Abstract
Coronary artery bypass grafting (CABG), one of the most common cardiac surgical procedures, is characterized by a burst of oxidative stress. 8-Oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG), produced following DNA repairing, is used as an indicator of oxidative DNA damage in humans. The effect of CABG on oxidative-induced DNA damage, evaluated through the measurement of urinary 8-oxodG by a developed and validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method in 52 coronary artery disease (CAD) patients, was assessed before (T0), five days (T1), and six months (T2) after CABG procedure. These results were compared with those obtained in 40 subjects with cardiovascular risk factors and without overt cardiovascular disease (CTR). Baseline (T0) 8-oxodG was higher in CAD than in CTR (p = 0.035). A significant burst was detected at T1 (p = 0.019), while at T2, 8-oxodG levels were significantly lower than those measured at T0 (p < 0.0001) and comparable to those found in CTR (p = 0.73). A similar trend was observed for urinary 8-iso-prostaglandin F2α (8-isoPGF2α), a reliable marker of oxidative stress. In the whole population baseline, 8-oxodG significantly correlated with 8-isoPGF2α levels (r = 0.323, p = 0.002). These data argue for CABG procedure in CAD patients as inducing a short-term increase in oxidative DNA damage, as revealed by 8-oxodG concentrations, and a long-term return of such metabolite toward physiological levels.
1. Introduction
Although coronary artery bypass grafting (CABG) is one of the most common cardiac surgical procedures, patients' morbidity and mortality, due to its adverse postoperative complications, are exceedingly high [1]. To investigate the mechanism underlying such adverse events, studies have assessed the biochemical effect of oxidative stress during this procedure [2, 3]. Reactive oxygen species (ROS), which are thought to be, at least in part, responsible for such adverse events, show a burst during CABG [4]. However, whilst the lipid peroxidation [5, 6] and the antioxidant defenses [7, 8] have been already evaluated, only limited information is available on oxidative DNA damage [9].
In human cells, the balance between DNA damage/repair is a finely regulated physiological process, resulting in approximately 104 oxidized DNA bases eliminated for cell for day in healthy subjects [10]. The oxidative modification of DNA by ROS can generate a variety of possible DNA lesions. Among nucleobases, guanine has the lowest redox potential, thus, being the most susceptible to oxidation [11]. Indeed, its stable urinary end-product, 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG), is one of the most widely recognized biomarkers of oxidative DNA damage [12, 13]. This metabolite, mirroring a ROS-damaged guanine mutagenic effect (due to its tendency to preferentially pair with adenine over cytosine during DNA replication, leading to G-to-T point mutation [14]), has been measured in several pathological conditions [15–18]. In particular, studies in patients with atherosclerotic cardiovascular disease highlight that 8-oxodG is significantly associated with both coronary artery disease (CAD) and other types of atherosclerotic diseases (stroke, peripheral artery disease, and carotid atherosclerosis) [19].
Little is known concerning 8-oxodG and cardiac surgery. Here, we report 8-oxodG levels in CAD patients undergoing CABG procedure, before and at different times after surgery, and in control subjects with cardiovascular risk factors and without overt cardiovascular disease (CTR).
As stated by the European Standards Committee on Urinary (DNA) Lesion Analysis (ESCULA), a fully recognized method to determine 8-oxodG is not defined so far [20]. Therefore, to measure this analyte, a simple and reliable liquid chromatography-tandem mass spectrometry (LC-MS/MS) method has been developed and validated and will be presented here. Finally, we report data on the association between urinary levels of 8-oxodG and 8-iso-prostaglandin F2α (8-isoPGF2α), a well-recognized index of lipid peroxidation [21].
2. Materials and Methods
2.1. Chemicals and Reagents
8-oxodG was purchased from Sigma-Aldrich (St. Louis, MO, USA); 15N5-8-oxodG was from Cambridge Isotope Laboratories, Inc. (Andover, MA, USA), and 8-iso-PGF2α and 8-iso-PGF2α-d4 were from Cayman Chemicals Co. (Ann Arbor, MI, USA). Amicon® Ultra centrifugal filters (Ultracel®-30 K) were purchased from Merck Millipore Ltd. (Cork, Ireland), and Sep-Pak® C18 solid-phase extraction (SPE) cartridges (3 cc, 500 mg) were purchased from Waters (Milford, MA, USA). Purified water was obtained from Milli-Q® Integral system (Merck Millipore Ltd., Cork, Ireland). All other chromatography-grade chemicals were obtained from Sigma-Aldrich.
2.2. Study Population
Over a six-month period (January 2011–June 2011), 52 consecutive CAD patients undergoing CABG surgery on extracorporeal circulation (ECC) were enrolled in the present study at Centro Cardiologico Monzino (CCM). Inclusion criteria were as follows: the need of an elective, isolated surgical procedure; age between 18 and 80 years; heart ejection fraction > 30%; normal sinus rhythm; no history of atrial fibrillation; coronary angiography carried out at least five days before the surgical procedure. Exclusion criteria were as follows: renal or liver disease; intake of antioxidant products (e.g., vitamins). Forty subjects with cardiovascular risk factors (e.g., diabetes, obesity, hypertension, smoking habit, and dyslipidemia without overt cardiovascular disease, on the basis of laboratory tests and clinical examination) acted as CTR group.
Informed consent to participate to this observational study, which was approved by CCM Institutional Review Board, was obtained by all subjects. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Institution's Human Research Committee.
