Abstract
Background
Phase III trials have revealed benefit of radiation therapy (ART) for men with adverse factors at radical prostatectomy, however, some patients have high progression risk despite ART. The role of systemic therapy with ART in this high-risk group remains to be defined.
Methods
Patients post-RP with PSA nadir > 0.2 and Gleason ≥ 7 or PSA nadir ≤ 0.2, Gleason ≥ 8 and ≥ pT3 received six months ADT + RT and six cycles of docetaxel. The primary objective was to assess whether addition of ADT/docetaxel to ART results in freedom from progression (FFP) ≥70% Vs. an expected rate of 50%. Multivariate logistic and Cox regression were used to model association of factors with outcomes.
Results
74 patients were enrolled. Median follow-up was 4.4 years. T classification: pT2 (4%), pT3 (95%) and pT4 (1%); Gleason 7 (18%) and ≥8 (82%); Post-RP PSA: ≤0.2 (53%) and >0.2 47%. Three year FFP was 73% (95% CI:61%–83%). Three year cumulative incidence of biochemical, distant, and local failure were 26%, 7%, and 0%. In multivariate models, post-prostatectomy PSA nadir was associated with three year FFP; Gleason score and PSA with biochemical failure. Grade 3–4 neutropenia was common however only three cases of febrile neutropenia occurred. Late toxicities were not impacted by addition of systemic therapy.
Conclusions
Combination of ADT, docetaxel, and ART for men with high-risk prostate cancer post-prostatectomy exceeded the pre-specified study endpoint of 70% three-year FFP. Phase III trials assessing combined local and systemic therapies for these high-risk patients are warranted.
Keywords: post-prostatectomy, radiation, androgen deprivation, chemotherapy, docetaxel, high risk
Introduction
Radical prostatectomy (RP) is common treatment for prostate cancer. Although a majority of patients with favorable risk features do well, those with high-risk features have a substantial risk of disease recurrence. Patients with persistently elevated PSA post-prostatectomy or who experience biochemical failure within three years of surgery have a significantly increased risk of death from prostate cancer.1
Radiation therapy is commonly used post-prostatectomy. Randomized trials of adjuvant radiation therapy (ART) showed improvement in progression-free survival, and with long-term follow-up an overall survival advantage.2–4 Despite this overall benefit, 50% of patients with pathologic T3 and Gleason ≥ 8 disease or Gleason ≥ 7 and PSA nadir > 0.2 ng/ml experienced treatment failure within three years. This subset of patients are failing despite both surgery and radiation therapy and therefore are expected to be at greater risk of death from prostate cancer than those who fail only surgery.
Optimal therapies for high risk patients despite radiotherapy in the post-prostatectomy setting remain to be defined. Standard care for high-risk patients receiving primary radiotherapy includes androgen deprivation therapy (ADT).5 Docetaxel has been shown to improve survival in men with metastatic prostate cancer6–8 and recently a potential survival benefit has been noted in treatment of high-risk clinically localized disease.9 The present study was designed to test the hypothesis that addition of docetaxel and ADT to radiation in men at high risk of failure despite ART would result in improved freedom from progression as compared with historical controls.
Methods
Patient Eligibility
Protocol approval was received from the Institutional Review Board at each site. Informed consent was obtained from patients prior to participation. Eligible patients had prostatic adenocarcinoma with either Gleason ≥ 7 at prostatectomy and PSA nadir > 0.2 ng/ml or Gleason ≥ 8 at prostatectomy, PSA nadir ≤ 0.2 ng/ml, and T classification ≥ pT3a. Patients were enrolled within one year of RP. Subjects had no lymph node or distant metastases determined by bone scan and magnetic resonance imaging or computer tomography of the pelvis.
A Zubrod performance status of ≤1 was required. PSA was obtained ≤ 6 weeks before registration. Absolute neutrophil count ≥ 2,000 cells/mm3, platelet count ≥ 100,000 cells/mm3, hemoglobin ≥ 8.0 g/dl, ALT or AST ≤ 1.5 times, alkaline phosphatase ≤ 2.5 times and total bilirubin ≤ 1.2 times the institutional upper normal limit were required.
Treatment
ADT including an LHRH agonist and non-steroidal anti-androgen (bicalutamide 50mg daily) were administered for six months beginning eight weeks prior to RT.
