Abstract
Objective
Ventilator-associated events (VAE) are associated with increased mortality, prolonged mechanical ventilation, and longer ICU stay. Given strong national interest in improving ventilated patient care, the NIH and AHRQ funded a two-state collaborative to reduce VAEs. We describe the collaborative’s impact on VAE rates in 56 ICUs.
Design
Longitudinal quasi-experimental study.
Setting
56 intensive care units (ICUs) at 38 hospitals in Maryland and Pennsylvania from October 2012 to March 2015.
Interventions
We organized a multifaceted intervention to improve adherence with evidence-based practices, unit teamwork and safety culture. Evidence-based interventions promoted by the collaborative included head-of-bed elevation, use of subglottic secretion drainage endotracheal tubes, oral care, chlorhexidine mouth care, and daily spontaneous awakening and breathing trials. Each unit established a multi-disciplinary quality improvement team. We coached teams to establish comprehensive unit-based safety programs through monthly teleconferences. Data were collected on rounds using a common tool and entered into a web-based portal.
Measurements and Results
ICUs reported 69,417 ventilated patient-days of intervention compliance observations and 1,022 unit-months of VAE data. Compliance with all evidence-based interventions improved over the course of the collaborative. The quarterly mean VAE rate significantly decreased from 7.34 to 4.58 cases per 1,000 ventilator-days after 24 months of implementation (p=0.007). During the same time period, infection-related ventilator-associated complication (IVAC) and possible and probable ventilator-associated pneumonia (PVAP) rates decreased from 3.15 to 1.56 and 1.41 to 0.31 cases per 1,000 ventilator-days (p=0.018, p=0.012), respectively.
Conclusions
A multifaceted intervention was associated with improved compliance with evidence-based interventions and decreases in VAE, IVAC and PVAP. Our study is the largest to date affirming that best practices can prevent VAEs.
Keywords: mechanical ventilation, ventilator-associated pneumonia, intensive care unit, quality improvement, patient safety, healthcare associated infections
Introduction
Approximately 800,000 hospitalized patients undergo mechanical ventilation each year in the United States, representing 2.7 episodes per 1,000 hospitalizations. These patients are disproportionately older and chronically ill (1–2). They are at risk for multiple complications including pneumonia, pulmonary edema, atelectasis, and acute respiratory distress syndrome. In January 2013, the Centers for Disease Control and Prevention (CDC) released new surveillance definitions for these complications, termed ventilator-associated events (VAE) (3). VAEs are associated with prolonged mechanical ventilation, and increased mortality, antimicrobial use, and intensive care unit (ICU) and hospital lengths of stay (4–7).
Given its high morbidity and mortality, VAE has been proposed as a potential quality metric for public reporting and pay-for-performance. However, there is a paucity of data demonstrating that VAEs are preventable (4,8–10). The National Heart, Lung, and Blood Institute and the Agency for Healthcare Research and Quality therefore funded a two-state collaborative to reduce VAEs by improving unit teamwork and safety culture and enhancing compliance with evidence-based interventions.
Methods
Collaborative Design
The collaborative was organized and led by the Johns Hopkins Armstrong Institute for Patient Safety and Quality in partnership with the Maryland Hospital Association and the Hospital and Healthsystem Association of Pennsylvania. All hospitals in Maryland and Pennsylvania with an adult ICU were invited to participate. ICUs were not compensated for participation. This was a pragmatic study; we did not conduct a power analysis. The Johns Hopkins University School of Medicine Institutional Review Board approved this quality improvement project.
The goals of the collaborative were to decrease VAEs by improving compliance with evidence-based interventions, unit teamwork and patient safety culture. We describe the collaborative’s results between October 2012 and March 2015.
Participating ICUs established multi-disciplinary quality improvement teams, including one physician champion, nurse champion, respiratory therapist, and administrator to implement the multifaceted intervention. Each team identified a leader as the contact point for the collaborative organizers (state leads from each hospital association and Armstrong Institute researchers). ICU teams were encouraged to engage frontline providers in all aspects of the intervention and meet monthly to review progress, identify obstacles, and develop solutions.
Multifaceted Intervention
The multifaceted intervention included training and coaching to improve adherence with evidence-based practices for VAE reduction, implementation of the Comprehensive Unit-based Safety Program (CUSP), and measurement and feedback of performance. It began with a Delphi process to elicit clinicians’ perceptions of the interventions that were most important to include in the prevention bundle. These clinicians did not participate in the collaborative. The structure and results of this process are reported elsewhere (11). We then organized bimonthly followed by monthly training and coaching webinars to educate providers about the six interventions identified by the Delphi process: head-of-bed elevation (HOB), use of subglottic suctioning for endotracheal tubes (Sub-G ETT), oral care (OC) six times per day, chlorhexidine mouth care (CHG) two times per day, performance of spontaneous awakening trials (SAT), and performance of spontaneous breathing trials (SBT).
