Abstract
Objective
Although counseling is a required part of office-based buprenorphine treatment of opioid use disorders, the nature of what constitutes appropriate counseling is unclear and controversial. We review the existing literature on the role, nature, and intensity of behavioral interventions in office-based buprenorphine treatment.
Method
Review of randomized controlled studies testing the efficacy of adding a behavioral intervention to buprenorphine maintenance treatment.
Results
Four key studies showed no benefit from adding a behavioral intervention to buprenorphine plus medical management, while 4 studies indicated some benefit for specific behavioral interventions, primarily contingency management. We examine the findings from the negative trials in the context of six questions: 1) Is buprenorphine that effective? 2) Is medical management that effective? 3) Are behavioral interventions that ineffective in this population? 4) How has research design affected the results of studies of buprenorphine plus behavioral treatment? 5) What do we know about subgroups of patients who do and do not seem to benefit from behavioral interventions? 6) What should clinicians aim for in terms of treatment outcome in buprenorphine maintenance?
Conclusions
High-quality medical management may suffice for some patients, but there are few data regarding the types of individuals for whom medical management is sufficient. Physicians should consider a stepped care model in which patients may begin with relatively non-intensive treatment, with increased intensity for patients who struggle early in treatment. Finally, with 6-month retention rates seldom exceeding 50% and poor outcomes following dropout, we must explore innovative strategies for enhancing retention in buprenorphine treatment.
Introduction
The Drug Abuse Treatment Act of 2000 allowed physicians, for the first time, to prescribe an opioid medication, the partial agonist buprenorphine, to treat patients with opioid dependence as part of office-based practice (1). Included in this important act was a proviso that when prescribing buprenorphine in office-based practice, "the practitioner has the capacity to refer the patients for appropriate counseling and other appropriate ancillary services." Sixteen years later, we address the question, "What is 'appropriate counseling and other appropriate ancillary services?' “
Four influential randomized trials evaluating the impact of adding counseling or other behavioral interventions to buprenorphine plus medical management have reported no difference in opioid use outcomes between patients receiving or not receiving additional counseling (2–5). These findings have sparked controversy and confusion among clinicians regarding the role of behavioral interventions in office-based buprenorphine treatment. Following an overview of the 4 trials, this commentary will build on recent reviews (6–8) by synthesizing what the existing literature reveals about the role of behavioral interventions in buprenorphine treatment as well as identifying important gaps in the literature. We will focus this review by considering six key questions raised by the four studies revealing no effect of adding counseling to buprenorphine plus medical management: 1) Is buprenorphine that effective? 2) Is medical management that effective? 3) Are behavioral interventions that ineffective in this population? 4) How has research design affected the results of studies of buprenorphine and behavioral treatment? 5) What do we know about subgroups of patients who do and do not seem to benefit from behavioral interventions? 6) What should clinicians aim for in terms of treatment outcome in buprenorphine treatment?
Overview of the four trials showing no benefit of adding counseling to buprenorphine plus medical management
In the first study to examine the role of behavioral interventions in office-based buprenorphine treatment, Fiellin and colleagues (5) randomized 166 patients dependent upon opioids (83% of whom used primarily heroin) to either 1) manual-based standard medical management plus weekly dispensing of buprenorphine-naloxone (referred to hereafter as simply ‘buprenorphine’); 2) standard medical management plus medication dispensing three times a week; or 3) enhanced medical management plus medication dispensing three times a week. All medical management sessions were delivered by primary care nurses with relatively little experience in treating substance-using individuals. Standard medical management sessions, which lasted approximately 20 minutes, discussed recent drug use, supported patients' attempts to reduce or stop drug use, encouraged abstinence, and recommended mutual-help groups such as Narcotics Anonymous. Enhanced medical management sessions lasted 45 minutes, covering similar topics more deeply. Outcomes improved in all three groups; the self-reported mean number of days of opioid use dropped from 5.3 days per week at baseline to 0.4 days per week during treatment. The percentage of opioid-negative urine tests ranged from 40–44% among the three treatment arms, with no significant differences. Forty-five percent of randomized patients completed the 24-week trial.
More recently, Fiellin and colleagues (4) examined the effect of adding cognitive-behavioral therapy (CBT) to buprenorphine plus standard medical management in a 24-week study of 141 primary care patients (64% primarily heroin users). Physicians delivered medical management weekly for two weeks, then biweekly for 4 weeks, then every 4 weeks. Patients randomized to medical management plus CBT were offered up to 12 CBT sessions in weeks 1–12. Again, there were significant improvements in frequency of self-reported opioid use, with mean reductions from 5.3 days of opioid use per week at baseline to 0.4 days during the second half of buprenorphine maintenance, with no difference between study conditions. Forty-one percent of randomized participants completed the trial.
Ling and colleagues (2) also tested CBT, contingency management, and their combination, versus medical management alone in a 32-week trial of 202 opioid-dependent patients (59% primarily heroin users). All participants received weekly medical management, and were randomized to receive either weekly CBT, twice-weekly contingency management (whereby patients with drug-free urine tests received a chance at receiving a cash reward ranging from $1–4), combined contingency management plus CBT, or medical management alone. Participants in all four conditions reported significant improvements in opioid use: days of heroin use in the past 30 days dropped from >20 in all conditions to 3.3–5.4 at the end of behavioral treatment, with no differences among groups. Fifty percent of the randomized participants completed the trial.
The largest study to examine the role of counseling in office-based buprenorphine treatment was the Prescription Opioid Addiction Treatment Study (POATS) (3), a 10-site trial (N=653) conducted by the National Drug Abuse Treatment Clinical Trials Network. This study focused on individuals dependent on prescription opioids, either exclusively (77%) or with a history of minimal, non-injection heroin use (23%). Using an adaptive treatment research design, POATS randomly assigned patients to weekly medical management or medical management plus individual drug counseling during 1) a 4-week taper and 2) for those who relapsed during the first phase, 12 weeks of buprenorphine stabilization. Rates of treatment “success” (abstinence or near-abstinence from opioids) were only 7% during the taper phase, but rose to 49% in the second phase of the trial (n=360) during the last four weeks of buprenorphine stabilization. Again, there was no difference in success rates between those who did or did not receive counseling. After completing Phase 2, participants were tapered off buprenorphine; only 9% of patients were abstinent after completing a second taper and 8 weeks of follow-up, again underscoring the importance of maintaining patients on buprenorphine for longer periods of time. The second phase of the trial included 360 of the 610 participants (59%) who did not succeed in Phase 1; 90% of Phase 2 participants completed the 12 weeks of buprenorphine stabilization treatment.
