Abstract
Background
Studies of obesity and survival among breast cancer patients show conflicting results, possibly due to heterogeneity by molecular subtype.
Methods
We examined whether the association of body mass index (BMI) at diagnosis with breast cancer recurrence and survival varies across subtypes defined by PAM50 gene expression. We included 1,559 Kaiser Permanente Northern California members with PAM50 assays aged 18-79 years who were diagnosed with AJCC stage I-III breast cancer from 1996 to 2013. Patients reported weight and height. Cox regression models adjusted for age, menopause, race/ethnicity, stage and chemotherapy.
Results
Over a median of 9-years (maximum 19-years), 378 women recurred and 312 died from breast cancer. Overall, BMI was not associated with breast cancer recurrence or survival controlling for subtype (e.g., the hazard ratio [HR] and 95% confidence interval [CI] per 5-kg/m2 was 1.05 [0.95, 1.15]) for breast cancer-specific death). However, associations varied by subtype. Among Luminal A cancers, women with class II/III obesity, but not class I obesity or overweight, had worse outcomes: comparing BMI≥35-kg/m2 versus 18.5-<25-kg/m2, the HR (95%CI) was 2.24 (1.22, 4.11) for breast cancer-specific death and 1.24 (1.00, 1.54) for recurrence. There was no association for Luminal B, Basal-like, or human epidermal growth factor over-expressing subtypes.
Conclusions
Among breast cancer patients with accurately classified subtypes based on gene expression, BMI≥35-kg/m2 was adversely associated with outcomes only among Luminal A cancers. Research is needed on whether tailoring recommendations for weight management to tumor characteristics will improve outcomes.
Keywords: Body mass index, obesity, breast cancer subtype, molecular classification, survival, recurrence, mortality, PAM50 gene expression assay
INTRODUCTION
Though pre-diagnosis obesity is an established risk factor for post-menopausal breast cancer, studies of obesity and breast cancer survival have heterogeneous results.1, 2 Potential reasons for these inconsistencies include methodological differences (e.g., exposure timing, control for confounding by physical activity or co-morbidities, etc.).1 Additionally, there may be threshold effects: associations are often strongest for (or do not emerge until) BMI≥35-kg/m2.3-6 Yet another consideration is that breast cancer is typically treated as a single disease, ignoring the biologic diversity in tumor characteristics that influences outcomes like recurrence and breast cancer death.7 The limited research within subtypes suggests heterogeneity in the influence of obesity on breast cancer outcomes, with obesity often appearing detrimental only for select subtypes.4, 8-16 This raises the possibility that certain subtypes may be more responsive to lifestyle intervention. To shed light on the biologic pathways through which obesity influences breast cancer outcomes, accurate classification of subtypes is essential.
Gene expression profiling, the gold standard for intrinsic subtyping, improves prognostication and predicts therapeutic response better than clinicopathologic subtypes.17-19 Without gene expression data, researchers often assign subtypes from estrogen receptor (ER) and progesterone receptor (PR) status, and/or human epidermal growth factor receptor 2 (Her2), proliferation markers or tumor grade. These subtypes do not always align with intrinsic subtypes classified by gene expression-based assays.17-19 PAM50 subtyping more accurately characterizes the spectrum of breast cancer biology and is more prognostic for outcome than immunohistochemistry (IHC) methods.19 Previously, we applied the PAM50 assay to archived tumor tissue from the Life After Cancer Epidemiology (LACE) and Pathways studies; the PAM50 assay is a real-time reverse-transcription PCR (qRT-PCR) intrinsic subtyping classifier that measures expression of 50 genes representative of 5 breast cancer intrinsic subtypes.17, 20
This is the largest study to evaluate the association of BMI and breast cancer outcomes within PAM50 subtypes16 and the first to control for confounders or examine whether comorbidities, physical activity or disease characteristics explain the association of BMI with breast cancer outcomes within subtypes. Understanding whether BMI influences breast cancer survival within accurately classified subtypes could inform a precision medicine approach which tailors lifestyle recommendations to the molecular characteristics of individual patient's tumor.
MATERIALS AND METHODS
Study Overview
We included AJCC stage I-III breast cancer patients from a population-based, prospective cohort at the Kaiser Permanente Northern California (KPNC) composed of the LACE21 and Pathways22 studies. LACE women were 18-79-years old at diagnosis (1996-2000) and completed chemotherapy by enrollment (<39-months post-diagnosis; on average, 23-months from diagnosis to enrollment). Pathways women were ≥21-years old at diagnosis (2005–2013) and enrolled on average 2-months post-diagnosis.
Women were followed until June 30, 2015, and censored at the recurrence date or death, ascertained combining self-report, the electronic medical record (EMR), and KPNC mortality files. Outcomes were verified by medical record review. Cause of death was from death certificates. Participants provided informed consent; KPNC institutional review boards approved the protocols.