Teams were encouraged to follow the translating research into practice (TRiP) framework to improve compliance with these interventions (12): 1) identify an evidence-based intervention, 2) identify implementation barriers, 3) measure baseline performance, and 4) ensure all patients receive the intervention by engaging and educating stakeholders, focusing on the local care delivery system rather than individual providers, and reporting performance back to staff and senior leaders.
Teams were also trained to implement CUSP on their unit. CUSP is a five-step iterative program, validated to improve unit safety culture and teamwork (13). Its adoption has led to improvements in previous ICU projects (14). Team leaders provided feedback to collaborative organizers regarding progress during individual quarterly coaching calls. Formal assessments, targeting a spectrum of providers on each unit, were used to dynamically address barriers, celebrate successes, and address education gaps (15). The application of TRiP combined with CUSP has been associated with decreased rates of hospital-acquired infections state- and country-wide (16–19).
A repository of the project materials was accessible to teams on a web-based platform to support implementation. Materials included literature reviews for the evidence-based interventions, brief evidence summaries or ‘fact sheets’ to help educate providers, the CUSP and TRiP toolkits, examples of protocols (e.g. SAT and SBT), and recorded project webinars.
Data Collection
Teams used a standard data collection tool developed by the project team to collect intervention data for all mechanically ventilated patients (Supplemental Figure A.1.). They were encouraged to collect performance data each day during multi-disciplinary morning rounds to maximize provider input and data accuracy. These were then entered or uploaded into a web-based portal weekly. Performance data collection started in October 2012.
VAE data were reported to the CDC National Healthcare Safety Network (NHSN) by infection preventionists. State leads extracted VAE data from NHSN and uploaded these into the data portal. VAE data collection started in January 2013 following the publication of the VAE definitions by the CDC.
Reports on intervention compliance and VAE rates over time with comparative rates from other ICUs were available on the data portal to teams and senior leaders. Teams were coached on effective ways to use these data to catalyze performance improvement.
Participating ICUs were asked to complete the annual Hospital Survey on Patient Safety Culture to evaluate teamwork and safety culture. Team leaders also provided feedback regarding team progress during quarterly implementation assessments. Formal assessments, targeting a spectrum of providers on each unit, were used to dynamically address barriers, celebrate successes, and address gaps in education. The results of these will be reported separately.
Intervention Exposure, Outcome Measures and Hypotheses
The exposure of interest was time since intervention implementation. We divided the study period into an early intervention phase (October 2012–March 2013) and late intervention phase (April 2013–March 2015) and compared performance in the late phase to the early phase. (Supplemental Table A.1). We divided the analysis in this fashion a priori to allow participants two quarters to learn how to use the data collection and reporting tools.
The primary outcomes were VAE, IVAC and PVAP incidence rates per 1,000 ventilator-days (Supplemental Table A.2;(3)). For the early and late intervention periods, compliance with each of the six evidence-based interventions was calculated as the proportion of opportunities for the intervention in which patients received the intervention (Supplemental Table A.3). Our primary hypotheses were that VAE, IVAC and PVAP rates would decrease with intervention implementation, and compliance with the evidence-based interventions would be higher in the late compared to the early intervention phase. Our secondary study hypothesis was that a higher composite measure compliance, defined as the percentage of ventilator-days during which there was compliance with the five interventions measured at patient-day level (HOB, OC, CHG, SAT, SBT), would be associated with decreased VAE rates.
Analysis
For the primary outcomes, we reported numbers of events, ventilator-days, and incidence rates for each event type. To explore the relationship between time since intervention and VAE, IVAC, PVAP rates, we used generalized linear mixed effects models with a log-link and Poisson variance for the quarterly numbers of events, including the log of ventilator-days as an offset term. We adjusted for state (binary), teaching status (binary), location (urban, suburban, rural), hospital size (≤99 beds, 100–499 beds, ≥500 beds) and ICU type (mixed, medical, surgical/trauma, cardiac). Unit was included as a random intercept to account for the longitudinal nature of the data. We modeled the exposure of interest (quarter) as a continuous variable and estimated average intervention effects per quarter.
The first quarter of 2013 was excluded from the primary analysis of VAE rates because teams were establishing infrastructure to collect VAE rates using the definitions introduced in January 2013. We conducted the following sensitivity analyses: including the first quarter of 2013, including only ICUs with complete VAE data, using data submitted prior to September 2014, using negative binomial variance, and for missing data, carrying forward last known values and multiple imputation (with five datasets), respectively.
We reported individual and composite measure compliance for the early and late intervention periods and compared these compliance rates using mixed effects logistic regression models, adjusting for the aforementioned facility characteristics. The relationship between VAE, IVAC, and PVAP rates and the composite measure was investigated using similar regression models as described above, in which the centered compliance rate (times 10) was added as a predictor. We used statistical software R 3.2.2 (20).
