Abstract
With the clinical impact of CTLA-4 and PD-1/PD-L1 immune checkpoint therapies, widespread interest in cancer immunotherapy has been ignited. However, the rate and extent of clinical responses to approved therapies are limited and often non-existent in many solid tumors. This is partially because immune checkpoint therapies are most effective against T-cell inflamed tumors, and non-T-cell inflamed or T-cell excluded tumors remain a significant barrier. New strategies are needed to overcome immune resistance mechanisms that arise during tumor development which result in T-cell exclusion. Approaches may need to be combined with conventional therapies such as chemotherapy, radiotherapy, and molecularly targeted therapy and many clinical trials are ongoing. This review discusses the challenge of T-cell exclusion and innate oncologic pathways that contribute to this problem, including β-catenin, STAT3, NF-κB, PTEN, and AXL tyrosine kinase. The GAS6/AXL pathway is of interest immunologically as it’s targeting can lead to greater antitumor immune responses after radiation. In addition, several targeted therapies that are selective and non-selective for AXL are in preclinical and clinical development in AML and renal cell cancer. There remains much to learn but the future is bright for anti-AXL therapies, though effective combinations and their impact may not be realized for years to come.
BACKGROUND
Resistance to Checkpoint Immunotherapy
Checkpoint Immunotherapy is quickly entering first line treatment of metastatic disease for multiple malignancies, but single agent responses to CTLA4 and PD1/PDL1 (PDCD1/CD274) antibodies (Ab) are limited to 10–30% (1–4). It is critical to understand factors that limit responses, which may be due to overlapping immune resistance mechanisms or the prevention of an initial immune response. Mechanisms of immune resistance after an initial immune response include but are not limited to the immunosuppressive role of FoxP3 transcription factor expressing regulatory T-cells (Tregs), the elevated expression of indoleamine-2,3-dioxygenase 1 (IDO1) that suppresses T-cell responses by decreasing tryptophan levels, or engagement of additional inhibitory receptors such as T-cell immunoglobulin and mucin-domain containing-3 (HAVCR2 or TIM3) that can be overexpressed in PD-1 resistance mediating T-cell exhaustion (5,6). The prevention of an initial T-cell mediated immune response can be attributed to deficient dendritic cell activation and prevention of a specific T-cell response by suppressive factors or the lack of tumor antigens to initiate clonal T-cell response. This results in a tumor microenvironment deficient in responsive T-cells commonly referred to non-T-cell inflamed or T-cell exclusion (7,8).
Resistant tumors with T-cell exclusion
A predictor of response to PD-1 and PD-L1 therapy is the presence of pretreatment CD8+ T-cell infiltration within the tumor. Non-responders may have scant infiltrating CD8+ T-cells or confinement to the tumor margin (9,10). It is suspected that an important consequence of tumor-derived factors resulting in immune resistance is T-cell exclusion, predominantly of CD8+ T-cells from the microenvironment (11). This topic is receiving more attention as CD3+ and/or CD8+ T-cell infiltrates correlate with prognosis in multiple cancers including melanoma, ovarian, and colon cancer (9,12–14). For example, Clark et al. reported that in clinical stage I melanoma, the presence of ‘brisk’ infiltrating CD3+ lymphocytes resulted in an adjusted odds ratio of 11.3 for survival at 8 years, meaning that patients with infiltrating lymphocytes had an 11.3 times higher odds of being alive at 8 years compared to absent lymphocytes (12). Recently, Tumeh et al. showed that pre-existing CD8+ T-cells negatively regulated by PD-1/PD-L1 have greater responses to anti-PD-1 therapy compared to tumors deficient in CD8+ T-cells (9).
