Abstract
Background
Clinical practice guidelines recommend use of fracture risk scores for screening and pharmacologic treatment decisions. The timing of occurrence of treatment-level (according to 2014 National Osteoporosis Foundation guidelines) or screening-level (according to 2011 US Preventive Services Task Force guidelines) fracture risk scores has not been estimated in postmenopausal women.
Methods
We conducted a retrospective competing risk analysis of new occurrence of treatment-level and screening-level fracture risk scores in postmenopausal women aged 50 and older, before receipt of pharmacologic treatment and before first hip or clinical vertebral fracture.
Results
In 54,280 postmenopausal women aged 50 to 64 without a bone mineral density test, the time for 10% to develop a treatment-level FRAX® could not be estimated accurately because of rare incidence of treatment-level scores. In 6096 women who had FRAX scores calculated with bone mineral density, the estimated unadjusted time to treatment-level FRAX ranged from 7.6 years (95% CI, 6.6, 8.7) for those aged 65 to 69 to 5.1 years (95% CI, 3.5, 7.5) for those aged 75 to 79 at baseline. Of 17,967 women aged 50 to 64 with a screening-level FRAX at baseline, 100 (0.6%) experienced a hip or clinical vertebral fracture by age 65.
Conclusions
Postmenopausal women with sub-threshold fracture risk scores at baseline were unlikely to develop a treatment-level FRAX score between ages 50 and 64. After age 65, the increased incidence of treatment-level fracture risk scores, osteoporosis and major osteoporotic fracture supports more frequent consideration of FRAX and bone mineral density testing.
Keywords: bone density, fractures, menopausal, osteoporosis/epidemiology, risk assessment
Introduction
Bone mineral density testing to screen for osteoporosis (lowest bone mineral density T-score ≤ -2.50 at femoral neck, total hip or lumbar spine) cannot identify all postmenopausal women who will have fractures. In an effort to identify candidates for antifracture treatment who may not have osteoporosis by bone density criteria, a scientific group led by investigators at University of Sheffield developed the Fracture Risk Assessment Tool (FRAX®) to estimate the 10-year probability of hip fracture or major osteoporotic (clinical spine, forearm, hip or proximal humerus) fracture based on individual patient models that included selected clinical risk factors for fracture and (optional) bone mineral density at the femoral neck.1 The National Osteoporosis Foundation adapted the FRAX fracture prediction algorithm to the US population2 and performed an economic analysis to identify thresholds of fracture risk above which it was estimated to be cost-effective to consider pharmacotherapy in the US.3 These US thresholds were 10-year absolute risks of ≥3% for hip fracture and ≥20% for major osteoporotic fracture in postmenopausal women and men.4
In 2011 the US Preventive Services Task Force (USPSTF) adapted the use of FRAX for identification of candidates for dual-energy x-ray absorptiometry (DXA) bone mineral density screening, proposing selection of postmenopausal women under age 65 if their calculated fracture risk was equal to or greater than that of a 65-year-old white woman who has no additional risk factors, i.e., 10-year risk of major osteoporotic fracture ≥9.3% according to the FRAX risk tool.5 Although a small observational study suggested that implementation of this strategy might decrease unnecessary testing,6 Crandall et al's large analysis of data from the Women's Health Initiative found that the USPSTF screening strategy was modestly better than chance and was inferior to simpler risk tools to identify postmenopausal women aged 50 to 64 with osteoporosis by bone mineral density criteria.7 Crandall's subsequent analysis suggested that neither the USPSTF strategy nor other fracture risk assessment tools adequately identified postmenopausal women aged 50 to 64 who went on to have major osteoporotic fracture within 10 years.8
To estimate a time frame for occurrence of clinically relevant FRAX scores by age, we conducted a retrospective competing risk analysis of serial FRAX scores in postmenopausal women aged 50 and older followed for up to 18.6 years in the Women's Health Initiative cohort. “Clinically relevant” scores were a treatment-level FRAX score to guide pharmacologic therapy according to National Osteoporosis Foundation Guidelines,4 or a screening-level score to select postmenopausal women under age 65 who should have DXA bone mineral density screening according to the USPSTF.5 Based on two earlier studies of postmenopausal women suggesting that the time to osteoporosis and time to fracture decrease with increasing age,9,10 we hypothesized that the time to treatment-level and screening-level FRAX scores would also decrease with increasing age, with a more rapid decrease after age 65.
