Abstract
Sphingolipids are structurally and functionally diverse molecules with significant physiologic functions and are found associated with cellular membranes and plasma lipoproteins. The cellular and plasma concentrations of sphingolipids are altered in several metabolic disorders and may serve as prognostic and diagnostic markers. Here, we discuss different sphingolipid transport mechanisms and highlight how changes in cellular and plasma sphingolipids levels contribute to cardiovascular disease, obesity, diabetes, insulin resistance and nonalcoholic fatty liver disease. Understanding mechanisms involved in intracellular transport, secretion and extracellular transport may provide novel information that might be amenable to therapeutic targeting in the treatment of various metabolic disorders.
Keywords: Sphingolipids, Lipoproteins, Metabolic disorders, Ceramide, Sphingomyelin, MTP
Background and scope of the review
Sphingolipids are both structural lipids and signaling molecules1. Their synthesis and degradation is regulated to maintain homeostasis. In de novo synthesis, ceramides are the key precursors for the synthesis of other sphingolipids. Ceramides can be glycosylated to form glycosphingolipids or acquire a polar head group to form sphingomyelin. They may also serve as precursors for other biologically active sphingolipids, including sphingosine and sphingosine-1-phosphate (S1P)1. Newly synthesized sphingolipids remain associated with cellular membranes or can be transported to plasma lipoproteins. Significant advances have been made in explaining intracellular transport and secretion of different sphingolipids. Further, new knowledge has been acquired regarding the role of different sphingolipids in metabolic diseases. Here, we will provide an overview of what is known about lipoproteins, lipid transport and sphingolipid biosynthesis. We will then discuss pathways known in the intracellular transport of sphingolipids and highlight deficiencies. Subsequently, we will summarize how alterations in plasma and tissue sphingolipid levels are associated with several metabolic disorders. Further, we highlight a few known molecular mechanisms involved in the pathogenesis of these disorders and discuss avenues for future research. Finally, we will discuss outstanding questions and provide some directions towards their resolution.
Overview of lipoproteins and lipid transport
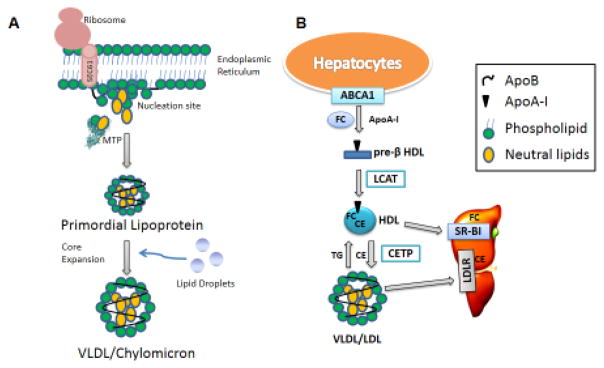
Lipoproteins transport insoluble lipids in the circulation2. Lipoproteins are classified based on their flotation densities into chylomicrons, very low density lipoproteins (VLDL), low density lipoproteins (LDL), and high density lipoproteins (HDL)2 (see Glossary). Chylomicrons, VLDL, and LDL share a common nonexchangeable structural apolipoprotein, apolipoprotein B (apoB), whereas HDL lacks this protein. VLDL and chylomicrons are assembled in the liver and intestine, respectively, and share a common two-step synthesis pathway: primordial lipoprotein formation and core expansion (Fig 1A). Assembly of these lipoproteins is dependent on microsomal triglyceride transfer protein (MTP). MTP interacts with the newly synthesized hydrophilic N-terminal domain of apoB and assists in its translocation and lipidation into primordial lipoproteins (pre-VLDL and pre-chylomicrons)3,4. In core expansion, primordial lipoproteins may fuse with lipid droplets to form VLDL or chylomicron sized particles3,4. Finally, chylomicrons and VLDL travel through the secretory pathway via special transport vesicles and are secreted into the lymph and blood stream, respectively5. LDL is formed after lipases hydrolyze triglycerides on VLDL for delivery of fatty acids to peripheral tissues.
Figure 1. Lipoprotein Assembly.
(A) VLDL and chylomicron follow similar biosynthesis pathways in the liver and intestine, respectively. While apoB is being translated, MTP physically binds apoB to possibly help it translocate into the endoplasmic reticulum lumen and to provide structural stability for further lipidation. As hydrophobic sequences are translated, apoB associates with the lumenal side of the ER membrane to form nucleation sites. MTP may transfer cholesteryl esters, triglycerides, free cholesterol, ceramide, and sphingomyelin to these nucleation sites and the translating apoB to form primordial lipoproteins. In the second step of lipoprotein assembly, the primordial lipoproteins fuse with lumenal lipid droplets to form VLDL or chylomicron that are secreted into the blood and lymph, respectively. (B) HDL Assembly. Apolipoprotein A-I (apoA-I) interacts with plasma membrane-embedded ABCA1 and accepts free cholesterol (FC) to form pre-β HDL extracellularly. Lecithin – cholesterol acyltransferase (LCAT) converts FC in pre-β HDL to cholesteryl esters (CE), which move to the core of HDL. HDL’s CE may be selectively delivered to the liver or steroidogenic tissues by scavenger receptor BI (SR-BI). Cholesteryl ester transfer protein (CETP) exchanges CE for triglycerides from HDL to apoB-containing lipoproteins. CE transferred to apoB-containing lipoproteins are delivered to the liver via receptor mediated endocytosis involving receptors such as LDL receptors.
HDL are also mainly produced by hepatocytes and enterocytes. Unlike VLDL and chylomicrons, their synthesis primarily occurs extracellularly. Apolipoproteins secreted from the liver and intestine interact with ATP-binding cassette transporter family A protein 1 (ABCA1) on the plasma membrane, accept unesterified cholesterol and phospholipids, and form discoidal pre-β HDL (Fig 1B). In the plasma, lecithin–cholesterol acyltransferase (LCAT) esterifies cholesterol in pre-β HDL to cholesteryl esters leading to a spherical HDL. ABCA1 and ABCG1 further promote cholesterol efflux to HDL. The cholesteryl esters are delivered to the liver and steroidogenic tissues after HDL interacts with the scavenger receptor B1 (SR-B1). HDL’s cholesteryl esters are also exchanged with triglycerides on apoB-lipoproteins by cholesteryl ester transfer protein (CETP) and are removed from circulation with apoB-lipoproteins via receptor-mediated endocytosis.
