Abstract
Background
Injury to cells adjacent to an intracerebral hemorrhage (ICH) is likely mediated at least in part by toxins released from the hematoma that initiate complex and interacting injury cascades. Pharmacotherapies targeting a single toxin or pathway, even if consistently effective in controlled experimental models, have a high likelihood of failure in a variable clinical setting. Nuclear factor erythroid-2 related factor 2 (Nrf2) regulates the expression of heme oxygenase-1 (HO-1) and multiple other proteins with antioxidant and anti-inflammatory effects, and may be a target of interest after ICH.
Methods
Studies that tested the effect of HO and Nrf2 in models relevant to ICH are summarized, with an effort to reconcile conflicting data by consideration of methodological limitations.
Results
In vitro studies demonstrated that Nrf2 activators rapidly increased HO-1 expression in astrocytes, and reduced their vulnerability to hemoglobin or hemin. Modulating HO-1 expression via genetic approaches yielded similar results. Systemic treatment with small molecule Nrf2 activators increased HO-1 expression in perivascular cells, particularly astrocytes. When tested in mouse or rat ICH models, Nrf2 activators were consistently protective, improving barrier function and attenuating edema, inflammation, neuronal loss and neurological deficits. These effects were mimicked by selective astrocyte HO-1 overexpression in transgenic mice.
Conclusion
Systemic treatment with Nrf2 activators after ICH is protective in rodents. Two compounds, dimethyl fumarate and hemin, are currently approved for treatment of multiple sclerosis and acute porphyria, respectively, and have acceptable safety profiles over years of clinical use. Further development of these drugs as ICH therapeutics seems warranted.
Keywords: astrocyte, heme, heme oxygenase, intracerebral hemorrhage, Nrf2, Stroke
Scope of the problem
Intracerebral hemorrhage (ICH) is the primary event in 10–15% of the 15 million strokes occurring annually worldwide [1]. This percentage is significantly higher Asians and African Americans, and increases in all populations with aging. Compared with ischemic stroke, ICH has a higher mortality rate and greater lifetime cost [2]. About half of ICH patients will be dead at one month; only 10% will be living independently at this time point, and only 20% will be independent at six months [3]. Despite its severity, ICH has been less intensively investigated than ischemic stroke, and injury mechanisms remain largely undefined. No effective pharmacotherapies are currently available, and no improvement in outcome has been observed over the past two decades [4].
Pathophysiology
Reduced blood flow without ischemia
ICH is produced by rupture of small penetrating arteries or arterioles. The immediate impact, at least in animal models that attempt to replicate arterial rupture, is transmission of a pressure wave from the vessel lumen to the parenchyma. The resulting increase in tissue pressure reduces blood flow in a manner that varies inversely with distance from the hematoma and time, and likely reflects impaired flow through lower pressure capillaries and venules [5, 6]. Although tissue injury after clinical ICH has classically been attributed to compressive ischemia, early blood flow reduction may not be of sufficient magnitude and duration to produce ischemic cell death. Perfusion imaging studies conducted in the acute phase (< 22 hours) after symptom onset suggest that perihematomal ischemia is absent in most patients; any decrease in perfusion is likely due reduced metabolic demand [7]. Consistent with these observations, no significant changes in cerebral oxygen extraction, CMR02, regional cerebral blood flow, glucose utilization or lactate production were detected at five hours after a massive experimental ICH in dogs [8]. However, ischemia cannot be excluded in a subset of patients with a particularly poor prognosis, although protocols for rapidly identifying these patients have not been established [9, 10].
Toxicity of extravascular blood
Since most injury after ICH cannot be directly attributed to ischemia, delineation of alternative pathophysiological pathways remains a primary focus of preclinical investigation. One hypothesis is that toxins released from a hematoma initiate injury cascades that contribute to cell loss and poor outcome [11]. Unfortunately, a hematoma contains many potential cytotoxins, complicating mechanistic investigation and reducing the likelihood that a single selective agent will have any efficacy in a complex clinical environment. In vitro studies may provide some insight into the predominant injury mechanisms that are induced as a hematoma evolves in proximity to neural cells. In primary cell cultures containing both glial cells and neurons, most cell loss produced by co-culture with a blood clot can be attributed to heme-mediated oxidative stress and excitoxicity [12, 13].
Hemoglobin Toxicity
Hemoglobin is a tetrameric protein containing four heme groups, and accounts for essentially all of the ~20 mM heme concentration in erythrocytes. The time course of erythrocyte lysis after experimental or clinical ICH has not been well-defined, nor has its predominant site, i.e. extracellular versus within microglia/macrophages after erythophagocytosis. In the rat blood injection ICH model, Perls’ staining demonstrated iron accumulation in neurons at 24 hours and in glial cells several days later [14], consistent with some early extracellular hemolysis. The extraneous redox activity of the heme groups of ferrous hemoglobin is limited by their location within hydrophobic pockets [15]. In the extracellular space, this protective structure is rapidly compromised as hemoglobin autoxidizes to its ferric form (methemoglobin) via a reaction that generates equimolar superoxide [16]. Hemoglobin autoxidation occurs in a predictable fashion after clinical ICH, and is the basis for estimating hematoma age via magnetic resonance imaging [17]. The heme moieties of methemoglobin are less tightly bound than those of ferrous hemoglobin and are readily released [18]. Free hemin, an oxidized form of heme, accumulates in intracranial hematomas in concentrations that reach the high micromolar range [19]. Its lipophilicity accounts for its tendency to concentrate in cell membranes [20].
At least three mechanisms contribute to the direct cytotoxicity of hemin. First, it directly and efficiently oxidizes membrane lipids by decomposing preformed lipid peroxides, thereby initiating free radical chain reactions [21, 22]. Second, it destabilizes membranes via an incompletely defined colloid osmotic mechanism that is not prevented by antioxidants [23]. Third, hemin breakdown by the heme oxygenase (HO) enzymes may produce an iron-dependent oxidative injury to cell populations with limited iron-sequestering capacity, such as neurons [24]. In addition, hemin also activates TLR4 and may thereby initiate an inflammatory response that indirectly contributes to secondary cell loss [25].
Excitotoxicity
Excessive activation of excitatory glutamate receptors contributes to ischemic CNS injury in multiple animal models [26], although clinical trials of receptor antagonists failed to demonstrate a significant benefit. Excitoxicity has received less attention as a therapeutic target after ICH, particularly after experimental and clinical studies suggesting that perihematomal tissue was not ischemic [7, 8]. However, extravascular blood is a very likely source of excitotoxic stress [27]. Blood lysate has an excitatory amino acid content in the high micromolar range, due predominately to the high glutamate and aspartate concentrations in erythrocytes [28, 29]. Extravascular hemolysis in the days after ICH will release a constant supply of these receptor ligands, and their neurotoxicity may be enhanced in a hemorrhagic environment [12]. In vitro, the NMDA receptor antagonist MK-801 was moderately protective per se against blood clot toxicity [13]. In vivo, striatal injection of lysed blood produced a rapid increase in [C14]-2-deoxyglucose utilization in adjacent tissue that was blocked by MK-801 and the AMPA receptor antagonist NBQX, indicating that the excitatory amino acid content in the lysate is sufficient to activate both receptor classes [30]. Studies testing the efficacy of glutamate receptor antagonists in ICH models have been relatively sparse. Memantine had some benefit as monotherapy after collagenase-induced ICH in rats, but was more effective when combined with the Cox-2 inhibitor celecoxib [31, 32]. The AMPA receptor antagonist YM872 had a modest benefit on neurological outcome in the rat collagenase ICH model, without affecting hematoma or lesion size [33].
The complexity of the injury initiated by an intracerebral hematoma is magnified in vivo by a secondary inflammatory response that is not prominent in simplified cell culture models. This response is activated at least in part by hemin, a TLR4 agonist [34], and thrombin, a serum protease generated by the coagulation cascade with multiple pro-inflammatory effects [35]. A selective pharmaceutical approach that targets a single mechanism, even if beneficial in a controlled experimental model, has a high probability of failure when tested in an inherently variable clinical setting. A single therapy that modulates multiple injury mechanisms after ICH may be preferred. Heme oxygenase-1 (HO-1) attenuates oxidative [36], excitotoxic [37] and inflammatory injury [38] in experimental models, so agents that upregulate this enzyme are of particular interest.
Pleiotropic effects of the heme oxygenases
The heme oxygenase enzymes catalyze the rate-limiting step of heme/hemin breakdown. Two isoforms, designated HO-1 and HO-2, are expressed by mammalian cells; a third, HO-3, was initially described but was in fact a pseudogene derived from HO-2 transcripts [39]. HO-1 and HO-2 are the products of separate genes (HMOX1 and HMOX2) and catalyze the same reaction, consuming three molecules of oxygen and seven electrons donated by NADPH to yield equimolar carbon monoxide, biliverdin and ferrous iron. The biological activity of these products may account for the diverse and sometimes discordant effects of HO activity in CNS injury models. Carbon monoxide is toxic at high atmospheric concentrations because its affinity for the heme moieties of hemoglobin and cytochrome c oxidase interferes with oxygen exchange and mitochondrial electron transport [40]. However, at the much lower concentrations associated with heme breakdown, it has immunomodulating and vasodilating effects that appear to be beneficial after acute CNS insults [38]. Biliverdin is converted to bilirubin by ubiquitously-expressed biiverdin reductase; both pigments are potent antioxidants [41]. Ferrous iron is oxidized by hydrogen peroxide to yield the highly reactive hydroxyl radical, but also is a very efficient inducer of ferritin, the primary cellular iron storage protein. Each ferritin molecule is a 24-mer heteropolymer constructed as a nanocage surrounding a mineral core that has an enormous capacity for iron sequestration (up to 4000 atoms [42]). An inducing stimulus usually increases iron binding capacity in excess of immediate need, thereby increasing cellular resistance to subsequent iron challenge [43, 44].
Initial studies evaluating the relationship between HO and outcome in models relevant to hemorrhagic stroke consistently reported very significant effects. However, the direction of these effects was contradictory and a source of considerable confusion. In hindsight, it is apparent that these studies were limited by the methods available at the time to modulate HO activity and expression, which primarily involved the use of either unconditional knockouts or nonspecific pharmacological inhibitors.
Unconditional knockout of HO-1, the inducible isoform that is expressed predominantly by glial cells in the CNS, has a combined prenatal and perinatal mortality rate exceeding 90% (unpublished observations). Adult HO-1 KO mice are therefore highly selected animals that may have compensatory but as yet unidentified survival mechanisms that are very unlikely to be expressed to a similar extent in wild-type controls. At baseline, HO-1 knockouts suffer from chronic anemia and a marked dysregulation of heme catabolism, resulting in a sevenfold increase in plasma hemoglobin and a fourfold increase in plasma heme [45]. Bone marrow and splenic macrophages are depleted, likely due to their increased vulnerability to heme uptake during erythrophagocytosis and erythropoiesis. An experimental hematoma in an HO-1 KO mouse will therefore contain less heme than a wild-type hematoma of equal volume, and will be delivered to a macrophage-deficient mouse that has been preconditioned by chronic elevation of extracellular heme. It is perhaps not surprising then, that unconditional HO-1 knockouts sustained less injury and inflammation after collagenase-induced ICH than their wild-type counterparts [46], despite the anti-inflammatory effects of HO-1 in wild-type animals [38, 47, 48].