Results
In Maryland and Pennsylvania, 56 ICUs from 38 hospitals joined the collaborative. Table 1 summarizes their characteristics. Of 56 participating ICUs, 52 submitted VAE outcomes data between January 2013 and March 2015, providing 1,022 unit-months and 120,519 ventilator-days. Of 416 eligible unit-quarters (52 units times 8 quarters), 113 (27%) were missing VAE data.
Table 1.
Characteristics of Participating Facilities
| Characteristics | Number (%) of Facilities |
|---|---|
| Hospitals | 38 (100) |
| State | |
| Maryland | 20 (53) |
| Pennsylvania | 18 (47) |
| Academic Facility | |
| No | 22 (58) |
| Yes | 16 (42) |
| Hospital size, beds | |
| a. Small (≤99 beds) | 5 (13) |
| b. Medium (100–499 beds) | 28 (74) |
| c. Large (≥500 beds) | 5 (13) |
| Urban/Rural Status | |
| a. Urban | 17 (45) |
| b. Suburban | 16 (42) |
| c. Rural | 5 (13) |
|
| |
| Units | 56 (100) |
| Unit Type | |
| Mixed | 24 (43) |
| Medical | 17 (30) |
| Surgical/Trauma | 9 (16) |
| Cardiac | 6 (11) |
The quarterly mean VAE rate significantly decreased from 7.34 cases per 1,000 ventilator-days during the first study quarter to 4.58 cases after 24 months of implementation (p=0.007; Table 2, Figure 1). During the same time period, IVAC and PVAP rates decreased from 3.15 to 1.56 cases per 1,000 ventilator-days (=0.018) and 1.41 to 0.31 cases per 1,000 ventilator-days (p=0.012), respectively.
Table 2.
Reporting and Ventilator-Associated Event-Related Data by Time Period of Participation
| Calendar Months | # Units Submitted Data | # Unit-Months | # Ventilator-Days | # VAE Events | # IVAC Events | # PVAP Events | VAE Rate | IVAC Rate | PVAP Rate |
|---|---|---|---|---|---|---|---|---|---|
| Apr–Jun 2013 | 52 | 152 | 18385 | 135 | 58 | 26 | 7.34 | 3.15 | 1.41 |
| Jul–Sep 2013 | 47 | 131 | 14116 | 106 | 44 | 18 | 7.51 | 3.12 | 1.28 |
| Oct–Dec 2013 | 36 | 107 | 13181 | 110 | 43 | 18 | 8.35 | 3.26 | 1.37 |
| Jan–Mar 2014 | 36 | 108 | 12868 | 88 | 27 | 9 | 6.84 | 2.1 | 0.7 |
| Apr–Jun 2014 | 36 | 108 | 12164 | 71 | 22 | 7 | 5.84 | 1.81 | 0.58 |
| Jul–Sep 2014 | 36 | 105 | 10900 | 71 | 22 | 7 | 6.51 | 2.02 | 0.64 |
| Oct–Dec 2014 | 35 | 102 | 11199 | 53 | 14 | 3 | 4.73 | 1.25 | 0.27 |
| Jan–Mar 2015 | 25 | 75 | 9608 | 44 | 15 | 3 | 4.58 | 1.56 | 0.31 |
Abbreviations: VAE, ventilator-associated event; IVAC, infection-related ventilator-associated complications; PVAP, probable and possible ventilator-associated pneumonia.
Figure 1. Cohort-level Ventilator-Associated Events Outcome Incidence Rates (per 1,000 ventilator-days) Over Time.
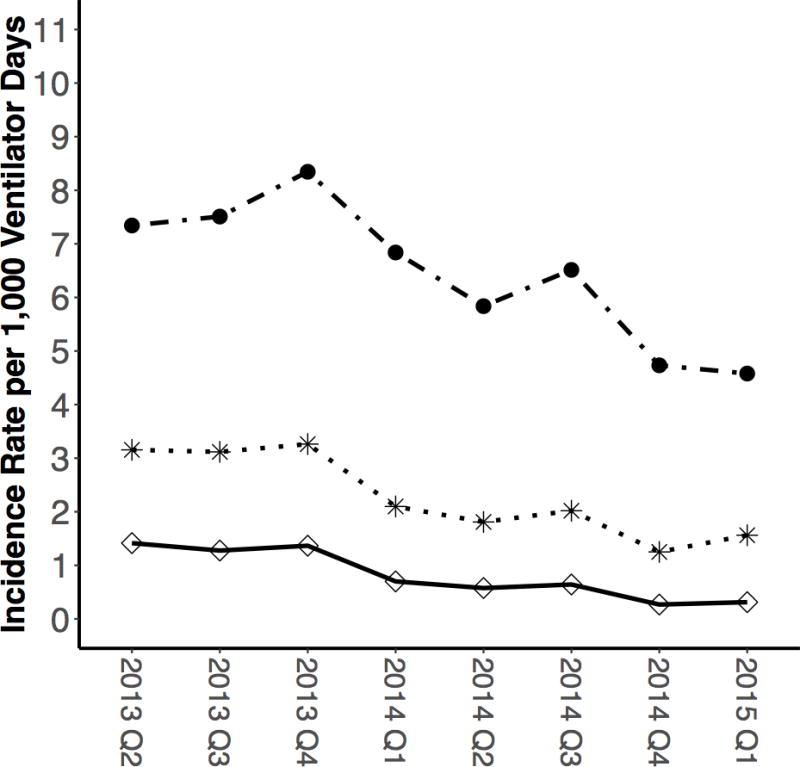

Ventilator-Associated Event (VAE)
Infection-Related Ventilator-Associated Complications (IVAC)
Probable and Possible Ventilator-Associated Pneumonia (PVAP)
Regression models demonstrated a statistically significant decrease in VAE rates over time, with an average 6% (95% CI [2%,9%], p=0.002) quarterly decrease in the incidence rate (Table 3). IVAC and PVAP rates demonstrated average quarterly decreases of 11% (95% CI [6%,16%], p<0.001) and 17% (95% CI [7%,25%], p<0.001), respectively. Sensitivity analyses, including the first quarter of VAE data, complete data, using data submitted prior to September 2014, using Negative Binomial variance, carrying forward last known values, and multiple imputation (Supplemental Tables A.4–A.9), were consistent with the primary analysis.
Table 3.
Estimated Ventilator-Associated Event Incidence Rate Ratios Based on Mixed Effects Poisson Regression Models
| Model Terms | VAE | IVAC | PVAP |
|---|---|---|---|
| Quarter | 0.94 [0.91, 0.98] | 0.89 [0.84, 0.94] | 0.83 [0.75, 0.93] |
|
| |||
| Maryland | Reference | Reference | Reference |
| Pennsylvania | 0.68 [0.32, 1.44] | 0.97 [0.50, 1.89] | 0.94 [0.39, 2.24] |
|
| |||
| Nonteaching | Reference | Reference | Reference |
| Teaching | 0.77 [0.29, 2.07] | 0.69 [0.29, 1.68] | 1.42 [0.48, 4.23] |
|
| |||
| Small (<99 beds) | Reference | Reference | Reference |
| Medium (100–499 beds) | 5.02 [0.92, 27.48] | 6.60 [1.04, 41.99] | NA |
| Large (>=500 beds) | 5.60 [0.90, 34.87] | 7.39 [1.04, 52.65] | NA |
|
| |||
| Urban | Reference | Reference | Reference |
| Suburban | 0.48 [0.21, 1.09] | 0.53 [0.25, 1.15] | 0.77 [0.26, 2.29] |
| Rural | 0.80 [0.19, 3.40] | 1.34 [0.37, 4.86] | 1.27 [0.25, 6.54] |
|
| |||
| Mixed | Reference | Reference | Reference |
| Medical | 0.53 [0.25, 1.11] | 0.33 [0.16, 0.67] | 0.38 [0.15, 0.96] |
| Surgical/Trauma | 1.16 [0.46, 2.94] | 1.58 [0.76, 3.30] | 1.75 [0.70, 4.35] |
| Cardiac | 0.60 [0.20, 1.81] | 0.71 [0.27, 1.87] | 0.44 [0.10, 1.89] |
Abbreviations: VAE, ventilator-associated event; IVAC, infection-related ventilator-associated complications; PVAP, probable and possible ventilator-associated pneumonia.
Of 56 participating ICUs, 51 submitted compliance intervention data between October 2012 and March 2015, supplying 796 unit-months and 69,417 ventilator-days. Of 51 participating units, 50 and 47 units contributed compliance data to the early phase (2 quarters) and the late phase (8 quarters), respectively, and 46 contributed compliance data to both time periods (Table 4). Data were reported for 75 (75%) unit-quarters in the early phase and for 226 (60%) unit-quarters in the late phase.
Table 4.
Compliance with Evidence-Based Interventions
| Intervention Period | Sub-G ETT | HOB | OC | CHG | SAT | SBT |
|---|---|---|---|---|---|---|
| Early Intervention | 29% | 97% | 63% | 79% | 72% | 67% |
| Late Intervention | 36% | 99% | 77% | 88% | 83% | 75% |
|
| ||||||
| P value‡ | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.024 |
Abbreviations: Sub-G ETT, subglottic suctioning endotracheal tubes; HOB, head of the bed; OC, oral care; CHG, Chlorhexidine gluconate; SAT, Spontaneous Awakening Trial; SAT, Spontaneous Breathing Trial.
