Abstract
Introduction
Gene-directed enzyme prodrug therapy (GDEPT) employing the cytosine deaminase (CD) gene, which encodes an enzyme that converts the nontoxic agent 5-fluorocytosine (5-FC) into the chemotherapeutic drug 5-fluorouracil (5-FU), has shown promise both in experimental animals and in clinical trials. Nevertheless, with the transfection systems available presently the percentage of tumor cells incorporating the desired gene is usually too low for successful therapy. We have examined the ability of photodynamic therapy (PDT) to enhance the efficacy of the metabolites, converted from 5-FC by CD gene transfected rat glioma cells.
Methods
Hybrid tumor cell spheroids consisting of CD positive and CD negative F98 glioma cells in varying ratios were used as in vitro tumor models. PDT was performed with the photosensitizer AlPcS2a and λ = 670nm laser irradiance, both before and after confrontation with 5-FC.
Results
PDT increased the toxicity of 5-FU either as pure drug or derived from monolayers of CD positive cells chalanged with 5-FC. PDT in combination with 5-FC resulted in a significantly enhanced inhibition of hybrid spheroid growth compared to non light treated controls. This was the case even at tumor to producer cell ratios as high as 40:1.
Conclusion
The results of the present study show that GDEPT and PDT interact in a synergistic manner over a range of prodrug concentration and tumor to transfected cell ratios. The degree of synergy was significant regardless if PDT treatment was given before or after 5-FC administration. The highest degree of interaction was observed though, when PDT was delivered prior to prodrug exposure.
Keywords: Glioblastoma multiforme, photodynamic therapy, photochemical internalization, suicide gene therapy, cytosine deaminase gene, 5-FU
Introduction
Glioblastoma multiforme (GBM, WHO grade IV) represents 60% of all malignant brain tumors but despite the recent technological advances in surgery and radio and chemo therapy, these procedures have not been able to significantly improve the prognosis of patients [1–3]. Improved treatment modalities such as gene therapy are therefore being developed and implemented. One form of gene therapy is gene-directed enzyme prodrug therapy (GDEPT), also referred to as suicide gene therapy. This involves transfection, into tumor cells, of non-mammalian genes encoding enzymes that convert nontoxic pro-drugs into toxic metabolites capable of inhibiting nucleic acid synthesis. GDEPT activating strategies allow a high concentration of toxic drug to be converted from the prodrug by the transfected cells at the tumor site, significantly reducing the side effects caused by systemic administration. Additionally the bystander effect, where activated drug is exported from the transfected cancer cells into the tumor microenvironment, plays an important role by inhibiting growth of adjacent non-transfected tumor cells.
GDEPT utilizing the induction of cytosine deaminase (CD), an enzyme which converts the antifungal agent 5-fluorocytosine (5-FC) into its antimetabolite 5-fluorouracil (5-FU) has been previously studied both experimentally and in ongoing clinical trials [4–9].
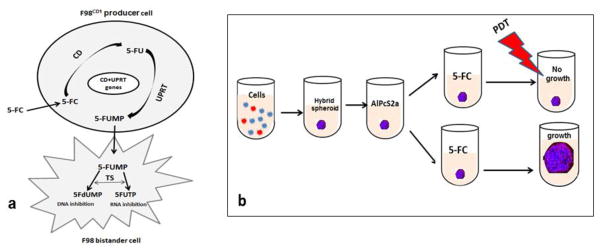
In addition to the CD gene, the uracil phosphoribosyl transferase (UPRT) gene which codes for the enzyme that converts 5-FU directly to 5-fluorouridine monophosphate (5-FUMP), has been used in combination in experimental protocols. This bypasses the inefficient intermediate steps in the conversion of 5-FU to toxic metabolites in mammalian cells, greatly enhancing the effects of therapy [10–12]. The basic steps in this process are illustrated in figure 1a.
Fig. 1. Conversion of prodrug to toxic metabolites via co-expression of CD and UPRT genes within the F98CD producer cell.
a. The cytosine deaminase (CD) enzyme catalyzes conversion of 5-fluorocytosine (5FC) to -5-fluorouracil (5-FU) and the uracil phosphoribosyl transferase (UPRT) enzyme directly converts 5-FU to 5-fluorouridine monophosphate (5-FUMP), bypassing the inefficient intermediate steps in the conversion of 5-FU to toxic metabolites in mammalian cells. 5-FUMP is then converted to the toxic metabolites 5-flurodeoxyuracilmonophosphate (5-FdUMP) and flurouracil-triphosphate (5-FUTP) resulting in a pronounced growth inhibitory bystander effect.
b. The basic experimental protocol. F98 (blue) and F98CD red cells are combined in vitro Following centrifugation and 24 hr. incubation hybrid glioma spheroids form. 18 hr. incubation with photosensitizer AlPcS2a Wash removal of photosensitizer PDT after; prodrug 5-FC added for 4 hrs. ; PDT before spheroids soak 4 hours PDT treatment, 5-FC added. Dark controls received no light Spheroid growth observed after 14 days of additional culture.
