Abstract
Social cognition is impaired in people with schizophrenia and these deficits are strongly correlated with social functioning. Oxytocin is a hypothalamic peptide that contributes to maternal infant bonding and has diverse pro-social effects in adults. This study tested the hypothesis that 12 weeks of intranasal oxytocin will improve social cognitive function in outpatients with schizophrenia and schizoaffective disorder. Sixty-eight eligible participants were randomized to oxytocin (24 IU twice daily) or placebo. Social cognitive function was assessed using the Emotion Recognition-40, Brüne Theory of Mind, Reading the Mind in the Eyes test, Trustworthiness task and Ambiguous Intentions Hostility Questionnaire at baseline, 6 weeks and 12 weeks. In addition, social function was assessed using the Specific Levels of Functioning Scale and a role-play test, and psychopathology was assessed using the Positive and Negative Syndrome Scale (PANSS). Fifty-five participants completed the 12-week trial. The study found no evidence for a differential advantage of oxytocin over placebo on social cognition. Among secondary outcomes, there was a modest advantage for oxytocin over placebo on a component of social functioning, although there was also evidence that the placebo group outperformed the oxytocin group on the role-play task. No between-group differences emerged on measures of psychopathology in pre-specified comparisons, but oxytocin showed significant within-group reduction in PANSS negative symptoms and significant between-group improvement in negative symptoms in the schizophrenia subgroup. Further testing is needed to clarify whether oxytocin has therapeutic potential for social cognitive deficits and/or negative symptoms in people with schizophrenia.
Keywords: oxytocin, social cognition, psychosis, schizophrenia, schizoaffective disorder, negative symptoms
1. Introduction
Multiple dimensions of psychopathology contribute to impaired social functioning in people with schizophrenia, including the socially isolating effects of positive symptoms, the lack of social drive associated with negative symptoms, and the disabling impact of cognitive deficits. Among the many cognitive domains that are impaired in people with schizophrenia, social cognition represents a key domain that is strongly correlated with social functioning (Fett et al., 2011). Social cognition represents the cognitive functions involved in facilitating social decision-making and associated behaviors. It incorporates emotion recognition (identifying other people’s emotional states by interpreting their facial expressions), attributional style (beliefs about the causes of events) and theory of mind (inferring the thoughts and feelings of others) (Pinkham et al., 2014). Studies indicate that each of these components of social cognition are impaired in people with schizophrenia (Bora et al., 2009; Kohler et al., 2010); however, antipsychotic medications provide no measurable benefit for social cognitive deficits (Penn et al., 2009).
Given the current limitations of available treatments for impairments in social function in schizophrenia, novel pharmacological options have been sought. The hypothalamic nonapeptide oxytocin has emerged as an intriguing candidate. Oxytocin has diverse pro-social effects including regulation of maternal-infant bonding, social affiliative behavior, social recognition and interpersonal trust (Heinrichs and Domes, 2008; Meyer-Lindenberg et al., 2011). In healthy volunteers, single-dose intranasal oxytocin administration enhances interpersonal trust (Baumgartner et al., 2008; Kosfeld et al., 2005) and improves recognition of internal mental states from subtle facial cues (Domes et al., 2007; Schulze et al., 2011). Similar effects have also been demonstrated in individuals with social deficits including in people with autism. Intravenous oxytocin has improved interpretation of emotional content of speech in people with autism (Hollander et al., 2007) and intranasal oxytocin has improved recognition of affective states from facial cues (Guastella et al., 2010).
There have been several smaller studies on the effects of intranasal oxytocin on social cognitive functioning in people with schizophrenia. For example, our group examined the effects of daily administration of oxytocin for 2 weeks (Pedersen et al., 2011) and 6 weeks (Gibson et al., 2014), while others have explored the impact of twice-weekly oxytocin for 6 weeks (Davis et al., 2014). These studies found modest improvements on several measures of social cognition in adults with chronic schizophrenia.
The current study sought to better understand the therapeutic potential of oxytocin on social cognition in people with schizophrenia and schizoaffective disorder by extending the treatment duration to 12 weeks in a larger cohort of participants than previously studied and examining a broader range of social cognitive assessments. Performance was evaluated on theory of mind, emotion perception and attributional style, as well as on the secondary outcomes of social skills, functional outcome and psychopathology. It was hypothesized that oxytocin will lead to differential improvements in social cognition when compared to placebo from baseline to 12 weeks.
2. Methods
2.1 Study Design
The study was conducted between June 2011 and September 2014 in the outpatient research clinics of an academic medical center and an affiliated state psychiatric hospital. The Biomedical Institutional Review Board of the University of North Carolina at Chapel Hill approved the study. In this double-blind, randomized study, stable outpatients with schizophrenia or schizoaffective disorder were randomized to receive 12 weeks of daily intranasal oxytocin or placebo.
2.2 Participants
Eligible subjects were 18–65 years of age; criteria for schizophrenia or schizoaffective disorder were met as determined by the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, 4th edition; duration of illness ≥1 year; clinically stable outpatient status and receiving antipsychotic medication with no change in antipsychotic agents or dose for one month prior to entry; concomitant medications were permitted (except as noted in exclusion criteria) if doses were unchanged for one month prior to entry; to enrich baseline deficits in social functioning, participants had to score < 24 on Reading the Mind in the Eyes Test (Eyes Test, a measure of theory of mind and emotion recognition, score<24 is 0.5 standard deviation (SD) below the mean in a large normative sample) OR score ≥3 on two or more of the following Positive and Negative Syndrome Scale (PANSS) items: suspiciousness/persecution, hostility, passive/apathetic social withdrawal, uncooperativeness, active social avoidance; women of childbearing potential and male participants had to use an acceptable method of birth-control; all subjects provided written informed consent.