P values are based on generalized linear mixed effects models with a logit-link and Binomial distribution variance, adjusting for state (binary), teaching status (binary), location (urban, suburban, rural), hospital size (≤99 beds, 100–499 beds, and ≥500 beds) and ICU type (mixed, medical, surgical/trauma, cardiac).
Compliance with each of the six interventions significantly increased from the early to the late phase (Table 4, Supplement Figures A.2 and A.10). The composite measure compliance increased from 14% to 20% from the early to the late phase (p<0.024). Contraindication rates for the interventions remained relatively stable with slight decreases for HOB and Oral Care, and slight increases for CHG and SAT (Supplemental Figure A.3, Tables A.11–A.12).
Regression models suggested that a 10 percentage point increase in the composite measure compliance rate was associated with a 12% VAE decrease (IRR=0.88 [0.78,0.99], p=0.032), and a 16% IVAC decrease (IRR=0.84 [0.71,1.01], p=0.066). No significant relationship between PVAP and the composite measure was observed.
Discussion
In this collaborative study, a multifaceted intervention was associated with improved compliance with six key interventions for ventilated patients and significantly lower VAE, IVAC, and PVAP rates. There was a significant inverse correlation between VAE rates and a composite measure of HOB, OC, CHG, SAT, and SBT. Our study is the largest to date affirming that best practices for ventilated patients can prevent VAEs.
The preventability of VAE and its clinical applicability have been questioned since the CDC published definitions for ventilator-associated complications (22,23). This study supports and adds to the nascent literature on VAE prevention strategies (4,8,21). Muscedere et al. demonstrated that increased concordance with their bundle was associated with decreases in ventilator-associated complications and VAP, and that increased compliance with SAT and SBT was associated with trends towards decreasing VAC (4). The Wake Up and Breathe collaborative demonstrated that coordinated SAT and SBT prevented VAE (21). And, Mekontso et al. showed that a fluid restrictive strategy could prevent VAC (8). Our study builds upon this literature and demonstrates that VAE is preventable in a large, diverse cohort of ICUs.
While care bundle implementation has been associated with improved outcomes, there are no published standards for bundle development. There is debate regarding bundles targeting mechanically ventilated patients (9). Current bundles vary widely with respect to care practices and development approaches. Our collaborative builds on prior work to advance the science of bundle development. This entailed a structured approach to distill numerous expert judgments regarding the interventions that were most important and feasible to implement (11). Our intervention bundle targeted VAP prevention because the evidence base for VAE prevention was immature at the time our study began. Our ventilator bundle aligns with the Society for Healthcare Epidemiology of America’s recommendations to prevent VAP (24).
It has been suggested that VAE and VAP are different complications and VAP prevention bundles cannot simply be applied to target VAE (9,25). We agree; decreasing VAE entails decreasing duration of mechanical ventilation and targeting primary conditions associated with VAE. Our intervention may have been successful because it targeted VAP, one of the most common causes of VAE (7,26–27), and because some of our key interventions, such as SATs and SBTs, can prevent multiple complications of mechanical ventilation (21).
We continue to modify our ventilator bundle based on emerging evidence. Early mobility and low tidal volume interventions are promising candidates to add to ventilator bundles. A recent meta-analysis, by contrast, suggests that CHG may not prevent VAP in non-cardiac patients (29). Further interventions to consider for future bundles include conservative fluid and transfusion strategies (9).
Determining which components of our multifaceted intervention led to its success is challenging, however, the differential improvement rates in bundle components offers some clues. HOB compliance remained high and Sub-G ETT compliance remained low throughout the collaborative, so these were unlikely significantly impactful. By default, then, we suspect OC, CHG, SAT, and SBT had the most impact. Additional analyses are underway to evaluate the relative importance of each intervention in the bundle on VAE rates.
It is possible that units’ participation in such a collaborative is associated with a reduction in VAE outcomes, beyond the effect of the improvement in bundle compliance due to the multifaceted nature of the collaborative. A second potential factor driving success was providing teams with a common surveillance system, including standard definitions for interventions, data collection tools, and a web-based portal with real-time access to performance reports. These tools were provided to providers in the context of a clinical community to foster peer learning, an important attribute of collaborative projects (30). Additional work is necessary to validate the interventions and measurement definitions, but this collaborative took the first step to establish definitions for use in diverse setting outside of research, without sophisticated technology or monetary resources. Prior to this collaborative, many patients were not receiving recommended interventions, despite published guidelines, because the field lacked practical definitions and a feasible measurement infrastructure (24). This hampered efforts to translate evidence into practice. Standard measures, along with scalable processes to collect and report performance data, are mechanisms to reduce preventable harm (31).