In light of the similar results from these four trials, which indicate improvement in opioid use with buprenorphine plus medical management but no benefit from additional behavioral interventions, we explore potential explanations below:
1. Is buprenorphine that effective?
Are the effects of buprenorphine so powerful as to overwhelm those of behavioral therapies? As is true with most pharmacotherapies for substance use disorders, one’s view of the effectiveness of buprenorphine depends on the question, “Compared to what?” There is no question that office-based buprenorphine treatment has been a significant advance in broadening the availability of an effective treatment for opioid dependence and has saved countless lives (9–12). Numerous reviews and meta-analyses underscore the strong effectiveness of buprenorphine in enhancing treatment retention and reducing illicit opioid use with respect to placebo or no treatment (13, 14). Buprenorphine’s important benefits also include reduced risk of HIV, sexually transmitted diseases, medical costs, and mortality (9).
However, there is clear room for improvement in buprenorphine treatment outcomes. In the POATS study (3), the sevenfold increase in rates of successful outcome for buprenorphine stabilization compared with a taper is a clear testament to its effectiveness; the fact that half of the participants were unsuccessful on buprenorphine stabilization highlights its limitations. Indeed, when compared to methadone maintenance, buprenorphine consistently demonstrates significantly lower rates of retention (13–17). For example, in a multisite clinical trial of 1267 individuals randomized to methadone or buprenorphine maintenance, six-month retention rates for buprenorphine and methadone were 46% and 74%, respectively (p<.01), with most dropout from buprenorphine occurring within the first month (18). A five-year follow-up study indicated continued superiority for methadone with respect to buprenorphine for both retention and rates of illicit opioid use (19).
Although it is possible that the superior retention for methadone over buprenorphine in this trial reflects individuals not being randomized to their preferred treatment, given that opioid-dependent individuals often have strong preferences for one over the other (20), a study by Pinto and colleagues (21) suggests an alternative explanation. They conducted a practical trial in which 361 opioid- dependent individuals in the United Kingdom were allowed to select whether they would receive either buprenorphine or methadone maintenance treatment. The sample included predominantly non-minority, unemployed heroin users with an average of 12 years of opioid dependence and substantial treatment histories. Most (63%) chose methadone over buprenorphine. Individuals who selected methadone tended to have more severe substance use problems and more psychological difficulties. Even with self-selection of treatment, 6-month retention rates were markedly higher with methadone versus buprenorphine (69.6% versus 42.5%, p<.001). Conversely, individuals who were retained in buprenorphine had significantly fewer opioid-positive urine specimens. Similar findings have been reported for large national data linkage studies (16). It should be noted that in the United Kingdom, both buprenorphine and methadone can be prescribed by physicians in office practice, whereas comparisons of buprenorphine vs. methadone in the United States involve differences not only in pharmacology (partial vs. full agonist) but in delivery system (buprenorphine most often via office-based treatment while methadone is only available through specialty opioid treatment programs).
Why might methadone be more effective than buprenorphine in retaining patients? In addition to pharmacologic differences, one factor in the United States may be the higher level of structure associated with methadone programs; most provide more regular contact, urine monitoring, and a larger array of counseling and services than office-based buprenorphine treatment (22). The substantial literature on outcomes for methadone maintenance programs indicates large differences in retention and outcome that vary with the intensity and quality of services provided by different programs (23). For example, in the landmark study on the effects of behavioral interventions and services in methadone maintenance treatment, McLellan and colleagues (24) randomly assigned 92 male opioid-dependent individuals who were stabilized on a therapeutic dose of methadone to either 1) no additional services, 2) standard services, including counseling, or 3) enhanced services, which provided counseling plus individually tailored, on-site medical, psychiatric, employment, and family therapy services. Although some individuals did well in the no-services condition, 69% had to be protectively transferred out of the study due to “unremitting use of opiates or cocaine, or medical/psychiatric emergencies.” Outcomes were significantly different across conditions, with best outcomes in the enhanced condition and poorest results in the no-services condition. The authors concluded, “The addition of basic counseling was associated with major increases in efficacy.” This study and many more have led to the general, though not universal (25–27), consensus that behavioral intervention is a key component of successful methadone treatment programs (28). Although the McLellan et al. study has not yet been replicated in the context of buprenorphine maintenance treatment, it raises the question of “Why would the lessons learned from methadone treatment not apply to buprenorphine?” One answer is the difference between the two medications in their delivery systems in the United States; since buprenorphine can be prescribed in a physician’s office, medical management should be, by definition, part of the process of buprenorphine treatment, whereas there is no analog in methadone treatment. Therefore, medical management itself could represent an effective behavioral treatment that obviates the need for further counseling for some patients.
2. Is medical management that effective?
The four studies suggesting no additional benefit of behavioral intervention with buprenorphine maintenance treatment all included a crucial component: regular medical management. In those studies, medical management typically involved regular appointments with a physician who assessed participant progress, medication efficacy and side effects, and consistently stressed simple messages including the importance of abstinence and the benefits of attending mutual-help support groups as well as regular (at least weekly) urine toxicology screens. It is impossible to determine how much the medical management contributed to patient outcomes in those studies; we are aware of no studies evaluating whether behavioral interventions might improve treatment outcomes when buprenorphine is delivered with no or very minimal medical management. It is possible that the effect of regular, structured, physician-delivered medical management plus urine monitoring was roughly parallel to the ‘standard methadone services’ provided within the McLellan et al. study described above, highlighting the potentially powerful influence of physician involvement on patient outcomes.
It is also likely that the medical management delivered in the four studies described above was offered more frequently and in a more structured way than is typical in many community settings (29, 30). It is unclear whether such frequent physician contact and urine monitoring is even feasible in most office-based settings (31, 32). Again, although there have been no studies comparing the effects of different intensities or types of medical management on buprenorphine outcomes, the variable levels of retention and outcome reported among community-based buprenorphine treatment programs suggests that the quality and intensity of medical management varies widely as well (33–37). Hence, in settings where medical management is not provided as intensively as it was in the four studies reviewed, additional behavioral intervention may have a greater effect on outcomes.
3. Are behavioral interventions ineffective in this population?
The four cited negative studies are countered by four other studies that do point to the efficacy of certain behavioral interventions in buprenorphine maintenance treatment. Bickel and colleagues (38) randomized 135 opioid-dependent individuals to one of 3 conditions in conjunction with buprenorphine plus thrice-weekly urine monitoring: (1) standard buprenorphine maintenance treatment, which included brief weekly counseling similar to that offered in methadone maintenance settings, (2) clinician-delivered community reinforcement approach, a validated multi-component approach (39) that included voucher-based contingency management for providing cocaine- and opioid-free urine toxicology screens (maximum possible earnings for complete abstinence $1316), or (3) community reinforcement approach provided via computer through the Therapeutic Education System, which also included the same voucher-based contingency management procedure. Rates of retention through 23 weeks were 58%, 53%, and 62%, respectively, and did not differ by condition. However, participants assigned to the two community reinforcement approach conditions had significantly longer periods of abstinence from both opioids and cocaine and more drug-negative urine specimens (70% for computer, 73% for therapist-delivered) compared with standard counseling (57%).
