Abstract
Purpose
Anxiety is common among cancer patients and their family caregivers (FCs) and is associated with poorer outcomes. Recently, associations between inflammation and anxiety were identified. However, the relationship between variations in cytokine genes and anxiety warrants investigation. Therefore, phenotypic and genotypic characteristics associated with trait and state anxiety were evaluated in a sample of 167 oncology patients with breast, prostate, lung, or brain cancer and 85 of their FCs.
Methods
Using multiple regression analyses, the associations between participants’ demographic and clinical characteristics, as well as variations in cytokine genes and trait and state anxiety were evaluated.
Results
In the bivariate analyses, a number of phenotypic characteristics were associated with both trait and state anxiety (e.g., age, functional status). However, some associations were specific only to trait anxiety (e.g., number of comorbid conditions) or state anxiety (e.g., participation with a FC). Variations in three cytokine genes (i.e., interleukin (IL) 1 beta, IL1 receptor 2 (IL1R2), nuclear factor kappa beta 2 (NFKB2)) were associated with trait anxiety and variations in two genes (i.e., IL1R2, tumor necrosis factor alpha (TNFA)) were associated with state anxiety.
Conclusions
These findings suggest that both trait and state anxiety need to be assessed in oncology patients and their FCs. Furthermore, variations in cytokine genes may contribute to higher levels of anxiety in oncology patients and their FCs.
Keywords: anxiety, radiation therapy, cytokines, single nucleotide polymorphisms, cancer, family caregiver, trait anxiety, state anxiety
INTRODUCTION
While anxiety is a common psychological symptom in oncology patients and their family caregivers (FC), compared to depression, it is studied less frequently. When evaluated, clinically significant anxiety occurs in 7% to 30% of oncology patients [1–5] and 20% to 40% of their FCs [1,2]. Most of these studies evaluated patients and FCs at the time of diagnosis or at the initiation of new treatments. In patients, higher levels of state anxiety were associated with increased levels of dyspnea [6], fatigue [7], nausea and pain [8,9], and decreased emotional, social, and cognitive function [10,11]. Moreover, increased anxiety was associated with decreased treatment adherence [12], longer hospital stays [13], and poorer quality of life (QOL) [10]. In a review of the symptom experience of FCs [14], higher anxiety scores were associated with higher levels of anger, depression, sleep disturbance, and fatigue, as well as poorer QOL.
A valid and reliable measure of anxiety is the Speilberger State-Trait Anxiety Inventory (STAI-T, STAI-S) [15]. Trait anxiety is defined as an individual’s predisposition to anxiety determined by his/her personality and estimates how a person generally feels [15]. Trait anxiety is considered by some to be a proxy for neuroticism [16]. State anxiety is defined as an individual’s transitory emotional response to a stressful situation [15]. While these two dimensions of anxiety are highly correlated [17], evidence suggests that they are distinct dimensions of anxiety [17].
Most studies of oncology patients and their FCs have evaluated state anxiety [18]. However, in the studies that evaluated trait anxiety in oncology patients [19–25], significant associations were found between higher levels of trait anxiety and depression [19,20], psychological distress [21], and pain [22], as well as decrements in health status [23], body image and sexual function [20], and QOL [24]. In addition, patients with higher trait anxiety expressed more negative emotions after diagnosis (e.g., concerns about cancer) as well as more negative perspectives on the future [21].
While the phenotypic characteristics that place oncology patients and their FCs at higher risk for clinically meaningful levels of trait and state anxiety require additional investigation, recent meta-analyses suggest that genetic factors may be involved in the development of anxiety disorders [26–31]. In addition, building on studies that suggest a role for inflammatory mediators in depressive disorders, a need exists to evaluate the role of cytokines in the pathogenesis of anxiety disorders [32,33]. Results of animal studies provide preliminary support for an association between cytokines and anxiety [34–37]. Furthermore, in a study of healthy volunteers who received endotoxin [38], higher anxiety scores were associated with increased levels of circulating pro-inflammatory cytokines.
In keeping with the findings in the literature that stress and inflammation are associated with higher levels of common symptoms, our research team has investigated the role of cytokine gene polymorphisms and increased risk for pain [39,40], depression [39,41], fatigue [39], and sleep disturbance [39,42] in oncology patients and their FCs. Based on these findings and the initial evidence that supports a role for inflammatory mediators in stress and anxiety [35,43], the purposes of this study, in the same sample of patients and FCs, who were evaluated prior to the initiation of the patient’s radiation therapy (RT), were: to evaluate for differences in trait and state anxiety between patients and FCs; to evaluate the relationships between select demographic and clinical characteristics and levels of trait and state anxiety; and to investigate the associations between pro- and anti-inflammatory cytokine genes and levels of trait and state anxiety.
