PAHs (Benowitz et al., 2015) (Table 6). The median concentration of the most common urinary PAH metabolite measured, 1-hydroxypyrene, was 5.3 ng/mg creatinine (IQR: 2.9–9.1) among study participants and 0.8 ng/mg creatinine (IQR: 0.6–1.2) among smokers. Additionally, for all PAH metabolites, the median of the minimum measurements for each individual in the present study were significantly greater than the median for smokers (Table 6).
Table 6.
Comparison of urinary biomarker levels in present study (n=23 women) to those of smokers and those exposed to secondhand smoke
| Biomarker | OH-PAH (ng/mg creatinine)
|
||
|---|---|---|---|
| Present Study Median (IQR) | Smokera (n=238) Median (IQR) | Second-hand smoke exposure | |
| 1-hydroxypyrene | 5.3 (2.9–9.1) | 0.8 (0.6–1.2)***,### | – |
| 2-naphthol | 34.3 (25.2–93.7) | 14.8 (9.4–21.5)***,### | – |
| Sum of hydroxyfluroenes | 8.9 (6.6–22.7) | 4.4 (3.0–6.2)***,### | – |
| Sum of hydroxyphenanthrenes | 11.3 (8.2–25.3) | 1.8 (1.3–2.6)***,### | – |
|
| |||
|
VOC
|
|||
| Biomarker | Present Study | Smokerb (n=347) (ng/mL) | Second-hand smoke exposurec (n=14)(ng/mg creatinine) |
| Mean±SD (ng/mL) Median (IQR) (ng/mg creatinine) | Mean±SD | Median (IQR) | |
|
| |||
| PMA (benzene) | 2.5±2.6 1.5 (0.8–4.0) | 0.92±2.11* | 0.38 (0.26–0.42)***,### |
| MHBMA-3 (1,3-butadiene) | 0.6±0.3 0.6 (0.4–0.8) | 36±34*** | 0.65 (0.43–0.87)### |
| HEMA (ethylene oxide) | 5.0±2.4 4.5 (3.7–5.8) | 1.90±3.70*** | 2.93 (2.19–5.12)***,## |
| MMA (methylating agents) | 141.4±126.4 94.7 (69.0–250.9) | 46.9 (33.3–121.0)***,# | |
| CNEMA (acrylonitrile) | 39.9±122.7 8.9 (5.8–25.4) | 187±181*** | 2.53 (2.10–2.88)***,### |
| 3HPMA (acrolein) | 555.1±632.6 369.0 (261.4–648.1) | 1546±1643*** | 150.2 (127.8–191.7)***,### |
| 2HPMA (propylene oxide) | 25.0±10.8 25.6 (20.3–29.2) | 185±235*** | 35.9 (17.1–63.6)***,### |
| AAMA (acrylamide) | 169.0±124.8 155.5 (108.0–199.6) | 196±180 | 50.0 (37.4–66.1)***,### |
| HPMMA (crotonaldehyde) | 357.2±350.5 239.2 (181.0–488.1) | 1,992±2,009*** | 154.7 (109.6–183.1)***,### |
|
| |||
|
Oxidative Stress (ng/mg creatinine)
|
|||
| Biomarker | Present Study Mean±SD | Smokerd (n=85) Mean±SD | Second-hand smoke exposure |
|
| |||
| isoprostane | 2.8±1.4 | 1.4±0.8***,# | – |
| 8-OHdG | 91.3±26.2 | 10.7±4.1***,### | – |
Metabolite concentrations compared to values of Chinese smokers (mean cigarettes per day = 18.0) from Benowitz et al. 2015 using the Wilcoxon signed-rank test.
Metabolite concentrations compared to values of American smokers (serum cotinine ≥ 10 ng/mL) from Alwis et al. 2012 using the t test.
Metabolite concentrations compared to values of people exposed to cigarette smoke within a car for an hour from St. Helen st al. 2014 using the Wilcoxon signed-rank test.