Understanding T-cell exclusion will depend upon insight into the evolutionary factors that begin early in malignancy, an idea commonly referred to as immunoediting (15). Factors that influence this include but are not limited to tumor antigens that develop from somatic mutations, classic oncologic pathways that suppress or exploit an immune response to its advantage, microenvironmental factors such as hypoxia and aberrant vasculature, and additional environmental factors such as exposure to microbes, viruses, or altered physiologic states. Though there is interest in each potential mechanism of T-cell exclusion, this review will focus on tumor cell-derived oncologic pathways.
Oncologic pathways can coordinate T-cell exclusion
Classic oncologic pathways critical for cellular transformation may be some of the most important determinants of T-cell exclusion and suppressing the antitumor immune response. For example, Hugo et al., reported that melanomas sensitive to anti-PD-1 therapy often possess mutations in the DNA repair gene BRCA2, and that resistant tumors have gene expression patterns associated with epithelial to mesenchymal transition, cell adhesion, matrix remodeling, angiogenesis, and wound healing (16). This study was important in providing genetic indicators for biologic resistance mechanisms beyond correlative analysis of infiltrating T-cells. Though it did not directly correlate BRCA2 and the various genes upregulated in resistance with T-cell exclusion, it can be inferred from Tumeh et al. and Herbst et al. that non-responders had greater T-cell exclusion (9,10). More studies will be important to biologically validate these findings and show how they influence an antitumor immune response.
A biologic pathway that impacts T-cell exclusion in melanoma recently identified is t β-catenin (CTNNB1) pathway (7). Following correlative studies with human data, the authors used a constitutively active β-catenin tumor model to demonstrate decreased production of chemokine’s that are important for recruiting dendritic cells to the microenvironment resulting in 3-fold decrease of T-cell infiltration. They suggest that lack of dendritic cell recruitment to the tumor microenvironment ultimately prevents development of a T-cell mediated antitumor response and results in resistance to anti-PD-L1 and anti-CTLA-4 checkpoint immunotherapy.
Additional factors that can influence immunosuppression and impact T-cell exclusion include signal transducer and activator of transcription 3 (STAT3), p53, NF-κB, and PTEN. The epidermal growth factor receptor (EGFR), vascular endothelial growth factor receptor (Flt1 or VEGFR1), and other receptors signal through the STAT-3 pathway that serves as a transcription activator after cytokine and growth factor receptor signaling. Tumor cell STAT3 signaling can promote immunosuppression, proinflammatory cytokines, and chemokines that recruit Tregs, suppress CD8+ T-cells, reduce neutrophil function, NK cell mediated cytotoxicity, and block dendritic cell maturation (17). Loss of STAT3 in a transgenic breast cancer model resulted in greater antitumor immune response, suggesting that STAT3 inhibitors would have immunomodulatory activity (18). In contrast to STAT3, the presence of wild-type p53 in tumor cells can lead to innate immune cell mediated antitumor response through chemokine signaling. The loss of p53 results in suppression of an antitumor immune response (19,20). In the case of NF-κB, reports suggest that tumor necrosis factor alpha (TNF) from surrounding tissues can stimulate NF-κB, thus promoting tumor progression. Although NF-κB has numerous downstream targets, the immune response induced through the NF-κB complex is likely due to chemokine production. However, the immune modulatory activity of NF-κB may be different in myeloid cells compared to tumor cells, making it difficult to target therapeutically (21,22). PTEN, an important tumor suppressor, which is often mutated, can result in decreased T-cell infiltration into tumors and T-cell mediated tumor killing through expression of immunosuppressive cytokines (23).