METHODS
Participants and Settings
This study is part of a larger, longitudinal study that evaluated multiple physical and psychological symptoms in patients who underwent primary or adjuvant RT and their FCs. A detailed description of the methods is published elsewhere [25,39,40,42]. In brief, participants were enrolled from two RT departments located in a Comprehensive Cancer Center and a community-based oncology program. Patients were eligible to participate if they were ≥18 years of age; were able to read, write, and understand English; had a self-reported Karnofsky Performance Status (KPS) score of ≥60; and were scheduled to receive primary or adjuvant RT. Patients were excluded if they had metastatic disease, more than one cancer diagnosis, or a diagnosed sleep disorder. FCs were eligible to participate if they were an adult (≥18 years of age); were able to read, write, and understand English; gave written informed consent; had a KPS score of ≥60; were living with the patient; and did not have a diagnosed sleep disorder.
Instruments
A demographic questionnaire obtained information on age, gender, marital status, education, ethnicity, employment status, and the presence of a number of co-morbid conditions. Patients’ medical records were reviewed for disease and treatment information.
The STAI-T and STAI-S consist of 20 items each that are rated from 1 to 4. The scores for each scale are summed and can range from 20 to 80. A higher score indicates greater anxiety. The STAI-T measures an individual’s predisposition to anxiety determined by his/her personality and estimates how a person generally feels. The STAI-S measures an individual’s transitory emotional response to a stressful situation. It evaluates the emotional responses of worry, nervousness, tension, and feelings of apprehension related to how a person feels “right now” in a stressful situation. In individuals with chronic medical conditions, cutoff scores of ≥31.8 and ≥32.2 indicate high levels of trait and state anxiety, respectively. The STAI-S and STAI-T inventories have well-established criterion and construct validity and internal consistency reliability coefficients [15,44,45]. In the current study, Cronbach’s alphas for the STAI-T and STAI-S were .92 and .95 for patients and .89 and .93 for FCs, respectively.
The Center for Epidemiological Studies-Depression scale (CESD) consists of 20 items selected to represent the major symptoms in the clinical syndrome of depression. Scores can range from 0 to 60, with scores of ≥16 indicating the need for individuals to seek clinical evaluation for major depression. The CES-D has well established concurrent and construct validity [46,47]. In the current study, the Cronbach’s alpha for the CES-D was .88 for patients and .84 for FCs.
Study Procedures
The study was approved by the Committee on Human Research at the University of California, San Francisco and at the second site. Approximately one week prior to the start of RT (i.e., simulation visit when the measurements for RT are made), patients were invited to participate in the study. If the FC was present, a research nurse explained the study protocol to both the patient and FC, determined eligibility, and obtained written informed consent. FCs who were not present were contacted by phone to determine their interest in participation. These FCs completed the enrollment procedures at home.
Phenotypic Data Analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) Version 21. Data collected at the enrollment visit were used in these analyses. Descriptive statistics and frequency distributions were generated on the sample characteristics and anxiety scores. Independent sample t-tests and Chi-square analyses were done to evaluate for differences in demographic and clinical characteristics between patients and FCs. Independent sample t-tests were used to evaluate for differences in anxiety scores between patients who participated with and without FCs. Bivariate analyses were performed to describe the relationships between trait and state anxiety scores and a number of demographic and clinical characteristics. In these bivariate analyses, correlations were used to analyze continuous variables and t-tests and analyses of variance (ANOVAs) were used for categorical variables.
Genomic Data Analysis
Blood collection and genotyping
Genomic deoxyribonucleic acid (DNA) was extracted from archived buffy coats using the PUREGene DNA Isolation System (Invitrogen, Carlsbad, CA). Of the 287 participants recruited, DNA was recovered from the archived buffy coats of 253 (i.e., 168 patients and 85 FCs).
DNA samples were quantitated with a Nanodrop Spectrophotometer (ND-1000) and normalized to a concentration of 50 ng/μL (diluted in 10 mM Tris/1 mM EDTA). Samples were genotyped using the GoldenGate genotyping platform (Illumina, San Diego, CA) and processed according to the standard protocol using GenomeStudio (Illumina, San Diego, CA). Signal intensity profiles and resulting genotype calls for each single nucleotide polymorphism (SNP) were visually inspected by two blinded reviewers.
Gene and SNP Selection
Genes for pro- and anti-inflammatory cytokines and cytokine receptors were selected for analysis (Supplementary Table 1). The pro-inflammatory cytokine genes included: interferon gamma 1 (IFNG1), IFNG receptor 1 (IFNGR1), interleukin (IL) 1, IL1R1, IL2, IL8, IL17A, and tumor necrosis factor alpha (TNFA). Anti-inflammatory cytokines included: IL1R2, IL4, IL10, and IL13. Of note, IFNG1, IL1B, and IL6 possess pro- and anti-inflammatory functions. Two genes in the nuclear factor-kappa beta (NFKB) family of transcription factors (i.e., NFKB1, NFKB2) were evaluated [48].
