Abstract
Infections caused by the bacterial pathogen Acinetobacter baumannii are a mounting concern for healthcare practitioners as widespread antibiotic resistance continues to limit therapeutic treatment options. The biological processes used by A. baumannii to cause disease are not well-defined, but recent research has indicated that secreted proteins may play a major role. A variety of mechanisms have now been shown to contribute to protein secretion by A. baumannii and other pathogenic species of Acinetobacter, including a type II and type VI secretion system, autotransporter, and outer membrane vesicles. In this review, we summarize the current knowledge of secretion systems in Acinetobacter species, and highlight their unique aspects that contribute to the pathogenicity and persistence of these emerging pathogens.
Keywords: Acinetobacter, secretion systems, T2SS, T6SS, autotransporters, outer membrane vesicles
Acinetobacter baumannii: a Drug-Resistant Pathogen
Hospital-acquired infections caused by opportunistic bacterial pathogens are a significant contributor to patient death and a major healthcare burden [1]. Coupled with an alarming increase in antibiotic resistance, and few new antibiotics in development, drug-resistant bacterial pathogens have quickly established themselves as a global threat to healthcare security. These infections are estimated to directly or indirectly claim the lives of 100,000 people and result in an additional $34 billion in health care costs each year in the US alone [2]. Due to the evolutionary capabilities of microorganisms, resistance to antibiotics is expected to occur; indeed, resistance has been detected since the beginning of widespread antibiotic use [3]. However, the sheer number of bacterial species that are now displaying multidrug resistance (MDR) to some of the ‘last line of defense’ antibiotics is of great concern [4]. Compared with decades past, there is no longer a fresh supply of novel antibiotics in the developmental pipeline, and thus the threat of a ‘post-antibiotic era’ is a real concern [4, 5]. Pan-drug resistant bacteria, which are resistant to every clinically relevant antibiotic, have already been isolated [6, 7]. In the absence of clear progress in advancing antimicrobial discovery, it is imperative to understand the molecular mechanisms used by bacteria to persist in hospital environments and cause infection so that alternative treatment strategies can be designed.
Acinetobacter baumannii has recently emerged as a one of the most concerning Gram-negative pathogens to infiltrate the hospital setting, and is among the few pathogens that have significantly increased as a cause of infection in US hospitals [8]. A. baumannii is responsible for more than 12,000 infections per year in the US and is considered a “serious” threat to healthcare systems (www.cdc.gov/drugresistance/threat-report-2013/). This threat is compounded by the frequent occurrence of MDR A. baumannii isolates. Patients who are immunocompromised are most at risk for contracting A. baumannii, with infections most commonly manifesting as pneumonia [9, 10]. New strategies for treating and managing MDR A. baumannii infections are urgently needed. Unfortunately, relatively little is known about the basic biological processes that have driven A. baumannii to successfully infiltrate the healthcare environment and cause disease.
Protein secretion through dedicated cell surface structures is essential for interactions of A. baumannii with the environment and host tissues, and therefore represent attractive targets for antibiotics and vaccines [11]. Here, we summarize the current knowledge on the secretory systems that have been studied in A. baumannii and other clinically relevant species of Acinetobacter. We particularly focus on the recently characterized type II and type VI secretion systems, as well as outer membrane vesicles and secretion of proteins that have been implicated in virulence but for which no dedicated secretory apparatus has been identified.
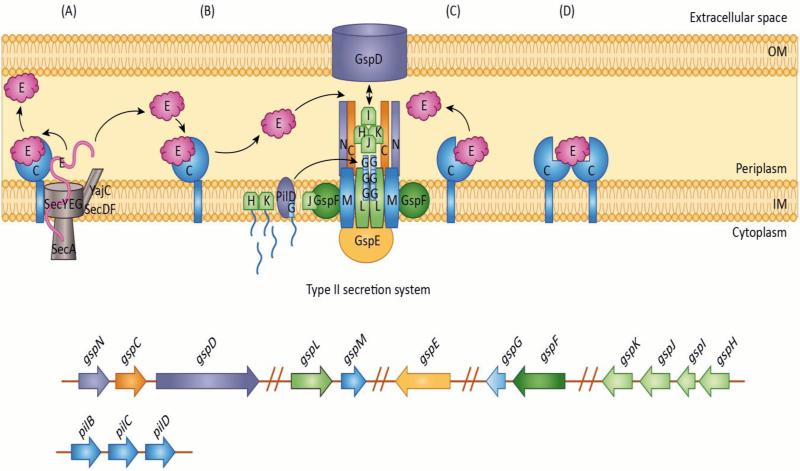
Type II Secretion
The type II secretion system (T2SS) is widespread among Gram-negative pathogens as well as environmental species capable of living in a variety of conditions [12, 13]. The T2SS is a two-step process, where proteins with an N-terminal secretion signal are translocated across the inner membrane by the general secretory pathway (Sec) or the Twin-arginine (Tat) system to the periplasmic space [13]. Following removal of the secretion signal, folded proteins are then secreted to the extracellular space by the T2SS machinery. The T2SS promotes in vivo survival and virulence of several pathogens, including Vibrio cholerae, enterotoxigenic Escherichia coli, Pseudomonas aeruginosa and Legionella pneumophila, through the activities of secreted toxins and hydrolytic enzymes aiding in nutrient acquisition [14-20]..
Eijkelkamp et al. were the first to report the presence of T2SS components in A. baumannii [21]. Subsequently, the T2SS of A. baumannii ATCC 17978, a commonly used laboratory strain, was shown to be active [22]. Mutagenesis of the assembly ATPase, gspE1, or secretin, gspD, in this strain abolished secretion of a putative lipase, LipA, identifying it as a T2SS effector. Biochemical characterization of LipA showed it possessed serine hydrolase activity, capable of hydrolyzing 4-nitrophenyl myristate. LipA was required for growth in minimal media supplemented with long-chain fatty acids as the sole carbon source, indicating an important role in nutrient acquisition [22]. Furthermore, the T2SS and T2SS-dependent lipid utilization were required for competitive colonization of a neutropenic murine model of infection [22]. Interestingly, A. baumannii ATCC 17978 encodes two assembly ATPase orthologs, where gspE1 is required for LipA secretion under laboratory conditions and gspE2 is dispensable [22]. The gene encoding inner membrane platform protein GspN is absent from many bacterial species with functional T2SS and is not required for secretion of the type II pullulanase in Klebsiella oxytoca [23, 24]. Neither gspN, encoding an inner membrane platform protein, nor gspE2 were required for secretion of LipA, suggesting they are not involved in the A. baumannii T2SS [22]. Additionally, an A. baumannii mutant lacking gspN was not defective for virulence in a mouse model of pneumonia [25]. This may suggest that GspE2 and/or GspN may play a role in T2SS under different conditions or may impact secretion of other effectors.
Harding et al. demonstrated T2SS activity in Acinetobacter strains A. nosocomialis M2 (formerly A. baumannii M2), A. pittii, A. calcoaceticus, and A. junii as well as A. baumannii, through heterologous expression and secretion of A. nosocomialis T2SS effectors [26]. Interestingly A. nosocomialis M2 encodes one prepillin peptidase, pilD, that is shared by the type IV pilus system and the T2SS. PilD cleaved leader peptides from both pre-pilins and pseudopilins, allowing formation of functional pilus structures [26]. Proteomic comparison of supernatants from wildtype A. nosocomialis M2 and an outer membrane secretin gspD mutant revealed numerous putative effector proteins, all with predicted N-terminal Sec signals [26]. Three of these effectors, CpaA, LipA and LipH, were confirmed as T2SS effectors, and LipA and LipH were confirmed as lipases through their ability to cleave para-nitrophenol palmitate. CpaA was previously shown to be a secreted zinc-dependent metallo-endopeptidase that was capable of degrading fibrinogen and factor V, deregulating blood coagulation [27]. The T2SS of A. nosocomialis was required for full virulence in Galleria mellonella and pulmonary murine models of infection [26]. Additionally, the T2SS-deficient strain did not disseminate to the liver or spleen as efficiently as the wildtype or complemented strains, suggesting it plays an important role in virulence. Other putative A. nosocomialis T2SS effectors include hypothetical proteins and a rhombotarget A protein. Confirmation that these proteins are secreted in a T2SS- dependent manner, characterization of their biochemical activities, and investigation into the specific role of each effector for pathogenicity of A. nosocomialis and other Acinetobacter spp. should be the focus of future work.
