Abstract
Man-made xenobiotics, whose potential toxicological effects are not fully understood, are oversaturating the already-contaminated environment. Due to the rate of toxicant accumulation, unmanaged disposal, and unknown adverse effects to the environment and the human population, there is a crucial need to screen for environmental toxicants. Animal models and in vitro models are ineffective models in predicting in vivo responses due to inter-species difference and/or lack of physiologically-relevant 3D tissue environment. Such conventional screening assays possess limitations that prevent dynamic understanding of toxicants and their metabolites produced in the human body. Organ-on-a-chip systems can recapitulate in vivo like environment and subsequently in vivo like responses generating a realistic mock-up of human organs of interest, which can potentially provide human physiology-relevant models for studying environmental toxicology. Feasibility, tunability, and low-maintenance features of organ-on-chips can also make possible to construct an interconnected network of multiple-organs-on-chip towards a realistic human-on-a-chip system. Such interconnected organ-on-a-chip network can be efficiently utilized for toxicological studies by enabling the study of metabolism, collective response, and fate of toxicants through its journey in the human body. Further advancements can address the challenges of this technology, which potentiates high predictive power for environmental toxicology studies.
Introduction
With the momentous advancement of technologies, introduction of man-made toxic xenobiotics, or toxicants, are accumulating in the environment that are poorly understood and/or not yet identified. The United States Centers for Disease Control and Prevention (CDC) reported over 80,000 chemicals used in 2012, which 2,000 chemicals are manufactured or imported into the U.S. in amounts of at least one million pounds per year, commonly referred to as high production volume (HPV) chemicals [1]. Due to the rate of toxicant accumulation, unmanaged disposal, and the unknown toxicological effects to the environment, there is a crucial need to quickly and efficiently evaluate the potential adverse health effects upon inevitable integration into the human body. Unfortunately, most of the previous research has concerned with identifying human exposure to HPV chemicals rather than addressing the need to understand toxicological effects in human physiology-relevant models.
One of the most well-known conventional screening methods is Toxicity Forecaster or ToxCast in short, which is a high throughput screening (HTS) based method employed by the U.S. Environmental Protection Agency's (EPA). ToxCast prioritizes HPV chemicals in in vitro models, of which over 1,800 chemicals have been at least partially analyzed, whose data is then compared to the results of animal studies. This method, however, remains time-consuming, costly, and still relatively low-throughput [2•,3]. In vitro models are limited in high predictive power due to significant shortcoming in the use of in vitro 2D models, which are incomparable to the complex, in vivo 3D microenvironment detailed in human physiology. The 3D microenvironment exhibits a well-organized architecture possessing intimate cell-cell interactions and cell-extracellular matrix (ECM) network that is essential for recapitulating the human physiology. In addition, toxicity studies from animal models may inaccurately portray toxicological effects in the human body due to obvious inter-species differences [2•,3].
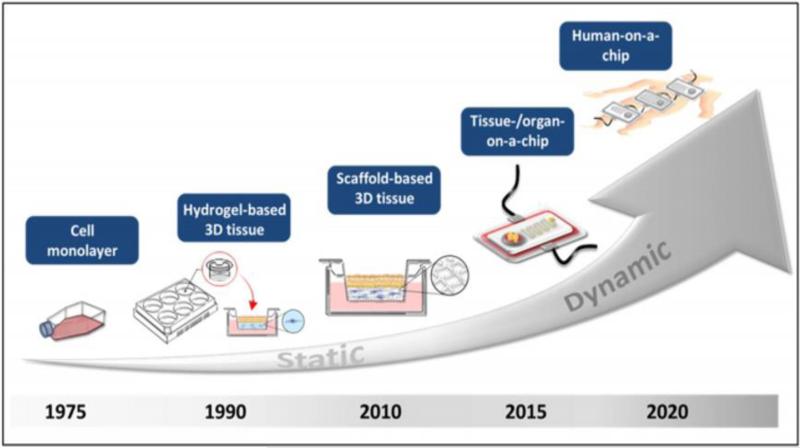
As illustrated in Figure 1, recent innovations in microfluidic technologies have produced organ-on-a-chip (OOC) platforms, which integrate advanced 3D tissue engineered constructs with microfluidic networks to minimize the shortcomings of in vitro 2D models [2•,4•]. Such cohesive platform enables important physiological cues, such as the vasculature and interstitial fluid flow, which improves mimicry of the in vivo physiological conditions for studying stem cell differentiation, metastasis, etc. In addition, inter-species differences can be eliminated through the use of human cells. Furthermore, OOC researchers have begun to investigate interconnecting multiple OOC systems into a network (Figure 1), in order to emulate inter-organ relationships and ultimately objectify human-body-like microphysiological systems [4•]. While OOC systems have primarily been utilized for stem cell, cancer, and drug testing, they can also be used towards environmental toxicology studies. In this mini-review, conventional environmental toxicology screening will first be summarized for select HPV toxicants. OOC technologies will then be discussed in regard to its potential for assessing environmental toxicants, in addition to what challenges must be addressed to produce a better alternative to in vitro 2D models and animal models.
Figure 1.
Evolution from in vitro models to multi-OOC systems. Figure reproduced from Planz et al., with permission from Elsevier [70].
Conventional Environmental Toxicology Screening
Conventional HTS relies on 2D cultured cells to evaluate the cytotoxicity to drugs or toxicants, whose responses differ from those obtained in vivo due to the lack of physical and humoral interactions provided by the ECM, cell-cell interactions, and other molecular components of the native organ [5]. Indeed animal models do reproduce organ complexity more accurately but deduction of toxicological responses may be ambiguous due to inter-species differences and thus irrelevant to human physiological responses. Also, the time consumption, cost, and ethical concerns of animal testing disfavors its use in toxicological research [5,6].
Microfluidic HTS systems (typically considered a precursor to OOC systems), where cells are cultured in microfluidic channels, do incorporate flow components in a miniaturized manner (leading to low fluid consumption, assay miniaturization, and parallel processing) [7,8,9,10]. Yet, they cannot assess detailed information regarding the effects of generated metabolites, bioaccumulation, cell-ECM interactions, and processing via organs as it travels throughout the human body.