RT was administered with either 3D-conformal radiation therapy (3DCRT) or intensity modulated radiation therapy (IMRT) using energies ≥ 6 MV. Total dose to the prostate bed was 6660 cGy (+/−180 cGy) including an initial pelvic field of 4500 cGy using daily fractions of 180 cGy. Pelvic fields included a superior border extending at a minimum to the bottom of the sacroiliac joint and at most superiorly to the L5-S1 interspace. Seminal vesicle remnants if present received a minimum of 5040 cGy and could receive full dose at the discretion of the treating physician.
Docetaxel was administered 3–6 weeks after completion of radiation therapy. Patients received six cycles of 75mg/m2 intravenously every three weeks. Pre-medication with dexamethasone was required. If granulocytes were ≤ 1,500 cell/mm3 or platelets ≤100,000 as measured within a day of docetaxel administration, treatment was held and counts repeated weekly with modification of subsequent docetaxel doses. If neutropenia/thrombocytopenia did not resolve to a point that allowed docetaxel administration by 15 days of scheduled chemotherapy, chemotherapy was discontinued. Docetaxel was also modified or held due to abnormal liver function tests. Dose was reduced by 25% for Grade 2 neuropathy without treatment delay or discontinued for Grade ≥ 3 neuropathy.
Follow-Up
Follow-up assessments occurred every three months for two years, every six months for three more years, then annually. PSA was obtained at each visit. PSA of ≥ 0.4 ng/ml was verified with a repeat level to confirm progression. Bone scans and CT or MRI were recommended at least annually after PSA progression to determine rates of metastatic progression.
Statistical Design and Analysis
The primary endpoint was freedom from progression (FFP), where failure was defined as PSA ≥ 0.4 ng/mL after end of RT confirmed by second higher PSA, non-protocol hormones, local-regional progression, distant metastasis, or death, within three years after registration. Per SWOG 8794, the expected FFP rate was 50% for patients treated with prostatectomy and RT. The experimental therapy was to be deemed effective if FFP rate was ≥ 70%. Per Fleming’s multiple testing procedure with three stages, 69 patients were required to test the null hypothesis (FFP ≤ 50%) against the alternative (FFP ≥ 70%) with 90% power and significance level 0.025. Allowing for 10% patient ineligibility or non-evaluability, total sample size was 76. At final analysis, if ≥ 44 of 69 had no FFP event, (i.e., were alive and progression-free), the null hypothesis would be rejected and we would conclude FFP rate is ≥ 0.7. The 95% confidence interval for FFP rate was calculated using the Clopper-Pearson method.
Secondary endpoints included FFP (at any time), local-regional progression, distant metastases, biochemical failure, overall survival, prostate cancer death, non-prostate-cancer death, and acute [within 90 days of treatment end (three weeks after last planned docetaxel dose)] and late (≥ 91 days after treatment end) treatment-related (definitely, probably, or possibly related to treatment) adverse events. Adverse events were scored using Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. FFP and survival rates were estimated by Kaplan-Meier method and all others by cumulative incidence method, with death prior to failure as competing risk. Age, Gleason score, PSA, TN stage, and surgical margins were correlated with outcomes by logistic or Cox regression.
Results
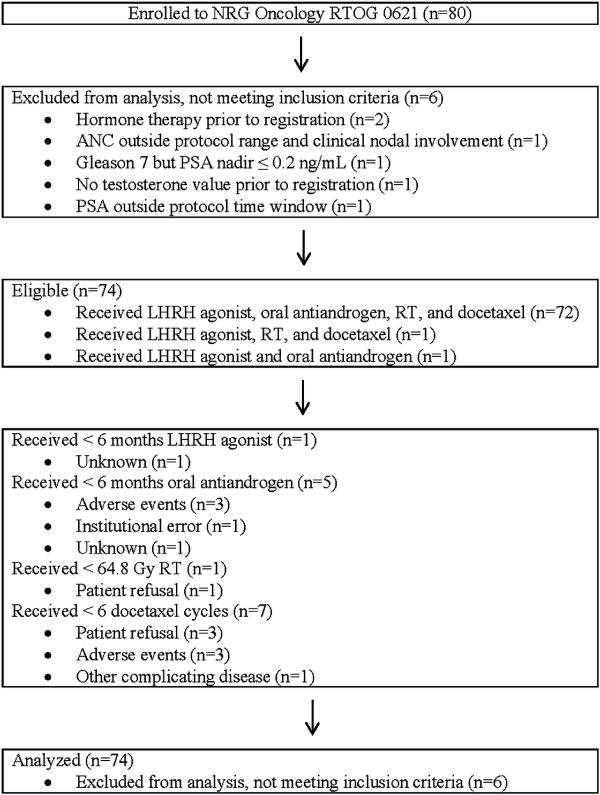
Eighty patients from 33 sites were enrolled between April, 2008 and September, 2010 of which 74 meeting eligibility requirements were included in analysis. Details of patient enrollment are shown in Figure 1. Patient characteristics are shown in Table 1. Post-RP PSA was ≤ 0.2 in 39 patients (52.7%) and >0.2 ng/ml in 35 (47.3%). Among patients with post-RP PSA > 0.2, the median was 0.60 (IQR 0.40 to 2.49). Surgical margins were positive in 43 patients (58.1%).