Two Acinetobacter T2SS effectors, LipA and CpaA, were found to require membrane bound chaperones, LipB and CpaB respectively, for their secretion [26, 28]. Topological prediction servers and bioinformatic analysis of these membrane bound chaperones suggested that their N-terminal transmembrane domains are imbedded in the inner membrane, with the C-terminal globular portions present in the periplasm. The requirement of T2SS chaperones is reminiscent of type III secretion systems, where the chaperones are soluble cytoplasmic proteins that interact with a specific effector or multiple effectors and aid in folding, stabilization, and regulating secretion of these effectors [29]. CpaB is the first membrane bound chaperone required for secretion of a T2SS protease. CpaB and LipB appear to be specific for CpaA and LipA, as neither was required for the secretion of LipH by various Acinetobacter species [26]. The impact of these chaperones on the entire secretome of A. nosocomialis remains elusive. LipB is similar to the Burkholderia glumae membrane bound chaperone lipase-specific foldase (Lif), which is required for production of enzymatically active lipase. The transmembrane domain of Lif is not required for its steric chaperone activity [30]. Understanding what the role of these membrane bound T2SS chaperones are, and specifically whether the membrane anchor is required for their function warrants investigation. There are several hypothetical mechanisms by which the chaperone may influence secretion of its effector (Figure 1). These chaperones could aid in the proper folding of their cognate effectors, sequester the effectors thereby regulating their secretion and/or impact effector secretion through protein-protein interactions with the Sec or T2SS machinery. Some chaperones bind effectors to protect the host bacterium against its enzymatic activity. It is possible that CpaB binds CpaA in order to protect periplasmic proteins from proteolysis. Alternatively, CpaA may be very specific for eukaryotic proteins such as fibrinogen and Factor V, eliminating the necessity of immunity. Understanding the extent of T2SS chaperones across bacterial genera is a necessary goal as they may have been overlooked in well-characterized T2SS of Pseudomonas, Vibrio, and Burkholderia species.
Figure 1. T2SS chaperone and effector interactions in A. nosocomialis M2.
In A. nosocomialis M2 the gsp genes required for T2SS are located in six separate operons. Secretion of type II effectors, CpaA and LipA, require specific membrane associated chaperones, CpaB and LipB. How these chaperones (C in figure) function in the secretion of their cognate effectors (E in figure) is unknown. This figure depicts potential chaperone-effector interaction mechanisms:
A) The effector is transported unfolded from the cytoplasm to the periplasmic space by the Sec secretion system. Once in the periplasm, the chaperone interacts with the effector initiating folding. The effector is then released into the periplasmic space, where it will eventually be secreted to the extracellular space by the T2SS. Alternatively, the effector may remain bound to the chaperone until it is released for secretion by the T2SS thereby preventing activity of the effector protein.
B) Upon entering the periplasmic space through the Sec secretion system, the effector folds. Once folded, it interacts with its chaperone. The interaction between the effector and chaperone may block activity of the effector protein through sequestration of the effector until it is released for secretion by the type II secretion system. C) The chaperone may function to guide the effector to the type II secretion machinery, through a secondary interaction with a component of the type II secretion system. D) The chaperone may function as a multimer when binding the effector protein.
OM: outer membrane, IM: Inner membrane.
Acinetobacter spp. can be found in the environment, the hospital setting as well as colonize the human host. It would be interesting to investigate whether secretion of specific effectors is dependent upon key environmental signals and what the specific contribution of each effector is on virulence, dissemination and/or survival of Acinetobacter in various environments. Little is known about the regulation of T2SS in Acinetobacter spp. The T2SS is active under laboratory conditions at mid-log phase in several species of Acinetobacter [22, 26], however the regulatory mechanism controlling this system and/or its effectors remains unknown. T2SS and/or production of effector proteins in P. aeruginosa and Burkholderia cepacia are regulated through quorum sensing [13, 31, 32]. The T2SS components are constitutively expressed in V. cholerae under laboratory conditions, however expression of the effectors is growth phase dependent [33]. In A. baumannii, expression of the T2SS was found to be slightly up regulated during exponential phase, and the presence of human serum increased expression of the LipH effector, suggesting environmental factors as well as growth phase may impact the regulation of T2SS in this organism [34]. It would be interesting to know whether the stress induced by human serum resulted in the change in gene expression, or if a component of the serum induced LipH expression due to altered nutrient availability.
It is clear that the Acinetobacter T2SS plays an important role in colonization and infection of mice. Whether T2SS effectors inducing tissue damage, aiding in nutrient acquisition, and fighting the human host defenses during colonization, or a combination of these mechanisms, causes this impact on virulence is yet to be elucidated.
Type VI Secretion
The export of antibacterial effectors by bacteria has recently emerged as a common mechanism for killing competitors [35, 36]. Many Gram-negative bacteria encode a type VI secretion system (T6SS), a multi-component secretion machine capable of mediating lethal injections of protein toxins into other bacteria in a contact-dependent manner [37-39]. The 13 ‘core’ structural proteins of the machinery are usually encoded in a single genetic locus and are generally well conserved across bacterial species [40]. Additional accessory components can be present, and frequently participate in regulatory aspects [41]. After assembly, the T6SS undergoes a rapid contraction event, releasing its effector cargo [42]. The majority of characterized T6SS effectors target other bacteria, with protection from self-intoxication provided by immunity proteins encoded nearby the effector [43]. Two structural components, Hcp and VgrG, are hallmarks of the T6SS, and both can be found in culture supernatants from bacteria with an active secretion system [44]. Detection of Hcp secretion is commonly used as a marker for T6SS activity. VgrG's can additionally encode effector functions, although this is not a universal property [45].
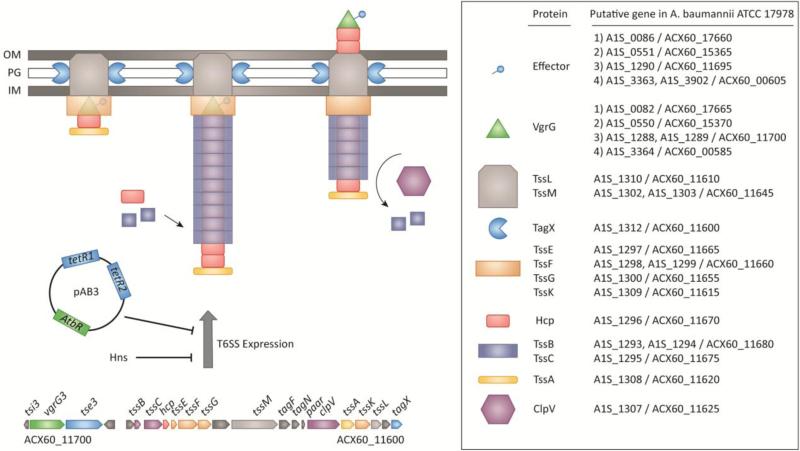
The core components of the Acinetobacter T6SS are well conserved and found in a single chromosomal locus, with vgrG, effector/immunity, and other accessory genes sporadically distributed across the chromosome [21, 46, 47]. Orthologs of the genes implicated in the biogenesis and assembly of the T6SS in other well-studied organisms are present in the main Acinetobacter T6SS locus. These include genes encoding the priming protein TssA [48], sheath components TssB and TssC [49], the tubule protein Hcp [50], baseplate components TssE, F, G, and K [51], membrane complex proteins TssL and M [52], and the ATPase ClpV [53]. A single PAAR protein, which, like VgrG's, can play both a structural and effector role [54], is present in the main cluster, with others distributed across the chromosome. One notable exception is the apparent absence of TssJ, a lipoprotein that has been shown to interact with TssL and TssM as part of the T6SS membrane complex [51, 55, 56]. A complete mutational analysis of the main T6SS cluster in A. baylyi has confirmed these core genes as being essential for T6SS function [46]. In addition to the 13 core components, the T6SS clusters of Acinetobacter spp. contain additional genes that play a role in T6SS function. Based on results from the mutational analysis in A. baylyi, at least three other genes in the T6SS cluster are essential for Hcp secretion. Two of these encode for hypothetical proteins, while the third encodes for the newly identified protein named TagX, which may be required for transit of the T6SS apparatus through the peptidoglycan layer. A second set of three additional genes, beyond the core components, seem to be important for regulatory aspects of T6SS, as mutation of these three genes resulted in increased Hcp secretion. Two of these genes are homologs of the previously identified T6SS accessory genes tagF and tagN, while the third does not seem to have any homologs outside of Acinetobacter [57, 58].