Metabolite concentrations compared to values of healthy smokers from Campos et al 2011 using the t test.
indicates significant difference between the mean metabolite concentration and that of comparison group and # indicates significant difference between the minimum metabolite concentration and that of the comparison group (*,#p < 0.05; **,##p < 0.01; ***,###p < 0.005)
Mean urinary 1-hydroxypyrene concentrations in study participants exposed to wood smoke was similar to urinary concentrations measured in workers exposed to coke ovens or aluminum production (Jongeneelen, 2001). Approximately 52% and 20% of women had urinary 1-hydroxypyrene concentrations that were higher than the occupational exposure limits in coke oven and aluminum production workers, respectively (Table 7). Urinary concentrations of 1-hydroxypyrene were particularly high among the seven sauna bath users. Six out of seven women had urinary 1-hydroxpyrene concentrations that were higher than the occupational exposure limit of workers exposed to coke ovens (4.43 ng/mg creatinine); 5 out of 7 women had urinary 1-hydroxpyrene concentrations that were higher than workers in aluminum production (9.26 ng/mg creatinine) (Jongeneelen, 2001). Excluding sauna measurements led to a modest reduction in the proportion of women with levels of 1-hydroxypyrene comparable to those of occupational exposure, however, a large proportion of women still had urinary concentrations greater than the occupational exposure limits from coke ovens (52%) and aluminum production (17%) (Table 7).
Table 7.
Proportion of study participants with minimum, mean and maximum urinary 1-hydroxypyrene concentrations greater than the occupational exposure limita
|
|
|||
|---|---|---|---|
| Occupational Exposure Limit | Minimum n (%) | Mean n (%) | Maximum n (%) |
| 9 (39) | 12 (52) | 15 (65) | |
| Coke ovens (4.44 ng/mg creatinine) | Excluding sauna measurements: | Excluding sauna measurements: | Excluding sauna measurements: |
| 9 (39) | 12 (52) | 14 (61) | |
| 2 (9) | 5 (22) | 9 (39) | |
| Aluminum production (9.45 ng/mg creatinine) | Excluding sauna measurements: | Excluding sauna measurements: | Excluding sauna measurements: |
| 2 (9) | 4 (17) | 7 (30) | |
Values for occupational exposure limits from Jongeneelen 2001.
Urinary concentrations of VOC metabolites from HAP were lower than those from smoking (Alwis et al., 2012) but higher than those from secondhand smoke (St Helen et al., 2014) (Table 6). Study participants had significantly higher urine concentrations than smokers for the metabolites of two VOCs, benzene (mean PMA concentration of 2.5 ng/mg creatinine (SD=2.6) among study participants compared to a mean of 0.92 ng/mg creatinine (SD=2.11) among smokers) and ethylene oxide (mean HEMA concentration of 5.0 ng/mg creatinine (SD=2.4) among study participants compared to a mean of 1.90 ng/mg creatinine (SD=3.70) among smokers). For these two biomarkers, 43% of women had minimum PMA concentrations and 65% had minimum HEMA concentrations that were higher than the mean levels in smokers (data not shown). The difference for AAMA (biomarker for acrylamide) was non-significant (Table 6), though over half of the women in the study (57%) had at least one measurement higher than the mean level of smokers (data not shown). The remaining VOC biomarkers were significantly higher among smokers.
Urinary concentrations of biomarkers for seven of nine VOC metabolites measured were higher in study participants than those exposed to secondhand smoke (St Helen et al., 2014) (Table 6). For these metabolites, the median lowest measured urinary concentration among participants was significantly higher than median levels in people exposed to secondhand smoke in a controlled exposure study (Table 6) (St Helen et al., 2014). Only urinary 2-HPMA was at a higher concentration after secondhand smoke exposure; MHBMA-3 a 1,3-butadiene metabolite, was not significantly different (Table 6). Despite no statistically significant difference between median levels of MHBMA-3 among those exposed to HAP and those exposed to second hand smoke, 70% of study participants had maximum measured concentrations of MHBMA-3 that exceeded the median exposure seen in secondhand smokers (data not shown).
Urinary markers of oxidative stress were higher in current study participants than among smokers (Campos et al., 2011) (Table 6). For both isoprostane and 8-OHdG the means of individual mean and lowest urinary concentrations were significantly higher than that found among smokers (Table 6) (Campos et al., 2011). Mean isoprostane (2.8 ng/mg creatinine, SD=1.4) and 8-OHdG (91.3 ng/mg creatinine, SD=26.2) within this study were two (1.4 ng/mg creatinine, SD=0.8) and eight (10.7 ng/mg creatinine, SD=4.1) times that found in smokers, respectively (Campos et al., 2011).