Exclusion criteria: Manic or hypomanic episode within the past 2 years for subjects with schizoaffective disorder; alcohol or substance abuse or dependence in the past 3 months (except caffeine or nicotine); simulant or chronic glucocorticoid use; unstable serious medical illness; major surgery/trauma in the past 4 months; pregnancy, childbirth in the past 6 months, or breast-feeding in the past 3 months; <5th grade reading level on the Wide Range Achievement Test (WRAT).
2.3 Intervention
Participants remained on their pre-study medications and doses over the course of the study. Intranasal study drug was self-administered twice daily (before breakfast and before dinner) for 12 weeks. Participants were trained on proper administration technique prior to baseline and administration was also observed at 2 and 6 weeks. Each dose consisted of six 0.1 mL insufflations (alternating every 30 seconds between the left and right nostril); each dose was approximately 24 international units (IU) of oxytocin (Syntocinon Spray, Novartis) or placebo (containing each ingredient in Syntocinon Spray except oxytocin). This dose was the same as in our pilot studies which suggested efficacy on several measures of social cognition (Gibson et al., 2014; Pedersen et al., 2011). Bottles containing study drug (50 mL solution) were weighed before dispensing to subjects and upon return. Bottle weights and a daily medication diary were used to assess adherence to study drug.
2.4 Randomization
Eligible subjects were randomly assigned in blocks of four, stratified by gender, to receive oxytocin or placebo in a 1:1 ratio. Randomization was performed using PROC PLAN in SAS Version 9.2. An interim analysis, which was performed after 19 subjects had completed the trial, revealed significantly uneven distribution in baseline PANSS scores between the two treatment groups. Given the target sample size (N=60), there was concern that the uneven distribution in baseline PANSS scores would not have time to even out over the remainder of enrollment. Therefore, subsequent randomization was also stratified by low (<63) versus high (≥63) baseline PANSS score based on a median split of total PANSS scores.
2.5 Primary Outcome Measures
Ratings of social cognitive function were administered at baseline, 6 and 12 weeks, as follows:
Emotion Recognition-40 Task (ER-40) – consists of 40 faces presented sequentially on a computer screen along with the choices of rating the face as happy, sad, anger, fear or no emotion (Kohler et al., 2004). It uses racially and ethnically diverse face images. Performance is indexed as the total number of correct responses.
Brüne Theory of Mind Stories Task – consists of a series of 6 sets of 4 cartoon pictures that illustrate interactions between two or more individuals (Brüne, 2003). The subject is asked to rearrange the pictures, initially presented in an illogical sequence, in an order that conveys a logical story. The duration of time the subject takes to complete the task and the accuracy of the sequencing is recorded. The subject is then asked questions about the characters’ own beliefs and beliefs of other characters in the cartoons. The subject’s interpretations of the characters’ beliefs are scored as correct or incorrect.
Reading the Mind in the Eyes Test (Eyes Test) – consists of 36 photographs and participants are asked to guess the mental state from among 4 choice words (Baron-Cohen et al., 2001). Each eye region is presented on a computer screen with the four-choice mental states shown in the four corners of the card or computer screen (one target word and three foil words). Performance is measured by the number of faces correctly discriminated.
Trustworthiness Task – comprises 42 faces of unfamiliar people (Adolphs et al., 1998). Participants are shown each picture individually on a computer screen and are asked to rate how much they would trust that person (i.e., with their money or their life) on a 7-point scale, ranging from −3 (very untrustworthy) to +3 (very trustworthy). Performance was indexed as the average rating for trustworthy and untrustworthy faces separately.
Ambiguous Intentions Hostility Questionnaire (AIHQ) – comprises 15 short vignettes that reflect negative events that vary in intentionality (i.e., obvious, accidental, and ambiguous intentions) (Combs et al., 2007). Participants are asked to read each vignette, to imagine the scenario happening to her/him (e.g., “You walk past a bunch of teenagers at a mall and you hear them start to laugh”), and to write down the reason why the other person(s) acted that way toward her/him, as a means of measuring attributions. Two independent raters code this written response for the purpose of computing a “hostility bias”. The participant then rates, on Likert scales, whether the other person(s) performed the action on purpose, how angry it would make her/him feel, and how much they would blame the other person(s). These three Likert ratings are combined into a single index, a “blame bias.” Because the hostility bias tends to show subpar test-retest reliability (Pinkham et al., 2016), we focused only on the blame bias scores.
2.6 Secondary Outcome Measures
2.6.1 Social function
The following assessments were performed at baseline and 12 weeks:
Specific Levels of Functioning Scale (SLOF) – 30-item questionnaire with 2 social functioning subsections (Interpersonal Relationships [IR] and Social Acceptability [SA]) and 2 community living skills subsections (Activities, Work Skills) (Schneider and Struening, 1983). The subject completes one version and an informant who knows the subject well completes another. Each item is rated on a 5-point Likert scale with anchors describing the frequency of the behavior and/or the subject’s level of independence.
Social Skills Performance Assessment (SSPA) – Social skill was evaluated with a variation of the SSPA (Patterson et al., 2001). The SSPA was modified to evaluate empathy in social situations (as part of a secondary study). Specifically, the modified SSPA consists of two 90-second role-plays. The first role-play is an unstructured conversation in which the research confederate plays the role of a new neighbor with whom the subject is instructed to strike up a conversation. In the second role-play, the research confederate plays an upset friend with whom the subject is instructed to attempt to console. The role-plays are recorded and later rated on the following factors: Verbal social skill (VSS; content, clarity, fluency, asks questions), nonverbal social skill (NVSS) (gaze, involvement, meshing, appropriate affect, flat affect), and global social skill (GSS; social anxiety [reverse scored] and overall social skill). Raters were trained to reliability by first watching several role-plays (from a previous study that used the same assessment) together with an advanced doctoral student, discussing their ratings and coming to a consensus. They then rated 20 role-plays on their own and reliability was calculated. Once they attained acceptable reliability (ICCs >0.6), they were permitted to rate the role-plays from this study. Interrater reliability was calculated on the ratings of the first 39 videos of the schizophrenia sample completed by the coders; ICCs for all items were above 0.7 with the exception of Appropriate Affect (0.654).