Genetic and functional studies have begun to unravel both the similar and unique aspects of the Acinetobacter T6SS (Figure 2). Hcp was detected in culture supernatants from wild type A. baummnnii ATCC 19606T, but was absent from a lipooligosaccharide (LOS) deficient, colistin resistant derivative of this strain [59]. Loss of Hcp secretion was concomitant with the down-regulation of several other T6SS genes, as well as the up-regulation of genes associated with membrane stability, suggesting an inability to form a functional apparatus in the membrane-compromised, LOS-deficient strain. Genetic analyses undertaken in A. baumannii ATCC 17978, A. baumannii DSM30011, and A. nosocomialis M2 confirmed the importance of structural genes tssM and tssC for Hcp secretion [60-62]. Additionally, the levels of Hcp secretion were shown to vary among different strains of A. baumannii [60]. As in other bacteria, the T6SS of A. nosocomialis M2 was shown to be essential for killing E. coli in a contact-dependent manner [61]. However, loss of essential T6SS genes in A. baumannii ATCC 17978 did not have an effect on anti-bacterial activity, although this result has now been clarified in a more recent study, which showed this strain could outcompete other bacteria when a fully T6SS active variant was isolated [63]. In addition to killing E. coli, A. baumannii with an active T6SS have been shown to kill K. pneumoniae and P. aeruginosa, and different strains of A. baumannii are also able to kill each other [62, 63]. A. baylyi ADP1 has also been determined to possess an active T6SS with anti-bacterial activity, and requires PAAR proteins for proper T6SS function [54]. These PAAR proteins are proposed to sit atop VgrG proteins and mediate cell puncturing, and in some case also encode effector functions. A. baylyi possesses 3 of these PAAR proteins, and although none are essential for T6SS activity when mutated alone, mutation of all 3 PAAR's results in a non-functional T6SS [54]. Whether these PAAR proteins are effectors, structural elements, or both remains to be determined. A recent bioinformatics analysis has shown that the number of paar genes varies among different strains of A. baumannii, similar to the variation seen in the number of vgrG genes [21]. The four vgrG genes encoded by A. baumannii ATCC 17978 play different roles in T6SS function and effector delivery. A single VgrG, VgrG1, plays an essential role for Hcp secretion, while the other three VgrG's seem to be important for mediating effector delivery [46]. These effectors include a putative nuclease (Tde) and cognate immunity protein (Tdi) pair, which require VgrG2 for T6SS-mediated killing of E. coli. Furthermore, this study identified the novel T6SS component TagX, which acts as an LD-endopeptidase that cleaves between L-Ala-D-Glu of peptidoglycan. TagX was essential for Hcp secretion in both A. baumannii and A. baylyi, and therefore was proposed to allow T6SS transit through the cell wall of the encoding organism. Analogous enzymes have been described for other secretion systems, but TagX represents the first peptidoglycan hydrolase required for T6SS biogenesis [46, 64].
Figure 2. Biogenesis and Regulation of the T6SS in A. baumannii ATCC 17978.
The predicted assembly process, and the proteins involved, in assembly of the T6SS are shown. The single T6SS operon from A. baumannii ATCC 17978 is shown, which contains the core genes for T6SS. The process of T6SS biogenesis has not been studied in detail for A. baumannii, but likely proceeds with the assembly of a membrane-spanning complex (TssL, M), baseplate components (TssE, F, G, K), and priming protein TssA followed by the polymerization of the VgrG-tipped Hcp tubule wrapped in the TssB/C sheath. The newly identified TagX peptidoglycan hydrolase may interact with the membrane complex to allow for localized hydrolysis of the sacculus, allowing the T6SS to pass through the periplasm. To date, only effectors secreted in a VgrG-dependent manner have been identified for A. baumannii. Gene identifiers (right side of figure) are listed for the model A. baumannii ATCC 17978 strain, but are conserved in other strains and species. The gene identifiers for both the original 17978 (accession: CP00521, A1S_XXXX) and the resequenced 17978 (accession: CP012004, ACX60_XXXXX) are shown. The TetR-like regulatory proteins encoded by genes on pAB3 and an Hns protein have been shown to repress expression of the T6SS in A. baumannii.
OM: outer membrane, PG: peptidoglycan, IM: inner membrane.
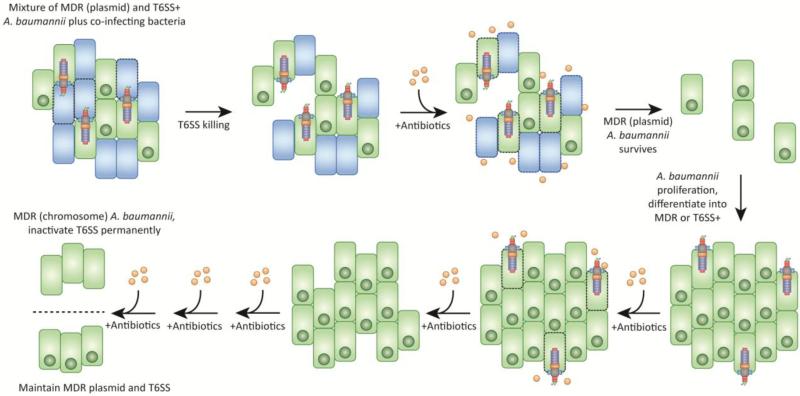
Several high-throughput screens to identify A. baumannii virulence factors have failed to implicate the T6SS in pathogenicity [25, 65, 66]. It remains to be determined what, if any, benefit the T6SS may provide to A. baumannii during infection. Galleria mellonella infected with a tssM mutant of A. baumannii DSM30011 did not succumb to infection as quickly as worms infected with wild-type, but ultimately were killed to the same extent at later time points [62]. In some recent clinical isolates of A. baumannii the T6SS cluster is absent or degraded, suggesting that it is not essential for causing disease [67]. It has also been noted that a general correlation exists between multi-drug resistance and an inactive T6SS, as multidrug resistant strains tend to repress the expression of their T6SS [62, 63]. We, and others, have suggested that this may represent a fitness trade-off between the two phenotypes: the cost of producing and firing the T6SS may be prohibitive when a strain also carries several antibiotic resistance genes [63, 67]. In environments where antibiotic resistance is key for survival, perhaps in a patient being treated for infection, it seems to be advantageous to either repress T6SS or delete the cluster altogether. A hypothetical model describing this scenario is presented in Figure 3. This may reflect a scenario where A. baumannii is less likely to encounter competitors under these conditions (due to high antibiotic levels), and therefore does not require T6SS-mediated killing. In this case, expression of antibiotic resistance genes provides the most fitness benefit to the organism.
Figure 3. Hypothetical Model for T6SS Deletion and MDR Maintenance in Clinical Isolates of A. baumannii.
The genome sequences of several MDR A. baumannii clinical isolates has shown they lack a full T6SS cluster. This model proposes that pressure to maintain drug resistance by antibiotic treatment may select for strains that either repress their T6SS or degrade the T6SS cluster, permanently inactivating the secretion system in favour of antibiotic resistance.