T-cell exclusion and immunosuppressive pathways impact the response to cancer therapy
It is commonly proposed that tumor cell death releases tumor antigens, thereby enabling adaptive immune responses. Cell death induced by chemotherapy and radiotherapy can lead to immune activation and overcoming T-cell exclusion. Such treatments can release molecules including high mobility group protein B1 (HMGB1), danger-associated molecular patterns (DAMPS), and ATP that increase dendritic cell antigen presentation and maturation (24). However, cell death can induce signals with opposing effects on the immune response through the induction of an antitumor immune response commonly referred to as immunogenic cell death (ICD) or cell death that fails to activate an immune response, known as tolerogenic cell death (TCD)(25). TCD is commonly associated with T-cell exclusion because an antitumor T-cell response is not elicited. In addition, various anticancer therapies can impact immune cell subsets; examples include the inhibition of Tregs by cyclophosphamide or inhibition of tumor promoting myeloid derived suppressor cells by gemcitabine. These and other cytotoxic treatments can temporarily overcome the immunosuppressive tumor microenvironment, enabling greater antitumor immune responses (26–28). Radiotherapy, can lead to ICD through effects that include the release of tumor antigens, exposure of heat shock proteins (HSP) on the cell surface, release of calreticulin, and the increased expression of MHCI, intracellular adhesion molecule 1 (ICAM1), and lymphocyte function–associated antigen 3 (LFA3)(29). Despite the promise of conventional therapies inducing ICD, factors promoting TCD need to be identified and evaluated.
Numerous preclinical studies have validated the combination of targeted therapy, chemotherapy, or radiotherapy with checkpoint immunotherapy to overcome limited responses. Key factors that enable radiation and checkpoint immunotherapy responses include highly immunogenic tumors, the induction of an adaptive T-cell mediated response, T-cell dependence of tumor eradication, and blocking tumor supporting immune cells (30–32). Chemotherapy and targeted therapies can augment immune responses similar to radiation, but depending upon the mechanism of action can complement or compete with immunotherapy (33–35). One example of competition is the finding that MET, a proto-oncogene often mutated or amplified, is important in the proper function of antitumor neutrophils, which limits therapeutic efficacy of targeted therapies against MET (35).
Identifying oncologic targets that influence T-cell exclusion and response to radiation
One approach to identify new targets that influence T-cell exclusion was to evaluate factors that impact the immune response to clonal tumors. In the transgenic PyMT breast cancer model tumors develop with different inflammatory microenvironments. Two tumor clones were evaluated, one clone exhibited a robust immune response with T-cell infiltration that was enhanced by radiation and the second clone had T-cell exclusion and was unresponsive even after radiotherapy. The receptor tyrosine kinase (RTK) AXL was identified as a potential mediator of T-cell exclusion after evaluating differences between the clones. AXL belongs to the TAM (TYRO3/AXL/MERTK) family of RTKs and is known to regulate epithelial to mesenchymal transition. Though the TAM family is important in the immune system in the regulation of macrophages and dendritic cells, AXL had not been identified as a mediator of immunosuppression by tumor cells. Genetic deletion of AXL resulted in up to twenty fold enhanced T-cell infiltration and sensitization of tumor cells to radiation and checkpoint immunotherapy. The mechanism appears to be due AXL’s role in suppressing antigen presentation, and producing myeloid supporting cytokines and chemokines, which results in a limited adaptive immune response and T-cell exclusion (36).
These data convey one example of how immunoediting can result in the suppression of an antitumor immune response and T-cell exclusion. AXL has received much attention for its role in invasion, metastasis, therapeutic resistance, and now immunotherapy resistance (37,38). A recent study investigating the genomic and transcriptomic profile of tumors resistant to PD-1 immunotherapy found that AXL transcript levels were significantly correlated with resistance to PD-1 immunotherapy. This was the first human data to suggest a biologic link between AXL and resistance to immunotherapies in humans and supports the biologic link defined in the PyMT tumors (16).
In addition to AXL’s immune function in tumor cells, its family member MERTK, the other known receptor for the GAS6 ligand, plays an important complementary role in TCD. MERTK is a mediator of apoptotic cell clearance by macrophages, promotes M2 anti-inflammatory cytokine production, suppresses an M1 pro-inflammatory response, and results in TCD (39–42). A study recently showed that after radiation, MERTK signaling in host myeloid cells leads to anti-inflammatory effects resulting in radiation resistance. Tumors became sensitive to radiation when implanted in MERTK knockout mice, though there was little effect on tumor growth in the absence of treatment (43). Although the investigators did not show that the therapeutic effect from the loss of MERTK resulted a greater antitumor T-cell response, it will be an important question to investigate further.