4. Discussion
This study indicates that solid fuel use in rural Guatemala presents high exposures to chemicals known to pose significant risks to health. Women participating in the study were exposed to levels of particulate matter far higher than the WHO air quality guidelines of 10 μg/m3 (WHO, 2014), which is particularly troubling given nearly all women used chimney stoves. The concentration of biomarkers of PAH and VOC exposure seen within this study are far higher than those seen in high income countries (Alshaarawy et al., 2016) and comparable to, or higher than, those seen in other studies in developing countries (Li et al., 2011; Pruneda-Alvarez et al., 2012; Riojas-Rodriguez et al., 2011). The urinary concentrations for 1-hydroxypyrene, a commonly used proxy for PAH exposure, were 0.083 ng/mg creatinine in NHANES (Alshaarawy et al., 2016), 2.5 ng/mg creatinine in Peru (Li et al., 2011), 7.68 ng/mg creatinine in Mexico (Pruneda-Alvarez et al., 2012), and 5.5 ng/mg creatinine in this study. Additionally, exposure to PAHs within this study are greater than that of heavy smokers (Benowitz et al., 2015) and, alarmingly, comparable to industrial exposure to coke ovens (Jongeneelen, 2001) even after excluding measurements taken after sauna use. VOC urinary metabolite concentrations in this study were greater than those seen in second-hand smokers (St Helen et al., 2014). Exposure to two carcinogenic volatile organic compounds, benzene and ethylene oxide (IARC, 2015), was higher among current study participants than smokers (Alwis et al., 2012). These exposures, however, were not associated with increased urinary markers of oxidative stress as has been shown in other studies (Commodore et al., 2013; Pilger and Rudiger, 2006). Overall, the concentration urinary markers of oxidative stress reported here were similar to those among women exposed to woodsmoke from cooking, though (Commodore et al., 2013).
The high concentrations of urinary metabolites from PAH and VOC exposure in this study are likely due to high levels of woodsmoke exposure from cooking since none of the women were smokers and only one lived with a smoker. The urinary concentrations of all PAH and some VOC metabolites were strongly, positively correlated kitchen particulate matter concentration, as has been previously shown in a study of urinary PAHs among solid fuel users in Peru (Li et al., 2011). It should be noted, however, that Li et al., 2011 found a significant correlation between urinary biomarker and particulate matter concentrations collected concurrently, whereas the correlation was significant only for the long-term average particulate matter concentration in the results presented here. More importantly, dietary factors were not correlated with urinary metabolite concentrations, possibly due to a lower intake of dietary PAHs relative to PAHs in woodsmoke. Previous studies on urinary PAH metabolites from woodsmoke found no association between food consumption and urinary PAH levels (Pruneda-Alvarez et al., 2012; Riojas-Rodriguez et al., 2011). A lack of statistical power from the small sample size or the low variability in the diet of these rural women cannot, however, be excluded as other potential reasons for the lack of correlation.
This study was nested within a larger study exploring effects of HAP on birth outcomes and child development. While urine samples were collected three months after birth, these levels were strongly correlated with the long-term airborne kitchen PM2.5 kitchen concentrations which included PM2.5 measurements taken during pregnancy. This indicates significant exposure to woodsmoke during the prenatal period, especially given the limited half-life of urinary PAHs (Li et al., 2012). As such, these levels are particularly striking given the strong effect of PAH exposure during the first trimester (Choi et al., 2012) on outcomes such as small for gestational age (Choi et al., 2008), preterm birth (Choi et al., 2008) and neural tube defects (Ren et al., 2011). Additionally, the exposure to only three VOCs from second-hand smoke has been found to double the estimated lifetime excess risk of cancer death (St Helen et al., 2014). The increased risk from exposure to household woodsmoke, however, could be far greater than this due to the higher exposure concentrations and longer exposure times. This represents a previously unidentified health risk as this was the first study to examine urinary metabolite levels from VOC exposure during cooking with solid fuels. Given the small study size, we could not make direct associations with adult or adverse birth outcomes. However, the magnitude of the PAH and VOC exposures found in this study represent a health risk to those who face chronic, daily exposures to household air pollution.