In contrast to HO-1, HO-2 knockouts breed well and are grossly indistinguishable from wild-type littermates; they do not differ from their wild-type counterparts in any hematological parameter [49]. However, while HO-2 is the predominant neuronal isoform, it is also constitutively expressed in glial and endothelial cells [50, 51], so an unconditional knockout will alter HO activity in multiple cell populations. In contrast to HO-1 KO, HO-2 knockout worsened outcome to a variable extent in the collagenase ICH model [52, 53], but was protective in the blood injection model [54], perhaps due to differences in the predominant injury mechanisms in these models. Collagenase disrupts multiple blood vessels near the injection site, and if sufficiently widespread may lead to ischemia. As mentioned above, generation of a hematoma by autologous blood injection does not reduce blood flow to ischemic levels [8]. The protective effect of HO-2 against collagenase-induced ischemia [55] may negate its deleterious effect against hemoglobin or hemin toxicity, and thereby account for the disparate impact of HO-2 knockout.
Diverging effects of HO on heme toxicity in different CNS cell populations are readily demonstrated in vitro. Primary neurons cultured from fetal mice express little HO-1, but uniformly express HO-2. Generation of reactive oxygen species and cell injury after hemin or hemoglobin treatment was markedly reduced by HO-2 knockout [56, 57], but HO-1 knockout had no effect [58]. However, when experiments were conducted in medium containing iron-poor transferrin (Neurobasal/B27), which protects against the iron-dependent component of heme toxicity [59], HO-2 knockout neurons were conversely more vulnerable to hemin [52]. The latter observation demonstrates that HO activity is beneficial as long as iron can be safely sequestered, likely due to the protective effects of other heme breakdown products, i.e. biliverdin/bilirubin and carbon monoxide. A deleterious effect of HO activity on heme-mediated injury appears to be limited to neurons, which express very little ferritin at baseline or with iron loading [60], and so are selectively vulnerable to low iron concentrations [61]. In contrast, knockout of either HO-1 or HO-2 increased the vulnerability of astrocytes to hemin or hemoglobin even in the absence of iron chelators [62, 63], reflecting their ability to rapidly increase ferritin expression and thereby detoxify iron [64]. HO-1 expression was also protective in models of pure excitotoxic [37] and inflammatory injury [65], which are also relevant injury mechanisms after ICH. The observed effect of unconditional HO knockout or nonselective HO inhibitors after ICH is likely a complex function of the local iron binding capacity and the injury mechanism that predominates in the model. The discordant results reported to date indicate that nonselective modulation of HO expression and activity is not an optimal therapeutic strategy after ICH; a more targeted approach is needed.
Overexpressing HO-1 protects cultured astrocytes
In mixed glial cultures containing >90% GFAP+ astrocytes, HO-1 expression and activity were increased four to six-fold by adenoviral gene transfer driven by either the CMV or GFAP promoters, with a transfection efficiency exceeding 80% [36, 66]. These cells were then protected from toxic concentrations of hemin, with significant reduction in protein carbonylation and cell death. While these studies provided a useful proof of concept, CNS gene therapies present major and perhaps unsolvable challenges related to vector delivery, toxicity, and timely gene expression, and may be difficult to implement or even test within hours of hemorrhagic stroke. A low molecular weight compound with CNS bioavailability that increases endogenous HMOX1 transcription may be preferred.
Nrf2 activation increases HO-1 and protects against hemin
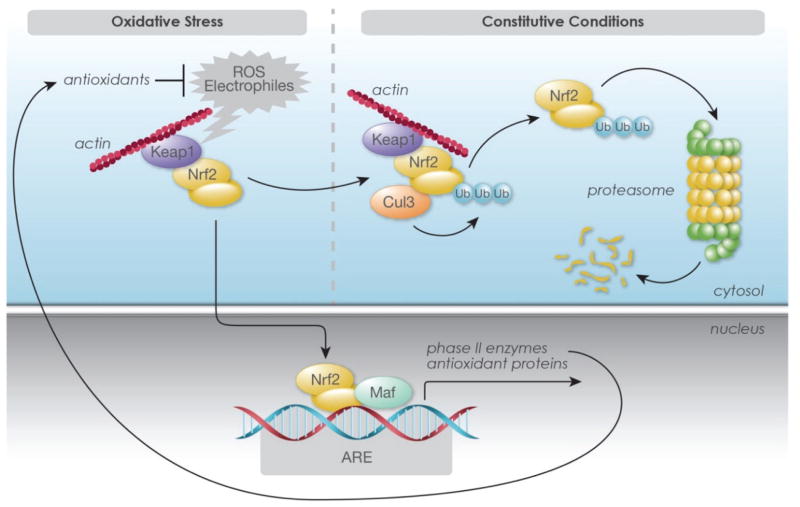
HO-1 expression is tightly regulated in cultured astrocytes. Basal levels are quite low but are increased within a few hours by hemin, hemoglobin or other oxidants, with rapid enzyme turnover [67–69]. Expression is positively regulated at the transcriptional level by nuclear factor erythroid-2 related factor 2 (Nrf2) (Fig. 1, see Baird and Dinkova-Kostova for review [70]). Under normal cell conditions, Nrf2 forms a complex with actin-bound Kelch like-ECH-associated protein 1 (Keap1) and Cullin 3. This binding sequesters it in the cytoplasm, where it is ubiquitinated and rapidly degraded by the 26S proteasome, resulting in low steady-state Nrf2 levels. The sulfhydryl groups of Keap1 cysteine residues are very efficient sensors of oxidative and electophilic stress, and their oxidation inhibits Nrf2 ubiquitination, permitting nuclear transport. After heterodimerization with small Maf proteins, the complex binds to antioxidant response elements (ARE) of HMOX1 and several other genes encoding proteins that may be beneficial after ICH (Table 1). This process is negatively regulated by Bach1, which competes with Nrf2 for MafK binding sites. Bach1-Maf heterodimers bind to HMOX1 enhancer sites and repress transcription. At least in some cell populations, release of Bach1 repression is a prerequisite for HMOX1 transcription [71].
Figure 1.
Schematic representation of Nrf2 activation by oxidative or electrophilic stress. Under constitutive conditions, Nrf2 binds to kelch-like ECH-associated protein 1 (Keap1) and is sequestered in the cytoplasm, where it is ubiquitinated and degraded. Reactive oxygen species (ROS) or electrophiles interact with Keap1 cysteine residues and mediate dissociation of Nrf2-Keap1 complex. This results in ubiquitination and proteasomal degradation of Keap1, while Nrf2 is stabilized and translocated to the nucleus. After heterodimerization with other transcription factors such as Maf, it binds to antioxidant response elements (ARE) in the promoter regions of heme oxygenase-1 and other target genes and activates transcription. ©Cayman Chemical, with permission.
Table 1.
Partial list of proteins upregulated by Nrf2 activation that may be relevant to intracerebral hemorrhage.
| Protein | Activity |
|---|---|
| Heme oxygenase-1 | Heme breakdown, CO and bilirubin production |
| H and L-Ferritin | Sequester and detoxify free iron |
| Haptoglobin | Binds hemoglobin |
| Metallothioneins | Metal-binding and detoxification |
| Thioredoxins | Direct antioxidants |
| Glutamate-cysteine ligase | Glutathione synthesis |
| Glutathione reductase | Reduces oxidized glutathione |
| Glucose-6-P dehydrogenase | NADPH generation |
| Catalase | Direct antioxidant |
| Superoxide dismutase | Direct antioxidant |
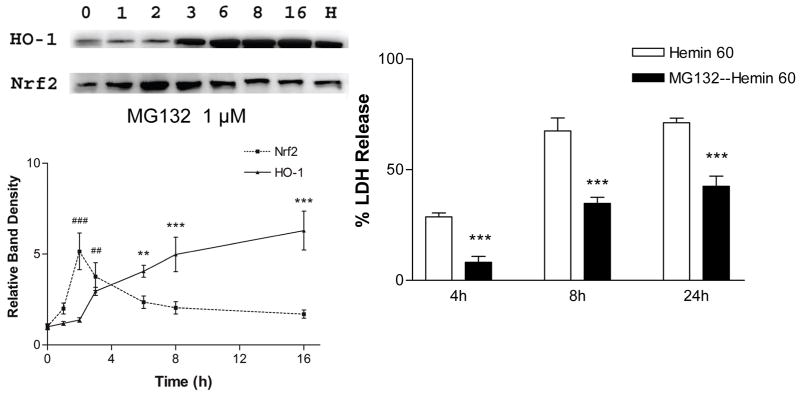
Nrf2 is rapidly increased in cultured cells by selective proteasome inhibitors, but not by other protease inhibitors [72]. MG132 is a widely used peptide aldehyde proteasome inhibitor (Z-Leu-Leu-Leu-CHO) that reversibly binds to the chymotrypsin-like site in the β subunit of the 20S core of the 26S proteasome complex [73]. When added to the medium of cortical mixed glial cultures (>90% GFAP+), it increased Nrf2 levels with an onset time of one hour and a peak effect at two hours, to a level that was five-fold that of vehicle-treated control cultures. HO-1 expression was significantly increased by 3 hours and remained elevated through the subsequent 13 hour observation period, yielding a six-fold increase over controls (Fig. 2) [74]. Cultures pretreated with MG132 were significantly protected from toxic concentrations of hemin. These results provided the first evidence that Nrf2 activation was protective in a model that was relevant to intracerebral hemorrhage. However, since Nrf2 regulates the expression of multiple antioxidant proteins as noted above, selectivity for HO-1 was not demonstrated.
Figure 2.
Proteasome inhibitor MG132 increases HO-1 expression and protects astrocytes from hemin. Glial cultures (>90% GFAP+) were treated with 1 μM MG-132 for indicated intervals; HO-1 and Nrf2 protein levels in lysates were then detected by Western blot analysis. “H” is protein from culture exposed to hemin (positive control). Glia pretreated with MG132 for 16 hours sustained less cell death after 4–24 h exposure to 60 μM hemin. From Chen and Regan, Current Neurovascular Research 2(3):189–96, with permission.