The final key collaborative component was pairing a clinical bundle with a practice behavior and culture change intervention (31). To implement the bundle, teams used a change model to translate research into practice (TRiP). TriP’s emphasis on the maintenance of local teams centralizes the technical work and ensures that those delivering care assume ownership of implementation. Executive engagement creates visibility and facilitates the procurement of resources to support the project. TRiP facilitates intervention execution by reducing complexity and providing tools to standardize care. It also encourages robust data collection and feedback to track performance and motivate performance.
To improve safety culture, communication, and teamwork, teams implemented CUSP. Establishing and sustaining a safety culture is a national public health priority (32). Multi-pronged programs like CUSP that promote executive engagement, multidisciplinary rounds, and use of communication tools, improve staff perceptions of safety and teamwork, along with care processes and outcomes (33–35). The implementation of other bundles, combined with TRiP and CUSP, have resulted in improvement in healthcare-associated infections, including central line-associated bloodstream infections and VAP (16–19). We are in the midst of evaluating the association of teamwork and safety culture, intervention performance and VAE rates.
Our study has limitations. First, we did not use a randomized study design, nor a concurrent control group. It is possible VAE decreases were unrelated to our intervention; our results may be influenced by temporal trends or other interventions. Second, we were not able to compare participating ICUs with non-participating ICUs which may have introduced an inclusion bias. Third, validating intervention compliance and VAE data was beyond the scope of this collaborative. Teams may have been motivated to demonstrate improvement and biased results. To minimize this, we standardized data collection techniques and sources and used the existing infrastructure of infection preventionists who routinely collect and report VAE data to NHSN. Fourth, we could not evaluate VAE rates pre- and post-intervention. There was no pre-intervention period because CDC first released VAE definitions in January 2013 and hospitals were not formally collecting these data previously. Fifth, there were missing data which could have biased our results; however, multiple sensitivity analyses (including the first quarter of VAE data, complete data, using data submitted prior to September 2014, using Negative Binomial variance, carrying forward last known values and multiple imputation) demonstrated consistent results. Fifth, we report VAE rates per 1,000 ventilator-days, not episodes, according to the NHSN definition. Interventions that decrease ventilator-days may paradoxically increase VAE rates and underestimate the impact of the intervention on VAE outcomes (9,21). Finally, we were unable to evaluate duration of mechanical ventilation, ICU and hospital lengths of stay, or mortality, though other studies have demonstrated improvements in some of these outcomes (8,21). We did not collect patient identifiers and were unable to reliably estimate total number of patients to calculate duration of mechanical ventilation per patient. In addition, the majority of hospitals lacked the infrastructure to collect these data and did not receive financial support to participate.
Conclusion
We implemented a collaborative that involved a multifaceted intervention across a large and diverse cohort of ICUs in Maryland and Pennsylvania that led to significant decreases in VAE rates. The six technical interventions, or bundle of evidence-based practices, were executed using models designed to change care practices and improve safety culture.
Supplementary Material
Acknowledgments
We thank Tara McFarlin and CECity for their support in data collection and analysis. We also thank the Maryland Hospital Association and the Hospital and Healthsystem Association of Pennsylvania for their leadership.
Financial Support: Funding provided by the Agency for Healthcare Research and Quality (HHSA29032002T) and National Heart, Lung and Blood Institute (1R01HL105903)
Dr. Rawat’s institution received funding from Agency for Healthcare Research and Quality (AHRQ) (HHSA29032002T) and National Institute of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) (1R01HL105903), received support for article research from the NIH and AHRQ, and disclosed paying CECity for being a vendor. Dr. Yang’s institution received funding from AHRQ, the NIH, PCORI, and the Moore Foundation, and she received support for article research from the NIH; she disclosed work for hire. Dr. Ali received support for article research from the NIH. Dr. O Farley received funding from Armstrong Institute, Johns Hopkins Medical School (independent consultant on the Armstrong research team). Dr. Cohen’s institution received funding from AHRQ and the NIH/NHLBI, and she received support for article research from the NIH and AHRQ. Dr. Lubomski’s institution received funding from AHRQ, NIH/NHLBI, and the American Medical Association, and she received support for article research from the NIH. Dr. Thompson’s institution received funding from AHRQ. Dr. Winters’s institution received funding from AHRQ. Dr. Cosgrove received support for article research from the NIH and AHRQ. Dr. Klompas’s institution received funding from AHRQ and the CDC. Dr. Speck’s institution received funding from AHRQ and the NIH/NHLBI, and she received support for article research from the NIH and AHRQ. Dr. Berenholtz’s institution received funding from AHRQ and the NIH/NHLBI, and he received support for article research from the NIH.
Footnotes
Institution Where Research Performed: Johns Hopkins University School of Medicine
Reprints will not be ordered
Copyright form disclosure: Dr. Catanzaro disclosed that she does not have any potential conflicts of interest.