Figure 1.
NRG Oncology RTOG 0621 CONSORT Flow Diagram
Table 1.
Patient and tumor characteristics (n=74)
| Age (years) | ||
| Median | 62 | |
| Minimum – maximum | 43 – 75 | |
| First quartile – third quartile | 55 – 66 | |
| ≤ 65 | 52 | 70.3% |
| > 65 | 22 | 29.7% |
| Race | ||
| American Indian or Alaskan native | 2 | 2.7% |
| Black or African-American | 6 | 8.1% |
| White | 65 | 87.8% |
| Unknown | 1 | 1.4% |
| Ethnicity | ||
| Hispanic or Latino | 1 | 1.4% |
| Not Hispanic or Latino | 66 | 89.2% |
| Unknown | 7 | 9.5% |
| Zubrod performance status | ||
| 0 | 69 | 93.2% |
| 1 | 5 | 6.8% |
| Prostatectomy margin | ||
| Positive | 43 | 58.1% |
| Negative | 31 | 41.9% |
| T stage, pathologic | ||
| pT2a | 1 | 1.4% |
| pT2c | 2 | 2.7% |
| pT3 (not otherwise specified) | 2 | 2.7% |
| pT3a | 28 | 37.8% |
| pT3b | 40 | 54.1% |
| pT4 | 1 | 1.4% |
| PSA (postoperative nadir, ng/mL) | ||
| ≤ 0.1 | 33 | 44.6% |
| > 0.1 to 0.2 | 6 | 8.1% |
| > 0.2 | 35 | 47.3% |
| PSA (study entry, ng/mL) | ||
| ≤ 0.1 | 27 | 36.5% |
| > 0.1 to 0.2 | 7 | 9.5% |
| > 0.2 | 40 | 54.1% |
| Gleason score, combined | ||
| 7 | 13 | 17.6% |
| 8 | 19 | 25.7% |
| 9 | 41 | 55.4% |
| 10 | 1 | 1.4% |
Freedom from progression in the first three years
Since final analysis is out of 74 patients, ≥ 46 patients with no FFP event (i.e., were alive and progression-free) were required to reject the null hypothesis (rather than 44). Fifty-four of 74 had no FFP event so the null hypothesis was rejected. Estimated FFP rate is 73.0% (95%CI 61.4–82.6). Post-RP PSA > 0.2 was associated with increased risk of failure in univariate [odds ratio 10.94 (95%CI 2.68–65.81), p<0.001] and multivariate analysis [odds ratio 16.27 (95%CI 3.51–108.17), p<0.001)] adjusted for Gleason score (8–10 vs. 7).
Time-to-event outcomes
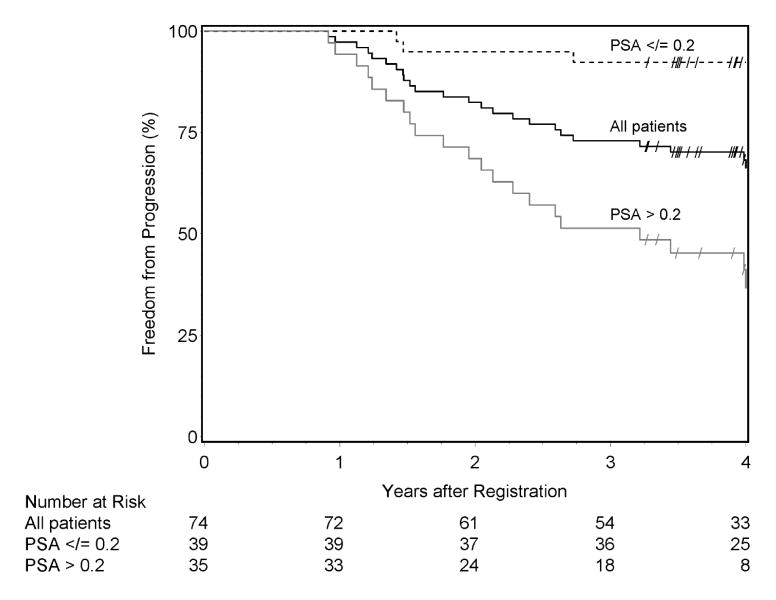
Median follow-up among surviving patients was 4.4 years (range 3.3–5.6). Three-year FFP was 73.0% (95%CI 62.9–83.1) (Figure 2). Twenty-six patients experienced treatment failure including 20 within three years. Biochemical failure was the first event noted in 24 (92.3%) with 2 (7.7%) presenting with metastases as the initial sign of treatment failure. Eleven subjects developed metastases. Three deaths including two from prostate cancer occurred. Three-year estimates are presented in Table 2. Gleason score 8–10 and post-RP PSA > 0.2 were associated with increased risk of failure for endpoints FFP and biochemical failure in multivariate analysis, but only PSA > 0.2 showed increased risk in univariate analysis (Table 3). Discrepant results for Gleason score between univariate and bivariate analysis are likely due to the eligibility criteria, where patients with Gleason 7 were only eligible with PSA > 0.2. In univariate analysis, post-RP PSA > 0.2 is also associated with increased risk of distant metastasis [hazard ratio 12.62 (95%CI 1.61–98.65), p=0.02)].
Figure 2.
Kaplan-Meier estimates of freedom from progression. Overall, the estimated three-year FFP rate is 73.0% (95%CI 62.9–83.1). Three-year FFP rates are 92.3% (95%CI 83.9–100) and 51.4% (95%CI 34.9–68.0) for patients with post-RP nadir PSA ≤ 0.2 ng/ml and > 0.2, respectively. Censored observations are denoted by “/”.
Table 2.
Three-year time-to-event estimates
| Endpoint | Number of events
|
3-year estimate (%) | 95% confidence interval (%) | |
|---|---|---|---|---|
| First 3 years | Total | |||
| Freedom from progression | 20 | 26 | 73.0 | 62.9 to 83.1 |
| Local-regional progression | 0 | 0 | 0.0 | Not applicable |
| Distant metastasis | 5 | 11 | 6.8 | 2.5 to 14.0 |
| Biochemical failure | 19 | 25 | 25.7 | 16.3 to 36.1 |
| Overall survival | 1 | 3 | 98.6 | 96.0 to 100 |
| Prostate cancer death | 0 | 2 | 0.0 | Not applicable |
| Non-prostate-cancer death | 1 | 1 | 1.4 | 0.1 to 6.5 |
Table 3.
Univariate and multivariate analysis of prognostic factors for freedom from progression and biochemical failure
| Endpoint | Variable | Hazard ratio | 95% CI | p-value |
|---|---|---|---|---|
| Freedom from progression | Univariate analysis | |||
| Gleason [1] | 0.87 | 0.33 to 2.32 | 0.78 | |
| PSA [2] | 13.45 | 4.00 to 45.22 | <0.001 | |
| Multivariate analysis | ||||
| Gleason [1] | 3.14 | 1.16 to 8.50 | 0.02 | |
| PSA [2] | 20.01 | 5.81 to 68.95 | <0.001 | |
|
| ||||
| Biochemical failure | Univariate analysis | |||
| Gleason [1] | 1.14 | 0.39 to 3.32 | 0.81 | |
| PSA [2] | 12.26 | 3.64 to 41.27 | <0.001 | |
| Multivariate analysis | ||||
| Gleason [1] | 4.13 | 1.39 to 12.28 | 0.01 | |
| PSA [2] | 19.72 | 5.72 to 67.99 | <0.001 | |
CI: confidence interval.
Gleason score, combined (8–10 vs. 7).
PSA (postoperative nadir, ng/mL) (> 0.2 vs. ≤ 0.2).
Treatment delivery
Sixty-six of 74 patients (89.2%) were scored by study chairs as per protocol or with acceptable variation for radiation therapy. Seventy of 74 (94.6%) were scored per protocol for chemotherapy, with 61 of 70 having no modifications or delays. 89.2% received IMRT, 9.5% 3DCRT, and one no RT. All 73 patients that started RT received 66.6Gy +/− 1.8. Sixty-seven of 74 patients (90.5%) received six cycles of docetaxel; one patient did not receive any docetaxel. All patients received LHRH agonist and all but one received oral antiandrogen.