Sphingolipid biosynthesis
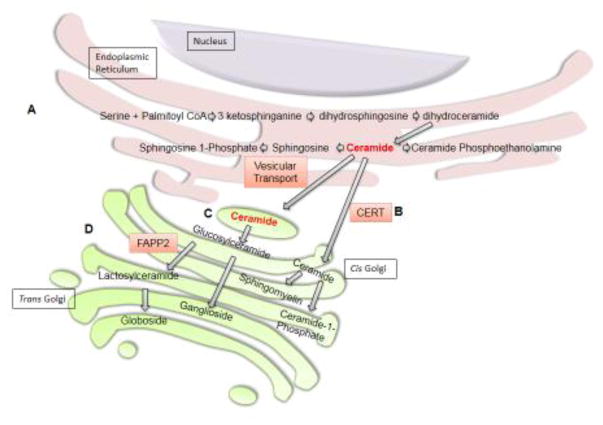
Sphingolipids are synthesized de novo in the endoplasmic reticulum (ER) (Fig 2A) and are derived from catabolism of other sphingolipids via the salvage pathway (not shown). Ceramides are the key precursors in the biosynthesis of different sphingolipids (Fig 2). In the ER, ceramides can be deacetylated to form sphingosine, which is phosphorylated to form S1P. Ceramides are also transported from the ER to Golgi via ceramide transfer protein (CERT) or transport vesicles and their mode of transfer may determine their fate6. Ceramide transfer from the ER to Golgi via CERT appears necessary for sphingomyelin synthesis as CERT-deficient mouse embryos have significantly reduced sphingomyelin levels7. Further, LY-A2 cells, which have dysfunctional CERT, have reduced sphingomyelin but normal glucosylceramide synthesis, supporting the notion that ceramide transfer via CERT predestines ceramide for sphingomyelin synthesis8. In the cis Golgi, sphingomyelin synthase 1 (SMS1) transfers the phosphorylcholine group from phosphatidylcholine to ceramide to form sphingomyelin (Fig 2B). Sphingomyelin is transported to the plasma membrane in distinct vesicles, where it forms lipid rafts with cholesterol9.
Figure 2. Sphingolipid Biosynthesis.
(A) De novo ceramide synthesis begins in the endoplasmic reticulum with the condensation of serine and palmitoyl CoA by serine palmitoyltransferase to form 3-ketosphinganine. Next, 3-ketosphinganine reductase catalyzes the reduction of 3-ketosphinganine to dihydrosphingosine which is N-acylated by ceramide synthases to form dihydroceramide. Finally, ceramide is formed by the dehydrogenation of dihydroceramide by dihydroceramide desaturase 1. Ceramides are precursors for the biosynthesis of more complex sphingolipids. In the ER, ceramides can be converted to ceramide phosphoethanolamine or deacylated by ceramidase to form sphingosine. Sphingosine can be phosphorylated to form sphingosine 1-phosphate. Ceramides can also be transferred from the endoplasmic reticulum to the Golgi by either vesicular transport or ceramide transfer protein (CERT). (B) Ceramide transfer by CERT predestines ceramide for sphingomyelin or ceramide 1-phosphate synthesis. In the Golgi, sphingomyelin synthase can convert ceramide to sphingomyelin. Alternatively, ceramide kinase can phosphorylate ceramide to form ceramide 1-phosphate. (C) Ceramide may also be transferred by vesicular transport to the cytoplasmic side of the Golgi, where it can be glycosylated to form glucosylceramide. Glucosylceramide can be transported to the lumenal side of the cis Golgi and converted to gangliosides. (D) FAPP2 can also transfer glucosylceramide from the cis Golgi to the trans Golgi, where it is converted to lactosylceramide and then to globosides.
Ceramides transported from the ER to the cytoplasmic side of the cis Golgi by transport vesicles are mainly glycosylated to form glucosylceramides (Fig 2C). Glucosylceramides are translocated from the cytoplasmic side to the Golgi lumen, possibly via transporters, for lactosylceramide synthesis6. Lactosylceramides are precursors for more complex glycosphingolipids such as globosides and gangliosides that are synthesized in the trans Golgi. Glucosylceramides can be transferred to the trans Golgi network by both vesicular and non-vesicular transport (Fig 2D)6. Four-phosphate adaptor protein 2 (FAPP2) knockdown specifically decreases globotriaosylceramide (Gb3)10, but not monosialodihexosylganglioside (GM3)6. Hence, it appears that Gb3 and GM3 are formed after glucosylceramides are transported to the trans Golgi network by FAPP2 and vesicular transport, respectively. Besides sphingomyelin and glucosylceramide, ceramides can also be phosphorylated to ceramide 1-phosphate (C1P) by ceramide kinase in the Golgi and delivered to the plasma membrane via ceramide-1-phosphate transfer protein11.
In the plasma membrane, regulation of sphingolipids involves hydrolysis and re-synthesis via different enzymes. Both acid and neutral sphingomyelinase (SMase) hydrolyze sphingomyelin to yield ceramide12. Ceramides can be converted back to sphingomyelin by SMS2. Alternatively, neutral ceramidases at the plasma membrane can convert ceramide to sphingosine. Sphingosine can be phosphorylated by types 1 and 2 sphingosine kinases to S1P.
Sphingolipid secretion and transport in the plasma
Plasma contains over 200 species of sphingolipids distributed on HDL, apoB-containing lipoproteins, and albumin13–15. Sphingolipids may associate with lipoproteins via several mechanisms. Plasma sphingolipids may associate with apoB-lipoproteins or apo-AI intracellularly prior to their secretion or effluxed from the plasma membrane to HDL, albumin or other extracellular acceptors. Sphingolipids may be transferred from one lipoprotein to another. Complex sphingolipids in lipoproteins may be degraded to other sphingolipids or synthesized from sphingolipids present on the lipoproteins. We summarize below known mechanisms and highlight deficiencies in our understanding of these pathways.
Ceramides
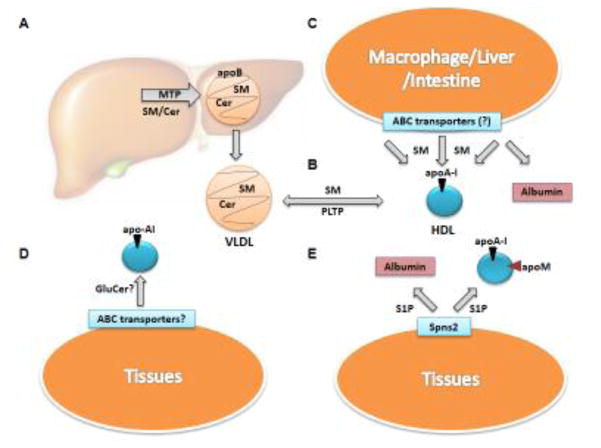
Ceramides (~3% of plasma sphingolipids) are present on VLDL, LDL and HDL15. They are distributed equally in apoB-containing lipoproteins and HDL. Within HDL subclasses, HDL2 (8.5 − 13 nm diameter) and HDL3 (7.3 − 8.5 nm diameter) carry 29% and 23%, respectively, of total ceramides in lipoproteins15. We observed that abetalipoproteinemia subjects who lack apoB-containing lipoproteins in their plasma exhibit ~80% less plasma ceramides compared to their parents and siblings and mice lacking hepatic and intestinal MTP (L-I-Mttp−/−) have >90% reduced ceramide plasma levels, highlighting the importance of MTP and apoB-lipoproteins in the control of plasma ceramides16. MTP may play a crucial role in ceramide transport to the plasma by helping apoB-lipoprotein assembly and by transferring ceramides to these lipoproteins (Fig 3A). It is unknown whether other proteins and mechanisms exist to enrich lipoproteins with ceramides.
Key Figure 3. Sphingolipid Transport to Plasma.
