Abstract
Background
A single institution prospective study was conducted to assess disease control and toxicity of proton therapy for patients with head and neck cancer.
Methods
Disease control, toxicity, functional outcomes and patterns of failure for the initial cohort of patients with oropharyngeal squamous carcinoma (OPC) treated with intensity modulated proton therapy (IMPT) were prospectively collected in two registry studies at a single institution. Locoregional failures were analyzed utilizing deformable image registration methodology.
Results
Fifty patients with OPC treated from 03/2011 to 07/2014 formed the cohort. Eight-four percent were male, 50% were never smokers, 98% were p16+, 98% had stage III/IV disease, 64% received concurrent and 35% received induction chemotherapy. Forty-four of 45 (98%) tumors tested for p16 were positive. All patients were treated with IMPT (multi-field optimization n=46; single-field optimization n=4). No CTC-AE grade 4/5 toxicities were observed. The most common grade 3 acute and late toxicities observed were acute mucositis and late dysphagia, in 58% and 12%, respectively. Eleven patients had a feeding gastrostomy placed during therapy, but none had a feeding tube at last follow up. With a median follow up of 29 months, 5 patients had disease recurrence: local in 1, local and regional in 1, regional in 2, and distant in 1. The 2-year actuarial overall and progression free survival were 94.5% and 88.6%, respectively.
Conclusion
The oncologic, toxicity and functional outcomes following IMPT for OPC are encouraging and provide the basis for ongoing and future clinical studies.
Keywords: Intensity-modulated proton therapy, oropharyngeal cancer, radiation therapy, chemoradiation, human papillomavirus, HPV
Introduction
Recent advances in proton therapy planning and delivery, particularly spot-scanning intensity modulated proton therapy (IMPT) techniques, have provided the ability to generate the dose distributions that are required to treat geometrically complex head and neck target volumes (1). Following development of planning techniques (2, 3), completion of necessary dosimetric comparison studies (4, 5), and implementation of plan quality assurance measurement procedures (6), our clinical proton therapy program for head and neck cancer was established.
The first pioneering clinical results of proton therapy for the treatment of oropharyngeal cancer (OPC) have been reported with passively scattered beams by the Loma Linda University Medical Center ten years ago as a boost in combination with photon radiotherapy (7). The technique has since then gained interest for numerous reasons (8). Most patients presenting with OPC today are younger, well-functioning, and have a largely favorable prognosis, particularly those with human papillomavirus-associated disease, and thus they have the potential to be greatly impacted by acute toxicities and to live for decades with the late effects of therapy. Toxicity reduction is now the current emphasis in numerous ongoing clinical studies in OPC, generally through treatment deintensification by total radiation therapy dose reduction (reduced tumor dose) and/or modification of systemic therapy (9).
Proton therapy offers another approach to investigate toxicity reduction in OPC. Given its reduction in integral dose to non-target structures compared to IMRT, particularly those anterior and posterior to the target volumes, proton therapy may reduce the “beam path toxicity” that has been observed with IMRT (10, 11). While we have previously described our initial clinical experience with spot-scanning IMPT using multi-field optimization for the treatment of head and neck tumors (1), detailed OPC-specific IMPT clinical outcomes reports are needed to inform patients, clinicians, and investigators.
Toward this end, the goals of the present study were to: 1) report the oncologic outcomes of our initial prospective cohort of patients treated with spot-scanning IMPT for OPC; 2) report the observed acute and late treatment-related toxicity and functional outcomes; 3) characterize the patterns of failure and analyze the patterns of locoregional failure; and 4) generate testable hypothesis for future study.
Methods
Patients
Adults receiving proton therapy for non-metastatic head and neck cancer at our institution were eligible for participation in an institutional review board–approved quality of life study where clinical outcomes were prospectively recorded. The first registry study was for all tumor sites (ClinicalTrials.org Identifiers: NCT XXXX) and collected baseline and follow-up clinical data. The second was specific to head and neck cancer patients (NCT XXXX) and also included the collection of patient reported outcomes (MD Anderson Dysphagia Inventory and Symptom Inventory, Xerostomia Questionnaire and the Functional Assessment of Cancer Therapy Head and Neck) on a weekly basis during treatment and every 3-6 months after treatment. Participants provided study-specific informed consent. No distinct clinical criteria were used for patient selection, and all patients with OPC treated with curative intent are generally considered candidates for IMRT or IMPT. The potential of treatment on other ongoing clinical trials at our institution and proton therapy institutional registries were considered and ultimate treatment decisions were made considering patient and physician preference as part of the shared decision process. For this analysis, we considered the initial 50 consecutive patients with OPC treated at our center with spot-scanning IMPT with curative intent, out of an estimated 600 OPC patients treated at our institution over the same time period. Prior to initiation of therapy, all patients underwent staging imaging, pathologic confirmation of disease, multidisciplinary evaluation within our institution, including evaluations by head and neck surgery, medical oncology, and dental oncology specialists, and all cases were presented at our head and neck cancer multidisciplinary tumor board for individualized treatment recommendations regarding sequence and combination of modalities.