Adverse events
Chemotherapy side effects were common but manageable and did not increase long-term toxicity. Thirty-five patients (47.3%) experienced at least one Grade 4 and an additional 23 (31.1%) at least one Grade 3 treatment-related adverse event at any time. Acute treatment-related adverse events are summarized in Table 4. The most common acute toxicities were hematologic including Grade 3 and 4 neutropenia (16.2 and 40.5%), leukopenia (35.1 and 13.5%) and lymphopenia (13.5 and 2.7%). However, only 4.1% of patients developed febrile neutropenia and infection was limited to a single case of a Grade 3 urinary tract infection. Grade 2 and 3 peripheral neuropathy occurred in 13.5% and 1.4% respectively. Late treatment related toxicities included six (8.1%) various Grade 3 and two (2.7%) cases of Grade 4 urinary incontinence (Table 5). Two-year cumulative incidence of Grade 3–4 late toxicity was 8.1% (95%CI 3.3–15.8).
Table 4.
Selected Treatment-Related Acute Adverse Events (n=74)
| CTCAE category | Grade 2 | Grade 3 | Grade 4 | |||
|---|---|---|---|---|---|---|
| CTCAE term | n | % | n | % | n | % |
| Maximum | 15 | 20.3 | 23 | 31.1 | 34 | 45.9 |
|
| ||||||
| Blood/bone marrow | ||||||
|
| ||||||
| Hemoglobin decreased | 12 | 16.2 | 0 | 0.0 | 0 | 0.0 |
| Leukopenia | 2 | 2.7 | 26 | 35.1 | 10 | 13.5 |
| Lymphopenia | 10 | 13.5 | 10 | 13.5 | 2 | 2.7 |
| Neutropenia | 2 | 2.7 | 12 | 16.2 | 30 | 40.5 |
|
| ||||||
| Infection | ||||||
|
| ||||||
| Febrile neutropenia | 0 | 0.0 | 2 | 2.7 | 1 | 1.4 |
| Urinary tract infection [with Grade 3–4 ANC] | 0 | 0.0 | 1 | 1.4 | 0 | 0.0 |
|
| ||||||
| Neurology | ||||||
|
| ||||||
| Peripheral sensory neuropathy | 10 | 13.5 | 1 | 1.4 | 0 | 0.0 |
|
| ||||||
| Constitutional symptoms | ||||||
|
| ||||||
| Fatigue | 42 | 56.8 | 1 | 1.4 | 0 | 0.0 |
|
| ||||||
| Gastrointestinal | ||||||
|
| ||||||
| Constipation | 8 | 10.8 | 1 | 1.4 | 0 | 0.0 |
| Diarrhea | 14 | 18.9 | 2 | 2.7 | 0 | 0.0 |
| Ileus | 0 | 0.0 | 1 | 1.4 | 0 | 0.0 |
| Nausea | 7 | 9.5 | 0 | 0.0 | 0 | 0.0 |
| Vomiting | 4 | 5.4 | 1 | 1.4 | 0 | 0.0 |
|
| ||||||
| Renal/genitourinary | ||||||
|
| ||||||
| Urinary frequency | 15 | 20.3 | 1 | 1.4 | 0 | 0.0 |
| Urinary incontinence | 15 | 20.3 | 0 | 0.0 | 1 | 1.4 |
CTCAE: Common Terminology Criteria for Adverse Events, version 3.0.
ANC: absolute neutrophil count.
Table 5.
Selected Treatment-Related Late Adverse Events (n=74)
| CTCAE category | Grade 2 | Grade 3 | Grade 4 | |||
|---|---|---|---|---|---|---|
| CTCAE term | n | % | n | % | n | % |
| Maximum | 36 | 48.6 | 6 | 8.1 | 2 | 2.7 |
|
| ||||||
| Blood/bone marrow | ||||||
|
| ||||||
| Lymphopenia | 0 | 0.0 | 1 | 1.4 | 0 | 0.0 |
|
| ||||||
| Infection | ||||||
|
| ||||||
| Sepsis [with normal or Grade 1–2 ANC] | 0 | 0.0 | 1 | 1.4 | 0 | 0.0 |
| Skin infection [with normal or Grade 1–2 ANC] | 0 | 0.0 | 1 | 1.4 | 0 | 0.0 |
| Urinary tract infection [with unknown ANC] | 1 | 1.4 | 0 | 0.0 | 0 | 0.0 |
|
| ||||||
| Pain | ||||||
|
| ||||||
| Pain [not otherwise specified] | 0 | 0.0 | 1 | 1.4 | 0 | 0.0 |
|
| ||||||
| Gastrointestinal | ||||||
|
| ||||||
| Proctitis | 2 | 2.7 | 0 | 0.0 | 0 | 0.0 |
|
| ||||||
| Renal/genitourinary | ||||||
|
| ||||||
| Cystitis | 2 | 2.7 | 1 | 1.4 | 0 | 0.0 |
| Ureteric obstruction | 0 | 0.0 | 2 | 2.7 | 0 | 0.0 |
| Urinary frequency | 6 | 8.1 | 0 | 0.0 | 0 | 0.0 |
| Urinary incontinence | 16 | 21.6 | 0 | 0.0 | 2 | 2.7 |
CTCAE: Common Terminology Criteria for Adverse Events, version 3.0.
ANC: absolute neutrophil count.
Discussion
Phase III trials studies have consistently demonstrated a benefit to the use of adjuvant radiation therapy for men with adverse pathologic findings following prostatectomy including extracapsular disease extension, seminal vesicle invasion, or positive margins.2–4 All three randomized studies showed benefit in progression-free survival and in SWOG 8794 with long-term follow-up an overall survival benefit was noted.
While adjuvant radiation post-prostatectomy will benefit many men, review of studies assessing post-prostatectomy radiation reveal subsets of patients at high risk of failure following both surgery and radiation. In a large series from Johns Hopkins, men who experienced treatment failure within three years of prostatectomy had a 15 year risk of prostate cancer specific mortality of 59% compared to 13% who experienced treatment failure at a longer period post-operatively. In design of the current study, a subgroup of patients from the SWOG 8794 study were identified with on average a 50% risk of failure by three years despite addition of adjuvant radiation. Given these patients are at high risk of failing two local therapies, the current study was designed to assess the potential benefit of adding docetaxel and ADT to radiation in this high-risk population.
While the roles of ADT and chemotherapy in combination with postoperative radiotherapy have yet to be defined, survival benefit has been noted with ADT and potential benefit with docetaxel in other high-risk settings. Addition of ADT to primary use of radiotherapy is standard in the setting of high-risk prostate cancer with established survival benefit. Phase III trials have also revealed survival benefit with docetaxel in metastatic prostate cancer, initially in the setting of castration resistant disease6,7 and more recently a large survival benefit was noted in the CHAARTED study when administered with initiation of ADT for newly diagnosed metastatic castration sensitive disease.8 The STAMPEDE trial showed benefit in metastatic disease and potential utility in the locally advanced setting and initial results of RTOG 0521 indicate there may be a survival benefit in primary treatment of high-risk patients.9,10 Benefit seen with use of docetaxel at earlier points in the course of prostate cancer supports investigation of its use in high-risk patients post-prostatectomy without frank evidence of metastases but likely to fail despite local therapy. The findings of the currently study support the hypothesis that use of systemic therapy benefits men without established metastatic disease but who are at high risk of local treatment failure despite both radical prostatectomy and post-operative RT. Three year freedom from progression was 73% compared to a projected rate of 50% observed among patients with high-risk features on the adjuvant radiation arm of SWOG 8794. While comparison between studies must be viewed with caution, on review of patient characteristics and known prognostic factors, subjects enrolled on the current study appear to be a higher risk cohort compared to the SWOG historical controls. 82% of subjects on the current study had Gleason 8–10 disease vs. 9% in the historical control series. PSA failed to nadir to ≤ 0.2 in 47% of subjects on the current study vs. 36% from SWOG 8794. 57% of subjects on the current study had seminal vesicle involvement vs. 31% from SWOG 8794 and fewer had positive margins. All these factors have been associated with greater risk of treatment failure in prior studies including SWOG 8794.11 Furthermore, both PSA nadir and Gleason score were associated with increased risk of treatment failure on multivariate analysis on the current study suggesting that the benefit to addition of systemic therapy in these very high-risk patients may exceed the estimated 23%.