Use of proteasome inhibitors in critically ill stroke patients may be limited by toxicity, which proceeds via multiple mechanisms and produces apoptotic, necrotic and autophagic cell death. This toxicity has led to development of these compounds as antitumor agents, with FDA approval of first-in-class bortezomib in 2008 to treat multiple myeloma [75, 76]. The search for alternate Nrf2 activators that are safe and effective has focused on compounds with a prior history of human use as pharmaceuticals for other indications or dietary consumption. One such compound is hemin, which has been the mainstay of treatment of acute porphyria attacks for several decades due to its feedback inhibition of δ-aminolevulinic acid synthase [77]. Although quite toxic when injected directly into the brain in concentrated solutions [78], low micromolar concentrations of hemin or a hemoprotein such as hemoglobin have potent conditioning effects [44, 69]. As an inducer of HO-1, hemin may have an advantage over other Nrf2 activators because it acts on both transcriptional activation and repression, accomplished via two related mechanisms. First, it increases nuclear translocation of Nrf2 and binding of Nrf2-Maf heterodimers to the HO-1 ARE, likely mediated by dissociation from Keap1 in an oxidative environment [79, 80]. Second, heme binds to Bach1, the primary HO-1 transcription repressor, displacing it from Maf proteins at upstream enhancer regions of the HO-1 gene [81, 82]. Subsequent Nrf2-Maf heterdimerization then facilitates transcription. In addition, hemin positively regulates expression of the iron binding protein ferritin, again by complementary mechanisms [83]. In addition to Nrf-2 mediated transcriptional activation, it antagonizes the binding of iron regulatory proteins to the iron responsive element of ferritin mRNA, thereby releasing their translational block [84].
A protective effect of hemin preconditioning was first reported in vitro by Balla et al [43]. Cultured aortic endothelial cells briefly treated with a nontoxic concentration of hemin became highly resistant to oxidative injury 16 hours later. This was associated with increased expression of HO-1, the inducible HO isoform, and ferritin. A very similar effect was subsequently reported in cultured CNS cells. Mixed glial cultures (>90% GFAP+ astrocytes) treated with 3–5 μM hemin or hemoglobin sustained no injury, and increased expression of heme oxygenase-1 and ferritin within two hours [44, 69]. These cells were then very resistant to hemin at concentrations (30–100 μM) toxic to cells that were not preconditioned. This effect could be prevented by blocking synthesis of both HO-1 and ferritin, and was reduced by protoporphyrin HO activity inhibitors. It is also noteworthy that the iron chelator deferoxamine, which is currently in clinical trials for ICH, also blocked the protective effect of heme preconditioning [85]. While having no effect on HO-1 expression, deferoxamine prevented ferritin upregulation. Taken together, these observations indicate that hemin protects cells by increasing expression of both HO-1 and ferritin, but neither protein alone is sufficient. Furthermore, they suggest that any benefit provided by hemin could be negated by continuous deferoxamine infusion. The compatibility of deferoxamine with other Nrf2 activators has not been defined.
Nrf2 knockout or antagonism worsens injury
A protective effect of Nrf2 in vivo after experimental ICH was first demonstrated by Wang et al. [86]. Nrf2 knockout mice are viable, although they do differ from wild-type counterparts in several hematopoietic parameters, most notably chronic anemia due to immune-mediated hemolysis [87]. Although this anemia would result in lower heme content in an induced intracerebral hematoma, Nrf2 knockout mice sustained greater neurological injury than wild-type mice in the collagenase ICH model. An increase in neutrophil infiltration, oxidative DNA damage, cytochrome C release, and lesion volume was also reported in knockouts. Exacerbation of neurological injury in knockouts was also reported by Zhao et al., using the blood injection ICH model [88].
Retinoic acid receptor-alpha (RARα) agonists decrease transcription of ARE-regulated genes in an oxidative environment by facilitating the formation of Nrf2-RARα complexes that cannot activate the ARE enhancer [89]. Consistent with the effect of Nrf2 knockout, rats treated with retinoic acid sustained greater neurological deficits after blood injection ICH, associated with reduced HO-1 expression [90, 91]. The multiple independent observations that ICH outcome was inversely related to Nrf2 expression and binding activity provided the rational basis for further testing of small molecule Nrf2 activators in ICH models.
Nrf2 activator testing in vivo
Protection by pre or post-injury treatment with Nrf2 activators has been reported in several acute CNS injury models, including both the collagenase and blood injection ICH models (Table 2). Of particular interest are the two compounds already in clinical use for other indications that may be repurposed for treatment of hemorrhagic stroke.
Table 2.
Studies testing Nrf2 activators in rodent blood injection or collagenase ICH models, demonstrating consistent benefit. TBHQ: tert-butylhydroquinone
| Drug | Species | Model | Reference |
|---|---|---|---|
| Sulforaphane | Rat, Mouse | Blood Inj | Zhao et al, Stroke 38:3280–6, 2007 |
| Curcumin | Mouse | Both | King et al., J Neurosurg 115:116–23, 2011 |
| Curcumin | Mouse | Blood Inj | Sun et al, J Neurotrauma 28:2513–21, 2011 |
| Hemin | Mouse | Both | Lu et al, Neurobiol Dis 70:245–51, 2014 |
| Dimethyl fumarate | Rat, Mouse | Blood Inj | Zhao et al, Stroke 46:1923–8, 2015 |
| Dimethyl fumarate | Mouse | Both | Iniaghe et al., Neurobiol Dis 82:349–58, 2015 |
| Sulforaphane | Rat | Blood Inj | Yin et al, Drug Dis Dev Ther 9:5973–86, 2015 |
| TBHQ | Mouse | Collagenase | Sukumari-Ramesh et al, J Mol Neurosci 58:525–531, 2016 |
Hemin
Hemin has been in clinical use for several decades to treat attacks of acute porphyria [92]. It is usually administered at a dose of 3–4 mg/kg i.v. daily for four consecutive days, although longer courses have been described. The major adverse effects are phlebitis and a transient coagulopathy, which are likely not due to hemin itself but to degradation products that spontaneously form when the lyophilized compound is dissolved in water [93]. Both phlebitis and coagulopathy are attenuated or prevented when hemin is stabilized by reconstitution in an albumin solution. A single case of acute but reversible kidney injury has also been reported when a large dose (1000 mg) was rapidly infused [94].
Initial preclinical studies of hemin for treatment of acute CNS injury tested its effects in models of acute spinal cord injury and global ischemia after cardiac arrest. Yamauchi and colleagues [95] pretreated mice with 0.45 μmoles/kg (0.29 mg/kg) hemin i.p. 24 hours before a moderate spinal cord contusion injury. This single injection alone was sufficient to increase spinal cord HO-1 expression, which was localized to the vicinity of blood vessels; the specific cell populations upregulating HO-1 were not defined. At 24 hours after trauma, blood-spinal cord barrier disruption and neutrophil infiltration were both significantly reduced in hemin-pretreated animals compared with vehicle controls. Zhang et al. [96] subsequently pretreated rats with 23 μmoles/kg (15 mg/kg) hemin i.p 12 hours before asphyxial cardiac arrest and resuscitation. Brain water content 1 hour after return of spontaneous circulation was significantly reduced in hemin-pretreated animals compared with untreated controls. Hemin pretreatment also increased CA1 neuronal survival at 4 and 14 days and improved neurological deficit scores.
These key studies demonstrated that peripheral administration of hemin before an acute traumatic or ischemic CNS insult reduced cell injury while improving barrier function and behavioral outcome. The clinical utility of hemin pretreatment is obviously quite limited, although it may be relevant to ischemia and trauma associated with vascular and neurosurgical procedures. Two subsequent studies suggest that initiating hemin therapy after acute traumatic spinal cord injury or ischemic stroke may offer benefit, although the time windows tested were brief. Diaz-Ruiz et al. treated rats with 0.45 μmoles/kg hemin i.p. at 2 and 8 hours after spinal cord contusion. This was sufficient to increase spinal cord HO activity 7.2-fold compared with vehicle-treated animals, and was associated with reduced tissue injury and improved motor scores [97]. Zhang et al. reported that hemin administered as a single 50 mg/kg dose one hour after permanent middle cerebral artery occlusion reduced mean infarct volume and neurological deficit scores compared with untreated controls [98].
Since a hematoma contains millimolar concentrations of heme, the benefit of administering hemin after ICH may not be apparent. Timing is likely the critical factor that determines its efficacy. After hemorrhage, heme is initially sequestered within the hydrophobic pockets of hemoglobin, which itself is sequestered within erythrocytes [15]. This heme is not available to provide a conditioning stimulus until erythrocytes lyse and cell-free hemoglobin oxidizes to methemoglobin, which has a lower affinity for its heme moieties and releases them to protein and lipid binding sites [18]. The entire process does not initiate for at least several hours and probably requires 2–3 days [99]. However, once it begins, toxic quantities of extracellular heme accumulate due to its very high concentration (~20 mM) within erythrocytes [19]. Delivery of a low, nontoxic concentration of hemin to perihematomal tissue prior to hemolysis provides a preconditioning stimulus, increasing cellular resistance to subsequent toxic hemin challenge. Since clinical ICH is rapidly symptomatic (headache, nausea, hemiparesis, altered mental status) and prompts patients to seek medical attention, this disease process may be particularly well-suited to a hemin conditioning approach.
Consistent with observations in spinal cord injury studies, mice injected on consecutive days with a single daily dose of hemin (26 mg/kg) increased brain HO-1 expression in perivascular cells [100]. Initiating treatment with hemin at 1–3 hours after experimental ICH modeled by either blood or collagenase injection improved barrier function as quantified by Evans blue and low molecular weight FITC dextran assays. Improvement was also noted in perihematomal cell viability, brain water content and neurological function. Hemin therapy therefore has consistent efficacy in hemorrhagic, ischemic, and traumatic CNS injury models.
Dimethyl fumarate
A mixture of fumaric acid esters including dimethyl fumarate (DMF) has been in clinical use since 1994 as an oral treatment for psoriasis (Fumaderm®), and an acceptable safety profile has been established with long-term use [101]. Purified DMF administered in a delayed-release formulation was subsequently demonstrated in Phase 3 clinical trials to reduce the relapse rate and MRI lesion frequency in patients with relapsing-remitting multiple sclerosis, while also increasing time to disability progression [102, 103]. Like Fumaderm®, adverse effects have been largely limited to flushing and mild to moderate gastrointestinal symptoms. However, fatal progressive multifocal leukoencephalopathy associated with moderate to severe lymphopenia has been reported in multiple sclerosis and psoriasis patients after long-term treatment with DMF [104, 105].
When administered orally, DMF is rapidly converted in the small bowel to monomethyl fumarate (MMF), its active metabolite [106]. DMF has similar efficacy when administered to rodents by either oral gavage or intraperitoneal injection [107], but safety of percutaneous administration of either DMF or MMF has not been established. Fumaric acid esters are electrophilic compounds that covalently modify cysteine residues of Keap 1 via a Michael addition, particularly Cys 151 [108]. This modification stabilizes Nrf2 and promotes its nuclear translocation and transcription of HMOX1 and other antioxidant genes relevant to heme-mediated injury, including haptoglobin, hemopexin and H-ferritin [109]. Although the benefits of DMF have been linked to Nrf2 activation in several models, it also has potent immunomodulatory effects that are not mediated by Nrf2 activation, and it was equally protective in wild-type and Nrf2 knockout mice in an experimental autoimmune encephalomyelitis (EAE) model [110]. DMF regulates transcription via both Nrf2-dependent and Nrf-2 independent mechanisms, which may be tissue-specific [111].