On the other hand, precision-cut organ models, where thin tissue slices are used rather than 2D cultured cells, demonstrate the sheer advantage of direct interspecies comparison with respect to metabolic capacity and sensitivity for toxicants [6], and therefore has been identified as useful models for toxicological assessment [5,11,12]. However, obtained tissue slices are largely constrained by the limited viability for toxicological testing, which inhibits long-term toxicity studies [5].
Known HPV Toxicants
Select known HPV toxicants of interest (especially prioritized by the CDC) are listed here: environmental phenols, polybrominated diphenyl ethers (PBDE), phthalates, and perfluorinated chemicals (PFCs) (Table 1).
Table 1.
Summary of select environmental HPV toxicants, shown together with how their adverse health concerns have been addressed with in vitro, animal, and human physiological response models. The most common health concerns of interest were those altering reproductive systems, altering developmental systems, inducing cancer, altering neurological systems, and inducing acute toxicity responses. Although there are statistical studies determining potential associations between existing adverse effects and human exposure of toxicants, those were not considered as human physiological response models.
| Group | Representative Chemical and Its Structure | Half-life | In vitro or Animal Model Available to Address Adverse Health Concerns? | Human Physiological Response Model Available to Address Adverse Health Concerns? |
|---|---|---|---|---|
| Environmental phenols |
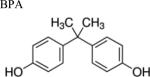
|
4-5 hours [78] | Reproductive: Y Developmental: Y Cancer: Y Neurological: Y Acute: Y *All from [78] |
Reproductive: Little Developmental: N Cancer: N Neurological: N Acute: N *All from [78] |
| PBDE |
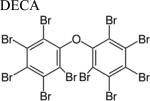
|
Average 15 days. Longer half-lives with decreasing bromides (37-91 days) [79] | Reproductive: Y [27] Developmental: Y [27] Cancer: Y [76] Neurological: Y [27] Acute: Y [18] |
Reproductive: Y [29,81] Developmental: N Cancer: N Neurological: Y [29,82] Acute: N |
| Phthalates |
|
12 hours [83] | Reproductive: Y [84] Developmental: Y [35,85] Cancer: Y [36] Neurological: N Acute: Y [36] |
Reproductive: N Developmental: N Cancer: N Neurological: N Acute: N |
| PFCs |

|
3.5 years [37,38] | Reproductive: Y [86] Developmental: Y [86,87] Cancer: Y [86] Neurological: Y [88] Acute: Y [89] |
Reproductive: N Developmental: N Cancer: N Neurological: N Acute: N |
Many environmental phenols, notably bisphenol A (BPA), serve as endocrine-disrupting chemicals (EDCs), which mimic or antagonize endogenous hormones due to similarities in their chemical structures [13,14]. Although the use of BPA has strictly been limited, BPA is ubiquitously prevalent in manufacturing plastics and frequently leaches into water sources, resulting in bioconcentration in the environment [14,15,16,17,18]. Alarmingly, BPA can induce endocrine-disrupting health effects at modest concentrations of nanograms per liter [19,20,21,22].
PBDEs, of which the most common form is decabromodiphenyl ether (DECA), are utilized as flame retardants in commercial products with well-documented varying effects in numerous animal organisms [23,24,25]. Human susceptibility to PBDEs through inhalation, dermal absorption, and ingestion is substantially high due to their lack of chemical binding to products [26]. Several limitations from previously conducted in vitro models include inaccurate use of PBDE dose-dependent concentrations [27] and evaluation of culture medium rather than quantifying PBDE accumulation in cells [28,29,30], which ultimately challenges translation of in vitro to in vivo results.
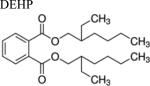
Like PBDEs, phthalates are industrial chemicals that also exhibit leaching behavior [31]. The most common phthalate toxicant is diethylhexyl phthalate (DEHP), which contaminates inhaled indoor air [32,33,34]. Majority of in vitro studies support phthalates as xenoestrogens [35], but remain unverified for in vivo studies. The plethora of in vitro toxicological studies have specifically yielded species-specific results that are not human physiology-relevant [36].
PFCs are used for protective coating of products, notably heat-resistant non-stick coatings. With limited water solubility and low volatility, PFCs, such as perfluorooctanoic acid (PFOA), bioaccumulate in the environment and in the body [37,38]. This slow elimination time challenges the determination of how lifestyle, diet, and other exposure-related factors influence physiological responses, and ultimately understanding the kinetics of the toxicant metabolism and subsequent removal from the body.
OOC Technologies
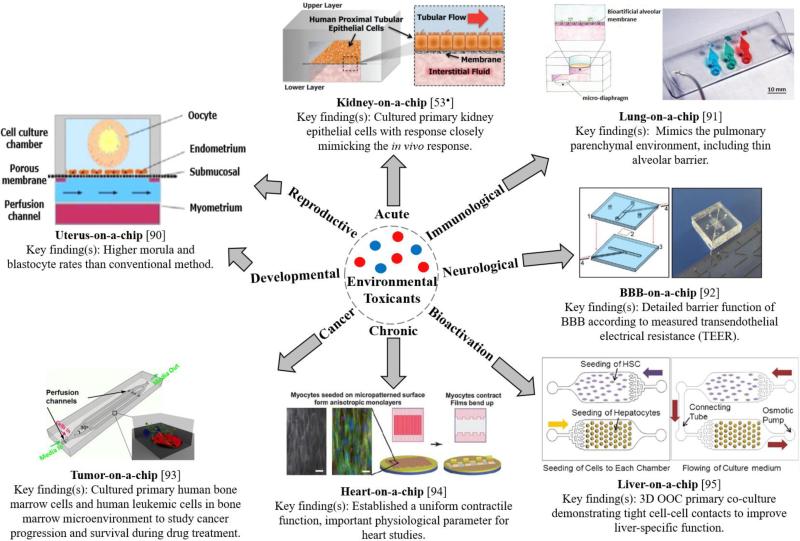
OOCs are microfluidic-based systems with advanced 3D tissue engineered constructs and cultured human cells to replicate a human organ of interest [2•]. Microfluidic channel networks are designed and fabricated to mimic the organ structure (e.g. liver sinusoid, nephron in a kidney, etc.). The channel surfaces are usually modified with the layers mimicking the ECM, allowing the human cells to adhere, spread, and proliferate within the channels (thus requiring tissue engineering technologies). Once OOCs are constructed, fluid flow is applied to generate mechanical forces that recapitulate the in vivo microenvironment experienced by cells [2•,39]. Specifically, organ-specific fluid flow enables gradient formations of molecular components and maintenance of cell-cell interactions [39,40•,41], which are vital to emulating human physiological responses. Previous research has demonstrated that incorporated ECM networks yielded in vivo-like behaviors such as apical-basal polarization [42], lumen formation [43], increased differentiation [44], and appropriate protein expression [45]. Finally, OOCs are significantly low-cost, possesses tunable properties, mass-producible, with low reagent consumption and waste production compared to the conventional 2D assays [46,47]. Figure 2 provides a summary of available OOC technologies that may be used to address significant health concerns of toxicants, although most of them are not designed to study them.
Figure 2.
Summary of existing OOC technologies. Figure of uterus-on-a-chip was adapted from [90] with permission from Elsevier. Figure of lung-on-a-chip was adapted from [90]. Tumor-on-a-chip figure was reproduced from [93]. Figure of heart-on-a-chip [94], kidney-on-a-chip [53•], BBB-on-a-chip [92], and liver-on-a-chip [95] were reproduced with permission from the Royal Society of Chemistry.
While many different OOC systems have already been demonstrated for various applications, we are particularly interested in kidney, liver, and lung OOC systems (referred to as kidney-on-a-chip, liver-on-a-chip, and lung-on-a-chip) due to their important roles in bioactivation, filtration, and susceptibility to the environmental toxicant exposures.
Kidney clears endogenous waste and exogenous toxicants from the body, and is highly susceptible to xenobiotic and metabolite-induced nephrotoxicity [46]. Unknown consumption of food disinfectants may induce oxidative stress to the human kidney [48,49,50]. Various existing literature demonstrates a strong promise of kidney-on-a-chip devices for studying drug-induced toxicity and drug interaction studies through recreating renal tubule microenvironment within microfluidic channels [51,52,53•,54] with appropriate transport functions [54], crucial for investigating filtration capabilities. Some fundamental requirements essential to reproducing efficient kidney-on-chips include the biocompatibility of chip materials, fabrication with non-cell-adhesive materials [55], and the control of fluid shear stress to facilitate tight monolayer formation.
Liver is responsible for drug bioactivation, drug clearance, and production of reactive metabolites that can interact with other downstream organs [56,57,58]. During these processes, liver is also susceptible to drug-induced injury. Multiple biomimetic liver-on-a-chip platforms have been established for drug toxicity testing [59,60,61,62]. Liver-on-a-chip is essential in multiple-OOC systems because of its high concentration of biotransformation enzymes that may bioactivate xenobiotics [63•]. A metabolically active liver model must be integrated into in vitro models in toxicology studies in order to determine toxicological effects of metabolites and serve as an ideal representation of human physiology [64], which is generally considered very challenging in 2D models. One such important interplay among organs is that of the hepatic and renal systems. According to human physiological processes and anatomical placements, the liver nearly receives all of the xenobiotic-containing blood perfused by the intestinal system (gut), whose bioactivated metabolites may compromise the renal system during subsequent hepatic first pass effect during circulation [63•]. A good alternative is the interconnected, multiple OOC systems (e.g., liver- and kidney-on-a-chip connected in a series), which is discussed later.
The respiratory tract is a significant entry port of the human body due to the thin mucosal barrier with adsorptive surface area of the alveoli, which enables rapid access to the bloodstream [2•]. Lung-on-chips engineered with appropriate alveolar-capillary interface and vacuum strain to mimic physiological breathing can be used to study aerosolized toxicants. The challenge resides with the reconstruction of a reliable alveolar-capillary barrier without a complex culturing process [65].
Since OOCs are easy to construct, small-scale, and flexible in changing their designs, they can be made into not only high-throughput systems but also interconnected, multi-OOC systems. Such multi-OOC systems can better simulate the overall physiological responses of human body, especially for toxicants. Such multi-OOC systems can be built into human-on-a-chip system, which will greatly improves in vivo physiological responses due to the better relationship modeling and correct anatomical placement (Figure 3).
Figure 3.
Schematics of interconnected multi-OOCs, with microfluidic circulatory system to ultimately yield an anticipated human-on-a-chip. As toxicants enter the integrated model via inhalation through the lungs, or ingested through the gut, an intimate study of toxicant bioactivation, metabolism, transport, and fate can be observed. Such interconnected system model is crucial for understanding potential in vivo responses to the toxicants from various organs. Figure was adapted by Huh et al. with permission from Elsevier [76••].
Use of OOCs towards Environmental Toxicology
Governmental funding programs are currently expanding worldwide on developing innovative drug screening tools, in particular, in vitro cell-based or tissue-based models reproducing human physiology [66]. OOCs are obviously superior platforms over those in vitro models, and better predict the in vivo-like responses. While OOCs have substantially been used for drug screening, several drug toxicity studies are currently emerging, which may be adapted to environmental toxicology assessment. For example, Homan et al. recapitulated human kidney's dose-dependent responses to a nephrotoxin on a 3D bioprinted proximal tubules on chip [67]. In addition, primitive OOC systems that utilize cell spheroids still serve as adequate alternatives to 2D in vitro models and are being tested for drug toxicity assessment. Ziolkowska et al. presented a microfluidic chip with carcinoma cell spheroids to investigate the efficacy and toxicity of an anticancer drug [68]. Wei et al. presented a similar concept using primary hepatocyte spheroids on innovative fibers, which achieved excellent prediction of in vivo drug clearance rate [69]. Albeit these preliminary studies have been demonstrated for drug assessment, they may be translated into environmental toxicology.
