Abstract
The extracellular domain of transmembrane alpha-Klotho (αKlotho, hereinafter simply called Klotho) is cleaved by secretases and released into the circulation as soluble Klotho. Soluble Klotho in the circulation starts to decline early in chronic kidney disease (CKD) stage 2 and urinary Klotho possibly even earlier in CKD stage 1. Therefore soluble Klotho could serve as an early and sensitive marker of kidney function decline. Moreover, preclinical animal data support Klotho deficiency is not just merely a biomarker, but a pathogenic factor for CKD progression and extrarenal CKD complications including cardiovascular disease and disturbed mineral metabolism. Prevention of Klotho decline, re-activation of endogenous Klotho production or supplementation of exogenous Klotho are all associated with attenuation of renal fibrosis, retardation of CKD progression, improvement of mineral metabolism, amelioration of cardiomyopathy, and alleviation of vascular calcification in CKD. Therefore Klotho is not only a diagnostic and/or prognostic marker for CKD, but the treatment of Klotho deficiency may be a promising strategy to prevent, retard, and decrease the burden of comorbidity in CKD.
1. INTRODUCTION
The Klotho gene was discovered in 1997 when mice with serendipitous silencing of this gene developed multiple organ dysfunction and failure with shortened life span [1]. Subsequently, two other paralogs βKlotho [2] and γKlotho [3] were identified, then Klotho was designated αKlotho [4]. For the sake of simplicity, αKlotho is referred hereinafter as Klotho throughout this manuscript.
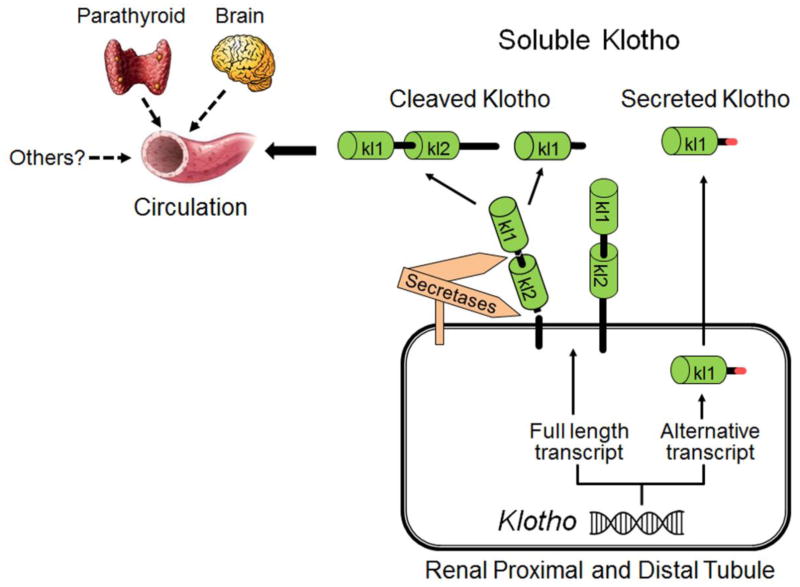
Klotho is highly expressed in the kidney, brain and to a lesser extent in other organs including parathryoid [1, 5]. The extracellular domain of membrane Klotho consisting of two repeat sequences (Kl1 and Kl2) can be shed by secretases and released into the circulation as cleaved Klotho [6–9] (Figure 1). Another form of Klotho protein in the circulation is Kl1 fragment which is generated by alternative transcript splicing called secreted Klotho (Figure 1) [1, 10–12]. In concert with cleaved Klotho, these are collectively termed soluble Klotho. But in this review, soluble Klotho is only strictedly used for cleaved full lengh of extracelluar domain of membrane Klotho. Soluble Klotho is a main functional form in the circulation [6, 13–16] and is also present in cerebrospinal fluid [17][16, 18–21] and urine of mammals [15, 22–24]. At physiologic condition, the kidney is a major contributor to maintaining soluble Klotho levels [6, 25], but other organs may participate in maintaining soluble Klotho in chronic kidney disease (CKD) and end-stage renal disease (ESRD) [23]. Soluble Klotho functions as a circulating substance exerting multiple biological actions on distant organs [26–31].
Figure 1. Source of soluble Klotho.
The kidney is the main source of circulating Klotho under physiological conditions. Both renal proximal and distal tubules express membrane Klotho protein and may also produce a secreted Klotho protein through alternative splicing. The secreted Klotho only contains Kl1 domain and is directly secreted into the blood circulation. But its biologic function is not clear yet. Extracellular domain of membrane Klotho containing Kl1 and Kl2 repeats is shed and cleaved by secretases into either full extracellular domain or Kl1 repeat. Both cleaved Klotho fragments are present in the circulation. A few extra-renal organs including parathyroid gland and brain express Klotho protein as well, but their contribution to circulating Klotho in CKD/ESRD (dash line) remains to be confirmed.
CKD is characterized by progressive deterioration of renal function with high risk of ESRD. CKD risk increases with age, and about half of the CKD stage ≥3 cases occurs in subjects >70 years old. CKD can be viewed as a state of accelerated aging [32, 33]. The relative risk for cardiovascular mortality of a 25 to 34-year-old dialysis patient is similar to a non-CKD patient of >75 years of age [34]. Cardiovascular disease is the principal killer in CKD and ESRD patients. The fact that Klotho-deficient mice and CKD subjects have similar phenotypes also suggests a potential pathogenic role of Klotho deficiency in CKD development and progression [4, 24, 35–37].
Since the kidney is the main origin for circulating Klotho [6, 25] [38, 39], it is not surprising that CKD and ESRD patients have low renal Klotho expression and low levels of circulating Klotho. Renal Klotho deficiency in early stages of CKD may be attributed mainly to suppression of Klotho expression rather than loss of viable renal tubules. Several intermediates are shown to be involved in the reduction of Klotho expression: high serum phosphate [40], hypermethylation [41–45] and hyper-deacetylation [46] in Klotho gene promoter induced by inflammatory cytokines and the uremic toxin, indoxyl sulfate (Figure 2). Furthermore, dialysis patients still have detectable circulating Klotho suggesting that renal Klotho expression is not completely suppressed, and Klotho may come from extra-renal source(s), although its origin is not clear to date (Figure 1) [23]. Establishing extra-renal sources of Klotho and characterizing how this can be up-regulated when renal production fails is of paramount importance.
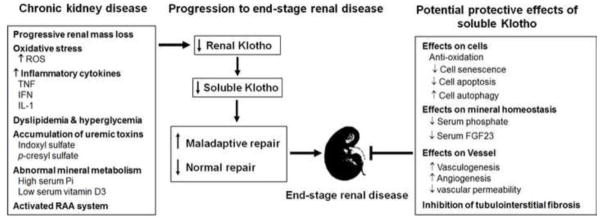
Figure 2. Potential mechanisms of Klotho downregulation in CKD, and beneficial effects of soluble Klotho on CKD.
Left panel: Loss of renal mass, over production of reactive oxygen species (ROS) as well as pro-inflammatory cytokines including tumor necrosis factor (TNF), interferon (IFN) and interleukin 1 (IL-1), dyslipidemia and hyperglycemia, and elevation of uremic toxins including indoxyl sulfate and p-cresyl sulfate may contribute to or participate in downregulation of renal Klotho. Furthermore, high serum phosphate and FGF23 as well as low serum 1,25-Vit.D3 inhibit renal Klotho expression. Low serum 1,25-Vit.D3 not only reduces Klotho expression, but also stimulates renin-aldosterone-angiotensin (RAA) system which further suppresses Klotho production. Middle panel: Reduced Klotho expression in the kidney would lead to endocrine Klotho deficiency in CKD. Low soluble Klotho promotes CKD progression to ESRD through impaired normal renal repair process and induction of maladaptive repair process. Right panel: Supplementation of soluble Klotho protein retards CKD progression through multiple biologic actions: (1) cytoprotection via anti-oxidation, reduction of cell senescence and apoptosis, and upregulation of autophagy, hence accelerating renal tubule regeneration; (2) correction of high serum phosphate and FGF23; (3) maintenance of peritubular capillary formation and function; and (4) inhibition of tubuloinsterstitial fibrosis.
Klotho deficiency is not only an early biomarker of CKD (to be discussed in detail below), but also a pathogenic intermediate for CKD development and progression (Figure 2), and extrarenal complications [24, 47, 48]. It has been shown that Klotho deficiency is associated with stem cell dysfunction and depletion which is part of normal aging [49]. Furthermore, Klotho deficiency in CKD could enhance renal tubular and vascular cell senescence induced by oxidative stress, uremic toxins such as indoxyl sulfate, and high phosphate (Figure 2) [50–57]. In addition, Klotho deficiency promotes renal fibrosis in several kidney disease models [58–60]. Klotho deficiency also results in defective endothelial function and impaired vasculogenesis [61], and Klotho protein protects vascular endothelium by inhibition of endothelial inflammation [62]. Klotho deficiency directly and indirectly contributes to uremic cardiomyopathy which can be prevented or attenuated by supplementation of soluble Klotho [47, 48, 63, 64]. Therefore, soluble Klotho protein may be a novel therapeutic agent for CKD patients. We will first discuss the recent literature about Klotho deficiency as a biomarker for CKD and its role in CKD-mineral and bone disorder (MBD) development, then summarize preclinical results about Klotho supplementation as a therapeutic agent for prevention of CKD progression and amelioration of cardiovascular disease (Figure 2).
