Abstract
Photothermal therapy (PTT), in which nanoparticles embedded within tumors generate heat in response to exogenously applied laser light, has been well documented as an independent strategy for highly selective cancer treatment. Gold-based nanoparticles are the main mediators of PTT because they offer: (1) biocompatibility, (2) small diameters that enable tumor penetration upon systemic delivery, (3) simple gold-thiol bioconjugation chemistry for the attachment of desired molecules, (4) efficient light-to-heat conversion, and (5) the ability to be tuned to absorb near-infrared light, which penetrates tissue more deeply than other wavelengths of light. In addition to acting as a standalone therapy, gold nanoparticle-mediated PTT has recently been evaluated in combination with other therapies, such as chemotherapy, gene regulation, and immunotherapy, for enhanced anti-tumor effects. When delivered independently, the therapeutic success of molecular agents is hindered by premature degradation, insufficient tumor delivery, and off-target toxicity. PTT can overcome these limitations by enhancing tumor- or cell-specific delivery of these agents or by sensitizing cancer cells to these additional therapies. All together, these benefits can enhance the therapeutic success of both PTT and the secondary treatment while lowering the required doses of the individual agents, leading to fewer off-target effects. Given the benefits of combining gold nanoparticle-mediated PTT with other treatment strategies, many exciting opportunities for multimodal cancer treatment are emerging that will ultimately lead to improved patient outcomes.
INTRODUCTION
Nanoparticle-mediated PTT has been rapidly developing as a standalone therapy for cancer because it enables selective hyperthermia of tumor tissue while avoiding damage to healthy tissue. In PTT, plasmonic nanoparticles (NPs) are delivered into tumors and are irradiated with laser light, which causes the NPs’ conduction band electrons to undergo synchronized oscillations that result in either the absorption or scattering of the applied light.1 The absorbed light is converted into heat, which irreversibly damages the surrounding diseased tissue (Figure 1(a)). While NPs made of various materials can be employed for PTT,2–4 gold-based nanoparticles (AuNPs), which we define here as those consisting either entirely or partially of gold (such as silica core/gold shell ‘nanoshells’), have emerged as the lead therapeutic platform because they offer several major benefits. First, AuNPs enable simple gold-thiol bioconjugation chemistry for surface functionalization with therapeutic molecules, targeting ligands, or passivating agents that enhance biocompatibility as described in detail below. Additionally, the optical properties of AuNPs” can be tuned by controlling their structural dimensions so that they maximally absorb near-infrared (NIR) light (λ ≈ 650–1350 nm), which is ideal for PTT because it can safely penetrate deeply through healthy tissue to reach AuNPs embedded within tumors. A third benefit of AuNPs is that they can passively accumulate within tumors via the enhanced permeability and retention (EPR) effect, which exploits the inherently leaky and unorganized tumor vasculature.5 Because of these benefits, AuNP-mediated PTT has been thoroughly investigated for tumor ablation in animal models and has even entered clinical trials.1,6–9 However, PTT as a standalone treatment is limited because it does not affect metastatic lesions or tumor cells outside of the area of irradiation, both of which may lead to disease recurrence. To overcome these limitations and enhance treatment outcome, researchers have recently begun to explore the use of AuNP-mediated PTT in combination with secondary therapeutic approaches that take advantage of the physiological and cellular changes within tumors afforded by PTT. Additionally, they have begun to utilize optical imaging techniques to guide and assess treatment in real-time. In this review, we first discuss the design features of AuNPs that are ideal for photothermal applications and then describe how PTT aids in the success of supplemental therapies. We also highlight the use of imaging to guide PTT. Finally, we discuss opportunities and challenges for the use of AuNP-mediated PTT in multimodal strategies for cancer management.
FIGURE 1.
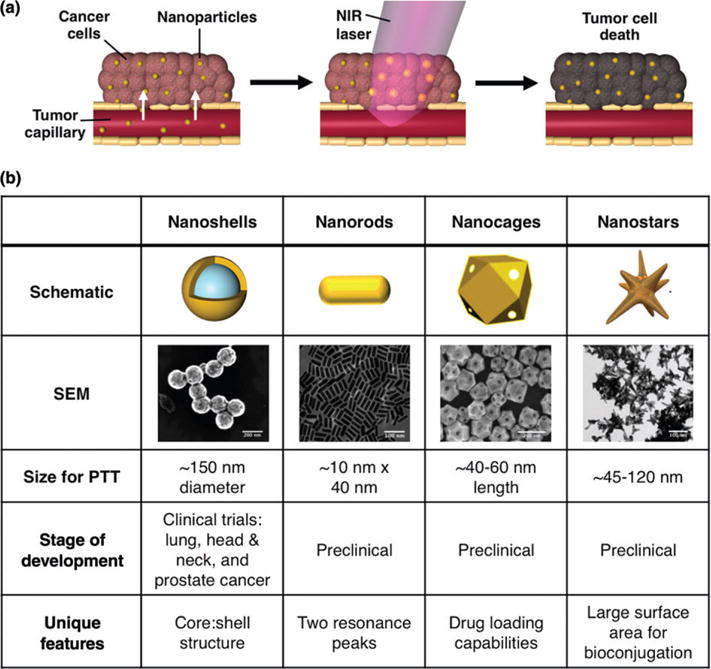
(a) In photothermal therapy, systemically-delivered nanoparticles accumulate within solid tumors via the enhanced permeability and retention effect, which exploits the leaky tumor vasculature. Once within the tumor, near-infrared light is applied to cause the nanoparticles to generate heat to kill the surrounding tumor tissue. (b) Four of the most commonly employed AuNPs for photothermal therapy include silica core/ gold shell nanoshells, gold nanorods, hollow gold nanocages, and nanostars. (Nanorod, nanocage, and nanostar images reprinted with permission from Ref 10. Copyright 2011 Wiley, Ref 11. Copyright 2007 Wiley-VCH Verlag GmbH & Co. KGaA, and Ref 12. Copyright 2015 Elsevier)
Design of Gold Nanoparticles for Photothermal Therapy
AuNPs used in photothermal applications must meet several design criteria such as having plasmon resonance tunability, high photothermal conversion efficiency,13,14 and simple surface functionalization or encapsulation chemistry. Based on these design criteria, nanoshells (NSs), nanorods (NRs), nanocages (NCs), and nanostars have emerged as the most common photothermal transducers. Here, we use these formulations as examples to discuss the desirable properties of AuNPs for PTT as depicted in Figure 1(b). This section is not meant to be a comprehensive overview, as others have discussed this topic elsewhere,1,15 but we highlight key features that researchers should consider as they plan to use PTT in individual or multimodal therapeutic strategies.
First, AuNPs used in PTT must be designed to absorb light within the first (650–850 nm) or second (950–1350 nm) NIR window because these wavelengths of light can safely and deeply penetrate healthy tissue to reach AuNPs embedded within tumors. An important feature of AuNPs is that their structural dimensions can be tuned to yield maximal absorption within one of these two regions of light. The majority of AuNPs have been designed to maximally absorb within the first NIR window, which can safely penetrate 2–3 cm of tissue. For example, the peak plasmon resonance of NSs, which consist of spherical silica cores with thin gold shells, can be tuned by adjusting their core diameter-to-shell thickness ratio. NSs with 120 nm diameter cores and 15 nm thick shells are typically used for PTT because they maximally absorb 800 nm light.16,17 Likewise, NRs have a short and long axis, thereby offering two absorption peaks corresponding to the transverse (λ ≈ 500–550 nm) and longitudinal (λ ≈ 650–850 nm) surface plasmon resonance (SPR).18,19 The rod length can be shortened or elongated to achieve peak longitudinal SPR in the NIR region.10 NRs on the order of 10 nm × ×40 nm maximally absorb ~800 nm light and are therefore the most common for PTT. While most AuNPs have been designed to maximally absorb within the first NIR window, more recently novel AuNP designs have emerged for PTT in the second NIR window. This is because these longer wavelengths can safely penetrate up to 10 cm of tissue to reach deeply embedded tumors.20–22 For example, Tchounwou et al. demonstrated the use of AuNP-coated single-walled carbon nanotubes as a multifunctional platform for imaging and PTT within the second NIR window.22 Although we expect that PTT utilizing second window NIR light will gain attention in the coming years, here we focus on PTT within the first NIR window because it has been more commonly studied in combination PTT applications.
In addition to absorbing NIR light, AuNPs intended for use in PTT should also display high photothermal conversion efficiency, which is dictated by the AuNPs’ structural dimensions (i.e., size and shape). The photothermal conversion efficiency of AuNPs of different sizes and shapes, and how this measurement can be determined, has been extensively studied in literature, and we point the reader towards these published works for more in-depth discussion.13,15,23,24 In general, for spherical AuNPs, those with smaller diameters have higher conversion efficiency than those with larger diameters. Additionally, non-spherical AuNP designs, including NRs, nanostars, and NCs, are more efficient photothermal transducers than their spherical counterparts like NSs due to their larger absorption cross sections.15,19
Notably, the size and shape of AuNPs also influence their ability to extravasate from vasculature and penetrate solid tumors, which will ultimately impact the success of PTT because it will influence how evenly heat is distributed throughout the tumor.25,26 For example, Perrault et al. compared the tumor penetrating abilities of 20 nm, 60 nm, and 100 nm solid gold spheres and found that larger size limits the distance NPs can travel away from blood vessels and into tumors.26 However, tumor retention of smaller AuNPs is challenging because they are rapidly cleared from the extracellular milieu. Therefore, it is critical to determine the optimal size for penetration and retention based on the tumor characteristics (i.e., the ‘leakiness’ of its vasculature, the organization of its lymphatic vessels, and the density of the tumor extracellular matrix). This is particularly important given that the EPR effect is now known to be heterogeneous in distinct tumor types.27 Intriguingly, PTT can be used to overcome these tumor penetration and retention limitations by amplifying tumor vessel leakiness and extracellular matrix permeability to enhance the delivery of more AuNPs or secondary therapeutic molecules, which we discuss later in this review.28,29
Another important consideration for AuNP design is their ability to encapsulate or be functionalized with biomolecules or drugs to enable combination therapy. The gold exterior of AuNPs is beneficial for bioconjugation because it enables simple gold-thiol bonding. One advantage of anisotropic materials like nanostars, which are spiked AuNPs, is that they offer a high surface-to-volume ratio for bioconjugation.30 This can be advantageous because loading density can influence how AuNPs interact with cells and tissues. One disadvantage of loading molecules on the exterior of solid AuNPs, however, is that it leaves them susceptible to immune recognition or degradation. Hollow, porous AuNPs, such as NCs, offer the ability to load therapeutic agents in the nanoparticle core.11 Since most hollow AuNPs destabilize upon NIR irradiation, the release of encapsulated molecules can be triggered ‘on demand.’31 Although this photothermal reshaping is desirable for drug delivery, it causes the AuNPs to lose their peak SPR to preclude their repeated use for PTT. Using NRs, researchers have demonstrated that one way to counteract this reshaping upon irradiation is by coating the NPs with a silica shell.32 A similar strategy could be employed for other AuNPs that are known to reshape upon irradiation.
Here, we focused on how AuNPs’ size, shape, and surface modifications can influence their heat generation and tumor penetration. In addition, these features also impact the AuNPs’ biodistribution and biocompatibility, as demonstrated in various cell and animal models.12,33–35 Similar to bulk gold, AuNPs are generally considered to be chemically inert and biocompatible.35 However, upon intravenous administration, AuNPs are rapidly coated with serum proteins that alter their biological identity and change how they are presented to cells.36–38 Further, AuNPs are quickly recognized by the mononuclear phagocytic system, also known as the reticuloendothelial system (RES), leading to their rapid clearance from the blood. AuNPs’ surface coating largely dictates the formation of the protein corona and clearance from the body. To reduce non-specific protein adsorption, extend circulation time, and enhance biocompatibility, AuNPs are often coated with passivating agents, such as poly(ethylene) glycol (PEG), in addition to targeting agents or other therapeutic molecules. The length and density of PEG on AuNPs is important, as it influences how well the AuNPs are shielded from protein corona formation and clearance from the body.37 Among the various types of AuNPs used for PTT, NSs coated with PEG (also known as AuroShells, which are being commercialized by Nanospectra Biosciences, Inc.) are the furthest along in development and are currently the only AuNP for PTT being evaluated in clinical trials. These trials are examining the safety and efficacy of nanoshell-mediated PTT against lung, head and neck, and prostate cancers.7–9 The first publication regarding these human trials indicates that PEG-coated NSs have an excellent clinical safety profile,39 supporting their continued use and providing evidence that PEG is a viable surface coating for AuNPs intended for human PTT. Although there are several clinical trials ongoing, efficacy data from these trials has not yet been published. In the Opportunities and Challenges section of this review, we further discuss ongoing clinical applications of AuNPs for PTT.
Overall, there are many features that contribute to whether a specific AuNP design will work well for PTT, and we have highlighted a few here. As previously mentioned, investigators have begun studying PTT in combination with other therapeutic strategies for overall enhanced therapeutic efficacy by exploiting the physiological and cellular level effects of PTT in tumors as described in the next section.
Physiological Effects of PTT and the Mechanism of Cell Death Induced by PTT
AuNP-mediated PTT causes several physiological and biological changes to the surrounding tumor environment that can maximize the cytotoxic effects of PTT itself, enhance the efficacy of secondary therapies, or decrease required treatment dosages for secondary agents.40–42 For example, the heat generated by AuNPs can increase the permeability of tumor vessels and cancer cell membranes to increase tumor and cellular uptake, respectively, of additional AuNPs or drugs (Figure 2(a)). We highlight some of the benefits of combination strategies that exploit these effects of PTT later in this review.
FIGURE 2.
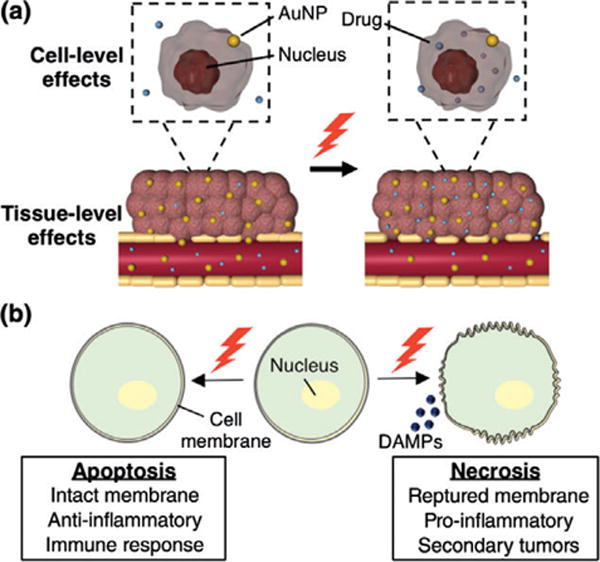
Schematics demonstrating the physiological and biological effects of photothermal therapy. (a) The heat generated by AuNPs in response to NIR light enhances the permeability of tumor vasculature and cell membranes to increase the accumulation of secondary therapies, such as chemotherapeutic drugs. (b) PTT can lead to either cellular necrosis or apoptosis, which cause different cellular and immune responses. The mechanism of cell death depends on the applied irradiation parameters.
In addition to increasing tumor vessel and cell membrane permeability, PTT also causes intracellular effects such as DNA damage and protein denaturation. Recently, researchers have begun to study the mechanisms of cell death triggered by PTT more closely because the mechanism of cell death (i.e., necrosis versus apoptosis) activates different cell signaling pathways that may influence treatment success and be beneficial for potential combination therapeutic strategies (Figure 2(b)). Conventional PTT uses high energy irradiation to cause rapid nanoparticle heating leading to cellular necrosis; while this strategy is effective for the ablation of established tumors,16,17,43 it can also induce undesirable effects. For example, high energy PTT resulting in cellular necrosis can trigger the release of cellular waste and damage-associated molecular patterns (DAMPs) that induce inflammation, which may lead to increased secondary tumor growths.44,45 Conversely, low energy PTT initiates cellular apoptosis, which may lead to beneficial immunogenic responses.44,45 For example, cellular apoptosis can potentially discourage inflammation by causing phagocytes to produce anti-inflammatory molecules such as TGF-β.46 Further, macrophages and dendritic cells (DCs) enter an anti-inflammatory state when in contact with apoptotic cells. The ability to induce apoptosis versus necrosis with PTT depends on many factors, including the AuNP photothermal properties, AuNP concentration in diseased tissue, and irradiation conditions. Given that initiating apoptosis versus necrosis with PTT can result in very distinct cellular effects, researchers should carefully control PTT parameters for their particular application. In addition, investigators should consider utilizing PTT in combination with secondary treatment strategies to capitalize on the tissue- or cellular- level effects induced by PTT. Below, we review how the physiological changes and mechanisms of cell death induced by PTT can be combined with chemotherapy, gene regulation, and immunotherapy for synergistic approaches to cancer treatment. We then describe how imaging can be used to guide and assess PTT in real-time.
ENHANCING CHEMOTHERAPY WITH GOLD NANOPARTICLE-MEDIATED PHOTOTHERMAL THERAPY
Although mortalities due to many cancers have not significantly improved in the past few decades, chemotherapy remains the current standard-of-care treatment. While cytotoxic at high doses, chemotherapy success is often hindered by acquired drug resistance and adverse side effects that limit the maximum administered dose. As previously mentioned, hyperthermia increases both vascular and cell membrane permeability, as well as extracellular matrix permeability.28 These physiological changes can amplify the effect of subsequently applied chemotherapy by increasing intratumoral and intracellular drug content.42,47 Further, drug cytotoxicity may be enhanced by heat application.48 One of the early indications of the synergy between hyperthermia and chemotherapy was demonstrated by Hahn et al., who showed optimal cytotoxicity by co-treating cells with doxorubicin and heat at 43°C.49 The clinical feasibility of widespread hyperthermia, however, is limited because it may increase drug cytotoxicity to healthy cells in addition to tumor cells. Comparatively, PTT-mediated by AuNPs can provide localized, tumor-specific heating, thereby avoiding the detrimental effects of widespread hyperthermia.
In one investigation of combined chemotherapy and PTT, Hauck et al. demonstrated that NR-mediated low intensity PTT initiates apoptosis in cancer cells, which in turn sensitizes the cells to cisplatin therapy and results in synergistic cytotoxicity.50 We recently demonstrated that NS-mediated PTT of breast cancer cells leads to enhanced cell permeability and therefore increased intracellular drug content leading to decreased viability compared to cells that do not receive PTT (Figure 3(a)).42 Notably, in addition to increasing the cellular uptake of drugs and the cellular response to drugs, hyperthermia can also be used to increase the amount of drugs that infiltrate tumors. This is because PTT can both increase tumors’ vascular permeability and alter the expression of proteins found on cancer cell membranes, thereby providing a handle for targeting of additional therapeutic agents. In an elegant example of this technique, Park et al. fabricated a cooperative system that targeted drug-loaded liposomes to the p32 protein, which is upregulated following NR-mediated PTT, to improve tumor delivery of the drugs and enhance treatment outcome versus PTT or chemotherapy individually (Figure 3(b)).29 Strategies such as this that exploit the physical effects of PTT for enhanced treatment outcome have outstanding promise for future applications.
FIGURE 3.
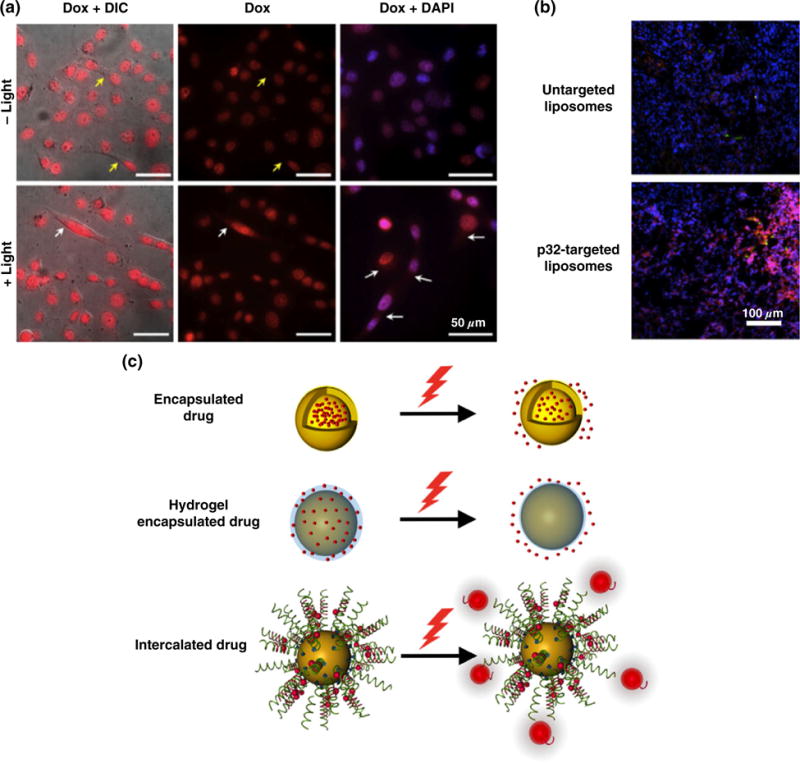
(a) Breast cancer cells treated with silica core/gold shell nanoshells and doxorubicin (red) and exposed to NIR light display increased drug uptake compared to cells that were not irradiated. Blue indicates nuclei, yellow arrows indicate cytoplasmic regions of cells not irradiated, and white arrows indicate enhanced doxorubicin content in the cytoplasm following irradiation. (Reprinted with permission from Ref 42. Copyright 2015 Dovepress) (b) Tumor tissue extracted from mice treated with nanorod-mediated hyperthermia and doxorubicin-loaded liposomes targeted to p32, a protein, that is upregulated in response to stress, shows enhanced liposome (green) and doxorubicin (red) distribution compared to mice treated with hyperthermia and untargeted liposomes. (Reprinted with permission from Ref 29. Copyright 2010 PNAS) (c) Schematics showing the mechanisms of light induced release of drugs from AuNPs.
While the aforementioned strategies delivered AuNPs and chemotherapy separately, researchers have begun to create more complex AuNP-drug conjugates for combination PTT and chemotherapy. This should reduce off-target toxicity by limiting the amount of chemotherapy that reaches normal tissues and enabling drug release only upon irradiation. In these systems, drugs are physically encapsulated within AuNPs or bound to their surfaces using linkers that hold them in place until the heat produced during light irradiation triggers their release (Figure 3(c)). Hollow AuNPs, such as NCs, are ideal for drug encapsulation and light-induced release strategies.31,51,52 For example, Wang et al. developed multifunctional NCs by encapsulating doxorubicin and coating the particles with hyaluronic acid (HA).52 In this design, the HA acted as both a targeting agent to bind CD44 receptors and as a biodegradable capping agent to avoid premature drug release. Upon irradiation, the encapsulated doxorubicin was released only following degradation of the HA, which resulted in a drastic decrease in cell viability relative to controls.52 Additional mechanisms of light-induced drug release include coating AuNPs with thermally responsive drug-containing hydrogels or oligonucleotides that contain intercalated drugs (Figure 3(c)). For instance, Strong et al. created NSs coated with a thin poly(N-isopropylacrylamide-co-acrylamide) hydrogel layer that remained swollen under physiological conditions, but expelled water and encapsulated drugs at higher temperatures achieved during NIR irradiation.53 Similarly, thiolated ligands attached to AuNPs can be used to carry intercalating agents that are released upon photothermal irradiation (Figure 3(c)).54,55 Overall, strategies for combination PTT and chemotherapy such as those reviewed here have great promise to increase treatment efficacy while minimizing off-target effects.
THERMALLY RESPONSIVE GOLD NANOPARTICLES FOR GENE REGULATION
Gene regulation is a technique that aims to treat cancer by using nucleic acids to inhibit the expression of genes that drive tumor progression, and it is particularly promising for targets that are considered ‘undruggable’ by small molecules due to lack of an effective binding site. The main type of nucleic acid used for gene regulation is small interfering RNA (siRNA), which suppresses gene expression by triggering degradation of targeted messenger RNA molecules inside the cell cytoplasm, thereby halting production of the encoded disease-associated protein.56–58 Unfortunately, several challenges have hindered the translation of gene regulation from the laboratory to the clinic. First, siRNAs and other nucleic acids are rapidly cleared from the bloodstream and quickly degraded in the presence of nucleases, making in vivo delivery difficult. Second, intracellular delivery of these negatively charged molecules requires the use of cationic transfections agents that display undesirable toxicity. Further, many siRNA delivery strategies are not cell-specific, and therefore may lead to uptake and gene silencing in all cell types. AuNPs have been exploited as tools for gene regulation because they offer simple bioconjugation chemistry, enabling them to carry siRNAs or other nucleic acids for enhanced stability, intracellular delivery, and gene regulation both in vitro and in vivo.59–61 However, a common challenge to achieving sufficient gene regulation with siRNA-AuNP conjugates is that they become trapped in intracellular compartments upon endocytosis.62 To improve upon this issue and enable gene regulation only in desired tissues, PTT can be used to enable endosomal escape and trigger gene silencing on demand (Figure 4(a) and (b)).62–65 A mechanistic study completed by Braun et al. demonstrated that hollow NSs conjugated with siRNA and Tat peptides for cellular uptake become trapped within endosomes following endocytosis. However, irradiating the samples with a pulsed NIR laser to induce NS heating caused the endosomes to rupture, enabling the release of the siRNA into the cytosol.62 Similarly, Huschka et al. demonstrated that silica core/gold shell NSs coated with GFP-silencing DNA or siRNA drastically decreased GFP expression only following irradiation.66
FIGURE 4.
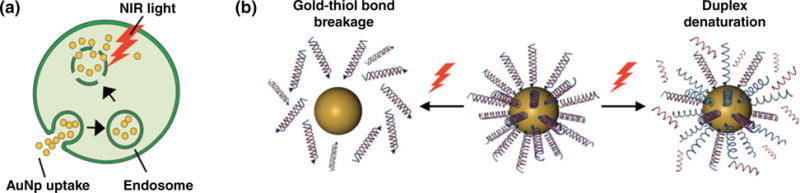
Mechanisms of light-triggered gene regulation using AuNPs. (a) Following cellular uptake, NIR-absorbing AuNPs functionalized with gene regulation agents become trapped within endosomes, limiting their ability to deliver the payload for effective gene silencing. Upon NIR light application, the AuNPs generate sufficient heat to rupture the surrounding endosome and release the AuNPs into the cell cytosol without harming overall cell integrity. (b) Schematic of the mechanisms of light induced release of oligonucleotides from AuNP surfaces. Under pulsed laser irradiation, the gold-thiol bond between the molecules and AuNPs breaks, releasing the entire duplex. Alternatively, continuous wave laser irradiation can denature the linkage between the two strands to release only single stranded oligonucleotides.
There are two proposed mechanisms for light-induced release of oligonucleotides from AuNP surfaces depending on the laser power and temperature reached during irradiation (Figure 4(b)).67 First, a pulsed laser is used to break the gold-thiol bond between the oligonucleotides and the AuNPs, releasing the entire molecule from the AuNP surface.62 The pitfall of this method is that the dissociation of the molecules from the NP surface results in NP instability, and the free exposed thiols can be detrimental to live cells.67 The second mechanism of release typically uses a continuous wave laser to denature the linkage between the sense and antisense strands of oligonucleotides with the goal of releasing only the unthiolated strand from the AuNP, although this may result in the gold-thiol bond breaking as well. This method requires longer irradiation times and is less efficient for release compared to the pulsed laser approach due to the lower laser powers used. However, researchers have optimized laser parameters to use the pulsed laser for duplex denaturation. For example, Poon et al. demonstrated that decreasing laser powers inhibited the gold-thiol bond breakage while still enabling duplex denaturation.67 In addition to understanding the role that light irradiation/heating conditions play in facilitating the release of oligonucleotides from AuNPs, it is also important to consider how particle shape may influence the delivery of gene regulatory agents. Gold NRs, e.g., have been vastly studied for photorelease because they melt and reshape into spherical particles following irradiation to trigger payload release.68–70
Here, we have briefly reviewed the use of PTT to trigger the release of oligonucleotides from AuNP surfaces, as well as the use of PTT to release AuNPs and oligonucleotides from endosomes, as both of these strategies result in enhanced gene regulation. A more detailed discussion of this topic can be found in the recent review article published by Kim et al.71 Looking forward, we encourage researchers to carefully study the signaling pathways that are activated in cells in response to PTT, and to investigate whether inhibiting these pathways via gene regulation can produce synergistic effects on tumor growth.
COMBINING PHOTOTHERMAL THERAPY WITH IMMUNOTHERAPY FOR PROLONGED ANTI-CANCER EFFECTS AND METASTASIS INHIBITION
As previously discussed, PTT as a standalone therapy faces risk of recurrence and is not suitable for treating disseminated metastatic disease. Combining PTT with immunotherapy may prevent tumor recurrence and inhibit metastases.2,55,72–77 Immunotherapy is a rapidly emerging, promising technique in which the host immune system is stimulated to recognize and kill cancer cells that have adapted various means of avoiding immune recognition.78 One way cancer cells avoid recognition is by secreting cytokines such as IL-10 that suppress DC maturation (mature DCs capture tumor antigens and present them to T cells for activation). Cancer cells also produce factors such as TRAIL and FasL that induce apoptosis of cytotoxic T cells (which find and destroy cancer cells), and down-regulate surface antigens and co-stimulatory molecules to decrease T-cell recognition and stimulation. Finally, cancer cells attract immune suppressive cells to the tumor microenvironment, including tumor-associated macrophages, myeloid derived suppressor cells, and T regulatory cells (Tregs, which suppress DCs and cytotoxic T cells by presenting inhibitory molecules such as cytotoxic lymphocyte antigen 4 (CTLA-4) and programmed cell death ligand 1 (PD-L1)). Immunotherapy counteracts these mechanisms by delivering tumor-specific T cells, immune checkpoint inhibitors (antibodies against CTLA-4 or PD-L1), or CpG oligodeoxynucleotides (which are recognized by toll-like receptor 9 (TLR9) on B cells and plasmacytoid DCs to stimulate an immune response). Each of these strategies has been evaluated in combination with PTT (mediated by both AuNPs and non-AuNPs), and the results indicate that dual PTT/immunotherapy is advantageous to either therapy alone.2,55,72–77
The rationale to combine PTT with immunotherapy is based on the fact that heat causes dying cancer cells to release antigens and heat shock proteins (HSPs) that are captured by antigen presenting cells such as DCs to mediate an immune response (Figure 5).72,73 Further, the immunostimulatory environment created by PTT can enhance immunotherapies to prolong anti-cancer effects and eliminate both primary tumors and metastatic disease. For example, Bear et al. demonstrated in a murine melanoma model that PTT mediated by hollow gold NSs promotes the expression of pro-inflammatory cytokines and chemokines that induce DC maturation in tumor-draining lymph nodes and that prime anti-tumor CD8+ effector T cells.74 Combining PTT with adoptive T-cell transfer (ATCT) decreased tumor recurrence by 21.8% compared to PTT alone and also suppressed distant, untreated contralateral tumors more effectively than either therapy alone.74 Thus, ATCT can help prevent primary tumor recurrence following PTT, and PTT can enhance the ability of ATCT to eradicate distant disease.
FIGURE 5.
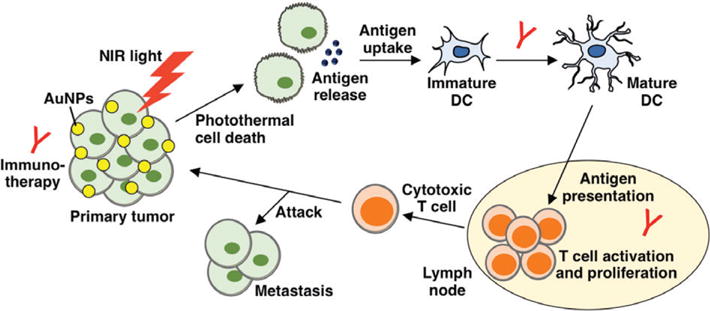
Schematic depicting the impact of combined photothermal therapy and immunotherapy on primary tumors and metastases. (Reprinted with minor modifications with permission from Ref 72. Copyright 2016 World Scientific Publishing Company)
To our knowledge, PTT mediated by AuNPs has not yet been evaluated in combination with immune checkpoint inhibitors, but a recent study by Wang et al. demonstrated that PTT mediated by single-walled carbon nanotubes (SWNTs) is synergistic with anti-CTLA-4 therapy.75 They observed that SWNT-mediated PTT increased DC maturation in tumor-draining lymph nodes, and the combination of PTT and anti-CTLA-4 therapy suppressed distant metastasis (either contralateral flank tumors or lung metastasis) in a murine model of breast cancer better than either therapy alone.75 Given the success of this study, it is likely that AuNP-mediated PTT would also result in impressive anti-tumor effects when combined with immune checkpoint inhibitors. Finally, CpG-mediated immunotherapy has also been tested in combination with PTT and appears promising.2,55 Tao et al., for instance, showed that NRs coated with CpGs that were intercalated with doxorubicin could enable in vivo combination chemotherapy, light-triggered drug release, hyperthermia, and immunotherapy.55 This is an excellent example of how AuNPs can be exploited for multimodal anticancer therapy.
While most research combining PTT with immunotherapy has used PTT to enhance the effect of immunotherapy, researchers have also begun to exploit immune cells to enhance PTT. Specifically, since immune cells such as antigen-specific cytotoxic T cells, cytokine-induced killer cells, and macrophages have a unique ability to ‘home’ to the tumor microenvironment in vivo, researchers have attached AuNPs such as NRs, nanoprisms, and NSs,79–82 as well as non-AuNPs such as Prussian blue NPs,77 to the surface of these cells or loaded them within the cells’ interior to enhance tumor delivery and improve the efficacy of PTT. This technique, which has been referred to as ‘cellular backpacking’ or ‘Trojan horse’ delivery, capitalizes on the natural homing ability of immune cells for enhanced treatment outcomes and shows great promise for further development.
Overall, there is great potential for combining AuNP-mediated PTT with immunotherapy, as it can enable treatment of both primary tumors and distant metastases and may also prevent long-term tumor recurrence. Given that PTT and immunotherapy are mutually beneficial, we anticipate that the field of photothermal immunotherapy will rapidly expand in the next decade to result in improved patient outcomes.
IMAGE-GUIDANCE AND ASSESSMENT OF PTT USING GOLD NANOPARTICLES
A key advantage of AuNPs is that their unique photonic properties enable them to be used as optical contrast agents, so imaging can be used to guide and assess PTT in real-time.83–85 Two imaging modalities that have been extensively studied for this purpose in pre-clinical settings are optical coherence tomography (OCT) and photoacoustic (PA) imaging, which exploit the AuNPs’ light scattering or absorption properties, respectively. Here, we describe each of these in detail. We also describe the use of magnetic resonance thermal imaging for image-guided PTT.
OCT uses a low coherence light source applied through the depth of a sample and detects the resultant reflection at micrometer-level resolution.86,87 Since AuNPs provide reflective signal beyond the native tissue itself, tumors or other tissues containing AuNPs display enhanced OCT signal compared to tissue not harvesting AuNPs (Figure 6(a)).87 Using NSs as contrast agents and photothermal transducers, Gobin et al. showed that OCT could be used to ensure sufficient NS accumulation within tumors prior to PTT. More specifically, they showed that systemically-delivered NSs enhanced the OCT signal in tumors but not in healthy tissue in a murine cancer model, and subsequent application of NIR light led to a reduction in tumor size.87 In this manner, researchers can utilize OCT for image-guided therapy to ensure ablation of the entire tumor. More recently, researchers have demonstrated that the absorptive properties of photothermally active NPs can be exploited to enhance contrast of phase-sensitive OCT (PSOCT) ex vivo. While conventional OCT generates contrast based on light scattering, PSOCT generates contrast based on small alterations in the sample’s optical path length, which is influenced by tissue temperature.87 Since AuNPs generate heat upon light irradiation, they can enhance PSOCT contrast in a technique known as photothermal OCT.89 Photo-thermal OCT has recently been applied in vivo, and has proven useful for analysis of highly-scattering tissues because it can separate the AuNPs from the scattering background.89 Overall, OCT has shown much promise as a tool to guide PTT in ex vivo and preclinical in vivo studies, but its low penetration depth may limit its clinical utility.
FIGURE 6.
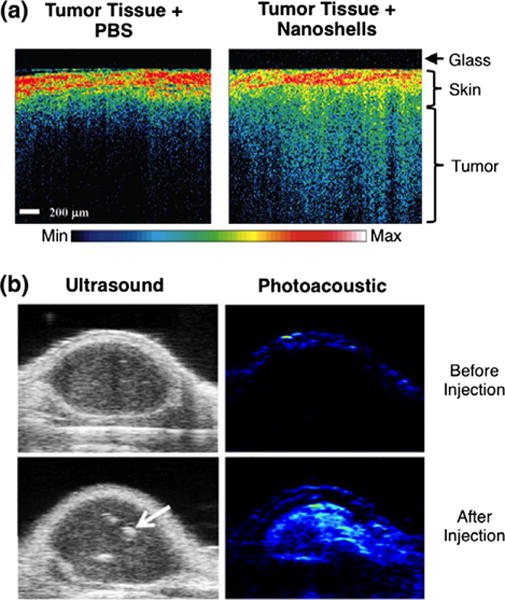
(a) Silica core/gold shell nanoshells accumulated within tumor tissue can be visualized with optical coherence tomography, as depicted by increased signal throughout the tissue depth. (b) In vivo ultrasonic and photoacoustic images demonstrate enhanced signal from biodegradable gold-based NPs accumulated within the tumor. NPs were composed of clusters of small gold nanoparticles that produced an ultrastrong plasmonic coupling effect for imaging and PTT. The arrow indicates NPs. ((a) Reprinted with permission from Ref 87. Copyright 2007 American Chemical Society and (b) Ref 88. Copyright 2013 Wiley-VCH Verlag GmbH & Co. KGaA)
An additional technique used for image guidance and assessment of PTT is photoacoustic (PA) imaging (Figure 6(b)).32,88 In PA imaging, tissue is irradiated with a pulsed laser and light absorption produces a temperature rise in the tissue leading to its subsequent thermo-elastic expansion. The pressure induced by this expansion leads to propagation of acoustic waves, which are detected by an ultrasound transducer at the surface of the body. AuNPs embedded in tissue provide increased PA signal because they convert light to heat more efficiently than native tissue itself. Therefore, PA imaging can be utilized to guide and assess PTT efficacy.90 Notably, since PA imaging incorporates an ultrasound transducer, this technology readily allows for acquisition of traditional ultrasound images. Overlaying PA images with ultrasound images can provide morphological information and reveal the distribution of AuNPs in tumor tissue. Further, as shown in Figure 6 (b), AuNPs can inherently increase contrast of both ultrasound and PA imaging.88 Another benefit of PA imaging is that it can also be used to remotely monitor the thermal dose applied to the tissue during PTT. This was demonstrated by Kim et al., who showed that PA imaging could be used to determine the distribution of NRs within subcutaneous human epithelial tumors in mice and to map the temperature distribution during irradiation.32 As previously mentioned, the temperature achieved during PTT will influence whether the cells undergo apoptosis or necrosis, which ultimately influences therapeutic success. Using techniques such as PA imaging to monitor the temperatures reached during PTT may allow researchers to optimize irradiation parameters during treatment to control the mechanism of cell death. Finally, a very exciting application of PA imaging is the analysis of micrometastases in lymph nodes.91,92 Since cancer typically spreads first to the lymph nodes prior to distant organs, the ability to detect this earliest stage of metastasis and provide PTT in a single setting holds much promise as an intervention strategy.
In addition to OCT and PA imaging, magnetic resonance thermal imaging (MRTI) has also been studied as a tool to non-invasively guide or assess AuNP-mediated PTT using pre-clinical in vivo models of human cancer.93–95 In MRTI, a temperature map is generated in real-time during irradiation that can be interpreted by physicians to control the thermal doses applied.93,94 In one example of this technique, Rylander et al. demonstrated the use of MRTI in prostate tumors and correlated the temperatures reached during irradiation with expression of heat shock proteins.95 Since MRTI temperature mapping can be used to control and optimize PTT parameters in real time, it may be possible to use this technology during PTT to control the mechanism of cell death and also to minimize any adverse impacts to adjacent healthy tissue. It should be noted that this technology is best applied to static tissues, as motion can easily corrupt the accuracy of MRTI.96
In this section, we have introduced three imaging techniques that are the most commonly studied for guidance and assessment of PTT. Confirming that AuNPs have adequately accumulated in tumors prior to irradiation, and that the desired temperature has been achieved upon irradiation, is critical to ensure success of PTT as a standalone therapy or in combination with chemotherapy, gene regulation, or immunotherapy. While the ability to guide PTT in humans with OCT, PA imaging, or MRTI has not yet been evaluated, we anticipate that this will occur in the near future as PTT progresses through clinical development. Indeed, MRTI is already used for temperature mapping during cryotherapy, radiofrequency ablation, and focused ultrasound ablation, so its implementation for clinical guidance of PTT should be straightforward.97 For a discussion of the clinical status and potential of each of these imaging modalities, we point the reader towards several recent papers that provide an excellent summary of the benefits and limitations of each of these imaging modalities in the clinical setting.98–101
ADDITIONAL APPLICATIONS OF PHOTOTHERMALLY ACTIVE GOLD NANOPARTICLES
While this review has emphasized the use of PTT in cancer therapy, PTT also poses opportunities for other biological applications outside cancer management. For example, since AuNPs can passively accumulate in atherosclerotic plaques, they have been used for image-guided PTT of atherosclerosis directed by intravascular photoacoustic imaging (IVPA).102,103 Additionally, PTT has been studied for the targeted ablation of bacterial cells to treat antibiotic-resistant infections such as methicillin-resistant Staphylococcus aureus (MRSA).104,105 Moreover, the heat-generating photo-thermal effect has also been investigated for global health and environmental applications. Neumann et al. demonstrated that specialized AuNPs designed to absorb light across the entire solar spectrum could produce sufficient heat in response to sunlight to generate steam for sterilization and sanitization purposes.106,107 These applications of photothermally active AuNPs are particularly interesting and important for developing countries that do not currently have access to clean energy and water supplies.
OPPORTUNITIES AND CHALLENGES
Excitingly, PTT has rapidly progressed from concept to clinical application, with PTT-mediated by NSs already in three distinct Phase 1 clinical trials (Figure 1(b))that focus on the treatment of lung, head and neck, and prostate cancers.7–9 While efficacy data has not yet been published, clinical trials evaluating safety of NSs are promising.39 In addition, PTT mediated by both NSs and NRs has begun to be applied to veterinarian medicine, with a special emphasis on treating canine and feline tumors.108,109 Although we do not focus on translatability here, a comprehensive overview of clinical trials that utilize AuNPs for photothermal applications has recently been published by Pedrosa et al.110 We anticipate that the next decade will witness the commencement of additional clinical trials of other more recently developed AuNPs, the expansion into applications beyond cancer, and the evaluation of multimodal strategies.
There are several critical opportunities and challenges that should be addressed as PTT continues to develop as a standalone therapy and as a component of multifunctional strategies for cancer management. Detailed studies should be performed to understand how adjusting irradiation conditions, AuNP types, and the temperatures achieved during PTT influence the underlying impact to cells, including the mechanism of cell death, as this is crucial for successful therapeutic outcome. Conventional PTT, while effective for tumor ablation with high laser energies and temperatures, is challenging as a standalone therapy because it may result in cellular necrosis and therefore increased inflammation and secondary tumor growth. Further, it is difficult to ensure complete ablation of all tumor cells, and PTT is not feasible as a treatment for metastatic disease. However, there are increasing opportunities to use PTT in combination with other therapies such as chemotherapy, gene regulation, and immunotherapy, to provide synergy for more thorough cancer treatment. The combination of PTT and these secondary techniques is mutually beneficial. That is, PTT can enhance secondary therapies by increasing molecular stability and tumor delivery, and these other therapies can enhance PTT by ensuring complete elimination of cells. Finally, PTT can be combined with imaging strategies to guide therapeutic application and monitor treatment success. Overall, using PTT in combination with imaging and/or second therapeutic strategies offers great potential for increasing tumor regression while decreasing off-target effects to healthy tissue.
CONCLUSION
AuNP-mediated PTT has been thoroughly investigated as a standalone therapy for cancer, but its recent use in combination with other imaging and treatment modalities presents improved therapeutic results. AuNPs are ideal photothermal transducers because their optical properties can be tuned by adjusting their structural dimensions so that they strongly absorb tissue-penetrating NIR light. Further, AuNPs provide several essential features for use in multimodal therapies including simple gold-thiol bioconjugation chemistry and efficient tumor accumulation and cellular uptake. PTT can exploit these qualities of AuNPs to provide controlled release of conjugated or encapsulated molecules specifically at diseased sites. Additionally, AuNP-mediated PTT can sensitize cancer cells to chemotherapy, gene regulation, and immunotherapy by increasing cell permeability and intracellular delivery. Controlled release and hyperthermia-induced sensitization can lower the required therapy dosages and increase localized efficacy. Lastly, PTT can modulate cellular apoptosis or necrosis depending on the laser power and resultant temperature reached during irradiation. The mechanism of cell death is important because necrotic cells can promote secondary tumor growth, while apoptotic cells may elicit an immune response to decrease the formation of secondary tumors. Therefore, it is critical to understand the mechanism of cell death to optimally design multimodal cancer therapies. Owing to the extensive synergy between hyperthermia and other treatment modalities, it is likely that AuNP-mediated PTT will become a hallmark of multifunctional cancer therapies.
Acknowledgments
This work was supported by the University of Delaware Research Foundation, by grant number IRG14-251-07-IRG from the American Cancer Society, by a Science and Engineering Research Grant from the W.M. Keck Foundation, and by the National Institutes of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) under award number R35GM119659 and through an Institutional Development Award (IDeA) from NIH NIGMS under grant number U54GM104941. The Delaware INBRE program, with a grant from NIGMS (P20-GM103446) from the NIH, also supported this project. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest: The authors have declared no conflicts of interest for this article.
References
- 1.Kennedy LC, Bickford LR, Lewinski NA, Coughlin AJ, Hu Y, Day ES, West JL, Drezek RA. A new era for cancer treatment: gold-nanoparticle-mediated thermal therapies. Small. 2011;7:169–183. doi: 10.1002/smll.201000134. [DOI] [PubMed] [Google Scholar]
- 2.Guo L, Yan D, Yang D, Li Y, Wang X, Zalewski O. Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles. ACS Nano. 2015;8:5670–5681. doi: 10.1021/nn5002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hashida Y, Tanaka H, Zhou S, Kawakami S, Yamashita F, Murakami T, Umeyama T, Imahori H, Hashida M. Photothermal ablation of tumor cells using a single-walled carbon nanotube-peptide composite. J Control Release. 2014;173:58–66. doi: 10.1016/j.jconrel.2013.10.039. [DOI] [PubMed] [Google Scholar]
- 4.Li M, Teh C, Ang CY, Tan SY, Luo Z, Qu Q, Zhang Y, Korzh V, Zhao Y. Near-infrared light-absorptive stealth liposomes for localized photothermal ablation of tumors combined with chemotherapy. Adv Funct Mater. 2015;25:5602–5610. [Google Scholar]
- 5.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 6.Blaney SM. Vascular-targeted photothermal therapy of an orthotopic murine glioma model. 2011 doi: 10.2217/nnm.11.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nanospectra Biosciences Inc. Pilot study of aurolase(tm) therapy in refractory and/or recurrent tumors of the head and neck. Available at: https://clinicaltrials.gov/ct2/show/NCT00848042?term=auroshell&rank=2. (Accessed May 23, 2016).
- 8.Nanospectra Biosciences Inc. Efficacy study of aurolase therapy in subjects with primary and/or metastatic lung tumors. Available at: https://clinicaltrials.gov/ct2/show/NCT01679470?term=auroshell&rank=3. (Accessed May 23, 2016).
- 9.Nanospectra Biosciences Inc. MRI/US fusion imaging and biopsy in combination with nanoparticle directed focal therapy for ablation of prostate tissue. Available at: https://clinicaltrials.gov/ct2/show/NCT02680535?term=auroshell&rank=1. (Accessed September 28, 2016).
- 10.Stone J, Jackson S, Wright D. Biological applications of gold nanorods. WIREs Nanomed Nanobiotechnol. 2011;3:100–109. doi: 10.1002/wnan.120. [DOI] [PubMed] [Google Scholar]
- 11.Skrabalak SE, Chen J, Au L, Lu X, Li X, Xia Y. Gold nanocages for biomedical applications. Adv Mater. 2007;19:3177–3184. doi: 10.1002/adma.200701972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dam DHM, Culver KSB, Kandela I, Lee RC, Chandra K, Lee H, Mantis C, Ugolkov A, Mazar AP, Odom TW. Biodistribution and in vivo toxicity of aptamer-loaded gold nanostars. Nanomed Nanotechnol Biol Med. 2015;11:671–679. doi: 10.1016/j.nano.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole JR, Mirin NA, Knight MW, Goodrich GP, Halas NJ. Photothermal efficiencies of nanoshells and nanorods for clinical therapeutic applications. J Phys Chem C. 2009;113:12090–12094. [Google Scholar]
- 14.Wang Y, Black K, Leuhmann H, Li W, Zhang Y, Cai X, Wan D, Liu S-Y, Li M, Kim P, et al. A comparison study of gold nanohexapods, nanorods, and nanocages for photothermal cancer treatment. ACS Nano. 2013;7:2068–2077. doi: 10.1021/nn304332s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abadeer NS, Murphy CJ. Recent progress in cancer thermal therapy using gold nanoparticles. J Phys Chem C. 2016;120:4691–4716. [Google Scholar]
- 16.Lowery AR, Gobin AM, Day ES, Halas NJ, West JL. Immunonanoshells for targeted photothermal ablation of tumor cells. Int J Nanomedicine. 2006;1:149–154. doi: 10.2147/nano.2006.1.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Day ES, Thompson PA, Zhang L, Lewinski NA, Ahmed N, Drezek RA, Blaney SM, West JL. Nano-shell-mediated photothermal therapy improves survival in a murine glioma model. J Neurooncol. 2011;104:55–63. doi: 10.1007/s11060-010-0470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc. 2006;128:2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 19.Mackey MA, Ali MRK, Austin LA, Near RD, El-sayed MA. The most effective gold nanorod size for plasmonic photothermal therapy: theory and in vitro experiments. J Phys Chem B. 2014;118:1319–1326. doi: 10.1021/jp409298f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Xu H, Ye J. Plasmonic rod-in-shell nanoparticles for photothermal therapy. Phys Chem Chem Phys. 2014;16:12275–12281. doi: 10.1039/c4cp00902a. [DOI] [PubMed] [Google Scholar]
- 21.Ding X, Liow CH, Zhang M, Huang R, Li C, Shen H, Liu M, Zou Y, Gao N, Zhang Z, et al. Surface plasmon resonance enhanced light absorption and photothermal therapy in the second near-infrared window. J Am Chem Soc. 2014;136:15684–15693. doi: 10.1021/ja508641z. [DOI] [PubMed] [Google Scholar]
- 22.Tchounwou C, Sinha SS, Viraka Nellore BP, Pramanik A, Kanchanapally R, Jones S, Chavva SR, Ray PC. Hybrid theranostic platform for second near-IR window light triggered selective two-photon imaging and photothermal killing of targeted melanoma cells. ACS Appl Mater Interfaces. 2015;7:20649–20656. doi: 10.1021/acsami.5b05225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang K, Smith DA, Pinchuk A. Size-dependent photo-thermal conversion efficiencies of plasmonically heated gold nanoparticles. J Phys Chem C. 2013;117:27073–27080. [Google Scholar]
- 24.Qin Z, Wang Y, Randrianalisoa J, Raeesi V, Chan WCW, Lipinski W, Bischof JC. Quantitative comparison of photothermal heat generation between gold nanospheres and nanorods. Sci Rep. 2016;6:29836. doi: 10.1038/srep29836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang K, Ma H, Liu J, Huo S, Kumar A, Wei T, Zhang X, Jin S, Gan Y, Wang PC, et al. Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano. 2012;6:4483–4493. doi: 10.1021/nn301282m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WCW. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009;9:1909–1915. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- 27.Prabhakar U, Maeda HK, Jain R, Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P, Blakey DC. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73:2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raeesi V, Chan WCW. Improving nanoparticle diffusion through tumor collagen matrix by photothermal gold nanorods. Nanoscale. 2016;8:12524–12530. doi: 10.1039/c5nr08463f. [DOI] [PubMed] [Google Scholar]
- 29.Park J-H, von Maltzahn G, Xu MJ, Fogal V, Kotamraju VR, Ruoslahti E, Bhatia SN, Sailor MJ. Cooperative nanomaterial system to sensitize, target, and treat tumors. Proc Natl Acad Sci U S A. 2010;107:981–986. doi: 10.1073/pnas.0909565107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen H, Zhang X, Dai S, Ma Y, Cui S, Achilefu S, Gu Y. Multifunctional gold nanostar conjugates for tumor imaging and combined photothermal and chemotherapy. Theranostics. 2013;3:633–649. doi: 10.7150/thno.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yavuz MS, Cheng Y, Chen J, Cobley CM, Zhang Q, Rycenga M, Xie J, Kim C, Song KH, Schwartz AG, et al. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nat Mater. 2009;8:935–939. doi: 10.1038/nmat2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim S, Chen YS, Luke G, Emelianov S. In-vivo ultrasound and photoacoustic image-guided photothermal cancer therapy using silica-coated gold nanorods. IEEE Trans Ultrason Ferroelectr Freq Control. 2014;61:891–897. doi: 10.1109/TUFFC.2014.6805702. [DOI] [PubMed] [Google Scholar]
- 33.Rotello VM. The role of surface functionality in determining nanoparticle cytotoxicity. Acc Chem Res. 2013;46:681–691. doi: 10.1021/ar3000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tagami T, Taki M, Ozeki T. Nanomaterials in pharmacology. Methods Pharmacol Toxicol. 2016;39:333–347. [Google Scholar]
- 35.Alkilany AM, Murphy CJ. Toxicity and cellular uptake of gold nanoparticles: what we have learned so far? J Nanopart Res. 2010;12:2313–2333. doi: 10.1007/s11051-010-9911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monopoli MP, Aberg C, Salvati A, Dawson KA. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol. 2012;7:779–786. doi: 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- 37.Dai Q, Walkey C, Chan WCW. Polyethylene glycol backfilling mitigates the negative impact of the protein corona on nanoparticle cell targeting. Angew Chem Int Ed Engl. 2014;53:5093–5096. doi: 10.1002/anie.201309464. [DOI] [PubMed] [Google Scholar]
- 38.Walkey CD, Chan WCW. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem Soc Rev. 2012;41:2780–2799. doi: 10.1039/c1cs15233e. [DOI] [PubMed] [Google Scholar]
- 39.Stern JM, Solomonov VVK, Sazykina E, Schwartz JA, Gad SC, Goodrich GP. Initial evaluation of the safety of nanoshell-directed photothermal therapy in the treatment of prostate disease. Int J Toxicol. 2015;35:38–46. doi: 10.1177/1091581815600170. [DOI] [PubMed] [Google Scholar]
- 40.Gormley AJ, Larson N, Banisadr A, Robinson R, Frazier N, Ray A, Ghandehari H. Plasmonic photothermal therapy increases the tumor mass penetration of HPMA copolymers. J Control Release. 2013;166:130–138. doi: 10.1016/j.jconrel.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gormley AJ, Greish K, Ray A, Robinson R, Gustafson JA, Ghandehari H. Gold nanorod mediated plasmonic photothermal therapy: a tool to enhance macromolecular delivery. Int J Pharm. 2011;415:315–318. doi: 10.1016/j.ijpharm.2011.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fay BL, Melamed JR, Day ES. Nanoshell-mediated photothermal therapy can enhance chemotherapy in inflammatory breast cancer cells. Int J Nanomedicine. 2015;10:6931–6941. doi: 10.2147/IJN.S93031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernardi RJ, Lowery AR, Thompson PA, Blaney SM, West JL. Immunonanoshells for targeted photothermal ablation in medulloblastoma and glioma: an in vitro evaluation using human cell lines. J Neurooncol. 2008;86:165–172. doi: 10.1007/s11060-007-9467-3. [DOI] [PubMed] [Google Scholar]
- 44.Melamed JR, Edelstein RS, Day ES. Elucidating the fundamental mechanisms of cell death triggered by photothermal therapy. ACS Nano. 2015;9:6–11. doi: 10.1021/acsnano.5b00021. [DOI] [PubMed] [Google Scholar]
- 45.Pérez-Hernández M, Del Pino P, Mitchell SG, Moros M, Stepien G, Pelaz B, Parak WJ, Gálvez EM, Pardo J, De La Fuente JM. Dissecting the molecular mechanism of apoptosis during photothermal therapy using gold nanoprisms. ACS Nano. 2015;9:52–61. doi: 10.1021/nn505468v. [DOI] [PubMed] [Google Scholar]
- 46.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Issels RD. Hyperthermia adds to chemotherapy. Eur J Cancer. 2008;44:2546–2554. doi: 10.1016/j.ejca.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 48.Park H, Yang J, Lee J, Haam S, Choi IH, Yoo KH. Multifunctional nanoparticles for combined doxorubicin and photothermal treatments. ACS Nano. 2009;3:2919–2926. doi: 10.1021/nn900215k. [DOI] [PubMed] [Google Scholar]
- 49.Hahn GM, Braun J, Har-Kedar I. Thermochemotherapy: synergism between hyperthermia (42–43 degrees) and adriamycin (or bleomycin) in mammalian cell inactivation. Proc Natl Acad Sci U S A. 1975;72:937–940. doi: 10.1073/pnas.72.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hauck TS, Jennings TL, Yatsenko T, Kumaradas JC, Chan WCW. Enhancing the toxicity of cancer chemotherapeutics with gold nanorod hyperthermia. Adv Mater. 2008;20:3832–3838. [Google Scholar]
- 51.Park J, Park J, Ju EJ, Park SS, Choi J, Lee JH, Lee KJ, Shin SH, Ko EJ, Park I, et al. Multifunctional hollow gold nanoparticles designed for triple combination therapy and CT imaging. J Control Release. 2015;207:77–85. doi: 10.1016/j.jconrel.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 52.Wang Z, Chen Z, Liu Z, Shi P, Dong K, Ju E, Ren J, Qu X. A multi-stimuli responsive gold nanocage-hyaluronic platform for targeted photothermal and chemotherapy. Biomaterials. 2014;35:9678–9688. doi: 10.1016/j.biomaterials.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 53.Strong LE, West JL. Hydrogel-coated near infrared absorbing nanoshells as light-responsive drug delivery vehicles. ACS Biomater Sci Eng. 2015;1:685–692. doi: 10.1021/acsbiomaterials.5b00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brann T, Patel D, Chauhan R, James KT, Bates PJ, Malik MT, Keynton RS, Toole MGO. Gold nanoplates as cancer-targeted photothermal actuators for drug delivery and triggered release. J Nanomater. 2016;2016:11. doi: 10.1155/2016/2036029. [DOI] [Google Scholar]
- 55.Tao Y, Ju E, Liu Z, Dong K, Ren J, Qu X. Engineered, self-assembled near-infrared photothermal agents for combined tumor immunotherapy and chemo-photothermal therapy. Biomaterials. 2014;35:6646–6656. doi: 10.1016/j.biomaterials.2014.04.073. [DOI] [PubMed] [Google Scholar]
- 56.Eloy JO, Petrilli R, Lopez RFV, Lee RJ. Stimuli-responsive nanoparticles for siRNA delivery. Curr Pharm Des. 2015;21:4131–4144. doi: 10.2174/1381612821666150901095349. [DOI] [PubMed] [Google Scholar]
- 57.Hannon GJ. RNA interference. Nature. 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 58.Kim HJ, Kim A, Miyata K, Kataoka K. Recent progress in development of siRNA delivery vehicles for cancer therapy. Adv Drug Deliv Rev. 2016;104:61–77. doi: 10.1016/j.addr.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 59.Jensen SA, Day ES, Ko CH, Hurley LA, Luciano JP, Kouri FM, Merkel TJ, Luthi AJ, Patel PC, Cutler JI, et al. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci Transl Med. 2013;5:209ra152. doi: 10.1126/scitranslmed.3006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giljohann DA, Seferos DS, Prigodich AE, Patel PC, Mirkin CA. Gene regulation with polyvalent siRNA—nanoparticle conjugates. JACS Commun. 2009;131:2072–2073. doi: 10.1021/ja808719p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kouri FM, Hurley LA, Daniel WL, Day ES, Hua Y, Hao L, Peng CY, Merkel TJ, Queisser MA, Ritner C, et al. MiR-182 integrates apoptosis, growth, and differentiation programs in glioblastoma. Genes Dev. 2015;29:732–745. doi: 10.1101/gad.257394.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Braun GB, Pallaoro A, Wu G, Missirlis D, Zasadzinski JA, Tirrell M, Reich NO. Laser-activated gene silencing via gold nanoshell-siRNA conjugates. ACS Nano. 2009;3:2007–2015. doi: 10.1021/nn900469q. [DOI] [PubMed] [Google Scholar]
- 63.Barhoumi A, Huschka R, Bardhan R, Knight MW, Halas NJ. Light-induced release of DNA from plasmon-resonant nanoparticles: towards light-controlled gene therapy. Chem Phys Lett. 2009;482:171–179. [Google Scholar]
- 64.Jones MR, Millstone JE, Giljohann DA, Seferos DS, Young KL, Mirkin CA. Plasmonically controlled nucleic acid dehybridization with gold nanoprisms. Chemphyschem. 2009;10:1461–1465. doi: 10.1002/cphc.200900269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu W, Zhang G, Zhang R, F LG, Ii, Huang Q, Gelovani JG, Li C. Tumor site-specific silencing of NF-κB p65 by targeted hollow gold nanosphere-mediated photothermal transfection. Cancer Res. 2010;70:3177–3189. doi: 10.1158/0008-5472.CAN-09-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huschka R, Barhoumi A, Liu Q, Roth JA, Ji L, Halas NJ. Gene silencing by gold nanoshell-mediated delivery and laser-triggered release of antisense oligonucleotide and siRNA. ACS Nano. 2012;6:7681–7691. doi: 10.1021/nn301135w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poon L, Zandberg W, Hsiao D, Erno Z, Sen D, Gates BD, Branda NR. Photothermal release of single-stranded DNA from the surface of gold nanoparticles through controlled denaturating and AU-S bond breaking. ACS Nano. 2010;4:6395–6403. doi: 10.1021/nn1016346. [DOI] [PubMed] [Google Scholar]
- 68.Lee SE, Liu GL, Kim F, Lee LP. Remote optical switch for localized and selective control of gene interference. Nano Lett. 2009;9:562–570. doi: 10.1021/nl802689k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takahashi H, Niidome Y, Yamada S. Controlled release of plasmid DNA from gold nanorods induced by pulsed near-infrared light. ChemComm. 2005;17:2247–2249. doi: 10.1039/b500337g. [DOI] [PubMed] [Google Scholar]
- 70.Wijaya A, Schaffer SB, Pallares IG, Hamad-Schifferli K. Selective release of multiple DNA oligonucleotides from gold nanorods. ACS Nano. 2009;3:80–86. doi: 10.1021/nn800702n. [DOI] [PubMed] [Google Scholar]
- 71.Kim J, Kim J, Jeong C, Kim WJ. Synergistic nanomedicine by combined gene and photothermal therapy. Adv Drug Deliv Rev. 2016;98:99–112. doi: 10.1016/j.addr.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 72.Zhou F, Nordquist RE, Chen WR. Photonics immunotherapy — a novel strategy for cancer treatment. J Innov Opt Health Sci. 2016;9:1–11. [Google Scholar]
- 73.Almeida JPM, Figueroa ER, Drezek RA. Gold nanoparticle mediated cancer immunotherapy. Nanomed NBM. 2014;10:503–514. doi: 10.1016/j.nano.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bear AS, Kennedy LC, Young JK, Perna SK, Mattos Almeida JP, Lin AY, Eckels PC, Drezek RA, Foster AE. Elimination of metastatic melanoma using gold nanoshell-enabled photothermal therapy and adoptive T cell transfer. PLoS One. 2013;8:e69073. doi: 10.1371/journal.pone.0069073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang C, Xu L, Liang C, Xiang J, Peng R, Liu Z. Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Adv Mater. 2014;26:8154–8162. doi: 10.1002/adma.201402996. [DOI] [PubMed] [Google Scholar]
- 76.Andersson H, Kim Y-S, O’Neill B, Shi Z-Z, Serda R. HSP70 promoter-driven activation of gene expression for immunotherapy using gold nanorods and near infrared light. Vaccines. 2014;2:216–227. doi: 10.3390/vaccines2020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burga RA, Patel S, Bollard CMY, Cruz CR, Fernandes R. Conjugating prussian blue nanoparticles onto antigen-specific T cells as a combined nanoimmunotherapy. Nanomedicine. 2016;11:1759–1767. doi: 10.2217/nnm-2016-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Munn DH, Bronte V. Immune suppressive mechanisms in the tumor microenvironment. Curr Opin Immunol. 2016;39:1–6. doi: 10.1016/j.coi.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Choi MR, Stanton-Maxey KJ, Stanley JK, Levin CS, Bardhan R, Akin D, Badve S, Sturgis J, Robinson JP, Bashir R, et al. A cellular trojan horse for delivery of therapeutic nanoparticles into tumors. Nano Lett. 2007;7:3759–3765. doi: 10.1021/nl072209h. [DOI] [PubMed] [Google Scholar]
- 80.Zhang J, Xia F, Yang Y, Yue C, Zhang C, Yang Y, Ma L, Alfranca G, Liu Y, Hou Y, et al. Human CIK cells loaded with gold nanoprisms as theranostic platform for targeted photoacoustic imaging and enhanced immuno-photothermal combined therapy. Nanoscale Res Lett. 2016;8:109–124. doi: 10.1186/s11671-016-1468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Immuno-photothermal E. Human CIK cells loaded with gold nanoprisms as theranostic platform for targeted photoacoustic imaging and enhanced immuno-photothermal combined therapy. Nano Biomed Eng. 2016;8:109–124. [Google Scholar]
- 82.Kennedy LC, Bear AS, Young JK, Lewinski NA, Kim J, Foster AE, Drezek RA. T cells enhance gold nanoparticle delivery to tumors in vivo. Nanoscale Res Lett. 2011;6:283. doi: 10.1186/1556-276X-6-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao S, Zhang L, Wang G, Yang K, Chen M, Tian R, Ma Q, Zhu L. Hybrid graphene/Au activatable theranostic agent for multimodalities imaging guided enhanced photothermal therapy. Biomaterials. 2016;79:36–45. doi: 10.1016/j.biomaterials.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 84.Liu Y, Ashton JR, Moding EJ, Yuan H, Register JK, Fales AM, Choi J, Whitley MJ, Zhao X, Qi Y, et al. A plasmonic gold nanostar theranostic probe for in vivo tumor imaging and photothermal therapy. Theranostics. 2015;5:946–960. doi: 10.7150/thno.11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Coughlin AJ, Ananta JS, Deng N, Larina IV, Decuzzi P, West JL. Gadolinium-conjugated gold nanoshells for multimodal diagnostic imaging and photothermal cancer therapy. Small. 2014;10:556–565. doi: 10.1002/smll.201302217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Loo C, Lin A, Hirsch L, Lee M-H, Barton J, Halas N, West J, Drezek R. Nanoshell-enabled photonics-based imaging and therapy of cancer. Technol Cancer Res Treat. 2004;3:33–40. doi: 10.1177/153303460400300104. [DOI] [PubMed] [Google Scholar]
- 87.Gobin AM, Lee MH, Halas NJ, James WD, Drezek RA, West JL. Near-infrared resonant nanoshells for combined optical imaging and photo-thermal cancer therapy. Nano Lett. 2007;7:1929–1934. doi: 10.1021/nl070610y. [DOI] [PubMed] [Google Scholar]
- 88.Huang P, Lin J, Li W, Rong P, Wang Z, Wang S, Wang X, Sun X, Aronova M, Niu G, et al. Biodegradable gold nanovesicles with an ultra-strong plasmonic coupling effect for photoacoustic imaging and photothermal therapy. Angew Chem Int Ed. 2013;52:13958–13964. doi: 10.1002/anie.201308986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tucker-Schwartz JM, Meyer TA, Patil CA, Duvall CL, Skala MC. In vivo photothermal optical coherence tomography of gold nanorod contrast agents. Biomed Opt Express. 2012;3:2881. doi: 10.1364/BOE.3.002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li W, Chen X. Gold nanoparticles for photoacoustic imaging. Nanomedicine (Lond) 2015;10:299–320. doi: 10.2217/nnm.14.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Luke GP, Bashyam A, Homan KA, Makhija S, Chen Y, Emelianov SY. Silica-coated gold nanoplates as stable photoacoustic contrast agents for sentinel lymph node imaging. Nanotechnology. 2013;24:455101. doi: 10.1088/0957-4484/24/45/455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luke GP, Myers JN, Emelianov SY, Sokolov K. Sentinel lymph node biopsy revisited: ultrasound-guided photoacoustic detection of micrometastases using molecularly targeted plasmonic nanosensors. Cancer Res. 2014;74:5397–5408. doi: 10.1158/0008-5472.CAN-14-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu W, Melancon MP, Xiong C, Huang Q, Elliott A, Song S, Zhang R, Flores LG, Gelovani JG, Wang LV, et al. Effects of photoacoustic imaging and photothermal ablation therapy mediated by targeted hollow gold nanospheres in an orthotopic mouse xenograft model of glioma. Cancer Res. 2011;71:6116–6121. doi: 10.1158/0008-5472.CAN-10-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Melancon MP, Elliott AM, Shetty A, Huang Q, Stafford RJ, Li C. Near-infrared light modulated photothermal effect increases vascular perfusion and enhances polymeric drug delivery. J Control Release. 2011;156:265–272. doi: 10.1016/j.jconrel.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rylander M, Stafford J, Hazle J, Whitney J, Diller K. Heat shock protein expression and temperature distribution in prostate tumours treated with laser irradiation and nanoshells. Int J Hyperthermia. 2011;27:791–801. doi: 10.3109/02656736.2011.607485. [DOI] [PubMed] [Google Scholar]
- 96.Stauffer P, Craciunescu O, Maccarini P, Wyatt C, Arunachalam K, Arabe O, Stakhursky V, Li Z, Soher B, MacFall J, et al. Clinical utility of magnetic resonance thermal imaging (MRTI) for realtime guidance of deep hyperthermia. Proc SPIE. 2009 doi: 10.1117/12.812188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sommer G, Bouley D, Gill H, Daniel B, Pauly KB, Diederich C. Focal ablation of prostate cancer: four roles for magnetic resonance imaging guidance. Can J Urol. 2013;20:6672–6681. [PMC free article] [PubMed] [Google Scholar]
- 98.Vakoc BJ, Fukumura D, Jain RK, Bouma BE. Cancer imaging by optical coherence tomography: preclinical progress and clinical potential. Nat Rev Cancer. 2012;12:363–368. doi: 10.1038/nrc3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Al-Mujaini A, Wali UK, Azeem S. Optical coherence tomography: clinical applications in medical practice. Oman Med J. 2013;28:86–91. doi: 10.5001/omj.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Adhi M, Duker JS. Optical coherence tomography— current and future applications. Curr Opin Ophthalmol. 2013;24:213–221. doi: 10.1097/ICU.0b013e32835f8bf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Valluru K, Wilson K, Willmann J. Photoacoustic imaging in oncology: translational preclinical and early clinical experience. Radiology. 2016;280(2):332–349. doi: 10.1148/radiol.16151414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kharlamov AN, Gabinsky JL. Plasmonic photothermic and stem cell therapy of atherosclerotic plaque as a novel nanotool for angioplasty and artery remodeling. Rejuvenation Res. 2012;15:222–230. doi: 10.1089/rej.2011.1305. [DOI] [PubMed] [Google Scholar]
- 103.Yeager D, Chen YS, Litovsky S, Emelianov S. Intra-vascular photoacoustics for image-guidance and temperature monitoring during plasmonic photothermal therapy of atherosclerotic plaques: a feasibility study. Theranostics. 2014;4:36–46. doi: 10.7150/thno.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khantamat O, Li CH, Yu F, Jamison AC, Shih WC, Cai C, Lee TR. Gold nanoshell-decorated silicone surfaces for the near-infrared (NIR) photothermal destruction of the pathogenic bacterium E. faecalis. ACS Appl Mater Interfaces. 2015;7:3981–3993. doi: 10.1021/am506516r. [DOI] [PubMed] [Google Scholar]
- 105.Millenbaugh NJ, Baskin JB, DeSilva MN, Elliott WR, Glickman RD. Photothermal killing of Staphylococcus aureus using antibody-targeted gold nanoparticles. Int J Nanomedicine. 2015;10:1953–1960. doi: 10.2147/IJN.S76150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Neumann O, Urban AS, Day J, Lal S, Nordlander P, Halas NJ. Solar vapor generation enabled by nanoparticles. ACS Nano. 2013;7:42–49. doi: 10.1021/nn304948h. [DOI] [PubMed] [Google Scholar]
- 107.Neumann O, Feronti C, Neumann AD, Dong A, Schell K, Lu B, Kim E, Quinn M, Thompson S, Grady N, et al. Compact solar autoclave based on steam generation using broadband light-harvesting nanoparticles. Proc Natl Acad Sci U S A. 2013;110:11677–11681. doi: 10.1073/pnas.1310131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ibrahim IM, Ali HR. Treatment of natural mammary gland tumors in canines and felines using gold nanorods-assisted plasmonic photothermal therapy to induce tumor apoptosis. Int J Nanomedicine. 2016;11:4849–4863. doi: 10.2147/IJN.S109470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schwartz JA, Shetty AM, Price RE, Stafford RJ, Wang JC, Uthamanthil RK, Pham K, McNichols RJ, Coleman CL, Payne JD. Feasibility study of particle-assisted laser ablation of brain tumors in orthotopic canine model. Cancer Res. 2009;69:1659–1667. doi: 10.1158/0008-5472.CAN-08-2535. [DOI] [PubMed] [Google Scholar]
- 110.Pedrosa P, Vinhas R, Fernandes A, Baptista P. Gold nanotheranostics: proof-of-concept or clinical tool? Nanomaterials. 2015;5:1853–1879. doi: 10.3390/nano5041853. [DOI] [PMC free article] [PubMed] [Google Scholar]