Treatment
The majority of OPC cases managed at our institution are treated with a radiation therapy-based approach (12). Our general OPC treatment philosophies, including integration of systemic therapy (13), target volume delineation (14), and post-radiation therapy neck management (15) have previously been described elsewhere. Regarding simulation for proton therapy, patients underwent non-contrast computed tomography (CT) simulation immobilized in the supine position using a posterior customized head, neck and shoulder mold, full length thermoplastic mask, and bite block with or without an oral stent. Target volumes and organs at risk were manually delineated on each axial CT slice following standard contouring guidelines. Following initial target volume delineation but prior to treatment planning, each patient underwent group physical examination, including flexible fiberoptic examination as appropriate; the proposed treatment strategy and target volumes were then peer-reviewed within our Head and Neck Radiation Oncology Planning and Development Clinic for quality assurance purposes (16). Target volumes were then finalized and treatment planning initiated.
IMPT doses were prescribed using a relative biological effectiveness (RBE) value of 1.1. Generally, 66 Gy (RBE) was prescribed for small volume disease and 70 Gy (RBE) for more advanced disease. IMPT plans used a simultaneous integrated boost technique, where lower daily doses were delivered to surrounding soft tissue and lymphatic regions at risk of harboring microscopic disease; depending on the estimated risk and number of fractions, the elective regions received 54 to 63 Gy (RBE). Regarding neck volumes, bilateral neck irradiation was pursued in all cases except for carefully selected patients with well-lateralized tonsil cancers, where ipsilateral neck irradiation was considered (17).
Eclipse proton therapy treatment planning system (version 8.9, Varian Medical Systems, Palo Alto, California) was used for IMPT planning. Typically, 3 beams were used for whole-field bilateral neck IMPT plans: a left and right anterior oblique and single posterior beam, as this beam arrangement was shown to optimize target coverage while minimizing dose to the brain, brainstem, spinal cord, oral cavity, salivary glands, and larynx (1, 18). Multi-field optimization was accomplished by simultaneously optimizing the spot intensities from all fields, with the objective of covering at least 95% of the target volumes with the prescribed doses while minimizing and appropriately balancing the dose among normal structures. The spot size ranged from 0.5cm-1.4cm in air at the isocenter. The robustness of each treatment plan was also considered in order to evaluate the sensitivity to uncertainties associated with variations in patient setup (3mm in every direction) and proton beam range (+/- 3.5%) in each patient (19, 20). For unilateral cases, single-field optimization was employed to achieve prescribed doses in the target volumes and minimum doses outside.
Prior to treatment delivery, plan-specific quality assurance was performed including measurements, independent dose calculation, and analysis of patient-specific treatment delivery log files with appropriate modifications, as described by Zhu et al (6). Daily orthogonal 2-dimensional kilovoltage x-ray images were compared with digitally reconstructed radiographs generated by the treatment planning system from simulation CT images to align the patient for image guidance. For additional verification, patients underwent verification CT simulation during week 1 and 4 of therapy or more frequently to determine the effects of weight loss or change in external contour of the patient due to disease regression. Adaptive re-planning was considered on a case-by-case basis and performed at the judgment of the treating physician.
Evaluations and data collection
Patients were evaluated weekly during treatment by the treating radiation oncologist. Feeding tube placement was based on a reactive approach in case of significant weight loss (5-10%), after a discussion involving the patient, treating radiation oncologist and dietician. Initial post-treatment evaluations were made at 8-12 weeks after therapy completion and subsequently every 2-3 months for the first year, every 3-4 months for the second year, and at least twice a year up to 5 years. Data were prospectively recorded according to predefined data collection forms, and included baseline patient, tumor and treatment characteristics, and oncologic outcomes. Additionally, at each visit, toxicity endpoints were assessed by the treating physician according to the Common Terminology Criteria for Adverse Events, version 4.03 scale (21). Acute toxicities were those observed between the start of radiotherapy and for 90 days after the end of radiotherapy, whereas late toxicities were those observed beyond 90 days.
Patterns of failure analysis
Cases where local and/or regional disease were recorded had their immediate post-failure diagnostic images exported as DICOM files from the clinical PACS system to the treatment planning system, where radiological evident recurrent gross disease (recGTV) was manually contoured. For each patient, the recurrence CT was co-registered with planning CT using deformable image registration (DIR) techniques. DIR was performed using Velocity AI v.3.01 commercial software (Varian Medical Systems, Atlanta, GA) validated previously by our group (22). Subsequently, the deformation vector fields were applied to recGTVs to convert them into ‘deformed recGTVs’ and transferred to the planning CT (supplementary figure 1). Evaluation of deformed recGTVs relative to original planning target volumes and prescribed radiation dose was done using both volumetric and dosimetric assessment (23, 24).