Methods
Study participants
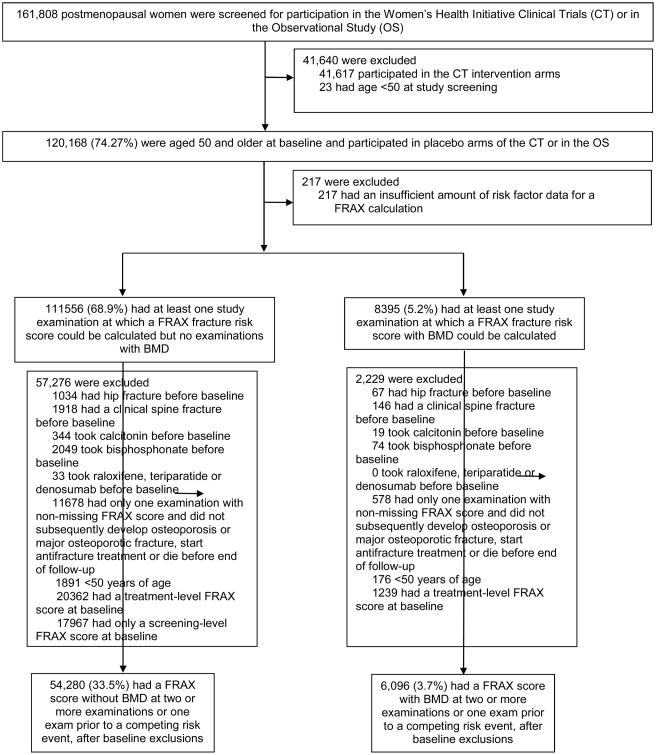
We studied 60,376 postmenopausal women (54,280 without and 6096 with bone mineral density measurements) aged 50 and older without a hip or clinical vertebral fracture, antifracture treatment or clinically relevant FRAX scores at baseline who participated in the Women's Health Initiative Observational Study or placebo arms of the WHI Hormone Therapy, Calcium/vitamin D and Dietary Modification Clinical Trials. We defined “clinically relevant” as FRAX scores that warrant treatment by the 2014 National Osteoporosis Foundation Guidelines4 (10-year risk ≥3% for hip fracture or ≥20% for major osteoporotic fracture) or warrant bone mineral density screening by the 2011 USPSTF Osteoporosis Screening Recommendation Statement5 (10-year risk ≥9.3% for major osteoporotic fracture). Because the competing risk framework requires concurrent follow-up of the primary endpoint and competing risks, the effective maximum length of follow-up for this study was 18.6 years for participants without DXA measurements and 11.2 years for those with DXA measurements. Details of the selection of the main cohort are shown in Figure 1.
Figure 1. Study population for primary analyses of time to treatment-level or screening-level FRAX risk score.
Of the 111,556 women who did not have a DXA bone density test, 57,276 were excluded, including those who had a past hip or clinical vertebral fracture or antifracture treatment (bisphosphonate or calcitonin) at their first study examination, or who did not have adequate data for a FRAX score calculation at two or more examinations, or one FRAX score and subsequent development of a competing risk. In the cohort of 54,280 women with adequate data for a FRAX-without-BMD score prior to censoring, two transitions were studied: transition to treatment-level FRAX score (10-year estimated risk of major osteoporotic fracture ≥20% or of hip fracture ≥3%), and transition to screening-level FRAX score (10-year estimated risk of major osteoporotic fracture ≥9.3%). By a similar exclusion process, 6096 eligible participants who had at least one bone mineral density test were identified and were studied for transition to treatment-level FRAX-with-BMD score. The 39,568 women excluded for screening-level or treatment-level FRAX scores at baseline could not participate in the main analysis, but their incidence of hip and clinical vertebral fracture was examined.
An additional 39,568 women had screening-level (17,967 without bone mineral density) or treatment-level (20,362 without and 1239 with bone mineral density) FRAX scores at baseline. Although these individuals were not eligible for the primary analysis, their fracture incidence was estimated.
The study protocol and consent forms for the WHI study were approved by the institutional review boards for all participating institutions. The analysis protocol was reviewed and approved by the Institutional Review Board of the University of North Carolina.
FRAX fracture risk scores
The FRAX calculation includes age, body mass index, history of previous low-trauma fracture, parent who fractured hip, current smoking, history of oral glucocorticoid use, rheumatoid arthritis, secondary cause of osteoporosis, alcohol use of 3 or more units per day according to the algorithm developed by the University of Sheffield.1 The FRAX version 3.8 multi-patient desktop application from the International Osteoporosis Foundation was used to calculate 10-year risks of major osteoporotic and hip fracture, with and without bone mineral density, in January 2014. All calculations were made using the US version of FRAX for self-reported race; for patients categorized as “other race” or with missing self-reported race, FRAX calculations for Caucasians were used. When applicable, femoral neck T-score based on young white female norms was used in FRAX-with-BMD calculations.11
Assessment of bone mineral density, fracture and medications
Women enrolled at the WHI Tucson/Phoenix, Pittsburgh and Birmingham clinical centers had DXA bone density scans at the hip and anteroposterior–lateral spine to measure femoral neck, total hip and lumbar spine bone mineral density using the Hologic DXA program. Standard protocols for positioning and analysis were used by technicians trained and certified by the DXA manufacturer and by the WHI DXA coordination center at the University of California at San Francisco.12 An ongoing quality assurance program monitored spine and hip phantom scans, reviewed a random sample and flagged problematic scans. Hardware and software changes were tracked with in vitro and in vivo cross-calibrations and by scans of calibration phantoms across instruments and clinical sites.