A few studies have addressed the regulation of the T6SS in A. baumannii. An hns deficient strain of A. baumannii ATCC 17978 was found to differentially express a number of genes, including genes for T6SS [68]. This report was prior to the finding that A. baumannii ATCC 17978 also contains a large plasmid, that, when lost, resulted in T6SS activation. The contribution of hns to T6SS regulation in the presence or absence of this plasmid, called pAB3, remains to be determined, but could represent an additional level of regulation. This large plasmid has been found in several recently isolated clinical strains, and in all cases tested thus far, can be lost under laboratory culture without selection [63]. The presence of the plasmid invariably resulted in T6SS repression, and when lost resulted in T6SS activation. Repression of T6SS was found to be linked to the presence of two plasmid-encoded tetR-like genes, which when expressed heterologously silenced T6SS in numerous A. baumannii strains and other species. Interestingly, the plasmid found in A. baumannii ATCC 17978, a strain isolated in the early 1950s, carried a single resistance cassette to sulfamethoxazole. The plasmids identified from more recent clinical isolates have acquired many additional resistance genes, with nearly a dozen identified on plasmid pAB04-1 from A. baumannii Ab04. The insertion of antibiotic resistance genes on this T6SS-controlling plasmid may therefore represent an alternative evolutionary strategy to maintain both the T6SS and multidrug resistance, rather than deleting the T6SS cluster. This could allow A. baumannii to transition from a “survival” mode when antibiotics are present, to a more aggressive “killing” mode when antibiotics are absent and other competing bacteria may encroach on its territory. The finding that this T6SS-controlling, resistance plasmid is conjugative may further allow the subset of cells that lose the plasmid to regain the plasmid, and antibiotic resistance, from sister cells later on. This also raises the interesting hypothesis that the plasmid acquired T6SS repressors in order to allow for its own propagation, as an active T6SS would kill potential recipients, inviting a more plasmid-centric view . Nonetheless, under laboratory conditions, when starting with an isogenic population of plasmid-containing cells, approximately 1-5% of the bacteria will lose the plasmid. Due to this frequent plasmid loss, we urge other researchers to carefully test their strains for the presence of this plasmid in order to avoid working with heterogeneous populations. This is particularly important for the commonly used laboratory strain A. baumannii ATCC 17978.
Ata Autotransporter
Acinetobacter spp. have also been shown to express a trimeric autotransporter, termed Ata, which is a representative member of the type Vc secretion system. Initial studies using A. baumannii ATCC 17978 found that Ata was indeed surface exposed and acted as an adhesin, binding multiple extracellular matrix components, particularly, collagen IV, collagen III and collagen V [69]. Ata was also implicated in biofilm formation/maintenance and virulence [69]. In a subsequent study, Ata from A. baumannii ATCC 19606T was found to be required for adherence to human endothelial cells under both static and dynamic flow conditions [70]. Interestingly, although most A. baumannii strains encode an Ata ortholog, Ata expression was found to be highly heterogeneous amongst a panel of clinical isolates, highlighting a complex regulatory network and the need for appropriate model strains [69]. In another study, Ata expression was found to be partially dependent on an hns-like gene as the disruption mutant displayed a roughly 10-fold increase in ata expression [71]. Similar studies using a different Acinetobacter species, Acinetobacter spp. Tol 5, identified the Acinetobacter trimeric autotransporter adhesin (AtaA) as an enormous nanofiber emanating from the outer membrane that was readily observed via electron microscopy [72]. As expected, AtaA was required for optimal adherence on many surfaces including collagen, polystyrene, polyvinylchloride, glass, and stainless steel. To demonstrate the universal role of AtaA in adherence to the aforementioned surfaces, AtaA was ectopically expressed in A. baylyi ADP1, which has a low propensity for cellular association with any of the tested substrata. ADP1 expressing AtaA demonstrated enhanced binding to all substrata tested when compared to the parental and empty vector controls, emphasizing the significant contribution AtaA can have on cellular adherence [72]. Subsequent structural and molecular studies on AtaA have provided the crystal structure of the C-terminal passenger domain of AtaA and identified a novel periplasmic protein, TpgA, required for anchoring AtaA to the outer membrane [73, 74]. Collectively, Acinetobacter trimeric autotransporters appear to be universally expressed by A. baumannii clinical isolates and many other Acinetobacter spp., playing a significant role in bacterial adherence and virulence.
Outer Membrane Vesicles and Other Secreted Proteins
Outer membrane vesicles (OMVs) are spherical nanocompartments ranging in size from 20-300 nanometers. OMVs are readily purified from culture supernatants of many Gram-negative bacteria, including Acinetobacter species; yet, their assembly mechanisms and cargo selection processes are contentious topics. Nevertheless, studies using Bacteroides, Porphorymonas, Haemophilus influenza and Salmonella enterica serovar Typhimurium have started to shed light on these dynamic processes [75-78]. Regardless of their biogenesis mechanisms, the role of OMVs in bacterial pathogenesis has been well documented and continues to be a topic of study [79].
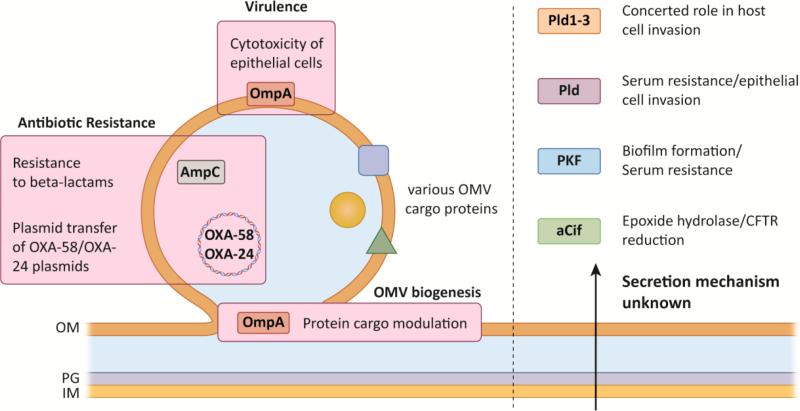
Similar to many other Gram-negative bacteria, Acinetobacter species have been shown to produce OMVs (Figure 4). Special emphasis has been placed on the role of A. baumannii OMVs, however, OMVs produced by A. nosocomialis [80], A. radioresistans [81], and A. baylyi [82] have also been studied for proteomic contents and virulence attributes. A. baumannii OMVs were originally identified as the secretion platform for outer membrane protein A (previously designated outer membrane protein 38), an important virulence factor and one of the more abundant outer membrane proteins in A. baumannii [83, 84]. Initial molecular and proteomic characterizations of A. baumannii OMVs defined their size range as 20-160nm [85]; however, a diverse array of OMV sizes, shapes, and compositions can be readily purified depending on what phase of growth the A. baumannii OMVs are captured. Cryo-electron tomographic analysis of A. baumannii ATCC 19606T at different stages of growth defined multiple sub-types of membrane vesicles, all of which may contribute to the phenotypes observed in OMV focused studies [86]. The first membrane vesicles observed were small prototypical outer membrane vesicles (less than 100 nm) budding from the outer-membrane of log phase A. baumannii cells. These vesicles appear to be the most commonly characterized OMVs from A. baumannii and seem to be the focus of many proteomic and pathogenic studies discussed below. Larger vesicles (ranging from 200-500 nm) were also observed emanating from the surface of A. baumannii; however, these large vesicles were only observed at the septa of dividing bacteria indicating they may be products of anomalous cellular division. Vesicles from bacteria in stationary phase contained prototypical OMVs but also displayed vesicles with both inner and outer membranes as well as a thin layer of peptidoglycan. These Inner-outer membrane vesicles (IOMVs), which are larger than prototypical OMVs (about 2.5 times larger), have yet to be fully characterized [86].
Figure 4. Alternative Mechanisms of Protein Secretion in Acinetobacter spp.