Statistical analysis
Categorical data are presented as crude numbers and percentages and continuous data are presented as median and range. Follow-up was calculated using the reverse Kaplan Meier Methods (25). Survival times were computed from the date of the end of radiotherapy to the occurrence of the first event. Events were death from any cause for overall survival and any recurrence or death for progression-free survival. Actuarial survival rates are computed and displayed using the Kaplan Meier method. Analyses were performed with JMP Pro v11 (SAS Institute Inc, Cary, North Carolina).
Results
Patient, tumor and treatment characteristics
From 03/2011 to 07/2014, 50 patients with OPC participated in our study and were treated with IMPT. Patient and tumor characteristics are displayed in Table 1, T- and N-categorization in Table 2, and treatment characteristics in Table 3. Induction chemotherapy regimens were in majority platinum and taxane based. One patient was treated postoperatively with concurrent chemo-IMPT following transoral robotic surgery and neck dissection. Two other patients had a neck excision/dissection prior to IMPT. Neck dissection following IMPT was performed in 6 patients due to residual nodal remnants, and three of these had viable residual disease on final pathology with subsequent regional control in all 6 patients. Doses to target volumes and organs at risk are reported in supplementary table 1 and two representative patient treatment plans are shown in figure 1. Nineteen patients (38%) have had an adaptive re-planning during IMPT due to weight loss and/or tumor volume changes, including one patient who required adaptive re-planning twice.
Table 1. Patient and tumor characteristics.
| n | % | |
|---|---|---|
| Sex | ||
| Male | 42 | 84% |
| Female | 8 | 16% |
| Race | ||
| White | 42 | 84% |
| Hispanic | 5 | 10% |
| African American | 1 | 2% |
| Asian | 1 | 2% |
| Other | 1 | 2% |
| Median age at diagnosis (range) | 61 years (37-84) | |
| Patient smoking status | ||
| Never | 25 | 50% |
| Current | 23 | 46% |
| Former | 2 | 4% |
| Primary sub-site | ||
| Tonsil | 27 | 54% |
| Base of tongue | 21 | 42% |
| Glossopharyngeal sulcus | 2 | 4% |
| Stage | ||
| I | 1 | 2% |
| II | 0 | 0% |
| III | 9 | 18% |
| IVA | 37 | 74% |
| IVB | 3 | 6% |
| Tumor p16 status | ||
| Positive | 44 | 88% |
| Unknown | 5 | 10% |
| Negative | 1 | 2% |
Table 2. T- and N-categorization.
| N0 | N1 | N2a | N2b | N2c | N3 | Total | |
|---|---|---|---|---|---|---|---|
| T1 | 1 | 5 | 5 | 4 | 0 | 0 | 15 |
| T2 | 0 | 1 | 1 | 17 | 5 | 1 | 25 |
| T3 | 0 | 3 | 0 | 2 | 1 | 0 | 6 |
| T4 | 0 | 0 | 0 | 1 | 2 | 1 | 4 |
| Total | 1 | 9 | 6 | 24 | 8 | 2 | 50 |
Table 3. Treatment characteristics.
| n | % | |
|---|---|---|
| Treatment strategy | ||
| Concurrent chemo-IMPT | 16 | 32% |
| IC → concurrent chemo-IMPT | 15 | 30% |
| IMPT alone | 13 | 26% |
| IC → IMPT alone | 5 | 10% |
| TORS → concurrent chemo-IMPT | 1 | 2% |
| Concurrent chemotherapy | ||
| Cisplatin | 13 | 41% |
| Carboplatin | 8 | 25% |
| Cetuximab | 11 | 34% |
| None | 18 | |
| Median dose (range) | 70 Gy (60-70) | |
| Median IMPT duration (range) | 45 days (36-57) | |
| Neck radiotherapy volume | ||
| Bilateral | 40 | 80% |
| Unilateral | 10 | 20% |
| Procedures/surgery prior to IMPT | ||
| Diagnostic tonsillectomy | 12 | 24% |
| Neck dissection | 1 | 2% |
| Tonsillectomy and neck dissection | 1 | 2% |
| TORS and neck dissection | 1 | 2% |
| None | 35 | 70% |
IC=induction chemotherapy; IMPT=intensity modulated proton therapy; Gy=Gray; TORS=transoral robotic surgery
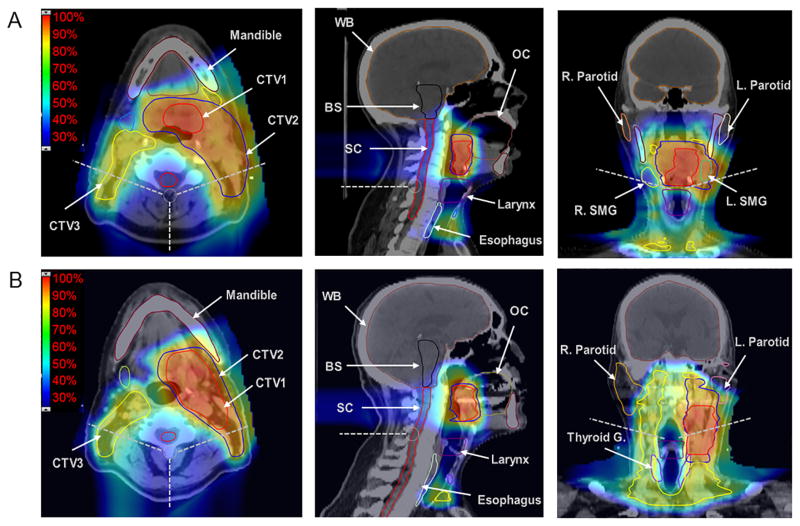
Figure 1. Axial and coronal views of two representative treatment plans for base of tongue (A) and tonsil carcinoma (B) patients.