Self-reported fractures were verified by review of radiology, magnetic resonance imaging, or operative reports by centrally trained physician adjudicators at the 3 WHI clinic sites that measured bone mineral density. For fracture sites other than hip, local clinic physician-adjudicated fractures were used. Final adjudication of hip fractures was performed centrally by blinded WHI physician adjudicators. The agreement between central and local adjudication for hip fracture was 94%.
Use of Food and Drug Administration–approved agents for the treatment of osteoporosis (bisphosphonates including alendronate, risedronate or zoledronate; calcitonin; raloxifene; parathyroid hormone) was assessed in the WHI Observational Study and WHI Extension Hormone Use Update medication follow-up surveys. Use of denosumab (FDA-approved for osteoporosis treatment in 2011) was not assessed.
Outcomes
Outcome metrics were based on Lee and Zelen's threshold method of defining an “optimal” screening interval as the testing interval that identifies a predetermined fraction of the expected number of cases in the screened population.13,14 By consensus decision, the clinician-investigator authors chose 10% as an acceptable threshold for asymptomatic risk scores that suggest higher fracture risk.
Accordingly, the primary outcome for our analysis was the time for 10% of women in seven age groups (50-54, 55-59, 60-64, 65-69, 70-74, 75-79, 80 and older) to develop a treatment-level FRAX score (estimated 10-year risk ≥20% for major osteoporotic fracture or ≥3% for hip fracture) prior to initiation of a Food and Drug Administration–approved antifracture treatment, sustaining a first hip or clinical vertebral fracture or dying. The secondary outcome was the estimated time for 10% of women aged 50 to 64 to develop a screening-level FRAX score (estimated 10-year risk ≥9.3% for major osteoporotic fracture) prior to initiation of antifracture treatment, sustaining a hip or clinical vertebral fracture or dying. Because the purpose of the USPSTF's screening-level FRAX score is to select postmenopausal women under age 65 who should be considered for DXA bone mineral density testing, screening scores were always calculated without bone mineral density.
Osteoporosis was defined by World Health Organization (WHO) diagnostic criteria as a bone mineral density T-score of –2.50 or below at the lumbar spine, femoral neck or total hip, calculated using the National Health and Nutrition Examination Study III bone mineral density norms for non-Hispanic white women aged 20-29.11,15,16 While an expanded definition of osteoporosis has been proposed elsewhere,17 we used osteoporosis by WHO bone mineral density criteria to allow differentiation between the competing risk of osteoporosis and a treatment-level FRAX score among the subset of WHI participants who underwent bone mineral density testing.
Statistical analysis
In women without clinically relevant FRAX scores at baseline, competing risk analyses were conducted to estimate the cumulative incidence functions for each age group for the time to development of a treatment-level or screening-level FRAX score before hip or clinical vertebral fracture, treatment initiation or death, and the corresponding intervals for 10% of participants to make the transition to a treatment-level or screening-level FRAX score. The competing risks were initiation of an antifracture agent, incident hip or clinical vertebral fracture and death. The time origin was the first study visit including adequate measurements to calculate a FRAX score, with follow-up continuing until the study examination preceding death or drop-out. Parametric regression models18 were fit to the cumulative incidence of fracture using naïve maximum likelihood analysis. The 10% time intervals and associated confidence intervals were based on the competing risks quantile methodology19 as implemented for parametric models by Lee and Fine.20 Covariate adjustments were not performed because the most important potential covariates were components of the FRAX risk score.
In women with treatment-level or screening-level FRAX scores at baseline, the time to clinically relevant FRAX score could not be estimated before study entry. Instead, we tabulated hip or clinical vertebral fracture incidence by end of follow-up in these participants.
All analyses were performed using Statistical Analysis Software (SAS) 9.4.21
Results
Characteristics of the main analytical cohort
Baseline characteristics of the 60,376 participants in the main cohort are shown in Table 1. The mean baseline FRAX-without-bone-mineral-density (FRAX-without-BMD) scores were below the treatment threshold, i.e. 10-year risks of 10.2% for major osteoporotic fracture and 1.75% for hip fracture for women aged 65 and older and lower mean scores for women aged 50 to 64. No participant reported use of raloxifene or parathyroid hormone during follow-up.