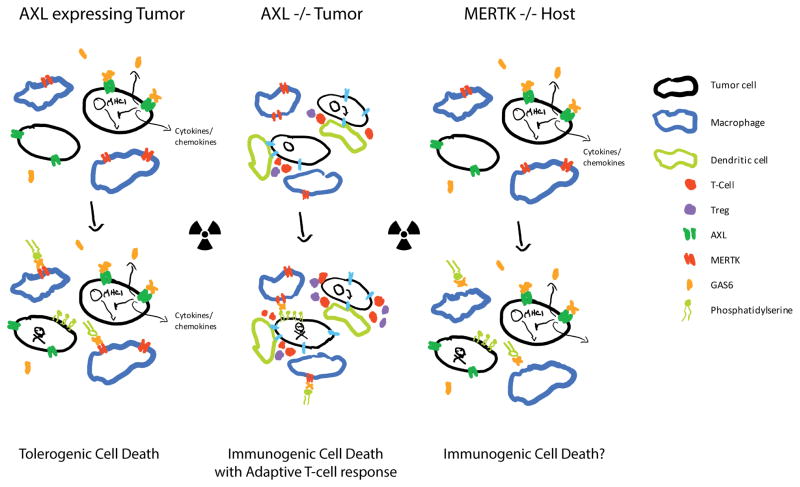
These reports suggest there is much to learn about TAM signaling in the tumor microenvironment but building evidence suggests that AXL, GAS6, and MERTK are important in supporting tumor growth with a role in cancer cells, infiltrating myeloid cells, and in suppressing ICD, thereby preventing dendritic cell licensing and promoting T-cell exclusion. Figure 1 depicts a representative tumor microenvironment in the presence and absence of AXL or MERTK and the response to radiation therapy. In the presence of AXL there is low expression of MHCI, cytokines and chemokines that support macrophages, and elevated levels of GAS6. MERTK favors TCD and the absence results in impaired clearance of apoptotic particles, which may lead to ICD.
Figure 1. Loss of AXL or MERTK in the tumor microenvironment results in an antitumor immune response to radiation.
Both AXL and MERTK play key roles in immunosuppression. Tumors with high AXL expression can be resistant to therapy through decreased MHCI expression, increased GAS6 production, and release of myeloid supporting cytokines and chemokines. Radiation results in tolerogenic cell death with GAS6 mediated signaling through MERTK and a limited adaptive immune response (Left). In the absence of AXL tumors are more immunogenic with evidence of an adaptive T-cell response and radiation further enhances the antitumor immune response (Center). Upon loss of MERTK there is a decrease in macrophage mediated phagocytosis and tolerogenic cell death is limited, which seems to result in a greater antitumor immune response after radiation (Right).
CLINICAL-TRANSLATIONAL ADVANCES
Clinical advances in radiation and immunotherapy as an approach to overcome T-cell exclusion
With the discovery of immune activating responses after chemotherapy or radiotherapy there is growing interest in combining these treatments with immune checkpoint therapy to enhance tumor responses. A dramatic, but uncommon example of the stimulating effects of radiation was highlighted in the case report by Postow et al. who showed widespread response to metastatic disease after palliative radiation to a single site in a young woman who was progressing on anti-CTLA-4 therapy Ipilimumab (44). In recent trials, there has not been convincing evidence that combining radiation and immunotherapy leads to greater systemic responses, and it remains unknown if radiation can consistently enhance immunotherapy (45–49). It may be that the combination is only synergistic in tumors with T-cell infiltration or a preexisting adaptive immune response. An example supporting this was demonstrated in a preclinical mouse model where tumors with T-cell exclusion and elevated levels of AXL had minimal response to radiation, checkpoint immunotherapy, and the combination. Sensitivity to the combination of therapies was attained in AXL knockout tumors by overcoming T-cell exclusion (36). The number of ongoing clinical trials with radiation and immunotherapy registered at clinicaltrials.gov is increasing, many of which were highlighted in recent reviews (50,51). Time will tell if a particular approach will offer more encouraging results than the early studies and whether barriers can be identified. But for now the true clinical benefit remains unknown and may depend upon the method of RT delivery, the timing, the disease targeted, the immunotherapeutic approach, or unidentified factors.