Case A: 50-year old male, left base of tongue, T1 Nx(2b). Mean doses (Gy RBE): ipsi parotid 23.4, contra parotid 16.8, ipsi SMG 63.6, contra SMG 34.6, OC 9, larynx 28.3, esophagus 29.5, man 27.4, SPC 54, MPC 59.9, IPC 34.9;
Case B: 75-year-old male, left tonsil, T2 N2b. Mean doses (Gy RBE): ipsi parotid 35.1, contra parotid 14.6, ipsi SMG 68.6, contra SMG 27.3, OC 13.4, larynx 29.9, esophagus 11.7, man 18.5, SPC 59.7, MPC 44.8, IPC 27.9
Abbreviations: BS, brainstem; contra, contralateral; CTV, clinical target volume; IPC, inferior pharyngeal constrictor; ipsi, ipsilateral; L, left; man, mandible; MPC, middle pharyngeal constrictor; OC, oral cavity; R, right; SC, spinal cord; SMG, submandibular gland; SPC, superior pharyngeal constrictor; Thyroid G, thyroid gland; WB, whole brain
Survival analysis
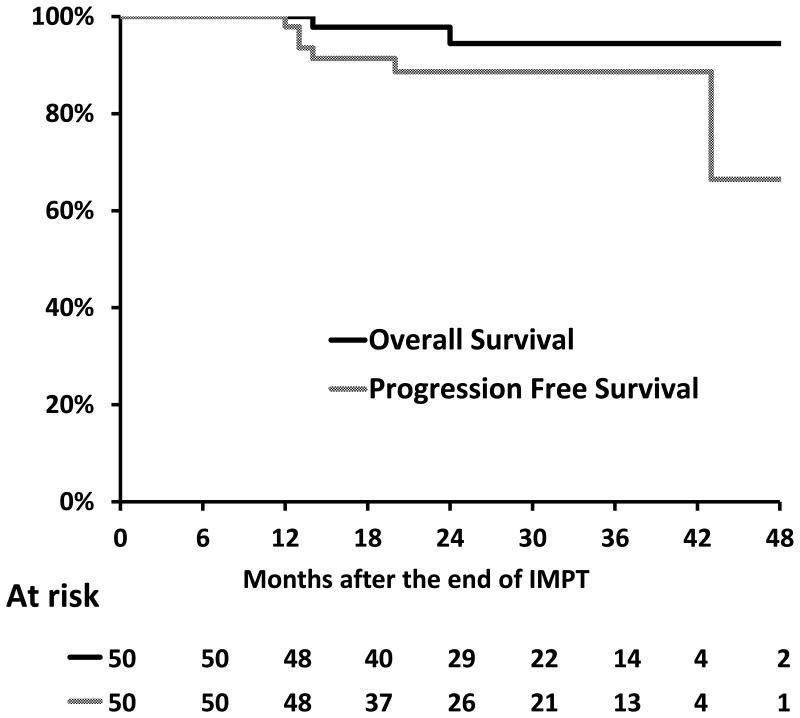
The median follow-up of the cohort is 29 months (range: 8-49), with 49 patients having more than 1 year follow-up. There were respectively 48, 26 and 13 patients alive and followed up more than one, two or three years. At last follow-up, 2 patients had died, 1 of unknown cause and 1 of locoregional progression. Forty-five patients were alive and disease free, 1 alive undergoing salvage therapy, 1 alive with active disease, and 1 was alive with an unknown disease status due to lack of follow up in our center. The Kaplan Meier curves for overall and progression free survival are displayed in Figure 2. The 2-year overall survival rate and progression free survival were 94.5% (95% Confidence interval (CI): 81.4, 98.5) and 88.6% (95% CI: 75.8, 95.1), respectively.
Figure 2. Actuarial overall and progression free survival.
Toxicity
The treatment-related acute and late toxicity profile is reported in Table 4. Acute side effects were dominated by dermatitis, mucositis and dysphagia. Sixteen patients (32%) had an unplanned visit to our emergency center during treatment followed by hospitalization in 10 patients (20%), in most cases due to dehydration and pain resulting from oral mucositis with subsequent acute odynophagia.
Table 4. Peak severity of acute and late toxicities observed per patient according to Common Terminology Criteria for Adverse Events, version 4.03.