DMF has been investigated in rodent ICH models by two research groups, with largely concordant results. Zhao et al. reported that i.p injection of 15 mg/kg DMF administered at two hours after striatal blood injection and then orally twice daily for three days reduced early neurological deficits and brain edema in rats, while increasing hematoma resolution [112]. In order to mechanistically link this effect with Nrf2 activation, additional experiments were conducted in wild-type and Nrf2 knockout mice. In wild-type mice, 15 mg/kg i.p. beginning 24 hours after blood injection and repeated daily for 3 days was sufficient to increase brain expression of several Nrf2 target genes at 48 hours after ICH, including HO-1, catalase, haptoglobin, and CD163, the receptor for hemoglobin and the hemoglobin-haptoglobin complex. Upregulation of these proteins was attenuated in Nrf2 knockout mice. Consistent with mitigation of the inflammatory response initiated by blood injection, DMF also reduced brain expression of iNOS and IL-1β while increasing IL-10. Surprisingly, the latter effects were also observed in Nrf2 knockouts, again indicating a mechanism independent of Nrf2 activation. However, Nrf2 activation mediated the improvement in functional outcome in DMF-treated mice in the week after hemorrhage, since it was not observed in knockouts. The lengthy therapeutic window (24 hours) identifies DMF as an excellent candidate for further clinical development, if confirmed in other models.
Iniaghe et al. evaluated low and high dose (10 mg/kg and 100 mg/kg) DMF in mice using both the collagenase and blood injection ICH models [113]. At the lower dose it was ineffective. However, 100 mg/kg i.p. administered one hour after striatal injection decreased neurological deficits and brain water content at 24–72 in both models. In the collagenase model, perihematomal microglial activation and expression of the adhesion molecule ICAM1 were reduced, and phosphorylated Nrf2 and MAFG were increased. However, in contrast to observations by Zhao et al., DMF did not accelerate hematoma resolution, which was evaluated only in the collagenase model. Casein kinase-2 inhibitors or MAFG siRNA pretreatment attenuated the effect of DMF on brain edema and neurological deficits, suggesting that it may be acting via casein kinase 2 phosphorylation of Nrf2.
Other Nrf2 activators
Beneficial effects of several Nrf2 activators in rodent ICH models have been reported. Some of these compounds were isolated from commonly consumed foods, and are currently in human use in an uncontrolled fashion as over-the-counter dietary supplements. However, their safety after parenteral administration at the relatively high doses needed in rodent studies has not been established, and they do not offer the efficiencies inherent in repurposing of drugs that are approved for other indications, which are already manufactured in pharmaceutical grade.
Sulforaphane (SFN) is the electrophilic isothiocyanate breakdown product of glucoraphanin, which is found in cruciferous vegetables such as broccoli, cauliflower and Brussels sprouts. The physical damage produced by chewing mixes glucoraphanin with myrosinase, which catalyzes its hydrolysis. SFN is available in microgram quantities in health food stores and online, without any proven efficacy for any indication. When administered to cultured cells, it reacts with cysteine 151 of Keap1 and thereby blocks Keap1-mediated Nrf2 degradation [114]. Rats receiving 5 mg/kg i.p. at 30 min after striatal blood injection had significantly lower neurological deficit scores at 10 days, associated with increased Phase II enzyme expression and reduced protein and lipid oxidation [88]. Neurological deficits were also mitigated in wild-type but not Nrf2 KO mice. The efficacy of SFN was independently confirmed using a lower dose (2 mg/kg/day i.p.) and a rat blood injection ICH model [91].
Curcumin, an electrophilic polyphenol isolated from the rhizomes of Curcuma longa (turmeric), has been consumed in Asia for millennia as a curry spice and herbal medicine. Like SFN, it is available in capsule form as a dietary supplement, although bioavailability after oral intake is very poor [115]. Curcumin is a pleiotropic molecule that modulates multiple signaling pathways, accounting for its putative anti-inflammatory, antioxidant, antineoplastic and antimicrobial properties [116]. However, its induction of HO-1 appears to be primarily mediated by releasing Keap1 inhibition of Nrf2 nuclear import, likely by direct interaction with Keap1 cysteine residues [117]. King et al. reported that 150 mg/kg curcumin administered i.p coincident with striatal collagenase injection attenuated edema, blood-brain barrier disruption, and neurological deficits in the first three days after ICH [118]. Hematoma volume was also reduced in both the collagenase and blood injection models, with a time window of three hours in the former. Using exclusively the blood injection ICH model, Sun et al. reported that curcumin administration at 15 minutes or 2 hours improved gait and neurological scores at 1–3 days, and also decreased blood-brain barrier injury and edema [119]. Subsequent studies independently confirmed these observations, and also demonstrated that curcumin reduced microglial activation, inflammatory cytokine production, lymphocyte infiltration and expression of aquaporin 4 and aquaporin 9 after experimental ICH [120–122].
Tert-butylhydroquinone (TBHQ) becomes electrophilic only when oxidized to tert-butylbenzoquinone, which then covalently binds to multiple cysteine residues of Keap1, activates Nrf2, and potently induces Phase II enzymes [123]. Unlike electrophilic Nrf2 activators, TBHQ does not interact with thiol groups or glutathione per se. It may therefore offer the advantage of less systemic toxicity, particularly if activated only or predominantly in the oxidative environment of its hemorrhagic target [124]. TBHQ 50 mg/kg i.p. divided into three doses over 24 hours beginning one hour after collagenase injection was sufficient to increase the DNA binding activity of Nrf2 in the mouse striatum [125]. Compared with vehicle, TBHQ treatment reduced perihematomal protein oxidation, microglial activation, IL-1β expression, and neuronal degeneration, and improved neurological outcome.
Cellular targets of Nrf2 activators
SFN is a lipophilic, low molecular weight compound that is widely distributed after systemic administration, but low brain tissue concentrations indicate a limited ability to cross an intact blood-brain barrier [126]. Although it is capable of inducing a Phase II response in the rodent brain after i.p. administration, it appears to be selective for astrocytes. At 16 hours after injection of high dose (50 mg/kg i.p.) SFN, Jazwa et al. [127] reported a modest ~1.5 fold increase in striatal HO-1 protein expression in mice by immunoblot analysis. However, immunostaining demonstrated that this increase was completely limited to GFAP+ astrocytes, with no change in expression in endothelial cells, microglia, or dopaminergic neurons. Approximately 30% of striatal astrocytes expressed HO-1 at this time point, compared with < 2% of saline-treated controls. Using a lower SFN dose (5 mg/kg i.p.) administered 1 hour before middle cerebral artery occlusion (MCAo)-induced stroke in rats, Alfieri et al. reported that SFN increased HO-1 expression in perivascular astrocytes in tissue surrounding the infarction, enhancing the effect of ischemia alone [128]. SFN-treated rats sustained less blood-brain barrier disruption, lesion progression, and neurological deficits. Perivascular astrocyte expression of HO-1 after SFN 5 mg/kg was also observed by Zhao et al., although cortical neuron expression was also reported [129].
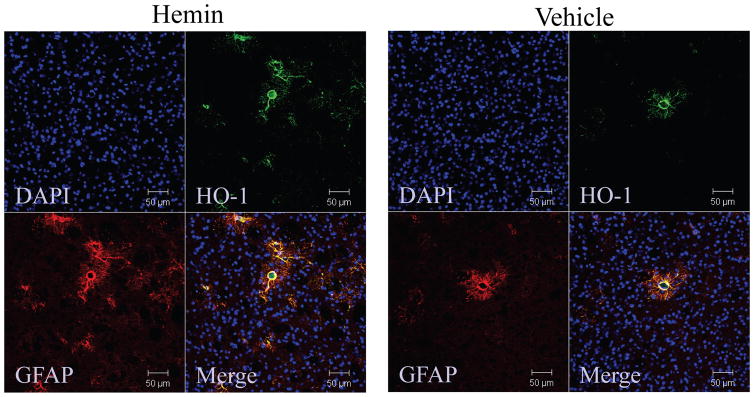
After parenteral administration, hemin binds to hemopexin or albumin and is largely cleared by the liver; only a minimal fraction is recovered from the brain [130]. However, this is apparently sufficient to induce HO-1 in the CNS, but expression is localized to the microvasculature [95, 100]. The latter studies did not determine the cell population expressing HO-1. We have recently observed that mice injected with 4 mg/kg hemin i.p. daily for 3 days overexpress HO-1 in perivascular astrocytes, mimicking the effect of SFN (Fig. 3).
Figure 3.
Systemic hemin treatment increases astrocyte HO-1 expression. Sections from mice treated with 4 mg/kg hemin or vehicle i.p. daily for 3 days, stained with antibodies to HO-1 and GFAP.
The selectivity of Nrf2 activators for astrocytes is consistent with observations that the Nrf2 pathway is substantially more active in this cell population than in neurons (see Baxter and Hardingham [131] for recent review). In cortical cell cultures under normal conditions, Nrf2 expression in astrocytes exceeds that in neurons by 2–3 orders of magnitude [132]. It is therefore not surprising that astrocytes robustly induce HO-1 and ferritin after hemin or hemoglobin treatment, while weaker expression is observed in neurons [44, 69, 133]. In vivo, even the profound oxidative stress produced by CNS hemorrhage induces HO-1 that is largely limited to glial cells [134, 135]. Nevertheless, Nrf2 activators clearly protect neurons in a variety of stroke and neurodegenerative disease models (Table 2), see also [136, 137]. Nrf2 overexpression in astrocytes via GFAP promoter-driven transgene expression provides greater specificity than treatment with activators, and is likewise sufficient to protect neurons [138, 139]. The precise signaling pathways that mediate the neuroprotective effect of astrocytes on neurons have not yet been defined. Localization of HO-1 expression to perivascular astrocytes after parenteral administration of Nrf2 activators suggests a primary benefit to barrier or microvascular function after acute injury [95, 100, 128, 140, 141].
Astrocyte HO-1 overexpression improves ICH outcome
In addition to activating Nrf2-regulated signaling, all activators have multiple off-target effects. Both agents currently in clinical use, hemin and DMF, bind nonselectively to thiol groups, a property which has been utilized in experimental models to deplete functional glutathione [142–144]. Adding to this nonspecificity, Nrf2 activation per se may regulate the expression of up to 600 target genes, based on ChIP-Seq and microarray analyses [145]. Consistent with these analyses, administration of hemin or DMF to mice alters the transcription of a plethora of genes via both Nrf2-dependent and independent mechanisms [111, 146]. Although astrocyte HO-1 overexpression and Nrf2 activators have similar effects on heme-mediated injury in vitro [36, 74], other proteins may contribute or predominate in a more complex in vivo environment.