2.3. Study Protocol and Sample Collection and Preparation
CTR's urinary samples were collected at the scheduled visit. To assess the effect of CABG intervention on oxidative-induced DNA damage in CAD patients, we collected urine samples before (T0), five days (T1), and six months after (T2) CABG procedure. Both in CTR and patients, all urine specimens for 8-oxodG measurement were collected early in the morning, aliquoted in tubes, and stored at −80°C until analysis. Frozen urine were thawed at room temperature, heated at 37°C for 10 minutes to redissolve possible analyte precipitates [22, 23], and then centrifuged at 1,700 ×g for 10 minutes. Subsequently, 200 μL aliquots were diluted with 200 μL of 15N5-8-oxodG internal standard solution (final concentration 5 ng/mL) and filtered through a 30,000 NMWL (nominal molecular weight limit) centrifugal filters at 10,000 ×g for 30 minutes. The filtrate was injected into the LC–MS/MS system.
For 8-iso-PGF2α, the processing of the collected urine was previously published [24].
2.4. 8-oxodG Measurement
An LC–MS/MS method was set up and validated to measure urinary 8-oxodG levels. The analytical instrument was an Accela HPLC System (Thermo Fisher Scientific, San Jose, CA, USA) coupled to a triple-quadrupole mass spectrometer TSQ Quantum Access (Thermo Fisher Scientific) outfitted with electrospray ionization (ESI) source operating in positive mode. The chromatographic separation was performed using a pentafluorophenyl Kinetex F5 100 Å analytical column (100 × 2.1 mm, Phenomenex, Torrance, CA, USA) packed with 2.6 μm core-shell particles, maintained at 30°C. The mobile phase was set at a flow rate of 0.25 mL/min using ammonium acetate 10 mmol/L (solvent A) and ammonium acetate 10 mmol/L in acetonitrile/water 50 : 50 v/v (solvent B). Samples (10 μL) were eluted with a gradient of mobile phase (Supplementary Table 1 available online at https://doi.org/10.1155/2017/9715898) during a total run time of 14 minutes. The selected reaction monitoring (SRM) was performed by monitoring the transitions m/z 284.0 ➔ m/z 168.1 (8-oxodG) and m/z 289.0 ➔ m/z 173.0 (15N5-8-oxodG). The operating conditions for MS analysis were the following: spray voltage, 2200 V; capillary temperature, 280°C; sheath gas, 25 UA; auxiliary gas, 10 UA. The Xcalibur® software, version 2.0 (Thermo Fisher Scientific), was used for system control, data acquisition, and processing. The method validation was based on U.S. Food and Drug Administration [25] and Matuszewski et al. [26] guidelines, including the evaluation of imprecision, linearity range, lower limit of quantification (LLOQ), limit of detection (LOD), relative matrix effect (ME), extraction recovery (RE), process efficiency (PE), and sample stability. Validation process details are reported in Supplementary Material.
2.5. 8-iso-PGF2α Measurement
8-Iso-PGF2α determination was performed by modifying a previously described LC-MS/MS method [24]. Briefly, the chromatographic separation was performed using an XBridge® C18 column (100 × 2.1 mm, particle size 3.5 μm; Waters Milford, MA, USA) maintained at 30°C. The mobile phase was composed by two solvents: water with 0.1% ammonium hydroxide (solvent A) and methanol : acetonitrile 50 : 50 v/v with 0.1% ammonium hydroxide (solvent B). The following gradient was used: 0 min—15% B, 14 min—50% B, 16 min—90% B, 22 min—15% B, and 40 min—15% B. SRM was performed in negative mode by monitoring the transitions m/z 353.1 ➔ m/z 192.8 (8-iso-PGF2α) and m/z 357.05 ➔ m/z 197.1 (8-iso-PGF2α-d4).
The estimated analyte values were corrected for the urinary creatinine levels, to control the variation in urinary output, and expressed as ng/mg of creatinine (8-oxodG) or pg/mg of creatinine (8-iso-PGF2α) [27]. Urinary creatinine was measured by a standard method in the clinical laboratory of CCM using Jaffe's reaction.
2.6. Statistical Analysis
Continuous variables are presented as mean ± standard deviation (SD) and were compared using the t-test for independent samples. Categorical variables were compared using chi-square test or Fisher's exact test, as appropriate.
As 8-oxodG and 8-iso-PGF2α values showed a nonnormal distribution, they were log transformed and their time courses were evaluated by covariance analysis for repeated measures. Data were adjusted with multivariable models for age and sex and expressed as geometric mean (GM) with 95% confidence intervals (95% CI). The Pearson correlation was used to detect correlations between 8-oxodG and 8-iso-PGF2α at T0. A p value <0.05 was considered statistically significant.
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina).
3. Results
3.1. Population
Demographic, clinical, and laboratory features of the two study groups are reported in Table 1. CAD patients were more hypercholesterolemic and hypertensive than CTR subjects; however, total cholesterol, LDL, and HDL were lower in the patient group as a result of the ongoing pharmacological treatments. There was no correlation between 8-oxodG levels and clinical parameters of the studied population.
Table 1.