(A) Ceramide (Cer) and sphingomyelin (SM) are incorporated into VLDL during lipoprotein assembly by microsomal triglyceride transfer protein (MTP). (B) Phospholipid transfer protein (PLTP) may transfer SM between VLDL and HDL. It is unknown whether PLTP can transfer Cer, glucosylceramide (GluCer), or other complex sphingolipids. (C) SM may also be incorporated into HDL via efflux from different cells. ATP-binding cassette transporters on the plasma membrane of macrophages, liver, intestine, or other peripheral tissues may efflux SM to free apolipoprotein (apoA-I), HDL, albumin or other acceptors. (D) The mechanism for GluCer incorporation into lipoproteins is not defined. It is likely that GluCer is incorporated into HDL during synthesis or effluxed from the plasma membrane. (E) In the plasma, sphingosine 1-phosphate (S1P) is present on HDL and albumin. It is effluxed from the plasma membrane of peripheral tissues, mainly erythrocytes and platelets, by Spinster2 (Spns2) to apoM-containing HDL and albumin.
Total dependence of ceramide transport on apoB-lipoproteins is surprising since all cells synthesize ceramides, whereas apoB-lipoprotein assembly is restricted to hepatocytes and enterocytes. It is likely that most cells make ceramides to synthesize other sphingolipids and not for secretion. Hepatocytes and enterocytes, on the other hand, may synthesize ceramides for delivery to other tissues. It is possible that transport of ceramides via lipoproteins could be a paracrine mechanism to regulate other cells.
Significant amounts of ceramides are also found on HDL. How ceramides are incorporated into HDL particles is unknown. Plasma lipid transfer proteins, such as PLTP and CETP, may transfer ceramides from apoB-lipoproteins to HDL. Sphingomyelin degradation by SMases may also contribute to HDL ceramides. HDL may directly efflux ceramides from plasma membranes. More studies are needed to evaluate these and other possibilities.
Sphingomyelin
Sphingomyelin is the most abundant sphingolipid in lipoproteins and makes up about 87% of total plasma sphingolipids15 and 20% of total plasma phospholipids17. Approximately 63–75% and 25–35% of sphingomyelin is associated with VLDL/LDL and HDL, respectively15. Sphingomyelin is slightly more abundant (13%) in large light HDL2 than in small dense HDL3 per ml plasma (three fold more per particle)15,18,19.
Sphingomyelin arrives in the plasma via different mechanisms. In abetalipoproteinemia subjects and L-I-Mttp−/− mice, sphingomyelin levels are reduced by ~40% and ~73% compared to controls, respectively16. Thus, apoB-lipoproteins are a major source of plasma sphingomyelin. Similar to ceramides, MTP also transfers sphingomyelin between vesicles in vitro, suggesting that MTP can add sphingomyelin into VLDL during biogenesis (Fig 3A)16.
Besides secretion with VLDL, HDL may bring sphingomyelin to the plasma compartment. Sphingomyelin may associate with apoA-I in the Golgi and secrete with nascent HDL or efflux to HDL or free apoA-I via ABC transporters extracellularly (Fig 3B). Reconstituted ABCA1 in liposomes transfers sphingomyelin from the cytoplasmic to exoplasmic leaflet20. HEK293 cells over-expressing ABCA1, ABCA7 or ABCG1 and incubated with apoA-I efflux sphingomyelin21–23. However, there is little evidence that their basal levels play a role in sphingomyelin release. Over-expression of transporters may restrict membrane lipid mobility favoring efflux. Therefore, there is a need for complementary approaches involving knockdown of these transporters. There is also some evidence that ABCA8 may stimulate SMS1 to enhance sphingomyelin production24. Further, in vivo studies are needed to establish physiological role of these transporters in sphingomyelin metabolism.
Macrophages express ABCA1 and are important in cholesterol efflux. Human monocyte-derived macrophages efflux sphingomyelin to apoA-I25. However, mouse RAW 264.7 macrophages did not efflux sphingomyelin to apoA-I or reconstituted HDL26. Similarly, the role of macrophage ABCA7 in sphingomyelin transport has been questioned as depletion of macrophage ABCA7 in Ldlr−/− mice did not affect phospholipid or cholesterol efflux to apoA-I or HDL. Further, there were no differences in foam cells or atherosclerotic lesions in these and wildtype mice27. Thus, the role of macrophage ABCA1 and ABCA7 in sphingomyelin metabolism is not well defined. More studies are needed to establish the role of macrophage ABCA1 and ABCA7 in sphingomyelin efflux and identify other contributor(s) to plasma sphingomyelin levels. For example, comprehensive studies in global and macrophage-specific knockout mice may provide novel information about the role of these and other transporters in sphingolipid metabolism.
Synthesis of sphingomyelin by SMS2 on the plasma membrane and transfer from VLDL by PLTP may also contribute to HDL sphingomyelin. Sms2−/− mice on chow and high fat diet have reduced plasma sphingomyelin levels and over expression of SMS2 increased plasma sphingomyelin. PLTP can transfer sphingomyelin between vesicles in vitro, and, Pltp−/− mice lack plasma sphingomyelin transfer activity28. HDL2 contains the same sphingomyelin species profile as VLDL suggesting that HDL2 may get its sphingomyelin from VLDL. Whereas HDL3 contains more sphingomyelin with longer fatty acid chains, suggesting that HDL3 may get its sphingomyelin during biogenesis or from efflux17.
Glycosphingolipids
Complex glycosphingolipids (>50 species) make up about 9–10% of plasma sphingolipids. The most abundant glucosylceramides and lactosylceramides are distributed on VLDL (8–14%), LDL (46–60%), and HDL (28–44%)15,29. Inhibition of glycosphingolipid synthesis reduces glycosphingolipid plasma concentrations and ameliorates atherosclerosis30; however, very little is known about the origin of glycosphingolipids in plasma lipoproteins. Unlike sphingomyelin and ceramide, MTP does not transfer glycosphingolipids between vesicles in vitro. Further, MTP deficiency in humans and mice has no effect on plasma glucosylceramide concentrations16. Instead, it is likely that the main source of glycosphingolipids in the plasma is HDL or yet unidentified protein(s) (Fig 3D) and that they are effluxed by yet unidentified mechanisms. Two possible transporters are ABCA12 or ABCC1. ABCC1 has been shown to transport GluCer in vitro31. ABCA12 deficiency is associated with Harlequin ichthyosis, a disease characterized by abnormal glucosylceramide distribution in keratinocytes leading to skin barrier disruptions32. It is also expressed in macrophages and its deficiency impairs cholesterol efflux33. Although ABCA12 has been implicated in glucosylceramide transport in keratinocytes, it is unknown whether it effluxes glucosylceramide to apoA1 or HDL or plays a role in plasma glycosphingolipid transport or metabolic disorders.
Sphingosine 1-Phosphate
S1P is a bioactive signaling molecule that modulates many physiological and pathophysiological processes by interacting with its receptors34. S1P is relatively more abundant in plasma than in tissues. Under normal conditions, S1P is mainly found on HDL (50–60%) and albumin (30–40%) with very little on LDL (~8%) or VLDL (2–3%)14,35. Contrary to sphingomyelin, lipoprotein-associated S1P is mainly carried on small dense HDL315,19. Erythrocytes, platelets and endothelial cells are significant sources of plasma S1P35. Bone marrow transplantation of sphingosine kinase deficient hematopoietic cells in wildtype mice showed over 90% reduction in plasma S1P, suggesting that hematopoietic cells are the major contributors of plasma S1P levels36. The liver may also be a significant source of plasma S1P as plasma S1P levels are reduced in carbon-tetrachloride-induced liver fibrosis in rats and in patients with chronic Hepatitis C who have high plasma hyaluronic acid concentrations, a surrogate marker of liver fibrosis37.