Classification of Intrinsic Subtypes by PAM50 Gene Expression
Of the 6,641 patients enrolled, 1,691 tumors underwent PAM50 molecular subtyping using a stratified case-cohort study design. ER, PR, and Her2 statuses (determined via IHC and fluorescence in situ hybridization [Her2]) defined the sampling strata. All ER− and PR− or Her2+ tumors were sampled, as well as a random 18% sample of common phenotypes (ER+ or PR+ and Her2−);17, 18 characteristics of the stratified random sample and the process of conducting the assays are published.17, 18, 20 In brief, qRT-PCR was conducted for 50 target genes comprising the PAM50 intrinsic subtype classified by laboratory personnel blinded to patient information. Applying centroid-based algorithms to the calibrated log-expression ratio for the 50 genes in the PAM50 assay resulted in five continuous-scale normalized subtype scores representing degree of Spearman correlation of gene expression with that of prototype Luminal A, Luminal B, Basal-like, and Her2 overexpressing (Her2-E) breast tumors. The subtype with the highest score became the predicted intrinsic subtype for that case. Compared to IHC classification, PAM50 subtyping distinguishes between Luminal A and B (ER+) tumors and more accurately classifies Basal-like and Her2-E tumors. Results from the GEICAM-9906 trial show that as part of this more accurate characterization of breast cancer biology, nearly one-third of tumors classified as triple-negative by IHC were classified as Her2-E by PAM50; approximately two-thirds of clinically Her2+ samples are Her2-E subtype by PAM50 with the remainder classified as Luminal B. Luminal B tumors are mainly distinguished from Luminal A as more proliferative and less frequently PR+.19
Body Mass Index and Covariates
On enrollment questionnaires, LACE women reported current height and recalled weight 1-year pre-diagnosis. Pathways women reported current height and weight at enrollment (<7-months post-diagnosis). Measured heights and weights were first electronically recorded in 2005; thus self-reported measures were used for “at-diagnosis” BMI. Women reported race/ethnicity and moderate/vigorous recreational physical activity in the preceding 6 months.23 Patient age, stage, co-morbidities in the Charlson Index,24 menopausal status and treatment information (receipt of adjuvant/neoadjuvant chemotherapy, hormonal or radiation therapy) were from questionnaires, EMR and the KPNC Cancer Registry.
Statistical Analysis
We excluded missing PAM50 or IHC data (n=56), normal-like subtypes (n=52), missing BMI (n=11) and BMI <18.5-kg/m2 (n=13), leaving n=1,559. Analyses were weighted to account for the stratified sampling design for unbiased estimation of population parameters and standard errors.25, 26 We computed cumulative incidence of breast cancer death by BMI at diagnosis (Figure 1). Descriptive statistics used the surveymeans procedure in SAS-9.3. We used Cox proportional hazards regression accounting for delays between diagnosis and cohort entry (0-3 years post-diagnosis) to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for continuous (5-kg/m2 increase) and categorical BMI (>18.5-<25-kg/m2, 25-<30-kg/m2, 30-<35-kg/m2, and ≥35-kg/m2) with recurrence and breast cancer-specific death.
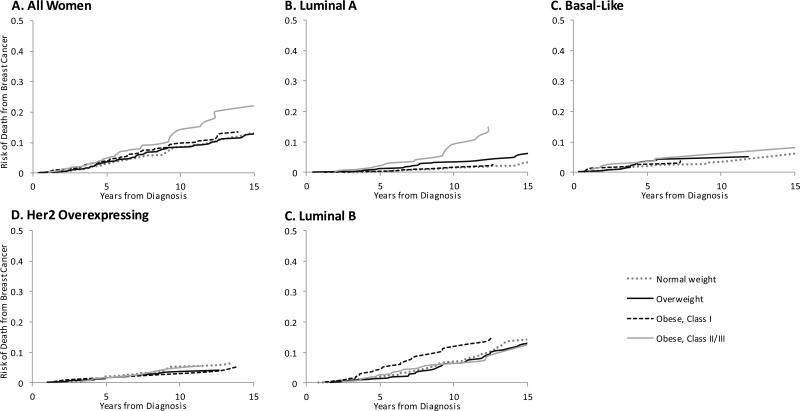
Figure 1.
Cumulative Risk of Death from Breast Cancer by Body Mass Index at Diagnosis (n=1,559)
We examined associations controlling for PAM50 subtype, then stratified analyses by subtype.17 Models adjusted for potential confounders selected a priori based on previous literature: age (<50-years, 50-<60-years, 60-<70-years or ≥70-years), menopausal status, race/ethnicity (non-Hispanic white, Asian/Pacific Islander, Hispanic/Latina or Black/African American), study, stage and any receipt of chemotherapy. As adjustment for radiation and hormone therapy did not alter the associations they were excluded from models. Due to limited power and common pathways (e.g., endogenous estrogen),27 we grouped Luminal versus non-Luminal breast cancers in sensitivity analyses. To explore mediators, we controlled for physical activity and co-morbidities at diagnosis in secondary analyses.