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Acute | ||||||||
| Dermatitis radiation | 1 | 2% | 5 | 10% | 21 | 42% | 23 | 46% |
| Oral mucositis | 2 | 4% | 1 | 2% | 18 | 36% | 29 | 58% |
| Dysphagia | 11 | 22% | 9 | 18% | 18 | 36% | 12 | 24% |
| Weight Loss | 25 | 50% | 20 | 40% | 4 | 8% | 1 | 2% |
| Dry Mouth | 38 | 76% | 7 | 14% | 4 | 8% | 1 | 2% |
| Dysgeusia | 13 | 26% | 13 | 26% | 24 | 48% | ||
| Late | ||||||||
| Dysphagia | 20 | 40% | 11 | 22% | 13 | 26% | 6 | 12% |
| Dry Mouth | 1 | 2% | 23 | 46% | 25 | 50% | 1 | 2% |
| Dysgeusia | 12 | 24% | 24 | 48% | 14 | 28% | ||
| Oral mucositis | 40 | 80% | 7 | 14% | 3 | 6% | 1 | 2% |
There were no grade 4/5 events observed.
Median weight loss was 7.4%, with 6 patients experiencing more than 10% weight loss and one more than 20% weight loss. One patient had a feeding tube placed before the start of treatment due to poor initial nutritional status, while 11 other patients have had a feeding gastrostomy tube placed during radiotherapy, with median tube duration of 82 days following treatment completion (range: 28-497). Regarding swallowing assessments and outcomes, symptomatic patients were referred for modified barium swallow studies (n=12 before IMPT, n=1 <90 days after IMPT, and n=9 between 90 days and 2 years post-IMPT). Aspiration was detected on modified barium swallow in 1 patient at baseline (Penetration-Aspiration Scale score = 8, “silent aspiration”) and persisted on follow up study in a 56 year old male who had an extensive T4N2b tonsil cancer invading adjacent swallowing-critical regions of the soft palate, and base of tongue.
Among significant late effects, five patients had their feeding tube for more than three months after radiotherapy. One patient required his feeding tube more than one year after radiotherapy and had it eventually removed 18 months after placement. No patient had persistent grade 3 or higher dysphagia at last follow-up. New onset, post-IMPT aspiration or esophageal stricture was not detected in any patient. One patient developed oropharyngeal mucosal ulceration 16 months after treatment completion with stabilization of the ulcer and improvement in symptoms following hyperbaric oxygen therapy. Fifty-two percent of patients had grade 2+ xerostomia at one moment during follow up. At last follow-up, among the 48 patients alive, 10 (21%) had grade 2 xerostomia, 32 (67%) grade 1 xerostomia, and 6 (12%) had grade 0. Of note, no myelitis, bone necrosis, trismus of any grade, grade 2+ subcutaneous fibrosis, nor grade 2+ fatigue were recorded in the long run and no patient required tracheotomy.
Patterns of failure
Patient, tumor, treatment and recurrence characteristics are detailed in supplementary table 2. Overall the pattern of failure was as follows: two regional relapses, one local relapse, one local and regional relapse and one biopsy proven distant relapse (lung). One additional patient had his local and/or regional relapse evaluated at an outside facility, details of which were not available. This patient initially presented with synchronous primaries of the oropharynx and hypopharynx, both classified as T4, and his relapse is felt to likely be in field as the treatment volume was required to be large. Pattern of failure analysis was therefore performed on the three remaining patients with locoregional relapse for whom imaging of the recurrence was available. The two patients with regional recurrences had in field relapses (three nodal volumes in these two patients), located within initial GTV or CTV 1 or 2. The sites of relapse are estimated to have received a minimum dose of 72.6, 66.4 and 65.2 Gy (RBE), respectively, and a dose to 95% of the recGTV of 73.2, 67.9 and 65.8 Gy (RBE), respectively. In the final patient the relapse is classified as out of field and could represent a second primary. Indeed this patient initially presented with a left tonsil T2N2b p16 positive tumor and then recurred 43 months after completion of treatment in the right (contralateral) glossopharyngeal sulcus associated with right level Ib adenopathy. Minimum dose and dose to 95% of the relapse volume in this case were 62.9 and 64.8 Gy (RBE) for the second primary tumor and 41.9 and 47.5 Gy (RBE) for the level Ib node. Failure analysis is illustrated in the supplementary figure 2.
Discussion
The disease control, acute and late toxicity, and swallowing functional outcomes following IMPT for OPC were largely favorable. The 2-year overall and progression free survival of 88.6% and crude locoregional failure rate of 8% reported here are comparable to the contemporary series of OPC treated with IMRT at our institution, where the 2-year overall and progression free survival rates were 92% and 88%, respectively (14). While all but one patient in our series had a minimum follow up of one year and the majority had more than two years, comparisons of longer-term mature results are needed.
The acute toxicity profile appears favorable, specifically placement of feeding tubes during therapy. Overall, 24% in this study had a feeding tube placed before or during therapy, where 47% required feeding tube placement in the aforementioned IMRT series (14). The potential of proton therapy to reduce the requirement for feeding tube placement during treatment compared to IMRT has been suggested in matched pair comparisons in both nasopharyngeal cancer (26) and OPC (27), where an approximate 50% or greater reduction in feeding tube requirement was seen with IMPT, likely related to dose reduction to the non-target structures. Of interest, feeding tube placement is considered a surrogate for grade 3 for numerous toxicities, and especially dysphagia, nausea/vomiting, xerostomia or weight loss. These studies might however be biased and need replication in prospective trials.