Left: OMVs generated by formation of outer membrane blebs can contain hundreds of proteins, including important virulence (OmpA) and antibiotic resistance factors (AmpC, plasmid DNA). OmpA may additionally play a role in modulating protein packaging into OMVs. Right: Proteins identified in culture supernatants that may contribute to virulence, but have no described secretory mechanism, are shown on right.
To start unraveling the roles of OMVs in Acinetobacter pathogenesis, specifically A. baumannii pathogenesis, several groups have taken a proteomics approach to identify the protein components associated with the OMVs. Multiple studies have characterized the proteomes of A. baumannii OMVs, collectively identifying hundreds of proteins associated with or in OMVs [85, 87-89]. As mentioned above, multiple sub-types of membrane vesicles are known to be secreted by A. baumannii; however, for the proteomic studies published, the majority of OMVs were prepared from bacteria grown to mid- to late log phase and were expected to contain prototypical OMVs (20-200nm with no inner membrane). Naturally, a preponderance of proteins known to associate with the outer membrane were localized to OMVs, including outer membrane protein H (OmpH) and W (OmpW), outer membrane protein/protective antigen OMA87, a 33-36 kDa outer membrane protein, and multiple putative outer membrane proteins [88]. Periplasmic, inner membrane, and cytosolic proteins were also identified in or associated with OMVs, bringing in to question whether some of these components are artifacts of dying cells or actively shepherded into OMVs [88, 89]. As such, a more comprehensive understanding on the molecular mechanisms of OMV biogenesis will help to unravel whether this is an active or passive process for Acinetobacter.
One of the most abundant proteins associated with OMVs is OmpA, which has been extensively studied for its role in Acinetobacter virulence [90]. Interestingly, deletion of ompA only marginally altered the outer membrane protein profile of A. baumannii ATCC 19606T, yet dramatically altered the protein composition of the OMVs, suggesting that OmpA may also play a role in Acinetobacter OMV formation and/or cargo selection [87]. For this study however, complementation tests would have provided the supportive information to conclude a direct role of OmpA in A. baumannii OMV biogenesis, as deletion of outer membrane proteins can have pleiotropic effects on membrane homeostasis. Nevertheless, it was shown that A. baumannii OMVs serve as the toxic protein delivery platforms for OmpA; specifically, Jin et al. demonstrated that human cell lines treated with A. baumannii OMVs were able to deliver the OmpA protein to the cytoplasm of these cells and in some cases nuclear localization was also observed [84]. Importantly, cytotoxicity associated with OMVs was dependent on the presence of OmpA as OMVs purified from an ompA mutant did not elicit a cytotoxic response. Many other known/putative virulence factors have also been identified in or associated with OMVs, like CsuA, a putative hemolysin, and a putative serine protease to name a few [84]; however, a definitive link between these proteins in OMVs and virulence has yet to be found.
As expected A. baumannii and A. nosocomialis OMVs elicit a pro-inflammatory cytokine response. Specifically, treatment of HEp-2 epithelial cells with A. baumannii OMVs also elicits pro-inflammatory cytokine release, including, the release of IL-1Beta, IL-6 MIP-1alpha, and MCP-1 [80, 91]. This response was found to be partially dependent on intact proteins associated with OMVs as proteinase k treatment decreased cytokine expression levels [91]. Lastly, OMVs intratracheally injected into mice were found to also elicit a pro-inflammatory cytokine response characterized by massive neutrophil infiltration [91]; however, although OMV-like structures were observed in tissue sections during a murine pulmonary infection model, the production of large quantities of OMVs in vivo by A. baumannii has not been observed [84].
Although Acinetobacter OMVs elicit pro-inflammatory responses and function as virulence factors delivering toxic proteins like OmpA to host cells, they also mediate bacterial survival when populations are threatened by antibiotics. Specifically, Acinetobacter OMVs can serve as plasmid delivery compartments transferring a carbepenamase-containing plasmid to other Acinetobacter isolates [92]. Interestingly, this phenomenon seemed to be independent of natural transformation as cultures treated with plasmid alone were unable to uptake the DNA, however, natural competence in A. baumannii has only been observed in a few strains. Furthermore, Acinetobacter OMVs can also reduce extracellular beta-lactam antibiotic concentrations through the action of both carabapenem-hydrolyzing class D beta-lactamases (CHDL) [93] and AmpC-like beta-lactamases [84]. The degradation of extracellular antibiotics is known as the “sheltering effect” and can protect susceptible bacteria from the action of antibiotics, but only when the CHDL is expressed at high levels [94].
Recently, OMVs have been gaining traction as viable, efficient vaccine delivery platforms given their endogenous adjuvanticity and ability to carry multiple, immunogenic proteins [95]. McConnell and colleagues demonstrated that an acellular vaccine using OMVs from A. baumannii elicited protective immunity in a murine sepsis infection model [96]. In depth characterization of vaccinated mice demonstrated significantly lower bacterial burdens as well as an attenuated pro-inflammatory cytokine response from OMV vaccinated mice. A subsequent study led by Huang and colleagues further demonstrated that vaccination of mice with OMVs from an A. baumannii clinical isolate was protective in a murine sepsis model, significantly increasing mice survival [97]. Furthermore, OMV vaccinated mice challenged intranasally with A. baumannii demonstrated reduced bacterial burdens, limited histological changes, and decreased pro-inflammatory cytokines within the lung environment when compared to non-vaccinated mice.
Acinetobacter spp. secrete several proteins that, as of yet, have not been associated with a particular secretion mechanism. Whether these proteins use secretory pathways that have not been studied in Acinetobacter, or are unidentified effectors of known systems, remains to be determined. Nonetheless, many of these proteins have biologically important roles. (Figure 4). A serine protease, designated PKF, was identified in culture supernatants from A. baumannii LK41 and subsequently shown to be partially required for resistance to human serum. Interestingly, the pfk mutant was also found to have increased biofilm-forming capabilities [98]. Another secreted protein identified in culture supernatants from A. baumannii and A. nosocomialis strains is the Cif protein (the cystic fibrosis transmembrane conductance regulator [CFTR] inhibitory factor), previously characterized in P. aeruginosa [99]. In a similar fashion to P. aeruginosa Cif, Acinetobacter Cif was shown catalyze the hydrolysis of multiple epoxide compounds and reduce apical levels of CFTR on polarized bronchial epithelial cells. Lastly, multiple reports have identified phospholipases, many of which are secreted in other Gram-negative bacteria, as important A. baumannii virulence factors. Specifically, a phospholipase D from A. baumannii 98-37-09 was found to be to mediate serum survival and epithelial cell cytotoxicity as well as being required for full virulence in a murine pneumonia model [100]. Subsequently, three different phospholipase D proteins were identified in A. baumannii ATCC 19606T and shown to act in concert to affect epithelial cell invasion efficiency and virulence in the Galleria mellonella model [101]. The absence of complementation experiments for the phospholipase D mutants however is required to assess the full potential of these proteins in A. baumannii virulence. Finally, a phospholipase C protein was found to be significantly up-regulated upon exposure to ethanol and also contributed to cytotoxicity of FaDu cells [102]. Although these phospholipases were shown to be act as virulence factors none of them have directly been shown through molecular techniques to be secreted.
Concluding Remarks
Decades of research has uncovered the clear importance of secretion systems for the survival and virulence of bacteria. Despite this, we have only recently begun to understand the roles, and mechanisms, of protein secretion for the genus Acinetobacter. Although infection-causing Acinetobacter spp. are often considered relatively low-grade pathogens, the studies detailed in this review show that these organisms possess multi-component secretion systems that are important mediators of virulence. The requirement of a T2SS for colonization and infection in mouse models suggests that effectors secreted by this mechanism play an important role in altering host physiology. Future experiments to identify additional effectors, and their enzymatic activity, will surely increase our understanding of the contribution T2SS plays during human infection. The finding that A. calcoaceticus, considered a non-pathogenic member of the genus, encodes a functional T2SS may suggest additional roles for the T2SS and its effectors in bacterial survival.