RESULTS
Table 1 shows characteristics by BMI category accounting for sampling weights. Most women had stage I (50%) or II (43%) breast cancer; 562 (54%) had Luminal A, 345 (20%) had Luminal B, 342 (15%) Her2-E, and 310 (11%) Basal-like subtypes. Mean (Standard Deviation, SD) BMI was 28 (8)-kg/m2. Mean (SD) age was 58 (15) years. Compared to normal-weight women, women with Class II/III obesity were more likely to have co-morbidities and Stage II/III cancer, and less likely to exercise or be non-Hispanic white. Of note, the stage distribution within BMI categories differed by subtype: among the Luminal breast cancers a smaller proportion of obese (versus non-obese) women were stage I (46% versus 54%); this pattern was less apparent among women with non-Luminal breast cancers where 40% were stage I regardless of obesity.
Table 1.
Weighted Participant Characteristics in PAM50 Sample by Body Mass Index at Breast Cancer Diagnosis, LACE and Pathways Studies
| All Women n=1,559 | Normal-weight, n=620 BMI: 18.5-<25-kg/m2 |
Overweight, n=468 BMI: 25-<30-kg/m2 |
Obese, Class I, n=271 BMI: ≥30-<35-kg/m2 |
Obese, Class II/III, n=200 BMI: ≥35-kg/m2 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unweighted N1 | Weighted Mean2 | SD | N | Weighted Mean2 | SD | N | Weighted Mean2 | SD | N | Weighted Mean2 | SD | N | Weighted Mean2 | SD | |
| Age, years | 1559 | 58.13 | 14.74 | 620 | 57.13 | 14.8 | 468 | 59.06 | 15.15 | 271 | 58.81 | 14.76 | 200 | 58.17 | 12.9 |
| BMI, kg/m2 | 1559 | 27.63 | 8.12 | 620 | 22.41 | 2.43 | 468 | 27.16 | 1.75 | 271 | 32.14 | 2.08 | 200 | 39.78 | 5.53 |
| Unweighted N1 | Weighted Proportion2 | N | Weighted Proportion | N | Weighted Proportion | N | Weighted Proportion | N | Weighted Proportion | |
|---|---|---|---|---|---|---|---|---|---|---|
| Physically active4 | 639 | 0.41 | 327 | 0.52 | 173 | 0.37 | 89 | 0.33 | 48 | 0.24 |
| Co-morbidities5 | 201 | 0.13 | 56 | 0.09 | 75 | 0.16 | 35 | 0.13 | 37 | 0.19 |
| Study | ||||||||||
| LACE | 860 | 0.47 | 386 | 0.54 | 261 | 0.45 | 125 | 0.41 | 88 | 0.34 |
| Pathways | 699 | 0.53 | 234 | 0.46 | 207 | 0.55 | 146 | 0.59 | 112 | 0.66 |
| Menopausal | ||||||||||
| Post-menopausal | 1030 | 0.67 | 398 | 0.66 | 325 | 0.68 | 175 | 0.67 | 132 | 0.70 |
| Pre-menopausal | 418 | 0.26 | 171 | 0.26 | 110 | 0.25 | 84 | 0.29 | 53 | 0.24 |
| Unknown | 111 | 0.07 | 51 | 0.08 | 33 | 0.07 | 12 | 0.04 | 15 | 0.06 |
| Race/ethnicity | ||||||||||
| Non-Hispanic white | 1127 | 0.72 | 459 | 0.76 | 349 | 0.73 | 195 | 0.72 | 124 | 0.58 |
| Black | 124 | 0.06 | 23 | 0.03 | 34 | 0.05 | 29 | 0.10 | 38 | 0.15 |
| Asian/Pacific Islander | 127 | 0.09 | 33 | 0.04 | 37 | 0.10 | 29 | 0.10 | 28 | 0.19 |
| Hispanic/Latino | 146 | 0.10 | 90 | 0.15 | 40 | 0.10 | 13 | 0.05 | 3 | 0.02 |
| Other | 35 | 0.02 | 15 | 0.02 | 8 | 0.01 | 5 | 0.03 | 7 | 0.05 |
| Treatment | ||||||||||
| Chemotherapy | 980 | 0.54 | 396 | 0.56 | 289 | 0.53 | 160 | 0.47 | 135 | 0.60 |
| Radiation therapy | 769 | 0.50 | 305 | 0.50 | 225 | 0.51 | 136 | 0.51 | 103 | 0.52 |
| Stage | ||||||||||
| I | 672 | 0.49 | 281 | 0.52 | 213 | 0.49 | 111 | 0.49 | 67 | 0.37 |
| II | 762 | 0.44 | 301 | 0.42 | 224 | 0.44 | 127 | 0.42 | 110 | 0.51 |
| III | 125 | 0.07 | 38 | 0.06 | 31 | 0.06 | 33 | 0.09 | 23 | 0.12 |
| PAM50 | ||||||||||
| Luminal A | 562 | 0.54 | 245 | 0.58 | 162 | 0.51 | 101 | 0.59 | 54 | 0.37 |
| Luminal B | 345 | 0.20 | 126 | 0.18 | 106 | 0.23 | 71 | 0.19 | 42 | 0.25 |
| Basal-Like | 310 | 0.11 | 102 | 0.09 | 94 | 0.11 | 56 | 0.12 | 58 | 0.20 |
| HER2-Overexpressing | 342 | 0.15 | 147 | 0.16 | 106 | 0.15 | 43 | 0.10 | 46 | 0.18 |
Sample sizes (raw “n”) are unweighted
Means, standard deviations and proportions are weighted to account for stratified case-cohort study design with strata defined as IHC clinical subtype
3 Metabolic Equivalent Task (MET) ≥13 hours/week from recreational activities
Co-morbidities represents the presence at diagnosis of >=1 of the co-morbidities that make up the Charlson Index
During a median follow-up of 9-years (maximum 19-years), there were 378 recurrences and 544 deaths, 312 from breast cancer. Figure 1 shows incidence of breast cancer-specific death by BMI category. The dose-response was clearest for Luminal A cancers, with breast cancer death increasing with BMI. There was a suggestion of an elevated risk with Class I obesity among Luminal B subtypes, and no indication of any increased risk with higher BMI for Her2-E or Basal-like subtypes.