Likewise, the observed late toxicity and swallowing functional outcomes were encouraging, in that all feeding tubes had been removed at last follow up, with median feeding tube duration of approximately two months from the completion of treatment, and no long term high grade aspiration nor any esophageal strictures have been observed to date. Preventive swallowing therapy emphasizing maximal use of the swallowing mechanism during IMPT (“eat” and “exercise”) routinely implemented during the study period likely also contributes to favorable functional outcomes (28). Just over 50% of patients experienced late grade 2+ xerostomia at some time point during follow up, but only 12 patients (25%) had grade 2 xerostomia at last follow-up. This is in agreement with the fact that long term xerostomia can improve over time (29), and even longer follow-up with detailed longitudinal reporting and dosimetric correlation will be of interest.
The inherent limitations of a single-institution series of modest power apply here. While we did focus on OPC, a head and neck anatomic site of particular interest to investigators, additional analyses and studies will seek to incorporate baseline and longitudinal patient reported outcomes, quantitative dosimetric factors, and serial objective toxicity and functional measures, such as longitudinal sialometry and routine swallowing evaluations and modified barium swallow studies.
Acknowledging these caveats, this prospective series represents the only OPC-specific report detailing the previously lacking disease control and toxicity outcomes with IMPT. The presented data have significant utility in that they demonstrate the feasibility and proof of principle of advanced proton therapy techniques delivering simultaneous integrated boost plans that have become standard with IMRT, thus laying the ground work for a direct head to head comparison study.
Regarding patterns of disease recurrence, aside from the patient with synchronous T4 primaries of the oropharynx and hypopharynx for who details of his recurrence where not available, no other patient developed a primary site recurrence within the targeted high-risk volume. We did code one failure as local for the purpose of this analysis, yet it could represent a contralateral second primary. The other two patients with locoregional recurrences had isolated regional recurrences within previously targeted regions, and underwent salvage surgical resection. We continue to follow our current neck evaluation and management paradigm, and incorporate post-radiotherapy neck dissection for persistent nodal disease after therapy, a surgical decision increasingly supplemented by PET/CT and/or US-FNA of nodal remnants (15). We continue to prospectively track and analyze disease control outcomes for quality improvement purposes. Overall, the pattern of failure reported provides further clinical validation of the robustness of the modulated dose distributions generated by IMPT, particularly at the primary tumor site.
On the basis of this initial experience and these encouraging disease control and toxicity results, and given the reduction of integral dose to non-target normal tissues afforded by IMPT (30), we are presently conducting a prospective randomized phase II/III trial comparing IMPT and IMRT for patients with stage III/IV oropharyngeal cancer, with the current primary endpoint of toxicity reduction (ClinicalTrials.gov Identifier: XXXX).
Supplementary Material
Supplementary Figure 1. Deformable image registration methodology for patterns of failure analysis: Contouring of recurrence GTV was done on patient's PET/CT documenting recurrence then deformable image registration (DIR) was applied to deform the PET/CT to the original planning CT. The deformation vector fields were then applied to rec-GTVs to convert it into ‘deformed rec-GTVs’ on the planning CT to calculate volumetric and dosimetric failure parameters.
Supplementary Figure 2: Failure analysis for those with locoregional failure and diagnostic imaging demonstrating the failure available for review.
A) Right level IIa nodal recurrence 12 months post-IMPT mapped to planning CT and dose where the centroid of the rec-GTV is located in GTV and minimum dose to rec-GTV was 72.6 Gy. B) Left level IIa nodal recurrence 20 months post-IMPT mapped to planning CT and dose where the centroid of the rec-GTV is located in CTV1 and minimum dose to rec-GTV was 66.4 Gy. C) Right (contralateral) glossopharyngeal sulcus recurrence 43 months post-IMPT mapped to planning CT and dose where the centroid of the rec-GTV is located outside target volumes and minimum dose to rec-GTV was 62.9 Gy.
Supplementary table 1. Dosimetric parameters for clinical target volumes (CTV) and selected organs at risk using IMPT for oropharyngeal carcinomas
Supplementary Table 2. Patient, disease, and treatment characteristics and outcomes for 5 patients who developed any disease recurrence following proton therapy for oropharyngeal cancer.