Christensen and colleagues (40) randomized 170 opioid-dependent individuals to buprenorphine with contingency management alone (maximum voucher value $997) or contingency management combined with the computerized Therapeutic Education system. The combined computerized therapeutic education system plus contingency management was associated with significantly better retention than contingency management alone through the 12-week trial (80% versus 64%) as well as significantly longer periods of abstinence (mean of 55 versus 49 days). Longer-term follow-up data were not reported for this study.
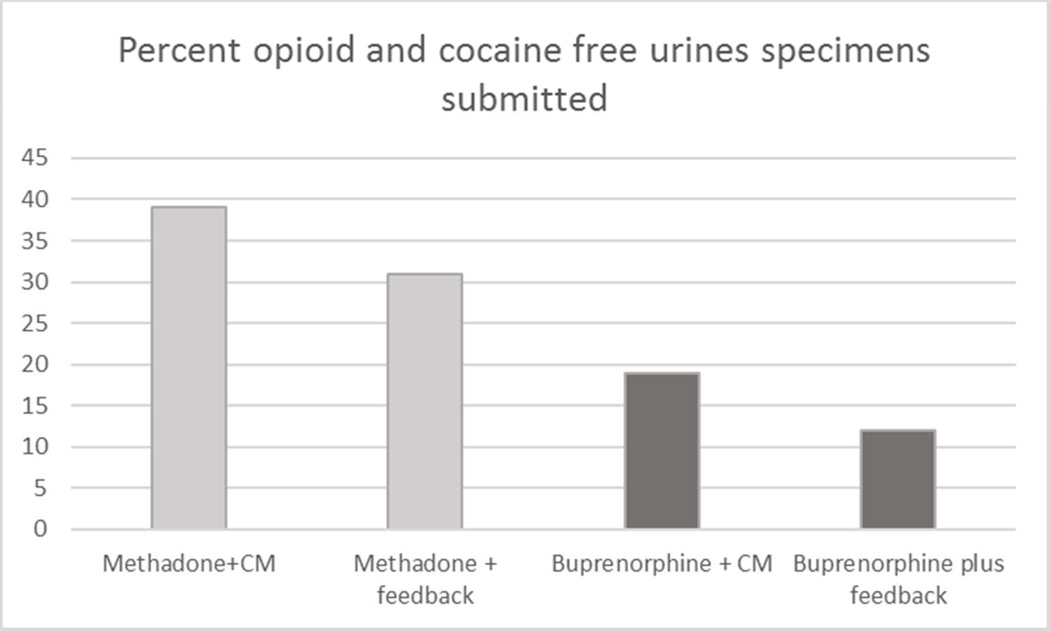
Schottenfeld and colleagues (41) randomized 162 cocaine- and opioid-dependent individuals to therapeutic doses of either methadone or buprenorphine and either voucher-based contingency management or performance feedback in a factorial (2×2) design. All participants received weekly individual counseling. In the contingency management system, the value of the vouchers escalated between weeks 1 and 12, then was reduced to a nominal value for weeks 13–24; the maximum possible total value was $1033. Over the course of the 24-week study, retention was significantly higher for those assigned to methadone versus buprenorphine (p<.05); similarly, those assigned to methadone had significantly longer periods of abstinence and submitted significantly more drug-free urine specimens (36 v. 19%; p<.05). The overall study retention rate through 24 weeks was 55%. There was a significant effect of contingency management on submission of drug-free urines during the first 12 weeks, the period during which the voucher values escalated (29% versus 21%, p<.05), although there were no significant effects for contingency management on retention or submission of drug-free urine specimens over the full 24 weeks. This study illustrates two key points: first, as in the larger body of literature on contingency management interventions, effects of contingency management procedures are most pronounced when escalating contingencies are in effect and tend to weaken once the contingencies are terminated (42). Second, the lower retention associated with buprenorphine may make it more difficult to detect effects of behavioral interventions due to ‘floor’ effects associated with poorer retention, as illustrated in Figure 1.
Figure 1.
Schottenfeld et al. (2005); Outcomes by treatment condition.
In addition to these three studies showing beneficial effects of behavioral interventions, one study demonstrated mixed results, with positive effects on retention but not on urine toxicology tests. Miotto and colleagues (43) randomized 94 opioid- dependent individuals to one of three treatment sites in which they received buprenorphine maintenance treatment over a 52-week period: (1) an opioid treatment program that provided supportive counseling by a certified counselor at the time of the medication visit (weekly during weeks 1–6 and monthly during weeks 7–52), (2) a primary care setting in which a physician provided brief counseling at the time of the study visits (also weekly during weeks 1–6 and monthly during weeks 7–52), or (3) behaviorally-oriented psychosocial treatment using the Matrix Model, in which cognitive-behavioral therapy groups were offered weekly for weeks 1–52. At week 20, there were significant differences by setting in terms of retention (21% in the opioid treatment program, 33% in the primary care setting, and 51% in the Matrix Model, p=.05). However, there were no differences by site in the rate of opioid-positive urine tests. It should be noted that there were differences in average dose of buprenorphine prescribed across settings, with physicians prescribing lower doses in the primary care setting.
4. Has research design affected the results of studies of buprenorphine and behavioral treatment?
As described above, four studies have indicated no additional benefit of adding a behavioral intervention to buprenorphine maintenance treatment, while four others have shown a potential benefit. Is it possible that the four negative studies were designed in a way that influenced the likelihood of finding a benefit of behavioral interventions? These were all rigorous randomized trials with multiple strengths, including comparatively large sample sizes, manualized interventions, and biological indicators of drug use outcomes (regular urine toxicology tests). However, when we compare the designs of the positive and negative trials, two key features may have influenced the outcomes: 1) the intensity and quality of medical management (both in terms of buprenorphine dose and medically-oriented counseling); and 2) the nature of the behavioral intervention itself (counseling of some sort versus contingency management). In particular, in all four negative studies (1–4), the behavioral interventions tested (counseling, CBT, or contingency management) were provided in addition to comparatively intensive medical management; three of the four studies included weekly medical management meetings. In contrast, three of the four positive studies did not describe a structured medical management condition.
The four negative studies also allowed for higher buprenorphine dosing than did the positive studies; the two Fiellin studies (4, 5) and the Ling study (2) allowed doses of up to 24 mg a day, while POATS (3) allowed doses of up to 32 mg a day, with dose adjustments occurring as part of medical management visits. The Christensen (23) and Schottenfeld studies (41) had maximum daily doses of 16 mg., while the Bickel study (38) had a maximum dose of 18 mg. a day unless the participant was in opioid withdrawal. Because there is some evidence that higher doses of buprenorphine are associated with better opioid use outcomes (15, 18), it is possible that some patients in the four negative studies benefited from their medical management sessions and more liberal dose adjustments, whereas the behavioral interventions in the positive studies played a more important role.