Overall population characteristics at baseline.
| Variables | CAD (n = 52) |
CTR (n = 40) |
p value |
|---|---|---|---|
| Demographic characteristics | |||
| Age, years | 63.5 ± 9.7 | 58.5 ± 7.0 | 0.004 |
| Male gender, number (%) | 40 (76.9) | 22 (55.0) | 0.014 |
| Clinical characteristics | |||
| Weight, kg | 75.6 ± 13.8 | 78.1 ± 13.7 | 0.390 |
| BMI, kg/m2 | 26 ± 5.2 | 27.5 ± 4.1 | 0.080 |
| Active smokers, number (%) | 6 (11.5) | 6 (15) | 0.630 |
| Biochemical | |||
| Total cholesterol, mg/dL | 190.7 ± 44.2 | 212.5 ± 29.0 | 0.004 |
| LDL cholesterol, mg/dL | 121.8 ± 35.6 | 139.5 ± 32.4 | 0.020 |
| HDL cholesterol, mg/dL | 48.0 ± 10.6 | 52.7 ± 12.9 | 0.004 |
| Triglycerides, mg/dL | 104.0 ± 41.6 | 130.3 ± 82.4 | 0.070 |
| Fasting glycemia, mg/dL | 117.7 ± 28.1 | 107.0 ± 31.3 | 0.190 |
| Creatinine, mg/dL | 149.9 ± 77.3 | 125.2 ± 51.8 | 0.070 |
| Comorbidities | |||
| Diabetes, number (%) | 7 (13.5) | 1 (2.5) | 0.080 |
| Hypertension, number (%) | 48 (92.3) | 17 (42.5) | 0.005 |
| Hypercholesterolemia, number (%) |
29 (55.8) | 9 (22.5) | 0.001 |
| Obesity, number (%) | 1 (1.9) | 5 (12.5) | 0.070 |
| Medications | |||
| Aspirin, number (%) | 33 (63.5) | 0 (0) | p < 0.001 |
| Statins, number (%) | 28 (53.9) | 4 (10) | p < 0.001 |
| Oral hypoglycemics, number (%) |
7 (13.5) | 1 (2.5) | 0.160 |
| Antihypertensive, number (%) |
48 (92.3) | 13 (32.5) | p < 0.001 |
Characteristics of the study groups: CAD patients (n = 52) and CTR subjects (n = 40). Values are presented as mean ± SD or in absolute numbers (percentage of total).
3.2. 8-oxodG Method Validation
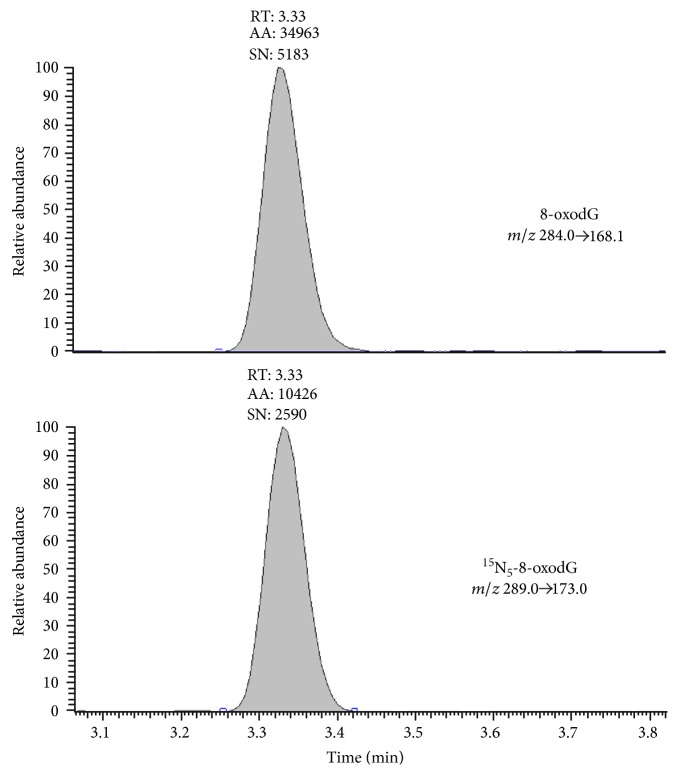
A representative chromatogram of 8-oxodG and of its internal standard 15N5-8-oxodG in urine pool sample, resulting from the chromatographic conditions and the selected transitions, is shown in Figure 1. The peaks eluted at 3.33 min, in a region of the chromatogram without any interfering background peaks.
Figure 1.
Representative chromatogram of 8-oxodG and of its internal standard 15N5-8-oxodG in urine pool sample.
The 10-point calibrator concentrations, plotted against the ratio of analyte/internal standard areas for five consecutive assays, showed linear and reproducible curves with the following nonzero forced linear regression equations: y = (0.1609 ± 0.006) x − (0.0143 ± 0.035) (r2 = 0.999). Over the entire concentration range of the curve, the mean-observed percentage deviation of back-calculated concentrations was between −0.7% and +3.8% with an imprecision coefficient of variation (CV) < 15%. However, as the human urine 8-oxodG concentration seldom exceeds 20 ng/mL, samples quantification was performed using a calibration curve in the range 0.25–25 ng/mL.
Intra-assay and interassay imprecisions were <10% for all quality controls (QC) tested; the LLOQ was 0.25 ng/mL while LOD value was 0.1 ng/mL. Detailed information are provided in Supplementary Table 2.
Supplementary Table 3 shows the relative ME, the RE, and the overall PE of the method. No relative matrix effect was observed at the three concentrations evaluated. Recovery and process efficiency values complied with the acceptability requirements, indicating a good reliability of the developed method.
The analyte was highly stable in urine at different temperatures of storage for at least 6 months and even throughout three freeze-thaw cycles (Supplementary Table 4).