Acknowledgments
Grant or financial support: Supported in part by the National Institutes of Health (NIH)/National Cancer Institute (NCI) Cancer Center Support (Core) Grant CA016672 and a U19 CA021239 to The University of Texas MD Anderson Cancer Center. Dr. Fuller received/receives grant and/or salary support from: the Paul Calabresi Clinical Oncology Award Program (K12 CA088084-06); NIH/NCI Clinician Scientist Loan Repayment Program (L30 CA136381-02); the SWOG/Hope Foundation Dr. Charles A. Coltman, Jr., Fellowship in Clinical Trials; a General Electric Healthcare/MD Anderson Center for Advanced Biomedical Imaging In-Kind Award: an Elekta AB/MD Anderson Department of Radiation Oncology Seed Grant: the Center for Radiation Oncology Research at MD Anderson Cancer Center; and the MD Anderson Institutional Research Grant Program. Dr Blanchard received funding from The Foundation Nuovo Soldati for Medical Research, the Philippe Foundation and the FRM grant SPE20150331822. Dr. Hutcheson receives grant support from the MD Anderson Institutional Research Grant Program and the National Cancer Institute (R03 CA188162). These listed funders/supporters played no role in the study design, collection, analysis, and interpretation of data, manuscript writing, or decision to submit the report for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Conflicts of interest: The authors have no financial conflicts of interest to disclose.
References
- 1.Frank SJ, Cox JD, Gillin M, et al. Multifield optimization intensity modulated proton therapy for head and neck tumors: A translation to practice. Int J Radiat Oncol Biol Phys. 2014;89:846–853. doi: 10.1016/j.ijrobp.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu XR, Poenisch F, Li H, et al. A single-field integrated boost treatment planning technique for spot scanning proton therapy. Radiat Oncol Lond Engl. 2014;9:202. doi: 10.1186/1748-717X-9-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu W, Frank SJ, Li X, et al. Effectiveness of robust optimization in intensity-modulated proton therapy planning for head and neck cancers. Med Phys. 2013;40:051711. doi: 10.1118/1.4801899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kandula S, Zhu X, Garden AS, et al. Spot-scanning beam proton therapy vs intensity-modulated radiation therapy for ipsilateral head and neck malignancies: A treatment planning comparison. Med Dosim. 2013;38:390–394. doi: 10.1016/j.meddos.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Quan EM, Liu W, Wu R, et al. Preliminary evaluation of multifield and single-field optimization for the treatment planning of spot-scanning proton therapy of head and neck cancer. Med Phys. 2013;40 doi: 10.1118/1.4813900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu XR, Li Y, Mackin D, et al. Towards effective and efficient patient-specific quality assurance for spot scanning proton therapy. Cancers. 2015;7:631–647. doi: 10.3390/cancers7020631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slater JD, Yonemoto LT, Mantik DW, et al. Proton radiation for treatment of cancer of the oropharynx: early experience at Loma Linda University Medical Center using a concomitant boost technique. Int J Radiat Oncol Biol Phys. 2005;62:494–500. doi: 10.1016/j.ijrobp.2004.09.064. [DOI] [PubMed] [Google Scholar]
- 8.Holliday EB, Frank SJ. Proton radiation therapy for head and neck cancer: A review of the clinical experience to date. Int J Radiat Oncol Biol Phys. 2014;89:292–302. doi: 10.1016/j.ijrobp.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 9.Masterson L, Moualed D, Masood A, et al. De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma. Cochrane Database Syst Rev. 2014;2 doi: 10.1002/14651858.CD010271.pub2. CD010271–CD010271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenthal DI, Chambers MS, Fuller CD, et al. Beam path toxicities to non-target structures during intensity-modulated radiation therapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;72:747–755. doi: 10.1016/j.ijrobp.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kocak-Uzel E, Gunn GB, Colen RR, et al. Beam path toxicity in candidate organs-at-risk: assessment of radiation emetogenesis for patients receiving head and neck intensity modulated radiotherapy. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2014;111:281–288. doi: 10.1016/j.radonc.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahlstrom KR, Calzada G, Hanby JD, et al. An evolution in demographics, treatment, and outcomes of oropharyngeal cancer at a major cancer center: a staging system in need of repair. Cancer. 2013;119:81–89. doi: 10.1002/cncr.27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garden AS, Kies MS, Morrison WH, et al. Outcomes and patterns of care of patients with locally advanced oropharyngeal carcinoma treated in the early 21st century. Radiat Oncol Lond Engl. 2013;8:21. doi: 10.1186/1748-717X-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garden AS, Dong L, Morrison WH, et al. Patterns of Disease Recurrence Following Treatment of Oropharyngeal Cancer With Intensity Modulated Radiation Therapy. Int J Radiat Oncol Biol Phys. 2012 doi: 10.1016/j.ijrobp.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Garden AS, Gunn GB, Hessel A, et al. Management of the lymph node-positive neck in the patient with human papillomavirus-associated oropharyngeal cancer. Cancer. 2014 doi: 10.1002/cncr.28831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenthal DI, Asper JA, Barker JL, et al. Importance of patient examination to clinical quality assurance in head and neck radiation oncology. Head Neck. 2006;28:967–973. doi: 10.1002/hed.20446. [DOI] [PubMed] [Google Scholar]