2.6.2 Psychopathology
Positive and Negative Syndrome Scale (PANSS) – The PANSS was administered at baseline, 2, 6, 9 and 12 weeks. The PANSS is a 30-item scale that provides information on positive and negative symptoms of psychosis as well as general psychopathology (Kay et al., 1987).
2.7 Laboratory/Safety Measures
Laboratory blood tests including complete blood count, electrolytes, standard measures of hepatic and renal function, TSH, as well as urine for urinalysis and urine toxicology, and an electrocardiogram were performed at screening, baseline, weeks 4,8,12.
2.8 Statistical Analysis
The efficacy of oxytocin was assessed in subjects who received at least one dose of study medication and had at least one post-baseline assessment of the primary outcome measures (modified intention-to-treat population, mITT). Efficacy outcomes were analyzed via a longitudinal mixed model with outcome of change from baseline and predictors for treatment group, visit, age, treatment-by-visit interaction, and baseline score for the outcome. In these models, visit was treated as a categorical variable with an unstructured covariance pattern in order to reflect correlation between visits within the same subject. Least squares means for change from baseline were estimated at each visit for each treatment group. Analyses of raw data is presented in the text and tables, standardized change data is also included in a Supplementary Table. Imputation was not performed when participants discontinued from the study early. Kenward Roger degrees of freedom were used in the denominator of significance tests of all predictors. Post hoc analyses were performed to assess for potential treatment interactions with gender, diagnosis, concomitant medication groups, age and baseline PANSS total score. Given the exploratory nature of this study, outcome measures were not adjusted for multiple comparisons.
3. Results
3.1 Randomization and Baseline Characteristics
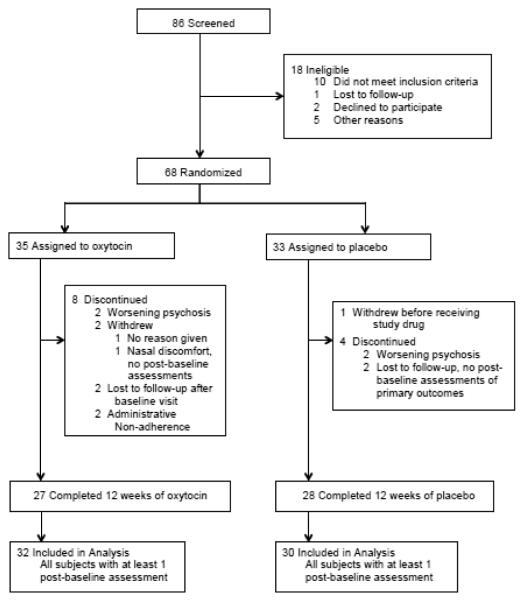
86 individuals were assessed for eligibility and 68 were found eligible and were randomized to study medication. Among randomized subjects, 35 were assigned oxytocin and 33 were assigned placebo. One subject in the placebo arm withdrew before receiving study medication. 32 subjects in the oxytocin arm and 30 subjects in the placebo arm completed at least one post-baseline assessment, constituting the mITT population. 27 subjects who received oxytocin and 28 subjects who received placebo completed 12 weeks of treatment. See Figure 1 for the CONSORT flowchart. Baseline demographic and clinical characteristics of the mITT population are presented in Table 1. No between-group differences emerged at baseline for any variables.
Figure 1.
Flowchart of subject participation and data analysis
Table 1.
Demographic and Clinical Characteristics at Baselinea
| Total N=62 |
Oxytocin N=32 |
Placebo N=30 |
|
|---|---|---|---|
| Age, years | 39.1 ± 12.5 | 41.9 ± 12.2 | 36.1 ± 12.3 |
| Gender, male, N (%) | 47 (75.8) | 24 (75.0) | 23 (76.7) |
| Race, N (%) | |||
| White | 31 (50.0) | 15 (46.9) | 16 (53.3) |
| Black | 26 (41.9) | 14 (43.8) | 12 (40.0) |
| Other | 5 (8.1) | 3 (9.3) | 2 (6.7) |
| Schizophrenia, N (%) | 39 (62.3) | 19 (59.4) | 20 (65.5) |
| Schizoaffective disorder, N (%) | 23 (37.7) | 13 (40.6) | 10 (34.5) |
| CGI-S | 4.0 ± 0.6 | 4.0 ± 0.7 | 3.9 ± 0.6 |
| PANSS total | 67.3 ± 12.2 | 65.8 ± 12.8 | 69.0 ± 11.5 |
| PANSS positive | 16.9 ± 4.9 | 16.6 ± 4.8 | 17.3 ± 5.0 |
| PANSS negative | 17.9 ± 4.6 | 17.4 ± 4.6 | 18.5 ± 4.7 |
| PANSS general psychopathology | 32.5 ± 6.4 | 31.8 ± 6.7 | 33.2 ± 6.1 |
| WRAT word reading standard score | 95.8 ± 14.4 | 93.6 ± 14.7 | 98.4 ± 14.0 |
| Concomitant medications, N (%) | |||
| Antipsychotics | 59 (95.1) | 30 (93.8) | 29 (96.7) |
| First-generation antipsychotics | 8 (12.9) | 4 (12.5) | 4 (13.3) |
| Second-generation antipsychotics | 53 (85.4) | 27 (84.3) | 26 (86.7) |
| Antidepressants | 25 (40.3) | 15 (46.8) | 10 (33.3) |
| Mood stabilizers | 17 (27.4) | 12 (37.5) | 5 (16.6) |
| Benzodiazepines | 15 (24.1) | 6 (18.7) | 9 (30.0) |
Abbreviations: CGI-S, Clinical Global Impressions-Severity scale; PANSS, Positive and Negative Syndrome Scale; WRAT, Wide Range Achievement Test
Data presented as mean ± standard deviation, unless otherwise indicated.