Although the results obtained so far are promising, one important limitation for GDEPT though is the inability of the gene to transfect a sufficient number of tumor cells. This in turn sets a limit to the amount of on-site conversion of prodrug to active drug. Methods to enhance the efficacy of the locally produced and exported drug would therefore offer a distinct advantage.
Photodynamic therapy, consisting of photosensitizers plus light irradiation, has the ability to kill cells directly via the production of reactive oxygen species and has also been shown to potentiate the efficacy of chemotherapy (13–15). In particular, the photosensitizer, disulfonated aluminum phthalocyanine (AlPcS2a) mediated PDT could enhance the effects of doxorubicin on mice bearing murine leukemia and lymphoma compared to chemotherapy alone [14]. It was therefore hypothesized that AlPcS2a PDT should be capable of increasing the efficacy of the compounds 5-FU and 5-FUMP converted from 5-FC by CD/UPRT gene transfected glioma cells, thus in effect reducing the number of transfected tumor cells and or prodrug concentration required for effective therapy.
In the experiments reported here a relatively small subpopulation of an engineered CD/UPRT gene positive glioma cell line, were incorporated into CD negative rat glioma spheroids as a simulation of limited gene penetration. The aim of the present research was designed to evaluate the ability of AlPcS2a PDT to enhance the effectiveness of suicide gene therapy to inhibit the growth of the hybrid multicell 3 dimensional glioma spheroids. The importance of the sequences, PDT before and PDT after 5-FC administration, were both examined in this study.
Materials & Methods
Cell lines
The rat glioma line F98 was obtained from the American Type Culture Collection (Manassas, VA, USA) and was maintained in Dulbecco’s Modified Eagle Medium (DMEM) with high glucose (Life Technologies Corp., Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS), 25 mM HEPES buffer (pH 7.4), penicillin (100 U ml-1) and streptomycin (100 μg ml-1) at 37°C and 5% CO2.
Generation of stable CD/UPRT gene positive cell lines
Generation of a stable F98cd cell line, expressing both the cytosine deaminase (CD) and uracil phosphoribosyl transferase (UPRT) genes has been described in detail previously [16]. Briefly, F98 cells were initially transfected with a plasmid consisting of the fusion plasmid pSELECT-zeo-Fcy::fur, (InvivoGen, San Diego, California) and the jetPEI carrier (Polyplus-transfection, Strasbourg France) following the manufacture’s protocols. Confirmation of the CD gene was done by fluorescent antibody staining and sensitivity to 5-FC. 100% of the F98CD1 cell line has been shown to express the gene and the gene product.
Production of active supernatants from 5-FC by F98CD1 cells
Monolayers of F98CD1 were grown to near confluence in T-42 flasks. 5-FC at a concentration of 12μg/ml was added to monolayers and four hours later the supernatant was harvested.
Spheroid generation
F98 or F98/F98CD hybrid spheroids were formed by a modification of the centrifugation method [17]. Briefly, either 4×103 F98 glioma cells or F98 and a variable number of F98CD1 cells (hybrid spheroids) in 200 μl of culture medium were alloquated into the wells of ultra-low attachment surface 96-well round-bottomed plates (Corning In., NY). The plates were centrifuged at 1000rpm for 30 minutes. Immediately following centrifugation the tumor cells formed into a disk shape. The plates were maintained at 37°C in a 5 % CO2 incubator for 24 h to allow them to take on the usual 3-dimensional spheroid form.
PDT enhanced toxicity of 5-FU or active supernatants on F98 spheroids
Twenty four hrs. after spheroid generation 0.5ug/ml of the photosensitizer disulfonated aluminum phthalocyanine (AlPcS2a, Frontier Scientific, Inc., Logan, UT) was added and 18 hrs. later the spheroids were washed 4 times and 5-FU as pure drug or active supernatant at increasing concentrations was added in fresh medium. Four hrs. after 5-FU or supernatant was added, light treatment from a diode laser (Intense; New Jersey USA) at an irradiance of 2.0 mW/cm2 was administered for 7.0 min (0.8J/cm2 , λ= 670 nm). Control cultures received no drug (PDT control) or drug but no illumination (drug only control). Following light treatment the plates were returned to the incubator. Typically, 8–16 spheroids were followed for each category in 3 individual trials for up to 14 days of incubation. Culture medium in the wells was exchanged every third day. Determination of spheroid growth was carried out by averaging two measured perpendicular diameters of each spheroid using a microscope with a calibrated eyepiece micrometer and their volume calculated assuming a perfect sphere.
Effects of 5-FC-GDEPT and PDT on hybrid spheroids
The basic experimental protocol is shown in fig 1b. Light treatment was administered either after or before prodrug addition to the spheroid cultures. Hybrid spheroids were formed as previously described. Twenty four hours after spheroid generation, 0.5ug/ml AlPcS2a,) was added and 18 hrs. later the spheroids were washed 4 times. For the PDT after protocol, immediately following the wash, 5-FC at increasing concentrations was then added in fresh medium. Four hours after 5-FC was added light treatment at an irradiance of 2 mW/cm2 was administered for 7.0 min (0.8J/cm2 λ= 670 nm). For the PDT before protocol, following the wash the spheroids were incubated for 4 hours in pure culture medium. Light treatment was administered as described previously. Immediately following the light exposure 5-FC at increasing concentrations was then added in fresh medium. Dark controls received both AlPcS2a and 5-FC but no light exposure. No further wash of the spheroids was done so the 5-FC remained in the culture medium. Following light treatment the plates were returned to the incubator. Typically, 8–16 spheroids were followed for each sequence category in 3 individual trials for up to 14 days of post treatment incubation.
Live/dead assay
Two weeks after 5-FC, PDT or 5-FC+ PDT treatment, 2–3 spheroids from the various experimental and control groups were transferred to glass bottomed imaging dishes, stained using a combination of Hoechst 33342 and Ethidium Homodimer 1 (Invitrogen H1399, Carlsbad, CA) for 1 h, washed and visualized using a two photon inverted Zeiss laser-scanning fluorescent microscope (LSM 410, Carl Zeiss, Jena, Germany). Fluorescent images were pseudo-colored blue (live) and red (dead).
Statistical analysis
All data was analyzed and graphed using Microsoft Excel. The arithmetic mean and standard error were used throughout to calculate averages and errors. Experimental data were analyzed using one-way ANalysis Of VAriance (ANOVA) at the significance level of P < 0.05 and presented as mean with standard error unless otherwise noted. In order to determine the degree of synergism between the toxic effects of PDT and the CD GDEPT produced chemo-agents the following equation was used (equation 1).
The numerator includes the product of the final spheroid volume (V) of the individual treatments separately compared to non-treated controls while the denominator includes the final spheroid volume compared to controls of the combined treatments. A value of α =1 indicates an additive effect. A value of α < 1 or α > 1 indicates an antagonistic or synergistic effect, respectively.
Results
Inhibitory effects of combined PDT and 5-FU or F98CD produced supernatants on F98 spheroids
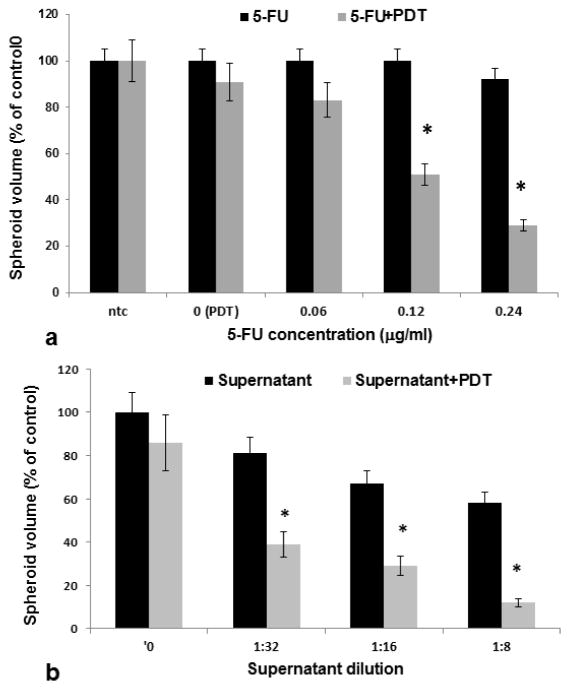
The ability of PDT to enhance the effecacy of 5-FU as measured by the growth inhibition of F98 spheroids is shown in fig 2a. Significant inhibition of PDT treated spheroids compared to drug alone was seen for 5-FU concentrations of 0.12 and 0.24μg/ml. A clear synergistic effect of PDT, with α values for these 2 drug concentrations of 1.6 and 3.1 respectivly, could be demonstrated.
Fig. 2. Inhibitory effects of PCI with 5-FU or CD/UPRT produced drug.
a. Spheroids incubated with1 μg/ml AlPcS2a for 18 h. Spheroids incubated with increasing concentrations of 5-FU. Light treatment (PCI) 0.8J/cm2, λ= 670nm. b F98 spheroids incubated together with photosensitizer and decreasing concentrations of active supernatants derived from 5-FC-F98CD1 co cultures (M&M). Light treatment as in a. Each data point represents spheroid volume after 2 weeks in culture as a % of untreated controls and the mean of 3 experiments. Error bars denote standard errors. Asterisks (*) denote significant differences (p<0.05) compared to control value.
Similar PDT spheroid experiments were performed emloying the F98CD1 supernatants (as described above) and harvested after 4 hrs of incubation with 5-FC. The results are shown in fig 2b. The supernatants were diluted with medium at a ratio of 1:8 to 1:32. The drug containing supernantants were inhibitory for spheroid growth at the lower dilutions. The spheroid growth inhibition was significantly enhanced by PDT, as shown in the figure for the 3 dilutions tested. α values 1.7 and 3.7 for dilutions of 1:32 or 1:8 resectivly. This clearly showed a synergistic response compared to drug or PDT applied seperatly. Surprisingly, F98CD1 cells exposed once to 5-FC, from which the supernatants were harvested, were capable of producing drug following a second 5-FC incubation, indicating that the cells were not dead but proved incapable of dividing. (data not shown).
Bystander effect of prodrug concentration and number of transfected F98CD cells
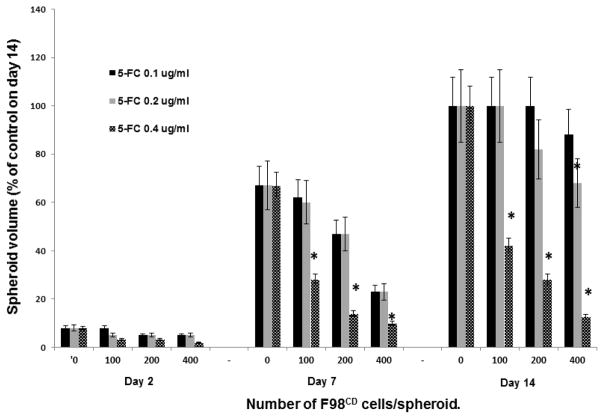
In order to explore the suboptimal prodrug concentration and number of transfected cells to be used in the subsequent combined treatment protocols, hybrid spheroid experiments were performed where both of these parameters were titrated. 5-FC concentrations of 0.1, 0.2 and 0.4 μg/ml and F98:F98CD ratios of 10:1–40:1 were tested. The kinetics of spheroid growth during a 14 day period is shown in figure 3. At a 5-FC concentration of 0.1μg/ml no significant growth inhibition was demonstrated at day 14. On the other hand, 5-FC concentration of 0.4μg/ml gave significant effects even in the absence of light treatment. Based on these results a F98:F98CD ratio of 10:1 was used for the prodrug titration and a 5-FC concentration of 0.2μg/ml was used for the producer cell titration in the subsequent combined treatment experiments.
Fig. 3. Effect of prodrug concentration and number of transfected F98CD cells on spheroid growth; bystander effect.
Hybrid F98:F98CD spheroids with constant number of F98 cells (4×103) and increasing number of prodrug converting F98CD cells (100, 200,400) incubated with a) 5-FC 0.1 μg/ml, b) 5-FC 0.2 μg/ml, c) 5-FC 0.4 μg/ml. The growth kinetics, calculated at 2, 7 and 14 days of incubation as a % of control values at day 14 are shown. Mean of 3 experiments, error bars denote standard errors. Asterisks (*) denote significant differences (p<0.05) compared to control value.
PDT mediated 5-FC effects on F98/F98CD hybrid spheroid growth
To ascertain the effects of the various treatment parameters on the growth and development of the hybrid spheroids following treatment, experiments were performed consisting of 4 groups: 1) no treatment controls. 2) PDT controls, 3) 5-FC, at various concentrations of drug or number of F98CD cells with no light treatment 4) 5-FC, at various concentrations of drug or number of F98CD cells + light treatment for both the light after and light before protocol sequences.
Effects of 5-FC concentration
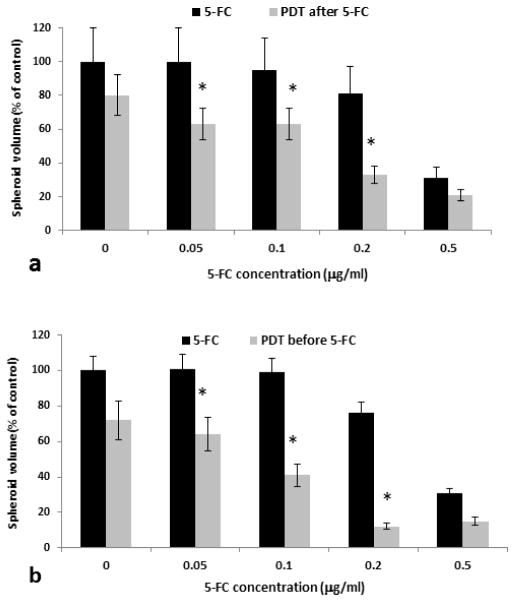
A cell ratio of F98:F98CD1 of 10:1 (i.e. 10% of transfected F98CDcells of the total cells at spheroid initiation) was used in these experiments. Figure 4a shows the average spheroid volume as a % of controls measured after a two week growth period for the sequence of PDT after drug addition. 5-FC concentrations of 0.05–0.5 μg/ml were examined. Three identical experiments were performed with 16 spheroids in each group per experiment. As seen in the figure, PDT significantly increased the efficacy of 5-FC at all prodrug concentrations examined (Fig. 4a). Figure 4b shows the results of the PDT before drug sequence. Although the effects of the two light-drug sequences demonstrated a synergistic effect, the light before sequence appeared to be more effective. The corresponding α values derived from experiments over a 5-FC concentration range of 0.025–0.2μg/ml are shown in table 1. α values greater than 1, indicating a synergistic response, were obtained for all the 5-FC concentrations, with the exception for the lowest value (0.025 μg/ml) tested for the light after sequence. At 5-FC concentrations greater than 1μg/ml or irradiance light levels greater than 1.5J/cm2, administered as single treatment, the growth inhibition was so pronounced that the additional effects of combined treatment were negligible (data not shown).
Fig. 4. Effects of prodrug 5-FC concentration and PDT on hybrid spheroids growth.
a Hybrid F98:F98CD spheroids (ratio of F98:F98CD = 4×103: 4×102 ) incubated together with AlPcS2a for 18 hrs. The spheroids were treated with increasing concentrations of 5-FC as indicated on the figure. PDT (0.8J/cm2 , λ= 670 nm). was performed after 5-FC was administered.
b Spheroids treated as in a but PDT was performed before 5-FC administration.
Table 1.
Synergistic PDT effect with increasing 5-FC concentration.
| 5-FC# | 0.025 | 0.05 | 0.1 | 0.2 |
| α value* light after | 0.96 | 1.3 | 1.4 | 1.7 |
| α value* light before | 1.0 | 1.3 | 1.9 | 3.2 |
μg/ml
α > 1 indicates synergistic response.
Effects of ratio of transfected to non-transfected tumor cells
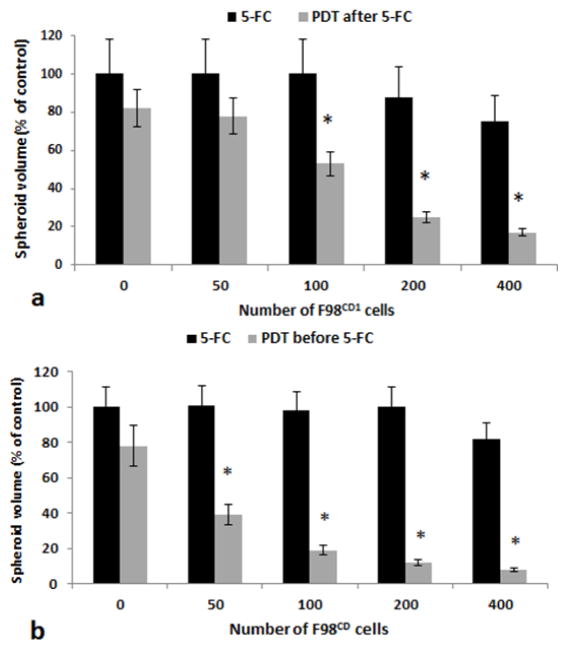
Besides the importance of the prodrug concentration available (as shown in fig 4) the number of transfected to non-transfected tumor cells is a critical factor. Experiments were therefore performed employing hybrid spheroids formed from 4×103 F98 cells in combination with a variable number of F98CD ranging from 50 to 400 CD/UPRT positive cells corresponding to a transfected subpopulation of 2.5–10%. A 5-FC concentration of 0.2μg/ml was used in all experiments. As seen in figure 5a for PDT after drug and fig 5b for PDT before sequences respectivly, significant growth inhibition was apparent even at a tumor to producer cell ratio of 40:1. The corresponding α values derived from the data shown in fig 5 are given in table 2. α values greater than 1 indicating a synergistic response were obtained for all ratios tested.
Figure 5. Effects of increasing the subpopulation of F98CD cells on hybrid spheroids growth.
a Hybrid F98:F98CD spheroids with constant number of F98 cells (4×103) and increasing number of prodrug converting F98CD cells as shown on the figure, incubated with 5-FC (0.2 μg/ml) with and without PDT. PDT was performed after 5-FC was administered. b PDT was performed before 5_FC was administered.
Light treatment in both cases was performed with 0.8J/cm2, λ= 670nm. Each data point represents spheroid volume after 2 weeks in culture as a % of controls at day 14. Mean of 3 experiments, error bars denote standard errors. Asterisks (*) denote significant differences (p<0.05) compared to control value.
Table 2.
Synergistic PDT effect with increasing number of F98CD1 producer cells.
| F98:F98CD1 ratio | 80:1 | 40:1 | 20:1 | 10:1 |
| α value light after* | 1.1 | 1.5 | 2.4 | 2.1 |
| α value light before | 1.6 | 2.4 | 2.5 | 3.2 |
α > 1 indicates synergistic response
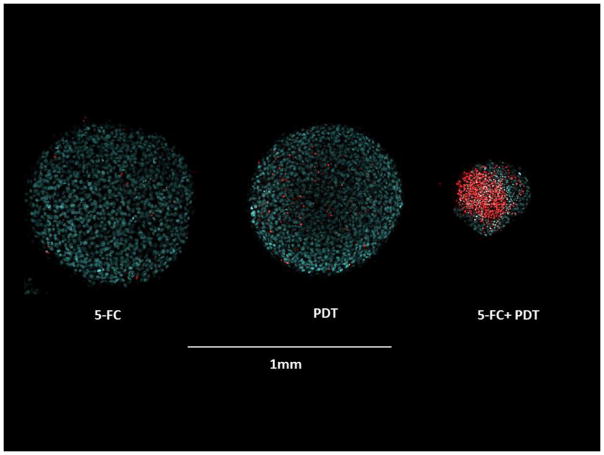
Two-photon fluorescence microscopy
Hybrid spheroids formed with a F98:F98CD ratio, were treated with 5-FC (0.1 μg/ml),and PDT treatment (light after sequence) 0.7J/cm2 at λ= 670nm. Sample spheroids were stained 14 days after treatment. The results of live/dead assays employing two-photon fluorescence images demonstrated enhanced toxicity of PDT + 5-FC combined treatment compared to 5-FC or PDT applied as single treatments (Figure 6). As seen in the figure few red (dead) cells were evident in the PDT or 5-FC prodrug exposure as single treatment. In contrast the spheroids receiving PDT+5-FC demonstrated a majority of dead cells and the spheroids had displayed little growth during the 14 day incubation period.
Fig. 6. Live/dead assay of hybrid F98:F98CD spheroids (ratio of F98:F98CD , 10:1).
Two-photon fluorescence microscopy images stained with Hoechst 33342 (blue: live) and Ethidium Homodimer (red: dead). Spheroid treatment; 5-FC (0.1 μg/ml), PDT treatment after 5-FC administration, 0.7J/cm2, λ= 670nm. Spheroids were stained 14 days after treatment. Scale bar = 1mm.
Discussion
Among the most studied GDEPT approaches, activation of ganciclovir (GCV) by herpes simplex virus thymidine kinase (HSV-TK) and activation of 5-FC by the CD gene have been and are now presently being tested in clinical trials for glioma treatment [8, 18]. For treatment of brain tumors it is important that the pro-drug can pass the blood brain barrier. 5-FC is a small molecule and due to its relatively low protein binding it readily penetrates into cerebrospinal fluid (CSF). CSF concentrations of 5-FC have been shown to obtain about 75% of serum concentration [19]. 5-FC has been used routinely for many years as treatment for fungal infection in the brain [20, 21]. PDT has also been evaluated in clinical trials for GBM although success has been limited [22–24].
The primary objective of this study was to evaluate the synergistic potential of combining PDT and CD- GDEPT. The results of the experiments reported here could clearly demonstrate that this was the case and that the effects of combined therapy were not simply additive (Table 1 and 2). An important observation, as shown in figure 5, was that the addition of PDT to CD-GDPDT greatly reduced the number of transfected tumor cells required for significant growth reduction to take place compared to CD-GDPDT alone. This pronounced effect was probably due to the existence of the UPRT gene as well as the CD gene in the transfected cells.
The two modalities PDT and CD- GDEPT kill cells by two distinctly different mechanisms. The basis mechanism for GDEPT toxicity is that the CD enzyme converts 5-FC to 5-FU and the UPRT enzyme converts 5-FU to 5-FUMP in the transfected cells. Both 5-FU and 5-FUMP are relatively small molecules and diffuse out of the transfected cells and are taken up by the surrounding bystander tumor cells. As illustrated in figure 1a, 5-FUMP will be taken up by the non-transfected bystander tumor cells and the metabolites, (5-fluoro-2′-deoxyuridine 5′-monophosphate [5-FdUMP] and 5-fluorouridine 5′-triphosphate) will be produced. These two toxic metabolites damage both DNA and RNA, respectively. 5-FUTP can be incorporated into RNA inhibiting transcription, whereas 5-FdUMP prevents DNA synthesis by irreversibly inhibiting thymidylate synthase. [ 25].
PDT on the other hand, especially following the protocols as were used in our experiments, are known to cause partial damage to lysosomes. This causes the release of hydrolases inducing apoptotic cell death [26–28]. In addition, low level PDT targeted towards intracellular organelles (endo-lysosomes) can activate autophagic process resulting in autophagic cell death [29, 30]. In the experiments described here the PDT effect was targeted towards intracellular organelles by removing excess photosensitizer from the medium via multiple wash cycles and allowing the spheroids to “soak” in pure medium for 4 hours before light treatment (fig 1b). This allowed the photosensitizer to leach out of the cell membrane, while remaining in the membranes of the intracellular endosomes and lysosomes.
Since these two distinct cytotoxic pathways are more or less independent of each other it might be that this is this mechanism that underlies the synergistic effect demonstrated in table 1 and 2. As is indicated in figure 4, the growth inhibitory effects of combined PDT and CD-GDEPT were also pronounced for the PDT before 5-FC sequence, indicating that PDT sensitized the tumor cell to the toxic metabolites converted from the prodrug. Similar effects were observed following brief exposure of CD positive tumor cells to 5-FC followed by ionizing radiation (equivalent to a “radiation after” sequence) [31]. These authors could demonstrate an enhanced effect of radiation both in vitro and in vivo, which they attributed to a sensitization to radiation by CD-GDEPT. Clearly, additional studies are required to explain in detail the mechanism of the observed synergistic effects of combined treatment protocols with CD-GDEPT.
A modified form of PDT, photochemical internalization (PCI) has been shown to greatly enhance gene insertion efficiency for both viral as well as nonviral gene transfection protocols [16, 32, 33]. Due to the rapid attenuation of light in tissue this enhanced transfection efficiency would be limited to the illuminated targeted tumor site, thus avoiding transfection of off target normal tissue. The physical site specificity of PDT would also apply to the increased drug efficacy converted by the transfected cells. Thus the light based therapies, PCI and PDT, can be used to both increase gene transfection efficiency as well as the efficacy of the compounds converted by GDEPT in a site and time specific manner.
Conclusion
The results of the present study show that GDEPT and PDT interact synergistically over a range of prodrug concentration and tumor to transfected cell ratios. The degree of interaction was significant for both of the sequences of drug and light treatment. The highest degree of interaction was observed though when PDT was delivered prior to prodrug exposure.