3.3. 8-oxodG Levels in CAD Patients Undergoing CABG Surgery
In Figure 2 the 8-oxodG levels in CAD and CTR are depicted. As shown, the values of 8-oxodG measured in CAD patients before surgery (T0) (GM: 2.66 ng/mg creatinine, 95% CI: 2.39–2.95), were significantly higher than those found in CTR (GM: 2.26 ng/mg creatinine, 95% CI: 2.00–2.56) (p = 0.035). In contrast, six months after the intervention (T2) (Figure 2), 8-oxodG levels of CAD patients were entirely comparable to those of CTR (GM: 2.04 ng/mg creatinine, 95% CI: 1.84–2.27 and 2.26 ng/mg creatinine, 95% CI: 2.00–2.56, resp.; p = 0.73).
Figure 2.
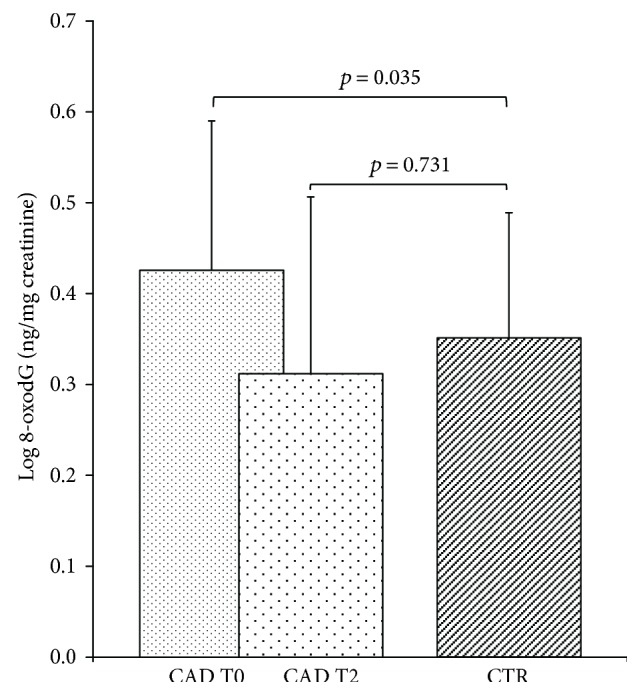
Levels of 8-oxodG measured in urine from CAD patients and CTR subjects. Data are represented as mean ± SD. Comparisons between groups were performed by covariance analysis, adjusting for age and sex.
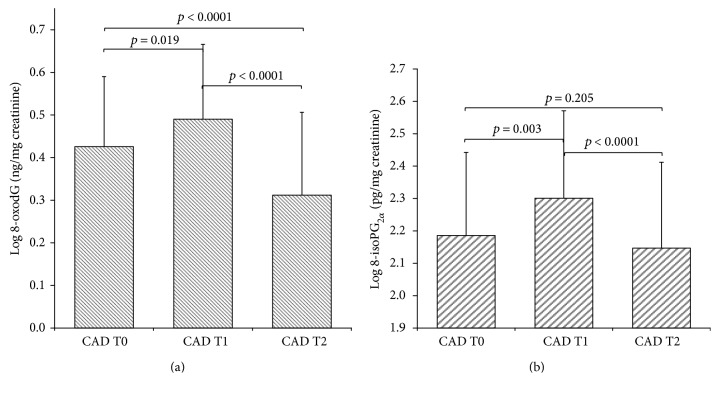
8-oxodG values at the different time points are shown in Figure 3(a). There was a statistical significant increase of short-term oxidative DNA damage after cardiac surgery (delta T1-T0, p = 0.019) and a significant decrease after six months from the intervention (delta T2-T1, p < 0.0001) with a full recovery at T2 (delta T2–T0, p < 0.0001), up to normal values.
Figure 3.
Time course of 8-oxodG (a) and of 8-isoPGF2α (b) following CABG surgery. Data are represented as mean ± SD.
No correlation between 8-oxodG levels and ECC or clamping time (r = 0.115, p = 0.42, and r = 0.205, p = 0.1, resp.) was observed.
3.4. 8-Iso-PGF2α Levels in CAD Patients Undergoing CABG Surgery
Levels of 8-iso-PGF2α in CAD patients undergoing surgery are depicted in Figure 3(b). Similar to 8-oxodG, an increase in 8-iso-PGF2α values was observed following the surgical procedure (delta T1-T0, p = 0.003) that returned to basal levels six months later (delta T2-T1, p < 0.0001). However, no significant difference was found before and six months after CABG procedure (delta T2–T0, p = 0.205).
At variance with 8-oxodG, no significant differences were found in 8-iso-PGF2α values between CTR (GM: 141.2 pg/mg creatinine 95% CI: 117.25–170.06) and CAD patients before surgery (T0) (GM: 155.06 pg/mg creatinine 95% CI: 132.45–181.53).
3.5. Correlation between 8-iso-PGF2α and 8-oxodG Levels
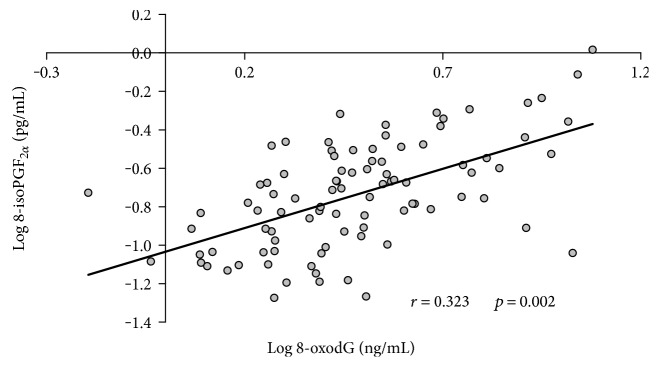
As shown in Figure 4, at baseline, a direct and positive correlation was found in the whole population studied (n = 92) between the two oxidative stress markers considered, 8-oxodG and 8-iso-PGF2α (r = 0.323, p = 0.002).