References
- 1.Wunsch H, Linde-Zwirble WT, Angus DC, et al. The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010 Oct;38(10):1947–53. doi: 10.1097/CCM.0b013e3181ef4460. [DOI] [PubMed] [Google Scholar]
- 2.Carson SS, Cox CE, Holmes GM, et al. The changing epidemiology of mechanical ventilation: a population-based study. J Intensive Care Med. 2006 May-Jun;21(3):173–82. doi: 10.1177/0885066605282784. [DOI] [PubMed] [Google Scholar]
- 3.Magill SS, Klompas M, Balk R, et al. Executive summary: Developing a new, national approach to surveillance for ventilator-associated events. Ann Am Thorac Soc. 2013 Dec;10(6):S220–3. doi: 10.1513/AnnalsATS.201309-314OT. [DOI] [PubMed] [Google Scholar]
- 4.Muscedere J, Sinuff T, Heyland DK, et al. The clinical impact and preventability of ventilator-associated conditions in critically ill patients who are mechanically ventilated. Chest. 2013 Nov;144(5):1453–60. doi: 10.1378/chest.13-0853. [DOI] [PubMed] [Google Scholar]
- 5.Boyer AF, Schoenberg N, Babcock H, et al. A prospective evaluation of ventilator-associated conditions and infection-related ventilator-associated conditions. Chest. 2015 Jan;147(1):68–81. doi: 10.1378/chest.14-0544. [DOI] [PubMed] [Google Scholar]
- 6.Zhu S, Cai L, Ma C, et al. The clinical impact of ventilator-associated events: A prospective multi-center surveillance study. Infect Control Hosp Epidemiol. 2015 Dec;36(12):1388–95. doi: 10.1017/ice.2015.200. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi Y, Morisawa K, Klompas M, et al. Toward improved surveillance: the impact of ventilator-associated complications on length of stay and antibiotic use in patients in intensive care units. Clin Infect Dis. 2013 Feb;56(4):471–7. doi: 10.1093/cid/cis926. [DOI] [PubMed] [Google Scholar]
- 8.Mekontso Dessap A, Katsahian S, Roche-Campo F, et al. Ventilator-associated pneumonia during weaning from mechanical ventilation: role of fluid management. Chest. 2014 Jul;146(1):58–65. doi: 10.1378/chest.13-2564. [DOI] [PubMed] [Google Scholar]
- 9.Klompas M. Potential Strategies to Prevent Ventilator-Associated Events. Am J Respir Crit Care Med. 2015 Sep 23; doi: 10.1164/rccm.201506-1161CI. [DOI] [PubMed] [Google Scholar]
- 10.Klompas M, Li L, Szumita P, et al. Associations between different sedatives and ventilator-associated events, length-of-stay, and mortality in mechanically ventilated patients. Chest. 2015 Oct 22; doi: 10.1378/chest.15-1389. [DOI] [PubMed] [Google Scholar]
- 11.Speck K, Rawat N, Weiner NC, et al. A systematic approach for developing a ventilator-associated pneumonia prevention bundle. Am J Infect Control. 2016 Feb 10; doi: 10.1016/j.ajic.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pronovost PJ, Berenholtz SM, Needham DM. Translating evidence into practice: a model for large scale knowledge translation. BMJ. 2008 Oct 6;337:a1714. doi: 10.1136/bmj.a1714. [DOI] [PubMed] [Google Scholar]
- 13.Sexton JB, Berenholtz SM, Goeschel CA, et al. Assessing and improving safety climate in a large cohort of intensive care units. Crit Care Med. 2011 May;39(5):934–9. doi: 10.1097/CCM.0b013e318206d26c. [DOI] [PubMed] [Google Scholar]
- 14.Pronovost PJ, Berenholtz SM, Goeschel C, et al. Improving patient safety in intensive care units in Michigan. J Crit Care. 2008 Jun;23(2):207–21. doi: 10.1016/j.jcrc.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Ali KJ, Farley DO, Speck K, et al. Measurement of implementation components and contextual factors in a two-state healthcare quality initiative to reduce ventilator-associated pneumonia. Infect Control Hosp Epidemiol. 2014 Oct;35(Suppl 3):S116–23. doi: 10.1086/677832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006 Dec 28;355(26):2725–32. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 17.Berenholtz SM, Pham JC, Thompson DA, et al. Collaborative cohort study of an intervention to reduce ventilator-associated pneumonia in the intensive care unit. Infect Control Hosp Epidemiol. 2011 Apr;32(4):305–14. doi: 10.1086/658938. [DOI] [PubMed] [Google Scholar]
- 18.Marsteller JA, Sexton JB, Hsu YJ, et al. A multicenter, phased, cluster-randomized controlled trial to reduce central line-associated bloodstream infections in intensive care units*. Crit Care Med. 2012 Nov;40(11):2933–9. doi: 10.1097/CCM.0b013e31825fd4d8. [DOI] [PubMed] [Google Scholar]