Spinster2 (Spns2), a cell surface membrane protein, exports S1P and contributes to plasma S1P levels (Fig 3E)38. Spns2−/− mice have decreased plasma S1P38. Besides Spns2, apolipoprotein M (apoM) is also important in the transport of S1P to plasma. ApoM−/− mice have 36% lower plasma S1P. Two and 10-fold over-expression of apoM increases plasma S1P levels by 71% and 246%, respectively39,40. ApoM may be required for S1P to associate with HDL34,41 or protect S1P from extracellular and intracellular degradation by lipid phosphate phosphatases39. PLTP has also been implicated in regulating plasma S1P levels. Despite similar levels of plasma apoM, Pltp−/− mice have reduced plasma S1P (~60%) compared to WT mice. Further, PLTP can transfer S1P from isolated murine red blood cells to HDL42. Thus, several proteins are involved in the regulation of plasma S1P levels.
Pathologies associated with abnormalities in plasma and tissue sphingolipids
Many experimental and clinical studies describe sphingolipid-dependent mechanisms in the pathogenesis of various metabolic diseases1. Some genetic disorders related to sphingolipid metabolism are also associated with increased risk of cardiovascular and metabolic diseases1. We summarize relationships between changes in tissue and plasma sphingolipid concentrations and metabolic disorders and explain mechanisms contributing to these pathologies.
Cardiovascular diseases
Besides traditional risk factors, changes in sphingolipids may contribute to the pathogenesis of cardiovascular disease1,43. Higher plasma levels of sphingomyelin are also associated with increased atherosclerosis and they have been proposed as independent risk factors for coronary heart disease in humans44. Four-fold higher plasma sphingomyelin levels are found in Apoe−/− mice compared to wildtype mice45. Significant alterations in plasma, erythrocytes and platelets sphingomyelin levels were observed in experimental myocardial infarction induced in male Wistar rats46. Sphingomyelin accumulates in atherosclerotic plaques in human and animal models47. LDL present in atherosclerotic plaques has higher sphingomyelin levels, mainly arising from de novo synthesis in the aorta, compared to plasma LDL48. SMases may hydrolyze LDL-sphingomyelin in the arterial wall increasing LDL-ceramide and aggregation of lipoproteins leading to initiation and progression of atherosclerosis49. Over expression of SMS2 in mice exaggerates atherosclerotic inflammation50, whereas inhibition of sphingolipid synthesis by myriocin reduces atherosclerosis51. Further, LDL receptor knockout mice transplanted with bone marrow cells derived from Sms2−/− mice show decreased atherosclerotic lesions52. These studies suggest that high sphingomyelin concentrations in the aorta could be deleterious.
Like sphingomyelin, increased plasma and aortic ceramide levels are also associated with increased risk of cardiovascular disease51. In fact, the ratio of certain ceramide species may be predictive for cardiovascular death in coronary artery disease independent of lipid markers and C-reactive protein53, perhaps because it promotes lipoprotein aggregation, plaque instability, inflammation, and apoptosis. Further, in vitro ischemia/reperfusion of rat hearts decrease sphingomyelin levels and significantly increase ceramide concentrations54. Similarly, myocardial ischemia/reperfusion in animals significantly increased ceramide levels in cardiomyocytes1. Increased ceramide concentrations may induce apoptosis in cardiomyocytes and contribute to the increased cardiomyopathy, mortality and morbidity in diabetic patients1. Some biologically active substances such as TNF-α induce synthesis of ceramide which promote cardiomyocytes apoptosis47.
Plasma glycosphingolipid concentrations are also elevated in patients at increased risk of atherosclerosis55. Furthermore, glycosphingolipids accumulate in atherosclerotic lesions in human and Apoe−/− mice56,57. However, inhibition of glycosphingolipid synthesis has been shown to have no effect or decrease atherosclerosis30,58. Thus, it remains to be determined whether elevated glycosphingolipids are pro-atherogenic.
In contrast to ceramide, glycosphingolipid and sphingomyelin, plasma S1P is believed to be cardioprotective1. Plasma S1P levels significantly decrease after myocardial infarction59 and increase in patients after percutaneous coronary intervention60. Low S1P levels are associated with impaired cell signaling and vasodilation. Both these defects can be corrected by loading HDL with S1P61 indicating that low S1P could be a contributing factor of HDL dysfunction and CAD. Thus increased plasma sphingomyelin, glycosphingolipids, and ceramide and decreased S1P below normal levels in healthy individuals can contribute to cardiovascular disease (Fig 4).
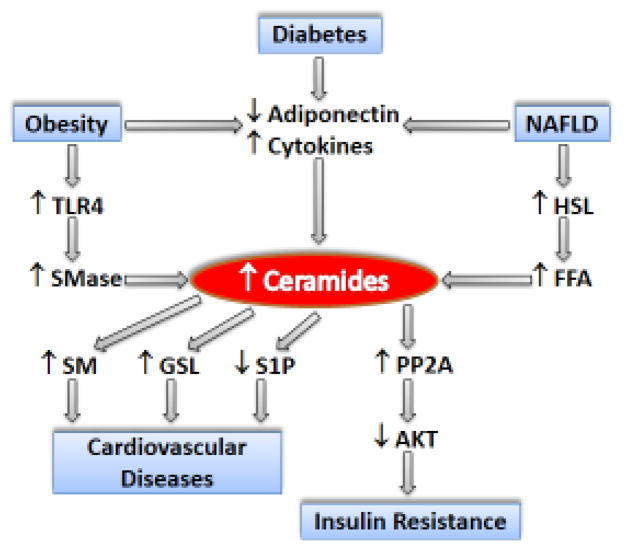
Figure 4. Schematic Diagram Depicting Molecular Events in Sphingolipid Associated Pathologies.
In diabetes, obesity and non-alcoholic fatty liver disease (NAFLD) decreased synthesis of anti-inflammatory adiponectin and increased production of pro-inflammatory cytokines lead to increased production of ceramides. Obesity also causes activation of TLR4 signaling pathway which leads to activation of sphingomyelinase (SMase). SMase hydrolyzes sphingomyelin (SM) to ceramide. In NAFLD, insulin is not able to inhibit hormone-sensitive lipase (HSL) activity in adipose tissue leading to increased production of free fatty acids (FFAs), specially palmitic acid, that are shunted for ceramides synthesis. Ceramides, in turn, enhance the activity of protein phosphatase 2A (PP2A) which causes dephosphorylation and inactivation of AKT leading to insulin resistance. Increased ceramides may be a substrate for increased SM and glycosphingolipids (GSL) synthesis that have been shown to increase the risk of cardiovascular diseases. Synthesis of sphinogsine 1-phosphate (S1P), which is cardioprotective, is decreased in cardiovascular diseases.
Obesity, diabetes and insulin resistance
Diabetic patients have elevated plasma ceramide levels, which have been implicated in insulin resistance1. Ceramides inactivate AKT in 3T3-L1 adipocytes and C2C12 myocytes62,63 by antagonizing phosphorylation and enhancing dephosphorylation by protein phosphatase 2A (PP2A)64. Furthermore, ceramides inhibit AKT translocation to membranes by activating PKCζ65. Thus, high ceramides contribute to insulin resistance by modulating different steps in the activation of AKT (Fig 4).