There was a significant association between time of collection and urinary biomarker concentrations. Though this has been previously identified (Li et al., 2010), other studies on exposure to woodsmoke have measured metabolite concentrations in first morning urine (Li et al., 2011; Pruneda-Alvarez et al., 2012). Quantifying the increased health risk from HAP using these measurements may thus underestimate the burden, given the markedly higher concentrations found in this study after wood-fired sauna bath use or after meals, the preparation of which exposes them to high levels of woodsmoke from cooking. While there are rapid increases in urinary metabolite concentrations from exposure to both airborne and dietary PAHs soon after exposure, they reach a maximum three to five hours after exposure (Li et al., 2012; Li et al., 2016). Samples were taken one to two hours following exposure and, thus, reported values may be an underestimate as there was not adequate time for urinary concentrations to reach a maximum.
This study highlights the need for the dissemination of cook stoves that completely combust cooking fuels in Guatemala. The Global Alliance for Clean Cookstoves was launched in 2010 with the goal to provide clean cook stoves to 100 million people by 2020; however, care must be taken to ensure that cook stoves reduce household smoke to acceptable levels. As evidenced by this study and others (Li et al., 2011; Riojas-Rodriguez et al., 2011; Torres-Dosal et al., 2008), even with “improved” solid fuel cook stoves urinary levels of PAHs can exceed levels found in tobacco smokers. Similarly, a recent laboratory study of improved stoves showed that despite large decreases in emissions compared to open fires, even the cleanest solid-fuel stove produced high levels of mutagenic emissions (Mutlu et al., 2016). Therefore, stoves that use cleaner burning fuels, such as liquid propane gas or electricity, should be encouraged. Liquid propane gas is associated with reduced concentrations of airborne PM and PAHs, even in unventilated kitchens (Titcombe and Simcik, 2011), and with reduced urinary concentrations of PAH metabolites (Pruneda-Alvarez et al., 2012). However, the availability of non-solid fuels in Guatemala has remained unchanged since 1990, especially among the rural population (World Bank, 2012), and, thus, greater accessibility is needed to reduce the burden of HAP within the country.
4.1. Limitations
This study had a small sample size (N=23) so observed trends may not be representative of a wider population. The small sample meant there was low power to see differences in PAH concentrations based on secondary stove use, burning of garbage or fuel type, which could explain the lack of significant differences. Future larger studies are required to verify the observed trends, to perform sub-group analyses and to investigate possible effects on birth and developmental outcomes.
Measurements of airborne particulate matter were only collected within the kitchen. Therefore, the contribution of other non-kitchen sources on urinary metabolite concentrations could not be investigated. Additionally, this could potentially explain the lack of correlation with the PM2.5 measurements taken concurrently with urine samples. Kitchen concentrations may not have accurately reflected personal exposures during the measurement period and, thus, did not correlate with urinary biomarker concentrations. However, the long-term average might have reduced intra-household variance, serving as a better measure of exposure and correlating with urinary biomarker concentrations.
The airborne PAH measures were of potentially low quality. This could be due to PAH losses from volatilization during field sampling, storage, transport, and lab extraction. There were poor correlations between duplicate PAH filter measurements. Additionally, in general, naphthalene is the most abundant PAH, whereas in this study we found it to be the least abundant. Thus, the poor airborne PAH measurements could have contributed to the weak correlations between airborne and urinary metabolite concentrations.
Urinary metabolites of PAHs, VOCs and oxidative stress were analyzed from the same sample. It has been shown that increases in urinary markers of oxidative stress lag several hours behind those of PAHs from dietary exposure (Chien and Yeh, 2010). Thus, since both were analyzed from the sample, there was no time for 8-OHdG concentration to reflect possible associated increases in oxidative stress from PAH exposure and possibly explaining the lack of correlation. The heterogeneity in results of this study and others may be due to the variety of confounding factors associated with urinary oxidant concentrations, such as age, physical activity and vitamin status (Pilger and Rudiger, 2006; Romanazzi et al., 2013).