The nature of the behavioral interventions themselves may have significantly influenced outcome. For example, in the first Fiellin study (5), both the standard and enhanced medical management sessions were delivered by primary care nurses and involved a difference of only 20 minutes per session. Although the second Fiellin study (4) included a somewhat less intensive medical management condition, CBT was offered only once a week, and participants completed an average of only 6.7 CBT sessions over the 12-week treatment period; the Ling (2) study also offered CBT only once a week. The drug counseling offered in POATS (3) was somewhat more intensive (twice weekly in Phase 1; twice weekly for 6 weeks, then weekly for 6 weeks in Phase 2); however, participants attended only 64.4% of potentially available counseling sessions in Phase 2, when the main outcome was measured. Thus, in all four of these negative trials, the difference between the medical management condition and the medical management plus behavioral treatment condition may not have been robust enough to make a difference in outcome. Moreover, all of the studies showing benefit from a behavioral intervention on urine test-confirmed opioid use outcomes entailed contingency management, either in the context of computerized or in-person counseling. Conversely, three of the four negative studies included counseling but no contingency management condition. The Ling study (2) was the only one in which contingency management offered no advantage; however, unlike the three positive contingency management studies, this study included weekly medical management visits, thus possibly mitigating the effect of other interventions. In addition, as effect sizes of behavioral interventions may be modest, it is not clear whether any of the studies other than the multisite POATS were adequately powered to detect a significant difference between the behavioral conditions.
Finally, handling of missing data and the timing of the measurement of primary outcomes may be important factors in interpreting outcomes from the negative trials. For almost all of the trials reviewed here, dropout rates were high, and data were not available from the roughly 50% of participants who were withdrawn or dropped out. Missing urine screens were assumed to be positive for drug use, and dropouts were assumed to be treatment ‘failures’; while this assumption remains a common approach for this emerging field, it should be noted that it is highly problematic from a missing data perspective (44) and subject to multiple biases. Multiple studies have demonstrated that it is possible to achieve high rates of follow-up in studies of opioid-dependent patients, and these studies demonstrate that outcomes vary widely across patients who drop out of a study, and include inpatient treatment, switch to a methadone maintenance program, and others (45, 46); thus the validity of the ‘missing equals failure’ assumption is questionable.
5. What do we know about subgroups of patients who do and do not seem to benefit from behavioral interventions?
One potentially useful way to frame this question may be “what types of patients respond well to medical management alone versus those patients who may require more intensive behavioral interventions?” To date, very few studies have addressed this issue. A secondary analysis of the POATS study focused on subgroups of participants who benefited from counseling in addition to buprenorphine and medical management, either due to greater problem severity or more exposure to counseling as a result of greater treatment adherence (47). Problem severity was measured alternately by a history of heroin use, a higher Addiction Severity Index (ASI) drug composite score (48), and presence of current chronic pain. Adequate treatment adherence was defined a priori as attending at least 60% of all offered sessions. The association between severity and outcome did not vary by treatment condition for chronic pain or ASI drug severity composite score. However, patients who had ever used heroin and received drug counseling were more likely to be successful (i.e., abstinent or nearly abstinent from opioids) than heroin users who received medical management alone, but only if they were adherent to treatment and thus received adequate exposure to counseling (p=0.03). This is notable because not only did heroin users as a group have worse overall outcomes in both the POATS main trial and in a 42-month follow-up study of the POATS population (45), but heroin users have repeatedly demonstrated worse treatment outcomes when compared with those who exclusively use prescription opioids (5, 49, 50). These findings emphasize the importance of treatment adherence, and suggest that patients with prescription opioid dependence are a heterogeneous group and that optimal treatment strategies may vary by subgroups (47).
A secondary analysis of the 2014 Fiellin study suggested that individuals who were dependent on prescription opioids had a better response to CBT, as measured by the percentage of urine tests negative for all drugs (p=03), with no differences between CBT and medical management for participants who were primary heroin users (51). We know of no other study that has reported on predictors of response to medical management alone versus additional behavioral interventions. However, the general literature on outcomes from buprenorphine-maintained patients in office-based settings suggests that better outcomes are associated with attendance at counseling (36, 52–55) or Alcoholics Anonymous/Narcotics Anonymous meetings (36, 54). For example, in a retrospective chart review study of a heterogeneous sample of 533 opioid-dependent individuals enrolled in a primary care center, Neumann and colleagues reported that retention in treatment through 6 months was associated with attendance at counseling and past physical trauma or injury (33).
6. What should clinicians aim for in terms of outcome in buprenorphine maintenance treatment?
The literature on buprenorphine maintenance treatment in office-based settings overwhelmingly emphasizes two outcomes: 1) treatment retention and 2) submission of urine toxicology tests negative for opioids and other drugs. While these are essential outcome indicators, they do not address other important areas of high significance to patients and their families (56, 57). There are no reliable data, for example, on the extent to which any of the treatment approaches reviewed here are successful in helping patients attain a sustained period of good functioning (which could be defined as having a stable place to live, a job, no criminal activity, and management or resolution of other medical or psychiatric problems). Trials should begin routinely assessing functional outcomes among buprenorphine-maintained patients (as problems in the area of housing, employment, illegal activity and medical/psychiatric functioning are likely to be associated with poorer buprenorphine outcomes) and consider treatment models in which we treat patients with a goal of achieving these outcomes.
Furthermore, as provision of intensive behavioral intervention may be neither necessary for all buprenorphine-treated patients nor feasible in many office-based settings, until we understand more about the types of patients for whom medical management is adequate versus those who require more intensive intervention, stepped care models (58) should be considered and evaluated systematically. Some patients with opioid use disorders do not want or accept counseling (59–61). Indeed, some behavioral interventions for this patient population have involved increased intensity of counseling as a negative consequence of continued substance use (62). A potential stepped-care model could involve starting those patients appropriate for office-based buprenorphine treatment at a level acceptable to them, which might be a basic level of medical management or medical management plus counseling, along with regular urine testing and monitoring; the treatment would consistently focus on what we call the ‘Five A’s’ of successful buprenorphine treatment: adherence, abstinence, attendance, alternative activities, and accessing support. For those patients who do well with this model and have adequate support, additional intervention may be needed episodically, during periods of stress, or not at all. However, patients who do poorly at any given level of treatment intensity should be offered a more intensive behavioral intervention. A secondary analysis from POATS (63) revealed that patients who use opioids during the first two weeks of buprenorphine treatment had only a 6% likelihood of achieving abstinence at 12 weeks; which is consistent with other research (52). Thus, the physician can tell early in treatment whether the level of behavioral intervention is adequate or needs strengthening.
For those individuals who show signs of needing additional intervention (missed visits, drug-positive urines, symptoms that cannot be managed in the office setting), more intensive intervention is indicated. A significant problem is that access to high quality, evidence-based interventions is limited in many areas of the country (11, 64–66). Alternate services and support, such as Alcoholics or Narcotics Anonymous may be more readily available, but there is virtually no information on how many buprenorphine-treated patients use such services or the benefits they may provide, given that there may be substantial variation in how buprenorphine-treated patients are received in some mutual-help groups (66, 67).
Summary and conclusions
To summarize in terms of the questions raised at the beginning of this review, first, is buprenorphine that effective? While there is no question that buprenorphine maintenance is far more effective than treatments that do not involve approved medications for opioid use disorders, retention in buprenorphine treatment appears significantly poorer than that of methadone maintenance treatment. Thus, while efforts to expand buprenorphine access are essential and urgent, there remains considerable room for improvement, given 6-month retention rates of about 50%, and the significantly higher risk of relapse, overdose, and death that is associated with dropout (16, 68, 69). Given these risks, we must find means of improving retention in office-based buprenorphine maintenance.
Second, is medical management that effective? Our review suggests that medical management, at the levels provided in the four negative studies reviewed, may reflect an adequate level of treatment for many patients. However, it is unclear whether the levels of intensity of medical management provided in these studies are routinely provided or even feasible in most community-based settings. Thus, efforts to identify subtypes of patients who respond to medical management versus those who require more intensive care should receive greater attention, in both completed and future studies.
Third, regarding the question of whether behavioral interventions are ineffective in this population, the four studies finding no benefit from behavioral interventions are countered by an equal number of trials demonstrating the efficacy of behavioral interventions, particularly contingency management. This underscores the idea that interventions with a stronger evidence base, such as contingency management, may play an important role in buprenorphine maintenance treatment, and suggests that the issue regarding the role of behavioral interventions is far from closed.
Fourth, in terms of the extent to which research design may have affected the results of studies of buprenorphine and behavioral treatment, two factors are notable. First, no study has compared a behavioral therapy to buprenorphine maintenance delivered without high-quality medical management. Second, the high dropout rate in these studies presents a general problem with this literature, related to the handling of significant levels of missing data. The assumption that missing data represent categorical failure can open these studies to multiple sources of bias and limit the strength of inferences that can be drawn from them (44, 70). Future studies should use proven methods to achieve high levels of follow-up (71–73), particularly from those who drop out of treatment.
Finally, in terms of understanding subgroups of patients who do and do not benefit from behavioral interventions as well as desirable outcomes from buprenorphine treatment, more data are needed on key issues including (1) the types of individuals for whom medical management with buprenorphine is adequate versus those who need more intensive care, (2) rates of buprenorphine-maintained patients who attend mutual-help groups and their positive and negative experiences with these important support groups, (3) outcome studies comparing stepped versus fixed models of care, (4) research on innovative approaches that both appeal to buprenorphine-maintained patients and improve outcomes beyond the current mark of 50% retention at 6 months, (5) the relationship between buprenorphine dose and patient outcome, and (6) optimal models of treatment to produce improvements in overall functioning for patients with opioid use disorders.
Table 1.
Overview of 8 studies evaluating behavioral interventions and buprenorphine maintenance
| Study | N/co nditi ons |
Exclusions | Length of trial | Frequency of medical management |
Behavioral therapy | Frequency of urine monitoring |
6-month retention rate, based on ITT |
% opioid-free urine specimens |
|---|---|---|---|---|---|---|---|---|
| Fiellin et al 2006 | 166/3 | No alcohol benzodiazepine, sedative dependence |
24 weeks | 1–3 times a week | Standard medication management=20 min Enhanced management=45 min. |
1× week | MM+1 =50% MM×3=41% EMM×3=44% *completion confounded w/attendance at counseling |
MM+1=44% MM×3=40% EMM×3=40% |
| Weiss et al 2011 | 653/2 | No heroin injection or history of heroin dependence, pain event within 6 months; no alcohol or other drug dependence requiring immediate medical attention |
24 weeks Phase 1=4-week taper, 8-week follow- up Phase 2=12 week maintenance+4 week taper+8 week follow-up |
Phase 1: 2×/week in week 1, then weeks 2,3,4,6,8 Phase 2: 2× week during week 1, 1× week weeks 2–16 |
Individual manualized drug counseling; In Phase 1, 2×/week in weeks1–4, bi-weekly in weeks 5–8 In Phase 2: Weeks 1–6, 2× week, weeks 7–12 1 visit/week; 0 visits weeks 12–16 |
1× week for weeks 1–16, 2× month weeks 16–24 |
Successful outcome: Composite of retention and abstinence; End of Phase 1 MM=7.4% MM+ODC==5.8% End of Phase 2 MM=46.7% MM+ODC==51.7% |
Not reported by group 42% positive at end of Phase II taper. |
| Ling et al 2013 | 202/4 | No alcohol or other drug dependence requiring immediate medical attention |
32 weeks Phase 1=16 week behavioral Phase 2=16 week medication maintenance only |
2× week for 16 weeks, checklist used |
CM 2× week; CBT 1× week Weeks 1–16 No behavioral therapy weeks 17–32 |
2× week for weeks 1–16, 1× week for weeks 17–24 |
CBT =49% CM=57% CBT+CM=49% NT=43% *32-week retention |
Not reported |
| Fiellin et al 2014 | 141/2 | No alcohol, cocaine, benzodiazepine dependence |
24 weeks 12 weeks with CBT 12 weeks no CBT |
1× week for first 2 weeks, then 2× month |
Clinician delivered CBT 1× week; |
1× week | MM =32/71=45% MM+CBT=27/70=38% Transfer to methadone MM=28/71=39% MM+CBT=19/70=27% |
Not reported |
| Bickel et al., 2008 | 135/3 | None | 23 weeks | Not described | Standard counseling 1× week; Computer CRA: 3× week Clinician CRA: 3× week |
3× week | Standard counseling: 58% Computer CRA: 62% Clinician CRA: 53% |
Standard counseling: 57% Computer CRA: 70% Clinician CRA: 73% |
| Christensen et al 2014 | 170/2 | None | 12 weeks | Not described | Computer CRA 3× week; All get CRA 3× week and 30 minutes counseling every 2 weeks |
3× week | CRA+CM:80% CM only 64% *12 week retention |
Not reported |
| Schottenfeld et al 2005 | 162/4 | No alcohol or sedative dependence |
24 weeks; contingencies change at week 12 |
Not described | Counseling: 2× week weeks 1–12, 1× week weeks 13–24 |
3× week | Meth+CM=60% Meth+PF=75% Bup+CM=45% Bup +PF=22% |
Meth+CM=55% Meth+PF=50% Bup+CM=37% Bup+PF=28% |
| Miotto et al 2012 | 94/3 | No benzodiazepine or other substance dependence |
20 week assessment 52 weeks total |
Flexible | ODC: 1× week weeks 1–6, 1× month weeks 7–52; Primary care: 1× week weeks 1–6, once monthly weeks 7–52 Matrix model, weekly groups |
1× weekly weeks 1–9, 1× monthly through week 52 |
20 week retention ODC: 21.4% Primary care: 33.3% Matrix model: 51.5% |
Not reported |
. Indicates study-specific exclusions, in addition to pregnancy as well standard medical or psychiatric exclusions
. MM=Medical management, EMM=Enhanced medical management, ODC=Outpatient drug counseling, CM=contingency management, CBT=cognitive behavioral therapy, NT=No treatment, CRA=Community Reinforcement Approach, PF=Performance feedback; Meth=methadone maintenance treatment
Acknowledgments
Dr. Carroll is a member in trust of CBT4CBT LLC. Dr. Weiss has consulted to Indivior, GW Pharmaceuticals, and US WorldMeds.
The authors acknowledge the help of David A. Fiellin, M.D., and Brent A. Moore, Ph.D., for their assistance in preparation of this manuscript.
Supported by Grants UG1DA015831 (Drs. Carroll and Weiss), P50 DA09241 (Dr. Carroll), and K24 DA022288 (Dr. Weiss) from the National Institute on Drub Abuse.
Footnotes
An earlier version of this paper was presented at the 39th Annual National Conference of the Association for Medical Education and Research in Substance Abuse, Washington D.C., November 5–7, 2015.
Dr. Carroll conflict of interest is managed by Yale.
References
- 1.Center for Substance Abuse Treatment (CSAT) Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction, TIP Series 40. Rockville, MD: SAMHSA; 2004. [Google Scholar]
- 2.Ling W, Hillhouse M, Ang A, Jenkins J, Fahey J. Comparison of behavioral treatment conditions in buprenorphine maintenance. Addiction. 2013;108(10):1788–1798. doi: 10.1111/add.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Archives of general psychiatry. 2011;68(12):1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiellin DA, Barry DT, Sullivan LE, Cutter CJ, Moore BA, O'Connor PG, et al. A randomized trial of cognitive behavioral therapy in primary care-based buprenorphine. The American journal of medicine. 2013;126(1):74 e11–74 e17. doi: 10.1016/j.amjmed.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiellin DA, Pantalon MV, Chawarski MC, Moore BA, Sullivan LE, O'Connor P, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355:365–374. doi: 10.1056/NEJMoa055255. [DOI] [PubMed] [Google Scholar]
- 6.Brady KT, McCauley JL, Back SE. Prescription Opioid Misuse, Abuse, and Treatment in the United States: An Update. Am J Psychiatry. 2016;173(1):18–26. doi: 10.1176/appi.ajp.2015.15020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dugosh K, Abraham A, Seymour B, McLoyd K, Chalk M, Festinger D. A Systematic Review on the Use of Psychosocial Interventions in Conjunction With Medications for the Treatment of Opioid Addiction. J Addict Med. 2016 doi: 10.1097/ADM.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Copenhaver MM, Bruce RD, Altice FL. Behavioral counseling for optimizing the use of buprenorphine treatment of opioid dependence in community based settings: A review of the empirical evidence. Am J Drug Alcohol Abuse. 2007;33(5):643–654. doi: 10.1080/00952990701522674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Shorter D, Kosten TR. Buprenorphine in the treatment of opioid addiction: opportunities, challenges and strategies. Expert Opin Pharmacother. 2014;15(15):2263–2275. doi: 10.1517/14656566.2014.955469. [DOI] [PubMed] [Google Scholar]
- 10.McCance-Katz EF. Office-based buprenorphine treatment for opioid-dependent patients. Harv Rev Psychiatry. 2004;12(6):321–338. doi: 10.1080/10673220490905688. [DOI] [PubMed] [Google Scholar]
- 11.Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and State Treatment Need and Capacity for Opioid Agonist Medication-Assisted Treatment. Am J Public Health. 2015;105(8):e55–e63. doi: 10.2105/AJPH.2015.302664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nosyk B, Anglin MD, Brissette S, Kerr T, Marsh DC, Schackman BR, et al. A call for evidence-based medical treatment of opioid dependence in the United States and Canada. Health Aff (Millwood) 2013;32(8):1462–1469. doi: 10.1377/hlthaff.2012.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas CP, Fullerton CA, Kim M, Montejano L, Lyman DR, Dougherty RH, et al. Medication-assisted treatment with buprenorphine: assessing the evidence. Psychiatric services. 2014;65(2):158–170. doi: 10.1176/appi.ps.201300256. [DOI] [PubMed] [Google Scholar]
- 14.Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11(9):1–171. iii–iv. doi: 10.3310/hta11090. [DOI] [PubMed] [Google Scholar]
- 15.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;2:CD002207. doi: 10.1002/14651858.CD002207.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell J, Trinh L, Butler B, Randall D, Rubin G. Comparing retention in treatment and mortality in people after initial entry to methadone and buprenorphine treatment. Addiction. 2009;104(7):1193–1200. doi: 10.1111/j.1360-0443.2009.02627.x. [DOI] [PubMed] [Google Scholar]
- 17.Fingerhood MI, King VL, Brooner RK, Rastegar DA. A comparison of characteristics and outcomes of opioid-dependent patients initiating office-based buprenorphine or methadone maintenance treatment. Subst Abus. 2014;35(2):122–126. doi: 10.1080/08897077.2013.819828. [DOI] [PubMed] [Google Scholar]
- 18.Hser YI, Saxon AJ, Huang D, Hasson A, Thomas C, Hillhouse M, et al. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction. 2014;109(1):79–87. doi: 10.1111/add.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hser YI, Evans E, Huang D, Weiss R, Saxon A, Carroll KM, et al. Long-term outcomes after randomization to buprenorphine/naloxone versus methadone in a multi-site trial. Addiction. 2015 doi: 10.1111/add.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gryczynski J, Jaffe JH, Schwartz RP, Dusek KA, Gugsa N, Monroe CL, et al. Patient perspectives on choosing buprenorphine over methadone in an urban, equal-access system. Am J Addict. 2013;22(3):285–291. doi: 10.1111/j.1521-0391.2012.12004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinto H, Maskrey V, Swift L, Rumball D, Wagle A, Holland R. The SUMMIT trial: a field comparison of buprenorphine versus methadone maintenance treatment. J Subst Abuse Treat. 2010;39(4):340–352. doi: 10.1016/j.jsat.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Gunderson EW, Fiellin DA. Office-based maintenance treatment of opioid dependence: how does it compare with traditional approaches? CNS Drugs. 2008;22(2):99–111. doi: 10.2165/00023210-200822020-00002. [DOI] [PubMed] [Google Scholar]
- 23.Ball JC, Ross A. The Effectiveness of Methadone Maintenance Treatment. New York: Springer-Verlag; 1991. [Google Scholar]
- 24.McLellan AT, Arndt IO, Metzger DS, Woody GE, O'Brien CP. The effects of psychosocial services in substance abuse treatment. JAMA : the journal of the American Medical Association. 1993;269(15):1953–1959. [PubMed] [Google Scholar]