2. KLOTHO DEFICIENCY AS A BIOMARKER FOR CKD
There is an urgent need to identify diagnostic and prognostic biomarkers for CKD which are both more sensitive (diagnostic value) and/or more specific (treatment effect value) than those currently used. The early identification of CKD onset and the risk-stratification of CKD progression and/or CKD-related complications are essential to ameliorate the comorbidity burden of CKD patients, particularly cardiovascular disease, and prevent the development of ESRD [65–67]. Certain characteristics are crucial for CKD biomarkers to be incorporated into clinical practice. These include the ability to improve current predictive clinical models of CKD onset or progression; the characterization of the severity of CKD stage; the reliability across species; and the accessibility to be measured in body fluids or tissues.
In this context, Klotho has emerged as both a promising biomarker and potential therapeutic agent for CKD [35]. Importantly, innovative translational efforts to confirm and validate animal data in different human CKD models are evolving. In the following sections, clinical data of Klotho measurements in CKD patients will be reviewed.
2.1. Klotho deficiency is associated with eGFR decline in CKD patients
CKD is a known state of Klotho deficiency [35, 68]. Klotho mRNA expression in kidney was found to be significantly lower in CKD patients than healthy controls and positively correlated with estimated glomerular filtration rate (eGFR) in a small number of CKD patients [69, 70]. Similarly, Klotho mRNA levels in parathyroid gland were shown to decline in parallel with decreasing eGFR [71]. In a larger sample of CKD patients (stages 1 to 5D) with available kidney biopsies, Klotho mRNA levels were also positively correlated with eGFR in multiple regression analysis that adjusted for age and CKD-MBD parameters, such as intact parathyroid hormone (iPTH), fibroblast growth factor 23 (FGF-23), 1,25-dihydroxy vitamin D3 (1,25 VitD3), corrected serum calcium, and serum phosphorus [72]. Importantly, Klotho mRNA in the kidney was the only independent predictor of serum Klotho across all CKD stages. Renal Klotho was significantly correlated with serum calcium, serum phosphorus, 1,25 VitD3, FGF23, and iPTH [72].
In another study, serum Klotho levels were significantly lower in CKD patients than in healthy controls and were shown to be progressively lower with more advanced CKD stage [73]. The adjusted mean serum Klotho decrease was 3.2 pg/mL for each 1 mL/min/1.73 m2 eGFR decrease. Age and eGFR were independently associated with serum Klotho levels [73]. A similar finding of progressive Klotho decline with worsening CKD was observed by Kim and coworkers in a post-hoc analysis of CKD patients [74]. Serum Klotho was independently associated with eGFR in multivariable linear regression analysis. In addition, serum Klotho was negatively correlated with FGF-23 and serum phosphate [74]. Several other studies have confirmed the positive correlation between Klotho levels (serum and urine) and eGFR in adult CKD patients [22, 24, 74–76]. Consistently, both serum and urine Klotho levels have been independently associated with eGFR in CKD patients [22, 74]. A similar positive correlation between plasma Klotho levels and eGFR was shown in children with CKD [77]. The decline in Klotho levels was associated with increased FGF23 and iPTH levels in this study [77]. Furthermore, serum Klotho levels in children on chronic peritoneal dialysis were significantly lower than in healthy controls [78].
The very early decline of serum Klotho levels in adults with incipient CKD i.e. CKD stages ≤2, suggests that this change antecedes the increase in serumFGF23, iPTH, and hyperphosphatemia. It may therefore constitute an early marker of progressive CKD and CKD-MBD disturbances [68, 74, 79, 80]. The early occurrence of Klotho deficiency in human CKD needs to be further confirmed with other assay(s) (the limitation of current ELISA kit will be discussed below) and its validity to predict adverse CKD-related outcome further evaluated.
2.2. Klotho deficiency and possible association with cardiovascular disease in CKD patients
A cross-sectional study of CKD patients with modest decline in renal function revealed that serum Klotho is associated with arterial stiffness measured by ankle-brachial pulse waive velocity [75]. This association was independent of age, gender, mean blood pressure, use of antihypertensive drugs, tobacco smoking, ethanol use, non-HDL cholesterol, statin use, diabetes status, eGFR, albuminuria, and hemoglobin [75]. In contrast, a larger cohort study of CKD stages 2 – 4 showed that plasma Klotho levels (highest vs lowest tertile) did not predict atherosclerotic or acute heart failure events or death at 2.6 years follow-up [81].
Serum Klotho levels were found to be significantly reduced in hypertensive (essential and renovascular) patients with mild CKD when compared to healthy controls, even after adjustment by eGFR [82]. The proposed cross-talk between the renin-angiotensin-aldosterone system and the vitamin D-FGF23-Klotho pathways supports the hypothesis that modulation of one system can have positive effects on the other [83, 84], [85]. In this context, a post hoc analysis of the ESCAPE trial in children with CKD (all received fixed dose of ramipril 6 mg/m2 per day) showed that 25 (OH)-D ≥50 nmol/L was associated with greater preservation of renal function. Interestingly, ramipril therapy significantly increased serum Klotho levels without any associated changes in serum calcium or phosphate [86].
Despite evolving experimental data showing that Klotho deficiency may be an intermediate mediator of the pathologic vascular calcification, endothelial dysfunction, cardiac remodeling, and cardiac hypertrophy observed in CKD [24, 47, 48, 64] an association between Klotho levels and cardiovascular disease has been only observed in small studies utilizing surrogate markers of cardiovascular disease rather than hard outcomes such as major cardiovascular events or death. Larger studies with well-defined cardiovascular disease outcome are needed to fully elucidate the role of Klotho as a prognostic marker of cardiovascular disease in human CKD.
2.3. Klotho deficiency may be a prognostic biomarker of progressive CKD
The role of Klotho as a predictor of adverse outcomes in CKD was examined in a post hoc cohort study of adult patients with CKD (stages 1 to 5). Patients with acute coronary syndrome, ischemic stroke or progressive CKD within 3 months prior to the study were excluded. Importantly, serum Klotho levels independently predicted the composite outcome of doubling serum creatinine (SCr), ESRD, or death in Cox regression time-to-event analysis that adjusted for age, diabetes, mean arterial pressure, eGFR, proteinuria, and iPTH [74]. If serum Klotho levels were ≤396.3 pg/mL, 35.2% of patients reached the composite outcome (doubling SCr, ESRD, or death), whereas only 15.7% of patients reached this adverse composite outcome if serum Klotho levels were >396.3 pg/mL. The areas under the curve (a measure of discrimination, that is, the ability of Klotho to correctly classify those with and without the outcome) for 1/serum Klotho to predict the composite outcome (doubling SCr, ESRD, or death) were 0.81, 0.78, and 0.72 at 12, 24, and 36 months [74]. Currently, these data constitute the most conclusive evidence of Klotho candidacy as a prognostic biomarker of progressive CKD or death. Although, these results are encouraging given the hard composite outcome utilized, this study was performed in a small sample of CKD patients and further validation is urgently needed.
3. CURRENT LIMITATIONS TO THE USE OF KLOTHO AS A BIOMARKER IN CKD
It is imperative to recognize that current Klotho clinical data are derived from small observational studies, despite being promising and somehow consistent, and still require subsequent extrinsic validation. Moreover, the utilization of hard cardiovascular and CKD outcomes will further test Klotho candidacy as a potential biomarker of CKD progression and/or CKD-related cardiovascular complications. There is a dire need for the development of collaborative research that expedite translational research in Klotho biology and kidney disease. Prospective studies with novel enrichment tools may be required to achieve adequate outcome events and the necessary power to test performance or reclassification metrics incorporating clinical and other biomarker data.
An additional difficulty that impaired progress in this field was the lack of a standardized assay to measure circulating Klotho, often yielding contradictory results [68, 87]. Immuno-Biological Laboratories Co generated ELISA kit that gave higher readings in fresh serum samples because Klotho ELISA kit may measure Klotho and other cross-reacting proteins in fresch samples, but gave lower readings in the stored samples when compared to immunoprecipitation-immunoblot assay [67] The development of a reliable assay is mandatory to accelerate human studies in CKD. Furthermore, the distinction and characterization of circulating free Klotho, complexed Klotho, cleaved Klotho, secreted Klotho, and the Kl1 and Kl2 Klotho sequences may be biologically necessary to fully unveil the role of Klotho in kidney disease. Finally, other clinical parameters known to influence Klotho, such as high phosphate diet, FGF-23, vitamin D supplementation and certain medications, cannot be addressed in observational studies and may exert influence in our interpretations. Prospective studies need to be carefully designed to address some of these difficulties.
Despite the stated caveats, Klotho has gained attention and raised a significant amount of enthusiasm in the nephrology community because of its candidacy as a potential biomarker and/or therapeutic agent in CKD. This is particularly important in an era of recurrent disillusionment when validating CKD biomarkers that are readily applicable in clinical practice.