Many researchers acknowledge that the future direction must entail influence of multi-organ crosstalk and ultimately reconstitute the human-on-a-chip that is capable of ideally replacing animal studies [70]. This multi-OOC feature is a very important aspect for assessing environmental toxicants, to fully and correctly emulate the inter-organ and systemic responses from human body. Toxicity studies with multi-OOC do exist, albeit for drug screening but not for environmental toxicology: Maschmeyer et al. connected and maintained four OOCs to profile drug metabolism and processing among organ cross-talking networks for 28 days [71••]. Oleaga et al. demonstrated a 14-day sustained multi-OOC model with functional cardiac, muscle, neuronal and liver activities [72••]. More significantly, they evaluated 5 drugs with known adverse health effects, whose results were in general agreement with available human and animal data [72••]. Such productive step towards human-on-a-chip may serve an ideal tool for assessing environmental toxicants.
However, the number of such multi-OOC toxicology studies remain minimal, and those small number of studies continue to focus primarily on drugs, not environmental toxicants. With the development of physiologically relevant OOC models, toxicant screening may become possible with reduced cost, time, and labor. Not to mention, further questions may be answered that current technologies have not been able to address with such multi-OOC models – for example, the effects of environmental toxicants on the development of allergies [15].
Nonetheless, there will be several challenges that need to be considered with advancing OOC technologies. The chip materials must be made tissue-compatible and optimized to closely capture the complexity of native tissues [73]. Use of primary cell culture is ideal, while they have a limited lifespan, undergo rapid phenotypic alterations, and show large variability over different batches of isolation [2•]. Because of these limitations, well–established, immortalized cell lines are most commonly used in OOC studies, while their immortalized trait often presents false tissue-specific functions [74••,75]. Additionally, current analytical methods for OOCs (mostly fluorescence microscopy-based) remain tedious, disruptive, time-consuming, and lack real-time in situ analysis capability [53•,73,76••]. Non-invasive monitoring tools for in situ OOC analysis has been previously demonstrated [47], again still quite small in number, which may facilitate the assay analysis with low costs.
Conclusion
There is an overwhelming burden of assessing numerous HPV toxicants present in the environment. In vitro models and animal models are inadequate for understanding the in vivo toxicological responses. In addition, they are severely limited in detecting additive or synergistic interactions of environmental toxicants occurring within the human body [77]. With the recent advances of OOC technologies that better recapitulate human physiology, adverse health effects of toxicants and assessment of multiple exposure of various toxicants can be evaluated. There are scarcely any conclusive studies of human responses to toxicants available with OOC technologies. In fact, the majority of OOC literature have been focused on preclinical studies of pharmaceutical drugs, but not on environmental toxicology. In this sense, we strongly suggest that OOC technologies should be employed for identifying and understanding environmental toxicants, which will significantly benefit the general public towards complete understanding on numerous environmental toxicants.
Highlights.
- Poorly understood xenobiotic toxicants are oversaturating the environment.
- Conventional methods are incapable of accurately predicting human physiological responses.
- Organ-on-a-chip systems may provide superior models in assessing environmental toxicants.
- Human-on-a-chip can serve as an ultimate platform for evaluating environmental toxicants.
Acknowledgements
This work was supported by the Cardiovascular Biomedical Engineering Training Grant from the U.S. National Institutes of Health (T32HL007955) and the Southwest Environmental Health Sciences Center (SWEHSC) at the University of Arizona, funded by U.S. National Institutes of Health (P30ES006694).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
There is no conflict of interest relating to this article.
References and recommended reading
Papers of particular interest published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Calafat AM, Ye X, Wong L-Y, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol:2003-2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2•.Bhise NS, Ribas J, Manoharan V, Zhang YS, Polini A, Massa S, Dokmeci MR, Khademhosseini A. Organ-on-a-chip platforms for studying drug delivery systems. J Control Release. 2014;190:82–93. doi: 10.1016/j.jconrel.2014.05.004. [Authors highlights the importance of integrating microfluidic networks with 3D in vitro models to produce OOCs as robust preclinical screening models.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang R, Xia M, Sakamuru S, Zhao J, Shahane SA, Attene-Ramos M, Zhao T, Austin CP, Simeonov A. Modelling the Tox21 10K chemical profiles for in vivo toxicity prediction and mechanism characterization. Nat Commun. 2016;7:10425. doi: 10.1038/ncomms10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4•.Khademhosseini A, Langer R. A decade of progress in tissue engineering. Nat Protoc. 2016;11:1775–1781. doi: 10.1038/nprot.2016.123. [Highlights significant findings that have motivated progressive research of tissue engineering, including microfluidic OOC models.] [DOI] [PubMed] [Google Scholar]