- 25.Schwartz RP, Highfield DA, Jaffe JH, Brady JV, Butler CB, Rouse CO, et al. A randomized controlled trial of interim methadone maintenance. Arch Gen Psychiatry. 2006;63(1):102–109. doi: 10.1001/archpsyc.63.1.102. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz RP, Kelly SM, O'Grady KE, Gandhi D, Jaffe JH. Randomized trial of standard methadone treatment compared to initiating methadone without counseling: 12-month findings. Addiction. 2012;107(5):943–952. doi: 10.1111/j.1360-0443.2011.03700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gruber VA, Delucchi KL, Kielstein A, Batki SL. A randomized trial of 6-month methadone maintenance with standard or minimal counseling versus 21-day methadone detoxification. Drug and alcohol dependence. 2008;94(1–3):199–206. doi: 10.1016/j.drugalcdep.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Institute on Drug Abuse. Principles of Drug Addiction Treatment: A Research Based Guide. Bethesda, MD: NIDA; 2007. [Google Scholar]
- 29.Arfken CL, Johanson CE, di Menza S, Schuster CR. Expanding treatment capacity for opioid dependence with office-based treatment with buprenorphine: National surveys of physicians. Journal of substance abuse treatment. 2010;39(2):96–104. doi: 10.1016/j.jsat.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Finch JW, Kamien JB, Amass L. Two-year Experience with Buprenorphine-naloxone (Suboxone) for Maintenance Treatment of Opioid Dependence Within a Private Practice Setting. J Addict Med. 2007;1(2):104–110. doi: 10.1097/ADM.0b013e31809b5df2. [DOI] [PubMed] [Google Scholar]
- 31.Magura S, Lee SJ, Salsitz EA, Kolodny A, Whitley SD, Taubes T, et al. Outcomes of buprenorphine maintenance in office-based practice. J Addict Dis. 2007;26(2):13–23. doi: 10.1300/J069v26n02_03. [DOI] [PubMed] [Google Scholar]
- 32.Walley AY, Alperen JK, Cheng DM, Botticelli M, Castro-Donlan C, Samet JH, et al. Office-based management of opioid dependence with buprenorphine: clinical practices and barriers. J Gen Intern Med. 2008;23(9):1393–1398. doi: 10.1007/s11606-008-0686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neumann AM, Blondell RD, Azadfard M, Nathan G, Homish GG. Primary care patient characteristics associated with completion of 6-month buprenorphine treatment. Addict Behav. 2013;38(11):2724–2728. doi: 10.1016/j.addbeh.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Alford DP, LaBelle CT, Kretsch N, Bergeron A, Winter M, Botticelli M, et al. Collaborative care of opioid-addicted patients in primary care using buprenorphine: five-year experience. Arch Intern Med. 2011;171(5):425–431. doi: 10.1001/archinternmed.2010.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki J, Zinser J, Klaiber B, Hannon M, Grassi H, Spinosa M, et al. Feasibility of Implementing Shared Medical Appointments (SMAs) for Office-Based Opioid Treatment With Buprenorphine: A Pilot Study. Subst Abus. 2015;36(2):166–169. doi: 10.1080/08897077.2014.998400. [DOI] [PubMed] [Google Scholar]
- 36.Parran TV, Adelman CA, Merkin B, Pagano ME, Defranco R, Ionescu RA, et al. Long-term outcomes of office-based buprenorphine/naloxone maintenance therapy. Drug Alcohol Depend. 2010;106(1):56–60. doi: 10.1016/j.drugalcdep.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liebschutz JM, Crooks D, Herman D, Anderson B, Tsui J, Meshesha LZ, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369–1376. doi: 10.1001/jamainternmed.2014.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bickel WK, Marsch LA, Buchhalter AR, Badger GJ. Computerized behavior therapy for opioid-dependent outpatients: a randomized controlled trial. Exp Clin Psychopharmacol. 2008;16(2):132–143. doi: 10.1037/1064-1297.16.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Budney AJ, Higgins ST. A Community Reinforcement Plus Vouchers Approach: Treating Cocaine Addiction. Rockville, MD: NIDA; 1998. [Google Scholar]
- 40.Christensen DR, Landes RD, Jackson L, Marsch LA, Mancino MJ, Chopra MP, et al. Adding an Internet-delivered treatment to an efficacious treatment package for opioid dependence. J Consult Clin Psychol. 2014;82(6):964–972. doi: 10.1037/a0037496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schottenfeld RS, Chawarski MC, Pakes JR, Pantalon MV, Carroll KM, Kosten TR. Methadone versus buprenorphine with contingency management or performance feedback for cocaine and opioid dependence. Am J Psychiatry. 2005;162(2):340–349. doi: 10.1176/appi.ajp.162.2.340. [DOI] [PubMed] [Google Scholar]
- 42.Higgins ST, Wong CJ, Badger GJ, Haug-Ogden DE, Dantona RL. Contingent reinforcement increases cocaine abstinence during outpatient treatment and one year follow-up. J Consult Clin Psychol. 2000;68:64–72. doi: 10.1037//0022-006x.68.1.64. [DOI] [PubMed] [Google Scholar]
- 43.Miotto K, Hillhouse M, Donovick R, Cunningham-Rathner J, Charuvastra C, Torrington M, et al. Comparison of buprenorphine treatment for opioid dependence in 3 settings. J Addict Med. 2012;6(1):68–76. doi: 10.1097/ADM.0b013e318233d621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Institute of Medicine Panel on Handling Data in Clinical Trials. The Prevention and Treatment of Missing Data in Clinical Trials. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 45.Weiss RD, Potter JS, Griffin ML, Provost SE, Fitzmaurice GM, McDermott KA, et al. Long-term outcomes from the National Drug Abuse Treatment Clinical Trials Network Prescription Opioid Addiction Treatment Study. Drug and alcohol dependence. 2015;150:112–119. doi: 10.1016/j.drugalcdep.2015.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hser Y, Hoffman V, Grella CE, Anglin MD. A 33-year follow-up of narcotics addicts. Archives of general psychiatry. 2001;58:503–508. doi: 10.1001/archpsyc.58.5.503. [DOI] [PubMed] [Google Scholar]