4. KLOTHO DEFICIENCY AS A PATHOGENIC INTERMEDIATE FOR BONE DISORDERS IN CKD
When Klotho was discoved as an aging suppressor, the association of Klotho deficiency with osteoporesis was readily documented [1]. Shortly Klotho deficiency was confirmed to lead to low-turnover bone metabolism and consequently cause oseoporesis and osteopenia [88–93]. Clinical observational studies showed that the severity of bone loss was associated with Klotho gene G395A and C1818T polymorphisms in Caucasian and Japanese postmenopausal women [94]. Reversal of renal Klotho by demethylation of Klotho gene promoter led to significant improvement in disturbed serum biochemistry and bone damage in CKD mice [95].
CKD-MBD is one of the striking features associated with high morbidity and mortality of cardiovascular events In CKD and ESRD [96–107]. Abnormal mineral metabolism including high serum phosphate, FGF23, and PTH as well as low serum 25- VitD3, and 1,25-VitD3D3, is not only a hallmark of CKD-MBD, but also an intiator or accelerator to bone disease development in CKD. Moreover, Klotho deficiency is proposed to be associated with or even induce high serum phosphate, FGF23, and PTH [35–37, 108–112]. On the other hand, high serum phosphate will further downregulate the renal Klotho expression [47]. These factors can exacerbate and also interact with each other. Together, they constitute a self-amplifying downhill vortex that drives disturbed mineral metabolism worse and worse. Given that CKD-MBD contributes to the high morbidity and mortality of cardiovascular disease in CKD/ESRD patients [97, 113–116], the increase in soluble Klotho in the circulation should aid in the disruption of this downhill vortex and improvement of CKD-MBD, and hence potentially reduces the risk for cardiovascular morbidfity and mortality [23, 117]. Therefore, FGF23-FGF receptor/Klotho pathway can be a new drug target for disorders of phosphate and bone metabolism [118].
5. POTENTIAL TREATMENT STRATEGY FOR CHRONIC KIDNEY DISEASE
The kidney is confirmed as the major organ that contributes to circulating Klotho [6, 25] and decreased Klotho production renders the kidney more susceptible to a variety of kidney insults [15, 30], retards kidney recovery from acute kidney injury (AKI) [85], promotes fibrosis [58] and AKI-to-CKD transition [119], and exacerbates uremic cardiomyopathy [47] and vascular calcification [24]. On the other hand, Klotho repletion through genetic manipulation, viral delivery, or administration of Klotho protein has been shown to improve multiple renal and extrarenal complications in both acute and chronic loss of kidney function [15, 24, 47, 58, 72, 74, 80, 120]. Therefore, Klotho deficiency may not only be a pathogenic intermediate for accelerating CKD progression, but also a major contributor to chronic complications such as secondary hyperparathyroidism and cardiovascular disease in CKD. Conceivably, any therapy that restores klotho levels through re-activation of endogenous Klotho or administration of exogenous Klotho might be a novel treatment strategy for CKD (Figure 2).
5.1. Re-Activation of Endogenous Klotho Expression dependent of epigenetic mechanism
5.1.1. Demethylation
Epigenetic modulation of Klotho was proposed to contribute to renal Klotho deficiency in certain instances including Klotho hypomorphic mice which was originally thought to result from interruption of the promoter of Klotho gene by the exogenous transgene [1], because there was aberrant Klotho promoter methylation due to exogenous transgene in kl/kl mice. The methylation of the Klotho gene promoter reduced its activity by 30–40%, whereas DNA demethylation increased Klotho expression 1.5- to 3.0-fold [44]. Moreover, uremic toxins – indoxyl sulfate or p-cresyl sulfate – induced hypermethylation of the Klotho gene, and decreased Klotho expression in renal tubules and kidney cell lines, which could be reversed by demethylation of the Klotho gene. Animal experiments clearly showed that demethylation of Klotho gene promoter remarkably reversed renal Klotho deficiency and reduced renal fibrosis [95]. Therefore, hypermethylation may be one of the mechanisms of Klotho gene expression inhibition in CKD [41, 42, 121].
5.1.2. Deacetylation
The hyperacetylation of histone in Klotho promoter was also proposed as a possible mechanism for Klotho silencing in many types of cancer cell lines [122, 123]. Furthermore, cytokines-induced elevation of histone acetylation is shown to be associated with downregulation of Klotho expression, because TNF and TNF-like weak inducer of apoptosis (TWEAK)-induced downregulation of Klotho expression in the kidney and kidney cell lines could be blunted by inhibition of histone deacetylase [46]. The effect of histone acetylation of Klotho promoter on the regulation of Klotho expression was shown to be implicated in several types of cancer development [45, 122, 123], but its role in renal Klotho deficiency and the therapeutic effect of inhibition of histone acetylation on upregulation of renal klotho is worthy to confirm in kidney disease subjects.
5.2. Re-Activation of Endogenous Klotho Expression Independently of Epigenetics
To date, several categories of drugs in the market including peroxisome proliferator-activated receptor-gamma (PPAR-γ) agonists [124–127], Angiotensin II-type I receptor antagonists [128–130], 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase inhibitors (statin) [131], vitamin D active derivatives [23, 85, 132–134], and intermedin [135, 136] have been shown to up-regulate Klotho expression in vivo and/or in vitro. Peritoneal injection of vitamin D receptor agonists robustly increased circulating Klotho levels, reduced serum creatinine, and attenuated vascular calcification in mice with CKD [23]. Hence, the up-regulation of Klotho doubtlessly has positive beneficial effect on target organs of experimental CKD models although the detailed molecular mechanisms whereby Klotho is upregulated remain to be explored [23].
Taken together, re-activation of Klotho production through demethylation and deacetylation of Klotho gene promoter, or other yet-to-be identified mechanism(s) may be an easy and useful strategy to restore renal Klotho production in patients with early CKD and consequently increase circulating Klotho levels. Higher Klotho in the kidney and the circulation is doubtlessly of help in maintenance of mineral metabolism homeostasis, prevention of CKD progression, and protection of vasculature and heart. Their clinical efficacy is worthy of being confirmed.
5.3. Delivery of Klotho cDNA
Klotho gene delivery via viral carrier was shown to effectively rescue many phenotypes observed in Klotho-deficient mice [137], attenuating the progression of hypertension and kidney damage in spontaneously hypertensive rats [138, 139], improving kidney function in AKI [120], ameliorating Angiotensin II-induced kidney injury [140], improving endothelial function [141], and protecting from uremic cardiomyopathy [142]. More recently, an injection of cDNA coding for full-length extracellular domain of rat Klotho under the control of the cytomegalovirus promoter was shown to ameliorate pathologic cardiac hypertrophy in Klotho-deficient CKD mice [61]. Although gene therapy is effective in animal studies, its safety is still questionable and human clinical application is not in the proximity.
5.4. Administration of soluble Klotho Protein
The supplementation of Klotho via gene delivery systems is not ready for clinical use, but it proves the concept that restoration of circulating Klotho is effective [117, 143]. The alternative option to increase circulating Klotho is the administration of soluble Klotho protein (cleaved full length of extracellular domain of membrane Klotho) which is more direct, safer, and an easier modality to restore endocrine Klotho deficiency than viral carrier delivery. Animal studies have already provided encouraging proof-of-concept data showing that bolus administration of soluble Klotho protein is safe and effective in protection against kidney injury [15] and induction of phosphaturia [144]. Furthermore, repeated administration of soluble Klotho protein attenuates AKI-to-CKD progression [119].
It has been shown that ischemia-reperfusion induced AKI is a state of acute Klotho deficiency. The single administration of soluble Klotho protein immediately after kidney injury effectively amelioratedkidney damage and preserves kidney function [15]. Furthermore, repeated administration of soluble Klotho protein post surgery inhibited renal fibrosis in a unilateral ureteral obstruction kidney injury model [58]. More recently, repeated administration of soluble Klotho protein starting one day after IRI-induced AKI (Scr at peak levels) promoted kidney recovery, suppressed renal fibrosis, accelerated the removal of deposited collagen through upregulation of autophagy, and consequently retarded AKI-to-CKD progression [119]. The effective therapeutic results in two different kidney disease models highly support the concept that Klotho protein administration is a promising strategy which can be used to prevent acute kidney injury when given early and also mitigate AKI-to-CKD progression when given post AKI. Thus, although Klotho protein administration has not been approved to treat AKI or CKD patients, the pre-clinical data clearly support the therapeutic potential of soluble Klotho protein to attenuate adverse renal outcome regardless of etiology.
The identification of AKI phenotypes (combining clinical and biomarker data) of patients at high risk of recurrent AKI or incomplete AKI recovery with rapid transition to CKD may help the identification of high-risk patients eligible for therapeutic Klotho trials in which renal and non-renal hard outcomes could be feasibly tested. The parallel development of Klotho therapeutic strategies (e.g., soluble Klotho protein administration) and more specific Klotho assays makes the design of Klotho trials foreseeable in the near future. Ongoing collaborative Klotho research in human AKI and CKD will aid confirmatory testing of circulating Klotho assays and the recognition of therapeutic windows for potential Klotho administration. In this context, recurrent soluble Klotho administration guided by serum or urine Klotho levels could be of paramount strategic importance. A highly relevant human model of disease is AKI-to-CKD progression in which Klotho administration could be initiated in the early post-AKI period in individuals at high risk of adverse renal outcome. It is expected that the development of Klotho therapies accelerates in the near future and therefore pragmatic clinical trials of Klotho administration in human disease are no longer utopic in the scientific community.
