Abstract
Objective
Both oxygenation and peak inspiratory pressure (PIP) are associated with mortality in pediatric ARDS. Since oxygenation and respiratory mechanics are linked, it is difficult to identify which variables, pressure or oxygenation, are independently associated with outcome. We aimed to determine whether respiratory mechanics (PIP, positive end-expiratory pressure [PEEP], ΔP [PIP minus PEEP], tidal volume, dynamic compliance [Cdyn]) or oxygenation (PaO2/FIO2) was associated with mortality.
Design
Prospective, observational, cohort study.
Setting
University affiliated pediatric intensive care unit.
Patients
Mechanically ventilated children with ARDS (Berlin).
Interventions
None.
Measurements and Main Results
PIP, PEEP, ΔP, tidal volume, Cdyn, and PaO2/FIO2 were collected at ARDS onset and at 24 hours in 352 children between 2011 and 2016. At ARDS onset, neither mechanical variables nor PaO2/FIO2 were associated with mortality. At 24 hours, PIP, PEEP, ΔP were higher, and Cdyn and PaO2/FIO2 lower, in non-survivors. In multivariable logistic regression, PaO2/FIO2 at 24 hours and ΔPaO2/FIO2 (change in PaO2/FIO2 over the first 24 hours) were associated with mortality, whereas pressure variables were not. Both oxygenation and pressure variables were associated with duration of ventilation in multivariable competing risk regression.
Conclusions
Improvements in oxygenation, but not in respiratory mechanics, were associated with lower mortality in pediatric ARDS. Future trials of mechanical ventilation in children should focus on oxygenation (higher PaO2/FIO2) rather than lower PIP or ΔP, as oxygenation was more consistently associated with outcome.
Keywords: Acute respiratory distress syndrome, pediatric, PaO2/FIO2, driving pressure
INTRODUCTION
Defined for adults (1, 2), acute respiratory distress syndrome (ARDS) affects 45,000 children in the United States annually (3), with mortality approaching 30% (4). Absent targeted therapies, lung-protective ventilation (5) remains the mainstay of treatment. In children, lack of therapies is compounded by uncertainty in management, as guidelines are extrapolated from adults, with suspect applicability. For example, unlike adults (5, 6), observational studies revealed no association between tidal volume (VT) and mortality in children (7). While VT ≤ 6 mL/kg have been recommended for pediatrics (8), ambiguity regarding utility of low VT has led to actual VT closer to 8 mL/kg (9, 10), and substantial utilization of VT > 10 mL/kg (9).
Unlike VT, peak inspiratory pressure (PIP) is consistently associated with mortality in pediatric ARDS (10, 11). Causality was not inferred in these observational studies, as elevated PIP may simply reflect worse ARDS. However, in light of re-analysis of adult ARDS trials (6), higher PIP may be causal for mortality via higher driving pressure. Additionally, oxygenation has a more consistent relationship with mortality in pediatric (10–12) than in adult ARDS (5). However, oxygenation and respiratory mechanics are linked, as changes to the ventilator which affect oxygenation simultaneously affect pressures. Therefore, it is difficult to identify which variable, pressure or oxygenation, is independently associated with outcome.
The aim of this study was to determine whether variables related to respiratory mechanics (PIP, positive end-expiratory pressure [PEEP], driving pressure [ΔP = PIP minus PEEP], VT, dynamic compliance [Cdyn = VT/ΔP]) or variables related to oxygenation (PaO2/FIO2) were independently associated with mortality in a cohort of children maintained on conventional mechanical ventilation for ≥ 24 hours after ARDS onset. We hypothesized that oxygenation, rather than pressure or volume variables, was the primary metric associated with mortality.
METHODS
Study Design and Patient Selection
This study was approved by the Children’s Hospital of Philadelphia’s Institutional Review Board, and requirement for informed consent waived. Eligibility criteria have been previously described in detail (12). Briefly, intubated children (> 1month and < 18 years) meeting American-European Consensus Conference criteria for acute lung injury (PaO2/FIO2 ≤ 300 with bilateral infiltrates) between July 1, 2011 and June 30, 2016 were enrolled. As the study was initiated prior to the 2012 Berlin definition (2), minimum PEEP was not specified; however, our unit does not utilize PEEP < 5 cmH2O, and all patients met Berlin criteria.
Data Collection and ARDS Management
We restricted primary analyses to patients on conventional ventilation over the first 24 hours of ARDS to assess the effect of variables during this early timeframe. We recorded demographics, ventilator settings and PaO2/FIO2 at ARDS onset and 24 hours, and treatments for the first 3 days. To avoid confounding by patient effort, ventilator pressures were recorded during periods of passive breathing under deep sedation or neuromuscular blockade (NMB) by data collectors (respiratory therapists) trained to identify spontaneous effort, most commonly by ensuring the set respiratory rate matched the observed rate.
Absent a standardized ventilator protocol, institutional practice is to initiate conventional ventilation with PEEP ≥ 5 cmH2O, and attempt to wean FIO2 to ≤ 0.60, keeping PaO2 ≥ 60 mmHg. Inability to wean FIO2 prompts PEEP escalation and subsequent efforts to wean FIO2. We exclusively utilize decelerating flow during conventional ventilation (either pressure control or pressure-regulated volume control [PRVC]). Persistently elevated PIP (≥ 35 cmH2O), hypercarbia (PaCO2 ≥ 80), or oxygenation difficulties (inability to wean FIO2 ≤ 0.60 despite increasing PEEP) prompted consideration for changing mode of ventilation, or escalating to extracorporeal membrane oxygenation (ECMO). Actual transition was left to the discretion of the attending physician. There was no standardization of ancillary therapies (inhaled nitric oxide [iNO], NMB, corticosteroids).
Equations and Definitions
PIP, PEEP, and ΔP (PIP minus PEEP), VT, and Cdyn were collected at the ventilator for most patients, using integrated software provided by the manufacturer (Dräger, Inc., Lübeck, Germany), adjusting for patient size. For VT < 100 mL, we utilized a sensor proximate to the patient at the endotracheal tube. We defined ΔP as PIP minus PEEP, rather than plateau pressure (Pplateau) minus PEEP, since we exclusively utilized decelerating flow waveforms, and inspiratory holds to collect Pplateau were not performed. VT was normalized to actual body weight. Cdyn was defined as VT/ΔP. Additionally, the difference between variables at 24 hours relative to at ARDS onset are reported (ΔPIP, ΔPEEP, ΔΔP, ΔVT, ΔCdyn and ΔPaO2/FIO2). The vasopressor score (13) was: dopamine (μg/kg/min) × 1 + dobutamine (μg/kg/min) × 1 + epinephrine (μg/kg/min) × 100 + norepinephrine (μg/kg/min) × 100 + phenylephrine (μg/kg/min) × 100 + vasopressin (U/kg/min) × 10,000 + milrinone (μg/kg/min) × 10. Non-pulmonary organ failures were identified using accepted definitions in children (14). The designation “immunocompromised” required presence of an immunocompromising diagnosis (oncologic, immunologic, rheumatologic, or transplant) and active immunosuppressive chemotherapy, or a congenital immunodeficiency (12, 15). Severity of illness score used was the Pediatric Risk of Mortality (PRISM) III at 12 hours.
Primary outcome was pediatric intensive care unit (PICU) mortality. Duration of ventilation was also recorded. All mention of “ventilation” in this study implies invasive ventilation; non-invasive support was not counted toward total ventilator days. “Day 1” was initiation of invasive ventilation. Liberation from invasive ventilation for > 24 hours defined duration of ventilation. Patients requiring re-initiation of invasive ventilation had the extra days counted towards total ventilator days.
Statistical Analysis
Data are expressed as percentages or median [interquartile range]. Categorical variables were analyzed by Fisher’s exact test and continuous variables by Wilcoxon rank sum. Given different scales of the variables, pressure and oxygenation variables were standardized to mean = 0 and standard deviation (SD) = 1 for multivariable analyses. For subgroup analyses, the cohort was divided by initial severity of lung injury (PaO2/FIO2 > 150 or PaO2/FIO2 ≤ 150) as in similar studies (16), and by direct or indirect ARDS (1).
Multivariable logistic regression was performed to test for independent association between pressure and oxygenation variables and mortality, after adjusting a priori for PRISM III, immunocompromised status, and PaO2/FIO2 at ARDS onset. We previously demonstrated that PRISM III and immunocompromised status are strongly associated with mortality (17, 18). PaO2/FIO2 at ARDS onset was included to control for baseline ARDS severity. Additional potential confounders with univariate association with mortality (p < 0.10, Table 1) were considered. Non-pulmonary organ failures, use of corticosteroids, vasopressor score, and fluid balance were co-linear with PRISM III, and were not included. Inclusion of either ARDS etiology or use of iNO did not improve the regression model by likelihood ratio test. Model fit was assessed using Hosmer-Lemeshow statistics.
Table 1.
Characteristics of the ARDS cohort.
| Variables | All patients (n = 352) |
Survived (n = 304) |
Died (n = 48) |
p value |
|---|---|---|---|---|
| Age (years) | 4.3 [1.5, 12.3] | 4.1 [1.5, 11.8] | 6.7 [2.6, 15] | 0.109 |
| Gender (% female) | 155 (44) | 136 (45) | 19 (40) | 0.534 |
| PRISM III at 12 hours | 11 [5, 16] | 9 [5, 15] | 17 [12, 30] | < 0.001 |
| Immunocompromised (%) | 73 (21) | 47 (15) | 26 (54) | < 0.001 |
| Cause of ARDS (%) | ||||
| Infectious pneumonia | 184 (52) | 167 (55) | 17 (35) | |
| Aspiration pneumonia | 32 (9) | 28 (9) | 4 (8) | 0.013 |
| Sepsis | 87 (25) | 74 (24) | 13 (27) | |
| Trauma | 22 (6) | 16 (5) | 6 (13) | |
| Other | 27 (8) | 19 (6) | 8 (17) | |
| Non-pulmonary organ failures at ARDS onset | 2 [1, 3] | 1 [1, 2] | 3 [2, 4] | < 0.001 |
| Berlin ARDS severity at onset (%) | ||||
| Mild | 135 (38) | 117 (38) | 18 (38) | 0.067 |
| Moderate | 167 (47) | 149 (49) | 18 (38) | |
| Severe | 50 (14) | 38 (13) | 12 (25) | |
| Ancillary therapy in first 72 hours (%) | ||||
| Neuromuscular blockade | 125 (35) | 106 (35) | 19 (40) | 0.520 |
| Inhaled nitric oxide | 89 (25) | 70 (23) | 19 (40) | 0.019 |
| Corticosteroids | 189 (54) | 156 (51) | 33 (69) | 0.029 |
| First 72 hours of ARDS | ||||
| Maximum vasopressor score | 9 [3, 18] | 8 [3, 16] | 12 [5, 35] | 0.005 |
| Fluid balance (mL/kg) | 93 [33, 165] | 87 [30, 153] | 146 [59, 257] | 0.002 |
| Any ECMO (%) | 6 (2) | 5 (2) | 1 (2) | 0.322 |
Because ventilator-free days (VFD) incorporates both mortality and length of ventilation, it is a problematic endpoint from which to identify variables associated predominantly with ventilator duration. Thus, rather than analyzing VFD, competing risk regression was used to test the association of pressure and oxygenation variables with duration of ventilation, using extubation as primary outcome, and death as a competing risk. Observations were censored at 28 days. Fine and Gray competing risk regression (19) calculates a subdistribution hazard ratio (SHR) for risk of extubation, accounting for the competing risk of death. Models were adjusted for PRISM III, immunocompromised status, and PaO2/FIO2 at ARDS onset. The proportional hazard assumption was assessed by testing for interaction with a time-dependent covariate.
RESULTS
Characteristics of the Cohort
During the study period, 459 children with ARDS were admitted. Of these, 107 were transitioned to either non-conventional ventilation (n = 93; 18 airway pressure release ventilation; 43 high frequency oscillation; 32 high frequency percussion) or ECMO (n = 10), or had died (n = 4), by 24 hours and were excluded from analyses. Of the remaining 352 children (Table 1), 277 (79%) were on volume-preset (PRVC) ventilation at both ARDS onset and 24 hours, 45 (13%) were on pressure control, and 30 (8%) alternated between modes. The median time from PICU admission to meeting ARDS criteria was 11 [IQR 6, 19] hours; median time from intubation to meeting ARDS criteria was 4 [0, 10] hours.
Respiratory and Oxygenation Variables
At ARDS onset, neither respiratory variables nor PaO2/FIO2 differed by survival status (Figure 1). At 24 hours, non-survivors had higher PIP, PEEP and ΔP (Figure 1A–B), and lower Cdyn and PaO2/FIO2 (Figure 1D–E). VT was similar between survivors and non-survivors at both timepoints (Figure 1C). When comparing the change in variables over the first 24 hours of ARDS, non-survivors had a higher ΔPEEP and a lower ΔPaO2/FIO2 (Supplementary Table 1). Pressure variables were modestly to highly correlated with each other and with oxygenation (Supplementary Figure 1).
Figure 1.
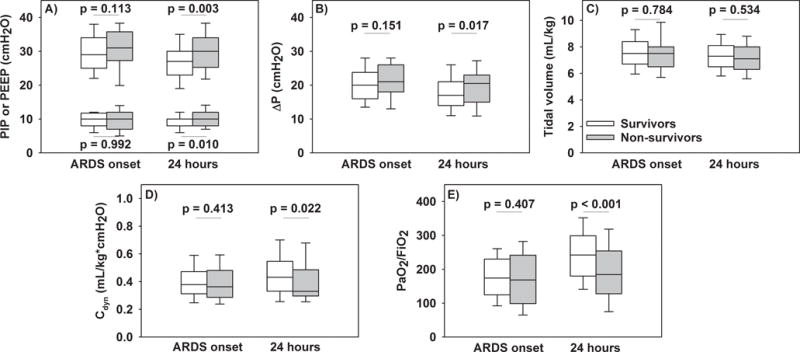
Respiratory variables (PIP, PEEP, ΔP, tidal volume, and Cdyn) and PaO2/FIO2 at ARDS onset and 24 hours after, stratified by PICU survivors (white) and non-survivors (gray). P values represent rank sum tests.
Multivariable Adjustment
Based on univariate analysis, we focused on the pressure variables (PIP, PEEP, ΔP, and Cdyn) and PaO2/FIO2 at 24 hours, and the change in these variables over the first 24 hours (ΔPIP, ΔPEEP, ΔΔP, ΔCdyn, and ΔPaO2/FIO2). We initially assessed independent contribution of these variables individually to mortality and duration of ventilation (Table 2). Increased PEEP and ΔPEEP were associated with increased mortality; increased PaO2/FIO2 at 24 hours and ΔPaO2/FIO2 were associated with lower mortality. Increases in PIP, PEEP, and ΔP were associated with longer duration of ventilation (i.e., decreased likelihood of extubation, SHR < 1), and increased Cdyn and PaO2/FIO2 associated with shorter duration of ventilation. Finally, pressure and oxygenation variables were entered simultaneously into the full model to assess which retained independent association with outcome (Table 3). In the full models, improved oxygenation retained association with reduced mortality, whereas pressure variables did not. Pressure and oxygenation retained association with duration of ventilation (Table 3, Figure 2).
Table 2.
Separate multivariate testing of association of respiratory variables with mortality (logistic regression) and successful extubation (competing risk regression)
| Variable (per increase of 1 SD) |
Mortality
|
Successful extubation
|
||
|---|---|---|---|---|
| Adjusted OR (95% CI) |
p value | Adjusted SHR (95% CI) |
p value | |
| PIP at 24 hours | 1.40 (0.99 to 1.97) | 0.052 | 0.71 (0.63 to 0.81) | < 0.001 |
| PEEP at 24 hours | 1.55 (1.07 to 2.23) | 0.020 | 0.79 (0.70 to 0.89) | < 0.001 |
| ΔP at 24 hours | 1.26 (0.89 to 1.79) | 0.195 | 0.76 (0.67 to 0.86) | < 0.001 |
| Cdyn at 24 hours | 0.80 (0.53 to 1.21) | 0.293 | 1.24 (1.12 to 1.36) | < 0.001 |
| PaO2/FiO2 at 24 hours | 0.54 (0.36 to 0.81) | 0.003 | 1.56 (1.36 to 1.76) | < 0.001 |
|
| ||||
| ΔPIP | 1.36 (0.98 to 1.90) | 0.068 | 0.72 (0.64 to 0.81) | < 0.001 |
| ΔPEEP | 1.53 (1.09 to 2.13) | 0.013 | 0.77 (0.68 to 0.86) | < 0.001 |
| ΔΔP | 1.21 (0.85 to 1.72) | 0.282 | 0.77 (0.69 to 0.87) | < 0.001 |
| ΔCdyn | 0.89 (0.62 to 1.29) | 0.546 | 1.19 (1.06 to 1.35) | 0.004 |
| ΔPaO2/FiO2 | 0.50 (0.31 to 0.79) | 0.003 | 1.65 (1.43 to 1.91) | < 0.001 |
Adjusted for PRISM III, immunocompromised status, and PaO2/FiO2 at ARDS onset
Table 3.
Full models testing association of respiratory variables with mortality (logistic regression) and successful extubation (competing risk regression) when pressure and oxygenation included in same model
| Mortality
|
Successful extubation
|
|||
|---|---|---|---|---|
| Variable (per increase of 1 SD) |
Adjusted OR (95% CI) |
p value | Adjusted SHR (95% CI) |
p value |
| PIP at 24 hours | 1.10 (0.74 to 1.64) | 0.625 | 0.83 (0.72 to 0.95) | 0.009 |
| PaO2/FiO2 at 24 hours | 0.57 (0.37 to 0.90) | 0.015 | 1.43 (1.24 to 1.66) | < 0.001 |
| PEEP at 24 hours | 1.34 (0.90 to 1.97) | 0.146 | 0.84 (0.74 to 0.95) | 0.006 |
| PaO2/FiO2 at 24 hours | 0.60 (0.40 to 0.91) | 0.015 | 1.52 (1.33 to 1.73) | < 0.001 |
| ΔP at 24 hours | 0.99 (0.66 to 1.49) | 0.961 | 0.89 (0.78 to 1.01) | 0.064 |
| PaO2/FiO2 at 24 hours | 0.54 (0.35 to 0.84) | 0.006 | 1.48 (1.28 to 1.70) | < 0.001 |
| Cdyn at 24 hours | 0.98 (0.64 to 1.50) | 0.940 | 1.12 (1.01 to 1.25) | 0.038 |
| PaO2/FiO2 at 24 hours | 0.55 (0.36 to 0.83) | 0.005 | 1.50 (1.31 to 1.72) | < 0.001 |
|
| ||||
| ΔPIP | 1.12 (0.77 to 1.64) | 0.540 | 0.81 (0.71 to 0.92) | 0.001 |
| ΔPaO2/FiO2 | 0.53 (0.32 to 0.87) | 0.012 | 1.54 (1.32 to 1.80) | < 0.001 |
| ΔPEEP | 1.32 (0.91 to 1.91) | 0.148 | 0.85 (0.76 to 0.96) | 0.008 |
| ΔPaO2/FiO2 | 0.57 (0.35 to 0.92) | 0.023 | 1.58 (1.36 to 1.84) | < 0.001 |
| ΔΔP | 1.02 (0.70 to 1.48) | 0.909 | 0.86 (0.75 to 0.98) | 0.019 |
| ΔPaO2/FiO2 | 0.50 (0.31 to 0.82) | 0.005 | 1.58 (1.36 to 1.84) | < 0.001 |
| ΔCdyn | 1.04 (0.70 to 1.55) | 0.843 | 1.07 (0.94 to 1.22) | 0.291 |
| ΔPaO2/FiO2 | 0.49 (0.31 to 0.80) | 0.004 | 1.63 (1.40 to 1.90) | < 0.001 |
All models include PRISM III, immunocompromised status, and PaO2/FiO2 at ARDS onset.
Figure 2.
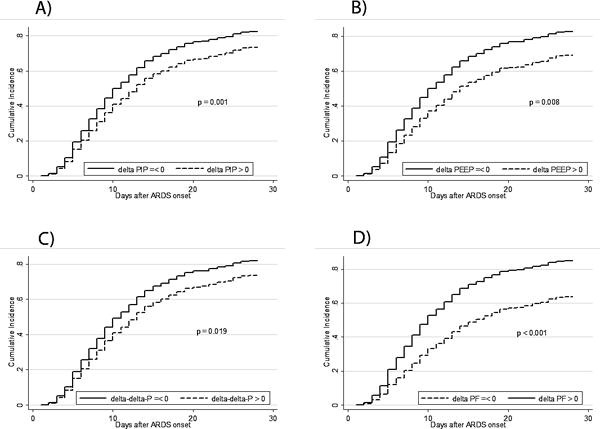
Predicted cumulative incidence of successful extubations in fully adjusted models testing pressure and oxygenation variables simultaneously. Increases in all three pressure variables (ΔPIP, ΔPEEP, ΔΔP), and decreases in ΔPaO2/FIO2, are associated with prolonged ventilation. Curves are shown for immunocompetent patients.
Subgroup Analyses
To assess whether the association between oxygenation and outcome differed depending on initial ARDS severity or by etiology of lung injury, we re-analyzed the cohort dichotomized by PaO2/FIO2 > 150 or PaO2/FIO2 ≤ 150 (Supplementary Table 2), and separately by direct or indirect ARDS (Supplementary Table 3). Results were consistent with the primary analysis demonstrating that oxygenation, rather than pressure, was more consistently associated with outcome. The association between oxygenation and mortality was only evident in patients with PaO2/FIO2 > 150, whereas oxygenation, but not pressure, was consistently associated with duration of ventilation irrespective of initial ARDS severity.
Sensitivity Analyses
We modeled PaO2/FIO2 in our primary analysis as this definition is least co-linear with pressure. However, PaO2/FIO2 does not incorporate degree of lung recruitment. Therefore, we repeated the analysis using oxygenation index (OI, mean airway pressure [mPaw] × FIO2 × 100)/PaO2) rather than PaO2/FIO2 (Supplementary Table 4). This analysis confirmed the association of oxygenation, rather than pressure, with both mortality and duration of ventilation.
Given the effects of iNO and continuous NMB on oxygenation and pressures, we repeated analyses excluding exposed to iNO (Supplementary Table 5) or NMB (Supplementary Table 6). Because of possible differences in children exposed to either volume or pressure controlled ventilation, we analyzed the 277 children exclusively on PRVC (Supplementary Table 7). Because of higher mortality for stem cell transplant patients, we repeated analysis excluding these children (Supplementary Table 8). Given the low mortality rate in immunocompetent children, we did not perform multivariable regression for mortality; however, we tested association of variables with duration of ventilation (Supplementary Table 9). Finally, to address possible confounding by bronchiolitis misdiagnosed as ARDS, we conducted analyses restricted to patients ≥ 2 years of age (Supplementary Table 10) and without detectable respiratory syncytial virus (Supplementary Table 11). Sensitivity analyses confirmed that oxygenation was more consistently associated with outcome than pressure.
DISCUSSION
PaO2/FIO2 at 24 hours and ΔPaO2/FIO2 over the first 24 hours of ARDS were independently associated with mortality, whereas PIP, PEEP, ΔP, and Cdyn, and changes in these variables over the first 24 hours, were not. Both oxygenation and pressure were associated with duration of ventilation. Thus, improvement in oxygenation, rather than improvement in pressure, was the primary variable associated with pediatric ARDS outcomes. Ours is the first study to demonstrate that after adjusting for PaO2/FIO2, the pressure variables PIP, PEEP, ΔP, and Cdyn were not associated with mortality.
In subgroup analysis, the association between oxygenation and mortality was limited to initial PaO2/FIO2 > 150. Potentially, children with severe ARDS are more likely to die of organ failures unrelated to ARDS; however, as there were only 23 deaths, we may be underpowered to detect an association with mortality. Even in children with PaO2/FIO2 ≤ 150, oxygenation, but not pressure, was associated with duration of ventilation, consistent with our primary analysis.
Re-analysis of adult ARDS trials (6) demonstrated that driving pressure (Pplateau minus PEEP) was associated with mortality. This was validated in the Large Observational Study to Understand the Global Impact of Severe Acute Respiratory Failure (LUNGSAFE)(20), which lacked explicit ventilation protocols. In our cohort, ΔP was not associated with mortality. Importantly, we defined ΔP as PIP minus PEEP, since this cohort was ventilated with decelerating flow. As PIP partly reflects resistance, it is an inherently more conservative measure of elastance than Pplateau. However, decelerating flow modes such as pressure control or PRVC are the predominant modes of ventilation in children (9, 11, 21), and PIP is more commonly tracked than Pplateau, making this more relevant for pediatrics. It is possible that re-defining ΔP as Pplateau minus PEEP would yield results more consistent with adult ARDS.
While our results are consistent with the hypothesis that oxygenation is causal for improved outcome, it is plausible that oxygenation is simply prognostic. That is, children more likely to survive demonstrate improved oxygenation, even if higher PaO2/FIO2 is not itself causal. Our study does not allow assignment of causality. However, the association between oxygenation (rather than PIP or ΔP) and survival suggests that oxygenation is a more appropriate surrogate outcome for future studies. The 2015 Pediatric Acute Lung Injury Consensus Conference recommendations for pediatric ARDS (22) suggest VT between 5 and 8 mL/kg, and lower (3 to 6 mL/kg) for severe disease, and to limit PIP, given prior associations with mortality (10, 11). Our results do not support such strict limitation on VT, PIP, and ΔP. Indeed, our results suggest that the upper limits of VT and PIP are currently unknown in pediatrics, and may be above what is conventionally recommended, as long as oxygenation is not compromised. Proof of this concept requires validation in a well-designed prospective trial.
Absent protocolized ventilation, we cannot determine whether the lack of association between pressure and outcome reflects pediatric physiology, or is confounded by peculiarities of pediatric ventilator management. However, our median PEEP (10 cmH2O) and PIP (30 cmH2O) are comparable to pressures reported in a re-analysis of adult PEEP trials (PEEP 11 cmH2O, Pplateau 29 cmH2O)(16), as well as the non-protocolized LUNGSAFE cohort (PEEP 8 cmH2O, PIP 27 cmH2O)(23), suggesting that our findings are not confounded by pediatric ventilator management substantially different from adults.
The lack of association between pressure and mortality in our cohort is consistent with reduced susceptibility to ventilator-induced lung injury (VILI) in juvenile animal models (24–26). The mechanism may be related to greater elasticity of developing lungs (27), reduced NF-κB after inflammatory stimuli (28, 29), and lower systemic inflammation after toll-like receptor activation (30, 31). VILI in children and pediatric animal models is only beginning to be studied, and its clinical relevance needs to be established in an appropriately designed trial.
Our study has several strengths. This is a large, prospective ARDS cohort from a large PICU, with detailed data collected using explicit methodology. Variables were measured absent patient effort and low risk of misclassification. However, our study has limitations. Although ARDS etiologies and severity were similar to others, generalizability may be reduced. However, as ventilation was not protocolized, the single center nature may limit heterogeneity of management. Ventilator management was somewhat lung protective, as 87% and 96% had PIP < 35 and 40 cmH2O, respectively, thus precluding a full assessment of the potential damage of elevated PIP and ΔP. Similarly, 71% and 96% of patients had VT < 8 and 10 mL/kg, respectively. However, it is difficult to find PIP and VT above these levels in modern pediatric ARDS (9, 32). Finally, as ventilation was not protocolized, lack of association between pressure and outcome may be confounded by clinician management. However, LUNGSAFE, which also lacked ventilator protocols, demonstrated an independent association between PIP and mortality (23), in contrast to our pediatric cohort, suggesting that our results may retain validity even absent protocolization.
CONCLUSION
Improvement in oxygenation, but not improvements in respiratory mechanics, was associated with lower mortality in pediatric ARDS. Future trials of ventilation in children should focus on oxygenation response (higher PaO2/FIO2) rather than lower PIP or ΔP, as oxygenation was more consistently associated with outcome, and is thus a more appropriate surrogate metric. The relevance of VILI to pediatric ARDS outcomes requires further study, as conclusions from adult ARDS extrapolate poorly to children.
Supplementary Material
Acknowledgments
Financial Support: NHLBI K12 HL-109009 (NY)
Footnotes
Reprints Planned: No
Copyright form disclosure: Dr. Yehya’s institution received funding from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute, and he received support for article research from the NIH. Dr. Thomas’s institution received funding from the FDA, and he received funding from Therabron and CareFusion.
References
- 1.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 2.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 3.Pediatric Acute Lung Injury Consensus Conference G. Pediatric acute respiratory distress syndrome: consensus recommendations from the pediatric acute lung injury consensus conference. Pediatr Crit Care Med. 2015;16(5):428–439. doi: 10.1097/PCC.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schouten LR, Veltkamp F, Bos AP, et al. Incidence and Mortality of Acute Respiratory Distress Syndrome in Children: A Systematic Review and Meta-Analysis. Crit Care Med. 2016;44(4):819–829. doi: 10.1097/CCM.0000000000001388. [DOI] [PubMed] [Google Scholar]
- 5.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 6.Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372(8):747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 7.de Jager P, Burgerhof JG, van Heerde M, et al. Tidal volume and mortality in mechanically ventilated children: a systematic review and meta-analysis of observational studies*. Crit Care Med. 2014;42(12):2461–2472. doi: 10.1097/CCM.0000000000000546. [DOI] [PubMed] [Google Scholar]
- 8.Randolph AG. Management of acute lung injury and acute respiratory distress syndrome in children. Crit Care Med. 2009;37(8):2448–2454. doi: 10.1097/CCM.0b013e3181aee5dd. [DOI] [PubMed] [Google Scholar]
- 9.Santschi M, Jouvet P, Leclerc F, et al. Acute lung injury in children: therapeutic practice and feasibility of international clinical trials. Pediatr Crit Care Med. 2010;11(6):681–689. doi: 10.1097/PCC.0b013e3181d904c0. [DOI] [PubMed] [Google Scholar]
- 10.Erickson S, Schibler A, Numa A, et al. Acute lung injury in pediatric intensive care in Australia and New Zealand: a prospective, multicenter, observational study. Pediatr Crit Care Med. 2007;8(4):317–323. doi: 10.1097/01.PCC.0000269408.64179.FF. [DOI] [PubMed] [Google Scholar]
- 11.Khemani RG, Conti D, Alonzo TA, et al. Effect of tidal volume in children with acute hypoxemic respiratory failure. Intensive Care Med. 2009;35(8):1428–1437. doi: 10.1007/s00134-009-1527-z. [DOI] [PubMed] [Google Scholar]