We are concerned that many cancers will prove to be resistant to the immune activating effects of radiation due to T-cell exclusion and suppressive factors such as GAS6/AXL. Clinical trials with RT and T-cell modulating therapeutics with sophisticated biologic analysis could provide insight into whether the combination therapy is more efficacious in T-cell infiltrated tumors compared to T-cell excluded tumors. We suspect that combination therapy will have minimal to no impact in tumors with T-cell exclusion. If this is the case, it will confirm the need to validate and translate therapies targeting drivers of immune resistance and T-cell exclusion such as AXL, etc.
A coming of age for targeted therapies for AXL and TAM RTKs in cancer
In recent years there have been efforts to develop a wide range of therapies targeting AXL and MERTK in cancer (37,52). These developments have complemented our growing understanding of their biology, role in cancer progression, and now their role immune resistance and T-cell exclusion. Most strategies are focused on small molecule inhibitors with a variety of specificities for AXL and other tyrosine kinases. The recently reported phase III study of Cabozantinib, which primarily targets VEGFR2, MET, and AXL (IC50 of 1.3, 0.035, and 5.2 nM respectively), nearly doubled median progression-free survival from 3.8 to 7.4 months in patients with advanced renal-cell carcinoma (53,54). A more specific AXL inhibitor by BerGenBio (BGB324) is currently being evaluated in early phase studies for acute myeloid leukemia with promising initial results showing tolerability and anti-leukemic activity (55). It has an IC50 of 14 nM and biochemical selectivity over Tie-2, MER, TYRO3, RET and FLT-4 of 3, 16, 14, 9, and 5.5 fold, respectively (56). Other AXL targeting approaches include blocking antibodies and a high affinity soluble AXL ‘decoy receptor’ that binds GAS6, which have shown promising results in animal models of metastasis (57,58).
The clinical impact and extent of therapeutic applications remains to be determined, but there is reason to be optimistic about drugs targeting TAM RTKs especially with their emerging role in immuno-oncology. However, it is becoming clear from the preclinical data that drugs and biologics targeting AXL may have a greater impact when combined with radiation, chemotherapy, and/or immunotherapy.
Future studies should be focused on preclinical determination of the optimal combination of cytotoxic and immune modulatory therapies, initiating innovative trials to test the most promising combinations, and evaluating the efficacy and toxicities of these therapeutic approaches. There is an excellent opportunity for anti-AXL therapies combined with radiotherapy and/or immunotherapy. With the potential to target the epithelial to mesenchymal transition, stimulate antigen presentation, and block immune suppressive macrophage recruitment to the tumor microenvironment clinically significant responses may be achieved.
Acknowledgments
Funding: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or funding organizations. The authors are supported by ASTRO Resident Seed Grant# RA2014-3 (TA), the Silicon Valley Foundation, the Sydney Frank Foundation, the Kimmelman Fund (AJG), and the National Cancer Institute of the National Institutes of health under award number T32CA121940 (TAA) and P01CA67166 and R35CA197713 (AJG).
Footnotes
Potential Conflicts of interest: AJG is a Director and Acting Chief Scientific Officer at Aravive Biologics, Inc.