Table 1. Baseline characteristics and follow-up time of the women studied to determine time to clinically relevant fracture risk score.
| Characteristics at baseline | Did Not Have BMD Test (n=54,280) | Had BMD Test (n=6096)* | All participants (n=60,376) | |
|---|---|---|---|---|
| Mean age at baseline, mean ± SD, y | 60.52 ± 6.33 | 61.55 ± 6.73 | 60.63 ± 6.38 | |
| BMI** kg/m2, mean ± SD | 28.62 ± 6.30 | 28.34 ± 5.98 | 28.60 ± 6.27 | |
| 25 | 17107 ± 31.52 | 1969 ± 32.30 | 19076 ± 31.60 | |
| ≥25 | 37173 ± 68.48 | 4127 ± 67.70 | 41300 ± 68.40 | |
| Race no./ total no. (%) | ||||
| White (non-Hispanic) | 41868/54159 (77.31%) | 4644/6087 (76.29%) | 46512/60246 (77.20%) | |
| African American | 6857/54159 (12.66%) | 953/6087 (15.66%) | 7810/60246 (12.96%) | |
| Asian | 2152/54159 (3.97%) | 24/6087 (0.39%) | 2176/60246 (3.61%) | |
| Hispanic | 2664/54159 (4.92%) | 437/6087 (7.18%) | 3101/60246 (5.15%) | |
| Other | 618/54159 (1.14%) | 29/6087 (0.48%) | 647/60246 (1.07%) | |
| Years of education no./ total no. (%) | ||||
| Less than high school | 2815/53830 (5.23%) | 559/6048 (9.24%) | 3374/59878 (5.63%) | |
| High school/some college | 28575/53830 (53.08%) | 3558/6048 (58.83%) | 32133/59878 (53.66%) | |
| College/some graduate school | 12289/53830 (22.83%) | 1048/6048 (17.33%) | 13337/59878 (22.27%) | |
| Completed graduate school | 10151/53830 (18.86%) | 883/6048 (14.60%) | 11034/59878 (18.43%) | |
| Previous fracture after age 55 no./ total no. (%) | ||||
| Yes | 1168/39365 (2.97%) | 411/4343 (9.46%) | 1579/43708 (3.61%) | |
| No | 38197/39365 (97.03%) | 3932/4343 (90.54%) | 42129/43708 (96.39%) | |
| Estrogen use no./ total no. (%) | ||||
| Yes | 7285/54244 (13.43%) | 891/6067 (14.69%) | 8176/60311 (13.56%) | |
| Past | 23571/54244 (43.45%) | 2521/6067 (41.55%) | 26092/60311 (43.26%) | |
| Never | 23388/54244 (43.12%) | 2655/6067 (43.76%) | 26043/60311 (43.18%) | |
| Current smoker no./ total no. (%) | ||||
| Current | 3504/53733 (6.52%) | 461/6012 (7.67%) | 3965/59745 (6.64%) | |
| Past | 23014/53733 (42.83%) | 2296/6012 (38.19%) | 25310/59745 (42.36%) | |
| Never | 27215/53733 (50.65%) | 3255/6012 (54.14%) | 30470/59745 (51.00%) | |
| Alcohol consumption (drinks per week) no./ total no. (%) | ||||
| 0 drinks per week | 5799/53917 (10.76%) | 1003/6045 (16.59%) | 6802/59962 (11.34%) | |
| <1 drinks per week | 17976/53917 (33.34%) | 1962/6045 (32.46%) | 19938/59962 (33.25%) | |
| 1 to 6 drinks per week | 13846/53917 (25.68%) | 1245/6045 (20.60%) | 15091/59962 (25.17%) | |
| ≥7 drinks per week | 6190/53917 (11.48%) | 499/6045 (8.25%) | 6689/59962 (11.16%) | |
| Past drinker | 10106/53917 (18.74%) | 1336/6045 (22.10%) | 11442/59962 (19.08%) | |
| History of parent with hip fracture, no. (%) | ||||
| Yes | 4874 (8.98%) | 1077 (17.67%) | 5951 (9.86%) | |
| No | 49406 (91.02%) | 5019 (82.33%) | 54425 (90.14%) | |
| Rheumatoid arthritis no./ total no. (%) | ||||
| Yes | 2463 (4.54%) | 309 (5.07%) | 2772 (4.59%) | |
| No | 51817 (95.46%) | 5787 (94.93%) | 57604 (95.41%) | |
| Current Oral glucocorticoid use, no. (%) | ||||
| Yes | 388 (0.71%) | 54 (0.89%) | 442 (0.73%) | |
| No | 53892 (99.29%) | 6042 (99.11%) | 59934 (99.27%) | |
| Menopause duration mean ± SD, y † | 12.89 ± 8.40 | 15.36 ± 8.93 | 13.13 ± 8.48 | |
| Baseline FRAX 10-year fracture risk scores, mean ± SD ‡ | ||||
| Major Osteoporotic Fracture | 7.24 ± 3.27 | 7.98 ± 4.02 | -- | |
| Age 50-64 at baseline | 5.76 ± 2.02 | 7.29 ± 4.00 | -- | |
| Age ≥65 at baseline | 10.23 ± 3.26 | 9.28 ± 3.73 | -- | |