3.2 Measures of Social Cognition
Table 2 summarizes results for each of the social cognition variables. Although several measures of social cognition demonstrated within-group changes for oxytocin and placebo groups, no measure (ER-40, Brüne Theory of Mind task, Eyes Test, Trustworthiness Task and AIHQ) demonstrated significant between-group change at 6 or at 12 weeks, see Table 2. To examine the impact of adherence, a post hoc analysis of only those subjects with at least 60% adherence to study drug (see section 3.5) showed no differential effect of oxytocin over placebo. No interactions emerged between study drug and gender, diagnosis, age or baseline PANSS on social cognitive outcomes. Small but significant interactions were found between AIHQ and treatment group for patients taking antidepressants (p=0.04) and between Brune-A total score and treatment group for patients taking benzodiazepines (p=0.045).
Table 2.
Change from baseline for outcomes of social cognitive function
| Oxytocin | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome Variablea | Time Point (wks) |
N | LS Mean (SE) |
P-Valueb Diff>0 |
N | LS Mean (SE) |
P-Valueb Diff>0 |
P-Valuec Trt Diff |
| Eyes Test total | 6 | 27 | −0.48 (0.59) |
0.422 | 27 | −1.09 (0.59) |
0.070 | 0.472 |
| 12 | 26 | −0.82 (0.67) |
0.227 | 27 | 0.17 (0.66) |
0.798 | 0.302 | |
| ER-40 total | 6 | 28 | 1.39 (0.55) |
0.014 | 29 | 0.83 (0.54) |
0.129 | 0.475 |
| 12 | 26 | 0.14 (0.50) |
0.782 | 29 | 0.93 (0.48) |
0.058 | 0.270 | |
| Brüne-A total | 6 | 28 | 3.28 (1.22) |
0.010 | 28 | 4.32 (1.19) |
0.001 | 0.552 |
| 12 | 26 | 4.52 (1.41) |
0.002 | 29 | 5.97 (1.36) |
0.000 | 0.469 | |
| AIHQ blame bias | 6 | 27 | 0.05 (0.56) |
0.926 | 29 | 0.34 (0.53) |
0.523 | 0.712 |
| 12 | 25 | −0.66 (0.54) |
0.228 | 29 | −0.53 (0.51) |
0.308 | 0.860 | |
| Trustworthiness task - | 6 | 27 | 0.22 (1.63) |
0.894 | 28 | 1.13 (1.58) |
0.479 | 0.694 |
| untrustworthy subscale | 12 | 25 | 0.86 (1.59) |
0.591 | 29 | 0.24 (1.50) |
0.875 | 0.779 |
| Trustworthiness task - | 6 | 27 | 1.22 (0.93) |
0.194 | 28 | −0.55 (0.90) |
0.546 | 0.183 |
| trustworthy subscale | 12 | 25 | −0.73 (1.02) |
0.479 | 29 | −0.29 (0.96) |
0.761 | 0.760 |
Abbreviations: LS, least squares; ER-40, Emotion Recognition-40 task; AIHQ, Ambiguous Intentions Hostility Questionnaire
Models adjusted for baseline value and age in addition to treatment group
Test for within-group change
Test for between-group change
3.3 Assessments of Social Function
Table 3 summarizes the results for the two measures of social functioning. For SLOF participant IP (interpersonal relationship) rating, the between-group comparison suggested an advantage for oxytocin over placebo at a statistical trend level (p=0.096). There were no other statistically significant group differences on the SLOF at either 6 or 12 weeks.
Table 3.
Change from baseline for outcomes of social functioning
| Oxytocin | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome Variablea | Time Point (wks) |
N | LS Mean (SE) |
P-Valueb Diff>0 |
N | LS Mean (SE) |
P-Valueb Diff>0 |
P-Valuec Trt Diff |
| SLOF-I IR | 12 | 17 | 0.64 (1.19) |
0.297 | 12 | 0.85 (1.42) |
0.275 | 0.455 |
| SLOF-P IR | 12 | 26 | −0.83 (0.96) |
0.195 | 27 | −2.61 (0.94) |
0.003 | 0.096 |
| SLOF-I SA | 12 | 17 | 0.38 (0.32) |
0.116 | 13 | 0.43 (0.36) |
0.122 | 0.463 |
| SLOF-P SA | 12 | 26 | 0.84 (0.51) |
0.050 | 28 | 0.44 (0.49) |
0.188 | 0.287 |
| SSPA Verbal SS | 6 | 26 | −0.09 (0.06) |
0.145 | 28 | 0.16 (0.06) |
0.008 | 0.005 |
| 12 | 23 | 0.03 (0.07) |
0.692 | 24 | 0.08 (0.07) |
0.277 | 0.631 | |
| SSPA Nonverbal SS | 6 | 26 | −0.00 (0.07) |
0.967 | 28 | 0.07 (0.07) |
0.316 | 0.472 |
| 12 | 23 | 0.01 (0.06) |
0.878 | 24 | 0.04 (0.06) |
0.537 | 0.748 | |
| SSPA Global SS | 6 | 26 | −0.27 (0.10) |
0.007 | 28 | 0.01 (0.09) |
0.950 | 0.045 |
| 12 | 23 | −0.14 (0.10) |
0.152 | 24 | −0.12 (0.10) |
0.233 | 0.847 | |
Abbreviations: LS, least squares; SLOF-I, Specific Levels of Functioning Scale - Informant; SLOF-P, SLOF-Participant; IR, Interpersonal Relationships; SA, Social Acceptability; SSPA, Social Skills Performance Assessment; SS, Social Skill
Models adjusted for baseline value and age in addition to treatment group.
Test for within-group change
Test for between-group change
On the SSPA modified role-play task, there were statistically significant group differences at 6 weeks only on the VSS and GSS in favor of placebo. No other group differences on the modified SSPA were observed at 6 or 12 weeks (Table 3).
3.4 Psychopathology
PANSS total, positive, negative and general psychopathology subscales demonstrated no significant between-group differences at any time point (Table 4). However, significant within-group negative symptom improvement in the oxytocin arm was seen at 2 weeks (p=0.024) and 6 weeks (p=0.044), with a statistical trend at 9 weeks (p=0.099) and 12 weeks (p=0.051), while the placebo group showed no within-group change at any time point (Table 4). When subjects were separated by diagnosis, a significant between-group advantage for oxytocin emerged on PANSS negative scores in subjects with schizophrenia at 6 weeks (p=0.029), and a statistical trend was seen at 9 weeks (p=0.096). In the schizoaffective disorder subgroup, no between-group advantage for oxytocin was seen at any point.
Table 4.
Change from baseline for PANSS following 12 weeks of treatment
| Oxytocin | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome Variablea | Time Point (wks) |
N | LS Mean (SE) |
P-Valueb Diff>0 |
N | LS Mean (SE) |
P-Valueb Diff>0 |
P-Valuec Trt Diff |
| PANSS Total | 2 | 32 | −4.10 (1.35) |
0.004 | 30 | −6.13 (1.40) |
0.000 | 0.310 |
| 6 | 28 | −3.23 (1.49) |
0.035 | 29 | −5.56 (1.49) |
0.000 | 0.279 | |
| 9 | 27 | −4.21 (1.69) |
0.016 | 29 | −4.77 (1.65) |
0.006 | 0.815 | |
| 12 | 26 | −3.54 (1.60) |
0.032 | 29 | −5.49 (1.54) |
0.001 | 0.389 | |
| PANSS Positive | 2 | 32 | −1.21 (0.43) |
0.007 | 30 | −1.40 (0.45) |
0.003 | 0.774 |
| 6 | 28 | −0.59 (0.55) |
0.292 | 29 | −1.68 (0.55) |
0.003 | 0.168 | |
| 9 | 27 | −1.39 (0.60) |
0.025 | 29 | −1.50 (0.59) |
0.014 | 0.894 | |
| 12 | 26 | −1.10 (0.56) |
0.056 | 29 | −1.49 (0.54) |
0.008 | 0.614 | |
| PANSS Negative | 2 | 32 | −1.20 (0.52) |
0.024 | 30 | −0.65 (0.54) |
0.232 | 0.467 |
| 6 | 28 | −1.28 (0.62) |
0.044 | 29 | −0.36 (0.62) |
0.558 | 0.303 | |
| 9 | 27 | −0.92 (0.55) |
0.099 | 29 | −0.13 (0.54) |
0.816 | 0.309 | |
| 12 | 26 | −1.18 (0.59) |
0.051 | 29 | −0.64 (0.57) |
0.261 | 0.521 | |
| PANSS General | 2 | 32 | −1.76 (0.84) |
0.042 | 30 | −4.02 (0.88) |
0.000 | 0.071 |
| 6 | 28 | −1.41 (0.79) |
0.077 | 29 | −3.46 (0.79) |
0.000 | 0.076 | |
| 9 | 27 | −1.92 (1.10) |
0.087 | 29 | −3.08 (1.08) |
0.006 | 0.460 | |
| 12 | 26 | −1.44 (0.93) |
0.127 | 29 | −3.28 (0.89) |
0.001 | 0.164 | |
Abbreviations: LS, least squares; PANSS, Positive and Negative Syndrome Scale
Models adjusted for baseline value and age in addition to treatment group.
Test for within-group change
Test for between-group change
3.5 Adherence to Treatment
In the mITT population, 75.0% of participants in the oxytocin arm and 81.2% in the placebo arm demonstrated excellent (80%-100%) or good (60%-80%) adherence to study drug based on bottle weights, patient adherence diaries and all other available information, with no between-group differences.
3.6 Adverse Events and Tolerability
Study drug was generally well tolerated. Six serious adverse events were reported; each was for a hospitalization (four with oxytocin, two with placebo). Three of the hospitalizations in the oxytocin arm were due to medical conditions considered unrelated to study participation, while one was due to psychotic exacerbation, considered unlikely related to study participation. One of the hospitalizations in the placebo arm was due to a medical condition and one was due to relapse of alcohol abuse prior to starting study drug, neither was considered related to study participation. Three participants experienced worsening psychotic symptoms during the trial, of whom two received oxytocin and one received placebo. For the two psychotic exacerbations in the oxytocin arm, one was considered “possibly” related and the other “unlikely” related to study drug. The exacerbation in the placebo arm was considered “possibly” related to study drug. Only one participant discontinued study participation because of intolerable side effects, reported as discomfort with the nasal spray.
4. Discussion
This study did not support the primary hypothesis that 12 weeks of intranasal oxytocin improves social cognition in people with schizophrenia and schizoaffective disorder. The primary outcomes of emotion perception, theory of mind, and attributional style did not demonstrate a differential advantage for oxytocin compared to placebo. This result is in apparent contrast to a number of smaller and shorter duration studies, including single-dose studies (Averbeck et al., 2012; Davis et al., 2013; Goldman et al., 2011; Guastella et al., 2015; Woolley et al., 2014), and multi-dose studies ranging from 2 weeks (Pedersen et al., 2011) to 6 weeks (Davis et al., 2014; Gibson et al., 2014), which have suggested an advantage for oxytocin on multiple dimensions of social cognition. However, the current results are consistent with another recent study in which 52 young people with early psychosis schizophrenia-spectrum illness received 6 weeks of intranasal oxytocin or placebo (Cacciotti-Saija et al., 2015). In that study, oxytocin was not associated with improvement on any of the primary or secondary measures of social cognition. These two negative findings may more accurately reflect the impact of longer consecutive-day oxytocin dosing on social cognition given their stronger statistical power compared to prior studies. However, there is also evidence from animal studies, including prairie voles and mice, demonstrating enhanced pro-social behaviors following single-dose oxytocin administration and that with chronic administration, the behaviors attenuate (Bales et al., 2013; Huang et al., 2014). These animal data provide a possible explanation for how positive effects on social cognitive function seen in single-dose and short-term studies in schizophrenia could diminish with longer administration. Studies that assess the impact and duration of oxytocin effects on social cognitive function in schizophrenia both acutely and after multi-day dosing regimens in longitudinal study designs are needed to better interpret findings to date.
Related to the issue of single versus multi-day regimens is the impact of the absolute dose of oxytocin on social cognitive function. Most studies of oxytocin in schizophrenia have used doses ranging from 10-40 IU for single-dose studies and from 20-40 IU twice daily for multi-day studies, (reviewed by Feifel et al., 2016). While oxytocin 24 IU twice daily in the current study was not effective on social cognitive function, it remains possible that lower or higher doses may provide benefits in longer-term studies, an issue requiring additional investigation.
Other potential moderators of social cognitive function by oxytocin in schizophrenia include genetic variance, gender and attachment history (Macdonald, 2012). In particular, genetic polymorphisms in the oxytocin and oxytocin receptor (OTR) genes have been identified (Bartholomeusz et al., 2015). The impact of genetic variants on the response to exogenous oxytocin in schizophrenia is not known; however, in healthy controls specific polymorphisms of the OTR appear to moderate the impact of oxytocin on subjective responses to infant faces (Marsh et al., 2012) and influence performance on the Eyes test (Lucht et al., 2013). Although speculative, social cognitive function in people with schizophrenia with specific genetic variants of oxytocin or OTR may represent subgroups of individuals who are differentially responsive to exogenous oxytocin. With regards to gender effects, a correlation was found between oxytocin blood levels and facial emotion perception in females, but not males, with schizophrenia (Rubin et al., 2011). However, in multi-day studies of oxytocin in people with schizophrenia, no gender effects on social cognitive outcomes have emerged, including in the current study.
Oxytocin was not associated with improvement in the total PANSS score or in the positive, negative or general psychopathology subscales in the main analysis. However, within-group analyses showed significant, or near significant, reductions in negative symptoms for the oxytocin group at each assessment point, while negative symptoms were unchanged at all points in the placebo group. Furthermore, exploratory subgroup analysis showed a significant advantage for oxytocin compared to placebo on negative symptoms in people with schizophrenia, but not schizoaffective disorder. While these findings need to be interpreted cautiously given the post hoc nature of the analyses, the data suggest a potential advantage for oxytocin on negative symptoms. This is also consistent with a recent meta-analysis which found that, among consecutive-day dosing studies (6 studies, N=192 participants), oxytocin demonstrated significant improvement in negative symptoms in people with schizophrenia (Oya et al., 2015). In addition, Cacciotti-Saija et al. (2014) reported a significant dose-response relationship between oxytocin and negative symptoms, suggesting a modest therapeutic effect that was not demonstrated for other symptom domains. A mechanism of action of oxytocin on negative symptoms is uncertain given limited insight for the neurobiological basis of negative symptoms (Kirkpatrick, 2014). Given that negative symptoms such as social withdrawal and poverty of speech often overlap with social cognitive deficits (Ochsner, 2008), a role for oxytocin in the treatment of negative symptoms is reasonable to hypothesize. Since currently available antipsychotic medications provide little to no benefit for primary negative symptoms (Kirkpatrick, 2014), further research into the potential role for oxytocin in the treatment of negative symptoms is warranted.
The potential role for oxytocin on social function was assessed as a secondary outcome measure using the SLOF and a modified role-play test. Overall, there was no evidence that oxytocin had a differential impact on measures of social functioning (and in fact, there was even a suggestion of worsening function in the placebo group compared to oxytocin). Given that social cognition and social functioning are highly related to one another (Fett et al., 2011), these negative findings on social function are not surprising.
A number of limitations to the current study should be considered. First, although this was the largest oxytocin trial in schizophrenia to date, the study was not adequately powered to test each of the outcome measures. Therefore, the outcomes should be considered exploratory. Second, only about 25% of the cohort was female, limiting the ability to distinguish potential gender-specific effects of oxytocin on emotion perception. Third, because oxytocin in human cerebrospinal fluid demonstrates diurnal variation (Amico et al., 1983), this could represent another source of variability. Most subjects in the current study were tested on social cognitive measures in the morning, but this was not standardized. Fourth, a number of symptom measures showed evidence of placebo response (as early as treatment week 2 for PANSS scores), suggesting that some subjects may not have been symptomatically stable at baseline. Future studies could benefit from a longer minimum period of antipsychotic dose stability prior to entry and a longer screening period during which symptom stability is established. Fifth, practice effects on social cognition rating scales represents another potential confound. The psychometric properties of the most widely used measures of social cognition were recently measured in the SCOPE study which found that the ER 40, Trustworthiness Task and AIHQ (but not the Eyes Test) showed evidence of small but consistent practice effects, ranging from 0.15 to 0.27 (Pinkham et al., 2016). The magnitude of these practice effects should have been too small to obscure a meaningful effect of oxytocin on social cognitive function, if present. However, for the Brüne task (not assessed in the SCOPE study), the larger parallel changes seen for placebo and oxytocin across 12 weeks suggest that the Brüne may be susceptible to larger practice effects. Sixth, because subjects were taking a broad background of antipsychotic and other psychotropic medications, the potential confounding impact of these medications is largely unknown. Greater standardization of concomitant medications could help reduce variability. Seventh, while an attempt was made to enrich the cohort with greater social cognitive impairment by using a minimum threshold score on the Eyes Test and a minimum threshold score on specific PANSS items thought to be associated with impaired social functioning (see inclusion criteria section 2.2), these selection criteria were not empirically based. Similar criteria were used in our pilot studies that suggested an advantage for oxytocin over placebo on social cognition (Gibson et al., 2014; Pedersen et al., 2011). Eighth, the intranasal drug delivery mechanism likely introduced additional variability that cannot be accounted for in terms of how much drug was absorbed at each dose for each patient. Even subjects who were fully adherent with all insufflations may not have experienced consistent day-to-day oxytocin dosing due to inadvertent loss or incomplete absorption through the nasal mucosa. Finally, other potential confounds such as smoking and illicit drug use may also have affected the results.
This double-blind randomized controlled study of 12 weeks of daily intranasal oxytocin in people with schizophrenia and schizoaffective disorder did not demonstrate efficacy for oxytocin on social cognitive function. The effect of oxytocin on psychopathology also did not separate from placebo, although post hoc analyses were suggestive of an effect of oxytocin on negative symptoms, especially in the subgroup with schizophrenia. This association must be interpreted with caution given the limited power and exploratory nature of these analyses. Intranasal oxytocin was generally well tolerated. Despite the overall negative outcome on social cognitive function, further clinical testing – including of various dosing strategies – is warranted to better understand the potential benefits of oxytocin on social cognition, social function and negative symptoms.
Supplementary Material
Acknowledgement
This paper is dedicated to Robert Hamer whose deep intellect, lack of pretense, and endless humor never ceased to inspire and make us laugh. We miss you dearly. RIP
Role of funding source
This work was supported by a grant from NIMH (grant number R01MH093529 – PIs DLP, CAP). NIMH had no role in study design, implementation, data collection, data interpretation or in the decision to submit for publication.
Abbreviations
- AIHQ
Ambiguous Intentions Hostility Questionnaire
- ER-40
Emotion Recognition-40 task
- Eyes Test
Reading the Mind in the Eyes Test
- GSS
Global social skill
- IU
International Units
- mITT
Modified intention-to-treat
- NVSS
Nonverbal social skill
- PANSS
Positive and Negative Syndrome Scale
- SLOF
Specific Levels of Functioning Scale
- SSPA
Social Skills Performance Assessment
- VSS
Verbal social skill
- WRAT
Wide Range Achievement Test
Footnotes
Contributors
DLP, CAP and RMH designed the study and wrote the protocol. LFJ wrote the first draft of the manuscript. JLJ performed statistical analyses. All authors contributed to data interpretation, meaningful manuscript revision, and all authors have approved the final manuscript.
Conflict of Interest
During the past 3 years, LFJ has received research grant support from Amgen and Teva/Auspex Pharmaceuticals, has served as a consultant for Roche and has served on a DSMB for Janssen. CAP, JLJ, SWR, TLE and DLP report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov identifier: NCT01394471
References
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393(6684):470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Amico JA, Tenicela R, Johnston J, Robinson AG. A time-dependent peak of oxytocin exists in cerebrospinal fluid but not in plasma of humans. J Clin Endocrinol Metab. 1983;57(5):947–951. doi: 10.1210/jcem-57-5-947. [DOI] [PubMed] [Google Scholar]
- Averbeck BB, Bobin T, Evans S, Shergill SS. Emotion recognition and oxytocin in patients with schizophrenia. Psychol Med. 2012;42(2):259–266. doi: 10.1017/S0033291711001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Perkeybile AM, Conley OG, Lee MH, Guoynes CD, Downing GM, Yun CR, Solomon M, Jacob S, Mendoza SP. Chronic intranasal oxytocin causes long-term impairments in partner preference formation in male prairie voles. Biol Psychiatry. 2013;74(3):180–188. doi: 10.1016/j.biopsych.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001;42(2):241–251. [PubMed] [Google Scholar]
- Bartholomeusz CF, Ganella EP, Labuschagne I, Bousman C, Pantelis C. Effects of oxytocin and genetic variants on brain and behaviour: Implications for treatment in schizophrenia. Schizophr Res. 2015;168(3):614–627. doi: 10.1016/j.schres.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58(4):639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Theory of mind impairment in schizophrenia: meta-analysis. Schizophr Res. 2009;109(1-3):1–9. doi: 10.1016/j.schres.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Brüne M. Social cognition and behaviour in schizophrenia. John Wiley & Sons; Chichester: 2003. [Google Scholar]
- Cacciotti-Saija C, Langdon R, Ward PB, Hickie IB, Scott EM, Naismith SL, Moore L, Alvares GA, Redoblado Hodge MA, Guastella AJ. A double-blind randomized controlled trial of oxytocin nasal spray and social cognition training for young people with early psychosis. Schizophr Bull. 2015;41(2):483–493. doi: 10.1093/schbul/sbu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs DR, Penn DL, Wicher M, Waldheter E. The Ambiguous Intentions Hostility Questionnaire (AIHQ): a new measure for evaluating hostile social-cognitive biases in paranoia. Cogn Neuropsychiatry. 2007;12(2):128–143. doi: 10.1080/13546800600787854. [DOI] [PubMed] [Google Scholar]
- Davis MC, Green MF, Lee J, Horan WP, Senturk D, Clarke AD, Marder SR. Oxytocin-augmented social cognitive skills training in schizophrenia. Neuropsychopharmacol. 2014;39(9):2070–2077. doi: 10.1038/npp.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MC, Lee J, Horan WP, Clarke AD, McGee MR, Green MF, Marder SR. Effects of single dose intranasal oxytocin on social cognition in schizophrenia. Schizophr Res. 2013;147(2-3):393–397. doi: 10.1016/j.schres.2013.04.023. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62(10):1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Feifel D, Shilling PD, MacDonald K. A Review of Oxytocin's Effects on the Positive, Negative, and Cognitive Domains of Schizophrenia. Biol Psychiatry. 2016;79(3):222–233. doi: 10.1016/j.biopsych.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35(3):573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Gibson CM, Penn DL, Smedley KL, Leserman J, Elliott T, Pedersen CA. A pilot six-week randomized controlled trial of oxytocin on social cognition and social skills in schizophrenia. Schizophr Res. 2014;156(2-3):261–265. doi: 10.1016/j.schres.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Gomes AM, Carter CS, Lee R. Divergent effects of two different doses of intranasal oxytocin on facial affect discrimination in schizophrenic patients with and without polydipsia. Psychopharmacology (Berl) 2011;216(1):101–110. doi: 10.1007/s00213-011-2193-8. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67(7):692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Ward PB, Hickie IB, Shahrestani S, Hodge MA, Scott EM, Langdon R. A single dose of oxytocin nasal spray improves higher-order social cognition in schizophrenia. Schizophr Res. 2015;168(3):628–633. doi: 10.1016/j.schres.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Domes G. Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans. Progress in brain research. 2008;170:337–350. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, Anagnostou E, Wasserman S. Oxytocin increases retention of social cognition in autism. Biol Psychiatry. 2007;61(4):498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Huang H, Michetti C, Busnelli M, Manago F, Sannino S, Scheggia D, Giancardo L, Sona D, Murino V, Chini B, Scattoni ML, Papaleo F. Chronic and acute intranasal oxytocin produce divergent social effects in mice. Neuropsychopharmacol. 2014;39(5):1102–1114. doi: 10.1038/npp.2013.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B. Progress in the study of negative symptoms. Schizophr Bull. 2014;40(Suppl 2):S101–106. doi: 10.1093/schbul/sbt158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler CG, Turner TH, Gur RE, Gur RC. Recognition of facial emotions in neuropsychiatric disorders. CNS Spectr. 2004;9(4):267–274. doi: 10.1017/s1092852900009202. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr Bull. 2010;36(5):1009–1019. doi: 10.1093/schbul/sbn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Lucht MJ, Barnow S, Sonnenfeld C, Ulrich I, Grabe HJ, Schroeder W, Volzke H, Freyberger HJ, John U, Herrmann FH, Kroemer H, Rosskopf D. Associations between the oxytocin receptor gene (OXTR) and “mind-reading” in humans--an exploratory study. Nordic J Psychiatry. 2013;67(1):15–21. doi: 10.3109/08039488.2012.700731. [DOI] [PubMed] [Google Scholar]
- Macdonald KS. Sex, receptors, and attachment: a review of individual factors influencing response to oxytocin. Front Neurosci. 2012;6:194. doi: 10.3389/fnins.2012.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Yu HH, Pine DS, Gorodetsky EK, Goldman D, Blair RJ. The influence of oxytocin administration on responses to infant faces and potential moderation by OXTR genotype. Psychopharmacol (Berl) 2012;224(4):469–476. doi: 10.1007/s00213-012-2775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature Rev Neurosci. 2011;12(9):524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Ochsner KN. The social-emotional processing stream: five core constructs and their translational potential for schizophrenia and beyond. Biol Psychiatry. 2008;64(1):48–61. doi: 10.1016/j.biopsych.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oya K, Matsuda Y, Matsunaga S, Kishi T, Iwata N. Efficacy and safety of oxytocin augmentation therapy for schizophrenia: an updated systematic review and meta-analysis of randomized, placebo-controlled trials. Eur Arch Psychiatry Clin Neurosci. 2015 doi: 10.1007/s00406-015-0634-9. [DOI] [PubMed] [Google Scholar]
- Patterson TL, Moscona S, McKibbin CL, Davidson K, Jeste DV. Social skills performance assessment among older patients with schizophrenia. Schizophr Res. 2001;48(2-3):351–360. doi: 10.1016/s0920-9964(00)00109-2. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Gibson CM, Rau SW, Salimi K, Smedley KL, Casey RL, Leserman J, Jarskog LF, Penn DL. Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophr Res. 2011;132(1):50–53. doi: 10.1016/j.schres.2011.07.027. [DOI] [PubMed] [Google Scholar]
- Penn DL, Keefe RS, Davis SM, Meyer PS, Perkins DO, Losardo D, Lieberman JA. The effects of antipsychotic medications on emotion perception in patients with chronic schizophrenia in the CATIE trial. Schizophr Res. 2009;115(1):17–23. doi: 10.1016/j.schres.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham AE, Penn DL, Green MF, Buck B, Healey K, Harvey PD. The social cognition psychometric evaluation study: results of the expert survey and RAND panel. Schizophr Bull. 2014;40(4):813–823. doi: 10.1093/schbul/sbt081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham AE, Penn DL, Green MF, Harvey PD. Social Cognition Psychometric Evaluation: Results of the Initial Psychometric Study. Schizophr Bull. 2016;42(2):494–504. doi: 10.1093/schbul/sbv056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Carter CS, Drogos L, Jamadar R, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Sex-specific associations between peripheral oxytocin and emotion perception in schizophrenia. Schizophr Res. 2011;130(1-3):266–270. doi: 10.1016/j.schres.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LC, Struening EL. SLOF: a behavioral rating scale for assessing the mentally ill. Social work research & abstracts. 1983;19(3):9–21. doi: 10.1093/swra/19.3.9. [DOI] [PubMed] [Google Scholar]
- Schulze L, Lischke A, Greif J, Herpertz SC, Heinrichs M, Domes G. Oxytocin increases recognition of masked emotional faces. Psychoneuroendocrinol. 2011;36(9):1378–1382. doi: 10.1016/j.psyneuen.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Woolley JD, Chuang B, Lam O, Lai W, O'Donovan A, Rankin KP, Mathalon DH, Vinogradov S. Oxytocin administration enhances controlled social cognition in patients with schizophrenia. Psychoneuroendocrinol. 2014;47:116–125. doi: 10.1016/j.psyneuen.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.