When all breast cancer cases were examined together with adjustment for subtype, BMI had no association with recurrence or breast cancer death (Table 2): the HR (95%CI) per 5-kg/m2 increase in BMI was 1.05 (0.95-1.15) for breast cancer death and 1.03 (0.92-1.13) for recurrence.
Table 2.
Body Mass Index at Diagnosis and Recurrence and Survival by PAM50 Subtype (n=1,559)
| Breast Cancer-Specific Survival | Recurrence | |||||||
|---|---|---|---|---|---|---|---|---|
| All women, adjusted | Deaths1 | HR2,3 | 95%CI | Recurrences | HR | 95%CI | ||
| 18.5-<25-kg/m2 | 119 | Reference | 147 | Reference | ||||
| 25-<30-kg/m2 | 90 | 0.94 | 0.71 | 1.24 | 111 | 0.94 | 0.69 | 1.29 |
| 30-<35-kg/m2 | 57 | 0.98 | 0.71 | 1.36 | 66 | 0.98 | 0.67 | 1.43 |
| ≥35-kg/m2 | 46 | 1.06 | 0.74 | 1.52 | 54 | 1.02 | 0.67 | 1.54 |
| Per 5-unit BMI | 312 | 1.05 | 0.95 | 1.15 | 378 | 1.03 | 0.92 | 1.14 |
| Luminal A | ||||||||
| 18.5-<25-kg/m2 | 43 | Reference | 52 | Reference | ||||
| 25-<30-kg/m2 | 31 | 1.36 | 0.84 | 2.19 | 39 | 1.43 | 0.78 | 2.62 |
| 30-<35-kg/m2 | 17 | 1.29 | 0.72 | 2.33 | 18 | 1.05 | 0.50 | 2.18 |
| ≥35-kg/m2 | 19 | 2.24 | 1.22 | 4.11 | 21 | 2.17 | 0.92 | 5.11 |
| Per 5-unit BMI | 110 | 1.31 | 1.11 | 1.54 | 130 | 1.24 | 1.00 | 1.54 |
| Luminal B | ||||||||
| 18.5-<25-kg/m2 | 32 | Reference | 39 | Reference | ||||
| 25-<30-kg/m2 | 26 | 0.62 | 0.36 | 1.06 | 33 | 0.67 | 0.27 | 1.71 |
| 30-<35-kg/m2 | 25 | 1.39 | 0.80 | 2.41 | 30 | 1.69 | 0.62 | 4.64 |
| ≥35-kg/m2 | 11 | 0.61 | 0.29 | 1.29 | 13 | 0.58 | 0.15 | 2.21 |
| Per 5-unit BMI | 94 | 0.99 | 0.83 | 1.18 | 115 | 0.98 | 0.71 | 1.35 |
| Basal-Like | ||||||||
| 18.5-<25-kg/m2 | 17 | Reference | 18 | Reference | ||||
| 25-<30-kg/m2 | 18 | 1.23 | 0.63 | 2.43 | 20 | 1.18 | 0.55 | 2.57 |
| 30-<35-kg/m2 | 8 | 0.52 | 0.22 | 1.26 | 10 | 0.72 | 0.14 | 3.68 |
| ≥35-kg/m2 | 9 | 0.67 | 0.28 | 1.59 | 11 | 0.75 | 0.25 | 2.25 |
| Per 5-unit BMI | 52 | 0.90 | 0.73 | 1.11 | 59 | 0.93 | 0.74 | 1.17 |
| Her2-Overexpressing | ||||||||
| 18.5-<25-kg/m2 | 27 | Reference | 38 | Reference | ||||
| 25-<30-kg/m2 | 15 | 0.78 | 0.40 | 1.51 | 19 | 0.66 | 0.17 | 2.50 |
| 30-<35-kg/m2 | 7 | 0.69 | 0.28 | 1.71 | 8 | 0.54 | 0.06 | 5.16 |
| ≥35-kg/m2 | 7 | 0.89 | 0.37 | 2.15 | 9 | 0.79 | 0.21 | 2.99 |
| Per 5-unit BMI | 56 | 0.92 | 0.71 | 1.20 | 74 | 0.87 | 0.53 | 1.41 |
Number of events is unweighted.