Highlights.
A method is proposed to enhance the efficacy of suicide gene therapy for cancer.
The therapy involves the enzymatic conversion of a non-toxic drug to a toxic one.
PDT has been demonstrated to further increase the toxicity of the converted drug.
Enhancement occurs for PDT treatment either before or after cell drug exposure.
Acknowledgments
The authors are grateful for the support from the Norwegian Radium Hospital Research Foundation. Portions of this work were made possible through access to the LAMMP Program NIBIB P41EB015890 at UCI
Footnotes
Compliance with Ethical Standards:
Funding: the Norwegian Radium Hospital Research Foundation. Grant nr 1503
Conflict of Interest: All of the authors declares that she/he has no conflict of interest.
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stupp R, Hegi ME, Mason WP. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 2.Brandes AA, Tosoni A, Franceschi E, Reni M, Gatta G, Vecht C. Glioblastoma in adults. Crit Rev Oncol Hematol. 2008;67:139–152. doi: 10.1016/j.critrevonc.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Petrecca K, Guiot MC, Panet-Raymond V, Souhami L. Failure pattern following complete resection plus radiotherapy and temozolomide is at the resection margin in patients with Glioblastoma. J Neurooncol. 2013;111:19–23. doi: 10.1007/s11060-012-0983-4. [DOI] [PubMed] [Google Scholar]
- 4.Mullen CA, Coale MM, Lowe R, Blase RM. Tumors expressing the cytosine-deaminase suicide gene can be eliminated in vivo with 5-fluorocytosine and induce protective immunity to wild-type tumor. Cancer Res. 1994;54:1503–1506. [PubMed] [Google Scholar]
- 5.Kai GE, Lingfei XU, Zheng Zhongcheng, et al. Transduction of cytosine deaminase gene makes rat gliomas cells highly sensitive to 5-fluorocytosine. Int J Cancer. 1997;71:675–679. doi: 10.1002/(sici)1097-0215(19970516)71:4<675::aid-ijc26>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Miller CR, Williams CR, Buchsbaum DJ, Gillespie GY. Intratumoral 5-fluorouracil produced by cytosine deaminase/5-fluorocytosine gene therapy is effective for experimental human glioblastomas. Cancer Res. 2002;62:773–780. [PubMed] [Google Scholar]
- 7.Ostertag D, Amundson KK, Lopez Espinoza F, Martin B, Buckley AP, et al. Brain tumor eradication and prolonged survival from intratumoral conversion of 5-fluorocytosine to 5-fluorouracil using a nonlytic retroviral replicating vector. Neurooncology. 2012;14:145–159. doi: 10.1093/neuonc/nor199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez OD, Logg CR, Hiraoka K, et al. Design and selection of Toca 511 for clinical use: modified retroviral replicating vector with improved stability and gene expression. Mol Ther. 2012 Sep;20(9):1689–98. doi: 10.1038/mt.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang TT, Parab S, Burnett R, et al. Intravenous administration of retroviral replicating vector, Toca 511, demonstrates therapeutic efficacy in orthotopic immune-competent mouse glioma model. Hum Gene Ther. 2015 Feb;26(2):82–93. doi: 10.1089/hum.2014.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams CW, Buchsbaum DJ, Miller CR. Uracil phosphoribosyltransferase potentiates 5-fluorouracil and cytosine deaminase/5-fluorocytosine cytotoxicity in prostate cancer. Proc Am Assoc Cancer Res. 2001;42:455. [Google Scholar]
- 11.Adachi Y, Tamiya T, Ichikawa T, Terada K, Ono Y, Matsumoto K, Furuta T, Hamada H, Ohmoto T. Experimental gene therapy for brain tumors using adenovirus-mediated transfer of cytosine deaminase gene and uracil phosphor-ribosyltransferase gene with 5-fluorocytosine. Hum Gene Ther. 2000;11:77–89. doi: 10.1089/10430340050016175. [DOI] [PubMed] [Google Scholar]
- 12.Erbs P, Regulier E, Kintz J, et al. In vivo cancer gene therapy by adenovirus-mediated transfer of abifunctional yeast cytosine deaminase/uracil phosphorribosyltransferase fusion gene. Cancer Res. 2000;60:3813–3822. [PubMed] [Google Scholar]
- 13.Postiglione I, Chiaviello A, Palumbo G. Enhancing Photodynamic Therapy Efficacy by Combination Therapy: Dated. Current and Oncoming Strategies Cancers. 2011;3:2597–2629. doi: 10.3390/cancers3022597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canti G, Nicolina A, Cubeddu R, Taroni P, Bandieramonte G, Valentini G. Antitumor efficacy of the combination of photodynamic therapy and chemotherapy in murine tumors. Cancer Lett. 1998;125:39–44. 37. doi: 10.1016/s0304-3835(97)00502-8. [DOI] [PubMed] [Google Scholar]