- 12.Yehya N, Servaes S, Thomas NJ. Characterizing degree of lung injury in pediatric acute respiratory distress syndrome. Crit Care Med. 2015;43(5):937–946. doi: 10.1097/CCM.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 13.Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 2010;11(2):234–238. doi: 10.1097/PCC.0b013e3181b806fc. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein B, Giroir B, Randolph A, et al. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 15.Yehya N, Topjian AA, Thomas NJ, et al. Improved oxygenation 24 hours after transition to airway pressure release ventilation or high-frequency oscillatory ventilation accurately discriminates survival in immunocompromised pediatric patients with acute respiratory distress syndrome. Pediatr Crit Care Med. 2014;15(4):e147–156. doi: 10.1097/PCC.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goligher EC, Kavanagh BP, Rubenfeld GD, et al. Oxygenation Response to Positive End-Expiratory Pressure Predicts Mortality in Acute Respiratory Distress Syndrome. A Secondary Analysis of the LOVS and ExPress Trials. Am J Respir Crit Care Med. 2014;190(1):70–76. doi: 10.1164/rccm.201404-0688OC. [DOI] [PubMed] [Google Scholar]
- 17.Yehya N, Servaes S, Thomas NJ, et al. Corticosteroid exposure in pediatric acute respiratory distress syndrome. Intensive Care Med. 2015;41(9):1658–1666. doi: 10.1007/s00134-015-3953-4. [DOI] [PubMed] [Google Scholar]
- 18.Yehya N, Bhalla AK, Thomas NJ, et al. Alveolar Dead Space Fraction Discriminates Mortality in Pediatric Acute Respiratory Distress Syndrome. Pediatr Crit Care Med. 2016;17(2):101–109. doi: 10.1097/PCC.0000000000000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 20.Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 21.Khemani RG, Newth CJ. The design of future pediatric mechanical ventilation trials for acute lung injury. Am J Respir Crit Care Med. 2010;182(12):1465–1474. doi: 10.1164/rccm.201004-0606CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rimensberger PC, Cheifetz IM. Ventilatory support in children with pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16(5 Suppl 1):S51–60. doi: 10.1097/PCC.0000000000000433. [DOI] [PubMed] [Google Scholar]
- 23.Laffey JG, Bellani G, Pham T, et al. Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: the LUNG SAFE study. Intensive Care Med. 2016;42(12):1865–1876. doi: 10.1007/s00134-016-4571-5. [DOI] [PubMed] [Google Scholar]
- 24.Copland IB, Martinez F, Kavanagh BP, et al. High tidal volume ventilation causes different inflammatory responses in newborn versus adult lung. Am J Respir Crit Care Med. 2004;169(6):739–748. doi: 10.1164/rccm.200310-1417OC. [DOI] [PubMed] [Google Scholar]
- 25.Kornecki A, Tsuchida S, Ondiveeran HK, et al. Lung development and susceptibility to ventilator-induced lung injury. Am J Respir Crit Care Med. 2005;171(7):743–752. doi: 10.1164/rccm.200408-1053OC. [DOI] [PubMed] [Google Scholar]
- 26.Smith LS, Gharib SA, Frevert CW, et al. Effects of age on the synergistic interactions between lipopolysaccharide and mechanical ventilation in mice. Am J Respir Cell Mol Biol. 2010;43(4):475–486. doi: 10.1165/rcmb.2009-0039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nardell EA, Brody JS. Determinants of mechanical properties of rat lung during postnatal development. Journal of applied physiology: respiratory, environmental and exercise physiology. 1982;53(1):140–148. doi: 10.1152/jappl.1982.53.1.140. [DOI] [PubMed] [Google Scholar]
- 28.Yang G, Abate A, George AG, et al. Maturational differences in lung NF-kappaB activation and their role in tolerance to hyperoxia. The Journal of clinical investigation. 2004;114(5):669–678. doi: 10.1172/JCI19300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvira CM, Abate A, Yang G, et al. Nuclear factor-kappaB activation in neonatal mouse lung protects against lipopolysaccharide-induced inflammation. Am J Respir Crit Care Med. 2007;175(8):805–815. doi: 10.1164/rccm.200608-1162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angelone DF, Wessels MR, Coughlin M, et al. Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-alpha production in vitro and in vivo. Pediatric research. 2006;60(2):205–209. doi: 10.1203/01.pdr.0000228319.10481.ea. [DOI] [PubMed] [Google Scholar]
- 31.Kollmann TR, Crabtree J, Rein-Weston A, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183(11):7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santschi M, Randolph AG, Rimensberger PC, et al. Mechanical ventilation strategies in children with acute lung injury: a survey on stated practice pattern*. Pediatr Crit Care Med. 2013;14(7):e332–337. doi: 10.1097/PCC.0b013e31828a89a2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