Uncategorized References
- 1.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine. 2012;366(26):2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. The New England journal of medicine. 2011;364(26):2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 5.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 2012;12(4):252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Science translational medicine. 2013;5(200):200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231–5. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 8.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348(6230):74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 9.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gajewski TF. The Next Hurdle in Cancer Immunotherapy: Overcoming the Non-T-Cell-Inflamed Tumor Microenvironment. Seminars in oncology. 2015;42(4):663–71. doi: 10.1053/j.seminoncol.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark WH, Jr, Elder DE, Guerry Dt, Braitman LE, Trock BJ, Schultz D, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81(24):1893–904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. The New England journal of medicine. 2003;348(3):203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 14.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58(16):3491–4. [PubMed] [Google Scholar]
- 15.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 16.Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell. 2016;165(1):35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nature reviews Immunology. 2007;7(1):41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 18.Jones LM, Broz ML, Ranger JJ, Ozcelik J, Ahn R, Zuo D, et al. STAT3 Establishes an Immunosuppressive Microenvironment during the Early Stages of Breast Carcinogenesis to Promote Tumor Growth and Metastasis. Cancer Res. 2016;76(6):1416–28. doi: 10.1158/0008-5472.CAN-15-2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445(7128):656–60. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iannello A, Thompson TW, Ardolino M, Lowe SW, Raulet DH. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J Exp Med. 2013;210(10):2057–69. doi: 10.1084/jem.20130783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431(7007):461–6. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 22.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118(3):285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov. 2016;6(2):202–16. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 25.Garg AD, Romano E, Rufo N, Agostinis P. Immunogenic versus tolerogenic phagocytosis during anticancer therapy: mechanisms and clinical translation. Cell death and differentiation. 2016;23(6):938–51. doi: 10.1038/cdd.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11(18):6713–21. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 27.Le DT, Jaffee EM. Regulatory T-cell modulation using cyclophosphamide in vaccine approaches: a current perspective. Cancer Res. 2012;72(14):3439–44. doi: 10.1158/0008-5472.CAN-11-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zitvogel L, Apetoh L, Ghiringhelli F, Andre F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? The Journal of clinical investigation. 2008;118(6):1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodge JW, Ardiani A, Farsaci B, Kwilas AR, Gameiro SR. The tipping point for combination therapy: cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. Seminars in oncology. 2012;39(3):323–39. doi: 10.1053/j.seminoncol.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11(2 Pt 1):728–34. [PubMed] [Google Scholar]
- 31.Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, et al. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol Res. 2015;3(4):345–55. doi: 10.1158/2326-6066.CIR-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. The Journal of clinical investigation. 2014 doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lake RA, Robinson BW. Immunotherapy and chemotherapy--a practical partnership. Nature reviews Cancer. 2005;5(5):397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 34.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nature reviews Cancer. 2012;12(4):237–51. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finisguerra V, Di Conza G, Di Matteo M, Serneels J, Costa S, Thompson AA, et al. MET is required for the recruitment of anti-tumoural neutrophils. Nature. 2015;522(7556):349–53. doi: 10.1038/nature14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aguilera TA, Rafat M, Castellini L, Shehade H, Kariolis MS, Hui AB, et al. Reprogramming the immunological microenvironment through radiation and targeting Axl. Nat Commun. 2016;7:13898. doi: 10.1038/ncomms13898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu X, Liu X, Koul S, Lee CY, Zhang Z, Halmos B. AXL kinase as a novel target for cancer therapy. Oncotarget. 2014;5(20):9546–63. doi: 10.18632/oncotarget.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graham DK, DeRyckere D, Davies KD, Earp HS. The TAM family: phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nature reviews Cancer. 2014;14(12):769–85. doi: 10.1038/nrc3847. [DOI] [PubMed] [Google Scholar]