- 47.Weiss RD, Griffin ML, Potter JS, Dodd DR, Dreifuss JA, Connery HS, et al. Who benefits from additional drug counseling among prescription opioid-dependent patients receiving buprenorphine-naloxone and standard medical management? Drug and alcohol dependence. 2014;140:118–122. doi: 10.1016/j.drugalcdep.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alterman AI, Brown LS, Zaballero A, McKay JR. Interviewer severity ratings and composite scores of the ASI: A further look. Drug Alcohol Depend. 1994;34:201–209. doi: 10.1016/0376-8716(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 49.Potter JS, Marino EN, Hillhouse MP, Nielsen S, Wiest K, Canamar CP, et al. Buprenorphine/naloxone and methadone maintenance treatment outcomes for opioid analgesic, heroin, and combined users: findings from starting treatment with agonist replacement therapies (START) J Stud Alcohol Drugs. 2013;74(4):605–613. doi: 10.15288/jsad.2013.74.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nielsen S, Hillhouse M, Thomas C, Hasson A, Ling W. A comparison of buprenorphine taper outcomes between prescription opioid and heroin users. J Addict Med. 2013;7(1):33–38. doi: 10.1097/ADM.0b013e318277e92e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cutter CJ, Moore BA, Barry D, Fiellin LE, Schottenfeld RS, Fiellin DA, et al. Cognitive behavioral therapy improves treatment outcome for prescription opioid users in primary care based buprenorphine/naltoxone treatment. Drug Alcohol Depend. 2015:e255. doi: 10.1016/j.jsat.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stein MD, Cioe P, Friedmann PD. Buprenorphine retention in primary care. J Gen Intern Med. 2005;20(11):1038–1041. doi: 10.1111/j.1525-1497.2005.0228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montoya ID, Schroeder JR, Preston KL, Covi L, Umbricht A, Contoreggi C, et al. Influence of psychotherapy attendance on buprenorphine treatment outcome. J Subst Abuse Treat. 2005;28(3):247–254. doi: 10.1016/j.jsat.2005.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mintzer IL, Eisenberg M, Terra M, MacVane C, Himmelstein DU, Woolhandler S. Treating opioid addiction with buprenorphine-naloxone in community-based primary care settings. Ann Fam Med. 2007;5(2):146–150. doi: 10.1370/afm.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haddad MS, Zelenev A, Altice FL. Integrating buprenorphine maintenance therapy into federally qualified health centers: real-world substance abuse treatment outcomes. Drug Alcohol Depend. 2013;131(1–2):127–135. doi: 10.1016/j.drugalcdep.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller PG, Miller WR. What should we be aiming for in the treatment of addiction? Addiction. 2009;104(5):685–686. doi: 10.1111/j.1360-0443.2008.02514.x. [DOI] [PubMed] [Google Scholar]
- 57.Green CA, McCarty D, Mertens J, Lynch FL, Hilde A, Firemark A, et al. A qualitative study of the adoption of buprenorphine for opioid addiction treatment. J Subst Abuse Treat. 2014;46(3):390–401. doi: 10.1016/j.jsat.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kakko J, Gronbladh L, Svanborg KD, von Wachenfeldt J, Ruck C, Rawlings B, et al. A stepped care strategy using buprenorphine and methadone versus conventional methadone maintenance in heroin dependence: a randomized controlled trial. Am J Psychiatry. 2007;164(5):797–803. doi: 10.1176/ajp.2007.164.5.797. [DOI] [PubMed] [Google Scholar]
- 59.Fox AD, Masyukova M, Cunningham CO. Optimizing psychosocial support during office-based buprenorphine treatment in primary care: Patients' experiences and preferences. Subst Abus. 2016;37(1):70–75. doi: 10.1080/08897077.2015.1088496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruetsch C, Cacciola J, Tkacz J. A national study of a telephone support service for patients receiving office-based buprenorphine medication-assisted treatment: study feasibility and sample description. J Subst Abuse Treat. 2010;39(4):307–317. doi: 10.1016/j.jsat.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 61.Barry DT, Irwin KS, Jones ES, Becker WC, Tetrault JM, Sullivan LE, et al. Integrating buprenorphine treatment into office-based practice: a qualitative study. J Gen Intern Med. 2009;24(2):218–225. doi: 10.1007/s11606-008-0881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brooner RK, Kidorf MS, King VL, Stoller KB, Peirce JM, Bigelow GE, et al. Behavioral contingencies improve counseling attendance in an adaptive treatment model. J Subst Abuse Treat. 2004;27(3):223–232. doi: 10.1016/j.jsat.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 63.McDermott KA, Griffin ML, Connery HS, Hilario EY, Fiellin DA, Fitzmaurice GM, et al. Initial response as a predictor of 12-week buprenorphine-naloxone treatment response in a prescription opioid-dependent population. J Clin Psychiatry. 2015;76(2):189–194. doi: 10.4088/JCP.14m09096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoffman KA, McCarty D. Improving the quality of addiction treatment. In: Miller PM, editor. Interventions for Addiction: Comprehensive Addictive Behaviors and Disorders. San Diego, CA: Academic Press; 2013. pp. 579–588. [Google Scholar]
- 65.Humphreys K, McLellan AT. A policy-oriented review of strategies for improving the outcomes of services for substance use disorder patients. Addiction. 2011;106(12):2058–2066. doi: 10.1111/j.1360-0443.2011.03464.x. [DOI] [PubMed] [Google Scholar]
- 66.Quest TL, Merrill JO, Roll J, Saxon AJ, Rosenblatt RA. Buprenorphine therapy for opioid addiction in rural Washington: the experience of the early adopters. J Opioid Manag. 2012;8(1):29–38. doi: 10.5055/jom.2012.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barry DT, Moore BA, Pantalon MV, Chawarski MC, Sullivan LE, O'Connor PG, et al. Patient satisfaction with primary care office-based buprenorphine/naloxone treatment. J Gen Intern Med. 2007;22(2):242–245. doi: 10.1007/s11606-006-0050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fiellin DA, Schottenfeld RS, Cutter CJ, Moore BA, Barry DT, O'Connor PG. Primary care-based buprenorphine taper vs maintenance therapy for prescription opioid dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(12):1947–1954. doi: 10.1001/jamainternmed.2014.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, Hickman M, et al. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction. 2011;106(1):32–51. doi: 10.1111/j.1360-0443.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- 70.Lavori PW. Clinical trials in psychiatry: Should protocol deviation censor patient data? Neuropsychopharmacology. 1992;6:39–48. [PubMed] [Google Scholar]
- 71.Hser YI. Predicting long-term stable recovery from heroin addiction: findings from a 33-year follow-up study. Journal of addictive diseases. 2007;26(1):51–60. doi: 10.1300/J069v26n01_07. [DOI] [PubMed] [Google Scholar]
- 72.Twitchell GR, Hertzog CA, Klein JL, Schuckit MA. The anatomy of a follow-up. Br J Addict. 1992;87:1327–1333. doi: 10.1111/j.1360-0443.1992.tb02741.x. [DOI] [PubMed] [Google Scholar]
- 73.Cottler LB, Compton WM, Ben-Abdallah A, Horne M. Achieving a 96.6 percent follow-up rate in a longitudinal study of drug abusers. Drug and alcohol dependence. 1996;41:209–217. doi: 10.1016/0376-8716(96)01254-9. [DOI] [PubMed] [Google Scholar]