- 5.Watson CY, Damiani F, Ram-Mohan S, Rodrigues S, Queiroz PD, Donaghey TC, Lichtenstein JHR, Brain JD, Krishnan R, Molina RM. Screening for chemical toxicity using cryopreserved precision cut lung slices. Toxicol Sci. 2016;150:225–233. doi: 10.1093/toxsci/kfv320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Kanter R, Monshouwer M, Meijer DK, Groothuis GM. Precision-cut organ slices as a tool to study toxicity and metabolism of xenobiotics with special reference to nonhepatic tissues. Curr Drug Metab. 2002;3:39–59. doi: 10.2174/1389200023338071. [DOI] [PubMed] [Google Scholar]
- 7.Hung PJ, Lee PJ, Sabounchi P, Lin R, Lee LP. Continuous perfusion microfluidic cell culture array for high-throughput cell-based assays. Biotechnol Bioeng. 2005;89:1–8. doi: 10.1002/bit.20289. [DOI] [PubMed] [Google Scholar]
- 8.van Duinen V, Trietsch SJ, Joore J, Vulto P, Hankemeir T. Microfluidic 3D cell culture: from tools to tissue models. Curr Opin Biotechnol. 2015;35:118–126. doi: 10.1016/j.copbio.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Slater K. Cytotoxicity tests for high-throughput drug discovery. Curr Opin Biotechnol. 2001;12:70–74. doi: 10.1016/s0958-1669(00)00177-4. [DOI] [PubMed] [Google Scholar]
- 10.Ju SM, Jang H-J, Kim K-B, Kim J. High-throughput cytotoxicity testing system of acetaminophen using a microfluidic device (MFD) in HepG2 cells. Toxicol Environ Health A. 2015;78:1063–1072. doi: 10.1080/15287394.2015.1068650. [DOI] [PubMed] [Google Scholar]
- 11.Balenga NA, Klichinsky M, Xie Z, Chan EC, Zhao M, Jude J, Laviolette M, Panettieri RA, Jr, Druey KM. A fungal protease allergen provokes airway hyper-responsiveness in asthma. Nat Commun. 2015;6:6963. doi: 10.1038/ncomms7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sauer UG, Vogel S, Aumann A, Hess A, Kolle SN, Ma-Hock L, Wohlleben W, Dammann M, Strauss V, Treumann S, Gröters S, Wiench K, van Ravenzwaay B, Landsiedel R. Applicability of rat precision-cut lung slices in evaluating nanomaterial cytotoxicity, apoptosis, oxidative stress, and inflammation. Toxicol Appl Pharmacol. 2014;276:1–20. doi: 10.1016/j.taap.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh N, Ali MA, Suresh K, Agrawal VV, Rai P, Sharma A, Malhotra BD, John R. In-situelectrosynthesized nanostructured Mn3O4-polyaniline nanofibers-biointerface for endocrine disrupting chemical detection. Sens Actuat B. 2016;236:781–793. [Google Scholar]
- 15.Robinson L, Miller R. The impact of bisphenol A and phthalates on allergy, asthma, and immune function: a review of latest findings. Curr Environ Health Rep. 2015;2:379–387. doi: 10.1007/s40572-015-0066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheong A, Zhang X, Cheung Y-Y, Tang W-y, Chen J, Ye S-H, Medvedovic M, Leung YK, Prins GS, Ho S-M. DNA methylome changes by estradiol benzoate and bisphenol A links early-life environmental exposures to prostate cancer risk. Epigenetics. 2016:1–16. doi: 10.1080/15592294.2016.1208891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Li H, Ran Y, Chan K. Distribution and bioconcentration of endocrine disrupting chemicals in surface water and fish bile of the Pearl River Delta, South China. Chemosphere. 2014;107:439–446. doi: 10.1016/j.chemosphere.2014.01.048. [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Chan KM. Evaluation of the toxic effects of brominated compounds (BDE-47, 99, 209, TBBPA) and bisphenol A (BPA) using a zebrafish liver cell line, ZFL. Aqua Toxicol. 2015;159:138–147. doi: 10.1016/j.aquatox.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Wu M, Pan C, Yang M, Xu B, Lei X, Ma J, Cai L, Chen J. Chemical analysis of fish bile extracts for monitoring endocrine disrupting chemical exposure in water: bisphenol A, alkylphenols, and norethindrone. Environ Toxicol Chem. 2016;35:182–190. doi: 10.1002/etc.3176. [DOI] [PubMed] [Google Scholar]
- 20.Diamanti-Kandarakis E, Bouguignon JP, Guidice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an endocrine society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nori F, Carbone P, Giordano F, Osborn J, Figa-Talamanca I. Endocrine disrupting chemicals and testicular cancer: a case-control study. Arch Environ Occup Health. 2006;61:87–95. doi: 10.3200/AEOH.61.2.87-95. [DOI] [PubMed] [Google Scholar]
- 22.Belcher SM, Gear RB, Kendig EL. Bisphenol A alters autonomic tone and extracellular matrix structure and induces sex-specific effects on cardiovascular function in male and female CD-1 mice. Endocrinology. 2015;156:882–895. doi: 10.1210/en.2014-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernie KJ, Shutt JL, Mayne G, Hoffman D, Letcher RJ, Drouillard KG, Ritchie IJ. Exposure to polybrominated diphenyl ethers (PBDEs): changes in thyroid, vitamin A, glutathione homeostasis, and oxidative stress in American Kestrels (Falco spaverius). Toxicol Sci. 2005;88:375–383. doi: 10.1093/toxsci/kfi295. [DOI] [PubMed] [Google Scholar]
- 24.Noyes PD, Lema SC, Macaulay LJ, Douglas NK, Stapleton HM. Low level exposure to the flame retardant BDE-209 reduces thyroid hormone levels and disrupts thyroid signaling in fathead minnows. Environ Sci Technol. 2013;47:10012–10021. doi: 10.1021/es402650x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vonderheide AP, Mueller KE, Meija J, Welsh GL. Polybrominated diphenyl ethers: causes for concern and knowledge gaps regarding environmental distribution, fate and toxicity. Sci Total Environ. 2008;400:425–436. doi: 10.1016/j.scitotenv.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Gill U, Chu I, Ryan JJ, Feeley M. Polybrominated diphenyl ethers: human tissue levels and toxicology. Rev Environ Contam Toxicol. 2004;183:55–97. doi: 10.1007/978-1-4419-9100-3_3. [DOI] [PubMed] [Google Scholar]