Hazard ratios and confidence intervals are weighted to account for stratified case-cohort study design with strata defined as IHC clinical subtype.
Adjusted for for age, menopausal status, race/ethnicity, study, stage and receipt of any chemotherapy.
Stratifying by subtype, BMI did not influence outcomes for Basal-like or HER2-E cancers. In Luminal B cancers there was a (non-significant) suggestion of an increased risk for Class I obesity. By contrast, among women with Luminal A cancers, each 5-kg/m2 higher BMI was associated with a 31% increased risk of death from breast cancer (HR=1.31; 95%CI: 1.11-1.54) and a 24% increased risk of recurrence (HR=1.24; 95%CI: 1.00-1.54). Associations among Luminal A cancers were driven by Class II/III obesity (BMI≥35-kg/m2): the HR (95%CI) was 2.24 (1.22-4.11) for breast-cancer death and 2.17 (0.92-5.11) for recurrence compared to normal-weight.
Sensitivity analyses grouped Luminal tumors: among Luminal subtypes, higher BMI increased risk of breast cancer recurrence (eTable 1) and death (eTable 1; Figure 2) in a dose-response fashion (HR=1.21; 95%CI: 1.04-1.42 for breast cancer death per 5-kg/m2). There was no apparent relationship between higher BMI and breast cancer outcomes among non-Luminal subtypes (HR=0.90; 95%CI: 0.71-1.14).
Figure 2.
Body Mass Index at Diagnosis and Breast Cancer-Specific Survival among Luminal and Non-Luminal Breast Cancers (n=1,559)
Hazard ratios and confidence intervals are weighted to account for stratified case-cohort study design with strata defined as IHC clinical subtype and adjusted for age, menopausal status, race/ethnicity, study, stage and chemotherapy.
Additional adjustment for the explanatory variables -co-morbidities and moderate/vigorous physical activity- did not alter the results (eTable 2).
DISCUSSION
This is the largest prospective cohort to examine BMI and breast cancer outcomes among breast cancer survivors separately by PAM50 subtype, and the first to do so adjusting for confounders. Among women with Luminal A subtypes, Class II/III obesity at diagnosis doubled the risk of breast cancer death. However, there was no significant association for overweight or mild obesity or for other subtypes. One prior study with PAM50 gene expression assays examined BMI,16 and this study did not adjust for any covariates when reporting associations by subtype: consistent with our results, Ligibel et al. found no significant associations with BMI among Luminal B, Basal-like or HER2-E subtypes.16 The unadjusted HR (95%CI) for recurrence-free survival in Luminal A cancers was 1.23 (1.08-1.40) per 5-kg/m2, very close to the unadjusted HR (95%CI) of 1.24 (1.06-1.46) in our study (multivariable-adjusted HR in Table 2). These studies demonstrate that stratifying tumors into biologically homogenous categories identifies patients whose cancer outcomes are influenced by obesity (Luminal).
One hypothesized mechanism linking obesity to breast cancer outcomes is increased endogenous estrogen.2, 27 Supporting the importance of estrogen, prospective cohort studies find post-menopausal obesity increases risk of invasive breast cancer for ER+/PR+ (not ER−/PR−) tumors, particularly among non-users of postmenopausal hormone therapy.28-31 The limited data examining BMI within IHC subtypes often identifies obesity as a prognostic factor for ER+/PR+ tumors only.10 While increased endogenous estrogen could fuel the growth of ER+ tumors, in our study BMI was only associated with breast cancer outcomes among Luminal A subtypes: there was no statistically significantly increased risk in Luminal B subtypes, which are also ER+ though to a lesser extent.32 However, stratifying by Luminal versus non-Luminal tumors (Figure 2), risk of breast cancer death increased with increasing BMI among Luminal cancers. This is consistent with an estrogen mechanism.
Additional hypotheses for how obesity influences prognosis include diagnosis at later stages and inflammation and insulin resistance, which contribute to disease progression.2, 27 Indeed, obese women with Luminal breast cancers tended to have later stage at diagnosis, which could indicate later detection (e.g., non-compliance with recommended screenings) or faster-growing tumors. However, the association of higher BMI with adverse outcomes in Luminal breast cancer remained after adjustment for stage (Table 2), suggesting stage does not explain the relationship. Adjustment for physical activity or co-morbidities at diagnosis (including diabetes) also did not alter the conclusions (eTable 2). While physical activity or co-morbidities are related to inflammation and insulin resistance they are not direct measurements: systemic, low-grade inflammation may still be a major pathway through which obesity influences outcomes in Luminal A breast cancer.