Serum sphingolipids may serve as biomarkers in obesity66. Ceramides are increased in the skeletal muscle, liver, and hypothalamus of obese rodents and humans67. Genetically obese mice have a significantly higher expression of enzymes promoting ceramide and sphingosine/S1P syntheses and decreased levels of enzymes that route ceramide to more complex metabolites68. Ceramide metabolism may be altered in obesity due to increased availability of plasma free fatty acids, pro-inflammatory cytokines, oxidative stress and hormones66. Fatty acids impact sphingolipid levels through both substrate supply and regulation of the enzymes involved in sphingolipid metabolism66.
During obesity, an expanded adipose tissue consisting of enlarged adipocytes and macrophages secretes a number of inflammatory cytokines, which elevates cellular levels of ceramide via increased hydrolysis of sphingomyelin and de novo synthesis. For example, TNFα and IL-1 increase ceramide synthesis by upregulating serine palmitoyltransferase (SPT) expression and activity in HepG2 cells69. Similarly, inflammatory cytokines stimulate the sphingolipid recycling pathway, in which ceramides are deacylated to form sphingosine70. Intraperitoneal administration of TNFα into C57BL/6J mice upregulates acid SMase, neutral SMase, and SPT to increase ceramide synthesis in adipose tissue68.
These cytokines also promote lipolysis in the adipose tissue which increases the delivery of fatty acids to other peripheral tissues. Elevated free fatty acids promote lipid accumulation in distal tissues and activate signaling pathways that interfere with insulin signaling and ultimately contribute to insulin resistance in adipose, muscle, and liver. Inhibition of ceramide synthesis improves obesity-induced insulin resistance71. Thus, it is likely that increased plasma and tissue ceramide concentrations contribute to insulin resistance in obese individuals.
These fatty acids may activate toll-like receptors (TLRs) in macrophages to produce various cytokines that regulate ceramide production47,72. There is an overlap between inflammatory status and ceramide production converging on the TLR4 pathway72. A subset of fatty acids that induce ceramide synthesis63 are similar to those that activate TLRs73. Further, saturated fat induces ceramide production and an inflammatory response in a TLR4-dependent manner. This increase was prevented in skeletal muscle and liver in mutant mice lacking functional TLR474. Lipopolysaccharide (LPS), which activates TLR4, induces accumulation of ceramide in serum, liver, kidney and spleen. The combination of LPS and palmitate synergistically activates ceramide production via TLR4-dependent and independent signaling, respectively75. MyD88, an essential component of TLR signaling pathway, activates SMase to increase ceramide production76. Thus, the conditions present in obesity stimulate ceramide production due to increased expression of the enzymes involved in sphingolipid metabolism involving TLR4-dependent and independent mechanisms.
Tissue ceramides may be increased in obesity and diabetes due to reduced plasma levels of adiponectin, an anti-inflammatory adipokine47. Adiponectin stimulates its receptor’s inherent ceramidase activity and the formation of sphingosine, which is phosphorylated by sphingosine kinase to produce S1P77. Metabolic disorders including diabetes, inflammation and atherosclerosis are alleviated by adiponectin47 which is attributed to its insulin-sensitizing and insulin-like effects in skeletal muscle and liver.
Non-alcoholic fatty liver disease
Non-alcoholic fatty liver disease (NAFLD) is one of the most common causes of liver disorders. A shift in one or more of the components of the sphingolipid pathway is associated with the pathogenesis of NAFLD. Transcripts that drive sphingolipid metabolism strongly correlate with liver fat78. Further, in obesity-induced insulin-resistant states common in NAFLD, insulin is unable to inhibit the activity of hormone-sensitive lipase in adipose tissues leading to increased shunting of free fatty acids for ceramide synthesis79. Ceramides can affect cellular processes that are linked to NAFLD, such as insulin resistance, steatosis, inflammation, and cholesterol levels80. For example, patients with chronic liver disease have a significantly higher serum levels of acid SMase and various ceramide species81, which positively correlate with cholesterol levels. Moreover, hepatic ceramide levels and the degree of steatosis demonstrate a positive correlation in ob/ob mice82. Inhibition of sphingolipid synthesis by myriocin reduced triglyceride accumulation, lobular inflammation, and mild centrilobular fibrosis in western diet fed Ldlr−/− mice, suggesting that ceramide synthesis may aggravate diet induced NAFLD51.
NAFLD subjects exhibit significantly greater adipose-derived cytokines and ceramides47,80. A correlation between adipose ceramide content and liver triglyceride in humans independent of obesity suggests a possible adipose-hepatic communication through which adipose ceramides and cytokines could contribute to NAFLD79. Patients with NAFLD have elevated plasma levels of TNFα83 and increased hepatic expression of TNFα receptor compared to healthy individuals84. Obese rodents show elevated levels of both hepatic sphingolipids and pro-inflammatory cytokines80. Hepatic expression of adipoR2, a predominant hepatic adiponectin receptor, and circulating adiponectin levels are reduced in diet-induced obese (DIO) mice85. Expression of adiponectin is inversely correlated with expression of SMase79 which is regulated by inflammatory stimuli, including TNFα47. Furthermore, LPS treatment upregulates hepatic Sptlc2 mRNA and activity leading to increased hepatic sphingomyelin and ceramide levels69. Taken together, these studies suggest that sphingolipids may play an important role in steatohepatitis.
7. Concluding remarks
As summarized above, deregulation of sphingolipid synthesis and transport is associated with cardiovascular disease, obesity, NAFLD, diabetes and other metabolic disorders. In some situations, mechanistic information has been gathered about how sphingolipids contribute to metabolic dysfunction. However, more mechanistic studies are needed to identify other critical sphingolipids, signaling pathways, the effects of specific sphingolipid species, and associated pathologies (Outstanding Questions). Plasma sphingolipid levels are emerging as biomarkers and prognostic indicators for many metabolic disorders86. Specific ceramide species have been linked to cardiovascular disease and insulin resistance87. Their ratios are emerging as prognostic indicators of mortality in metabolic diseases. In fact, ceramide measurements may become routine in the clinic allowing the identification of high risk cardiovascular patients based on ceramide plasma levels53. Further, large scale measurements of different plasma sphingolipid species may advance their association with various other pathologies.
Outstanding Questions Box.
How does sphingomyelin associate with HDL?
How does ceramide reach HDL after entering plasma with apoB-lipoproteins?
How do glucosylceramides arrive in the plasma compartment?
What happens to sphingolipids during the hydrolysis of triglycerides in apoB-containing lipoproteins?
Do PLTP and CETP play a role in redistributing sphingolipids amongst different lipoproteins?
Do sphingolipids present in lipoproteins affect cellular function? If they do, does this require cellular uptake?
What is the differential contribution of extracellular and intracellular ceramides and other sphingolipids in the pathogenesis of metabolic disorders?
Will targeting of sphingolipid synthesis and transport proteins alleviate some metabolic disorders?
Although significant information is available about different sphingolipids and their presence with lipoproteins, there is paucity of information about mechanisms involved in their transport to the plasma compartment. It is likely that disruptions in these mechanisms could contribute to metabolic disorders and modulation of these pathways may prove therapeutic. Therefore, more efforts are needed to understand mechanisms involved in the transport of different sphingolipids to plasma. Several sphingolipid synthesis genes have been targeted as potential therapeutics for various metabolic disorders, such as atherosclerosis. Similarly, identification and targeting of specific sphingolipid transport proteins may provide additional targets for therapeutics.