In order to determine if selective astrocyte HO-1 per se is protective after ICH, transgenic mice expressing human HO-1 driven by the GFAP promoter were tested in the blood injection ICH model [147]. HO-1 expression in ipsilateral striata at 7 hours after blood injection was increased over sevenfold in transgenics, and was localized predominantly to perivascular astrocytes. A dramatic reduction in mortality (34.8% v. 0%) was observed after experimental ICH in transgenics, associated with increased striatal cell viability and reduced blood-brain barrier disruption and neurological deficits. These observations demonstrate that astrocyte HO-1 overexpression per se is robustly protective after ICH, and highlight the therapeutic potential of drug therapies that activate the Nrf2-HO-1 axis in this cell population.
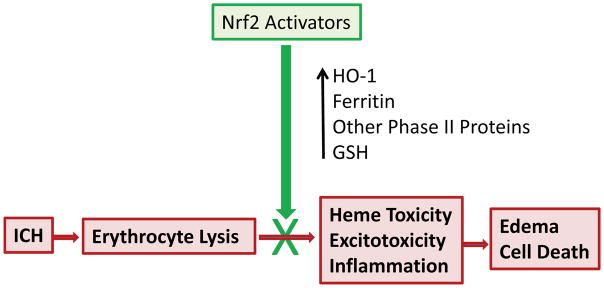
Conclusion
Considerable experimental evidence indicates that systemic treatment with Nrf2 activators after ICH is sufficient to increase expression of HO-1 and other Phase II proteins that may attenuate perihematomal injury cascades (Fig. 4). Nrf2 activators have been tested in eight preclinical ICH trials to date, using both the blood injection and collagenase models in rats and mice. All eight have reported positive effects, providing a compelling proof of concept. Two of the agents used in these studies, dimethyl fumarate and hemin, are already approved for use in other clinical conditions, and pharmaceutical-grade products are available to facilitate the repurposing of these compounds. Future studies should focus on establishing their optimal dose, time window, and treatment duration in rodent and large animal ICH models. Given the poor prognosis and limited therapeutic options available to ICH patients, and the acceptable safety profile of both DMF and hemin established over many years of use, progression to clinical trials should receive the highest priority.
Figure 4.
Summary of effects of Nrf2 activators on injury mediated by ICH and subsequent erythrocyte lysis.
Acknowledgments
This work was supported by NIH grants RO1NS079500, R01NS095205 and R21NS088986. The authors report no conflicts of interest.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor TN, Davis PH, Torner JC, Holmes J, Meyer JW, Jacobson MF. Lifetime cost of stroke in the United States. Stroke. 1996;27:1459–66. doi: 10.1161/01.str.27.9.1459. [DOI] [PubMed] [Google Scholar]
- 3.Broderick JP, Adams HP, Jr, Barsan W, Feinberg W, Feldmann E, Grotta J, Kase C, Krieger D, Mayberg M, Tilley B, Zabramski JM, Zuccarello M. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Statement for Healthcare Professionals From a Special Writing Group of the Stroke Council, American Heart Association. Stroke. 1999;30:905–915. doi: 10.1161/01.str.30.4.905. [DOI] [PubMed] [Google Scholar]
- 4.Rincon F, Mayer SA. The Epidemiology of Intracerebral Hemorrhage in the United States from 1979 to 2008. Neurocrit Care. 2013;19:95–102. doi: 10.1007/s12028-012-9793-y. [DOI] [PubMed] [Google Scholar]
- 5.Mendelow AD, Bullock R, Teasdale GM, Graham DI, McCulloch J. Intracranial haemorrhage induced at arterial pressure in the rat. Part 2: Short term changes in local cerebral blood flow measured by autoradiography. Neurol Res. 1984;6:189–93. doi: 10.1080/01616412.1984.11739688. [DOI] [PubMed] [Google Scholar]
- 6.Bullock R, Mendelow AD, Teasdale GM, Graham DI. Intracranial haemorrhage induced at arterial pressure in the rat. Part 1: Description of technique, ICP changes and neuropathological findings. Neurol Res. 1984;6:184–8. doi: 10.1080/01616412.1984.11739687. [DOI] [PubMed] [Google Scholar]
- 7.Zazulia AR, Diringer MN, Videen TO, Adams RE, Yundt K, Aiyagari V, Grubb RL, Jr, Powers WJ. Hypoperfusion without ischemia surrounding acute intracerebral hemorrhage. J Cereb Blood Flow Metab. 2001;21:804–10. doi: 10.1097/00004647-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Qureshi AI, Wilson DA, Hanley DF, Traystman RJ. No evidence for an ischemic penumbra in massive experimental intracerebral hemorrhage. Neurology. 1999;52:266–72. doi: 10.1212/wnl.52.2.266. [DOI] [PubMed] [Google Scholar]
- 9.Kidwell CS, Saver JL, Mattiello J, Warach S, Liebeskind DS, Starkman S, Vespa PM, Villablanca JP, Martin NA, Frazee J, Alger JR. Diffusion-perfusion MR evaluation of perihematomal injury in hyperacute intracerebral hemorrhage. Neurology. 2001;57:1611–7. doi: 10.1212/wnl.57.9.1611. [DOI] [PubMed] [Google Scholar]
- 10.Aguilar MI, Brott TG. Update in intracerebral hemorrhage. Neurohospitalist. 2011;1:148–59. doi: 10.1177/1941875211409050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 12.Regan RF, Panter SS. Hemoglobin potentiates excitotoxic injury in cortical cell culture. J Neurotrauma. 1996;13:223–231. doi: 10.1089/neu.1996.13.223. [DOI] [PubMed] [Google Scholar]
- 13.Jaremko KM, Chen-Roetling J, Chen L, Regan RF. Accelerated Hemolysis and Neurotoxicity in Neuron-Glia-Blood Clot Co-cultures. J Neurochem. 2010;114:1063–73. doi: 10.1111/j.1471-4159.2010.06826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J, Hua Y, Keep RF, Nakemura T, Hoff JT, Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke. 2003;34:2964–2969. doi: 10.1161/01.STR.0000103140.52838.45. [DOI] [PubMed] [Google Scholar]
- 15.Hebbel RP, Eaton JW. Pathobiology of heme interaction with the erythrocyte membrane. Sem Hematol. 1989;26:136–149. [PubMed] [Google Scholar]
- 16.Alyash A. Redox and radical reactions of hemoglobin solutions: toxicities and protective strategies. In: Winslow R, editor. Blood Substitutes. Academic Press; London: 2006. pp. 197–205. [Google Scholar]
- 17.Bradley WG., Jr MR appearance of hemorrhage in the brain. Radiology. 1993;189:15–26. doi: 10.1148/radiology.189.1.8372185. [DOI] [PubMed] [Google Scholar]
- 18.Bunn HF, Jandl JH. Exchange of heme along hemoglobins and between hemoglobin and albumin. J Biol Chem. 1968;243:465–475. [PubMed] [Google Scholar]
- 19.Letarte PB, Lieberman K, Nagatani K, Haworth RA, Odell GB, Duff TA. Hemin: levels in experimental subarachnoid hematoma and effects on dissociated vascular smooth muscle cells. J Neurosurg. 1993;79:252–255. doi: 10.3171/jns.1993.79.2.0252. [DOI] [PubMed] [Google Scholar]
- 20.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford University Press; Oxford: 1999. [Google Scholar]
- 21.Gutteridge JMC, Smith A. Antioxidant protection by haemopexin of haem-stimulated lipid peroxidation. Biochem J. 1988;256:861–865. doi: 10.1042/bj2560861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vincent SH, Grady RW, Shaklai N, Snider JM, Muller-Eberhard U. The influence of heme-binding proteins in heme-catalyzed oxidations. Arch Biochem Biophys. 1988;265:539–50. doi: 10.1016/0003-9861(88)90159-2. [DOI] [PubMed] [Google Scholar]
- 23.Chou AC, Fitch CD. Mechanism of hemolysis induced by ferriprotoporphyrin IX. J Clin Invest. 1981;68:672–677. doi: 10.1172/JCI110302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldstein L, Teng ZP, Zeserson E, Patel M, Regan RF. Hemin induces an iron-dependent, oxidative injury on human neuron-like cells. J Neurosci Res. 2003;73:113–121. doi: 10.1002/jnr.10633. [DOI] [PubMed] [Google Scholar]
- 25.Figueiredo RT, Fernandez PL, Mourao-Sa DS, Porto BN, Dutra FF, Alves LS, Oliveira MF, Oliveira PL, Graca-Souza AV, Bozza MT. Characterization of heme as activator of Toll-like receptor 4. J Biol Chem. 2007;282:20221–9. doi: 10.1074/jbc.M610737200. [DOI] [PubMed] [Google Scholar]
- 26.Lai TW, Zhang S, Wang YT. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol. 2014;115:157–88. doi: 10.1016/j.pneurobio.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Qureshi AI, Ali Z, Suri MF, Shuaib A, Baker G, Todd K, Guterman LR, Hopkins LN. Extracellular glutamate and other amino acids in experimental intracerebral hemorrhage: an in vivo microdialysis study. Crit Care Med. 2003;31:1482–9. doi: 10.1097/01.CCM.0000063047.63862.99. [DOI] [PubMed] [Google Scholar]
- 28.D’Eufemia P, Finocchiaro R, Lendvai D, Celli M, Viozzi L, Troiani P, Turri E, Giardini O. Erythrocyte and plasma levels of glutamate and aspartate in children affected by migraine. Cephalalgia. 1997;17:652–7. doi: 10.1046/j.1468-2982.1997.1706652.x. [DOI] [PubMed] [Google Scholar]
- 29.Divino Filho JC, Hazel SJ, Furst P, Bergstrom J, Hall K. Glutamate concentration in plasma, erythrocyte and muscle in relation to plasma levels of insulin-like growth factor (IGF)-I, IGF binding protein-1 and insulin in patients on haemodialysis. J Endocrinol. 1998;156:519–27. doi: 10.1677/joe.0.1560519. [DOI] [PubMed] [Google Scholar]
- 30.Ardizzone TD, Lu A, Wagner KR, Tang Y, Ran R, Sharp FR. Glutamate receptor blockade attenuates glucose hypermetabolism in perihematomal brain after experimental intracerebral hemorrhage in rat. Stroke. 2004;35:2587–91. doi: 10.1161/01.STR.0000143451.14228.ff. [DOI] [PubMed] [Google Scholar]
- 31.Lee ST, Chu K, Jung KH, Kim J, Kim EH, Kim SJ, Sinn DI, Ko SY, Kim M, Roh JK. Memantine reduces hematoma expansion in experimental intracerebral hemorrhage, resulting in functional improvement. J Cereb Blood Flow Metab. 2006;26:536–44. doi: 10.1038/sj.jcbfm.9600213. [DOI] [PubMed] [Google Scholar]
- 32.Sinn DI, Lee ST, Chu K, Jung KH, Song EC, Kim JM, Park DK, Kim M, Roh JK. Combined neuroprotective effects of celecoxib and memantine in experimental intracerebral hemorrhage. Neurosci Lett. 2007;411:238–42. doi: 10.1016/j.neulet.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 33.Terai K, Suzuki M, Sasamata M, Yatsugi S, Yamaguchi T, Miyata K. Effect of AMPA receptor antagonist YM872 on cerebral hematoma size and neurological recovery in the intracerebral hemorrhage rat model. Eur J Pharmacol. 2003;467:95–101. doi: 10.1016/s0014-2999(03)01572-3. [DOI] [PubMed] [Google Scholar]
- 34.Lin S, Yin Q, Zhong Q, Lv FL, Zhou Y, Li JQ, Wang JZ, Su BY, Yang QW. Heme activates TLR4-mediated inflammatory injury via MyD88/TRIF signaling pathway in intracerebral hemorrhage. J Neuroinflammation. 2012;9:46. doi: 10.1186/1742-2094-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ebrahimi S, Jaberi N, Avan A, Ryzhikov M, Keramati MR, Parizadeh MR, Hassanian SM. Role of Thrombin in the Pathogenesis of Central Nervous System Inflammatory Diseases. J Cell Physiol. 2016 doi: 10.1002/jcp.25501. [DOI] [PubMed] [Google Scholar]
- 36.Benvenisti-Zarom L, Regan RF. Astrocyte-specific heme oxygenase-1 hyperexpression attenuates heme-mediated oxidative injury. Neurobiol Dis. 2007;26:688–95. doi: 10.1016/j.nbd.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmad AS, Zhuang H, Dore S. Heme oxygenase-1 protects brain from acute excitotoxicity. Neuroscience. 2006;141:1703–8. doi: 10.1016/j.neuroscience.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 38.Schallner N, Pandit R, LeBlanc R, 3rd, Thomas AJ, Ogilvy CS, Zuckerbraun BS, Gallo D, Otterbein LE, Hanafy KA. Microglia regulate blood clearance in subarachnoid hemorrhage by heme oxygenase-1. J Clin Invest. 2015;125:2609–25. doi: 10.1172/JCI78443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayashi S, Omata Y, Sakamoto H, Higashimoto Y, Hara T, Sagara Y, Noguchi M. Characterization of rat heme oxygenase-3 gene. Implication of processed pseudogenes derived from heme oxygenase-2 gene. Gene. 2004;336:241–50. doi: 10.1016/j.gene.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Miro O, Casademont J, Urbanomarquez A, Cardellach F. Mitochondrial cytochrome C oxidase inhibition during carbon monoxide poisoning. Pharmacology and Toxicology. 1998;82:199–202. doi: 10.1111/j.1600-0773.1998.tb01425.x. [DOI] [PubMed] [Google Scholar]
- 41.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–6. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 42.Santambrogio P, Levi S, Cozzi A, Corsi B, Arosio P. Evidence that the specificity of iron incorporation into homopolymers of human L- and H- chains is conferred by the nucleation and ferroxidase centres. Biochem J. 1996;314:139–44. doi: 10.1042/bj3140139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, Vercellotti GM. Ferritin: A cytoprotective strategem of endothelium. J Biol Chem. 1992;267:18148–18153. [PubMed] [Google Scholar]
- 44.Regan RF, Kumar N, Gao F, Guo YP. Ferritin induction protects cortical astrocytes from heme-mediated oxidative injury. Neuroscience. 2002;113:985–994. doi: 10.1016/s0306-4522(02)00243-9. [DOI] [PubMed] [Google Scholar]
- 45.Fraser ST, Midwinter RG, Coupland LA, Kong S, Berger BS, Yeo JH, Andrade OC, Cromer D, Suarna C, Lam M, Maghzal GJ, Chong BH, Parish CR, Stocker R. Heme oxygenase-1 deficiency alters erythroblastic island formation, steady-state erythropoiesis and red blood cell lifespan in mice. Haematologica. 2015;100:601–10. doi: 10.3324/haematol.2014.116368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, Doré S. Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain. 2007;130:1643–52. doi: 10.1093/brain/awm095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryter SW, Otterbein LE. Carbon monoxide in biology and medicine. Bioessays. 2004;26:270–80. doi: 10.1002/bies.20005. [DOI] [PubMed] [Google Scholar]
- 48.Shih RH, Yang CM. Induction of heme oxygenase-1 attenuates lipopolysaccharide-induced cyclooxygenase-2 expression in mouse brain endothelial cells. J Neuroinflammation. 2010;7:86. doi: 10.1186/1742-2094-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poss KD, Thomas MJ, Ebralidze AK, TJOD, Tonegawa S. Hippocampal long-term potentiation is normal in heme oxygenase-2 mutant mice. Neuron. 1995;15:867–873. doi: 10.1016/0896-6273(95)90177-9. [DOI] [PubMed] [Google Scholar]
- 50.Chen J, Regan RF. Heme oxygenase-2 gene deletion increases astrocyte vulnerability to hemin. Biochem Biophys Res Commun. 2004;318:88–94. doi: 10.1016/j.bbrc.2004.03.187. [DOI] [PubMed] [Google Scholar]
- 51.Basuroy S, Bhattacharya S, Tcheranova D, Qu Y, Regan RF, Leffler CW, Parfenova H. HO-2 provides endogenous protection against oxidative stress and apoptosis caused by TNF-alpha in cerebral vascular endothelial cells. Am J Physiol Cell Physiol. 2006;291:C897–908. doi: 10.1152/ajpcell.00032.2006. [DOI] [PubMed] [Google Scholar]
- 52.Wang J, Zhuang H, Doré S. Heme oxygenase 2 is neuroprotective against intracerebral hemorrhage. Neurobiol Dis. 2006;22:473–476. doi: 10.1016/j.nbd.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 53.Chen-Roetling J, Lu X, Regan KA, Regan RF. A rapid fluorescent method to quantify neuronal loss after experimental intracerebral hemorrhage. J Neurosci Methods. 2013;216:128–36. doi: 10.1016/j.jneumeth.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qu Y, Chen-Roetling J, Benvenisti-Zarom L, Regan RF. Attenuation of oxidative injury after induction of experimental intracerebral hemorrhage in heme oxygenase-2 knockout mice. J Neurosurg. 2007;106:428–35. doi: 10.3171/jns.2007.106.3.428. [DOI] [PubMed] [Google Scholar]
- 55.Dore S, Sampei K, Goto S, Alkayed NJ, Guastella D, Blackshaw S, Gallagher M, Traystman RJ, Hurn PD, Koehler RC, Snyder SH. Heme oxygenase-2 is neuroprotective in cerebral ischemia. Mol Med. 1999;5:656–63. [PMC free article] [PubMed] [Google Scholar]
- 56.Rogers B, Yakopson V, Teng ZP, Guo Y, Regan RF. Heme oxygenase-2 knockout neurons are less vulnerable to hemoglobin toxicity. Free Rad Biol Med. 2003;35:872–881. doi: 10.1016/s0891-5849(03)00431-3. [DOI] [PubMed] [Google Scholar]
- 57.Regan RF, Chen J, Benvenisti-Zarom L. Heme oxygenase-2 gene deletion attenuates oxidative stress in neurons exposed to extracellular hemin. BMC Neurosci. 2004;5:34. doi: 10.1186/1471-2202-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen-Roetling J, Regan RF. Effect of heme oxygenase-1 on the vulnerability of astrocytes and neurons to hemoglobin. Biochem Biophys Res Commun. 2006;350:233–7. doi: 10.1016/j.bbrc.2006.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen-Roetling J, Chen L, Regan RF. Apotransferrin protects cortical neurons from hemoglobin toxicity. Neuropharmacology. 2011;60:423–31. doi: 10.1016/j.neuropharm.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moos T, Morgan EH. The metabolism of neuronal iron and its pathogenic role in neurological disease: review. Ann N Y Acad Sci. 2004;1012:14–26. doi: 10.1196/annals.1306.002. [DOI] [PubMed] [Google Scholar]
- 61.Kress GJ, Dineley KE, Reynolds IJ. The relationship between intracellular free iron and cell injury in cultured neurons, astrocytes, and oligodendrocytes. J Neurosci. 2002;22:5848–55. doi: 10.1523/JNEUROSCI.22-14-05848.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen-Roetling J, Benvenisti-Zarom L, Regan RF. Cultured astrocytes from heme oxygenase-1 knockout mice are more vulnerable to heme-mediated oxidative injury. J Neurosci Res. 2005;82:802–10. doi: 10.1002/jnr.20681. [DOI] [PubMed] [Google Scholar]
- 63.Schipper HM. Heme oxygenase-1: transducer of pathological brain iron sequestration under oxidative stress. Ann N Y Acad Sci. 2004;1012:84–93. doi: 10.1196/annals.1306.007. [DOI] [PubMed] [Google Scholar]
- 64.Hohnholt MC, Dringen R. Uptake and metabolism of iron and iron oxide nanoparticles in brain astrocytes. Biochem Soc Trans. 2013;41:1588–92. doi: 10.1042/BST20130114. [DOI] [PubMed] [Google Scholar]
- 65.Wiesel P, Patel AP, DiFonzo N, Marria PB, Sim CU, Pellacani A, Maemura K, LeBlanc BW, Marino K, Doerschuk CM, Yet S-F, Lee M-E, Perrella MA. Endotoxin-induced mortality is related to increased oxidative stress and end-organ dysfunction, not refractory hypotension, in heme oxygenase-1–deficient mice. Circulation. 2000;102:3015–3022. doi: 10.1161/01.cir.102.24.3015. [DOI] [PubMed] [Google Scholar]
- 66.Teng ZP, Chen J, Chau LY, Galunic N, Regan RF. Adenoviral transfer of the heme oxygenase-1 gene protects cortical astrocytes from heme-mediated oxidative injury. Neurobiol Dis. 2004;17:179–187. doi: 10.1016/j.nbd.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 67.Dwyer BE, Nishimura RN, De Vellis J, Yoshida T. Heme oxygenase is a heat shock protein and PEST protein in rat astroglial cells. Glia. 1992;5:300–5. doi: 10.1002/glia.440050407. [DOI] [PubMed] [Google Scholar]
- 68.Dwyer BE, Nishimura RN, Lu SY. Differential expression of heme oxygenase-1 in cultured cortical neurons and astrocytes determined by the aid of a new heme oxygenase antibody. Response to oxidative stress. Brain Res Mol Brain Res. 1995;30:37–47. doi: 10.1016/0169-328x(94)00273-h. [DOI] [PubMed] [Google Scholar]
- 69.Regan RF, Guo YP, Kumar N. Heme oxygenase-1 induction protects murine cortical astrocytes from hemoglobin toxicity. Neurosci Lett. 2000;282:1–4. doi: 10.1016/s0304-3940(00)00817-x. [DOI] [PubMed] [Google Scholar]
- 70.Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol. 2011;85:241–72. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 71.Reichard JF, Motz GT, Puga A. Heme oxygenase-1 induction by NRF2 requires inactivation of the transcriptional repressor BACH1. Nucleic Acids Res. 2007;35:7074–86. doi: 10.1093/nar/gkm638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stewart D, Killeen E, Naquin R, Alam S, Alam J. Degradation of transcription factor Nrf2 via the ubiquitin-proteasome pathway and stabilization by cadmium. J Biol Chem. 2003;278:2396–402. doi: 10.1074/jbc.M209195200. [DOI] [PubMed] [Google Scholar]