- 39.Zizzo G, Hilliard BA, Monestier M, Cohen PL. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. Journal of immunology. 2012;189(7):3508–20. doi: 10.4049/jimmunol.1200662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Camenisch TD, Koller BH, Earp HS, Matsushima GK. A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. Journal of immunology. 1999;162(6):3498–503. [PubMed] [Google Scholar]
- 41.Tibrewal N, Wu Y, D’Mello V, Akakura R, George TC, Varnum B, et al. Autophosphorylation docking site Tyr-867 in Mer receptor tyrosine kinase allows for dissociation of multiple signaling pathways for phagocytosis of apoptotic cells and down-modulation of lipopolysaccharide-inducible NF-kappaB transcriptional activation. J Biol Chem. 2008;283(6):3618–27. doi: 10.1074/jbc.M706906200. [DOI] [PubMed] [Google Scholar]
- 42.Wallet MA, Sen P, Flores RR, Wang Y, Yi Z, Huang Y, et al. MerTK is required for apoptotic cell-induced T cell tolerance. J Exp Med. 2008;205(1):219–32. doi: 10.1084/jem.20062293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crittenden MR, Baird J, Friedman D, Savage T, Uhde L, Alice A, et al. Mertk on tumor macrophages is a therapeutic target to prevent tumor recurrence following radiation therapy. Oncotarget. 2016 doi: 10.18632/oncotarget.11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. The New England journal of medicine. 2012;366(10):925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang C, Welsh JW, de Groot P, Massarelli E, Chang JY, Hess KR, et al. Ipilimumab with stereotactic ablative radiation therapy: Phase I results and immunologic correlates from peripheral T-cells. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–7. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kiess AP, Wolchok JD, Barker CA, Postow MA, Tabar V, Huse JT, et al. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. International journal of radiation oncology, biology, physics. 2015;92(2):368–75. doi: 10.1016/j.ijrobp.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slovin SF, Higano CS, Hamid O, Tejwani S, Harzstark A, Alumkal JJ, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013;24(7):1813–21. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184–043): a multicentre, randomised, double-blind, phase 3 trial. The Lancet Oncology. 2014;15(7):700–12. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crittenden M, Kohrt H, Levy R, Jones J, Camphausen K, Dicker A, et al. Current clinical trials testing combinations of immunotherapy and radiation. Seminars in radiation oncology. 2015;25(1):54–64. doi: 10.1016/j.semradonc.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer. 2016;4:51. doi: 10.1186/s40425-016-0156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cummings CT, Deryckere D, Earp HS, Graham DK. Molecular pathways: MERTK signaling in cancer. Clin Cancer Res. 2013;19(19):5275–80. doi: 10.1158/1078-0432.CCR-12-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, et al. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. The New England journal of medicine. 2015;373(19):1814–23. doi: 10.1056/NEJMoa1510016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10(12):2298–308. doi: 10.1158/1535-7163.MCT-11-0264. [DOI] [PubMed] [Google Scholar]
- 55.Loges S, Gjertsen BT, Heuser M, Ben-Batalla I, Micklem D, Jorg C, Kebenko M, Fiedler WM, Cortes JE. J Clin Oncol. Chicago Il: 2016. A first-in-patient phase I study of BGB324, a selective Axl kinase inhibitor in patients with refractory/relapsed AML and high-risk MDS. p suppl; abstr 2561. [Google Scholar]
- 56.Holland SJ, Pan A, Franci C, Hu Y, Chang B, Li W, et al. R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res. 2010;70(4):1544–54. doi: 10.1158/0008-5472.CAN-09-2997. [DOI] [PubMed] [Google Scholar]
- 57.Ye X, Li Y, Stawicki S, Couto S, Eastham-Anderson J, Kallop D, et al. An anti-Axl monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies. Oncogene. 2010;29(38):5254–64. doi: 10.1038/onc.2010.268. [DOI] [PubMed] [Google Scholar]
- 58.Kariolis MS, Miao YR, Jones DS, 2nd, Kapur S, Mathews II, Giaccia AJ, et al. An engineered Axl ‘decoy receptor’ effectively silences the Gas6-Axl signaling axis. Nat Chem Biol. 2014;10(11):977–83. doi: 10.1038/nchembio.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]