| Hip fracture | 0.91 ± 0.75 | 0.71 ± 0.71 | -- | |
| Age 50-64 at baseline | 0.49 ± 0.31 | 0.49 ± 0.55 | -- | |
| Age ≥65 at baseline | 1.75 ± 0.67 | 1.12 ± 0.79 | -- | |
| Follow-up time, y | ||||
| Mean ± SD | 12.91 ± 3.84 | 13.16 ± 4.13 | 12.94 ± 3.87 | |
| Minimum | 1 day | 7 days | 1 day | |
| Maximum | 18.64 | 18.53 | 18.64 | |
: Femoral neck, total hip and lumbar spine bone mineral density (BMD) was measured
: For 108 subjects, BMI was recorded after first bone mineral density test
: Based on 47248 responses, 4981 responses and 52229 responses. “Major osteoporotic fracture” includes clinical spine, forearm, hip or proximal humerus fracture.
: FRAX scores without BMD and with BMD for the respective groups without clinically relevant FRAX scores at baseline
Women aged 50 to 64 without clinically relevant FRAX scores at baseline
Estimated time to treatment-level FRAX-without-BMD score
Time to treatment-level FRAX-without-BMD could not be estimated accurately in women aged 50 to 64 but was likely longer than 18.6 years because it was an extrapolation beyond the maximum follow-up time.
Estimated time to treatment-level FRAX-with-BMD score
The estimated times for 10% of women aged 50 to 64 without osteoporosis or hip or clinical vertebral fracture to develop a treatment-level FRAX-with-BMD was likely longer than 11.2 years because rare endpoint events led to extremely extrapolated values with confidence intervals that excluded 11.2 years.
Estimated time to screening-level FRAX-without-BMD score
Time to screening-level FRAX-without-BMD could not be estimated accurately in women aged 50 to 54, but was likely longer than 18.6 years because it was an extrapolation beyond the maximum length of follow-up. The estimated time to a screening-level FRAX was 15.9 years (95% CI, 14.8, 17.0) for women aged 55 to 59 and 6.3 years (95% CI, 5.9, 6.7) for women aged 60 to 64 (Table 2).
Table 2. Time to clinically relevant FRAX fracture risk scores in WHI participants without BMD test.
| Baseline age ranges (years) | N for stratum | Time for 10% of participants to have the event (years), accounting for competing risks* | |
|---|---|---|---|
| Screening-level fracture risk score without BMD† | Treatment-level fracture risk score without BMD‡ | ||
| 50 to 54 | 12027 | -- | -- |
| 55 to 59 | 12881 | 15.87 (14.80, 17.03) | -- |
| 60 to 64 | 11340 | 6.29 (5.91, 6.70) | -- |
| 65 to 69 | 13842 | N/A | 5.43 (5.14, 5.73) |
| 70 to 74 | 3892 | N/A | 2.94 (2.64, 3.27) |
| 75 to 79 | 298 | N/A | 3.67 (2.44, 5.53) |
| ≥80 | 0 | N/A | -- |
competing risks: treatment, first hip or clinical vertebral fracture or death. Estimates greater than the maximum length of follow-up (18.6 years) with 95% CIs excluding the maximum are not presented.
(for baseline age 50 to 64 only) screening-level FRAX score of 10-year risk ≥9.3% for major osteoporotic fracture (hip, clinical spine, proximal humerus, or wrist), calculated without BMD
treatment-level FRAX score of 10-year risk ≥20% for major osteoporotic fracture or ≥3% for hip fracture (whichever is first) calculated without BMD
Women aged 65 and older without clinically relevant FRAX scores at baseline
Estimated time to treatment-level FRAX-without-BMD score
The estimated time to a treatment-level FRAX-without-BMD score was 5.4 years (95% CI, 5.1, 5.7) for women age 65 to 69, 2.9 years (95% CI, 2.6, 3.3) for those age 70 to 74 and 3.7 (95% CI, 2.4, 5.5) for those aged 75 to 79 (Table 2).