6. SUMMARY
Several beneficial roles of Klotho have been proposed in clinical nephrology based on pre-clinical studies (Table 1): (1) Klotho may be a sensitive marker for early diagnosis of CKD and a reliable prognostic marker for the prediction of CKD progression, the development of ESRD, and CKD-related cardiovascular disease. However, the biomarker candidacy of serum or urine Klotho needs to be further evaluated; (2) Klotho could serve as a prophylactic agent to prevent AKI in patients at high risk for this complication such as those with underlying CKD; (3) Klotho holds a promising therapeutic effect to mitigate CKD progression, AKI-to-CKD progression, and alleviate cardiovascular disease, being the latter the principal culprit of elevated mortality in CKD/ESRD patients. However, the efficacy and safety of Klotho therapy in different stages of human CKD still needs to be demonstrated.
Table 1.
Potential applications of circulating Klotho in clinical nephrology
| Biomarker | Therapeutic agent | ||
|---|---|---|---|
|
|
|
||
| Diagnostic |
|
Prophylactic |
|
| Prognostic |
|
Therapeutic |
|
AKI: acute kidney injury; CKD: chronic kidney disease; CKD-MBD: chronic kidney disease-mineral and bone disorder; CVD: cardiovascular disease; ESRD: end-stage renal disease
The molecular mechanisms of Klotho’s renoprotection and cardioprotection are still being unraveled. Whether the effect of Klotho on the kidney and heart is organ-specific or shares similar signaling pathways is not known. Obviously, better understanding of the molecular mechanism(s) of Klotho curing kidney disease and preventing cardiovascular disease will help in developing and implementing novel therapeutic strategies.
Klotho therapy to arrest or attenuate the development or progression of CKD or CKD-related complications is definitely in the pipeline of development. Collaborative and translational research are crucial to enhance bench-to-bedside transition. Novel circulating Klotho assays should be tested for generalizability across the spectrum of kidney disease and heterogeneity of sample characteristics. Exogenous soluble Klotho administration may not be the sole agent to prevent or ameliorate the burden of CKD but definitely could be a key player in conjunction with FGF-23 antagonism, vitamin D supplementation and phosphate control to restore mineral metabolism homeostasis and halt CKD-related complications, because high phoshage, low serum vitamin D and and high FGF23 are negative regulators for Klotho expression in the kidney and circulating Klotho [16,33~35,85]. A key human disease model that can potentially unveil the pathobiology of Klotho in renal disease is AKI-to-CKD progression. Longitudinal follow-up of AKI patients at high risk of CKD should be carefully instituted for state-of-the-art patient care and research utilizing biological samples and clinical data. The time has come for translational and clinical research to support the vast animal data in Klotho biology and renal disease.
Table 2.
Potential strategies to upregulate Klotho for treatment of human CKD
| Epigenetic increase of endogenous Klotho |
|
| Non-epigenetic increase of endogenous Klotho |
|
| Delivery of Kotho gene in vivo |
|
| Administration of exogenous Klotho protein |
|
CKD: chronic kidney disease;
Highlights.
CKD is a state of pan-Klotho deficiency
Klotho deficiency is a biomarker for CKD and a predictor for CKD progression
Klotho deficiency is an intermediate for CKD progression and CKD-MBD
Soluble Klotho protein is novel and promising therapeutic agent for CKD/ESRD
Acknowledgments
The authors appreciate Dr. Orson Moe for helpful interactions in the editing of this chapter. The research work in MC Hu’s research laboratory is in part supported by the NIH (R01-DK091392, R01-DK092461, R01-DK092461-S1), The George M. O’Brien Kidney Research Center (P30-DK-07938), the Charles and Jane Pak Foundation, and the Pak Center Innovative Research Support. JA Neyra is in part supported by the Ben J. Lipps Research Fellowship Program of American Society of Nephrology Foundation for Kidney Research and the Truelson Fellowship Fund at the Charles and Jane Pak Center of Mineral Metabolism and Clinical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 2.Ito S, Kinoshita S, Shiraishi N, Nakagawa S, Sekine S, Fujimori T, Nabeshima YI. Molecular cloning and expression analyses of mouse betaklotho, which encodes a novel Klotho family protein. Mech Dev. 2000;98(1–2):115–9. doi: 10.1016/s0925-4773(00)00439-1. [DOI] [PubMed] [Google Scholar]
- 3.Ito S, Fujimori T, Hayashizaki Y, Nabeshima Y. Identification of a novel mouse membrane-bound family 1 glycosidase-like protein, which carries an atypical active site structure. Biochim Biophys Acta. 2002;1576(3):341–5. doi: 10.1016/s0167-4781(02)00281-6. [DOI] [PubMed] [Google Scholar]
- 4.Hu MC, Shiizaki K, Kuro-o M, Moe OW. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol. 2013;75:503–33. doi: 10.1146/annurev-physiol-030212-183727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato Y, Arakawa E, Kinoshita S, Shirai A, Furuya A, Yamano K, Nakamura K, Iida A, Anazawa H, Koh N, Iwano A, Imura A, Fujimori T, Kuro-o M, Hanai N, Takeshige K, Nabeshima Y. Establishment of the anti-Klotho monoclonal antibodies and detection of Klotho protein in kidneys. Biochem Biophys Res Commun. 2000;267(2):597–602. doi: 10.1006/bbrc.1999.2009. [DOI] [PubMed] [Google Scholar]
- 6.Hu MC, Shi M, Zhang J, Addo T, Cho HJ, Barker SL, Ravikumar P, Gillings N, Bian A, Sidhu SS, Kuro-o M, Moe OW. Renal Production, Uptake, and Handling of Circulating αKlotho. Journal of the American Society of Nephrology. 2015 doi: 10.1681/ASN.2014101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CD, Tung TY, Liang J, Zeldich E, Tucker Zhou TB, Turk BE, Abraham CR. Identification of cleavage sites leading to the shed form of the anti-aging protein klotho. Biochemistry. 2014;53(34):5579–87. doi: 10.1021/bi500409n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A. 2007;104(50):19796–801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloch L, Sineshchekova O, Reichenbach D, Reiss K, Saftig P, Kuro-o M, Kaether C. Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett. 2009;583(19):3221–4. doi: 10.1016/j.febslet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242(3):626–30. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 11.Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, Nagai R, Kuro-o M, Nabeshima Y. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998;424(1–2):6–10. doi: 10.1016/s0014-5793(98)00127-6. [DOI] [PubMed] [Google Scholar]
- 12.Tohyama O, Imura A, Iwano A, Freund JN, Henrissat B, Fujimori T, Nabeshima Y. Klotho is a novel beta-glucuronidase capable of hydrolyzing steroid beta-glucuronides. J Biol Chem. 2004;279(11):9777–84. doi: 10.1074/jbc.M312392200. [DOI] [PubMed] [Google Scholar]
- 13.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309(5742):1829–33. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu MC, Shi M, Zhang J, Quinones H, Griffith C, Kuro OM, Moe OW. Klotho Deficiency Causes Vascular Calcification in Chronic Kidney Disease. J Am Soc Nephrol. 2010 doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu MC, Shi M, Zhang J, Quinones H, Kuro OM, Moe OW. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010;78(12):1240–1251. doi: 10.1038/ki.2010.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565(1–3):143–7. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 17.Kunert SK, Hartmann H, Haffner D, Leifheit-Nestler M. Klotho and fibroblast growth factor 23 in cerebrospinal fluid in children. J Bone Miner Metab. 2016 doi: 10.1007/s00774-016-0746-y. [DOI] [PubMed] [Google Scholar]