- 27.Costa LG, de Laat R, Tagliaferri S, Pellacani C. A mechanistic view of polybrominated diphenyl ether (PBDE) developmental neurotoxicity. Toxicol Lett. 2014;230:282–294. doi: 10.1016/j.toxlet.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang SC, Giordano G, Costa LG. Comparative cytotoxicity and intracellular accumulation of five polybrominated diphenyl ether congeners in mouse cerebellar granule neurons. Toxicol Sci. 2010;114:124–132. doi: 10.1093/toxsci/kfp296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schreiber T, Gassmann K, Götz C, Hübenthal U, Moors M, Krause G, Merk HF, Nguyen NH, Scanlan TS, Abel J, et al. Polybrominated diphenyl ethers induce developmental neurotoxicity in a human in vitro model: evidence for endocrine disruption. Environ Health Perspect. 2010;118:572–578. doi: 10.1289/ehp.0901435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei RG, Zhao YX, Liu PY, Qin ZF, Yan SS, Li Y, Qin XF, Xia XJ, Xu XB, Yan MC. Determination of environmentally relevant exposure concentrations of polybrominated diphenyl ethers for in vitro toxicological studies. Toxicol In Vitro. 2010;24:1078–1085. doi: 10.1016/j.tiv.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 31.Wormuth M, Scheringer M, Vollenweider M, Hungerbühler K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans. Risk Anal. 2006;26:803–824. doi: 10.1111/j.1539-6924.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- 32.Koch HM, Lorber M, Christensen KL, Pälmke C, Koslitz S, Brüning T. Identifying sources of phthalate exposure with human biomonitoring: results of a 48 h fasting study with urine collection and personal activity patterns. Int J Hyg Environ Health. 2013;216:672–681. doi: 10.1016/j.ijheh.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Whyatt RM, Perzanowski MS, Just AC, Rundle AG, Donohue KM, Calafat AM, Hoepner LA, Perera FP, Miller RL. Asthma in inner-city children at 5-11 years of age and prenatal exposure to phthalates: the Columbia Center for Children's Environmental Health Cohort. Environ Health Perspect. 2014;122:1141–1146. doi: 10.1289/ehp.1307670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolarik B, Naydenov K, Larsson M, Bornehag C-G, Sundell J. The association between phthalates in dust and allergic diseases among Bulgarian children. Environ Health Perspect. 2008;6:98–103. doi: 10.1289/ehp.10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coldham NG, Dave M, Silvapathasundaram S, McDonnell DP, Connor C, Sauer MJ. Evaluation of a recombinant yeast cell estrogen screening assay. Environ Health Perspect. 1997;105:734–742. doi: 10.1289/ehp.97105734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rusyn I, Peters JM, Cunningham ML. Modes of action and species-specific effects of di-(2-ethylhexyl)phthalate in the liver. Crit Rev Toxicol. 2006;36:459–479. doi: 10.1080/10408440600779065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olsen GW, Burris JM, Ehresman DJ, Forehlich JW, Seacat AM, Butenhoff JL, Zobel LR. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115:1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steenland K, Fletcher T, Savitz DA. Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA). Environ Health Perspect. 2010;118:1100–1108. doi: 10.1289/ehp.0901827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inamdar NK, Borenstein JT. Microfluidic cell culture models for tissue engineering. Curr Opin Biotechnol. 2011;22:681–689. doi: 10.1016/j.copbio.2011.05.512. [DOI] [PubMed] [Google Scholar]
- 40•.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [Design, fabrication, and testing of lung-on-a-chip was demonstrated for drug screening.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim HJ, Huh D, Hamilton G, Ingber DE. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 2012;12:2165–2174. doi: 10.1039/c2lc40074j. [DOI] [PubMed] [Google Scholar]
- 42.Schoenenberger C, Zuk A, Zinkl GM, Kendall D, Matiln KS. Integrin expression and localization in normal MDCK cells and transformed MDCK cells lacking apical polarity. J Cell Sci. 1994;107:527–541. doi: 10.1242/jcs.107.2.527. [DOI] [PubMed] [Google Scholar]
- 43.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 44.Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking. Antibodies. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin CQ, Bissell MJ. Multi-faceted regulation of cell differentiation by extracellular matrix. FASEB J. 1993;7:737–743. doi: 10.1096/fasebj.7.9.8330681. [DOI] [PubMed] [Google Scholar]
- 46.Nieskens TTG, Wilmer MJ. Kidney-on-a-chip technology for renal proximal tubule tissue reconstruction. Eur J Pharmacol. 2016;790:46–56. doi: 10.1016/j.ejphar.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 47.Cho S, Islas-Robles A, Nicolini AM, Monks TJ, Yoon J-Y. In situ, dual-mode monitoring of organ-on-a-chip with smartphone-based fluorescence microscope. Biosens Bioelectron. 2016;86:697–705. doi: 10.1016/j.bios.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karuppasamy K, Yadav AS, Saxena GK. Thermal inactivation of salmonella enteritidison chicken skin previously exposed to acidifed sodium chlorite or tri-sodium phosphate. J Food Sci Technol. 2015;52:8236–8243. doi: 10.1007/s13197-015-1922-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prado-Silva L, Cadavez V, Gonzales-Barron U, Rezende ACB, Sant'Ana AS. Meta-analysis of the effects of sanitizing treatments on salmonella, Escherichia coliO157:H7, and listeria monocytogenes inactivation in fresh produce. Appl Environ Microbiol. 2015;81:8008–8021. doi: 10.1128/AEM.02216-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ali SN, Mahmood R. Sodium chlorite increases production of reactive oxygen species that impair the antioxidant system and causes morphological changes in human erythrocytes. Environ Toxicol. 2016 doi: 10.1002/tox.22328. doi: 10.1002/tox.22328. [DOI] [PubMed] [Google Scholar]
- 51.Choucha Snouber S, Jacques S, Monge M, Legallais C, Leclerc E. Transcriptomic analysis of the effect of ifosfamide on MDCK cells cultivated in microfluidic biochips. Genomics. 2012;100:27–34. doi: 10.1016/j.ygeno.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 52.DesRochers TM, Suter L, Roth A, Kaplan DL. Bioenegineered 3D human kidney tissue, a platform for the determination of nephrotoxicity. PLoS ONE. 2013;8:e59219. doi: 10.1371/journal.pone.0059219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53•.Jang KJ, Mehr AP, Hamilton GA, McPartlin LA, Chung S, Suh KY, Ingber DE. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr Biol. 2013;5:1119–1129. doi: 10.1039/c3ib40049b. [A membrane-based kidney-on-a-chip with primary human kidney proximal tubular epithelial cells was demonstrated, with preliminary studies on nephrotoxicology.] [DOI] [PubMed] [Google Scholar]