Figure 4.
Correlation between baseline levels of 8-oxodG and 8-iso-PGF2α in the whole population analyzed (n = 92).
4. Discussion
The aim of this study was to assess the effect of CABG procedure on the oxidative stress status in CAD patients. To reach this goal, we decided to measure 8-oxodG, a marker of oxidative DNA damage, 5 days and 6 months after CABG surgery. For this purpose, we developed an LC-MS/MS method to quantify urinary 8-oxodG level. Although LC-MS/MS is the gold standard technique to measure 8-oxodG [27–29], and various methods are currently employed (as stated by ESCULA), none of them is, up to now, overall accepted [20]. The LC-MS/MS method that we have developed and validated is simple, sensitive, and reliable and can be proposed in routine determination of 8-oxodG levels.
Following the application of this method, we show that, before CABG procedure, CAD patients have significantly higher urinary 8-oxodG levels as compared to CTR. Previously published studies in different matrices (serum [30], DNA extracts from leukocyte or lymphocytes [31, 32], and urine [33, 34]) showed higher than normal 8-oxodG concentrations in atherosclerotic patients. Moreover, a previously published meta-analysis [19] documented a statistically significant higher concentrations of 8-oxodG in CAD patients than in control subjects. In keeping with this, enhanced 8-oxodG concentrations have been reported in atherosclerotic plaques compared to the underlying media or to nonatherosclerotic mammary arteries [35] and to elevated DNA strand breaks in cells isolated from the atherosclerotic lesions [36]. 8-oxodG measurement in cardiovascular disease allows to evaluate oxidative damage from a different point of view, catching a picture of ROS-induced mutagenic effect. Indeed, as previously discussed, 8-oxodG reflects mutagenic guanine damage that may mispair with adenine, leading to a G-to-T point mutation during replication [14].
The return of 8-oxodG concentrations toward control values in CAD patients six months after cardiac surgery reveals the recovery of oxidative stress toward physiological levels. It is conceivable that CABG procedures, probably in association with pharmacological therapy, reduce oxidative stress and, in turn, ischemia-related DNA damage in CAD patients. Established atherogenic risk factors such as hypertension, smoking habits, and diabetes mellitus have been associated to an increased oxidative stress [37]; however, the pre- and postsurgical schedules for the treatment of these risk factors did not change in the present setting.
The present data also provide evidence for a short-term burst of 8-oxodG associated with CABG surgery, highlighting a significant increase of this metabolite 5 days after procedure. A higher than normal oxidative stress has been associated to CABG, and it is considered a risk factor for postoperative complications such as morbidity and mortality, in this very frequent cardiac intervention [1]. The observed increase in oxidative DNA damage and oxidative stress at T1 is likely to reflect the overproduction of ROS due to surgical trauma and/or ischemia-reperfusion process. The contact of blood with polymers of the extracorporeal circuit may also induce neutrophil activation and, in turn, ROS production [38]. However, we did not observe any correlation between ECC or clamping time and 8-oxodG levels in the present setting. Karahalil et al. assessed the short-term effects of CABG surgery on 8-oxodG serum levels [9], but they did not observe any difference in the levels of 8-oxodG in samples obtained before versus after surgery. Differences in the matrices and in the collection times employed may well explain the discrepancies between the present data and those obtained by Karahalil et al.
Our results clearly highlight that CABG surgery causes a burst of oxidative damage both in DNA and lipids (as depicted by the increase of 8-oxodG and 8-isoPGF2α, resp.). In line with other studies in this area [39–41], we have found a direct positive baseline correlation between urinary 8-isoPGF2α and 8-oxodG in samples obtained from CAD patients and CTR. However, England and coworkers showed no correlation between these two markers in healthy subjects [42]. These authors measured these metabolites in white cells (as to 8-oxodG) and in plasma (as to 8-isoPGF2α), leading to the hypothesis that measuring both analytes in the same matrix may probably give a more reliable assessment of the in vivo oxidative stress status and an explanation for the discrepancy between the two data.
In addition to similarities, these two markers of oxidative stress also show potential differences in the oxidative stress evaluation. The fact that, at variance with 8-oxodG, 8-iso-PGF2α levels were entirely comparable to those obtained 6 months after surgery deserves some considerations. Being an index of lipid peroxidation, 8-iso-PGF2α levels are likely to be influenced by plasma cholesterol levels and by the ongoing treatments on total and LDL cholesterol [43]. Table 1 indicates that plasma total, LDL, and HDL cholesterol are significantly higher in CTR than in CAD patients, a finding consistent with an ongoing, probably long-term, statin treatment (as also reported in Table 1) and a more intensive lipid-lowering strategy of (pharmacological/nutritional) intervention in the present CAD setting. In addition, hypertension [44], smoking habits, [45] and diabetes [46] (recognized cardiovascular risk factors) show a positive correlation with 8-iso-PGF2α and their presence in the CTR could mask the differences between the groups.