Trends Box.
Deregulation of plasma sphingolipids are implicated in many metabolic disorders and are emerging as biomarkers and prognostic indicators.
The importance of plasma sphingolipids and recent technological advances in lipidomics has spurred investigation into sphingolipid transport mechanisms and the effects of specific sphingolipid species in disease.
Identification of proteins involved in sphingolipid efflux and transport may provide additional therapeutic targets for metabolic disorders.
Acknowledgments
This work was supported in part by US National Institutes of Health grants HL-95924, R56DK046900-17A1 and VA Merit Award (BX001728) to MMH. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Abbreviations used
- ABCA1
ATP-binding cassette transporter family A protein 1
- ABCA7
ATP-binding cassette transporter family A protein 7
- ABCG1
ATP-binding cassette transporter family G protein 1
- ApoA-I
apolipoprotein A-I
- ApoB
apolipoprotein B
- ApoE
apolipoprotein E
- ApoM
apolipoprotein M
- C1P
ceramide 1-phosphate
- CERT
ceramide transfer protein
- CETP
cholesteryl ester transfer protein
- ER
endoplasmic reticulum
- FAPP2
four-phosphate adaptor protein 2
- Gb3
globotriaosylceramide
- GM3
monosialodihexosylganglioside
- HDL
high density lipoproteins
- LCAT
lecithin–cholesterol acyltransferase
- LDL
low density lipoproteins
- LPS
lipopolysaccharide
- MTP
microsomal triglyceride transfer protein
- NAFLD
non-alcoholic fatty liver disease
- PLTP
phospholipid transfer protein
- PP2A
protein phosphatase 2A
- S1P
sphingosine-1-phosphate
- SMS
sphingomyelin synthase
- SMase
sphingomyelinase
- Spns2
spinster2
- SPT
serine palmitoyltransferase
- SR-B1
scavenger receptor B1
- TLR
toll-like receptor
- VLDL
very low density lipoproteins
Glossary
- Abetalipoproteinemia
A disorder associated with loss of function mutation in the microsomal triglyceride transfer protein gene. These individuals have very low plasma lipids and lack apoB-containing lipoportiens
- Apolipoproteins
Proteins found associated with lipoproteins. They are designated as A, B, C etc. and are sometimes followed with numbers that convey different meanings. ApoB is non-exchangeable and is always found associated with lipoproteins. All other apolipoproteins are exchangeable proteins that exist free or associated with lipoproteins
- Atherosclerosis
A disease where accumulation of fat causes narrowing and hardening of large arteries
- Chylomicrons
Very large, triglyceride-rich lipoproteins synthesized by the intestine to transport dietary fat
- Diabetes
A disease associated with high plasma glucose levels
- HDL
High density lipoproteins are mainly involved in the reverse cholesterol transport from peripheral tissues to the liver. They also have anti-inflammatory and anti-oxidative properties and are negatively correlated with atherosclerosis. HDL are further subdivided into HDL2 and HDL3 etc. based on density
- LDL
Catabolic products of VLDL that stay in plasma for long periods and their high levels are a major risk factor for heart disease
- Lipid rafts
Detergent insoluble regions of the plasma membrane that contain high levels of cholesterol, sphingomyelin, and glycosphingolipids
- Lipoproteins
Complexes of lipids and proteins that transport lipids from the liver and intestine to peripheral tissues. They consist of a neutral lipid core and a surface monolayer consisting of cholesterol, phospholipids and sphingolipids that harbors apolipoproteins
- NAFLD
Accumulation of fat in the liver that is independent of alcohol intake
- Sphingolipids
Sphingolipids are composed of a sphingosine (an 18-carbon amino alcohol) backbone that is N-acylated with various fatty acids
- VLDL
Very low density lipoproteins that are mainly synthesized by the liver to transport endogenous fat
Footnotes
Conflicts of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Borodzicz S, et al. Sphingolipids in cardiovascular diseases and metabolic disorders. Lipids Health Dis. 2015;14:55. doi: 10.1186/s12944-015-0053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feingold KR, Grunfeld C. Introduction to Lipids and Lipoproteins. In: De Groot LJ, Chrousos G, Dungan K, et al., editors. Endotext [Internet] South Dartmouth (MA): MDText.com, Inc; 2000. Updated 2015 Jun 10. Available from: https://www.ncbi.nlm.nih.gov/books/NBK305896/ [Google Scholar]
- 3.Walsh MT, Hussain MM. Targeting microsomal triglyceride transfer protein and lipoprotein assembly to treat homozygous familial hypercholesterolemia. Crit Rev Clin Lab Sci. 2016:1–23. doi: 10.1080/10408363.2016.1221883. [DOI] [PubMed] [Google Scholar]
- 4.Hussain MM, et al. Multiple functions of microsomal triglyceride transfer protein. Nutr Metab (Lond) 2012;9:14. doi: 10.1186/1743-7075-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tiwari S, Siddiqi SA. Intracellular trafficking and secretion of VLDL. Arterioscler Thromb Vasc Biol. 2012;32:1079–1086. doi: 10.1161/ATVBAHA.111.241471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Angelo G, et al. Vesicular and non-vesicular transport feed distinct glycosylation pathways in the Golgi. Nature. 2013;501:116–120. doi: 10.1038/nature12423. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, et al. Mitochondrial degeneration and not apoptosis is the primary cause of embryonic lethality in ceramide transfer protein mutant mice. J Cell Biol. 2009;184:143–158. doi: 10.1083/jcb.200807176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanada K, et al. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–809. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- 9.Deng Y, et al. Sphingomyelin is sorted at the trans Golgi network into a distinct class of secretory vesicle. Proc Natl Acad Sci U S A. 2016;113:6677–6682. doi: 10.1073/pnas.1602875113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Angelo G, et al. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature. 2007;449:62–67. doi: 10.1038/nature06097. [DOI] [PubMed] [Google Scholar]
- 11.Simanshu DK, et al. Non-vesicular trafficking by a ceramide-1-phosphate transfer protein regulates eicosanoids. Nature. 2013;500:463–467. doi: 10.1038/nature12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussain MM, et al. Mechanisms involved in cellular ceramide homeostasis. Nutr Metab (Lond) 2012;9:71. doi: 10.1186/1743-7075-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quehenberger O, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51:3299–3305. doi: 10.1194/jlr.M009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammad SM, et al. Sphingosine 1-phosphate distribution in human plasma: associations with lipid profiles. J Lipids. 2012;2012:180705. doi: 10.1155/2012/180705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammad SM, et al. Blood sphingolipidomics in healthy humans: impact of sample collection methodology. J Lipid Res. 2010;51:3074–3087. doi: 10.1194/jlr.D008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iqbal J, et al. Microsomal triglyceride transfer protein transfers and determines plasma concentrations of ceramide and sphingomyelin but not glycosylceramide. J Biol Chem. 2015;290:25863–25875. doi: 10.1074/jbc.M115.659110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nilsson A, Duan RD. Absorption and lipoprotein transport of sphingomyelin. J Lipid Res. 2006;47:154–171. doi: 10.1194/jlr.M500357-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Kontush A, et al. Preferential sphingosine-1-phosphate enrichment and sphingomyelin depletion are key features of small dense HDL3 particles: relevance to antiapoptotic and antioxidative activities. Arterioscler Thromb Vasc Biol. 2007;27:1843–1849. doi: 10.1161/ATVBAHA.107.145672. [DOI] [PubMed] [Google Scholar]