- 54.Lawrence ML, Chang CH, Davies JA. Transport of organic anions and cations in murine embryonic kidney development and in serially-reaggregated engineered kidneys. Sci Rep. 2015;5:9092. doi: 10.1038/srep09092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Midwoud PM, Janse A, Merema MT, Groothuis GM, Verpoorte E. Comparison of biocompatibility and adsorption properties of different plastics for advanced microfluidic cell and tissue culture models. Anal Chem. 2012;84:3938–3944. doi: 10.1021/ac300771z. [DOI] [PubMed] [Google Scholar]
- 56.Picollet-D'hahan N, Dolega ME, Liguori L, Marquette C, Le Gac S, Gidrol X, Martin DK. A 3D toolbox to enhance physiological relevance of human tissue models. Trends Biotechnol. 2016;34:757–769. doi: 10.1016/j.tibtech.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 57.Ma C, Zhao L, Zhou E-M, Xu J, Shen S, Wang J. On-chip construction of liver lobule-like microtissue and its application for adverse drug reaction assay. Anal Chem. 2016;88:1719–1727. doi: 10.1021/acs.analchem.5b03869. [DOI] [PubMed] [Google Scholar]
- 58.No DY, Lee KH, Lee J, Lee SH. 3D liver models on a microplatform: well-defined culture, engineering of liver tissue and liver-on-a-chip. Lab Chip. 2015;15:3822–3837. doi: 10.1039/c5lc00611b. [DOI] [PubMed] [Google Scholar]
- 59.Wagner I, Materne EM, Brincker S, Sussbier U, Fradrich C, Busek M, Sonntag F, Sakharov DA, Trushkin EV, Tonevitsky AG, Lauster R, Marx U. A dynamic multi-organ chip for long-term cultivation and substance testing proven by 3D human liver and skin tissue co-culture. Lab Chip. 2013;13:3538–3547. doi: 10.1039/c3lc50234a. [DOI] [PubMed] [Google Scholar]
- 60.Toh YC, Lim TC, Tai D, Xiao G, van Noort D, Yu H. A microfluidic 3D hepatocyte chip for drug toxicity testing. Lab Chip. 2009;9:2026–2035. doi: 10.1039/b900912d. [DOI] [PubMed] [Google Scholar]
- 61.van Midwoud PM, Merema MT, Verpoorte E, Groothuis GM. A microfluidic approach for in vitro assessment of interorgan interactions in drug metabolism using intestinal and liver slices. Lab Chip. 2010;10:2778–2786. doi: 10.1039/c0lc00043d. [DOI] [PubMed] [Google Scholar]
- 62.Goral VN, Hsieh YC, Petzold ON, Clark JS, Yuen PK, Faris RA. Perfusion-based microfluidic device for three-dimensional dynamic primary human hepatocyte cell culture in the absence of biological or synthetic matrices or coagulants. Lab Chip. 2010;10:3380–3386. doi: 10.1039/c0lc00135j. [DOI] [PubMed] [Google Scholar]
- 63•.Chang SY, Weber EJ, Van Ness KP, Eaton DL, Kelly EJ. Liver and kidney on chips: microphysiological models to understand transporter function. Clin Pharmacol Therap. 2016;100:464–478. doi: 10.1002/cpt.436. [Authors investigated the metabolic roles of bioactivating and transporter enzymes using liver-on-a-chip.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skardal A, Shupe T, Atala A. Organoid-on-a-chip and body-on-a-chip systems for drug screening and disease modeling. Drug Discov Today. 2016;21:1399–1411. doi: 10.1016/j.drudis.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumar Mahto S, Tenenbaum-Katan J, Sznitman J. Respiratory physiology on a chip. Scientifica. 2012;2012:364054. doi: 10.6064/2012/364054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luni C, Serena E, Elvassore N. Human-on-chip for therapy development and fundamental science. Curr Opin Biotechnol. 2014;25:45–50. doi: 10.1016/j.copbio.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 67.Homan KA, Kolesky DB, Skylar-Scott MA, Herrmann J, Obuobi H, Moisan A, Lewis JA. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci Rep. 2016;6:34845. doi: 10.1038/srep34845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ziolkowska K, Stelmachowska A, Kwapiszewski R, Chudy M, Dybko A, Brzόzka Z. Long-term three-dimensional cell culture and anticancer drug activity evalutation in a microfluidic chip. Biosens Bioelectron. 2013;40:68–74. doi: 10.1016/j.bios.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 69.Wei J, Lu J, Liu Y, Yan S, Li X. Spheroid culture of primary hepatocytes with short fibers as a predictable in vitro model for drug screening. J Mater Chem B. 2016;4:7155–7167. doi: 10.1039/c6tb02014c. [DOI] [PubMed] [Google Scholar]
- 70.Planz V, Lehr C-M, Windbergs M. In vitro models for evaluating safety and efficacy of novel technologies for skin drug delivery. J Control. Release. 2016 doi: 10.1016/j.jconrel.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 71••.Maschmeyer I, Lorenz AK, Schimek K, Hasenberg T, Ramme AP, Hübner J, Lidner M, Drewell C, Bauer S, Thomas A, et al. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip. 2015;15:2688–2699. doi: 10.1039/c5lc00392j. [An efficient study of four interconnected OOCs that shows promise of developing an anticipated human-on-a-chip. Such physiologically relevant innovation may serve as a powerful predictive tool in determining detailed and intricate effects of environmental toxicants as it journeys through multiple organ systems.] [DOI] [PubMed] [Google Scholar]
- 72••.Oleaga C, Bernabini C, Smith AST, Srinivasan B, Jackson M, McLamb W, Platt V, Bridges R, Cai Y, Santhanam N, et al. Multi-organ toxicity demonstration in a functional human in vitro system composed of four organs. Sci Rep. 2016;6:20030. doi: 10.1038/srep20030. [A 4-organ system (with cardiac, muscle, neuronal and liver modules) was demonstrated and maintained for 14 days under continuous flow. Pharmacological relevance were evaluated to 5 known drugs. Multi-OOC exhibited appropriate phenotypic culture and provides significant progress towards human-on-a-chip.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Uto K, Tsui JH, DeForest CA, Kim D-H. Dynamically tunable cell culture platforms for tissue engineering and mechanobiology. Prog Polym Sci. 2016 doi: 10.1016/j.progpolymsci.2016.09.004. doi: 10.1016/j.progpolymsci.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74••.Esch MB, King TL, Shuler ML. The role of body-on-a-chip devices in drug and toxicity studies. Annu Rev Biomed Eng. 2011:55–72. doi: 10.1146/annurev-bioeng-071910-124629. [Comprehensive review of body-on-a-chip models for toxicity studies, and what challenges need to be addressed. Possible solutions are also listed.] [DOI] [PubMed] [Google Scholar]