Estimated time to treatment-level FRAX-with-BMD score
The unadjusted estimated time for 10% of women to develop a treatment-level FRAX-with-BMD score ranged from 7.6 years (95% CI, 6.6, 8.7) for those aged 65-69 to 5.1 years (95% CI, 3.5, 7.5) for those aged 75 to 79, and could not be estimated for the 1 participant aged 80 or older (Table 3).
Table 3. Time to clinically relevant FRAX fracture risk scores in WHI participants with at least one BMD test.
| Baseline age ranges (years) | N for stratum | Time for 10% of participants to develop treatment-level fracture risk score with BMD, accounting for competing risks* |
|---|---|---|
| 50 to 54 | 1128 | -- |
| 55 to 59 | 1341 | -- |
| 60 to 64 | 1497 | -- |
| 65 to 69 | 1312 | 7.60 (6.64, 8.70) |
| 70 to 74 | 664 | 6.94 (5.70, 8.44) |
| 75 to 79 | 153 | 5.13 (3.53, 7.46) |
| ≥80 | 1 | -- |
treatment-level FRAX score of 10-year risk ≥20% for major osteoporotic fracture or ≥3% for hip fracture calculated with BMD. Estimates greater than the maximum length of follow-up (11.2 years) with 95% CIs excluding the maximum are not presented.
Competing risks: osteoporosis by BMD criteria, treatment, first hip or clinical vertebral fracture or death.
Women with clinically relevant FRAX scores at baseline
An additional 39,568 women (not included in the tables) were not eligible for the main analysis because they had a treatment-level or screening-level FRAX score at baseline. Fracture incidence results for these women are presented by age group immediately below:
Women aged 50 to 64
Of the 255 women aged 50 to 64 with treatment-level FRAX scores at baseline, 26 (10.2%) had a hip or clinical vertebral fracture by end of study follow-up. Of the 17,967 women aged 50 to 64 with screening-level FRAX scores at baseline, 100 (0.6%) experienced a hip or clinical vertebral fracture by age 65; their estimated time for 10% to develop a treatment-level FRAX score was 19.4 years (95% CI 17.3, 21.7) for those aged 55 to 59, and 6.1 years (95% CI 5.7, 6.6) for those aged 60 to 64. (This time could not be calculated for those aged 50 to 54 due to rare incidence of the endpoint.)
Women aged 65 and older
Among the 21,346 women aged 65 and older with treatment-level FRAX-with-BMD scores at baseline, 1517 (7.1%) had a hip or clinical vertebral fracture during follow-up.
Discussion
We conducted a competing risk analysis of new occurrence of clinically relevant FRAX scores in postmenopausal women aged 50 and older. The estimated time for 10% of women to develop treatment-level FRAX fracture risk scores before osteoporosis or major osteoporotic fracture was shorter when scores were calculated without bone mineral density compared to with bone mineral density. Using FRAX-without-BMD for conservative time estimates, approximately 10% of women 65 and older developed a treatment-level FRAX within 3 to 5 years. Consistent with past studies, screening-level FRAX scores in postmenopausal women aged 50 to 64 had low predictive ability due in large part to very low rates of incident treatment-level risk scores within this age group.
Treatment-level FRAX scores in women aged 65 and older
We used a 10% fracture threshold to allow comparison to previously published estimates of time to osteoporosis in women over age 65 with serial DXA bone mineral density testing.10 Among women aged 65 and older, the 3-to-5-year estimate of time to treatment-level FRAX is similar to the bone mineral density screening intervals of 5 years or less for women with bone mineral density T-scores ≤ -1.50 in the previous study.10 These findings might offer a rationale for concurrent use of DXA screening and FRAX scoring to maximize identification of female treatment candidates aged 65 and older.
Screening-level FRAX scores in postmenopausal women aged 50 to 64
Previous studies suggested that the screening-level FRAX threshold recommended by the US Preventive Services Task Force has low sensitivity to detect osteoporosis in postmenopausal women aged 50 to 64.7,22 Accordingly, we found that postmenopausal women aged 50 to 64 who developed a screening-level FRAX were unlikely to have a hip or clinical vertebral fracture before age 65. Like past studies,7,8 our results do not support routine use of FRAX to identify candidates for screening in postmenopausal women aged 50 to 64.
FRAX has been incorporated into subspecialty and clinical practice guidelines with recommended use in conjunction with bone mineral density testing.23,4 In 2011, the US Preventive Services Task Force (USPSTF) recommended use of the screening-level FRAX score in postmenopausal women under age 65 but did not endorse the use of treatment-level FRAX scores in the absence of randomized controlled trial data regarding this treatment strategy.5 We did not find data regarding US clinicians' familiarity with and use of FRAX; however a 2013 mailed survey in Belgium suggested that one-third of general practitioners who were assessed knew of the FRAX tool but less than 20% used it in their daily clinical practice.24 The lack of RCT data to test the ability of FRAX-guided treatment to reduce fracture events is a potentially correctable barrier to promoting broader awareness and consideration of use of the FRAX tool in primary care practice settings.