Another potential explanation for our findings is that aggressive tumor subtypes operate through faster-acting pathways that are weakly to BMI: aggressive subtypes like Luminal B, Basal-like and Her2-E have high risk of early recurrence (<5 years),17 and the underlying biology of these highly proliferative tumors may not be readily modifiable. Meanwhile, Luminal A cancers have a higher risk of late recurrence (>5 years)17 and BMI may more readily effect tumor growth through estrogen and other pathways in this more indolent cancer. Of note, even in ER+ tumors with good prognosis, greater proliferation markers distinguish those at risk of late recurrence.33 A prior study in our PAM50 cohort found that Class II obesity was associated with a higher proliferation score,20 suggesting that even within a given subtype obese women have more proliferative tumors and thus greater probability of recurrence/death. This hypothesis is consistent with our observation that among Luminal A tumors, the cumulative incidence curves for breast cancer death diverged around 5 years post-diagnosis (Figure 1B), with obese women assuming the highest risk. Further, a study examining ER+ breast cancers found that class II/III obesity were associated with increased risk of late recurrence: HRs (95%CIs): 1.40 (1.05-1.86) and 1.41 (1.02-1.93), respectively.4 While it is possible that cumulative exposure to adiposity over a woman's lifetime defines tumor characteristics and therefore its effects on breast cancer outcomes are not modifiable, it is also possible that for slower-growing tumors (e.g., Luminal A) at-diagnosis BMI influences tumor progression.27
The lack of a significant association of BMI with cancer outcomes among more aggressive subtypes raises the question of whether lifestyle interventions will be equally effective for these patients, a question that cannot be answered by examining a BMI at a single time-point as in our study. While an ongoing randomized controlled trial of weight loss among overweight/obese breast cancer survivors may partially answer this question, the trial excludes Her2+ cancers.34 An alternative approach would be to tailor lifestyle intervention to those patients most likely to derive benefit. Supporting the potential of a precision oncology approach targeted by tumor subtype, a previous study in our cohort found that Luminal A cancers were preferentially responsive to exercise.35 These results provide additional support for the idea that the impact of obesity and obesity-related behaviors on breast cancer outcomes could differ according to the molecular features of the tumor.
A limitation of the present study is that BMI does not distinguish lean from fat mass, nor describe fat distribution; this is especially true among older adults with chronic diseases.36, 37 Higher BMI at diagnosis could indicate larger muscle reserves to withstand the catabolic demands of an aggressive tumor and accompanying treatment. While we had limited power for rarer subtypes, this is consistent with the non-significantly protective relationship of BMI and breast cancer outcomes among Her2-E cancers in our study. This suggests BMI does not adequately measure adiposity in the setting of catabolic disease; of note, studies with alternative measures of adiposity such as waist-to-hip ratio found more consistent mortality associations within and across IHC subtypes.9 Body composition assessment could yield biologically relevant measures of fat and lean mass that predict breast cancer outcomes among women with aggressive subtypes where BMI cannot.
This study measured BMI at a single time-point, yet changes in weight and body composition over time may have the greatest influence on cancer outcomes. The extent of these changes differs by subtype and BMI at diagnosis: we previously found in a large cohort of early-stage breast cancer survivors that post-diagnosis weight loss was common (20% of women), disproportionately experienced by obese women and women with non-Luminal subtypes, and associated with reduced survival.38 Pre and post-diagnosis weight changes could explain the null association of obesity with outcomes among women with aggressive subtypes: the normal-weight group might include women who lost weight pre-diagnosis and migrated down BMI categories, and the obese group might contain women who go on to lose weight after diagnosis.
This is the largest study to examine the association of BMI with breast cancer outcomes by PAM50 subtype with adjustment for confounders such as race/ethnicity and disease stage. Further, our long duration of follow-up (up to 19 years) enabled observation of late recurrence in Luminal A subtypes. This research contributes new information by examining whether physical activity or co-morbidities explain BMI-survival associations. Importantly, though the point estimates for the rarer, non-Luminal subtypes did not suggest an adverse association of BMI with breast cancer outcomes, we had low power to detect associations within these subtypes. Due to limited sample size we also did not evaluate associations within subgroups defined by both subtype and menopausal status. While 72% of women in our study were non-Hispanic white, this cohort has more racial/ethnic diversity than previous studies, enhancing generalizability. Additionally, women were recruited from medical centers, reducing confounding by health care access but potentially limiting generalizability to the medically-insured. Though weights were self-reported, they correlated well with measured weights among women for whom both were available, overall (n=490; r=0.98) and within subgroups. In all observational research, unmeasured confounding is possible, e.g., weight history prior to diagnosis could partially explain the association of at-diagnosis obesity and breast cancer outcomes.
CONCLUSION
For patients with Luminal A breast cancer, extreme obesity at diagnosis is associated with increased risk of recurrence and breast cancer death. Future research should seek to understand both the impacts of weight change by subtype on breast cancer outcomes and whether direct measurement of body composition outperforms BMI as a predictor of breast cancer outcomes across different biologic subtypes.