- 17.Chronowski GM, Garden AS, Morrison WH, et al. Unilateral radiotherapy for the treatment of tonsil cancer. Int J Radiat Oncol Biol Phys. 2012;83:204–209. doi: 10.1016/j.ijrobp.2011.06.1975. [DOI] [PubMed] [Google Scholar]
- 18.Zhu XR, Wu T, Yeh C, et al. Comparison of two different planning techniques for IMPT of Head-and-Neck cancers. Int J Part Ther; Proc. 1st Annual Meeting Part. Ther. Coop. Group – N. Am. PTCOG-NA; Winter. 2014. pp. 759–824. [Google Scholar]
- 19.Lomax AJ. Intensity modulated proton therapy and its sensitivity to treatment uncertainties 1: the potential effects of calculational uncertainties. Phys Med Biol. 2008;53:1027–1042. doi: 10.1088/0031-9155/53/4/014. [DOI] [PubMed] [Google Scholar]
- 20.Lomax AJ, Pedroni E, Rutz H, et al. The clinical potential of intensity modulated proton therapy. Z Für Med Phys. 2004;14:147–152. doi: 10.1078/0939-3889-00217. [DOI] [PubMed] [Google Scholar]
- 21.Anon. Common Terminology Criteria for Adverse Events (CTCAE) - CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 22.Mohamed ASR, Ruangskul M-N, Awan MJ, et al. Quality assurance assessment of diagnostic and radiation therapy-simulation CT image registration for head and neck radiation therapy: anatomic region of interest-based comparison of rigid and deformable algorithms. Radiology. 2015;274:752–763. doi: 10.1148/radiol.14132871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohamed AS, Aristophanous M, Awan M, et al. Biological and Dosimetric Analysis of Locoregional Failure After IMRT for Head and Neck Cancer. Int J Radiat Oncol • Biol • Phys. 2014;90:S571–S572. [Google Scholar]
- 24.Mohamed AS, Awan M, Kocak E, et al. Methods for Analysis and Reporting the Patterns of Locoregional Failure in the Era of IMRT for Head and Neck Cancer: Deformable Image Registration–Based Quality Assurance Workflow. Int J Radiat Oncol • Biol • Phys. 2014;90:S569–S570. [Google Scholar]
- 25.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 26.Holliday EB, Garden AS, Rosenthal DI, et al. Proton Therapy Reduces Treatment-Related Toxicities for Patients with Nasopharyngeal Cancer: A Case-Match Control Study of Intensity-Modulated Proton Therapy and Intensity-Modulated Photon Therapy. Int J Part Ther. 2015;2:19–28. [Google Scholar]
- 27.Frank SJ, Rosenthal DI, Ang K, et al. Gastrostomy Tubes Decrease by Over 50% With Intensity Modulated Proton Therapy (IMPT) During the Treatment of Oropharyngeal Cancer Patients: A Case–Control Study. Int J Radiat Oncol • Biol • Phys. 2013;87:S144. [Google Scholar]
- 28.Hutcheson KA, Bhayani MK, Beadle BM, et al. Eat and exercise during radiotherapy or chemoradiotherapy for pharyngeal cancers: use it or lose it. JAMA Otolaryngol -- Head Neck Surg. 2013;139:1127–1134. doi: 10.1001/jamaoto.2013.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisbruch A, Harris J, Garden AS, et al. Multi-institutional trial of accelerated hypofractionated intensity-modulated radiation therapy for early-stage oropharyngeal cancer (RTOG 00-22) Int J Radiat Oncol Biol Phys. 2010;76:1333–1338. doi: 10.1016/j.ijrobp.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Der Laan HP, Van De Water TA, Van Herpt HE, et al. The potential of intensity-modulated proton radiotherapy to reduce swallowing dysfunction in the treatment of head and neck cancer: A planning comparative study. Acta Oncol. 2013;52:561–569. doi: 10.3109/0284186X.2012.692885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Deformable image registration methodology for patterns of failure analysis: Contouring of recurrence GTV was done on patient's PET/CT documenting recurrence then deformable image registration (DIR) was applied to deform the PET/CT to the original planning CT. The deformation vector fields were then applied to rec-GTVs to convert it into ‘deformed rec-GTVs’ on the planning CT to calculate volumetric and dosimetric failure parameters.
Supplementary Figure 2: Failure analysis for those with locoregional failure and diagnostic imaging demonstrating the failure available for review.
A) Right level IIa nodal recurrence 12 months post-IMPT mapped to planning CT and dose where the centroid of the rec-GTV is located in GTV and minimum dose to rec-GTV was 72.6 Gy. B) Left level IIa nodal recurrence 20 months post-IMPT mapped to planning CT and dose where the centroid of the rec-GTV is located in CTV1 and minimum dose to rec-GTV was 66.4 Gy. C) Right (contralateral) glossopharyngeal sulcus recurrence 43 months post-IMPT mapped to planning CT and dose where the centroid of the rec-GTV is located outside target volumes and minimum dose to rec-GTV was 62.9 Gy.
Supplementary table 1. Dosimetric parameters for clinical target volumes (CTV) and selected organs at risk using IMPT for oropharyngeal carcinomas
Supplementary Table 2. Patient, disease, and treatment characteristics and outcomes for 5 patients who developed any disease recurrence following proton therapy for oropharyngeal cancer.