A combination of tag-SNPs and literature driven SNPs were selected for analysis. Tagging SNPs were required to be common (defined as having a minor allele frequency ≥.05) in public databases (e.g., HapMap). In order to ensure robust genetic association analyses, quality control filtering of SNPs was performed. SNPs with call rates of <95% or Hardy-Weinberg p-values of <.001 were excluded.
A total of 92 SNPs among the 15 candidate genes (IFNG1: 5 SNPs, IFNGR1: 1 SNP; IL1B: 12 SNPs; IL1R1: 5 SNPs; IL1R2: 3 SNPs; IL2: 5 SNPs; IL4: 8 SNPs; IL6: 9 SNPs; IL8: 3 SNPs; IL10: 8 SNPs; IL13: 4 SNPs; IL17A: 5 SNPs; NFKB1: 11 SNPs; NFKB2: 4 SNPs; TNFA: 9 SNPs) passed all quality control filters and were included in the genetic association analyses (see Supplementary Table 1). Potential functional roles of SNPs were examined using PUPAS uite 2.0 [49].
Statistical Analyses for the Genetic Data
Allele and genotype frequencies were determined by gene counting. Hardy-Weinberg equilibrium was assessed by the Chi-square or Fisher Exact tests. Measures of linkage disequilibrium (i.e., D’ and r2) were computed from the participants’ genotypes with Haploview 4.2. Linkage disequilibrium (LD)-based haplotype block definition was based on D’ confidence interval [50].
For SNPs that were members of the same haploblock, haplotype analyses were conducted in order to localize the association signal within each gene and to determine if haplotypes improved the strength of the association with the phenotype. Haplotypes were constructed using the program PHASE version 2.1 [51]. In order to improve the stability of haplotype inference, the haplotype construction procedure was repeated five times using different seed numbers with each cycle. Only haplotypes that were inferred with probability estimates of ≥.85, across the five iterations, were retained for downstream analyses. Haplotypes were evaluated assuming a dosage model (i.e., analogous to the additive model).
Ancestry informative markers (AIMS) were used to minimize confounding due to population stratification [52–54]. Homogeneity in ancestry among participants was verified by principal component analysis [55], using Helix Tree (Golden Helix, Bozeman, MT). Briefly, the number of principal components (PCs) was sought that distinguished the major racial/ethnic groups in the sample by visual inspection of scatter plots of orthogonal PCs (i.e., PC 1 versus PC2, PC2 versus PC3). This procedure was repeated until no discernible clustering of participants by their self-reported race/ethnicity was possible (data not shown). One hundred and six AIMs were included in the analysis. The first three PCs were selected to adjust for potential confounding due to population substructure (i.e., race/ethnicity) by including these three covariates in all regression models.
For association tests, using Independent Student’s t-tests or ANOVAs, three genetic models were assessed for each SNP: additive, dominant, and recessive. Barring trivial improvements (i.e., delta <10%), the genetic model that best fit the data, by maximizing the significance of the p-value was selected for each SNP.
Linear regression analysis that controlled for significant covariates, as well as genomic estimates of and self-reported race/ethnicity, was used to evaluate the associations between genotype and anxiety scores. Only those genetic associations identified as significant from the bivariate analyses were evaluated in the multivariate analyses. A backwards stepwise approach was used to create a parsimonious model. Except for self-reported and genomic estimates of race/ethnicity, only predictors with a p-value of <.05 were retained in the final model. Genetic model fit and both unadjusted and covariate-adjusted coefficients were estimated using STATA version 13.
As was done in our previous studies [39,40,42] based on recommendations in the literature [56,57], the implementation of rigorous quality controls for genomic data, the non-independence of SNPs/haplotypes in LD, and the exploratory nature of the analyses, adjustments were not made for multiple testing. In addition, significant SNPs identified in the bivariate analyses were evaluated further using regression analyses that controlled for differences in phenotypic characteristics, potential confounding due to population stratification, and variation in other SNPs/haplotypes within the same gene. Only those SNPs that remained significant were included in the final presentation of the results. Therefore, the significant independent associations reported are unlikely to be due solely to chance. Unadjusted (bivariate) associations are reported for all SNPs passing quality control criteria in Supplementary Table 1 to allow for subsequent comparisons and meta-analyses.
RESULTS
Participant Characteristics
A detailed description of the demographic and clinical characteristics of the participants is published elsewhere [42]. In brief, as shown in Table 1, the majority of participants were female, Caucasian, and well educated. