Abstract
Oxysterols are oxygenated derivatives of cholesterol formed in the human body or ingested in the diet. By modulating the activity of many proteins, e.g., liver X receptors, oxysterol-binding proteins, or some ATP binding cassette transporters, oxysterols can affect many cellular functions and influence various physiological processes, e.g., cholesterol metabolism, membrane fluidity regulation, intracellular signaling pathways. Therefore, the role of oxysterols is also important in pathological conditions, e.g., atherosclerosis, diabetes mellitus type 2, neurodegenerative disorders. Finally, current evidence suggests that oxysterols play a role in malignancies, such as breast, prostate, colon, and bile duct cancer.
This review summarizes the physiological importance of oxysterols in the human body, with a special emphasis on their roles in various tumors.
Keywords: oxysterols, cholesterol, disease, cancer
Underlying principles
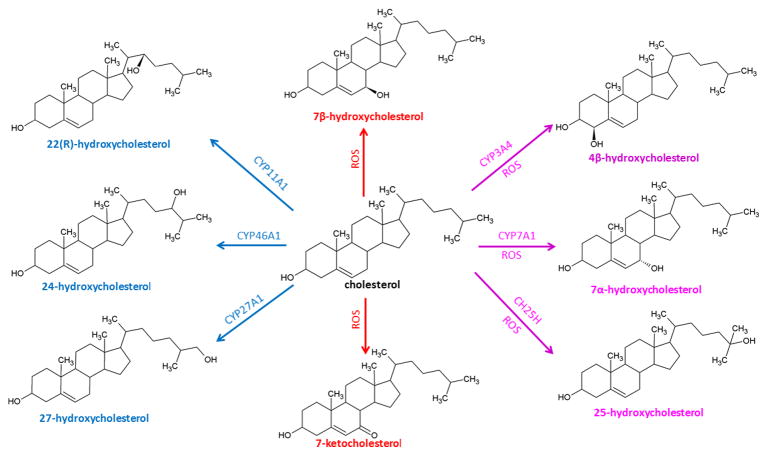
Oxysterols are 27-carbon derivatives of cholesterol created by enzymatic or radical oxidation [1] (Figure 1). Oxygenated forms of plant sterols (phytosterols) and cholesterol precursors may also be considered oxysterols. The oxygenation of cholesterol occurs either on the side chain or on the sterol nucleus. Side chain oxidation generates 24-hydroxycholesterol (24-HC), 25-hydroxycholesterol (25-HC), 27-hydroxycholesterol (27-HC), or other products while oxidation of the steroid nucleus gives rise to ring oxysterols, mostly ring-B oxysterols, e.g., 6-hydroxycholesterol (6-HC), 7α/β-hydroxycholesterol (7α/β-HC), 7-ketocholesterol (7-KC).
Figure 1. Structures of common oxysterols.
Oxysterols are formed by both enzymatic and non-enzymatic reactions. The enzymatic pathways are mediated by enzymes from the cytochrome P450 (CYP) family and cholesterol-25-hydroxylase (CH25H). Enzymatic oxidation of cholesterol gives rise to side-chain oxysterols (blue), and ring-oxysterols are generated by non-enzymatic oxidation mediated by reactive oxygen species (ROS, red). Some oxysterols are generated by both enzymatic and non-enzymatic reactions (purple).
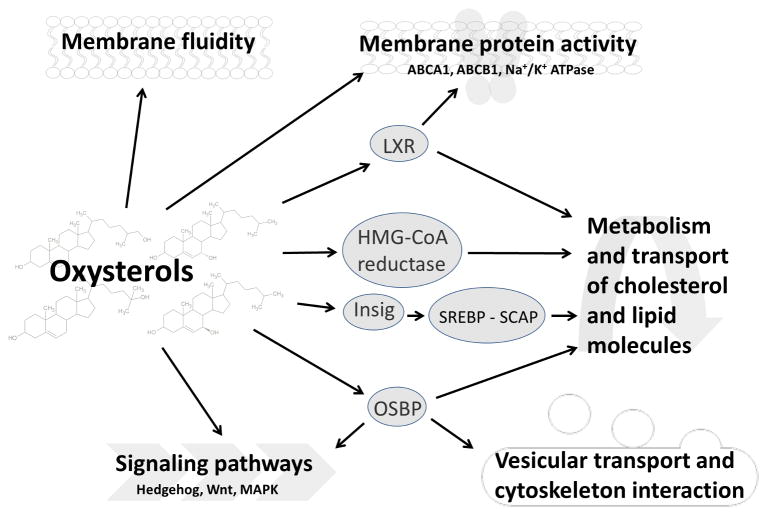
In very low concentrations [2, 3], oxysterols are natural components of the human body and mediate many physiological functions. They take part in the regulation of cholesterol metabolism (Figure 2, see Boxes 1 and 2). Oxysterols influence several signaling pathways, membrane fluidity, and activity of some membrane proteins (Figure 2). However, oxysterol action is also connected with human pathologies, e.g., atherosclerosis, Alzheimer’s disease, or Parkinson’s disease [4] (Box 3). Apart from that, oxysterols influence carcinogenesis and cancer progression. This review focuses especially on their roles in disease onset and therapy outcome, with special regard to different types of cancer.
Figure 2. The role of oxysterols in physiological cellular processes.
Oxysterols are important regulators of many biological processes in the cell. The regulation of sterol metabolism is mediated by various ways – through transcription factors (liver X receptors - LXRs, or sterol regulatory binding proteins - SREBPs), by interaction with oxysterol-binding proteins - OSBPs, or by regulation of 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase, a key enzyme of cholesterol metabolism. Oxysterols influence the biophysical properties of lipid membranes, such as the ordering of the bilayer [4] and play role in vesicular transport. Oxysterols also interact with integral or peripheral membrane proteins and are capable of modulation of their activity. They modulate the activity, e.g., of ABC transporters, or Na+/K+ ATPase. Oxysterols also interact with signaling pathway proteins and may modulate the Hedgehog, Wnt, or MAP kinase signaling.
Box 1. Oxysterols and cholesterol metabolism.
The regulation of cholesterol synthesis is mediated by both cholesterol and oxysterols. Modulation by oxysterols includes both regulation at the level of gene expression and direct regulation of the protein activity. The gene expression of key cholesterol metabolizing enzymes is regulated by transcription factors termed sterol regulatory element binding proteins (SREBPs) (SREPB1; OMIM: 184756 and SREBP2; OMIM: 600481) [81]. If the cellular level of cholesterol is high, these factors localize in the membrane of the endoplasmic reticulum, where they form a complex with SCAP protein (SREBP-cleavage activating protein; OMIM: 601510). When the cholesterol level decreases, the SREBP-SCAP complex translocates to the Golgi apparatus. Next, the SREBP-SCAP complex is cleaved and the released transcription factor SREBP moves to the nucleus and activates the expression of appropriate genes [81]. In the presence of cholesterol, the sterol-sensing domain of SCAP protein interacts with Insig (insulin-induced gene; INSIG1; OMIM: 602055 and INSIG2; OMIM: 608660) proteins and the SREBP-SCAP complex remains in the endoplasmic reticulum. Both cholesterol and oxysterols regulate the SREBP activation – cholesterol by direct binding to SCAP and oxysterols through interaction with Insig protein [81].
Cholesterol metabolism is also modulated through 3-hydroxy-3-methylglutarylCoA (HMG-CoA) reductase (HMGCR; OMIM: 142910), a key enzyme of the cholesterol synthesis pathway. HMG-CoA reductase contains a sterol-sensing domain and thus can bind the Insig protein, leading to increased degradation of the HMG-CoA reductase protein [82]. Many oxysterols, such as 24-HC, 25-HC, 27-HC, 19-HC, 24(S),25-epoxycholesterol, and 5-cholesten-3β,16β,27-triol, contribute to the ubiquitination of the HMG-CoA reductase protein and its degradation in the proteasome [83].
Regulation of cholesterol level is modulated also by liver X receptors (LXRs). First, LXRs form dimers with retinoid X receptors (RXRs) and act as transcription factors, which can activate gene expression of sterol transporters, e.g., ABCA1 (OMIM: 600046), ABCG1 (OMIM: 603076), ABCG5 (OMIM: 605459), and ABCG8 (OMIM: 605460) [18]. Secondly, protein-protein interactions between LXR/RXR dimers and the ABCA1 transporter also influence LXR-mediated cholesterol levels. Oxysterols bind to LXRs and cause their dissociation from ABCA1 and activation of this cholesterol efflux transporter [84]. The most potent activators of LXRs are side-chain oxysterols, such as 24-HC, 25-HC, or 27-HC [18].
Box 2. Oxysterol-binding proteins.
The family of oxysterol-binding proteins (OSBPs) consists at least of 12 members, which play a role, particularly in the endoplasmic reticulum and in the Golgi apparatus, where they take part in the regulation of metabolism and trafficking of cholesterol and lipid molecules. For example, OSBP1 protein influence sterol metabolism via negative regulation of ABCA1 transporter through modulation of gene expression and protein destabilization [85]. The modulation of cholesterol metabolism may be mediated through transcription factors ORP8 was shown to regulate the activity of SREBP, presumably by an indirect mechanism [86] and ORP2 binds LXRs and is required for expression of LXR target genes [87].
The OSBP family also plays roles also in other cellular processes, such as vesicular trafficking [88, 89]. OSPBs also act as scaffolds in cell signaling pathways, or participate in cytoskeleton organization and cell adhesion [11, 88].
In addition, there is also evidence connecting OSBPs with cell proliferation and carcinogenesis. The level of various OSBPs is altered in tumors or cancer cells on mRNA or protein level, suggesting a role of these proteins in tumorigenesis. Moreover, ORP inhibitors (named ORPphilins), which are capable of attenuating OSBP or ORP4L, act as anti-proliferative agents and inhibit tumor growth [88]. There are many studies revealing the putative role of OSBPs in various cancer types (see the main text).
Box 3. Oxysterols and human disease.
Besides the physiological effects, indications of linkage exist between oxysterols and various cardiovascular, metabolic, neurodegenerative, and cancerous pathologies.
Oxysterols influence a typical cardiovascular disease, atherosclerosis. An increased level of various oxysterols, such as 27-HC, 7-KC, 7α-HC, and 7β-HC, was found in atherosclerotic lesions and foam cells. Moreover, the evidence suggests that oxysterols, e.g. 27-HC, promote atherogenesis by upregulating the inflammatory pathways in vascular cells and increasing the accumulation of macrophages in vascular wall [4].
Moreover, oxysterol levels are associated with some metabolic disorders, e.g., the level of oxidized cholesterol derivatives is altered in patients with abnormal lipid metabolism disorders, such as dyslipidemia or hypercholesterolemia, and presents a risk factor for other diseases. Thus, higher oxysterol level in hypercholesteremic patients may further impact other metabolic pathologies, e.g., diabetes mellitus type 2, and contribute to the disease progression. LXRs, which regulate several metabolic pathways, have been proposed as potential therapeutic targets of lipid metabolism disorders and type 2 diabetes [90].
Additionally, oxysterols present interesting targets in various neurodegenerative disorders, such as multiple sclerosis, Huntington’s disease, Parkinson’s disease, and Alzheimer’s disease [4]. 24-HC is predominantly formed in brain tissue and known as cerebrosterol; it appears to be the most potent oxysterol that can contribute to the development of neurological diseases. The presence of 24-HC, together with 27-HC, caused changes in the level of Parkinson’s disease-associated proteins in cell culture models in vitro [91] and enhanced β-amyloidogenesis, which is associated with Alzheimer’s disease [92]. Oxysterols also play a role in hereditary disorders, such as Niemann-Pick disease type C, which is accompanied by cholesterol accumulation in various tissues and presents with neurological defects such as dementia or ataxia. Consequently, oxysterols such as 7-KC or cholestane-3β,5α,6β-triol (CT) were postulated as potential diagnostic and predictive biomarkers of this disease [93]. Altered oxysterol levels have been observed in patients with Smith-Lemli-Opitz syndrome (SLOS), a disease resulting from mutation in 7-dehydrocholesterol reductase (DHCR7; OMIM: 602858), the enzyme responsible for the reduction of 7-dehydrocholesterol to cholesterol. Thus, SLOS presents with accumulation of oxysterols formed during 7-dehydrocholesterol oxidation, e.g., 3β,5α-dihydroxycholest-7-en-6-one, a putative biomarker of this oxidation [94].
Cholesterol metabolism and carcinogenesis
Numerous genetic and environmental factors are implicated in the etiology of cancer. For instance, obesity increases the risk of postmenopausal ER-positive breast cancer [5], perhaps due to the estrogen production by adipose tissue, enhanced level of insulin and insulin-like growth factors, and high cholesterol levels, which often accompany obesity. Congruently, hypercholesterolemia leads to an increased risk of breast, prostate, and colon cancer, but it is also connected to a decreased risk of liver and stomach cancers [6]. Additionally, a high level of dietary cholesterol increases the risk of breast cancer in postmenopausal women and the risk of cancers of the stomach, colon, rectum, pancreas, lung, kidney, and bladder and non-Hodgkin lymphoma [7].
It is currently understood that oxysterols may modify cancer risk in various ways. However, it remains to evaluate the exact influence of presence of oxysterols on carcinogenesis, or cancer progression, because the effect of oxysterols on various cell lines seems very complex. There are many studies declaring the pro-cancerous and pro-proliferative role of oxysterols (see below), which could be explained, e.g., by modulation of inflammatory [8, 9] and signaling pathways (e.g., Wnt or Hedgehog pathways [10, 11]), or through oxysterol-binding proteins [12, 13] (Box 2).
Interestingly, oxysterols also have a pro-apoptotic and cytotoxic effects on tumor cells. The pro-apoptotic effect of oxysterols is connected to overproduction of reactive oxygen species, changes of calcium level in the cell, or modifications of mitochondrial membrane [4]. Oxysterol-induced apoptosis is mediated by both intrinsic mitochondrial pathways [14, 15] and an extrinsic death receptor-dependent pathway [16].
Targeting LXR receptors (see Glossary) presents an additional putative mechanism of oxysterol-mediated pro-apoptotic effects. Several studies have demonstrated that LXRs are also able to inhibit cell proliferation, e.g., in colorectal, ovarian, breast, prostate and gallbladder cancer, glioblastoma, melanoma and leukemia [17, 18]. Because LXRs are lipid-sensing receptors that modulate cholesterol metabolism (Box 1), the anti-proliferative role of LXRs may be connected to reduction of cellular cholesterol levels, because cholesterol is needed for the cell growth (e.g., for membrane biogenesis) and many studies suggest its role in cancer development [19]. In consistence, LXR mediated expression is decreased in highly proliferating cells and cancer cells, e.g., by oxysterol deprivation or inactivation by sulfation [18] and the addition of LXR ligands inhibits the proliferation. Accordingly, LXRs are considered to be potential therapeutic targets for cancers [17, 18].
Moreover, LXRs can also inhibit the inflammatory signaling [11], but on the other hand, tumor-derived oxysterols are potent in recruitment of pro-tumor neutrophils and inhibition of anti-tumor immune response and thus exert other pro-cancerous actions, both by LXR-dependent and LXR-independent mechanism [20].
The role of oxysterols in breast cancer
Not only cholesterol, but also oxysterols, may play a role in breast cancer pathology and progression. For example, oxysterols produced by osteoblast-like MG63CM cells promote migration of MCF7 and MDA-MB-231 breast cancer cells, suggesting that oxysterols are important navigators that stimulate the bone metastasis formation in breast cancer patients [21, 22]. Moreover, after exposure to 27-HC, breast carcinoma MCF7 cells showed lower expression of E-cadherin and β-catenin molecules, indicating the role of 27-HC in the process of epithelial-mesenchymal transition [23]. 27-HC also promotes cell growth and proliferation in the MCF7 breast cancer cell line [24, 25].
Oxysterols and hormonal therapy of breast cancer
Apart from a role in the progression of breast cancer, oxysterols are suspected of interfering with hormonal therapy in breast cancer patients and thus may affect the breast cancer treatment outcome. Lappano et al., showed 25-HC to enhance cell proliferation in MCF7 breast cancer cell line via induction of estrogen receptor α (ESR1; OMIM: 133430) target genes [26] and Umetani et al., reported that 27-HC binds to the ER and modulates its activity [27]. 27-HC affects both ER regulation of transcription and the non-genomic (rapid-cytoplasmic) action of ER protein [27]. In addition to a series of synthetic selective estrogen receptor modulators (SERMs) used for breast cancer treatment, 27-HC is the first endogenous SERM identified, whereas 25-HC acts rather as an agonistic ligand [26].
The presence of 25-HC and 27-HC may be associated with resistance to aromatase inhibitors, which block estrogen synthesis, but do not influence the ER protein. However, the ER protein may bind not only estrogens, but also oxysterols, which modulates its activity and may affect tumor progression. Both 25-HC and 27-HC increased the transcription of ER target genes in breast cancer cell lines MCF7 and HCC1428, which were long-term estrogen deprived, suggesting that these oxysterols can substitute estrogen in activation of ER mediated expression and can play a potential role in resistance to the therapy [28]. Interestingly, breast cancer patients treated with aromatase inhibitors had significantly increased plasma level of 27-HC and moderately increased the level of 25-HC after 28 days of treatment [29], supporting the potential role of 25-HC and 27-HC level and therapy outcome of patients.
27-HC acts as a partial agonist of ERs and has different effects on various cell types. In the ER-expressing breast cancer cell line MCF7, it functions as an activator of ER, but the activation slightly differs from the activation by estrogen itself, namely in that 27-HC binding to the ER causes a unique conformational change, which is not seen with estrogen or synthetic SERMs. In the MCF7 breast cancer line, this change is followed by transcriptional activation of genes leading to cell growth and proliferation, e.g., cyclin D1 or E2F transcription factor [30]. Congruently, 27-HC promotes faster growth and proliferation of mouse mammary gland tumors. Tumors grew faster in a knock-out mouse model lacking the 27-HC-metabolizing cytochrome P450 enzyme CYP7B1 (OMIM: 603711) than in the model with a defect in the 27-HC synthesizing cytochrome P450 enzyme CYP27A1 (OMIM: 606530) [31].
Because 27-HC modulates the activity of ER, its role in hormonal therapy outcome or association with the risk of the disease relapse may be expected. 27-HC is one of the most abundant oxysterols in humans and its levels tend to increase with age and are positively correlated with the level of cholesterol [32]. Taken together, postmenopausal and obese women have the highest risk of breast cancer relapse linked to 27-HC. Patient studies have yielded similar results as a study in a mouse model, i.e., an association between low CYP7B1 expression in ER-expressing tumors and poor disease-free survival in breast cancer patients [30, 31]. Moreover, higher expression levels of CYP27A1 were found among patients with high tumor grade, i.e., with less differentiated tumor cells [31].
CYP27A1 is a potential target for breast carcinoma treatment and is a subject of interest. Mast et al. tested the effects of marketed drugs used for treatment of various disorders on CYP27A1 enzyme activity. Interestingly, four strong inhibitors of CYP27A1 (inhibition ≥ 75 %) were found, including two breast cancer drugs anastrozole and fadrozole. Moreover, the inhibitory effect of anastrozole treatment was confirmed in a mouse model in vivo [33].
Additionally, it has been shown that 27-HC acts not only by affecting the ER protein but also through interaction with the LXR protein. In this case, 27-HC is not important for tumor growth but rather for metastasis formation. By affecting gene expression via the LXR protein, 27-HC contributes to the epithelial-mesenchymal transition of cancer cells [31]. The role of 27-HC as a SERM is even more interesting because this oxysterol is synthesized in macrophages. Macrophages infiltrate the tumor environment in breast cancer patients and higher levels of macrophages in tumors are connected to poorer prognosis [34].
Some oxysterols bind to anti-estrogen binding sites (AEBS). Hormonal therapeutics, e.g., tamoxifen and toremifene, as well as B-ring oxysterols, such as 7-KC, 7-ketocholestanol, and 6-ketocholestanol [35] bind to AEBS. Binding of AEBS ligands results in decreased cholesterol levels and, conversely, increased levels of the intermediates of cholesterol synthesis [36]. Accumulation of these intermediates affects cell growth by causing cell cycle arrest in the G0 – G1 phase [37] and stimulates apoptosis or autophagy [38], possibly by increasing the level of reactive oxygen species and auto-oxidized sterols. Antiestrogens and oxysterols also inhibit cholesterol epoxide hydrolase (ChEH) activity. Since the substrates of ChEH, cholestane-5α,6α-epoxy-3β-ol (α-CE) and cholestane-5β,6β-epoxy-3β-ol (β-CE) induce cell differentiation and death [39], and inversely, the product of ChEH metabolism, cholestane-3β,5α,6β-triol (CT), is mutagenic [40], inhibition of this activity presents another antitumor effect of hormonal therapeutics, as well as oxysterols.
Oxysterols and prostate cancer
Prostate carcinoma is also potentially influenced by oxysterols. Prostate cancer belongs to the group of hormonally-dependent tumors; in this case, it is dependent on androgens. Similar to estrogens, androgen action is mediated via a receptor protein, the androgen receptor (AR; OMIM: 313700). AR triggers proliferation of prostate cancer cells, and a high level of AR has been connected to poor disease-free survival of patients [41]. Additionally, AR is able to down-regulate the activity of the LXR protein [42] suggesting a potential modulatory role of oxysterols. Moreover, androgens also modulate expression of the cholesterol efflux ABCA1 (OMIM: 600046) transporter and thus influence the levels of cholesterol and oxysterols in prostate tissue [43].
27-HC may influence prostate carcinoma by stimulation of proliferation of prostatic cells, enhancing the expression of prostate specific antigen (PSA) and also by increasing AR transcriptional activity [44]. Moreover, 27-HC can suppress apoptosis induced by docetaxel [44] and thus evoke drug resistance. On the other hand, CT reduced colony formation and cell proliferation in prostate cancer cell models, LNCaP CDXR-3, DU-145, and PC-3, and the authors of the study proposed CT as a promising therapy to metastatic prostate carcinomas [45]. Previously CT was showed to be mutagenic [40].
Oxysterols and digestive tract malignancies
Due the fact that dietary oxysterols pass through the digestive tract and are absorbed by the intestine, it is not surprising that these compounds are suspected to influence malignancies arising in the gastrointestinal tract. In addition, because some oxysterols are generated as intermediates of bile acid synthesis pathway, they can influence the progression of gallbladder cancer and bile duct cancer, i.e., cholangiocarcinoma.
The available evidence suggests that oxysterols exert anti-proliferative and pro-apoptotic effects in colon adenocarcinoma cell lines, e.g., 7β-HC inhibits cell growth and induces apoptotic process in CaCo-2 cell cultures [46]. Application of a diet-representative mixture of oxysterols to CaCo-2 cells caused pro-apoptotic effects, but only in a spontaneously differentiated subtype of the cell line [47]. Treatment with a mixture of 7α-HC, 7β-HC, 7-KC, α-CE, and β-CE caused activation of caspases 3 and 7 and release of cytochrome c in vitro [47, 48]. In addition, 25-HC is able to induce anoikis, a programmed cell death in response to loss of anchorage, in DLD-1 colon tumor cell line [49].
Similarly to other cancers, oxysterols have an anti-proliferative effect via targeting LXR receptors in colorectal cancer, as the activation of LXR proteins inhibited proliferation of both colorectal cancer cell lines and colorectal tumors in a mouse model [17].
However, oxysterols also exert pro-inflammatory effects [47, 48, 50], potentially leading to development of cancer in some tissues, including the colon. Moreover, the presence of oxysterols modulates the expression of the transcription factor TGF-β1 in fibroblasts and macrophages and thus influences the TGF-β1 signaling pathway. Since these cells also reside in intestinal tissue and compose the tumor stroma, TGF-β1 activation may promote dedifferentiation and clonal expansion of neoplastic cells in the colon, leading to carcinogenesis [51]. A pro-cancerous effect of oxidized sterols has also been shown on the mouse model with induced intestinal pre-neoplasia. Mice fed by dietary oxysterols showed increased cell proliferation and aberrant crypt foci formation [52].
As some oxysterols arise directly in human body, the investigation of key enzymes of cholesterol metabolism is also important. The expression level of oxysterol metabolizing cytochrome P450 proteins was evaluated in colorectal carcinoma samples and the overexpression of some enzymes and association with cancer prognosis was found, e.g., the levels of the 27-HC metabolizing enzymes CYP27A1 and CYP7B1 showed similar association to patient prognosis as in breast cancer patients – higher expression of CYP27A1 was associated with poor outcome of patients and increased level of CYP7B1 was associated with good prognosis of patients [53], suggesting a potential role of 27-HC in patients with colorectal carcinoma.
Cholangiocarcinoma, a malignant cancer of bile duct epithelium, may also be modulated by oxysterols. For example, oxysterols stabilize cyclooxygenase-2 mRNA and thus increase its expression, contributing to tumor progression [8]. Moreover, the presence of oxidative derivatives, including oxysterols in biliary ducts, has a direct link to infection and consequently can further impact on disease progression to cancer. Oxysterols were found in fresh bile samples, as well as in gallstones, and the occurrence of these oxysterols correlated with the presence of bacteria in the same samples [54]. It has been hypothesized that leukocytes are responsible for oxysterol formation in this case [55], connecting these oxysterols with carcinogenesis of gallbladder or bile duct cells. Moreover, the increased level of several oxysterol-binding proteins (OSBPs) was found in cholangiocarcinoma patients, suggesting that oxysterols take part in progression of this cancer via OSBPs and that OSBPs play a potential role as molecular markers of cholangiocarcinoma [56].
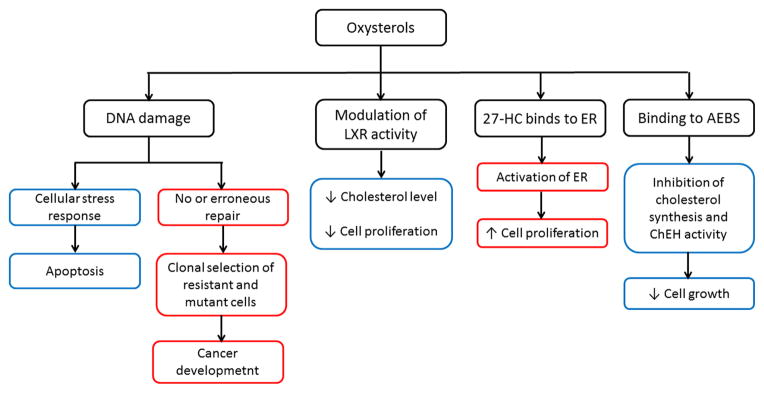
An increase of some oxysterols was seen in hamsters infected by Opisthorchis viverrini (so called Southeast Asian liver fluke), which infects the biliary tract in humans [57], indirectly linking oxysterols to the development of cholangiocarcinoma. Oxysterols induced DNA fragmentation and apoptosis in the human cholangiocyte cell line MMNK-1, and the authors concluded that oxysterols, together with reactive oxygen species and lipid peroxidation products, caused DNA damage leading either to apoptosis or DNA repair. Incorrect or deficient DNA repair frequently leads to carcinogenesis [57, 58] (Figure 3). Similarly, oxysterols induced apoptosis in canine gallbladder cells in vitro, suggesting that they may play a role in pathogenesis of gallbladder cancer [59].
Figure 3. The role of oxysterols in carcinogenesis and modulation of cell proliferation.
The model of pro-apoptotic and pro-cancerous role of oxysterols is adapted from the proposed scheme of cholangiocarcinogenesis by Jusakul et al.,[58], suggesting that this model could be applied also to other cancers. The scheme is specifically focused on oxysterol effects in breast carcinoma where oxysterols can interfere with hormonal therapy by binding to anti-estrogen binding sites (AEBS) and estrogen receptor (ER) molecules. The anti-proliferative and pro-apoptotic action of oxysterols is marked by blue rectangles and the pro-proliferative and pro-cancerous effect by red ones.
Oxysterol levels were also elevated in a rat model of hepatocellular carcinoma [60]. Consistent with this observation, the levels of 4β-HC, 7α-HC, and 25-HC were elevated by 29, 236, and 44 %, respectively, in patients with chronic hepatitis type C, and the previously established higher risk of hepatocellular carcinoma in these patients also points to a potential contribution of oxysterols to liver carcinogenesis [61]. On the other hand, the oxysterol-binding protein ORP8 seems to promote Fas-ligand mediated apoptosis [62] and also mediates the cytotoxicity of 25-HC in hepatoma cell lines [63]. The apoptosis activation and dampening the tumor growth by ORP8 was also shown in gastric cancer cells [64].
Oxysterols also influence carcinogenesis in pancreatic tissue, e.g., the development of pancreatic neuroendocrine tumor (pNET; islet cell tumor). The tumorigenesis of pNET is accompanied by high expression of the 24-HC forming enzyme CYP46A1 (OMIM: 604087), and 24-HC is suggested to recruit pro-tumor neutrophils and thus promote the angiogenesis [65]. Additionally, in various pancreatic cell lines, the expression of ORP5 has been associated with invasiveness and patients with the ORP5-expressing pancreatic tumors have poor prognosis [66].
Oxysterols and lung cancer
A study evaluating 7α-HC, 7β-HC, α-CE, β-CE, CT, and 7-KC found a positive association between 7β-HC levels and lung cancer risk after adjustment for physical activity, suggesting its potential predictive value [67]. Congruently, 7β-HC had anti-proliferative effects and induced apoptosis via activation of the intrinsic apoptotic pathway in the lung cancer cell line NCI-H460 [14]. On the other hand, there is also a suggested role of LXRs in lung carcinoma etiology. Inactivation of genes coding LXRα and LXRβ leads to chronic inflammation of lung tissue and squamous cell carcinoma-like lesions in mice [68]. Similarly, LXRα was shown to be a potential prognostic factor of better survival in non-small-cell lung carcinoma patients [69].
Recently, another role of oxysterols in lung cancer promotion was identified, namely the mediation of the activity of OSBPs, evidenced by the observed higher expression of ORP5 protein in lung cancer cases with metastatic disease compared to patients with locally advanced tumors, suggesting a diagnostic potential for OPR5 in lung cancer patients [13].
Other malignancies
In addition to the carcinomas mentioned above, there are other tumors with a potential link to oxysterols. In the C6 glioblastoma cell line, 7β-HC slowed the cell cycle and caused cell death [70, 71]. On the other hand, expression of cholesterol 25-hydroxylase (CH25H; OMIM: 604551) was upregulated by the inflammatory cytokines TNFα and IL1β while the 25-HC synthesized by CH25H modulated migration of tumor-associated macrophages/monocytes [72]. In BG-1 ovarian cancer cell line, 25-HC induced cell proliferation [26]. Similarly, ORP4 variant, ORP4L was shown to promote the proliferation of cervical cancer cell lines [12].
The pro-apoptotic or cytostatic activity of oxysterols observed in various model cancer cell lines, e.g., murine lymphoma [73], human lymphoma and leukemic cells [74–76], as well as in non-tumor cells, e.g., thymocytes [73], retina-derived cells [77], adipose tissue-derived mesenchymal stem cells [78] and rat and human vascular smooth muscle cells [15, 16], suggests that these effects have universal importance.
Concluding Remarks and Future Perspectives
Despite rather low concentrations of most oxysterols found in the circulation, these compounds are very important regulators in the human body. Apart from physiological functions, many studies provide evidence that oxysterols play roles in the development and progression of various cancer types. Oxysterols influence inflammation by modulating diverse signaling pathways and reactive oxygen species formation, conditions previously linked to a higher risk of carcinogenesis.
Various studies have shown strong associations between oxysterols and apoptosis, inhibition of proliferation, and cell cycle arrest. Jusakul et al., provided an explanation of this phenomenon in the case of cholangiocarcinoma formation. Oxysterols mediate oxidative DNA damage, which needs to be repaired. In the absence of or erroneous DNA repair these changes lead either to cell death or carcinogenesis [58]. Additionally, the anti-proliferative role may also be modulated by oxysterol receptors, the LXRs.
Nevertheless, various oxysterols exert different actions in various cell models. For instance, a mutagenic effect of CT was shown using the Ames test. Treatment of Chinese hamster ovary epithelial cells (CHO-K1) with CT also increased reactive oxygen species formation and chromosome aberrations [40]. On the other hand, the CT precursor cholestane-5α,6α-epoxy-3β-ol had anti-proliferative effects, causing cell cycle arrest in human leukemic cell lines HL-60 and Molt-4 [79]. In addition, the cytotoxicity of oxysterols differs in various cell lines, e.g., IC50 values of 25-HC and 7β-HC were variable in eight human leukemia cell lines (5 – 40 μM and 10 – 32 μM, respectively) [76].
Oxysterols produce different effects when applied separately or in mixtures. For example, a mixture of 7β-HC and 25-HC had weaker pro-apoptotic effects compared to the application of 7β-HC alone [75]. The cell-specific effect of oxysterol action was seen in U-937 cells, but not in a HC-60 monocytic cell line. The different effects of oxysterols administered individually or in a mixture have also been described in other studies [80]. Therefore, studies on dietary representative combinations of oxysterols should become more informative.
Certain proteins related to oxysterol action are already considered as potential targets for design of cancer therapeutics. For instance, activating LXR receptors could help inhibit proliferation. Also, targeting CYP27A1 or CYP7B1, which regulate the level of 27-HC in cells, may affect roles of LXRs, for example in breast carcinoma. However, the area of genetics of these markers is rather underexplored, and the missing functional link to both the circulating and target tissue oxysterol levels in human cancer patients precludes the translation of these results in clinical practice.
In summary, further studies of oxysterols are needed to elucidate their roles in carcinogenesis and their potential in cancer prevention or treatment. Such studies need to employ genomic, proteomic, and metabolomic profiling of a series of samples from the circulation and target tissues of patients with different pathologies to address the clinical utility of oxysterols in diagnosis and therapy (see Outstanding Questions Box).
Outstanding Questions.
Oxysterols, intrinsic products of cholesterol metabolism and exogenous substances ingested in the diet, interfere with a plethora of signaling pathways in cell- and tissue-specific manner implicating their role in human pathology. A key issue is to distinguish between positive and negative roles of oxysterols bearing in mind concentration ranges and compositions of oxysterol and other sterol mixtures in context with variety of cell compartments and tissue biology.
For answering the above questions, the development and validation of precise methods for detection of oxysterols and optimal sample handling protocols are necessary. Recent advances in lipidomics show that such approaches exist and may help to delineate clues about inter- and intra-individual variations in circulating and target tissue concentrations of oxysterols, their circadian rhythm dependence, and changes with age, gender, and dietary status of healthy and diseased individuals. These crucial conditions are currently unknown. After filling these gaps we can finally raise the prudent question: Can we generate knowledge about circulating or tissue oxysterol spectra enabling its use for prediction the disease risk, its prevention, improving its diagnosis and prognosis or even therapy?
Equally important are questions about relations between oxysterols and genotype and phenotype of cholesterol and oxysterol signaling and sensing genes. Can we link their hereditary variations or sporadic mutations in target tissues with individual differences in oxysterols levels? Are among them any invasive (tissue, blood) or noninvasive (saliva, urine, feces) markers useful for prediction the pathology risk? Are there markers suggesting a potential preventive measures, i.e., the use of oxysterols for decreasing the risk of disease? And finally, can some genes or proteins from the oxysterol pathway be used as targets for individualized therapies?
Trends.
Oxysterols are oxidative derivatives of cholesterol, which are a natural component of a diet, or arise enzymatically or non-enzymatically in human organism.
By interaction with some proteins and transcription factors, e.g., liver X receptors, oxysterol-binding proteins, or Insig protein, oxysterols take part in regulation of cholesterol metabolism, enzyme activity, and signaling pathways as Hedgehog, Wnt, or MAP kinase pathway.
A role of oxysterols in some pathological, e.g., atherosclerosis and neurodegenerative disorder conditions, has recently been suggested. Oxysterols are also able to modulate cancerous conditions by influencing cell proliferation or apoptosis.
25- and 27-hydroxycholesterol increase the transcription of estrogen receptor target genes in breast cancer models in vitro, suggesting their potential role in resistance to hormonal therapy and implicating clinical applications.
Acknowledgments
This work was supported by projects of the Czech Science Foundation no.: 13-25222J and the National Sustainability Program I provided by the Ministry of Education Youth and Sports of the Czech Republic, no.: LO1503. FPG acknowledges support from United States National Institutes of Health Grant R01 GM118122. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare that they have no conflict of interest.
Glossary Box
- Anti-estrogen binding sites (AEBS)
sites composed of two cholesterol metabolizing enzymes – 3β-hydroxycholesterol-Δ7-reductase (DHCR7; OMIM: 602858) and 3β-hydroxycholesterol-Δ8Δ7-isomerase (EBP; OMIM: 300205). Additionally, the AEBS complex is also reported to have cholesterol epoxide hydrolase (ChEH) activity.
- Cholesterol epoxide hydrolase (ChEH)
has enzymatic activity that converts cholestane-5α,6α-epoxy-3β-ol (α-CE) and cholestane-5β,6β-epoxy-3β-ol (β-CE) to cholestane-3β,5α,6β-triol (CT). ChEH activity is localized to endoplasmic reticulum; it is present in most mammalian tissues with the highest abundance in liver.
- Liver X receptors (LXRs)
a group of proteins influenced by the presence of oxysterols (also called oxysterol receptors). LXRα/NR1H3 (OMIM: 602423) and LXRβ/NR1H2 (OMIM: 600380) form heterodimers with retinoid X receptors (RXRs). These heterodimers sense the level of sterols in cells and influence cholesterol adsorption, cholesterol transport, and bile acid synthesis. Both regulation of gene expression and protein-protein interactions take part in this mechanism.
- Oxysterol binding proteins (OSBPs)
proteins that mediate the oxysterol activity in cells. The OSBP family contains at least 12 members, which play a role in lipid metabolism, cell signaling, or vesicle transport in response to ligand binding. The OSBP family is characterized by conserved domain (OHD – OSBP homology domain) that can bind oxysterols, cholesterol, or phospholipids. The first member of OSBP group is OSBP1 (OMIM: 167040) protein. Other members are ORPs (OSPB-related proteins) or OSBPLs (OSBP-like proteins).
- Selective estrogen receptor modulators (SERMs)
drugs commonly used as a treatment of estrogen receptor (ER) positive breast cancer patients. SERMs bind to the ER protein and modulate its activity resulting in inhibition of ER-related proliferation. The most used SERM is tamoxifen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown AJ, Jessup W. Oxysterols: Sources, cellular storage and metabolism, and new insights into their roles in cholesterol homeostasis. Mol Aspects Med. 2009;30:111–122. doi: 10.1016/j.mam.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Griffiths WJ, et al. Methods for oxysterol analysis: past, present and future. Biochem Pharmacol. 2013;86:3–14. doi: 10.1016/j.bcp.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Mutemberezi V, et al. Oxysterols: From cholesterol metabolites to key mediators. Prog Lipid Res. 2016;64:152–169. doi: 10.1016/j.plipres.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Kulig W, et al. Cholesterol oxidation products and their biological importance. Chem Phys Lipids. 2016;199:144–160. doi: 10.1016/j.chemphyslip.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Vrieling A, et al. Adult weight gain in relation to breast cancer risk by estrogen and progesterone receptor status: a meta-analysis. Breast Cancer Res Treat. 2010;123:641–649. doi: 10.1007/s10549-010-1116-4. [DOI] [PubMed] [Google Scholar]
- 6.Kitahara CM, et al. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol. 2011;29:1592–1598. doi: 10.1200/JCO.2010.31.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu J, et al. Dietary cholesterol intake and cancer. Ann Oncol. 2012;23:491–500. doi: 10.1093/annonc/mdr155. [DOI] [PubMed] [Google Scholar]
- 8.Yoon JH, et al. Oxysterols induce cyclooxygenase-2 expression in cholangiocytes: implications for biliary tract carcinogenesis. Hepatology. 2004;39:732–738. doi: 10.1002/hep.20125. [DOI] [PubMed] [Google Scholar]
- 9.Huang JD, et al. 7-Ketocholesterol-induced inflammation signals mostly through the TLR4 receptor both in vitro and in vivo. PLoS ONE. 2014;9:e100985. doi: 10.1371/journal.pone.0100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Weille J, et al. Oxysterols in cancer cell proliferation and death. Biochem Pharmacol. 2013;86:154–160. doi: 10.1016/j.bcp.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 11.Olkkonen VM, et al. Oxysterols and their cellular effectors. Biomolecules. 2012;2:76–103. doi: 10.3390/biom2010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li JW, et al. Oxysterol-binding protein-related protein 4L promotes cell proliferation by sustaining intracellular Ca2+ homeostasis in cervical carcinoma cell lines. Oncotarget. 2016;7:65849–65861. doi: 10.18632/oncotarget.11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagano K, et al. Identification and evaluation of metastasis-related proteins, oxysterol binding protein-like 5 and calumenin, in lung tumors. Int J Oncol. 2015;47:195–203. doi: 10.3892/ijo.2015.3000. [DOI] [PubMed] [Google Scholar]
- 14.Kang KA, et al. Cytotoxic effect of 7β-hydroxycholesterol on human NCI-H460 lung cancer cells. Biol Pharm Bull. 2005;28:1377–1380. doi: 10.1248/bpb.28.1377. [DOI] [PubMed] [Google Scholar]
- 15.Appukuttan A, et al. Oxysterol-induced apoptosis of smooth muscle cells is under the control of a soluble adenylyl cyclase. Cardiovasc Res. 2013;99:734–742. doi: 10.1093/cvr/cvt137. [DOI] [PubMed] [Google Scholar]
- 16.Lee T, Chau L. Fas/Fas ligand-mediated death pathway is involved in oxLDL-induced apoptosis in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2001;280:C709–C718. doi: 10.1152/ajpcell.2001.280.3.C709. [DOI] [PubMed] [Google Scholar]
- 17.De Boussac H, et al. Oxysterol receptors and their therapeutic applications in cancer conditions. Expert Opin Ther Targets. 2013;17:1029–1038. doi: 10.1517/14728222.2013.820708. [DOI] [PubMed] [Google Scholar]
- 18.Bovenga F, et al. Uncoupling nuclear receptor LXR and cholesterol metabolism in cancer. Cell Metab. 2015;21:517–526. doi: 10.1016/j.cmet.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Kuzu OF, et al. The Role of Cholesterol in Cancer. Cancer Res. 2016;76:2063–2070. doi: 10.1158/0008-5472.CAN-15-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raccosta L, et al. Cholesterol metabolites and tumor microenvironment: the road towards clinical translation. Cancer Immunol Immunother. 2016;65:111–117. doi: 10.1007/s00262-015-1779-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva J, et al. Osteoblast-derived oxysterol is a migration-inducing factor for human breast cancer cells. J Biol Chem. 2003;278:25376–25385. doi: 10.1074/jbc.M301233200. [DOI] [PubMed] [Google Scholar]
- 22.Silva J, et al. Lipids isolated from bone induce the migration of human breast cancer cells. J Lipid Res. 2006;47:724–733. doi: 10.1194/jlr.M500473-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Torres CG, et al. 27-Hydroxycholesterol induces the transition of MCF7 cells into a mesenchymal phenotype. Oncol Rep. 2011;26:389–397. doi: 10.3892/or.2011.1284. [DOI] [PubMed] [Google Scholar]
- 24.Wu Q, et al. 27-Hydroxycholesterol promotes cell-autonomous, ER-positive breast cancer growth. Cell Rep. 2013;5:637–645. doi: 10.1016/j.celrep.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raza S, et al. The cholesterol metabolite 27-hydroxycholesterol regulates p53 activity and increases cell proliferation via MDM2 in breast cancer cells. Mol Cell Biochem. 2015;410:187–195. doi: 10.1007/s11010-015-2551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lappano R, et al. The cholesterol metabolite 25-hydroxycholesterol activates estrogen receptor α-mediated signaling in cancer cells and in cardiomyocytes. PLoS ONE. 2011;6:e16631. doi: 10.1371/journal.pone.0016631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Umetani M, et al. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med. 2007;13:1185–1192. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- 28.Simigdala N, et al. Cholesterol biosynthesis pathway as a novel mechanism of resistance to estrogen deprivation in estrogen receptor-positive breast cancer. Breast Cancer Res. 2016 doi: 10.1186/s13058-016-0713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dalenc F, et al. Circulating oxysterol metabolites as potential new surrogate markers in patients with hormone receptor-positive breast cancer: Results of the OXYTAM study. J Steroid Biochem Mol Biol. 2016 doi: 10.1016/j.jsbmb.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 30.DuSell CD, et al. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol. 2008;22:65–77. doi: 10.1210/me.2007-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson ER, et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342:1094–1098. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burkard I, et al. Lipoprotein distribution and biological variation of 24S- and 27-hydroxycholesterol in healthy volunteers. Atherosclerosis. 2007;194:71–78. doi: 10.1016/j.atherosclerosis.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 33.Mast N, et al. Marketed drugs can inhibit cytochrome P450 27A1, a potential new target for breast cancer adjuvant therapy. Mol Pharmacol. 2015;88:428–436. doi: 10.1124/mol.115.099598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leek RD, et al. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–4629. [PubMed] [Google Scholar]
- 35.Hwang P, Matin A. Interactions of sterols with antiestrogen-binding sites - structural requirements for high-affinity binding. J Lipid Res. 1989;30:239–245. [PubMed] [Google Scholar]
- 36.Gylling H, et al. Tamoxifen and toremifene lower serum cholesterol by inhibition of delta 8-cholesterol conversion to lathosterol in women with breast cancer. J Clin Oncol. 1995;13:2900–2905. doi: 10.1200/JCO.1995.13.12.2900. [DOI] [PubMed] [Google Scholar]
- 37.Payré B, et al. Microsomal antiestrogen-binding site ligands induce growth control and differentiation of human breast cancer cells through the modulation of cholesterol metabolism. Mol Cancer Ther. 2008;7:3707–3718. doi: 10.1158/1535-7163.MCT-08-0507. [DOI] [PubMed] [Google Scholar]
- 38.de Medina P, et al. Tamoxifen and AEBS ligands induced apoptosis and autophagy in breast cancer cells through the stimulation of sterol accumulation. Autophagy. 2009;5:1066–1067. doi: 10.4161/auto.5.7.9820. [DOI] [PubMed] [Google Scholar]
- 39.Segala G, et al. 5,6-Epoxy-cholesterols contribute to the anticancer pharmacology of tamoxifen in breast cancer cells. Biochem Pharmacol. 2013;86:175–189. doi: 10.1016/j.bcp.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 40.Cheng YW, et al. Cholesterol-3-β, 5-α, 6-β-triol induced genotoxicity through reactive oxygen species formation. Food Chem Toxicol. 2005;43:617–622. doi: 10.1016/j.fct.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Lee D. High androgen receptor levels are predictive of decreased survival in prostate cancer. Clin Prostate Cancer. 2003;2:13–14. doi: 10.1016/s1540-0352(11)70012-9. [DOI] [PubMed] [Google Scholar]
- 42.Krycer JR, Brown AJ. Cross-talk between the androgen receptor and the liver X receptor: implications for cholesterol homeostasis. J Biol Chem. 2011;286:20637–20647. doi: 10.1074/jbc.M111.227082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fukuchi J, et al. Androgenic suppression of ATP-binding cassette transporter A1 expression in LNCaP human prostate cancer cells. Cancer Res. 2004;64:7682–7685. doi: 10.1158/0008-5472.CAN-04-2647. [DOI] [PubMed] [Google Scholar]
- 44.Raza S, et al. 27-Hydroxycholesterol stimulates cell proliferation and resistance to docetaxel-induced apoptosis in prostate epithelial cells. Med Oncol. 2016 doi: 10.1007/s12032-015-0725-5. [DOI] [PubMed] [Google Scholar]
- 45.Lin CY, et al. Cholestane-3β, 5α, 6β-triol suppresses proliferation, migration, and invasion of human prostate cancer cells. PLoS ONE. 2013;8:e65734. doi: 10.1371/journal.pone.0065734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roussi S, et al. Different apoptotic mechanisms are involved in the antiproliferative effects of 7β-hydroxysitosterol and 7β-hydroxycholesterol in human colon cancer cells. Cell Death Differ. 2005;12:128–135. doi: 10.1038/sj.cdd.4401530. [DOI] [PubMed] [Google Scholar]
- 47.Biasi F, et al. Pro-oxidant and proapoptotic effects of cholesterol oxidation products on human colonic epithelial cells: a potential mechanism of inflammatory bowel disease progression. Free Radic Biol Med. 2009;47:1731–1741. doi: 10.1016/j.freeradbiomed.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 48.Biasi F, et al. Evidence of cell damage induced by major components of a diet-compatible mixture of oxysterols in human colon cancer CaCo-2 cell line. Biochimie. 2013;95:632–640. doi: 10.1016/j.biochi.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka AR, et al. p38MAPK and Rho-dependent kinase are involved in anoikis induced by anicequol or 25-hydroxycholesterol in DLD-1 colon cancer cells. Biochem Biophys Res Commun. 2013;430:1240–1245. doi: 10.1016/j.bbrc.2012.12.067. [DOI] [PubMed] [Google Scholar]
- 50.Mascia C, et al. Proinflammatory effect of cholesterol and its oxidation products on CaCo-2 human enterocyte-like cells: effective protection by epigallocatechin-3-gallate. Free Radic Biol Med. 2010;49:2049–2057. doi: 10.1016/j.freeradbiomed.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 51.Biasi F, et al. The contribution of animal fat oxidation products to colon carcinogenesis, through modulation of TGF-β1 signaling. Carcinogenesis. 2008;29:890–894. doi: 10.1093/carcin/bgn106. [DOI] [PubMed] [Google Scholar]
- 52.Kendall CW, et al. Effect of dietary oxidized cholesterol on azoxymethane-induced colonic preneoplasia in mice. Cancer Lett. 1992;66:241–248. doi: 10.1016/0304-3835(92)90253-r. [DOI] [PubMed] [Google Scholar]
- 53.Swan R, et al. Characterisation of the oxysterol metabolising enzyme pathway in mismatch repair proficient and deficient colorectal cancer. Oncotarget. 2016;7:46509–46527. doi: 10.18632/oncotarget.10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haigh WG, Lee SP. Identification of oxysterols in human bile and pigment gallstones. Gastroenterology. 2001;121:118–123. doi: 10.1053/gast.2001.25513. [DOI] [PubMed] [Google Scholar]
- 55.Haigh WG, et al. The production of oxysterols in bile by activated human leukocytes. Biochem Biophys Res Commun. 2006;343:467–469. doi: 10.1016/j.bbrc.2006.02.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loilome W, et al. Expression of oxysterol binding protein isoforms in opisthorchiasis-associated cholangiocarcinoma: a potential molecular marker for tumor metastasis. Parasitol Int. 2012;61:136–139. doi: 10.1016/j.parint.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 57.Jusakul A, et al. Liver fluke-induced hepatic oxysterols stimulate DNA damage and apoptosis in cultured human cholangiocytes. Mutat Res. 2012;731:48–57. doi: 10.1016/j.mrfmmm.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 58.Jusakul A, et al. Anti-apoptotic phenotypes of cholestan-3β,5α,6β-triol-resistant human cholangiocytes: characteristics contributing to the genesis of cholangiocarcinoma. J Steroid Biochem Mol Biol. 2013;138:368–375. doi: 10.1016/j.jsbmb.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seo DW, et al. Oxysterols from human bile induce apoptosis of canine gallbladder epithelial cells in monolayer culture. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1247–G1256. doi: 10.1152/ajpgi.00013.2004. [DOI] [PubMed] [Google Scholar]
- 60.Hirayama T, et al. Hypercholesterolemia in rats with hepatomas: increased oxysterols accelerate efflux but do not inhibit biosynthesis of cholesterol. Hepatology. 2006;44:602–611. doi: 10.1002/hep.21291. [DOI] [PubMed] [Google Scholar]
- 61.Ikegami T, et al. Increased serum oxysterol concentrations in patients with chronic hepatitis C virus infection. Biochem Biophys Res Commun. 2014;446:736–740. doi: 10.1016/j.bbrc.2014.01.176. [DOI] [PubMed] [Google Scholar]
- 62.Zhong W, et al. Oxysterol-binding protein-related protein 8 (ORP8) increases sensitivity of hepatocellular carcinoma cells to Fas-mediated apoptosis. J Biol Chem. 2015;290:8876–8887. doi: 10.1074/jbc.M114.610188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li J, et al. Oxysterol binding protein-related protein 8 mediates the cytotoxicity of 25-hydroxycholesterol. J Lipid Res. 2016;57:1845–1853. doi: 10.1194/jlr.M069906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo X, et al. Oxysterol binding protein-related protein 8 inhibits gastric cancer growth through induction of ER stress, inhibition of Wnt signaling and activation of apoptosis. Oncol Res. 2016 doi: 10.3727/096504016X14783691306605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soncini M, et al. 24-Hydroxycholesterol participates in pancreatic neuroendocrine tumor development. Proc Natl Acad Sci USA. 2016;113:E6219–E6227. doi: 10.1073/pnas.1613332113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koga Y, et al. Oxysterol binding protein-related protein-5 is related to invasion and poor prognosis in pancreatic cancer. Cancer Sci. 2008;99:2387–2394. doi: 10.1111/j.1349-7006.2008.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Linseisen J, et al. Plasma 7β-hydroxycholesterol as a possible predictor of lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11:1630–1637. [PubMed] [Google Scholar]
- 68.Dai YB, et al. Ablation of Liver X receptors α and β leads to spontaneous peripheral squamous cell lung cancer in mice. Proc Natl Acad Sci USA. 2016;113:7614–7619. doi: 10.1073/pnas.1607590113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Melloni G, et al. Prognostic role of liver X receptor-alpha in resected stage II and III non-small-cell lung cancer. Clin Respir J. 2016 doi: 10.1111/crj.12522. [DOI] [PubMed] [Google Scholar]
- 70.Clarion L, et al. 7β-Hydroxycholesterol-induced energy stress leads to sequential opposing signaling responses and to death of C6 glioblastoma cells. Biochem Pharmacol. 2012;83:37–46. doi: 10.1016/j.bcp.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 71.de Weille J, et al. Similar pyruvate kinase modifications in glioblastoma cells by 7β-hydroxycholesterol and glutamine withdrawal. Biochem Pharmacol. 2013;86:161–167. doi: 10.1016/j.bcp.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 72.Eibinger G, et al. On the role of 25-hydroxycholesterol synthesis by glioblastoma cell lines. Implications for chemotactic monocyte recruitment. Exp Cell Res. 2013;319:1828–1838. doi: 10.1016/j.yexcr.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Christ M, et al. Apoptosis induced by oxysterols in murine lymphoma cells and in normal thymocytes. Immunology. 1993;78:455–460. [PMC free article] [PubMed] [Google Scholar]
- 74.Rosa Fernandes L, et al. 7-Ketocholesterol overcomes drug resistance in chronic myeloid leukemia cell lines beyond MDR1 mechanism. J Proteomics. 2017;151:12–23. doi: 10.1016/j.jprot.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 75.Aupeix K, et al. Oxysterol-induced apoptosis in human monocytic cell lines. Immunobiology. 1995;194:415–428. doi: 10.1016/S0171-2985(11)80108-7. [DOI] [PubMed] [Google Scholar]
- 76.Lim HK, et al. Oxysterols induce apoptosis and accumulation of cell cycle at G(2)/M phase in the human monocytic THP-1 cell line. Life Sci. 2003;72:1389–1399. doi: 10.1016/s0024-3205(02)02377-9. [DOI] [PubMed] [Google Scholar]
- 77.Pfeffer BA, et al. Differential cytotoxic effects of 7-dehydrocholesterol-derived oxysterols on cultured retina-derived cells: Dependence on sterol structure, cell type, and density. Exp Eye Res. 2016;145:297–316. doi: 10.1016/j.exer.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Silva SF, et al. Oxysterols in adipose tissue-derived mesenchymal stem cell proliferation and death. J Steroid Biochem Mol Biol. 2016 doi: 10.1016/j.jsbmb.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 79.Ishimaru C, et al. Inhibitory effects of cholesterol derivatives on DNA polymerase and topoisomerase activities, and human cancer cell growth. Lipids. 2008;43:373–382. doi: 10.1007/s11745-007-3149-y. [DOI] [PubMed] [Google Scholar]
- 80.Biasi F, et al. Oxysterol mixtures prevent proapoptotic effects of 7-ketocholesterol in macrophages: implications for proatherogenic gene modulation. FASEB J. 2004;18:693–695. doi: 10.1096/fj.03-0401fje. [DOI] [PubMed] [Google Scholar]
- 81.Gill S, et al. Sterol regulators of cholesterol homeostasis and beyond: the oxysterol hypothesis revisited and revised. Prog Lipid Res. 2008;47:391–404. doi: 10.1016/j.plipres.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 82.Sever N, et al. Insig-dependent ubiquitination and degradation of mammalian 3-hydroxy-3-methylglutaryl-CoA reductase stimulated by sterols and geranylgeraniol. J Biol Chem. 2003;278:52479–52490. doi: 10.1074/jbc.M310053200. [DOI] [PubMed] [Google Scholar]
- 83.Song BL, DeBose-Boyd RA. Ubiquitination of 3-hydroxy-3-methylglutaryl-CoA reductase in permeabilized cells mediated by cytosolic E1 and a putative membrane-bound ubiquitin ligase. J Biol Chem. 2004;279:28798–28806. doi: 10.1074/jbc.M402442200. [DOI] [PubMed] [Google Scholar]
- 84.Hozoji M, et al. Direct interaction of nuclear liver X receptor-β with ABCA1 modulates cholesterol efflux. J Biol Chem. 2008;283:30057–30063. doi: 10.1074/jbc.M804599200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bowden K, Ridgway ND. OSBP negatively regulates ABCA1 protein stability. J Biol Chem. 2008;283:18210–18217. doi: 10.1074/jbc.M800918200. [DOI] [PubMed] [Google Scholar]
- 86.Zhou T, et al. OSBP-related protein 8 (ORP8) regulates plasma and liver tissue lipid levels and interacts with the nucleoporin Nup62. PLoS ONE. 2011;6:e21078. doi: 10.1371/journal.pone.0021078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Escajadillo T, et al. Oxysterol-related-binding-protein related Protein-2 (ORP2) regulates cortisol biosynthesis and cholesterol homeostasis. Mol Cell Endocrinol. 2016;427:73–85. doi: 10.1016/j.mce.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weber-Boyvat M, et al. Oxysterol-binding proteins: functions in cell regulation beyond lipid metabolism. Biochem Pharmacol. 2013;86:89–95. doi: 10.1016/j.bcp.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 89.Olkkonen VM, et al. Oxysterol-binding proteins-emerging roles in cell regulation. Eur J Lipid Sci Technol. 2012;114:634–643. [Google Scholar]
- 90.Steffensen K, Gustafsson J. Liver X receptors: new drug targets to treat Type 2 diabetes? Future Lipidol. 2006;1:181–189. [Google Scholar]
- 91.Prabhakara J, et al. Differential effects of 24-hydroxycholesterol and 27-hydroxycholesterol on tyrosine hydroxylase and alpha-synuclein in human neuroblastoma SH-SY5Y cells. J Neurochem. 2008;107:1722–1729. doi: 10.1111/j.1471-4159.2008.05736.x. [DOI] [PubMed] [Google Scholar]
- 92.Gamba P, et al. Up-regulation of β-amyloidogenesis in neuron-like human cells by both 24- and 27-hydroxycholesterol: protective effect of N-acetyl-cysteine. Aging Cell. 2014;13:561–572. doi: 10.1111/acel.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boenzi S, et al. Evaluation of plasma cholestane-3β,5α,6β-triol and 7-ketocholesterol in inherited disorders related to cholesterol metabolism. J Lipid Res. 2016;57:361–367. doi: 10.1194/jlr.M061978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu L, et al. An oxysterol biomarker for 7-dehydrocholesterol oxidation in cell/mouse models for Smith-Lemli-Opitz syndrome. J Lipid Res. 2011;52:1222–1233. doi: 10.1194/jlr.M014498. [DOI] [PMC free article] [PubMed] [Google Scholar]