- 19.DePalo VA, McNicoll L, Cornell M, et al. The Rhode Island ICU collaborative: a model for reducing central line-associated bloodstream infection and ventilator-associated pneumonia statewide. Qual Saf Health Care. 2010 Dec;19(6):555–61. doi: 10.1136/qshc.2009.038265. [DOI] [PubMed] [Google Scholar]
- 20.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. https://www.R-project.org/ [Google Scholar]
- 21.Klompas M, Anderson D, Trick W, et al. The preventability of ventilator-associated events. The CDC Prevention Epicenters Wake Up and Breathe Collaborative. Am J Respir Crit Care Med. 2015 Feb 1;191(3):292–301. doi: 10.1164/rccm.201407-1394OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lilly CM, Ellison RT., 3rd Quality measures for critically ill patients: where does ventilator-associated condition fit in? Chest. 2013 Nov;144(5):1429–30. doi: 10.1378/chest.13-1887. [DOI] [PubMed] [Google Scholar]
- 23.Raoof S, Baumann MH. An official multi-society statement: ventilator-associated events–the new definition. Ann Am Thorac Soc. 2014 Jan;11(1):99–100. doi: 10.1513/AnnalsATS.201311-401OT. [DOI] [PubMed] [Google Scholar]
- 24.Yokoe D, Anderson D, Berenholtz S, et al. Compendium of strategies to prevent healthcare-associated infections in acute care hospitals: 2014 update. Infect control Hosp Epidemiol. 2014 Aug;35(8):367–377. doi: 10.1086/677216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis SC, Li L, Murphy MV, et al. Risk factors for ventilator-associated events: a case-control multivariable analysis. Crit Care Med. 2014 Aug;42(8):1839–48. doi: 10.1097/CCM.0000000000000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klompas M, Khan Y, Kleinman K, et al. Multicenter evaluation of a novel surveillance paradigm for complications of mechanical ventilation. PLoS One. 2011 Mar 22;6(3):e18062. doi: 10.1371/journal.pone.0018062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein Klouwenberg PM, van Mourik MS, Ong DS, et al. Electronic implementation of a novel surveillance paradigm for ventilator-associated events. Feasibility and validation. Am J Respir Crit Care Med. 2014 Apr 15;189(8):947–55. doi: 10.1164/rccm.201307-1376OC. [DOI] [PubMed] [Google Scholar]
- 28.CUSP for Mechanically Ventilated Patients – Ventilator Associated Pneumonia. Armstrong Institute for Patient Safety and Quality Portal website. https://jh.community360.net/cusp4mvp.aspx. Published 2012. Accessed March 23, 2016.
- 29.Klompas M, Speck K, Howell MD, et al. Reappraisal of routine oral care with chlorhexidine gluconate for patients receiving mechanical ventilation: systematic review and meta-analysis. JAMA Intern Med. 2014 May;174(5):751–61. doi: 10.1001/jamainternmed.2014.359. [DOI] [PubMed] [Google Scholar]
- 30.Aveling EL, Martin G, Armstrong N, Banerjee J, Dixon-Woods M. Quality improvement through clinical communities: eight lessons for practice. J Health Organ Manag. 2012;26(2):158–74. doi: 10.1108/14777261211230754. [DOI] [PubMed] [Google Scholar]
- 31.Pronovost PJ, Cleeman JI, Wright D, et al. Fifteen years after To Err is Human: A success story to learn from. BMJ Qual Saf. 2015 Dec 15; doi: 10.1136/bmjqs-2015-004720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Patient Safety Foundation. Free from Harm: Accelerating Patient Safety Improvement Fifteen Years after To Err is Human. Boston, MA: National Patient Safety Foundation; 2015. [Google Scholar]
- 33.Weaver SJ, Lubomksi LH, Wilson RF, et al. Promoting a culture of safety as a patient safety strategy: a systematic review. Ann Intern Med. 2013 Mar 5;158(5 Pt 2):369–74. doi: 10.7326/0003-4819-158-5-201303051-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vigorito MC, McNicoll L, Adams L, et al. Improving safety culture results in Rhode Island ICUs: lessons learned from the development of action-oriented plans. Jt Comm J Qual Patient Saf. 2011 Nov;37(11):509–14. doi: 10.1016/s1553-7250(11)37065-1. [DOI] [PubMed] [Google Scholar]
- 35.Sacks GD, Shannon EM, Dawes AJ, et al. Teamwork, communication and safety climate: a systematic review of interventions to improve surgical culture. BMJ Qual Saf [Epub] 2015 May;0:1–10. doi: 10.1136/bmjqs-2014-003764. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