- 19.Camont L, et al. Small, dense high-density lipoprotein-3 particles are enriched in negatively charged phospholipids: relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti-inflammatory, and antiapoptotic functionalities. Arterioscler Thromb Vasc Biol. 2013;33:2715–2723. doi: 10.1161/ATVBAHA.113.301468. [DOI] [PubMed] [Google Scholar]
- 20.Quazi F, Molday RS. Differential phospholipid substrates and directional transport by ATP-binding cassette proteins ABCA1, ABCA7, and ABCA4 and disease-causing mutants. J Biol Chem. 2013;288:34414–34426. doi: 10.1074/jbc.M113.508812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang N, et al. ATP-binding cassette transporter A7 (ABCA7) binds apolipoprotein A-I and mediates cellular phospholipid but not cholesterol efflux. J Biol Chem. 2003;278:42906–42912. doi: 10.1074/jbc.M307831200. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi A, et al. Efflux of sphingomyelin, cholesterol, and phosphatidylcholine by ABCG1. J Lipid Res. 2006;47:1791–1802. doi: 10.1194/jlr.M500546-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Hotta N, et al. Preferential incorporation of shorter and less unsaturated acyl phospholipids into high density lipoprotein-like particles in the ABCA1- and ABCA7-mediated biogenesis with apoA-I. Chem Phys Lipids. 2015;187:1–9. doi: 10.1016/j.chemphyslip.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Kim WS, et al. ABCA8 stimulates sphingomyelin production in oligodendrocytes. Biochem J. 2013;452:401–410. doi: 10.1042/BJ20121764. [DOI] [PubMed] [Google Scholar]
- 25.Schifferer R, et al. ApoA-I induces a preferential efflux of monounsaturated phosphatidylcholine and medium chain sphingomyelin species from a cellular pool distinct from HDL(3) mediated phospholipid efflux. Biochim Biophys Acta. 2007;1771:853–863. doi: 10.1016/j.bbalip.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Toledo JD, et al. Effect of reconstituted discoidal high-density lipoproteins on lipid mobilization in RAW 264.7 and CHOK1 cells. J Cell Biochem. 2012;113:1208–1216. doi: 10.1002/jcb.23453. [DOI] [PubMed] [Google Scholar]
- 27.Meurs I, et al. Effects of deletion of macrophage ABCA7 on lipid metabolism and the development of atherosclerosis in the presence and absence of ABCA1. PLoS One. 2012;7:e30984. doi: 10.1371/journal.pone.0030984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, et al. Sphingomyelin synthase 2 is one of the determinants for plasma and liver sphingomyelin levels in mice. Arterioscler Thromb Vasc Biol. 2009;29:850–856. doi: 10.1161/ATVBAHA.109.185223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scherer M, et al. Sphingolipid profiling of human plasma and FPLC-separated lipoprotein fractions by hydrophilic interaction chromatography tandem mass spectrometry. Biochim Biophys Acta. 2011;1811:68–75. doi: 10.1016/j.bbalip.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Chatterjee S, et al. Inhibition of glycosphingolipid synthesis ameliorates atherosclerosis and arterial stiffness in apolipoprotein E−/ − mice and rabbits fed a high-fat and -cholesterol diet. Circulation. 2014;129:2403–2413. doi: 10.1161/CIRCULATIONAHA.113.007559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raggers RJ, et al. The human multidrug resistance protein MRP1 translocates sphingolipid analogs across the plasma membrane. J Cell Sci. 1999;112( Pt 3):415–422. doi: 10.1242/jcs.112.3.415. [DOI] [PubMed] [Google Scholar]
- 32.Ishibashi Y, et al. New insights on glucosylated lipids: metabolism and functions. Biochim Biophys Acta. 2013;1831:1475–1485. doi: 10.1016/j.bbalip.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Fu Y, et al. ABCA12 regulates ABCA1-dependent cholesterol efflux from macrophages and the development of atherosclerosis. Cell Metab. 2013;18:225–238. doi: 10.1016/j.cmet.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Proia RL, Hla T. Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. J Clin Invest. 2015;125:1379–1387. doi: 10.1172/JCI76369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ksiazek M, et al. Sources, metabolism, and regulation of circulating sphingosine-1-phosphate. J Lipid Res. 2015;56:1271–1281. doi: 10.1194/jlr.R059543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pappu R, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 37.Ikeda H, et al. Plasma concentration of bioactive lipid mediator sphingosine 1-phosphate is reduced in patients with chronic hepatitis C. Clin Chim Acta. 2010;411:765–770. doi: 10.1016/j.cca.2010.02.063. [DOI] [PubMed] [Google Scholar]
- 38.Nagahashi M, et al. Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels, and the lymphatic network. FASEB J. 2013;27:1001–1011. doi: 10.1096/fj.12-219618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurano M, et al. Liver involvement in sphingosine 1-phosphate dynamism revealed by adenoviral hepatic overexpression of apolipoprotein M. Atherosclerosis. 2013;229:102–109. doi: 10.1016/j.atherosclerosis.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 40.Liu M, et al. Hepatic apolipoprotein M (apoM) overexpression stimulates formation of larger apoM/sphingosine 1-phosphate-enriched plasma high density lipoprotein. J Biol Chem. 2014;289:2801–2814. doi: 10.1074/jbc.M113.499913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christoffersen C, et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc Natl Acad Sci U S A. 2011;108:9613–9618. doi: 10.1073/pnas.1103187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu Y, et al. Phospholipid transfer protein deficiency decreases the content of S1P in HDL via the loss of its transfer capability. Lipids. 2014;49:183–190. doi: 10.1007/s11745-013-3850-y. [DOI] [PubMed] [Google Scholar]
- 43.Sasset L, et al. Sphingolipid De Novo Biosynthesis: A Rheostat of Cardiovascular Homeostasis. Trends Endocrinol Metab. 2016;27:807–819. doi: 10.1016/j.tem.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang XC, et al. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler Thromb Vasc Biol. 2000;20:2614–2618. doi: 10.1161/01.atv.20.12.2614. [DOI] [PubMed] [Google Scholar]
- 45.Jeong T, et al. Increased sphingomyelin content of plasma lipoproteins in apolipoprotein E knockout mice reflects combined production and catabolic defects and enhances reactivity with mammalian sphingomyelinase. J Clin Invest. 1998;101:905–912. doi: 10.1172/JCI870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knapp M, et al. Myocardial infarction differentially alters sphingolipid levels in plasma, erythrocytes and platelets of the rat. Basic Res Cardiol. 2012;107:294. doi: 10.1007/s00395-012-0294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang SC, et al. Sphingolipid metabolism and obesity-induced inflammation. Front Endocrinol (Lausanne) 2013;4:67. doi: 10.3389/fendo.2013.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guyton JR, Klemp KF. Development of the lipid-rich core in human atherosclerosis. Arterioscler Thromb Vasc Biol. 1996;16:4–11. doi: 10.1161/01.atv.16.1.4. [DOI] [PubMed] [Google Scholar]