Our study has some potential limitations. The controls employed do not entirely match the clinical characteristics of the 52 subjects that underwent CABG surgery. However, consecutive patients were chosen, and the inclusion/exclusion criteria employed were stringent enough to avoid major biases. Another possible limitation is the small number of subjects enrolled. Future investigation in the area, with larger numbers of patients, will help to confirm and extend the clinical impact of the present findings.
5. Conclusion
The LC-MS/MS method developed and validated in our laboratory allows monitoring the oxidative DNA damage without interfering with patient's postoperative progress, being urine as one of the simplest biological matrices. The results obtained in this study highlight a short-term oxidative DNA damage induced by CABG procedure in CAD patients and a complete recovery of this oxidative burst six months after the procedure.
Overall, our results argue for normalization of oxidative DNA damage vis-à-vis correction of ischemia-related ROS damage in CAD patients.
Supplementary Material
8-oxodG method validation procedures. Supplemental Table 1. Mobile phase gradient. Supplemental Table 2. Imprecision, LLOQ and LOD of the LC-MS/MS method. Supplemental Table 3. Matrix effect, recovery and process efficiency data for 8-oxodG in urine. Supplemental Table 4. Stability of 8-oxodG in urine.
Abbreviations
- 8-isoPGF2α:
8-Iso-prostaglandin F2α
- 8-oxodG:
8-Oxo-7,8-dihydro-2′-deoxyguanosine
- CABG:
Coronary artery bypass grafting
- CAD:
Coronary artery disease
- CCM:
Centro Cardiologico Monzino
- CI:
Confidence interval
- CTR:
Control subject
- ECC:
Extracorporeal circulation
- GM:
Geometric mean
- HDL:
High-density lipoprotein
- LC–MS/MS:
Liquid chromatography-tandem mass spectrometry
- LDL:
Low-density lipoprotein
- LLOQ:
Lower limit of quantification
- LOD:
Limit of detection
- ME:
Matrix effect
- PE:
Process efficiency
- QC:
Quality control
- RE:
Extraction recovery
- SD:
Standard deviation
- SRM:
Selected reaction monitoring.
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
Linda Turnu and Alessandro Di Minno equally contributed to the present report.
References
- 1.Bravata D. M., Gienger A. L., McDonald K. M., et al. Systematic review: the comparative effectiveness of percutaneous coronary interventions and coronary artery bypass graft surgery. Annals of Internal Medicine. 2007;147(10):703–716. doi: 10.7326/0003-4819-147-10-200711200-00185. [DOI] [PubMed] [Google Scholar]
- 2.Gerritsen W. B., Aarts L. P., Morshuis W. J., Haas F. J. Indices of oxidative stress in urine of patients undergoing coronary artery bypass grafting. European Journal of Clinical Chemistry and Clinical Biochemistry. 1997;35(10):737–742. doi: 10.1515/cclm.1997.35.10.737. [DOI] [PubMed] [Google Scholar]
- 3.Lazzarino G., Raatikainen P., Nuutinen M., et al. Myocardial release of malondialdehyde and purine compounds during coronary bypass surgery. Circulation. 1994;90(1):291–297. doi: 10.1161/01.CIR.90.1.291. [DOI] [PubMed] [Google Scholar]
- 4.Cavalca V., Tremoli E., Porro B., et al. Oxidative stress and nitric oxide pathway in adult patients who are candidates for cardiac surgery: patterns and differences. Interactive Cardiovascular and Thoracic Surgery. 2013;17(6):923–930. doi: 10.1093/icvts/ivt386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-de-la-Asuncion J., Pastor E., Perez-Griera J., et al. Oxidative stress injury after on-pump cardiac surgery: effects of aortic cross clamp time and type of surgery. Redox Report. 2013;18(5):193–199. doi: 10.1179/1351000213Y.0000000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavalca V., Sisillo E., Veglia F., et al. Isoprostanes and oxidative stress in off-pump and on-pump coronary bypass surgery. The Annals of Thoracic Surgery. 2006;81(2):562–567. doi: 10.1016/j.athoracsur.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Luyten C. R., van Overveld F. J., De Backer L. A., et al. Antioxidant defence during cardiopulmonary bypass surgery. European Journal of Cardio-Thoracic Surgery. 2005;27(4):611–616. doi: 10.1016/j.ejcts.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Vogt S., Sattler A., Sirat A. S., et al. Different profile of antioxidative capacity results in pulmonary dysfunction and amplified inflammatory response after CABG surgery. The Journal of Surgical Research. 2007;139(1):136–142. doi: 10.1016/j.jss.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Karahalil B., Kesimci E., Emerce E., Gumus T., Kanbak O. The impact of OGG1, MTH1 and MnSOD gene polymorphisms on 8-hydroxy-2′-deoxyguanosine and cellular superoxide dismutase activity in myocardial ischemia-reperfusion. Molecular Biology Reports. 2011;38(4):2427–2435. doi: 10.1007/s11033-010-0378-6. [DOI] [PubMed] [Google Scholar]