5. Conclusions
Exposure to woodsmoke represents a major risk factor for deleterious health outcomes around the world. Participants in this study in rural Guatemala are exposed daily to high levels of particulate matter from wood-fired cookstoves in their kitchens, which were associated with high levels of urinary PAH and VOC metabolites. These exposures to PAHs and VOCs from incomplete combustion of solid fuels are dependent on time of day and activity (e.g. cooking or sauna bath use) and were found to be comparable to other studies of smokers or industrial workers. Given the effects of these pollutants on fetal development during pregnancy, our findings indicate the importance of reducing exposure to woodsmoke among women cooking with solid fuels. In this study, women used chimney stoves for cooking, thus, highlighting a need to disseminate and encourage the use of cleaner burning cooking fuels in Guatemala and in other countries similarly burdened by solid fuel use for daily cooking. In addition to the potential health effects, in areas where liquid propane gas is available, switching to this fuel has ancillary benefits including potential reduced fuel costs among those who purchase wood, time savings, and improved diet (Anderman et al., 2015).
Acknowledgments
This work was supported by National Center for Research Resources (No. KL2RR024130); and the National Institutes of Health (No. S10 RR026437 and No. P30 DA012393). The authors are very grateful to Biruk Temru and Charles Perrino at UC Berkeley; in Guatemala, Eduardo Canuz, Maritza Barrios, Expedita Ramirez, Domitila Velasquez Ambrosio and study participants. The authors also thank Margaret Peng for performing analytical chemistry and for critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer:
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Competing interests: We declare that we have no financial or non-financial competing interests related to the study.
References
- Alshaarawy O, Elbaz HA, Andrew ME. The association of urinary polycyclic aromatic hydrocarbon biomarkers and cardiovascular disease in the US population. Environ Int. 2016:89–90. doi: 10.1016/j.envint.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwis KU, Blount BC, Britt AS, Patel D, Ashley DL. Simultaneous analysis of 28 urinary VOC metabolites using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI/MSMS) Anal Chim Acta. 2012;750:152–160. doi: 10.1016/j.aca.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderman TL, DeFries RS, Wood SA, Remans R, Ahuja R, Ulla SE. Biogas Cook Stoves for Healthy and Sustainable Diets? A Case Study in Southern India. Front Nutr. 2015;2:28. doi: 10.3389/fnut.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Gan Q, Goniewicz ML, Lu W, Xu J, Li X, Jacob P, 3rd, Glantz S. Different profiles of carcinogen exposure in Chinese compared with US cigarette smokers. Tob Control. 2015;24:e258–263. doi: 10.1136/tobaccocontrol-2014-051945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos C, Guzman R, Lopez-Fernandez E, Casado A. Urinary biomarkers of oxidative/nitrosative stress in healthy smokers. Inhal Toxicol. 2011;23:148–156. doi: 10.3109/08958378.2011.554460. [DOI] [PubMed] [Google Scholar]
- Chatfield M, Mander A. The Skillings-Mack test (Friedman test when there are missing data) Stata J. 2009;9:299–305. [PMC free article] [PubMed] [Google Scholar]
- Chien YC, Yeh CT. Excretion characteristics of urinary 8-hydroxydeoxyguanosine after dietary exposure to polycyclic aromatic hydrocarbons. Environ Mol Mutagen. 2010;51:243–250. doi: 10.1002/em.20536. [DOI] [PubMed] [Google Scholar]