Our study had several limitations. Because FRAX is validated for use in untreated patients only,25 and because patients who are treated or who already have osteoporosis or fragility fracture no longer meet the epidemiological definition of screened individuals,26 women with past antifracture treatment, existing osteoporosis or hip or clinical vertebral fracture were not eligible for the main analysis. We used US FRAX score thresholds; the results are not generalizable to countries that use different score thresholds for treatment or screening decisions. We could not calculate time estimates for women under age 55 because of their rare incidence of clinically relevant FRAX scores; however the extreme extrapolations in this group suggest that their time estimates would be longer than the maximum follow-up time of 18.6 years (without bone mineral density) or 11.2 years (with bone mineral density) in this study. The time estimates are means within age strata and do not reflect every woman within the stratum, e.g., women with scores close to the cut point for screening-level or treatment-level FRAX would have a shorter time to clinically relevant scores than women with lower scores. FRAX scores were calculated using FRAX version 3.8. Since then in versions 3.9 and 3.10 (current), models were added for 5 additional countries, but the US version has remained the same. Strengths of the analysis include the large size of the cohort, the long follow-up period including women with and without bone mineral density measurements, use of time-to-event analysis to study the timing of a screening test, and consideration of longitudinal FRAX scores instead of baseline scores only.
Conclusions
In conclusion, our results suggest that 10% of women aged 65 and older without treatment-level FRAX scores at baseline may develop a treatment-level FRAX score within 3 to 5 years. Although screening-level FRAX scores were common among postmenopausal women aged 50 to 64, rare incidence of hip and clinical spine fractures before age 65 suggests that routine FRAX scoring would be low-yield in this age range. A randomized trial of routine use of FRAX scoring versus usual care could help clarify whether FRAX-guided therapy can reduce fracture in postmenopausal women without osteoporosis by bone mineral density criteria.
Clinical Significance.
Ten percent of women aged ≥65 developed a treatment-level FRAX by 3 to 5 years withoutbone density measurements, and 5 to 7 years with bone density measurements.
Screening-level FRAX had low predictive ability in postmenopausal women aged 50 to 64.
FRAX scores calculated without bone density were higher-risk than FRAX scores calculatedwith bone density.
Acknowledgments
We acknowledge Carrie Gartland for her assistance with manuscript preparation.
Funding/support: The project described was funded by the National Institute on Aging through Grant Number R01AG046294 and by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR000083. The Women's Health Initiative (WHI) program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. The sponsors had no role in study design; in the collection, analysis, and interpretation of data; in writing the report; or in the decision to submit the article for publication.
Footnotes
Conflicts of interest/disclosures: No conflicts of interest were reported for the submitted work.
Authors' roles: All authors had access to the data and a role in writing the manuscript.
Trial registration: The Women's Health Initiative is registered as ClinicalTrials.gov number NCT00000611.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization Collaborating Centre for Metabolic Bone Diseases, University of Sheffield, UK. University of Sheffield; Sheffield, UK: [Accessed December 27, 2016]. FRAX: WHO Fracture Risk Assessment Tool [Internet] Available from http://www.shef.ac.uk/FRAX/ [Google Scholar]
- 2.Dawson-Hughes B, Tosteson AN, Melton LJ, 3rd, et al. Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2008;19(4):449–458. doi: 10.1007/s00198-008-0559-5. [DOI] [PubMed] [Google Scholar]
- 3.Tosteson AN, Melton LJ, 3rd, Dawson-Hughes B, et al. Cost-effective osteoporosis treatment thresholds: the United States perspective. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2008;19(4):437–447. doi: 10.1007/s00198-007-0550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosman F, de Beur SJ, LeBoff MS, et al. Clinician's Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25(10):2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Screening for osteoporosis: U.S. preventive services task force recommendation statement. Ann Intern Med. 2011;154(5):356–364. doi: 10.7326/0003-4819-154-5-201103010-00307. [DOI] [PubMed] [Google Scholar]