- 10.van Loon B., Markkanen E., Hubscher U. Oxygen as a friend and enemy: how to combat the mutational potential of 8-oxo-guanine. DNA Repair. 2010;9(6):604–616. doi: 10.1016/j.dnarep.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Devasagayam T. P., Steenken S., Obendorf M. S., Schulz W. A., Sies H. Formation of 8-hydroxy(deoxy)guanosine and generation of strand breaks at guanine residues in DNA by singlet oxygen. Biochemistry. 1991;30(25):6283–6289. doi: 10.1021/bi00239a029. [DOI] [PubMed] [Google Scholar]
- 12.Kasai H. Analysis of a form of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutation Research. 1997;387(3):147–163. doi: 10.1016/S1383-5742(97)00035-5. [DOI] [PubMed] [Google Scholar]
- 13.Beckman K. B., Ames B. N. Oxidative decay of DNA. The Journal of Biological Chemistry. 1997;272(32):19633–19636. doi: 10.1074/jbc.272.32.19633. [DOI] [PubMed] [Google Scholar]
- 14.Cheng K. C., Cahill D. S., Kasai H., Nishimura S., Loeb L. A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G–T and A–C substitutions. The Journal of Biological Chemistry. 1992;267(1):166–172. [PubMed] [Google Scholar]
- 15.Serdar M., Sertoglu E., Uyanik M., et al. Comparison of 8-hydroxy-2′-deoxyguanosine (8-oxodG) levels using mass spectrometer and urine albumin creatinine ratio as a predictor of development of diabetic nephropathy. Free Radical Research. 2012;46(10):1291–1295. doi: 10.3109/10715762.2012.710902. [DOI] [PubMed] [Google Scholar]
- 16.Broedbaek K., Weimann A., Stovgaard E. S., Poulsen H. E. Urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine as a biomarker in type 2 diabetes. Free Radical Biology & Medicine. 2011;51(8):1473–1479. doi: 10.1016/j.freeradbiomed.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Siomek A., Tujakowski J., Gackowski D., et al. Severe oxidatively damaged DNA after cisplatin treatment of cancer patients. International Journal of Cancer. 2006;119(9):2228–2230. doi: 10.1002/ijc.22088. [DOI] [PubMed] [Google Scholar]
- 18.Di Minno A., Turnu L., Porro B., et al. 8-Hydroxy-2-deoxyguanosine levels and heart failure: a systematic review and meta-analysis of the literature. Nutrition, Metabolism, and Cardiovascular Diseases. 2017;27(3):201–208. doi: 10.1016/j.numecd.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Di Minno A., Turnu L., Porro B., et al. 8-Hydroxy-2-deoxyguanosine levels and cardiovascular disease: a systematic review and meta-analysis of the literature. Antioxidants & Redox Signaling. 2016;24(10):548–555. doi: 10.1089/ars.2015.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans M. D., Olinski R., Loft S., Cooke M. S. Toward consensus in the analysis of urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine as a noninvasive biomarker of oxidative stress. FASEB Journal. 2010;24(4):1249–1260. doi: 10.1096/fj.09-147124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delanty N., Reilly M. P., Pratico D., et al. 8-epi PGF2α generation during coronary reperfusion. A potential quantitative marker of oxidant stress in vivo. Circulation. 1997;95(11):2492–2499. doi: 10.1161/01.CIR.95.11.2492. [DOI] [PubMed] [Google Scholar]
- 22.Andreoli R., Manini P., De Palma G., et al. Quantitative determination of urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine, 8-oxo-7,8-dihydroguanine, 8-oxo-7,8-dihydroguanosine, and their non-oxidized forms: daily concentration profile in healthy volunteers. Biomarkers. 2010;15(3):221–231. doi: 10.3109/13547500903434501. [DOI] [PubMed] [Google Scholar]
- 23.Bogdanov M. B., Beal M. F., McCabe D. R., Griffin R. M., Matson W. R. A carbon column-based liquid chromatography electrochemical approach to routine 8-hydroxy-2′-deoxyguanosine measurements in urine and other biologic matrices: a one-year evaluation of methods. Free Radical Biology & Medicine. 1999;27(5-6):647–666. doi: 10.1016/S0891-5849(99)00113-6. [DOI] [PubMed] [Google Scholar]
- 24.Cavalca V., Minardi F., Scurati S., et al. Simultaneous quantification of 8-iso-prostaglandin-F2α and 11-dehydro thromboxane B2 in human urine by liquid chromatography-tandem mass spectrometry. Analytical Biochemistry. 2010;397(2):168–174. doi: 10.1016/j.ab.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 25.U.D.o.H.a.H.S. Food and Drug Administration F. Guidance for Industry: Bioanalytical Method Validation. 2013. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm368107.pdf.