- 18.Chen CD, Li H, Liang J, Hixson K, Zeldich E, Abraham CR. The anti-aging and tumor suppressor protein klotho enhances differentiation of a human oligodendrocytic hybrid cell line. J Mol Neurosci. 2015;55(1):76–90. doi: 10.1007/s12031-014-0336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Degaspari S, Tzanno-Martins CB, Fujihara CK, Zatz R, Branco-Martins JP, Viel TA, de Buck HS, Orellana AM, Bohmer AE, de Sa Lima L, Andreotti DZ, Munhoz CD, Scavone C, Kawamoto EM. Altered KLOTHO and NF-kappaB-TNF-alpha Signaling Are Correlated with Nephrectomy-Induced Cognitive Impairment in Rats. PLoS One. 2015;10(5):e0125271. doi: 10.1371/journal.pone.0125271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emami Aleagha MS, Siroos B, Ahmadi M, Balood M, Palangi A, Haghighi AN, Harirchian MH. Decreased concentration of Klotho in the cerebrospinal fluid of patients with relapsing-remitting multiple sclerosis. J Neuroimmunol. 2015;281:5–8. doi: 10.1016/j.jneuroim.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Semba RD, Moghekar AR, Hu J, Sun K, Turner R, Ferrucci L, O’Brien R. Klotho in the cerebrospinal fluid of adults with and without Alzheimer’s disease. Neurosci Lett. 2014;558:37–40. doi: 10.1016/j.neulet.2013.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akimoto T, Yoshizawa H, Watanabe Y, Numata A, Yamazaki T, Takeshima E, Iwazu K, Komada T, Otani N, Morishita Y, Ito C, Shiizaki K, Ando Y, Muto S, Kuro-o M, Kusano E. Characteristics of urinary and serum soluble Klotho protein in patients with different degrees of chronic kidney disease. BMC Nephrol. 2012;13:155. doi: 10.1186/1471-2369-13-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau WL, Leaf EM, Hu MC, Takeno MM, Kuro-o M, Moe OW, Giachelli CM. Vitamin D receptor agonists increase klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate diet. Kidney Int. 2012;82(12):1261–70. doi: 10.1038/ki.2012.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu MC, Shi M, Zhang J, Quinones H, Griffith C, Kuro-o M, Moe OW. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22(1):124–36. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindberg K, Amin R, Moe OW, Hu MC, Erben RG, Ostman Wernerson A, Lanske B, Olauson H, Larsson TE. The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol. 2014;25(10):2169–75. doi: 10.1681/ASN.2013111209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu MC, Shi M, Cho HJ, Zhang J, Pavlenco A, Liu S, Sidhu S, Huang LJ, Moe OW. The erythropoietin receptor is a downstream effector of Klotho-induced cytoprotection. Kidney Int. 2013;84(3):468–81. doi: 10.1038/ki.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng MF, Chen LJ, Niu HS, Yang TT, Lin KC, Cheng JT. Signals mediating Klotho-induced neuroprotection in hippocampal neuronal cells. Acta Neurobiol Exp (Wars) 2015;75(1):60–71. [PubMed] [Google Scholar]
- 28.Sun S, Cheng B, Sun PG, Wu XH, Wu QQ, He P. RTEF-1 protects against oxidative damage induced by H2O2 in human umbilical vein endothelial cells through Klotho activation. Exp Biol Med (Maywood) 2015 doi: 10.1177/1535370215587914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravikumar P, Ye J, Zhang J, Pinch SN, Hu MC, Kuro-o M, Hsia CC, Moe OW. alpha-Klotho protects against oxidative damage in pulmonary epithelia. Am J Physiol Lung Cell Mol Physiol. 2014;307(7):L566–75. doi: 10.1152/ajplung.00306.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panesso MC, Shi M, Cho HJ, Paek J, Ye J, Moe OW, Hu MC. Klotho has dual protective effects on cisplatin-induced acute kidney injury. Kidney Int. 2014;85(4):855–70. doi: 10.1038/ki.2013.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Chen L, Huang G, He D, He J, Xu W, Zou C, Zong F, Li Y, Chen B, Wu S, Zhao W, Wu J. Klotho sensitizes human lung cancer cell line to cisplatin via PI3k/Akt pathway. PLoS One. 2013;8(2):e57391. doi: 10.1371/journal.pone.0057391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stenvinkel P, Larsson TE. Chronic kidney disease: a clinical model of premature aging. Am J Kidney Dis. 2013;62(2):339–51. doi: 10.1053/j.ajkd.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 33.Kooman JP, Broers NJ, Usvyat L, Thijssen S, van der Sande FM, Cornelis T, Levin NW, Leunissen KM, Kotanko P. Out of control: accelerated aging in uremia. Nephrol Dial Transplant. 2013;28(1):48–54. doi: 10.1093/ndt/gfs451. [DOI] [PubMed] [Google Scholar]
- 34.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(5 Suppl 3):S112–9. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 35.Hu MC, Kuro-o M, Moe OW. Renal and extrarenal actions of Klotho. Semin Nephrol. 2013;33(2):118–29. doi: 10.1016/j.semnephrol.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu MC, Kuro-o M, Moe OW. Klotho and chronic kidney disease. Contrib Nephrol. 2013;180:47–63. doi: 10.1159/000346778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu MC, Kuro-o M, Moe OW. Secreted klotho and chronic kidney disease. Adv Exp Med Biol. 2012;728:126–57. doi: 10.1007/978-1-4614-0887-1_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ponte B, Trombetti A, Hadaya K, Ernandez T, Fumeaux D, Iselin C, Martin PY, de Seigneux S. Acute and long term mineral metabolism adaptation in living kidney donors: a prospective study. Bone. 2014;62:36–42. doi: 10.1016/j.bone.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 39.Akimoto T, Kimura T, Watanabe Y, Ishikawa N, Iwazu Y, Saito O, Muto S, Yagisawa T, Kusano E. The impact of nephrectomy and renal transplantation on serum levels of soluble Klotho protein. Transplant Proc. 2013;45(1):134–6. doi: 10.1016/j.transproceed.2012.07.150. [DOI] [PubMed] [Google Scholar]
- 40.Hu MC, Shi M, Cho HJ, Adams-Huet B, Paek J, Hill K, Shelton J, Amaral AP, Faul C, Taniguchi M, Wolf M, Brand M, Takahashi M, Kuro OM, Hill JA, Moe OW. Klotho and Phosphate Are Modulators of Pathologic Uremic Cardiac Remodeling. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2014050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young GH, Wu VC. KLOTHO methylation is linked to uremic toxins and chronic kidney disease. Kidney Int. 2012;81(7):611–2. doi: 10.1038/ki.2011.461. [DOI] [PubMed] [Google Scholar]
- 42.Sun CY, Chang SC, Wu MS. Suppression of Klotho expression by protein-bound uremic toxins is associated with increased DNA methyltransferase expression and DNA hypermethylation. Kidney Int. 2012;81(7):640–50. doi: 10.1038/ki.2011.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.King GD, Rosene DL, Abraham CR. Promoter methylation and age-related downregulation of Klotho in rhesus monkey. Age (Dordr) 2012;34(6):1405–19. doi: 10.1007/s11357-011-9315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Azuma M, Koyama D, Kikuchi J, Yoshizawa H, Thasinas D, Shiizaki K, Kuro-o M, Furukawa Y, Kusano E. Promoter methylation confers kidney-specific expression of the Klotho gene. FASEB J. 2012;26(10):4264–74. doi: 10.1096/fj.12-211631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee J, Jeong DJ, Kim J, Lee S, Park JH, Chang B, Jung SI, Yi L, Han Y, Yang Y, Kim KI, Lim JS, Yang I, Jeon S, Bae DH, Kim CJ, Lee MS. The anti-aging gene KLOTHO is a novel target for epigenetic silencing in human cervical carcinoma. Mol Cancer. 2010;9:109. doi: 10.1186/1476-4598-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moreno JA, Izquierdo MC, Sanchez-Nino MD, Suarez-Alvarez B, Lopez-Larrea C, Jakubowski A, Blanco J, Ramirez R, Selgas R, Ruiz-Ortega M, Egido J, Ortiz A, Sanz AB. The inflammatory cytokines TWEAK and TNFalpha reduce renal klotho expression through NFkappaB. J Am Soc Nephrol. 2011;22(7):1315–25. doi: 10.1681/ASN.2010101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu MC, Shi M, Cho HJ, Adams-Huet B, Paek J, Hill K, Shelton J, Amaral AP, Faul C, Taniguchi M, Wolf M, Brand M, Takahashi M, Kuro-o M, Hill JA, Moe OW. Klotho and Phosphate Are Modulators of Pathologic Uremic Cardiac Remodeling. Journal of the American Society of Nephrology. 2015;26(6):1290–302. doi: 10.1681/ASN.2014050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie J, Yoon J, An SW, Kuro-o M, Huang CL. Soluble Klotho Protects against Uremic Cardiomyopathy Independently of Fibroblast Growth Factor 23 and Phosphate. J Am Soc Nephrol. 2015;26(5):1150–60. doi: 10.1681/ASN.2014040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317(5839):803–6. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 50.Verbeke F, Van Biesen W, Vanholder R. The role of collagen metabolism in CKD-associated arterial senescence: underestimated and underappreciated. Nephrol Dial Transplant. 2011;26(9):2726–8. doi: 10.1093/ndt/gfr421. [DOI] [PubMed] [Google Scholar]
- 51.Carracedo J, Buendia P, Merino A, Soriano S, Esquivias E, Martin-Malo A, Aljama P, Ramirez R. Cellular senescence determines endothelial cell damage induced by uremia. Exp Gerontol. 2013;48(8):766–73. doi: 10.1016/j.exger.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Small DM, Bennett NC, Roy S, Gabrielli BG, Johnson DW, Gobe GC. Oxidative stress and cell senescence combine to cause maximal renal tubular epithelial cell dysfunction and loss in an in vitro model of kidney disease. Nephron Exp Nephrol. 2012;122(3–4):123–30. doi: 10.1159/000350726. [DOI] [PubMed] [Google Scholar]
- 53.Clements ME, Chaber CJ, Ledbetter SR, Zuk A. Increased cellular senescence and vascular rarefaction exacerbate the progression of kidney fibrosis in aged mice following transient ischemic injury. PLoS One. 2013;8(8):e70464. doi: 10.1371/journal.pone.0070464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamada S, Tatsumoto N, Tokumoto M, Noguchi H, Ooboshi H, Kitazono T, Tsuruya K. Phosphate binders prevent phosphate-induced cellular senescence of vascular smooth muscle cells and vascular calcification in a modified, adenine-based uremic rat model. Calcif Tissue Int. 2015;96(4):347–58. doi: 10.1007/s00223-014-9929-5. [DOI] [PubMed] [Google Scholar]
- 55.Niwa T, Shimizu H. Indoxyl sulfate induces nephrovascular senescence. J Ren Nutr. 2012;22(1):102–6. doi: 10.1053/j.jrn.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 56.Tsirpanlis G. Cellular senescence, cardiovascular risk, and CKD: a review of established and hypothetical interconnections. Am J Kidney Dis. 2008;51(1):131–44. doi: 10.1053/j.ajkd.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 57.de Oliveira RM. Klotho RNAi induces premature senescence of human cells via a p53/p21 dependent pathway. FEBS Lett. 2006;580(24):5753–8. doi: 10.1016/j.febslet.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 58.Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, Shiizaki K, Gotschall R, Schiavi S, Yorioka N, Takahashi M, Boothman DA, Kuro-o M. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. 2011;286(10):8655–65. doi: 10.1074/jbc.M110.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou L, Li Y, Zhou D, Tan RJ, Liu Y. Loss of Klotho contributes to kidney injury by derepression of Wnt/beta-catenin signaling. J Am Soc Nephrol. 2013;24(5):771–85. doi: 10.1681/ASN.2012080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Satoh M, Nagasu H, Morita Y, Yamaguchi TP, Kanwar YS, Kashihara N. Klotho protects against mouse renal fibrosis by inhibiting Wnt signaling. Am J Physiol Renal Physiol. 2012;303(12):F1641–51. doi: 10.1152/ajprenal.00460.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shimada T, Takeshita Y, Murohara T, Sasaki K, Egami K, Shintani S, Katsuda Y, Ikeda H, Nabeshima Y, Imaizumi T. Angiogenesis and vasculogenesis are impaired in the precocious-aging klotho mouse. Circulation. 2004;110(9):1148–55. doi: 10.1161/01.CIR.0000139854.74847.99. [DOI] [PubMed] [Google Scholar]
- 62.Maekawa Y, Ishikawa K, Yasuda O, Oguro R, Hanasaki H, Kida I, Takemura Y, Ohishi M, Katsuya T, Rakugi H. Klotho suppresses TNF-alpha-induced expression of adhesion molecules in the endothelium and attenuates NF-kappaB activation. Endocrine. 2009;35(3):341–6. doi: 10.1007/s12020-009-9181-3. [DOI] [PubMed] [Google Scholar]
- 63.Song S, Gao P, Xiao H, Xu Y, Si LY. Klotho suppresses cardiomyocyte apoptosis in mice with stress-induced cardiac injury via downregulation of endoplasmic reticulum stress. PLoS One. 2013;8(12):e82968. doi: 10.1371/journal.pone.0082968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xie J, Cha SK, An SW, Kuro OM, Birnbaumer L, Huang CL. Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nature communications. 2012;3:1238. doi: 10.1038/ncomms2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Kottgen A, Levey AS, Levin A. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382(9887):158–69. doi: 10.1016/S0140-6736(13)60439-0. [DOI] [PubMed] [Google Scholar]
- 66.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80(1):17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 67.Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, Wiebe N, Tonelli M Alberta Kidney Disease N. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303(5):423–9. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 68.Barker SL, Pastor J, Carranza D, Quinones H, Griffith C, Goetz R, Mohammadi M, Ye J, Zhang J, Hu MC, Kuro-o M, Moe OW, Sidhu SS. The demonstration of alphaKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant. 2015;30(2):223–33. doi: 10.1093/ndt/gfu291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koh N, Fujimori T, Nishiguchi S, Tamori A, Shiomi S, Nakatani T, Sugimura K, Kishimoto T, Kinoshita S, Kuroki T, Nabeshima Y. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun. 2001;280(4):1015–20. doi: 10.1006/bbrc.2000.4226. [DOI] [PubMed] [Google Scholar]
- 70.Asai O, Nakatani K, Tanaka T, Sakan H, Imura A, Yoshimoto S, Samejima K, Yamaguchi Y, Matsui M, Akai Y, Konishi N, Iwano M, Nabeshima Y, Saito Y. Decreased renal alpha-Klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. Kidney Int. 2012;81(6):539–47. doi: 10.1038/ki.2011.423. [DOI] [PubMed] [Google Scholar]
- 71.Krajisnik T, Olauson H, Mirza MA, Hellman P, Akerstrom G, Westin G, Larsson TE, Bjorklund P. Parathyroid Klotho and FGF-receptor 1 expression decline with renal function in hyperparathyroid patients with chronic kidney disease and kidney transplant recipients. Kidney Int. 2010;78(10):1024–32. doi: 10.1038/ki.2010.260. [DOI] [PubMed] [Google Scholar]
- 72.Sakan H, Nakatani K, Asai O, Imura A, Tanaka T, Yoshimoto S, Iwamoto N, Kurumatani N, Iwano M, Nabeshima Y, Konishi N, Saito Y. Reduced renal alpha-Klotho expression in CKD patients and its effect on renal phosphate handling and vitamin D metabolism. PLoS One. 2014;9(1):e86301. doi: 10.1371/journal.pone.0086301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pavik I, Jaeger P, Ebner L, Wagner CA, Petzold K, Spichtig D, Poster D, Wuthrich RP, Russmann S, Serra AL. Secreted Klotho and FGF23 in chronic kidney disease Stage 1 to 5: a sequence suggested from a cross-sectional study. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2013;28(2):352–9. doi: 10.1093/ndt/gfs460. [DOI] [PubMed] [Google Scholar]
- 74.Kim HR, Nam BY, Kim DW, Kang MW, Han JH, Lee MJ, Shin DH, Doh FM, Koo HM, Ko KI, Kim CH, Oh HJ, Yoo TH, Kang SW, Han DS, Han SH. Circulating alpha-klotho levels in CKD and relationship to progression. Am J Kidney Dis. 2013;61(6):899–909. doi: 10.1053/j.ajkd.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 75.Kitagawa M, Sugiyama H, Morinaga H, Inoue T, Takiue K, Ogawa A, Yamanari T, Kikumoto Y, Uchida HA, Kitamura S, Maeshima Y, Nakamura K, Ito H, Makino H. A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS One. 2013;8(2):e56695. doi: 10.1371/journal.pone.0056695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ozeki M, Fujita S, Kizawa S, Morita H, Sohmiya K, Hoshiga M, Ishizaka N. Association of serum levels of FGF23 and alpha-Klotho with glomerular filtration rate and proteinuria among cardiac patients. BMC Nephrol. 2014;15:147. doi: 10.1186/1471-2369-15-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wan M, Smith C, Shah V, Gullet A, Wells D, Rees L, Shroff R. Fibroblast growth factor 23 and soluble klotho in children with chronic kidney disease. Nephrol Dial Transplant. 2013;28(1):153–61. doi: 10.1093/ndt/gfs411. [DOI] [PubMed] [Google Scholar]
- 78.Cano FJ, Freundlich M, Ceballos ML, Rojo AP, Azocar MA, Delgado IO, Ibacache MJ, Delucchi MA, Lillo AM, Irarrazabal CE, Ugarte MF. Longitudinal FGF23 and Klotho axis characterization in children treated with chronic peritoneal dialysis. Clin Kidney J. 2014;7(5):457–63. doi: 10.1093/ckj/sfu074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rotondi S, Pasquali M, Tartaglione L, Muci ML, Mandanici G, Leonangeli C, Sales S, Farcomeni A, Mazzaferro S. Soluble alpha -Klotho Serum Levels in Chronic Kidney Disease. Int J Endocrinol. 2015;2015:872193. doi: 10.1155/2015/872193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shimamura Y, Hamada K, Inoue K, Ogata K, Ishihara M, Kagawa T, Inoue M, Fujimoto S, Ikebe M, Yuasa K, Yamanaka S, Sugiura T, Terada Y. Serum levels of soluble secreted alpha-Klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis. Clin Exp Nephrol. 2012;16(5):722–9. doi: 10.1007/s10157-012-0621-7. [DOI] [PubMed] [Google Scholar]