Similarly, the distribution of the T6SS among many Acinetobacter spp. suggests it primary function is for inter-bacterial interactions. We now know many of the genes which contribute to T6SS biogenesis in these organisms, but several hypothetical proteins essential for T6SS activity remain to be characterized. Effector/immunity proteins have mainly been characterized or suggested for A. baumannii, and additional studies to define the full-range of T6SS effectors and their roles are needed to understand the significance of this secretion system for Acinetobacter spp. survival. The novel regulatory mechanisms for T6SS in A. baumannii, and the loss of the T6SS in recent clinical isolates, suggests that this system may not have a prominent role during infection. More work to genetically and biochemically characterize additional secreted proteins and OMVs, as well as their secretory mechanisms, will facilitate a deeper understanding of the pathogenic potential of Acinetobacter.
Trends box.
-Known mechanisms for protein secretion by A. baumannii include a T2SS, T6SS, autotransporters and OMVs.
-The T2SS secretes several effector proteins including lipases and proteases and is required for pathogenesis.
-A new family of T2SS effector chaperones that appear to be widespread among Gram negative pathogens has been identified.
-In some A. baumannii, T6SS is repressed by a plasmid that carries antibiotic resistance genes, and this plasmid can be spontaneously lost leading to T6SS activation with loss of resistance.
-A. baumannii T6SS assembly requires a dedicated peptidoglycanase named TagX.
-A. baumannii OMVs contain virulence factors and may be useful as vaccines.
Outstanding questions.
-What is the full effector repertoire for the T2SS and T6SS? Are more recent clinical isolates acquiring novel effectors and losing others?
-What are the roles of the T2SS chaperones?
-Is the T2SS a viable target for antibacterial therapy?
-Is there a fitness trade-off between antibiotic resistance and T6SS? Do certain environments favor the T6SS over antibiotic resistance?
-Why is the T6SS apparently being lost or inactivated by some clinical isolates?
-What are the mechanisms of export for proteins with no clear association to known secretion systems?
-Is there a need for an A. baumannii vaccine, and, if so, are OMVs a viable option?
What would be the target population for an A. baumannii vaccine?
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boucher HW, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;48(1):1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 2.Infectious Diseases Society of, A et al. Combating antimicrobial resistance: policy recommendations to save lives. Clin Infect Dis. 2011;52(Suppl 5):S397–428. doi: 10.1093/cid/cir153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fowler T, et al. The risk/benefit of predicting a post-antibiotic era: is the alarm working? Ann N Y Acad Sci. 2014;1323:1–10. doi: 10.1111/nyas.12399. [DOI] [PubMed] [Google Scholar]
- 4.Spellberg B, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(2):155–64. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 5.Talbot GH, et al. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis. 2006;42(5):657–68. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 6.Valencia R, et al. Nosocomial outbreak of infection with pan-drug-resistant Acinetobacter baumannii in a tertiary care university hospital. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2009;30(3):257–63. doi: 10.1086/595977. [DOI] [PubMed] [Google Scholar]
- 7.Falagas ME, et al. Pandrug-resistant Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii infections: characteristics and outcome in a series of 28 patients. Int J Antimicrob Agents. 2008;32(5):450–4. doi: 10.1016/j.ijantimicag.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Sievert DM, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2013;34(1):1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 9.Peleg AY, Hooper DC. Hospital-acquired infections due to Gram-negative bacteria. The New England journal of medicine. 2010;362(19):1804–13. doi: 10.1056/NEJMra0904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaynes R, Edwards JR. Overview of nosocomial infections caused by Gram-negative bacilli. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005;41(6):848–54. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 11.Grandi G. Bacterial surface proteins and vaccines. F1000 Biol Rep. 2010;2:36. doi: 10.3410/B2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nivaskumar M, Francetic O. Type II secretion system: a magic beanstalk or a protein escalator. Biochim Biophys Acta. 2014;1843(8):1568–77. doi: 10.1016/j.bbamcr.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 13.Sandkvist M. Type II secretion and pathogenesis. Infect Immun. 2001;69(6):3523–35. doi: 10.1128/IAI.69.6.3523-3535.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DebRoy S, et al. Legionella pneumophila type II secretome reveals unique exoproteins and a chitinase that promotes bacterial persistence in the lung. Proc Natl Acad Sci U S A. 2006;103(50):19146–51. doi: 10.1073/pnas.0608279103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCoy-Simandle K, et al. Legionella pneumophila type II secretion dampens the cytokine response of infected macrophages and epithelia. Infect Immun. 2011;79(5):1984–97. doi: 10.1128/IAI.01077-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandkvist M, et al. A protein required for secretion of cholera toxin through the outer membrane of Vibrio cholerae. Gene. 1993;123(1):81–6. doi: 10.1016/0378-1119(93)90543-c. [DOI] [PubMed] [Google Scholar]
- 17.Sikora AE, et al. Proteomic analysis of the Vibrio cholerae type II secretome reveals new proteins, including three related serine proteases. J Biol Chem. 2011;286(19):16555–66. doi: 10.1074/jbc.M110.211078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho TD, et al. Type 2 secretion promotes enterohemorrhagic Escherichia coli adherence and intestinal colonization. Infect Immun. 2008;76(5):1858–65. doi: 10.1128/IAI.01688-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baldi DL, et al. The type II secretion system and its ubiquitous lipoprotein substrate, SslE, are required for biofilm formation and virulence of enteropathogenic Escherichia coli. Infect Immun. 2012;80(6):2042–52. doi: 10.1128/IAI.06160-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jyot J, et al. Type II secretion system of Pseudomonas aeruginosa: in vivo evidence of a significant role in death due to lung infection. J Infect Dis. 2011;203(10):1369–77. doi: 10.1093/infdis/jir045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eijkelkamp BA, et al. Comparative analysis of surface-exposed virulence factors of Acinetobacter baumannii. BMC Genomics. 2014;15:1020. doi: 10.1186/1471-2164-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson TL, et al. Acinetobacter baumannii Is Dependent on the Type II Secretion System and Its Substrate LipA for Lipid Utilization and In Vivo Fitness. J Bacteriol. 2016;198(4):711–9. doi: 10.1128/JB.00622-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Douzi B, et al. On the path to uncover the bacterial type II secretion system. Philos Trans R Soc Lond B Biol Sci. 2012;367(1592):1059–72. doi: 10.1098/rstb.2011.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Possot OM, et al. Multiple interactions between pullulanase secreton components involved in stabilization and cytoplasmic membrane association of PulE. J Bacteriol. 2000;182(8):2142–52. doi: 10.1128/jb.182.8.2142-2152.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang N, et al. Genome-wide identification of Acinetobacter baumannii genes necessary for persistence in the lung. MBio. 2014;5(3):e01163–14. doi: 10.1128/mBio.01163-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harding CM, et al. Medically Relevant Acinetobacter Species Require a Type II Secretion System and Specific Membrane-Associated Chaperones for the Export of Multiple Substrates and Full Virulence. PLoS Pathog. 2016;12(1):e1005391. doi: 10.1371/journal.ppat.1005391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tilley D, et al. CpaA a novel protease from Acinetobacter baumannii clinical isolates deregulates blood coagulation. FEMS Microbiol Lett. 2014;356(1):53–61. doi: 10.1111/1574-6968.12496. [DOI] [PubMed] [Google Scholar]