- 6.Edwards FD, Grover ML, Cook CB, Chang YH. Use of FRAX as a Determinant for Risk-Based Osteoporosis Screening May Decrease Unnecessary Testing While Improving the Odds of Identifying Treatment Candidates. Womens Health Issues. 2014;24(6):629–634. doi: 10.1016/j.whi.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Crandall CJ, Larson J, Gourlay ML, et al. Osteoporosis Screening in Postmenopausal Women 50-64 Years-Old: Comparison of U.S. Preventive Services Task Force Strategy and Two Traditional Strategies in the Women's Health Initiative. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014 Jan 16; doi: 10.1002/jbmr.2174. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crandall CJ, Larson JC, Watts NB, et al. Comparison of Fracture Risk Prediction by the US Preventive Services Task Force Strategy and Two Alternative Strategies in Women 50-64 Years Old in the Women's Health Initiative. J Clin Endocrinol Metab. 2014;99(12):4514–4522. doi: 10.1210/jc.2014-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gourlay ML, Overman RA, Fine JP, et al. Baseline age and time to major fracture in younger postmenopausal women. Menopause. 2015;22(6):589–597. doi: 10.1097/GME.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gourlay ML, Fine JP, Preisser JS, et al. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366(3):225–233. doi: 10.1056/NEJMoa1107142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Looker A, Wahner H, Dunn W, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–489. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 12.Jackson RD, LaCroix AZ, Cauley JA, McGowan J. The Women's Health Initiative calcium-vitamin D trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13(9 Suppl):S98–106. doi: 10.1016/s1047-2797(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 13.Lee S, Zelen M. Scheduling periodic examinations for the early detection of disease: applications to breast cancer. J Am Stat Assoc. 1998;93:1271–1281. [Google Scholar]
- 14.Lee S, Huang H, Zelen M. Early detection of disease and scheduling of screening examinations. Stat Methods Med Res. 2004;13:443–456. doi: 10.1191/0962280204sm377ra. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. WHO Technical report series no 843. Geneva; World Health Organization: 1994. Assessment of osteoporotic fracture risk and its role in screening for postmenopausal osteoporosis. [PubMed] [Google Scholar]
- 16.Looker AC, Melton LJ, 3rd, Borrud LG, Shepherd JA. Lumbar spine bone mineral density in US adults: demographic patterns and relationship with femur neck skeletal status. Osteoporos Int. 2012;23(4):1351–1360. doi: 10.1007/s00198-011-1693-z. [DOI] [PubMed] [Google Scholar]
- 17.Siris ES, Adler R, Bilezikian J, et al. The clinical diagnosis of osteoporosis: a position statement from the National Bone Health Alliance Working Group. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2014;25(5):1439–1443. doi: 10.1007/s00198-014-2655-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeong JH, Fine JP. Parametric regression on cumulative incidence function. Biostatistics. 2007;8(2):184–196. doi: 10.1093/biostatistics/kxj040. [DOI] [PubMed] [Google Scholar]
- 19.Peng L, Fine J. Nonparametric Quantile Inference with Competing-Risks Data. Biometrika. 2007;94:735–744. [Google Scholar]
- 20.Lee M, Fine JP. Inference for cumulative incidence quantiles via parametric and nonparametric approaches. Stat Med. 2011;30(27):3221–3235. doi: 10.1002/sim.4349. [DOI] [PubMed] [Google Scholar]
- 21.SAS Institute Inc. SAS/STAT 9.4 User's Guide. Cary, NC: SAS Institute Inc; 2013. [Google Scholar]
- 22.Bansal S, Pecina JL, Merry SP, et al. US Preventative Services Task Force FRAX threshold has a low sensitivity to detect osteoporosis in women ages 50-64 years. Osteoporos Int. 2015;26(4):1429–1433. doi: 10.1007/s00198-015-3026-0. [DOI] [PubMed] [Google Scholar]
- 23.Lewiecki EM, Compston JE, Miller PD, et al. Official Positions for FRAX(R) Bone Mineral Density and FRAX(R) simplification from Joint Official Positions Development Conference of the International Society for Clinical Densitometry and International Osteoporosis Foundation on FRAX(R) J Clin Densitom. 2011;14(3):226–236. doi: 10.1016/j.jocd.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Bruyere O, Nicolet D, Compere S, et al. Perception, knowledge, and use by general practitioners of Belgium of a new WHO tool (FRAX) to assess the 10-year probability of fracture. Rheumatol Int. 2013;33(4):979–983. doi: 10.1007/s00296-012-2461-x. [DOI] [PubMed] [Google Scholar]
- 25.Siris ES, Baim S, Nattiv A. Primary care use of FRAX: absolute fracture risk assessment in postmenopausal women and older men. Postgrad Med. 2010;122(1):82–90. doi: 10.3810/pgm.2010.01.2102. [DOI] [PubMed] [Google Scholar]
- 26.Sackett D, Straus S, Richardson W, Rosenberg W, Haynes R. Evidence-based medicine: how to practice and teach EBM. 2nd. New York: Harcourt; 2000. Diagnosis and screening; p. 261. [Google Scholar]