- 81.Seiler S, Rogacev KS, Roth HJ, Shafein P, Emrich I, Neuhaus S, Floege J, Fliser D, Heine GH. Associations of FGF-23 and sKlotho with cardiovascular outcomes among patients with CKD stages 2–4. Clin J Am Soc Nephrol. 2014;9(6):1049–58. doi: 10.2215/CJN.07870713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park MY, Herrmann SM, Saad A, Eirin A, Tang H, Lerman A, Textor SC, Lerman LO. Biomarkers of kidney injury and klotho in patients with atherosclerotic renovascular disease. Clin J Am Soc Nephrol. 2015;10(3):443–51. doi: 10.2215/CJN.07290714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang B, Umbach AT, Chen H, Yan J, Fakhri H, Fajol A, Salker MS, Spichtig D, Daryadel A, Wagner CA, Foller M, Lang F. Up-regulation of FGF23 release by aldosterone. Biochem Biophys Res Commun. 2016;470(2):384–90. doi: 10.1016/j.bbrc.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 84.Zhou X, Chen K, Wang Y, Schuman M, Lei H, Sun Z. Antiaging Gene Klotho Regulates Adrenal CYP11B2 Expression and Aldosterone Synthesis. J Am Soc Nephrol. 2016;27(6):1765–76. doi: 10.1681/ASN.2015010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Borst MH, Vervloet MG, ter Wee PM, Navis G. Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. J Am Soc Nephrol. 2011;22(9):1603–9. doi: 10.1681/ASN.2010121251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shroff R, Aitkenhead H, Costa N, Trivelli A, Litwin M, Picca S, Anarat A, Sallay P, Ozaltin F, Zurowska A, Jankauskiene A, Montini G, Charbit M, Schaefer F, Wuhl E, Group ET. Normal 25-Hydroxyvitamin D Levels Are Associated with Less Proteinuria and Attenuate Renal Failure Progression in Children with CKD. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014090947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Devaraj S, Syed B, Chien A, Jialal I. Validation of an immunoassay for soluble Klotho protein: decreased levels in diabetes and increased levels in chronic kidney disease. Am J Clin Pathol. 2012;137(3):479–85. doi: 10.1309/AJCPGPMAF7SFRBO4. [DOI] [PubMed] [Google Scholar]
- 88.Yamashita T, Nifuji A, Furuya K, Nabeshima Y, Noda M. Elongation of the epiphyseal trabecular bone in transgenic mice carrying a klotho gene locus mutation that leads to a syndrome resembling aging. J Endocrinol. 1998;159(1):1–8. doi: 10.1677/joe.0.1590001. [DOI] [PubMed] [Google Scholar]
- 89.Kawaguchi H, Manabe N, Miyaura C, Chikuda H, Nakamura K, Kuro-o M. Independent impairment of osteoblast and osteoclast differentiation in klotho mouse exhibiting low-turnover osteopenia. J Clin Invest. 1999;104(3):229–37. doi: 10.1172/JCI5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kawaguchi H, Manabe N, Chikuda H, Nakamura K, Kuroo M. Cellular and molecular mechanism of low-turnover osteopenia in the klotho-deficient mouse. Cell Mol Life Sci. 2000;57(5):731–7. doi: 10.1007/s000180050037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yamashita T, Nabeshima Y, Noda M. High-resolution micro-computed tomography analyses of the abnormal trabecular bone structures in klotho gene mutant mice. J Endocrinol. 2000;164(2):239–45. doi: 10.1677/joe.0.1640239. [DOI] [PubMed] [Google Scholar]
- 92.Yamashita T, Yoshitake H, Tsuji K, Kawaguchi N, Nabeshima Y, Noda M. Retardation in bone resorption after bone marrow ablation in klotho mutant mice. Endocrinology. 2000;141(1):438–45. doi: 10.1210/endo.141.1.7252. [DOI] [PubMed] [Google Scholar]
- 93.Kashimada K, Yamashita T, Tsuji K, Nifuji A, Mizutani S, Nabeshima Y, Noda M. Defects in growth and bone metabolism in klotho mutant mice are resistant to GH treatment. J Endocrinol. 2002;174(3):403–10. doi: 10.1677/joe.0.1740403. [DOI] [PubMed] [Google Scholar]
- 94.Kawano K, Ogata N, Chiano M, Molloy H, Kleyn P, Spector TD, Uchida M, Hosoi T, Suzuki T, Orimo H, Inoue S, Nabeshima Y, Nakamura K, Kuro-o M, Kawaguchi H. Klotho gene polymorphisms associated with bone density of aged postmenopausal women. J Bone Miner Res. 2002;17(10):1744–51. doi: 10.1359/jbmr.2002.17.10.1744. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Q, Liu L, Lin W, Yin S, Duan A, Liu Z, Cao W. Rhein reverses Klotho repression via promoter demethylation and protects against kidney and bone injuries in mice with chronic kidney disease. Kidney Int. 2016 doi: 10.1016/j.kint.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 96.Langman CB, Brooks ER. Renal osteodystrophy in children: a systemic disease associated with cardiovascular manifestations. Growth Horm IGF Res. 2006;16(Suppl A):S79–83. doi: 10.1016/j.ghir.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 97.Chen Z, Qureshi AR, Ripsweden J, Wennberg L, Heimburger O, Lindholm B, Barany P, Haarhaus M, Brismar TB, Stenvinkel P. Vertebral bone density associates with coronary artery calcification and is an independent predictor of poor outcome in end-stage renal disease patients. Bone. 2016;92:50–57. doi: 10.1016/j.bone.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 98.Moorthi RN, Moe SM. CKD-mineral and bone disorder: core curriculum 2011. Am J Kidney Dis. 2011;58(6):1022–36. doi: 10.1053/j.ajkd.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Davidovich E, Davidovits M, Peretz B, Shapira J, Aframian DJ. The correlation between dental calculus and disturbed mineral metabolism in paediatric patients with chronic kidney disease. Nephrol Dial Transplant. 2009;24(8):2439–45. doi: 10.1093/ndt/gfp101. [DOI] [PubMed] [Google Scholar]
- 100.London GM, Marchais SJ, Guerin AP, Metivier F. Arteriosclerosis, vascular calcifications and cardiovascular disease in uremia. Curr Opin Nephrol Hypertens. 2005;14(6):525–31. doi: 10.1097/01.mnh.0000168336.67499.c0. [DOI] [PubMed] [Google Scholar]
- 101.Kestenbaum B, Belozeroff V. Mineral metabolism disturbances in patients with chronic kidney disease. Eur J Clin Invest. 2007;37(8):607–22. doi: 10.1111/j.1365-2362.2007.01840.x. [DOI] [PubMed] [Google Scholar]
- 102.Kaisar M, Isbel N, Johnson DW. Cardiovascular disease in patients with chronic kidney disease. A clinical review. Minerva Urol Nefrol. 2007;59(3):281–97. [PubMed] [Google Scholar]
- 103.Wesseling-Perry K. Defective skeletal mineralization in pediatric CKD. Curr Osteoporos Rep. 2015;13(2):98–105. doi: 10.1007/s11914-015-0253-4. [DOI] [PubMed] [Google Scholar]
- 104.van Ballegooijen AJ, Rhee EP, Elmariah S, de Boer IH, Kestenbaum B. Renal Clearance of Mineral Metabolism Biomarkers. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014121253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Obi Y, Hamano T, Isaka Y. Prevalence and prognostic implications of vitamin D deficiency in chronic kidney disease. Disease markers. 2015;2015:868961. doi: 10.1155/2015/868961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fernandez-Martin JL, Martinez-Camblor P, Dionisi MP, Floege J, Ketteler M, London G, Locatelli F, Gorriz JL, Rutkowski B, Ferreira A, Bos WJ, Covic A, Rodriguez-Garcia M, Sanchez JE, Rodriguez-Puyol D, Cannata-Andia JB C group. Improvement of mineral and bone metabolism markers is associated with better survival in haemodialysis patients: the COSMOS study. Nephrol Dial Transplant. 2015 doi: 10.1093/ndt/gfv099. [DOI] [PubMed] [Google Scholar]
- 107.Siomou E, Stefanidis CJ. FGF-23 in children with CKD: a new player in the development of CKD-mineral and bone disorder. Nephrol Dial Transplant. 2012;27(12):4259–62. doi: 10.1093/ndt/gfs315. [DOI] [PubMed] [Google Scholar]
- 108.Takenaka T, Inoue T, Miyazaki T, Hayashi M, Suzuki H. Xeno-Klotho Inhibits Parathyroid Hormone Signaling. J Bone Miner Res. 2016;31(2):455–62. doi: 10.1002/jbmr.2691. [DOI] [PubMed] [Google Scholar]
- 109.Kuro OM. The FGF23 and Klotho system beyond mineral metabolism. Clin Exp Nephrol. 2016 doi: 10.1007/s10157-016-1357-6. [DOI] [PubMed] [Google Scholar]
- 110.Salanova Villanueva L, Sanchez Gonzalez C, Sanchez Tomero JA, Aguilera A, Ortega Junco E. Bone mineral disorder in chronic kidney disease: Klotho and FGF23; cardiovascular implications. Nefrologia. 2016;36(4):368–75. doi: 10.1016/j.nefro.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 111.Yamada S, Giachelli CM. Vascular calcification in CKD-MBD: Roles for phosphate, FGF23, and Klotho. Bone. 2016 doi: 10.1016/j.bone.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hu MC, Kuro-o M, Moe OW. The emerging role of Klotho in clinical nephrology. Nephrol Dial Transplant. 2012;27(7):2650–7. doi: 10.1093/ndt/gfs160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jiang Y, Shen Z, Zhang J, Xing C, Zha X, Shen C, Zeng M, Yang G, Mao H, Zhang B, Yu X, Sun B, Ouyang C, Ge Y, Zhang L, Cheng C, Zhang J, Yin C, Chen H, Wang N. Parathyroidectomy Increases Heart Rate Variability and Leptin Levels in Patients with Stage 5 Chronic Kidney Disease. Am J Nephrol. 2016;44(3):245–54. doi: 10.1159/000449018. [DOI] [PubMed] [Google Scholar]