- 49.Schissel SL, et al. Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. Proposed role for arterial-wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. J Clin Invest. 1996;98:1455–1464. doi: 10.1172/JCI118934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao YR, et al. Sphingomyelin synthase 2 over-expression induces expression of aortic inflammatory biomarkers and decreases circulating EPCs in ApoE KO mice. Life Sci. 2012;90:867–873. doi: 10.1016/j.lfs.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 51.Kasumov T, et al. Ceramide as a mediator of non-alcoholic Fatty liver disease and associated atherosclerosis. PLoS One. 2015;10:e0126910. doi: 10.1371/journal.pone.0126910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu J, et al. Macrophage sphingomyelin synthase 2 deficiency decreases atherosclerosis in mice. Circ Res. 2009;105:295–303. doi: 10.1161/CIRCRESAHA.109.194613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laaksonen R, et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J. 2016;37:1967–1976. doi: 10.1093/eurheartj/ehw148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cordis GA, et al. HPTLC analysis of sphingomylein, ceramide and sphingosine in ischemic/reperfused rat heart. J Pharm Biomed Anal. 1998;16:1189–1193. doi: 10.1016/s0731-7085(97)00260-4. [DOI] [PubMed] [Google Scholar]
- 55.Dawson G, et al. Distribution of glycosphingolipids in the serum lipoproteins of normal human subjects and patients with hypo- and hyperlipidemias. J Lipid Res. 1976;17:125–131. [PubMed] [Google Scholar]
- 56.Breckenridge WC, et al. Increase of gangliosides in atherosclerotic human aortas. Lipids. 1975;10:256–259. doi: 10.1007/BF02532490. [DOI] [PubMed] [Google Scholar]
- 57.Garner B, et al. Increased glycosphingolipid levels in serum and aortae of apolipoprotein E gene knockout mice. J Lipid Res. 2002;43:205–214. [PubMed] [Google Scholar]
- 58.Glaros EN, et al. Reduction of plasma glycosphingolipid levels has no impact on atherosclerosis in apolipoprotein E-null mice. J Lipid Res. 2008;49:1677–1681. doi: 10.1194/jlr.E800005-JLR200. [DOI] [PubMed] [Google Scholar]
- 59.Knapp M, et al. Sustained decrease in plasma sphingosine-1-phosphate concentration and its accumulation in blood cells in acute myocardial infarction. Prostaglandins Other Lipid Mediat. 2013;106:53–61. doi: 10.1016/j.prostaglandins.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 60.Egom EE, et al. Serum sphingolipids level as a novel potential marker for early detection of human myocardial ischaemic injury. Front Physiol. 2013;4:130. doi: 10.3389/fphys.2013.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sattler K, et al. Defects of High-Density Lipoproteins in Coronary Artery Disease Caused by Low Sphingosine-1-Phosphate Content: Correction by Sphingosine-1-Phosphate-Loading. J Am Coll Cardiol. 2015;66:1470–1485. doi: 10.1016/j.jacc.2015.07.057. [DOI] [PubMed] [Google Scholar]
- 62.Summers SA, et al. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol Cell Biol. 1998;18:5457–5464. doi: 10.1128/mcb.18.9.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chavez JA, et al. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem. 2003;278:10297–10303. doi: 10.1074/jbc.M212307200. [DOI] [PubMed] [Google Scholar]
- 64.Dobrowsky RT, et al. Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem. 1993;268:15523–15530. [PubMed] [Google Scholar]
- 65.Powell DJ, et al. Intracellular ceramide synthesis and protein kinase Czeta activation play an essential role in palmitate-induced insulin resistance in rat L6 skeletal muscle cells. Biochem J. 2004;382:619–629. doi: 10.1042/BJ20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Russo SB, et al. Sphingolipids in obesity, type 2 diabetes, and metabolic disease. Handb Exp Pharmacol. 2013:373–401. doi: 10.1007/978-3-7091-1511-4_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choi S, Snider AJ. Sphingolipids in high fat diet and obesity-related diseases. Mediators Inflamm. 2015;2015:520618. doi: 10.1155/2015/520618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Samad F, et al. Altered adipose and plasma sphingolipid metabolism in obesity: a potential mechanism for cardiovascular and metabolic risk. Diabetes. 2006;55:2579–2587. doi: 10.2337/db06-0330. [DOI] [PubMed] [Google Scholar]
- 69.Memon RA, et al. Endotoxin and cytokines increase hepatic sphingolipid biosynthesis and produce lipoproteins enriched in ceramides and sphingomyelin. Arterioscler Thromb Vasc Biol. 1998;18:1257–1265. doi: 10.1161/01.atv.18.8.1257. [DOI] [PubMed] [Google Scholar]
- 70.Sultan I, et al. Regulation of the sphingosine-recycling pathway for ceramide generation by oxidative stress, and its role in controlling c-Myc/Max function. Biochem J. 2006;393:513–521. doi: 10.1042/BJ20051083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holland WL, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 72.Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev. 2008;29:381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holland WL, et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest. 2011;121:1858–1870. doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schilling JD, et al. Palmitate and lipopolysaccharide trigger synergistic ceramide production in primary macrophages. J Biol Chem. 2013;288:2923–2932. doi: 10.1074/jbc.M112.419978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davis CN, et al. IL-1beta induces a MyD88-dependent and ceramide-mediated activation of Src in anterior hypothalamic neurons. J Neurochem. 2006;98:1379–1389. doi: 10.1111/j.1471-4159.2006.03951.x. [DOI] [PubMed] [Google Scholar]
- 77.Holland WL, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Greco D, et al. Gene expression in human NAFLD. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1281–G1287. doi: 10.1152/ajpgi.00074.2008. [DOI] [PubMed] [Google Scholar]
- 79.Kolak M, et al. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes. 2007;56:1960–1968. doi: 10.2337/db07-0111. [DOI] [PubMed] [Google Scholar]
- 80.Ilan Y. Compounds of the sphingomyelin-ceramide-glycosphingolipid pathways as secondary messenger molecules: new targets for novel therapies for fatty liver disease and insulin resistance. Am J Physiol Gastrointest Liver Physiol. 2016;310:G1102–G1117. doi: 10.1152/ajpgi.00095.2016. [DOI] [PubMed] [Google Scholar]
- 81.Grammatikos G, et al. Serum acid sphingomyelinase is upregulated in chronic hepatitis C infection and non alcoholic fatty liver disease. Biochim Biophys Acta. 2014;1841:1012–1020. doi: 10.1016/j.bbalip.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 82.Yetukuri L, et al. Bioinformatics strategies for lipidomics analysis: characterization of obesity related hepatic steatosis. BMC Syst Biol. 2007;1:12. doi: 10.1186/1752-0509-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jarrar MH, et al. Adipokines and cytokines in non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;27:412–421. doi: 10.1111/j.1365-2036.2007.03586.x. [DOI] [PubMed] [Google Scholar]
- 84.Feldstein AE, et al. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–194. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 85.Peng Y, et al. Downregulation of adiponectin/AdipoR2 is associated with steatohepatitis in obese mice. J Gastrointest Surg. 2009;13:2043–2049. doi: 10.1007/s11605-009-1032-2. [DOI] [PubMed] [Google Scholar]
- 86.Upadhyay RK. Emerging risk biomarkers in cardiovascular diseases and disorders. J Lipids. 2015;2015:971453. doi: 10.1155/2015/971453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Turpin SM, et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014;20:678–686. doi: 10.1016/j.cmet.2014.08.002. [DOI] [PubMed] [Google Scholar]