- Choi H, Rauh V, Garfinkel R, Tu Y, Perera FP. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and risk of intrauterine growth restriction. Environ Health Perspect. 2008;116:658–665. doi: 10.1289/ehp.10958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Wang L, Lin X, Spengler JD, Perera FP. Fetal window of vulnerability to airborne polycyclic aromatic hydrocarbons on proportional intrauterine growth restriction. PLoS One. 2012;7:e35464. doi: 10.1371/journal.pone.0035464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborators G.B.D.R.F. Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, Burnett R, Casey D, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:2287–2323. doi: 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commodore AA, Zhang JJ, Chang Y, Hartinger SM, Lanata CF, Mausezahl D, Gil AI, Hall DB, Aguilar-Villalobos M, Vena JE, Wang JS, Naeher LP. Concentrations of urinary 8-hydroxy-2′-deoxyguanosine and 8-isoprostane in women exposed to woodsmoke in a cookstove intervention study in San Marcos, Peru. Environ Int. 2013;60:112–122. doi: 10.1016/j.envint.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MD, Saparbaev M, Cooke MS. DNA repair and the origins of urinary oxidized 2′-deoxyribonucleosides. Mutagenesis. 2010;25:433–442. doi: 10.1093/mutage/geq031. [DOI] [PubMed] [Google Scholar]
- Hammond SK, Leaderer BP. A diffusion monitor to measure exposure to passive smoking. Environ Sci Technol. 1987;21:494–497. doi: 10.1021/es00159a012. [DOI] [PubMed] [Google Scholar]
- Haussmann HJ. Use of hazard indices for a theoretical evaluation of cigarette smoke composition. Chem Res Toxicol. 2012;25:794–810. doi: 10.1021/tx200536w. [DOI] [PubMed] [Google Scholar]
- Institute for Health Metrics and Evaluation (IHME) GBD Compare Data Visualization. Seattle, WA: IHME, University of Washington; 2015. Available from http://vizhub.healthdata.org/gbd-compare. [Google Scholar]
- International Agency for Research on Cancer (IARC) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Lyon, France. 2015. [PMC free article] [PubMed] [Google Scholar]
- Jacob P, 3rd, Wilson M, Benowitz NL. Determination of phenolic metabolites of polycyclic aromatic hydrocarbons in human urine as their pentafluorobenzyl ether derivatives using liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79:587–598. doi: 10.1021/ac060920l. [DOI] [PubMed] [Google Scholar]
- Jongeneelen FJ. Benchmark guideline for urinary 1-hydroxypyrene as biomarker of occupational exposure to polycyclic aromatic hydrocarbons. Ann Occup Hyg. 2001;45:3–13. [PubMed] [Google Scholar]
- Lam N, Nicas M, Ruiz-Mercado I, Thompson LM, Romero C, Smith KR. Noninvasive measurement of carbon monoxide burden in Guatemalan children and adults following wood-fired temazcal (sauna-bath) use. J Environ Monit. 2011;13:2172–2181. doi: 10.1039/c1em10172b. [DOI] [PubMed] [Google Scholar]
- Li J, Lu S, Liu G, Zhou Y, Lv Y, She J, Fan R. Co-exposure to polycyclic aromatic hydrocarbons, benzene and toluene and their dose-effects on oxidative stress damage in kindergarten-aged children in Guangzhou, China. Sci Total Environ. 2015:524–525. doi: 10.1016/j.scitotenv.2015.04.020. [DOI] [PubMed] [Google Scholar]
- Li Z, Romanoff L, Bartell S, Pittman EN, Trinidad DA, McClean M, Webster TF, Sjodin A. Excretion profiles and half-lives of ten urinary polycyclic aromatic hydrocarbon metabolites after dietary exposure. Chem Res Toxicol. 2012;25:1452–1461. doi: 10.1021/tx300108e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Romanoff LC, Lewin MD, Porter EN, Trinidad DA, Needham LL, Patterson DG, Jr, Sjodin A. Variability of urinary concentrations of polycyclic aromatic hydrocarbon metabolite in general population and comparison of spot, first-morning, and 24-h void sampling. J Expo Sci Environ Epidemiol. 2010;20:526–535. doi: 10.1038/jes.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Sjodin A, Romanoff LC, Horton K, Fitzgerald CL, Eppler A, Aguilar-Villalobos M, Naeher LP. Evaluation of exposure reduction to indoor air pollution in stove intervention projects in Peru by urinary biomonitoring of polycyclic aromatic hydrocarbon metabolites. Environ Int. 2011;37:1157–1163. doi: 10.1016/j.envint.2011.03.024. [DOI] [PubMed] [Google Scholar]