- 73.Cui W, Bai Y, Luo P, Miao L, Cai L. Preventive and therapeutic effects of MG132 by activating Nrf2-ARE signaling pathway on oxidative stress-induced cardiovascular and renal injury. Oxid Med Cell Longev. 2013;2013:306073. doi: 10.1155/2013/306073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen J, Regan RF. Increasing expression of heme oxygenase-1 by proteasome inhibition protects astrocytes from heme-mediated oxidative injury. Curr Neurovasc Res. 2005;2:189–96. doi: 10.2174/1567202054368344. [DOI] [PubMed] [Google Scholar]
- 75.Vlachostergios PJ, Voutsadakis IA, Papandreou CN. Mechanisms of proteasome inhibitor-induced cytotoxicity in malignant glioma. Cell Biol Toxicol. 2013;29:199–211. doi: 10.1007/s10565-013-9248-z. [DOI] [PubMed] [Google Scholar]
- 76.Goldberg AL. Development of proteasome inhibitors as research tools and cancer drugs. J Cell Biol. 2012;199:583–8. doi: 10.1083/jcb.201210077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lamon JM, Frykholm BC, Hess RA, Tschudy DP. Hematin therapy for acute porphyria. Medicine (Baltimore) 1979;58:252–69. doi: 10.1097/00005792-197905000-00005. [DOI] [PubMed] [Google Scholar]
- 78.Huang FP, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J Neurosurg. 2002;96:287–293. doi: 10.3171/jns.2002.96.2.0287. [DOI] [PubMed] [Google Scholar]
- 79.Kim YC, Masutani H, Yamaguchi Y, Itoh K, Yamamoto M, Yodoi J. Hemin-induced activation of the thioredoxin gene by Nrf2. A differential regulation of the antioxidant responsive element by a switch of its binding factors. J Biol Chem. 2001;276:18399–406. doi: 10.1074/jbc.M100103200. [DOI] [PubMed] [Google Scholar]
- 80.Nakaso K, Yano H, Fukuhara Y, Takeshima T, Wada-Isoe K, Nakashima K. PI3K is a key molecule in the Nrf2-mediated regulation of antioxidative proteins by hemin in human neuroblastoma cells. FEBS Lett. 2003;546:181–4. doi: 10.1016/s0014-5793(03)00517-9. [DOI] [PubMed] [Google Scholar]
- 81.Sun J, Hoshino H, Takaku K, Nakajima O, Muto A, Suzuki H, Tashiro S, Takahashi S, Shibahara S, Alam J, Taketo MM, Yamamoto M, Igarashi K. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002;21:5216–24. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun J, Brand M, Zenke Y, Tashiro S, Groudine M, Igarashi K. Heme regulates the dynamic exchange of Bach1 and NF-E2-related factors in the Maf transcription factor network. Proc Natl Acad Sci U S A. 2004;101:1461–6. doi: 10.1073/pnas.0308083100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hintze KJ, Theil EC. DNA and mRNA elements with complementary responses to hemin, antioxidant inducers, and iron control ferritin-L expression. Proc Natl Acad Sci U S A. 2005;102:15048–52. doi: 10.1073/pnas.0505148102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin JJ, Daniels-McQueen S, Patino MM, Gaffield L, Walden WE, Thach RE. Derepression of ferritin messenger RNA by hemin in vitro. Science. 1990;247:74–77. doi: 10.1126/science.2294594. [DOI] [PubMed] [Google Scholar]
- 85.Chen-Roetling J, Sinanan J, Regan RF. Effect of iron chelators on methemoglobin and thrombin preconditioning. Transl Stroke Res. 2013;3:452–9. doi: 10.1007/s12975-012-0195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang J, Fields J, Zhao C, Langer J, Thimmulappa RK, Kensler TW, Yamamoto M, Biswal S, Dore S. Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radic Biol Med. 2007;43:408–14. doi: 10.1016/j.freeradbiomed.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee JM, Chan K, Kan YW, Johnson JA. Targeted disruption of Nrf2 causes regenerative immune-mediated hemolytic anemia. Proc Natl Acad Sci U S A. 2004;101:9751–6. doi: 10.1073/pnas.0403620101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao X, Sun G, Zhang J, Strong R, Dash PK, Kan YW, Grotta JC, Aronowski J. Transcription Factor Nrf2 Protects the Brain From Damage Produced by Intracerebral Hemorrhage. Stroke. 2007;38:3280–3286. doi: 10.1161/STROKEAHA.107.486506. [DOI] [PubMed] [Google Scholar]
- 89.Wang XJ, Hayes JD, Henderson CJ, Wolf CR. Identification of retinoic acid as an inhibitor of transcription factor Nrf2 through activation of retinoic acid receptor alpha. Proc Natl Acad Sci U S A. 2007;104:19589–94. doi: 10.1073/pnas.0709483104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yin XP, Zhou J, Wu D, Chen ZY, Bao B. Effects of that ATRA inhibits Nrf2-ARE pathway on glial cells activation after intracerebral hemorrhage. Int J Clin Exp Pathol. 2015;8:10436–43. [PMC free article] [PubMed] [Google Scholar]
- 91.Yin XP, Chen ZY, Zhou J, Wu D, Bao B. Mechanisms underlying the perifocal neuroprotective effect of the Nrf2-ARE signaling pathway after intracranial hemorrhage. Drug Des Devel Ther. 2015;9:5973–86. doi: 10.2147/DDDT.S79399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dhar GJ, Bossenmaier I, Petryka ZJ, Cardinal R, Watson CJ. Effects of hematin in hepatic porphyria. Further studies. Ann Intern Med. 1975;83:20–30. doi: 10.7326/0003-4819-83-1-20. [DOI] [PubMed] [Google Scholar]
- 93.Anderson KE, Bloomer JR, Bonkovsky HL, Kushner JP, Pierach CA, Pimstone NR, Desnick RJ. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Intern Med. 2005;142:439–50. doi: 10.7326/0003-4819-142-6-200503150-00010. [DOI] [PubMed] [Google Scholar]
- 94.Dhar GJ, Bossenmaier I, Cardinal R, Petryka ZJ, Watson CJ. Transitory renal failure following rapid administration of a relatively large amount of hematin in a patient with acute intermittent porphyria in clinical remission. Acta Med Scand. 1978;203:437–43. doi: 10.1111/j.0954-6820.1978.tb14903.x. [DOI] [PubMed] [Google Scholar]
- 95.Yamauchi T, Lin Y, Sharp FR, Noble-Haeusslein LJ. Hemin induces heme oxygenase-1 in spinal cord vasculature and attenuates barrier disruption and neutrophil infiltration in the injured murine spinal cord. J Neurotrauma. 2004;21:1017–30. doi: 10.1089/0897715041651042. [DOI] [PubMed] [Google Scholar]
- 96.Zhang B, Wei X, Cui X, Kobayashi T, Li W. Effects of heme oxygenase 1 on brain edema and neurologic outcome after cardiopulmonary resuscitation in rats. Anesthesiology. 2008;109:260–8. doi: 10.1097/ALN.0b013e31817f5c2e. [DOI] [PubMed] [Google Scholar]
- 97.Diaz-Ruiz A, Maldonado PD, Mendez-Armenta M, Jimenez-Garcia K, Salgado-Ceballos H, Santander I, Rios C. Activation of heme oxygenase recovers motor function after spinal cord injury in rats. Neurosci Lett. 2013;556:26–31. doi: 10.1016/j.neulet.2013.08.067. [DOI] [PubMed] [Google Scholar]
- 98.Zhang B, Ji X, Zhang S, Ren H, Wang M, Guo C, Li Y. Heminmediated neuroglobin induction exerts neuroprotection following ischemic brain injury through PI3K/Akt signaling. Mol Med Rep. 2013;8:681–5. doi: 10.3892/mmr.2013.1523. [DOI] [PubMed] [Google Scholar]
- 99.Xi GH, Keep RF, Hoff JT. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J Neurosurg. 1998;89:991–996. doi: 10.3171/jns.1998.89.6.0991. [DOI] [PubMed] [Google Scholar]
- 100.Lu X, Chen-Roetling J, Regan RF. Systemic hemin therapy attenuates blood-brain barrier disruption after intracerebral hemorrhage. Neurobiol Dis. 2014;70C:245–251. doi: 10.1016/j.nbd.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hoefnagel JJ, Thio HB, Willemze R, Bouwes Bavinck JN. Long-term safety aspects of systemic therapy with fumaric acid esters in severe psoriasis. Br J Dermatol. 2003;149:363–9. doi: 10.1046/j.1365-2133.2003.05433.x. [DOI] [PubMed] [Google Scholar]
- 102.Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, Yang M, Raghupathi K, Novas M, Sweetser MT, Viglietta V, Dawson KT. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367:1087–97. doi: 10.1056/NEJMoa1206328. [DOI] [PubMed] [Google Scholar]
- 103.Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, Tornatore C, Sweetser MT, Yang M, Sheikh SI, Dawson KT. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367:1098–107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 104.Rosenkranz T, Novas M, Terborg C. PML in a patient with lymphocytopenia treated with dimethyl fumarate. N Engl J Med. 2015;372:1476–8. doi: 10.1056/NEJMc1415408. [DOI] [PubMed] [Google Scholar]
- 105.Hoepner R, Faissner S, Klasing A, Schneider R, Metz I, Bellenberg B, Lukas C, Altmeyer P, Gold R, Chan A. Progressive multifocal leukoencephalopathy during fumarate monotherapy of psoriasis. Neurol Neuroimmunol Neuroinflamm. 2015;2:e85. doi: 10.1212/NXI.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Linker RA, Gold R. Dimethyl fumarate for treatment of multiple sclerosis: mechanism of action, effectiveness, and side effects. Curr Neurol Neurosci Rep. 2013;13:394. doi: 10.1007/s11910-013-0394-8. [DOI] [PubMed] [Google Scholar]
- 107.Kunze R, Urrutia A, Hoffmann A, Liu H, Helluy X, Pham M, Reischl S, Korff T, Marti HH. Dimethyl fumarate attenuates cerebral edema formation by protecting the blood-brain barrier integrity. Exp Neurol. 2015;266:99–111. doi: 10.1016/j.expneurol.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 108.Linker RA, Lee DH, Ryan S, van Dam AM, Conrad R, Bista P, Zeng W, Hronowsky X, Buko A, Chollate S, Ellrichmann G, Bruck W, Dawson K, Goelz S, Wiese S, Scannevin RH, Lukashev M, Gold R. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134:678–92. doi: 10.1093/brain/awq386. [DOI] [PubMed] [Google Scholar]