- 28.Zheng X, et al. Probing the molecular determinant of the lipase-specific foldase Lif26 for the interaction with its cognate Lip26. Int J Biol Macromol. 2013;53:54–61. doi: 10.1016/j.ijbiomac.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Page AL, Parsot C. Chaperones of the type III secretion pathway: jacks of all trades. Mol Microbiol. 2002;46(1):1–11. doi: 10.1046/j.1365-2958.2002.03138.x. [DOI] [PubMed] [Google Scholar]
- 30.El Khattabi M, et al. Role of the lipase-specific foldase of Burkholderia glumae as a steric chaperone. J Biol Chem. 2000;275(35):26885–91. doi: 10.1074/jbc.M003258200. [DOI] [PubMed] [Google Scholar]
- 31.Chapon-Herve V, et al. Regulation of the xcp secretion pathway by multiple quorum-sensing modulons in Pseudomonas aeruginosa. Mol Microbiol. 1997;24(6):1169–78. doi: 10.1046/j.1365-2958.1997.4271794.x. [DOI] [PubMed] [Google Scholar]
- 32.Lewenza S, et al. Quorum sensing in Burkholderia cepacia: identification of the LuxRI homologs CepRI. J Bacteriol. 1999;181(3):748–56. doi: 10.1128/jb.181.3.748-756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zielke RA, et al. The type II secretion pathway in Vibrio cholerae is characterized by growth phase-dependent expression of exoprotein genes and is positively regulated by sigmaE. Infect Immun. 2014;82(7):2788–801. doi: 10.1128/IAI.01292-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobs AC, et al. Characterization of the Acinetobacter baumannii growth phase-dependent and serum responsive transcriptomes. FEMS Immunol Med Microbiol. 2012;64(3):403–12. doi: 10.1111/j.1574-695X.2011.00926.x. [DOI] [PubMed] [Google Scholar]
- 35.Ruhe ZC, et al. Bacterial contact-dependent growth inhibition. Trends Microbiol. 2013;21(5):230–7. doi: 10.1016/j.tim.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell AB, et al. Type VI secretion system effectors: poisons with a purpose. Nature reviews. Microbiology. 2014;12(2):137–48. doi: 10.1038/nrmicro3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pukatzki S, et al. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(5):1528–33. doi: 10.1073/pnas.0510322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mougous JD, et al. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science. 2006;312(5779):1526–30. doi: 10.1126/science.1128393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hood RD, et al. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell host & microbe. 2010;7(1):25–37. doi: 10.1016/j.chom.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boyer F, et al. Dissecting the bacterial type VI secretion system by a genome wide in silico analysis: what can be learned from available microbial genomic resources? BMC genomics. 2009;10:104. doi: 10.1186/1471-2164-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silverman JM, et al. Structure and regulation of the type VI secretion system. Annual review of microbiology. 2012;66:453–72. doi: 10.1146/annurev-micro-121809-151619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basler M, et al. Type VI secretion requires a dynamic contractile phage tail-like structure. Nature. 2012;483(7388):182–6. doi: 10.1038/nature10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russell AB, et al. Type VI secretion delivers bacteriolytic effectors to target cells. Nature. 2011;475(7356):343–7. doi: 10.1038/nature10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pukatzki S, et al. The type VI secretion system: translocation of effectors and effector-domains. Current opinion in microbiology. 2009;12(1):11–7. doi: 10.1016/j.mib.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Pukatzki S, et al. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(39):15508–13. doi: 10.1073/pnas.0706532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weber BS, et al. Genetic Dissection of the Type VI Secretion System in Acinetobacter and Identification of a Novel Peptidoglycan Hydrolase, TagX, Required for Its Biogenesis. MBio. 2016;7(5):e01253–16. doi: 10.1128/mBio.01253-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Berardinis V, et al. A complete collection of single-gene deletion mutants of Acinetobacter baylyi ADP1. Molecular systems biology. 2008;4:174. doi: 10.1038/msb.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zoued A, et al. Priming and polymerization of a bacterial contractile tail structure. Nature. 2016;531(7592):59–63. doi: 10.1038/nature17182. [DOI] [PubMed] [Google Scholar]
- 49.Bonemann G, et al. Remodelling of VipA/VipB tubules by ClpV-mediated threading is crucial for type VI protein secretion. The EMBO journal. 2009;28(4):315–25. doi: 10.1038/emboj.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruiz FM, et al. Crystal Structure of Hcp from Acinetobacter baumannii: A Component of the Type VI Secretion System. PLoS One. 2015;10(6):e0129691. doi: 10.1371/journal.pone.0129691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brunet YR, et al. The Type VI Secretion TssEFGK-VgrG Phage-Like Baseplate Is Recruited to the TssJLM Membrane Complex via Multiple Contacts and Serves As Assembly Platform for Tail Tube/Sheath Polymerization. PLoS Genet. 2015;11(10):e1005545. doi: 10.1371/journal.pgen.1005545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Durand E, et al. Biogenesis and structure of a type VI secretion membrane core complex. Nature. 2015;523(7562):555–60. doi: 10.1038/nature14667. [DOI] [PubMed] [Google Scholar]
- 53.Pietrosiuk A, et al. Molecular basis for the unique role of the AAA+ chaperone ClpV in type VI protein secretion. The Journal of biological chemistry. 2011;286(34):30010–21. doi: 10.1074/jbc.M111.253377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shneider MM, et al. PAAR-repeat proteins sharpen and diversify the type VI secretion system spike. Nature. 2013;500(7462):350–3. doi: 10.1038/nature12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Felisberto-Rodrigues C, et al. Towards a structural comprehension of bacterial type VI secretion systems: characterization of the TssJ-TssM complex of an Escherichia coli pathovar. PLoS pathogens. 2011;7(11):e1002386. doi: 10.1371/journal.ppat.1002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aschtgen MS, et al. SciN is an outer membrane lipoprotein required for type VI secretion in enteroaggregative Escherichia coli. Journal of bacteriology. 2008;190(22):7523–31. doi: 10.1128/JB.00945-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aschtgen MS, et al. Anchoring the type VI secretion system to the peptidoglycan: TssL, TagL, TagP... what else? Virulence. 2010;1(6):535–40. doi: 10.4161/viru.1.6.13732. [DOI] [PubMed] [Google Scholar]
- 58.Silverman JM, et al. Separate inputs modulate phosphorylation-dependent and -independent type VI secretion activation. Molecular microbiology. 2011;82(5):1277–90. doi: 10.1111/j.1365-2958.2011.07889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henry R, et al. Colistin-resistant, lipopolysaccharide-deficient Acinetobacter baumannii responds to lipopolysaccharide loss through increased expression of genes involved in the synthesis and transport of lipoproteins, phospholipids, and poly-beta-1,6-N-acetylglucosamine. Antimicrobial agents and chemotherapy. 2012;56(1):59–69. doi: 10.1128/AAC.05191-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weber BS, et al. Genomic and functional analysis of the type VI secretion system in Acinetobacter. PLoS One. 2013;8(1):e55142. doi: 10.1371/journal.pone.0055142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carruthers MD, et al. Acinetobacter baumannii utilizes a type VI secretion system for bacterial competition. PloS one. 2013;8(3):e59388. doi: 10.1371/journal.pone.0059388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Repizo GD, et al. Differential Role of the T6SS in Acinetobacter baumannii Virulence. PLoS One. 2015;10(9):e0138265. doi: 10.1371/journal.pone.0138265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weber BS, et al. A multidrug resistance plasmid contains the molecular switch for type VI secretion in Acinetobacter baumannii. Proc Natl Acad Sci U S A. 2015;112(30):9442–7. doi: 10.1073/pnas.1502966112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scheurwater EM, Burrows LL. Maintaining network security: how macromolecular structures cross the peptidoglycan layer. FEMS Microbiol Lett. 2011;318(1):1–9. doi: 10.1111/j.1574-6968.2011.02228.x. [DOI] [PubMed] [Google Scholar]