Supplementary Material
Acknowledgments
Funding: Supported by National Cancer Institute awards R01CA129059, R01CA105274 and U01 CA195565.
Footnotes
Conflicts of interest: Dr. Bernard is an inventor of the PAM50 gene expression signature, which was licensed to Nanostring for commercialization of the Prosigna breast test.
Contributions: All authors contributed to study conception and design, data interpretation, and approved the manuscript. EMCF wrote the manuscript. EMCF and EKW analyzed the data.
In the largest prospective study to examine the association of obesity and breast cancer outcomes separately by PAM50 subtype, extreme obesity doubled the risk of breast cancer death among women with Luminal A tumors; there was no association of BMI and breast cancer outcomes for other subtypes.
Future research should investigate the potential of a precision oncology approach that targets lifestyle intervention according to individual characteristics of the patient and disease.
REFERENCES
- 1.Chan DS, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25:1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast cancer research and treatment. 2010;123:627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 3.Ligibel JA, Winer E, Hudis CA, Barry WT. Response. J Natl Cancer Inst. 2016:108. doi: 10.1093/jnci/djv334. [DOI] [PubMed] [Google Scholar]
- 4.Nechuta S, Chen WY, Cai H, et al. A pooled analysis of post-diagnosis lifestyle factors in association with late estrogen-receptor–positive breast cancer prognosis. International Journal of Cancer. 2015 doi: 10.1002/ijc.29940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwan ML, Quesenberry CP, Jr., Caan BJ. RE: Body Mass Index, PAM50 Subtype, and Outcomes in Node-Positive Breast Cancer: CALGB 9741. J Natl Cancer Inst. 2016:108. doi: 10.1093/jnci/djv333. [DOI] [PubMed] [Google Scholar]
- 6.Kwan ML, Chen WY, Kroenke CH, et al. Pre-diagnosis body mass index and survival after breast cancer in the After Breast Cancer Pooling Project. Breast cancer research and treatment. 2012;132:729–739. doi: 10.1007/s10549-011-1914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turkoz FP, Solak M, Petekkaya I, et al. The prognostic impact of obesity on molecular subtypes of breast cancer in premenopausal women. J BUON. 2013;18:335–341. [PubMed] [Google Scholar]
- 9.Sun X, Nichols HB, Robinson W, Sherman ME, Olshan AF, Troester MA. Post-diagnosis adiposity and survival among breast cancer patients: influence of breast cancer subtype. Cancer Causes Control. 2015;26:1803–1811. doi: 10.1007/s10552-015-0673-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sparano JA, Wang M, Zhao F, et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer. 2012;118:5937–5946. doi: 10.1002/cncr.27527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson PJ, Bell RJ, Davis SR. Obesity is associated with a poorer prognosis in women with hormone receptor positive breast cancer. Maturitas. 2014;79:279–286. doi: 10.1016/j.maturitas.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Mowad R, Chu QD, Li BD, Burton GV, Ampil FL, Kim RH. Does obesity have an effect on outcomes in triple-negative breast cancer? J Surg Res. 2013;184:253–259. doi: 10.1016/j.jss.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 13.Mazzarella L, Disalvatore D, Bagnardi V, et al. Obesity increases the incidence of distant metastases in oestrogen receptor-negative human epidermal growth factor receptor 2-positive breast cancer patients. Eur J Cancer. 2013;49:3588–3597. doi: 10.1016/j.ejca.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Dawood S, Lei X, Litton JK, Buchholz TA, Hortobagyi GN, Gonzalez-Angulo AM. Impact of body mass index on survival outcome among women with early stage triple-negative breast cancer. Clin Breast Cancer. 2012;12:364–372. doi: 10.1016/j.clbc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Ademuyiwa FO, Groman A, O'Connor T, Ambrosone C, Watroba N, Edge SB. Impact of body mass index on clinical outcomes in triple-negative breast cancer. Cancer. 2011;117:4132–4140. doi: 10.1002/cncr.26019. [DOI] [PubMed] [Google Scholar]
- 16.Ligibel JA, Cirrincione CT, Liu M, et al. Body Mass Index, PAM50 Subtype, and Outcomes in Node-Positive Breast Cancer: CALGB 9741 (Alliance). J Natl Cancer Inst. 2015:107. doi: 10.1093/jnci/djv179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caan BJ, Sweeney C, Habel LA, et al. Intrinsic subtypes from the PAM50 gene expression assay in a population-based breast cancer survivor cohort: prognostication of short- and long-term outcomes. Cancer Epidemiol Biomarkers Prev. 2014;23:725–734. doi: 10.1158/1055-9965.EPI-13-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sweeney C, Bernard PS, Factor R, et al. Molecular subtypes from PAM50 in a breast cancer cohort: differences by patient characteristic, reproducibility. Cancer Research. 2012;72:1670–1670. [Google Scholar]