- 109.Belcher JD, Chen C, Nguyen J, Zhang P, Abdulla F, Nguyen P, Killeen T, Xu P, O’Sullivan G, Nath KA, Vercellotti GM. Control of oxidative stress and inflammation in sickle cell disease with the Nrf2 activator dimethyl fumarate. Antioxid Redox Signal. 2016 doi: 10.1089/ars.2015.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schulze-Topphoff U, Varrin-Doyer M, Pekarek K, Spencer CM, Shetty A, Sagan SA, Cree BA, Sobel RA, Wipke BT, Steinman L, Scannevin RH, Zamvil SS. Dimethyl fumarate treatment induces adaptive and innate immune modulation independent of Nrf2. Proc Natl Acad Sci U S A. 2016;113:4777–82. doi: 10.1073/pnas.1603907113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brennan MS, Patel H, Allaire N, Thai A, Cullen P, Ryan S, Lukashev M, Bista P, Huang R, Rhodes KJ, Scannevin RH. Pharmacodynamics of Dimethyl Fumarate Are Tissue Specific and Involve NRF2-Dependent and -Independent Mechanisms. Antioxid Redox Signal. 2016 doi: 10.1089/ars.2015.6622. [DOI] [PubMed] [Google Scholar]
- 112.Zhao X, Sun G, Zhang J, Ting SM, Gonzales N, Aronowski J. Dimethyl fumarate protects brain from damage produced by intracerebral hemorrhage by mechanism involving Nrf2. Stroke. 2015;46:1923–8. doi: 10.1161/STROKEAHA.115.009398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Iniaghe LO, Krafft PR, Klebe DW, Omogbai EK, Zhang JH, Tang J. Dimethyl fumarate confers neuroprotection by casein kinase 2 phosphorylation of Nrf2 in murine intracerebral hemorrhage. Neurobiol Dis. 2015;82:349–58. doi: 10.1016/j.nbd.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol. 2003;23:8137–51. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kurita T, Makino Y. Novel curcumin oral delivery systems. Anticancer Res. 2013;33:2807–21. [PubMed] [Google Scholar]
- 116.Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–95. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.King MD, McCracken DJ, Wade FM, Meiler SE, Alleyne CH, Jr, Dhandapani KM. Attenuation of hematoma size and neurological injury with curcumin following intracerebral hemorrhage in mice. J Neurosurg. 2011;115:116–23. doi: 10.3171/2011.2.JNS10784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sun Y, Dai M, Wang Y, Wang W, Sun Q, Yang GY, Bian L. Neuroprotection and sensorimotor functional improvement by curcumin after intracerebral hemorrhage in mice. J Neurotrauma. 2011;28:2513–21. doi: 10.1089/neu.2011.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang Z, Zhao T, Zou Y, Zhang JH, Feng H. Curcumin inhibits microglia inflammation and confers neuroprotection in intracerebral hemorrhage. Immunol Lett. 2014;160:89–95. doi: 10.1016/j.imlet.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 121.Wang BF, Cui ZW, Zhong ZH, Sun YH, Sun QF, Yang GY, Bian LG. Curcumin attenuates brain edema in mice with intracerebral hemorrhage through inhibition of AQP4 and AQP9 expression. Acta Pharmacol Sin. 2015;36:939–48. doi: 10.1038/aps.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu W, Yuan J, Zhu H, Zhang X, Li L, Liao X, Wen Z, Chen Y, Feng H, Lin J. Curcumin reduces brain-infiltrating T lymphocytes after intracerebral hemorrhage in mice. Neurosci Lett. 2016;620:74–82. doi: 10.1016/j.neulet.2016.03.047. [DOI] [PubMed] [Google Scholar]
- 123.Abiko Y, Miura T, Phuc BH, Shinkai Y, Kumagai Y. Participation of covalent modification of Keap1 in the activation of Nrf2 by tert-butylbenzoquinone, an electrophilic metabolite of butylated hydroxyanisole. Toxicol Appl Pharmacol. 2011;255:32–9. doi: 10.1016/j.taap.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 124.Satoh T, McKercher SR, Lipton SA. Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radic Biol Med. 2013;65:645–57. doi: 10.1016/j.freeradbiomed.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sukumari-Ramesh S, Alleyne CH., Jr Post-injury administration of tert-butylhydroquinone attenuates acute neurological injury after intracerebral hemorrhage in mice. J Mol Neurosci. 2016;58:525–31. doi: 10.1007/s12031-016-0722-y. [DOI] [PubMed] [Google Scholar]
- 126.Clarke JD, Hsu A, Williams DE, Dashwood RH, Stevens JF, Yamamoto M, Ho E. Metabolism and tissue distribution of sulforaphane in Nrf2 knockout and wild-type mice. Pharm Res. 2011;28:3171–9. doi: 10.1007/s11095-011-0500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jazwa A, Rojo AI, Innamorato NG, Hesse M, Fernandez-Ruiz J, Cuadrado A. Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxid Redox Signal. 2011;14:2347–60. doi: 10.1089/ars.2010.3731. [DOI] [PubMed] [Google Scholar]
- 128.Alfieri A, Srivastava S, Siow RC, Cash D, Modo M, Duchen MR, Fraser PA, Williams SC, Mann GE. Sulforaphane preconditioning of the Nrf2/HO-1 defense pathway protects the cerebral vasculature against blood-brain barrier disruption and neurological deficits in stroke. Free Radic Biol Med. 2013;65:1012–22. doi: 10.1016/j.freeradbiomed.2013.08.190. [DOI] [PubMed] [Google Scholar]
- 129.Zhao J, Kobori N, Aronowski J, Dash PK. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci Lett. 2006;393:108–12. doi: 10.1016/j.neulet.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 130.Linden IB, Tokola O, Karlsson M, Tenhunen R. Fate of haem after parenteral administration of haem arginate to rabbits. J Pharm Pharmacol. 1987;39:96–102. doi: 10.1111/j.2042-7158.1987.tb06952.x. [DOI] [PubMed] [Google Scholar]
- 131.Baxter PS, Hardingham GE. Adaptive regulation of the brain’s antioxidant defences by neurons and astrocytes. Free Radic Biol Med. 2016 doi: 10.1016/j.freeradbiomed.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bell KF, Al-Mubarak B, Martel MA, McKay S, Wheelan N, Hasel P, Markus NM, Baxter P, Deighton RF, Serio A, Bilican B, Chowdhry S, Meakin PJ, Ashford ML, Wyllie DJ, Scannevin RH, Chandran S, Hayes JD, Hardingham GE. Neuronal development is promoted by weakened intrinsic antioxidant defences due to epigenetic repression of Nrf2. Nat Commun. 2015;6:7066. doi: 10.1038/ncomms8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Regan RF, Chen M, Li Z, Zhang X, Benvenisti-Zarom L, Chen-Roetling J. Neurons lacking iron regulatory protein-2 are highly resistant to the toxicity of hemoglobin. Neurobiol Dis. 2008;31:242–249. doi: 10.1016/j.nbd.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Matz P, Turner C, Weinstein PR, Massa SM, Panter SS, Sharp FR. Heme-oxygenase-1 induction in glia throughout rat brain following experimental subarachnoid hemorrhage. Brain Res. 1996;713:211–222. doi: 10.1016/0006-8993(95)01511-6. [DOI] [PubMed] [Google Scholar]
- 135.Nakaso K, Kitayama M, Mizuta E, Fukuda H, Ishii T, Nakashima K, Yamada K. Co-induction of heme oxygenase-1 and peroxiredoxin I in astrocytes and microglia around hemorrhagic region in the rat brain. Neurosci Lett. 2000;293:49–52. doi: 10.1016/s0304-3940(00)01491-9. [DOI] [PubMed] [Google Scholar]
- 136.Shih AY, Li P, Murphy TH. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J Neurosci. 2005;25:10321–35. doi: 10.1523/JNEUROSCI.4014-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Johnson DA, Johnson JA. Nrf2--a therapeutic target for the treatment of neurodegenerative diseases. Free Radic Biol Med. 2015;88:253–67. doi: 10.1016/j.freeradbiomed.2015.07.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen PC, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, Johnson JA. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: Critical role for the astrocyte. Proc Natl Acad Sci U S A. 2009;106:2933–8. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vargas MR, Johnson DA, Sirkis DW, Messing A, Johnson JA. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci. 2008;28:13574–81. doi: 10.1523/JNEUROSCI.4099-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Leffler CW, Parfenova H, Fedinec AL, Basuroy S, Tcheranova D. Contributions of astrocytes and CO to pial arteriolar dilation to glutamate in newborn pigs. Am J Physiol Heart Circ Physiol. 2006;291:H2897–904. doi: 10.1152/ajpheart.00722.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gesuete R, Orsini F, Zanier ER, Albani D, Deli MA, Bazzoni G, De Simoni MG. Glial cells drive preconditioning-induced blood-brain barrier protection. Stroke. 2011;42:1445–53. doi: 10.1161/STROKEAHA.110.603266. [DOI] [PubMed] [Google Scholar]
- 142.Shviro Y, Shaklai N. Glutathione as a scavenger of free hemin. A mechanism of preventing red cell membrane damage. Biochem Pharmacol. 1987;36:3801–3807. doi: 10.1016/0006-2952(87)90441-2. [DOI] [PubMed] [Google Scholar]
- 143.Pellmar TC, Roney D, Lepinski DL. Role of glutathione in repair of free radical damage in hippocampus in vitro. Brain Res. 1992;583:194–200. doi: 10.1016/s0006-8993(10)80024-1. [DOI] [PubMed] [Google Scholar]
- 144.Laird MD, Wakade C, Alleyne CH, Jr, Dhandapani KM. Hemin-induced necroptosis involves glutathione depletion in mouse astrocytes. Free Radic Biol Med. 2008;45:1103–14. doi: 10.1016/j.freeradbiomed.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 145.Malhotra D, Portales-Casamar E, Singh A, Srivastava S, Arenillas D, Happel C, Shyr C, Wakabayashi N, Kensler TW, Wasserman WW, Biswal S. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res. 2010;38:5718–34. doi: 10.1093/nar/gkq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Correa-Costa M, Azevedo H, Amano MT, Goncalves GM, Hyane MI, Cenedeze MA, Renesto PG, Pacheco-Silva A, Moreira-Filho CA, Camara NO. Transcriptome analysis of renal ischemia/reperfusion injury and its modulation by ischemic pre-conditioning or hemin treatment. PLoS One. 2012;7:e49569. doi: 10.1371/journal.pone.0049569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen-Roetling J, Song W, Schipper HM, Regan CS, Regan RF. Astrocyte overexpression of heme oxygenase-1 improves outcome after intracerebral hemorrhage. Stroke. 2015;46:1093–8. doi: 10.1161/STROKEAHA.115.008686. [DOI] [PMC free article] [PubMed] [Google Scholar]