Additional study of docetaxel in high-risk patients post-prostatectomy is indicated noting both the potential benefits and toxicities. High rates of hematologic toxicities including Grade 3 and 4 neutropenia and leukopenia occurred on the current study. These rates appear higher than previously reported series assessing use of docetaxel in treatment of metastatic prostate cancer likely reflecting initiation of docetaxel three to six weeks after the completion of pelvic radiation.7,11–13 The impact of timing of initiation of docetaxel following radiation on hematologic toxicities warrants further investigation. While concurrent use of docetaxel with radiation may have sensitizing effects as commonly employed in treatment of other malignancies, the rates of hematologic toxicity noted on the present study support the sequential use of pelvic radiation and docetaxel in this clinical setting. It is important to note that while rates of these laboratory toxicities were high, rates of febrile neutropenia and infection were similar to previously reported series.7,12 Rates of other toxicities commonly associated with docetaxel including peripheral neuropathy were in line with expected rates.13 Notably use of docetaxel and ADT in the current study was not associated with increased long term toxicities including exacerbation of genitourinary and gastrointestinal side effects common with radiation therapy.4,14
It is important to note limitations of the current study. Due to the non-randomized phase II design, the impact of each intervention including radiation, ADT, and docetaxel on improvement in freedom from progression compared to historical controls remains to be defined. Improved surgical techniques and changes in Gleason grading since completion of SWOG 8794 might have accounted for some of the benefit seen on NRG Oncology RTOG 0621. However, this concern is significantly mitigated by the comparatively higher risk factors of subjects enrolled on NRG Oncology RTOG 0621 including more subjects with persistently detectable PSA post-operatively. It is unlikely that change in Gleason grading over time, can account for the large discrepancy in numbers of high risk (Gleason ≥ 8) subjects between studies. The current study also differed from prior phase III trials of adjuvant radiation therapy in the use of an initial pelvic field and prescribed dose of 6660 cGy as compared to 6000–6400 used in previous phase III trials including SWOG 8794, the comparator for the present series. The results of a DART 01/05 trial indicating benefit to extended ADT treatment in addition to dose escalated radiation in primary treatment of high-risk patients suggests the benefit seen in the current study is unlikely due to the use of pelvic radiation therapy and slightly higher doses as commonly used currently.15 Use of ADT may also provide a lead time bias in defining treatment failure due to time required for testosterone recovery. Ultimately, the contribution of docetaxel and ADT, and whether extended duration of ADT would provide further benefit are questions that only a phase III trial can definitively answer.
The optimal approach to management of high-risk patients remains an important question to be answered in the post-prostatectomy setting. NRG Oncology RTOG 0621 has revealed a very high risk subset of patients post-prostatectomy at near term risk of disease progression even with combined local and systemic therapy. The results of the current study strongly support further investigation of integration of systemic therapies including both ADT and chemotherapy with post-operative radiation in this patient population.
Conclusions
Addition of docetaxel and ADT to ART for men at high-risk of failure despite both surgery and radiation therapy resulted in significant improvement in three-year freedom from progression as compared to historical controls treated with radiation alone. While past randomized trials have proven the efficacy of ART in selected subsets of men after radical prostatectomy, the individual contribution of hormonal therapy and docetaxel in this setting will require additional appropriately designed prospective randomized studies.
Precis.
Addition of docetaxel and ADT to ART for men at high-risk of failure despite both surgery and radiation therapy resulted in significant improvement in three-year freedom from progression as compared to historical controls treated with radiation alone.
Acknowledgments
This project was supported by grants U10CA21661, U10CA180868, U10CA180822, U10 CA37422, U24CA180803 from the National Cancer Institute (NCI) and Sanofi-Aventis.
Footnotes
Conflicts of interest: Mark D. Hurwitz reports non-financial support and other from RTOG/NRG Oncology, during the conduct of the study; personal fees from Medivation and Astellas, outside the submitted work. Oliver Sartor reports personal fees from Sanofi, grants from Sanofi, outside the submitted work. Howard M. Sandler reports personal fees from AstraZeneca, personal fees from Medivation, grants from Myriad, from Janssen, personal fees from Blue Earth Diagnostics, personal fees from Ferring, personal fees from Bayer, personal fees from eviti, outside the submitted work.
Previous presentation: The study was present at the Clinical trials session of the 56th annual ASTRO meeting in September 2014.
Author Contributions
Mark D. Hurwitz, MD: conception and design of the study; acquisition, analysis, and interpretation of data, drafting and revision of the manuscript and approval of the final manuscript.
Jonathan Harris, MS: design of the study, analysis and interpretation of data, drafting and revision of the manuscript and approval of the final manuscript.
Oliver Sartor, MD: conception and design of the study; acquisition, analysis, and interpretation of data, drafting and revision of the manuscript and approval of the final manuscript.
Ying Xiao, PhD: design of the study; acquisition, analysis, and interpretation of data, drafting and revision of the manuscript and approval of the final manuscript.
Bobby Shayegan, MD: design of the study; acquisition, analysis, and interpretation of data, drafting and revision of the manuscript and approval of the final manuscript.
Paul W. Sperduto, MD: acquisition of data, revision of the manuscript and approval of the final manuscript.
Kasra R. Badiozamani, MD: acquisition of data, revision of the manuscript and approval of the final manuscript.
Colleen A. F. Lawton, MD: design of the study; acquisition of data, revision of the manuscript and approval of the final manuscript.