- 15.Tahmasebia H, Khoshgardb K, Sazgarniac A, Mostafaied A, Taghi Eivaziba M. Enhancing the efficiency of 5-aminolevulinic acid-mediatedphotodynamic therapy using 5-fluorouracil on human melanoma cells. Photodiagnosis and Photodynamic Therapy. 2016;13:297–302. doi: 10.1016/j.pdpdt.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Wang F, Zamora G, Sun CH, Trinidad A, Chun C, Kwon YJ, Berg K, Madsen SJ, Hirschberg H. Increased sensitivity of glioma cells to 5-fluorocytosine following photo-chemical internalization enhanced nonviral transfection of the cytosine deaminase suicide gene. J Neurooncol. 2014 May;118(1):29–37. doi: 10.1007/s11060-014-1410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathews MS, Blickenstaff JW, Shih EC, Zamora G, Vo V, Sun CH, Hirschberg H, Madsen SJ. Photochemical internalization of bleomycin for glioma treatment. J Biomed Opt. 2012;17(5):058001. doi: 10.1117/1.JBO.17.5.058001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11:2389–401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 19.Fass RJ, Perkins RL. 5-fluorocytosine in the treatment of cryptococcal and candida mycoses. Ann Intern Med. 1971;74:535–539. doi: 10.7326/0003-4819-74-4-535. [DOI] [PubMed] [Google Scholar]
- 20.Morse GD, Shelton MJ, O’Donnell AM. Comparative pharmacokinetics of antiviral nucleoside analogues. Clin Pharmacokinet. 1993;24:101–23. doi: 10.2165/00003088-199324020-00002. [DOI] [PubMed] [Google Scholar]
- 21.Barriere SL. Pharmacology and pharmacokinetics of traditional systemic antifungal agents. Pharmacotherapy. 1990;10:134S–140S. [PubMed] [Google Scholar]
- 22.Stylli SS, Kaye AH. Photodynamic therapy of cerebral glioma – A review Part II – Clinical studies. J Clin Neurosci. 2006 Aug;13(7):709–17. doi: 10.1016/j.jocn.2005.11.012. Review. [DOI] [PubMed] [Google Scholar]
- 23.Quirk BJ, Brandal G, et al. Photodynamic therapy (PDT) for malignant brain tumors — Where do we stand? Photodiagnosis and Photodynamic Therapy. 2015;12:530–540. doi: 10.1016/j.pdpdt.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Eljamel MS, Goodman C, Moseley H. ALA and Photofrin fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: a single centre Phase III randomised controlled trial. Lasers Med Sci. 2008;23:361–7. doi: 10.1007/s10103-007-0494-2. [DOI] [PubMed] [Google Scholar]
- 25.Tiraby M, Cazaux C, Baron B, Drocourt D, Reynes JP, Tiraby G. Concomitant expression of E coli cytosine deaminase and uracil phosphoribosyltransferase improves the cytotoxicity of 5-fluorocytosine. FEMS Microbiol Letters. 1998;167:41–49. doi: 10.1111/j.1574-6968.1998.tb13205.x. [DOI] [PubMed] [Google Scholar]
- 26.Guicciardi ME, Leist M, Gores GJ. Lysosomes in cell death. Oncogene. 2004;23:2881–2890. doi: 10.1038/sj.onc.1207512. [DOI] [PubMed] [Google Scholar]
- 27.Kessel D, Vicente MGH, Reiners JJ. Initiation of apoptosis and autophagy by photodynamic therapy. Autophagy. 2006;2:289–290. doi: 10.4161/auto.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez ME, Zhang P, Azizuddin KJC, et al. Structural factors and mechanisms underlying the improved photodynamic cell killing with silicon phthalocyanine photosensitizers directed to lysosomes versus mitochondria. Photochem Photobiol. 2009;85:1189–1200. doi: 10.1111/j.1751-1097.2009.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inguscio V, Panzarini E, Dini L. Autophagy contributes to the death/survival balance in cancer photodynamic therapy. Cells 2012. 2012;1:464–491. doi: 10.3390/cells1030464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reiners JJ, Agostinis P, Berg K, Oleinick NL, Kessel D. Assessing autophagy in the context of photodynamic therapy. Autophagy. 2010;6:7–18. doi: 10.4161/auto.6.1.10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi M, Valdes G, et al. Radio-sensitization of gliomas by intracellular generation of 5-fluorouracil potentiates prodrug activator gene therapy with a retroviral replicating vector. Cancer Gene Ther. 2014 Oct;21(10):405–410. doi: 10.1038/cgt.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prasmickaite L, Høgset A, Olsen VM, Kaalhus O, Mikalsen SO, Berg K. Photochemically enhanced gene transfection increases the cytotoxicity of the herpes simplex virus thymidine kinase gene combined with ganciclovir. Cancer Gene Ther. 2004;11(7):514–23. doi: 10.1038/sj.cgt.7700720. [DOI] [PubMed] [Google Scholar]
- 33.Zamora G, Wang F, Sun CH, Trinidad A, Kwon YJ, Cho SK, Berg K, Madsen SJ, Hirschberg H. Photochemical internalization-mediated nonviral gene transfection: polyamine core-shell nanoparticles as gene carrier. J Biomed Opt. 2014;19(10):105009. doi: 10.1117/1.JBO.19.10.105009. [DOI] [PMC free article] [PubMed] [Google Scholar]