- 114.Lee SA, Lee MJ, Ryu GW, Jhee JH, Kim HW, Park S, Jung SY, Oh HJ, Park JT, Han SH, Kang SW, Yoo TH. Low serum intact parathyroid hormone level is an independent risk factor for overall mortality and major adverse cardiac and cerebrovascular events in incident dialysis patients. Osteoporos Int. 2016;27(9):2717–26. doi: 10.1007/s00198-016-3636-1. [DOI] [PubMed] [Google Scholar]
- 115.Seifert ME, de las Fuentes L, Rothstein M, Dietzen DJ, Bierhals AJ, Cheng SC, Ross W, Windus D, Davila-Roman VG, Hruska KA. Effects of phosphate binder therapy on vascular stiffness in early-stage chronic kidney disease. Am J Nephrol. 2013;38(2):158–67. doi: 10.1159/000353569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shoji T, Marubayashi S, Shigematsu T, Iseki K, Tsubakihara Y Committee of Renal Data R, Japanese Society for Dialysis T. Use of vitamin D receptor activator, incident cardiovascular disease and death in a cohort of hemodialysis patients. Ther Apher Dial. 2015;19(3):235–44. doi: 10.1111/1744-9987.12274. [DOI] [PubMed] [Google Scholar]
- 117.Hum JM, O’Bryan LM, Tatiparthi AK, Cass TA, Clinkenbeard EL, Cramer MS, Bhaskaran M, Johnson RL, Wilson JM, Smith RC, White KE. Chronic Hyperphosphatemia and Vascular Calcification Are Reduced by Stable Delivery of Soluble Klotho. J Am Soc Nephrol. 2016 doi: 10.1681/ASN.2015111266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fukumoto S. FGF23-FGF Receptor/Klotho Pathway as a New Drug Target for Disorders of Bone and Mineral Metabolism. Calcif Tissue Int. 2016;98(4):334–40. doi: 10.1007/s00223-015-0029-y. [DOI] [PubMed] [Google Scholar]
- 119.Shi M, Flores B, Gillings N, Bian A, Cho HJ, Yan S, Liu Y, Levine B, Moe OW, Hu MC. αKlotho Mitigates Progression of AKI to CKD through Activation of Autophagy. Journal of the American Society of Nephrology. 2015 doi: 10.1681/ASN.2015060613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sugiura H, Yoshida T, Tsuchiya K, Mitobe M, Nishimura S, Shirota S, Akiba T, Nihei H. Klotho reduces apoptosis in experimental ischaemic acute renal failure. Nephrol Dial Transplant. 2005;20(12):2636–45. doi: 10.1093/ndt/gfi165. [DOI] [PubMed] [Google Scholar]
- 121.Chen J, Zhang X, Zhang H, Lin J, Zhang C, Wu Q, Ding X. Elevated Klotho promoter methylation is associated with severity of chronic kidney disease. PLoS One. 2013;8(11):e79856. doi: 10.1371/journal.pone.0079856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rubinek T, Shulman M, Israeli S, Bose S, Avraham A, Zundelevich A, Evron E, Gal-Yam EN, Kaufman B, Wolf I. Epigenetic silencing of the tumor suppressor klotho in human breast cancer. Breast cancer research and treatment. 2012;133(2):649–57. doi: 10.1007/s10549-011-1824-4. [DOI] [PubMed] [Google Scholar]
- 123.Xie B, Zhou J, Yuan L, Ren F, Liu DC, Li Q, Shu G. Epigenetic silencing of Klotho expression correlates with poor prognosis of human hepatocellular carcinoma. Hum Pathol. 2013;44(5):795–801. doi: 10.1016/j.humpath.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 124.Yang HC, Deleuze S, Zuo Y, Potthoff SA, Ma LJ, Fogo AB. The PPARgamma agonist pioglitazone ameliorates aging-related progressive renal injury. J Am Soc Nephrol. 2009;20(11):2380–8. doi: 10.1681/ASN.2008111138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang R, Zheng F. PPAR-gamma and aging: one link through klotho? Kidney Int. 2008;74(6):702–4. doi: 10.1038/ki.2008.382. [DOI] [PubMed] [Google Scholar]
- 126.Zhang H, Li Y, Fan Y, Wu J, Zhao B, Guan Y, Chien S, Wang N. Klotho is a target gene of PPAR-gamma. Kidney Int. 2008;74(6):732–9. doi: 10.1038/ki.2008.244. [DOI] [PubMed] [Google Scholar]
- 127.Chen LJ, Cheng MF, Ku PM, Lin JW. Rosiglitazone increases cerebral klotho expression to reverse baroreflex in type 1-like diabetic rats. BioMed research international. 2014;2014:309151. doi: 10.1155/2014/309151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou Q, Lin S, Tang R, Veeraragoo P, Peng W, Wu R. Role of Fosinopril and Valsartan on Klotho Gene Expression Induced by Angiotensin II in Rat Renal Tubular Epithelial Cells. Kidney Blood Press Res. 2010;33(3):186–192. doi: 10.1159/000316703. [DOI] [PubMed] [Google Scholar]
- 129.Yoon HE, Ghee JY, Piao S, Song JH, Han DH, Kim S, Ohashi N, Kobori H, Kuro-o M, Yang CW. Angiotensin II blockade upregulates the expression of Klotho, the anti-ageing gene, in an experimental model of chronic cyclosporine nephropathy. Nephrol Dial Transplant. 2011;26(3):800–13. doi: 10.1093/ndt/gfq537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Karalliedde J, Maltese G, Hill B, Viberti G, Gnudi L. Effect of renin-angiotensin system blockade on soluble Klotho in patients with type 2 diabetes, systolic hypertension, and albuminuria. Clin J Am Soc Nephrol. 2013;8(11):1899–905. doi: 10.2215/CJN.02700313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Narumiya H, Sasaki S, Kuwahara N, Irie H, Kusaba T, Kameyama H, Tamagaki K, Hatta T, Takeda K, Matsubara H. HMG-CoA reductase inhibitors up-regulate anti-aging klotho mRNA via RhoA inactivation in IMCD3 cells. Cardiovasc Res. 2004;64(2):331–6. doi: 10.1016/j.cardiores.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 132.Ritter CS, Zhang S, Delmez J, Finch JL, Slatopolsky E. Differential expression and regulation of Klotho by paricalcitol in the kidney, parathyroid, and aorta of uremic rats. Kidney Int. 2015;87(6):1141–52. doi: 10.1038/ki.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, Hsiao LL. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125(18):2243–55. doi: 10.1161/CIRCULATIONAHA.111.053405. [DOI] [PubMed] [Google Scholar]
- 134.Forster RE, Jurutka PW, Hsieh JC, Haussler CA, Lowmiller CL, Kaneko I, Haussler MR, Kerr Whitfield G. Vitamin D receptor controls expression of the anti-aging klotho gene in mouse and human renal cells. Biochem Biophys Res Commun. 2011;414(3):557–62. doi: 10.1016/j.bbrc.2011.09.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chang JR, Guo J, Wang Y, Hou YL, Lu WW, Zhang JS, Yu YR, Xu MJ, Liu XY, Wang XJ, Guan YF, Zhu Y, Du J, Tang CS, Qi YF. Intermedin1–53 attenuates vascular calcification in rats with chronic kidney disease by upregulation of alpha-Klotho. Kidney Int. 2016;89(3):586–600. doi: 10.1016/j.kint.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 136.Hu MC. Klotho connects intermedin1–53 to suppression of vascular calcification in chronic kidney disease. Kidney Int. 2016;89(3):534–7. doi: 10.1016/j.kint.2015.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shiraki-Iida T, Iida A, Nabeshima Y, Anazawa H, Nishikawa S, Noda M, Kuro-o M, Nabeshima Y. Improvement of multiple pathophysiological phenotypes of klotho (kl/kl) mice by adenovirus-mediated expression of the klotho gene. J Gene Med. 2000;2(4):233–42. doi: 10.1002/1521-2254(200007/08)2:4<233::AID-JGM110>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 138.Wang Y, Sun Z. Antiaging gene Klotho regulates endothelin-1 levels and endothelin receptor subtype B expression in kidneys of spontaneously hypertensive rats. J Hypertens. 2014;32(8):1629–36. doi: 10.1097/HJH.0000000000000233. discussion 1636. [DOI] [PubMed] [Google Scholar]
- 139.Wang Y, Sun Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension. 2009;54(4):810–7. doi: 10.1161/HYPERTENSIONAHA.109.134320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mitani H, Ishizaka N, Aizawa T, Ohno M, Usui S, Suzuki T, Amaki T, Mori I, Nakamura Y, Sato M, Nangaku M, Hirata Y, Nagai R. In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension. 2002;39(4):838–43. doi: 10.1161/01.hyp.0000013734.33441.ea. [DOI] [PubMed] [Google Scholar]
- 141.Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, Kuro-o M, Nabeshima Y, Kurabayashi M, Nagai R. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun. 2000;276(2):767–72. doi: 10.1006/bbrc.2000.3470. [DOI] [PubMed] [Google Scholar]
- 142.Xie J, Yoon J, An SW, Kuro OM, Huang CL. Soluble Klotho Protects against Uremic Cardiomyopathy Independently of Fibroblast Growth Factor 23 and Phosphate. J Am Soc Nephrol. 2015;26(5):1150–60. doi: 10.1681/ASN.2014040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hum JM, O’Bryan L, Smith RC, White KE. Novel functions of circulating Klotho. Bone. 2016 doi: 10.1016/j.bone.2016.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, Moe OW. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010;24(9):3438–50. doi: 10.1096/fj.10-154765. [DOI] [PMC free article] [PubMed] [Google Scholar]