- 26.Matuszewski B. K., Constanzer M. L., Chavez-Eng C. M. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Analytical Chemistry. 2003;75(13):3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 27.Barregard L., Moller P., Henriksen T., et al. Human and methodological sources of variability in the measurement of urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine. Antioxidants & Redox Signaling. 2013;18(18):2377–2391. doi: 10.1089/ars.2012.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu C. W., Wu M. T., Chao M. R., et al. Comparison of analyses of urinary 8-hydroxy-2′-deoxyguanosine by isotope-dilution liquid chromatography with electrospray tandem mass spectrometry and by enzyme-linked immunosorbent assay. Rapid Communications in Mass Spectrometry. 2004;18(4):505–510. doi: 10.1002/rcm.1367. [DOI] [PubMed] [Google Scholar]
- 29.Rossner P., Jr., Mistry V., Singh R., Sram R. J., Cooke M. S. Urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine values determined by a modified ELISA improves agreement with HPLC-MS/MS. Biochemical and Biophysical Research Communications. 2013;440(4):725–730. doi: 10.1016/j.bbrc.2013.09.133. [DOI] [PubMed] [Google Scholar]
- 30.Xiang F., Shuanglun X., Jingfeng W., et al. Association of serum 8-hydroxy-2′-deoxyguanosine levels with the presence and severity of coronary artery disease. Coronary Artery Disease. 2011;22(4):223–227. doi: 10.1097/MCA.0b013e328344b615. [DOI] [PubMed] [Google Scholar]
- 31.Gackowski D., Kruszewski M., Jawien A., Ciecierski M., Olinski R. Further evidence that oxidative stress may be a risk factor responsible for the development of atherosclerosis. Free Radical Biology & Medicine. 2001;31(4):542–547. doi: 10.1016/S0891-5849(01)00614-1. [DOI] [PubMed] [Google Scholar]
- 32.Kaya Y., Cebi A., Soylemez N., Demir H., Alp H. H., Bakan E. Correlations between oxidative DNA damage, oxidative stress and coenzyme Q10 in patients with coronary artery disease. International Journal of Medical Sciences. 2012;9(8):621–626. doi: 10.7150/ijms.4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arao K., Yasu T., Umemoto T., et al. Effects of pitavastatin on fasting and postprandial endothelial function and blood rheology in patients with stable coronary artery disease. Circulation Journal. 2009;73(8):1523–1530. doi: 10.1253/circj.CJ-08-0917. [DOI] [PubMed] [Google Scholar]
- 34.Jaruga P., Rozalski R., Jawien A., Migdalski A., Olinski R., Dizdaroglu M. DNA damage products (5'R)- and (5′S)-8,5′-cyclo-2′-deoxyadenosines as potential biomarkers in human urine for atherosclerosis. Biochemistry. 2012;51(9):1822–1824. doi: 10.1021/bi201912c. [DOI] [PubMed] [Google Scholar]
- 35.Martinet W., Knaapen M. W., De Meyer G. R., Herman A. G., Kockx M. M. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation. 2002;106(8):927–932. doi: 10.1161/01.CIR.0000026393.47805.21. [DOI] [PubMed] [Google Scholar]
- 36.Botto N., Masetti S., Petrozzi L., et al. Elevated levels of oxidative DNA damage in patients with coronary artery disease. Coronary Artery Disease. 2002;13(5):269–274. doi: 10.1097/00019501-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Vassalle C., Petrozzi L., Botto N., Andreassi M. G., Zucchelli G. C. Oxidative stress and its association with coronary artery disease and different atherogenic risk factors. Journal of Internal Medicine. 2004;256(4):308–315. doi: 10.1111/j.1365-2796.2004.01373.x. [DOI] [PubMed] [Google Scholar]
- 38.Kevin L. G., Novalija E., Stowe D. F. Reactive oxygen species as mediators of cardiac injury and protection: the relevance to anesthesia practice. Anesthesia and Analgesia. 2005;101(5):1275–1287. doi: 10.1213/01.ANE.0000180999.81013.D0. [DOI] [PubMed] [Google Scholar]
- 39.Basili S., Tanzilli G., Mangieri E., et al. Intravenous ascorbic acid infusion improves myocardial perfusion grade during elective percutaneous coronary intervention: relationship with oxidative stress markers. JACC Cardiovascular Interventions. 2010;3(2):221–229. doi: 10.1016/j.jcin.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 40.Harman S. M., Liang L., Tsitouras P. D., et al. Urinary excretion of three nucleic acid oxidation adducts and isoprostane F2α measured by liquid chromatography-mass spectrometry in smokers, ex-smokers, and nonsmokers. Free Radical Biology & Medicine. 2003;35(10):1301–1309. doi: 10.1016/j.freeradbiomed.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Yamano Y., Miyakawa S., Nakadate T. Association of arteriosclerosis index and oxidative stress markers in school children. Pediatrics International. 2015;57(3):449–454. doi: 10.1111/ped.12545. [DOI] [PubMed] [Google Scholar]
- 42.England T., Beatty E., Rehman A., et al. The steady-state levels of oxidative DNA damage and of lipid peroxidation (F2-isoprostanes) are not correlated in healthy human subjects. Free Radical Research. 2000;32(4):355–362. doi: 10.1080/10715760000300351. [DOI] [PubMed] [Google Scholar]
- 43.Lee T. M., Chou T. F., Tsai C. H. Association of pravastatin and left ventricular mass in hypercholesterolemic patients: role of 8-iso-prostaglandin F2α formation. Journal of Cardiovascular Pharmacology. 2002;40(6):868–874. doi: 10.1097/00005344-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Rodrigo R., Prat H., Passalacqua W., Araya J., Guichard C., Bachler J. P. Relationship between oxidative stress and essential hypertension. Hypertension Research. 2007;30(12):1159–1167. doi: 10.1291/hypres.30.1159. [DOI] [PubMed] [Google Scholar]
- 45.Lowe F. J., Gregg E. O., McEwan M. Evaluation of biomarkers of exposure and potential harm in smokers, former smokers and never-smokers. Clinical Chemistry and Laboratory Medicine. 2009;47(3):311–320. doi: 10.1515/CCLM.2009.069. [DOI] [PubMed] [Google Scholar]
- 46.Devangelio E., Santilli F., Formoso G., et al. Soluble RAGE in type 2 diabetes: association with oxidative stress. Free Radical Biology & Medicine. 2007;43(4):511–518. doi: 10.1016/j.freeradbiomed.2007.03.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
8-oxodG method validation procedures. Supplemental Table 1. Mobile phase gradient. Supplemental Table 2. Imprecision, LLOQ and LOD of the LC-MS/MS method. Supplemental Table 3. Matrix effect, recovery and process efficiency data for 8-oxodG in urine. Supplemental Table 4. Stability of 8-oxodG in urine.