- 65.Subashchandrabose S, et al. Acinetobacter baumannii Genes Required for Bacterial Survival during Bloodstream Infection. mSphere. 2016;1(1):e00013–15. doi: 10.1128/mSphere.00013-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Umland TC, et al. In vivo-validated essential genes identified in Acinetobacter baumannii by using human ascites overlap poorly with essential genes detected on laboratory media. MBio. 2012;3(4):e00113–12. doi: 10.1128/mBio.00113-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wright MS, et al. New insights into dissemination and variation of the health care-associated pathogen Acinetobacter baumannii from genomic analysis. mBio. 2014;5(1):e00963–13. doi: 10.1128/mBio.00963-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eijkelkamp BA, et al. H-NS plays a role in expression of Acinetobacter baumannii virulence features. Infection and immunity. 2013;81(7):2574–83. doi: 10.1128/IAI.00065-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bentancor LV, et al. Identification of Ata, a multifunctional trimeric autotransporter of Acinetobacter baumannii. J Bacteriol. 2012;194(15):3950–60. doi: 10.1128/JB.06769-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weidensdorfer M, et al. Analysis of Endothelial Adherence of Bartonella henselae and Acinetobacter baumannii Using a Dynamic Human Ex Vivo Infection Model. Infect Immun. 2016;84(3):711–22. doi: 10.1128/IAI.01502-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eijkelkamp BA, et al. H-NS plays a role in expression of Acinetobacter baumannii virulence features. Infect Immun. 2013;81(7):2574–83. doi: 10.1128/IAI.00065-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ishikawa M, et al. AtaA, a new member of the trimeric autotransporter adhesins from Acinetobacter sp. Tol 5 mediating high adhesiveness to various abiotic surfaces. PLoS One. 2012;7(11):e48830. doi: 10.1371/journal.pone.0048830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koiwai K, et al. Structural Basis for Toughness and Flexibility in the C-terminal Passenger Domain of an Acinetobacter Trimeric Autotransporter Adhesin. J Biol Chem. 2016;291(8):3705–24. doi: 10.1074/jbc.M115.701698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ishikawa M, et al. Discovery of a novel periplasmic protein that forms a complex with a trimeric autotransporter adhesin and peptidoglycan. Mol Microbiol. 2016;101(3):394–410. doi: 10.1111/mmi.13398. [DOI] [PubMed] [Google Scholar]
- 75.Elhenawy W, et al. Preferential packing of acidic glycosidases and proteases into Bacteroides outer membrane vesicles. MBio. 2014;5(2):e00909–14. doi: 10.1128/mBio.00909-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haurat MF, et al. Selective sorting of cargo proteins into bacterial membrane vesicles. J Biol Chem. 2011;286(2):1269–76. doi: 10.1074/jbc.M110.185744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roier S, et al. A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria. Nat Commun. 2016;7:10515. doi: 10.1038/ncomms10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Elhenawy W, et al. LPS Remodeling Triggers Formation of Outer Membrane Vesicles in Salmonella. MBio. 2016;7(4):e00940–16. doi: 10.1128/mBio.00940-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ellis TN, Kuehn MJ. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev. 2010;74(1):81–94. doi: 10.1128/MMBR.00031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nho JS, et al. Acinetobacter nosocomialis secretes outer membrane vesicles that induce epithelial cell death and host inflammatory responses. Microb Pathog. 2015;81:39–45. doi: 10.1016/j.micpath.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 81.Fulsundar S, et al. Molecular characterization of outer membrane vesicles released from Acinetobacter radioresistens and their potential roles in pathogenesis. Microb Pathog. 2015;83-84:12–22. doi: 10.1016/j.micpath.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 82.Fulsundar S, et al. Gene transfer potential of outer membrane vesicles of Acinetobacter baylyi and effects of stress on vesiculation. Appl Environ Microbiol. 2014;80(11):3469–83. doi: 10.1128/AEM.04248-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choi CH, et al. Outer membrane protein 38 of Acinetobacter baumannii localizes to the mitochondria and induces apoptosis of epithelial cells. Cell Microbiol. 2005;7(8):1127–38. doi: 10.1111/j.1462-5822.2005.00538.x. [DOI] [PubMed] [Google Scholar]
- 84.Jin JS, et al. Acinetobacter baumannii secretes cytotoxic outer membrane protein A via outer membrane vesicles. PLoS One. 2011;6(2):e17027. doi: 10.1371/journal.pone.0017027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kwon SO, et al. Proteome analysis of outer membrane vesicles from a clinical Acinetobacter baumannii isolate. FEMS Microbiol Lett. 2009;297(2):150–6. doi: 10.1111/j.1574-6968.2009.01669.x. [DOI] [PubMed] [Google Scholar]
- 86.Koning RI, et al. Cryo-electron tomography analysis of membrane vesicles from Acinetobacter baumannii ATCC19606 T. Res Microbiol. 2013;164(5):397–405. doi: 10.1016/j.resmic.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 87.Moon DC, et al. Acinetobacter baumannii outer membrane protein A modulates the biogenesis of outer membrane vesicles. J Microbiol. 2012;50(1):155–60. doi: 10.1007/s12275-012-1589-4. [DOI] [PubMed] [Google Scholar]
- 88.Mendez JA, et al. Extracellular proteome of a highly invasive multidrug-resistant clinical strain of Acinetobacter baumannii. J Proteome Res. 2012;11(12):5678–94. doi: 10.1021/pr300496c. [DOI] [PubMed] [Google Scholar]
- 89.Li ZT, et al. Outer membrane vesicles isolated from two clinical Acinetobacter baumannii strains exhibit different toxicity and proteome characteristics. Microb Pathog. 2015;81:46–52. doi: 10.1016/j.micpath.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 90.Cerqueira GM, Peleg AY. Insights into Acinetobacter baumannii pathogenicity. IUBMB Life. 2011;63(12):1055–60. doi: 10.1002/iub.533. [DOI] [PubMed] [Google Scholar]
- 91.Jun SH, et al. Acinetobacter baumannii outer membrane vesicles elicit a potent innate immune response via membrane proteins. PLoS One. 2013;8(8):e71751. doi: 10.1371/journal.pone.0071751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rumbo C, et al. Horizontal transfer of the OXA-24 carbapenemase gene via outer membrane vesicles: a new mechanism of dissemination of carbapenem resistance genes in Acinetobacter baumannii. Antimicrob Agents Chemother. 2011;55(7):3084–90. doi: 10.1128/AAC.00929-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liao YT, et al. Acinetobacter baumannii Extracellular OXA-58 Is Primarily and Selectively Released via Outer Membrane Vesicles after Sec-Dependent Periplasmic Translocation. Antimicrob Agents Chemother. 2015;59(12):7346–54. doi: 10.1128/AAC.01343-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liao YT, et al. Sheltering effect and indirect pathogenesis of carbapenem-resistant Acinetobacter baumannii in polymicrobial infection. Antimicrob Agents Chemother. 2014;58(7):3983–90. doi: 10.1128/AAC.02636-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Price NL, et al. Glycoengineered Outer Membrane Vesicles: A Novel Platform for Bacterial Vaccines. Sci Rep. 2016;6:24931. doi: 10.1038/srep24931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McConnell MJ, et al. Outer membrane vesicles as an acellular vaccine against Acinetobacter baumannii. Vaccine. 2011;29(34):5705–10. doi: 10.1016/j.vaccine.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 97.Huang W, et al. Immunization against multidrug-resistant Acinetobacter baumannii effectively protects mice in both pneumonia and sepsis models. PLoS One. 2014;9(6):e100727. doi: 10.1371/journal.pone.0100727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.King LB, et al. Serine protease PKF of Acinetobacter baumannii results in serum resistance and suppression of biofilm formation. J Infect Dis. 2013;207(7):1128–34. doi: 10.1093/infdis/jis939. [DOI] [PubMed] [Google Scholar]
- 99.Bahl CD, et al. Signature motifs identify an Acinetobacter Cif virulence factor with epoxide hydrolase activity. J Biol Chem. 2014;289(11):7460–9. doi: 10.1074/jbc.M113.518092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jacobs AC, et al. Inactivation of phospholipase D diminishes Acinetobacter baumannii pathogenesis. Infect Immun. 2010;78(5):1952–62. doi: 10.1128/IAI.00889-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stahl J, et al. Acinetobacter baumannii Virulence Is Mediated by the Concerted Action of Three Phospholipases D. PLoS One. 2015;10(9):e0138360. doi: 10.1371/journal.pone.0138360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Camarena L, et al. Molecular mechanisms of ethanol-induced pathogenesis revealed by RNA-sequencing. PLoS Pathog. 2010;6(4):e1000834. doi: 10.1371/journal.ppat.1000834. [DOI] [PMC free article] [PubMed] [Google Scholar]