- Li Z, Trinidad D, Pittman EN, Riley EA, Sjodin A, Dills RL, Paulsen M, Simpson CD. Urinary polycyclic aromatic hydrocarbon metabolites as biomarkers to woodsmoke exposure — results from a controlled exposure study. J Expo Sci Environ Epidemiol. 2016;26:241–248. doi: 10.1038/jes.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken JP, Grajeda L, Reyes A, Sajquim E. Burden of Disease due to Household Air Pollution in Guatemala. Report for the Public Health Institute; Oakland, CA USA: 2015. April 13, 2015. [Google Scholar]
- Milne GL, Musiek ES, Morrow JD. F2-isoprostanes as markers of oxidative stress in vivo: an overview. Biomarkers. 2005;10(Suppl 1):S10–23. doi: 10.1080/13547500500216546. [DOI] [PubMed] [Google Scholar]
- Mutlu E, Warren SH, Ebersviller SM, Kooter IM, Schmid JE, Dye JA, Linak WP, Gilmour MI, Jetter JJ, Higuchi M, DeMarini DM. Mutagenicity- and Pollutant-Emission Factors of Solid-Fuel Cookstoves: Comparison to Other Combustion Sources. Environ Health Perspect; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health (NIH) RFA-HL-16-012: Household Air Pollution (HAP) Health Outcomes Trial (UM1) UM1 Funding Opportunity; 2015. [Google Scholar]
- Nuernberg AM, Boyce PD, Cavallari JM, Fang SC, Eisen EA, Christiani DC. Urinary 8-isoprostane and 8-OHdG concentrations in boilermakers with welding exposure. J Occup Environ Med. 2008;50:182–189. doi: 10.1097/JOM.0b013e31815cf6cc. [DOI] [PubMed] [Google Scholar]
- Padula AM, Noth EM, Hammond SK, Lurmann FW, Yang W, Tager IB, Shaw GM. Exposure to airborne polycyclic aromatic hydrocarbons during pregnancy and risk of preterm birth. Environ Res. 2014;135:221–226. doi: 10.1016/j.envres.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patelarou E, Kelly FJ. Indoor exposure and adverse birth outcomes related to fetal growth, miscarriage and prematurity-a systematic review. International journal of environmental research and public health. 2014;11:5904–5933. doi: 10.3390/ijerph110605904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilger A, Rudiger HW. 8-Hydroxy-2′-deoxyguanosine as a marker of oxidative DNA damage related to occupational and environmental exposures. Int Arch Occup Environ Health. 2006;80:1–15. doi: 10.1007/s00420-006-0106-7. [DOI] [PubMed] [Google Scholar]
- Poulsen HE, Nadal LL, Broedbaek K, Nielsen PE, Weimann A. Detection and interpretation of 8-oxodG and 8-oxoGua in urine, plasma and cerebrospinal fluid. Biochim Biophys Acta. 2014;1840:801–808. doi: 10.1016/j.bbagen.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Pruneda-Alvarez LG, Perez-Vazquez FJ, Salgado-Bustamante M, Martinez-Salinas RI, Pelallo-Martinez NA, Perez-Maldonado IN. Exposure to indoor air pollutants (polycyclic aromatic hydrocarbons, toluene, benzene) in Mexican indigenous women. Indoor Air. 2012;22:140–147. doi: 10.1111/j.1600-0668.2011.00750.x. [DOI] [PubMed] [Google Scholar]
- Ren A, Qiu X, Jin L, Ma J, Li Z, Zhang L, Zhu H, Finnell RH, Zhu T. Association of selected persistent organic pollutants in the placenta with the risk of neural tube defects. Proc Natl Acad Sci U S A. 2011;108:12770–12775. doi: 10.1073/pnas.1105209108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riojas-Rodriguez H, Schilmann A, Marron-Mares AT, Masera O, Li Z, Romanoff L, Sjodin A, Rojas-Bracho L, Needham LL, Romieu I. Impact of the improved patsari biomass stove on urinary polycyclic aromatic hydrocarbon biomarkers and carbon monoxide exposures in rural Mexican women. Environ Health Perspect. 2011;119:1301–1307. doi: 10.1289/ehp.1002927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanazzi V, Pirro V, Bellisario V, Mengozzi G, Peluso M, Pazzi M, Bugiani M, Verlato G, Bono R. 15-F(2)t isoprostane as biomarker of oxidative stress induced by tobacco smoke and occupational exposure to formaldehyde in workers of plastic laminates. Sci Total Environ. 2013;442:20–25. doi: 10.1016/j.scitotenv.2012.10.057. [DOI] [PubMed] [Google Scholar]