Eric M. Horwitz, MD: design of the study; acquisition of data, revision of the manuscript and approval of the final manuscript.
Jeff M. Michalski, MD: design of the study; acquisition of data, revision of the manuscript and approval of the final manuscript.
Kevin Roof, MD: acquisition of data, revision of the manuscript and approval of the final manuscript.
David C. Beyer, MD: acquisition of data, revision of the manuscript and approval of the final manuscript.
Qiang Zhang, PhD: design of the study; acquisition, analysis, and interpretation of data, drafting and revision of the manuscript and approval of the final manuscript.
Howard Sandler, MD: conception and design of the study; acquisition, analysis, and interpretation of data, drafting and revision of the manuscript and approval of the final manuscript.
References
- 1.Freedland SJ, Humphreys EB, Mangold LA, et al. Time to prostate specific antigen recurrence after radical prostatectomy and the risk of prostate cancer specific mortality. J Urol. 2006;176(4):1404–1408. doi: 10.1016/j.juro.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 2.Swanson GP, Goldman B, Tangen CM, et al. Southwest Oncology Group 8794 Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. J Urol. 2008;180(6):2453–2457. doi: 10.1016/j.juro.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van der Kwast TH, Bolla M, Van Poppel HV, et al. Identification of patients with prostate cancer who benefit from immediate postoperative radiotherapy: EORTC 22911. J Clin Oncol. 2007;25(27):4178–4186. doi: 10.1200/JCO.2006.10.4067. [DOI] [PubMed] [Google Scholar]
- 4.Wiegel T, Bartkowiak D, Bottke D, et al. Adjuvant Radiotherapy Versus Wait-and-See After Radical Prostatectomy: 10-year Follow-up of the ARO 96-02/AUO AP 09/95 Trial. Eur Urol. 2014 Mar 21; doi: 10.1016/j.eururo.2014.03.011. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.D’Angelillo RM, Franco P, De Bari B, Fiorentino A, Arcangeli S, Alongi F. Combination of androgen deprivation therapy and radiotherapy for localized prostate cancer in the contemporary era. Crit Rev Oncol Hematol. 2015 Feb;93(2):136–48. doi: 10.1016/j.critrevonc.2014.10.003. Epub 2014 Oct 17. [DOI] [PubMed] [Google Scholar]
- 6.Petrylak DP, Tangen CM, Hussain M, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 7.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 8.Sweeney CJ, Chamberlain D. Insights into E3805: the CHAARTED trial. Future Oncol. 2015;11(6):897–9. doi: 10.2217/fon.14.310. [DOI] [PubMed] [Google Scholar]
- 9.Sandler H, Hu C, Rosenthal SA, et al. A phase III protocol of androgen suppression (AS) and 3DCRT/IMRT versus AS and 3DCRT/IMRT followed by chemotherapy (CT) with docetaxel and prednisone for localized, high-risk prostate cancer (RTOG 0521) J Clin Oncol. 2015;33 suppl; abstr LBA5002. [Google Scholar]
- 10.James ND, Sydes MR, Clarke NW, et al. STAMPEDE investigators Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2015 Dec 21; doi: 10.1016/S0140-6736(15)01037-5. pii: S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hervonen P, Joensuu H, Joensuu T, et al. Biweekly docetaxel is better tolerated than conventional three-weekly dosing for advanced hormone-refractory prostate cancer. Anticancer Res. 2012;32(3):953–956. [PubMed] [Google Scholar]
- 12.Kelly WK, Halabi S, Carducci M, et al. Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J Clin Oncol. 2012;30(13):1534–1540. doi: 10.1200/JCO.2011.39.4767. Epub 2012 Mar 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKeage K. Docetaxel: a review of its use for the first-line treatment of advanced castration-resistant prostate cancer. Drugs. 2012;72(11):1559–1577. doi: 10.2165/11209660-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Goenka A, Magsanoc JM, Pei X, et al. Improved toxicity profile following high-dose postprostatectomy salvage radiation therapy with intensity modulated radiation therapy. Eur Urol. 2011;60(6):1142–1148. doi: 10.1016/j.eururo.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Zapatero A, Guerrero A, Maldonado X, et al. High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): a randomised, controlled, phase 3 trial. Lancet Oncol. 2015 Mar;16(3):320–327. doi: 10.1016/S1470-2045(15)70045-8. Epub 2015 Feb 19. Erratum in: Lancet Oncol. 2015 Jun;16(6):e262. [DOI] [PubMed] [Google Scholar]