- 75.Wilkening S, Stahl F, Bader A. Comparison of primary human hepatocytes and hepatoma cell line Hepg2 with regard to their biotransformation properties. Drug Metab Dispos. 2003;31:1035–1042. doi: 10.1124/dmd.31.8.1035. [DOI] [PubMed] [Google Scholar]
- 76••.Huh D, Hamilton GA, Ingber DE. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011;21:745–754. doi: 10.1016/j.tcb.2011.09.005. [This review accentuates the advances in organ-on-a-chip technologies towards accelerating drug development.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen H, Liu Z, Zhang X, Jia X, Li Q, Su Q, Wang W. Assessment of synergistic thyroid disrupting effects of a mixture of EDCs in ovariectomized rats using factorial analysis and dose addition. Toxicol Res. 2016 doi: 10.1039/c6tx00193a. doi: 10.1039/C6TX00193A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Office of Environmental Health Hazard Assessment (OEHHA) Toxicological Profile for Bisphenol A. California Environmental Protection Agency; 2009. http://www.opc.ca.gov/webmaster/ftp/project_pages/MarineDebris_OEHHA_ToxProfiles/Bisphenol%20A%20Final.pdf. [Google Scholar]
- 79.Thuresson K, Höglund P, Hagmar L, Sjödin A, Bergman A, Jakobsson K. Apparent half-lives of hepta- to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environ Health Perspect. 2006;114:176–181. doi: 10.1289/ehp.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Z-H, Liu X-Y, Wang N, Chen J-S, Chen Y-H, Huang J-T, Su C-H, Xie F, Yu B, Chen D-J. Effects of decabrominated diphenyl ether (PBDE-209) in regulation of growth and apoptosis of breast, ovarian, and cervical cancer cells. Environ Health Perspect. 2012;120:541–546. doi: 10.1289/ehp.1104051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harley K, Marks A, Hevrier J, Bradman A, Sjödin A, Eskenzai B. PBDE concentrations in women's serum and fecundability. Environ Health Perspect. 2010;118:699–704. doi: 10.1289/ehp.0901450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roberts SC, Bianco AC, Stapleton HM. Disruption of type 2 iodothyronine deiodinase activity in cultured human glial cells by polybrominated diphenyl ethers. Chem Res Toxicol. 2015;28:1265–1274. doi: 10.1021/acs.chemrestox.5b00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hoppin JA, Brock JW, Davis BJ, Baird DD. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environ Health Perspect. 2002;110:515–518. doi: 10.1289/ehp.02110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lovecamp-Swan T, Davis BJ. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ Health Perspect. 2003;111:139–145. doi: 10.1289/ehp.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harris CA, Henttu P, Parker MG, Sumpter JP. The estrogenic activity of phthalate esters in vitro. Environ Health Perspect. 1997;105:802–811. doi: 10.1289/ehp.97105802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Biegel LB, Hurtt ME, Frame SR, O'Connor JC, Cook JC. Mechanisms of extrahepatic tumor induction by peroxisome proliferators in male CD rats. Toxicol Sci. 2001;60:44–55. doi: 10.1093/toxsci/60.1.44. [DOI] [PubMed] [Google Scholar]
- 87.Kennedy GL, Jr, Butenhoff JL, Olsen GW, O'Connor JC, Seacat AM, Perkins RG, Biegel LB, Murphy SR, Farrar DG. The toxicology of perfluorooctanoate. Crit Rev Toxicol. 2004;34:351–384. doi: 10.1080/10408440490464705. [DOI] [PubMed] [Google Scholar]
- 88.Lau C, Butenhoff JL, Rogers JM. The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicol Appl Pharmacol. 2004;198:231–241. doi: 10.1016/j.taap.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 89.Slotkin TA, MacKillop EA, Melnick RL, Thayer KA, Seidler FJ. Developmental neurotoxicity of perfluorinated chemicals modeled in vitro. Environ Health Perspect. 2008;116:716–722. doi: 10.1289/ehp.11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li W-X, Liang G-T, Yan W, Zhang Q, Wang W, Zhou X-M, Liu D-Y. Artificial uterus on a microfluidic chip. Chinese J Anal Chem. 2013;41:467–472. [Google Scholar]
- 91.Stucki AO, Stucki JD, Hall SRR, Felder M, Mermoud Y, Schmid RA, Geiser T, Guenat OT. A lung-on-a-chip array with an integrated bio-inspired respiration mechanism. Lab Chip. 2015;15:1302–1310. doi: 10.1039/c4lc01252f. [DOI] [PubMed] [Google Scholar]
- 92.Griep LM, Wolbers F, de Wagenaar B, ter Braak PM, Weksler BB, Romero IA, Couraud PO, Vermes I, van der Meer AD, van den Berg A. BBB on chip: microfluidic platform to mechanically and biochemically modulate blood-brain barrier function. Biomed Microdev. 2013;15:145–150. doi: 10.1007/s10544-012-9699-7. [DOI] [PubMed] [Google Scholar]
- 93.Bruce A, Evans R, Mezan R, Shi L, Moses BS, Martin KH, Yang Y. Three-dimensional microfluidic tri-culture model of the bone marrow microenvironment for study of acute lymphoblastic leukemia. PLoS ONE. 2015;10:e0140506. doi: 10.1371/journal.pone.0140506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grosberg A, Alford PW, McCain ML, Parker KK. Ensembles of engineered cardiac tissues for physiological and pharmacological study: heart on a chip. Lab Chip. 2011;11:4165–4173. doi: 10.1039/c1lc20557a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee S-A, No DY, Kang E, Ju J, Kim D-S, Lee S-H. Spheroid-based three-dimensional liver-on-a-chip to investigate hepatocyte-hepatic stellate cell interactions and flow effects. Lab Chip. 2013;13:3529–3537. doi: 10.1039/c3lc50197c. [DOI] [PubMed] [Google Scholar]