- Smith KR, McCracken JP, Thompson L, Edwards R, Shields KN, Canuz E, Bruce N. Personal child and mother carbon monoxide exposures and kitchen levels: methods and results from a randomized trial of woodfired chimney cookstoves in Guatemala (RESPIRE) J Expo Sci Environ Epidemiol. 2010;20:406–416. doi: 10.1038/jes.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, McCracken JP, Weber MW, Hubbard A, Jenny A, Thompson LM, Balmes J, Diaz A, Arana B, Bruce N. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. Lancet. 2011;378:1717–1726. doi: 10.1016/S0140-6736(11)60921-5. [DOI] [PubMed] [Google Scholar]
- Sorensen M, Andersen AM, Raaschou-Nielsen O. Non-occupational exposure to paint fumes during pregnancy and fetal growth in a general population. Environ Res. 2010;110:383–387. doi: 10.1016/j.envres.2010.02.011. [DOI] [PubMed] [Google Scholar]
- St Helen G, Jacob P, 3rd, Peng M, Dempsey DA, Hammond SK, Benowitz NL. Intake of toxic and carcinogenic volatile organic compounds from secondhand smoke in motor vehicles. Cancer Epidemiol Biomarkers Prev. 2014;23:2774–2782. doi: 10.1158/1055-9965.EPI-14-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwan-ampai P, Navas-Acien A, Strickland PT, Agnew J. Involuntary tobacco smoke exposure and urinary levels of polycyclic aromatic hydrocarbons in the United States, 1999 to 2002. Cancer Epidemiol Biomarkers Prev. 2009;18:884–893. doi: 10.1158/1055-9965.EPI-08-0939. [DOI] [PubMed] [Google Scholar]
- Svecova V, Rossner P, Jr, Dostal M, Topinka J, Solansky I, Sram RJ. Urinary 8-oxodeoxyguanosine levels in children exposed to air pollutants. Mutat Res. 2009;662:37–43. doi: 10.1016/j.mrfmmm.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Thompson LM, Bruce N, Eskenazi B, Diaz A, Pope D, Smith KR. Impact of reduced maternal exposures to wood smoke from an introduced chimney stove on newborn birth weight in rural Guatemala. Environ Health Perspect. 2011a;119:1489–1494. doi: 10.1289/ehp.1002928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LM, Clark M, Cadman B, Canuz E, Smith KR. Exposures to high levels of carbon monoxide from wood-fired temazcal (steam bath) use in highland Guatemala. Int J Occup Environ Health. 2011b;17:103–112. doi: 10.1179/107735211799030979. [DOI] [PubMed] [Google Scholar]
- Titcombe ME, Simcik M. Personal and indoor exposure to PM(2).(5) and polycyclic aromatic hydrocarbons in the southern highlands of Tanzania: a pilot-scale study. Environ Monit Assess. 2011;180:461–476. doi: 10.1007/s10661-010-1799-3. [DOI] [PubMed] [Google Scholar]
- Torres-Dosal A, Perez-Maldonado IN, Jasso-Pineda Y, Martinez Salinas RI, Alegria-Torres JA, Diaz-Barriga F. Indoor air pollution in a Mexican indigenous community: evaluation of risk reduction program using biomarkers of exposure and effect. Sci Total Environ. 2008;390:362–368. doi: 10.1016/j.scitotenv.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Vanker A, Barnett W, Nduru PM, Gie RP, Sly PD, Zar HJ. Home environment and indoor air pollution exposure in an African birth cohort study. Sci Total Environ. 2015;536:362–367. doi: 10.1016/j.scitotenv.2015.06.136. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zheng Y, Zhao B, Zhang Y, Liu Z, Xu J, Chen Y, Yang Z, Wang F, Wang H, He J, Zhang R, Abliz Z. Human metabolic responses to chronic environmental polycyclic aromatic hydrocarbon exposure by a metabolomic approach. Journal of proteome research. 2015;14:2583–2593. doi: 10.1021/acs.jproteome.5b00134. [DOI] [PubMed] [Google Scholar]
- WHO. Population using solid fuels (estimates): Data by country. Global Health Observatory data repository 2013 [Google Scholar]
- WHO. WHO Indoor Air Quality Guidelines: Household Fuel Combustion. World Health Organization; Geneva: 2014. [PubMed] [Google Scholar]
- WHO and the International Agency for Research on Cancer (IARC) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 92. 2010. Some Non-heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures. [PMC free article] [PubMed] [Google Scholar]
- World Bank. Access to non-solid fuel (% of population) World Development Indicators; 2012. [Google Scholar]