- 19.Bastien RR, Rodriguez-Lescure A, Ebbert MT, et al. PAM50 breast cancer subtyping by RT-qPCR and concordance with standard clinical molecular markers. BMC Med Genomics. 2012;5:44. doi: 10.1186/1755-8794-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwan ML, Kroenke CH, Sweeney C, et al. Association of high obesity with PAM50 breast cancer intrinsic subtypes and gene expression. BMC Cancer. 2015;15:278. doi: 10.1186/s12885-015-1263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caan B, Sternfeld B, Gunderson E, Coates A, Quesenberry C, Slattery ML. Life After Cancer Epidemiology (LACE) Study: a cohort of early stage breast cancer survivors (United States). Cancer Causes & Control. 2005;16:545–556. doi: 10.1007/s10552-004-8340-3. [DOI] [PubMed] [Google Scholar]
- 22.Kwan ML, Ambrosone CB, Lee MM, et al. The Pathways Study: a prospective study of breast cancer survivorship within Kaiser Permanente Northern California. Cancer Causes & Control. 2008;19:1065–1076. doi: 10.1007/s10552-008-9170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sternfeld B, Weltzien E, Quesenberry CP, et al. Physical activity and risk of recurrence and mortality in breast cancer survivors: findings from the LACE study. Cancer Epidemiology Biomarkers & Prevention. 2009;18:87–95. doi: 10.1158/1055-9965.EPI-08-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Borgan O, Langholz B, Samuelsen SO, Goldstein L, Pogoda J. Exposure stratified case-cohort designs. Lifetime data analysis. 2000;6:39–58. doi: 10.1023/a:1009661900674. [DOI] [PubMed] [Google Scholar]
- 26.Langholz B, Jiao J. Computational methods for case-cohort studies. Computational Statistics & Data Analysis. 2007;51:3737–3748. [Google Scholar]
- 27.Goodwin PJ, Ambrosone CB, Hong C-C. Improving Outcomes for Breast Cancer Survivors: Springer; 2015. Modifiable Lifestyle Factors and Breast Cancer Outcomes: Current Controversies and Research Recommendations. pp. 177–192. [DOI] [PubMed] [Google Scholar]
- 28.Rosner B, Glynn RJ, Tamimi RM, et al. Breast cancer risk prediction with heterogeneous risk profiles according to breast cancer tumor markers. Am J Epidemiol. 2013;178:296–308. doi: 10.1093/aje/kws457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahn J, Schatzkin A, Lacey JV, Jr., et al. Adiposity, adult weight change, and postmenopausal breast cancer risk. Arch Intern Med. 2007;167:2091–2102. doi: 10.1001/archinte.167.19.2091. [DOI] [PubMed] [Google Scholar]
- 30.Colditz GA, Rosner BA, Chen WY, Holmes MD, Hankinson SE. Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst. 2004;96:218–228. doi: 10.1093/jnci/djh025. [DOI] [PubMed] [Google Scholar]
- 31.Neuhouser ML, Aragaki AK, Prentice RL, et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the women's health initiative randomized clinical trials. JAMA oncology. 2015;1:611–621. doi: 10.1001/jamaoncol.2015.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ades F, Zardavas D, Bozovic-Spasojevic I, et al. Luminal B breast cancer: molecular characterization, clinical management, and future perspectives. Journal of Clinical Oncology. 2014;32:2794–2803. doi: 10.1200/JCO.2013.54.1870. [DOI] [PubMed] [Google Scholar]
- 33.Bianchini G, Pusztai L, Karn T, et al. Proliferation and estrogen signaling can distinguish patients at risk for early versus late relapse among estrogen receptor positive breast cancers. Breast cancer research. 2013;15:1. doi: 10.1186/bcr3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alliance for Clinical Trials in Oncology [August 8, 2016]; NCT02750826. Breast Cancer WEight Loss Study (BWEL Study) Available from URL: https://clinicaltrials.gov/ct2/show/record/NCT02750826.
- 35.Jones LW, Kwan ML, Weltzien EK, et al. Exercise and Prognosis on the Basis of Clinicopathologic and Molecular Features in Early Stage Breast Cancer: The LACE and Pathways Studies. Cancer Res. 2016 doi: 10.1158/0008-5472.CAN-15-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prado CM, Gonzalez MC, Heymsfield SB. Body composition phenotypes and obesity paradox. Current Opinion in Clinical Nutrition & Metabolic Care. 2015;18:535–551. doi: 10.1097/MCO.0000000000000216. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez MC, Pastore CA, Orlandi SP, Heymsfield SB. Obesity paradox in cancer: new insights provided by body composition. The American journal of clinical nutrition. 2014;99:999–1005. doi: 10.3945/ajcn.113.071399. [DOI] [PubMed] [Google Scholar]
- 38.Cespedes Feliciano EM, Kroenke CH, Bradshaw PT, et al. Post-diagnosis Weight Change and Survival Following a Diagnosis of Early Stage Breast Cancer. Cancer Epidemiology, Biomarkers and Prevention. 2016 doi: 10.1158/1055-9965.EPI-16-0150. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.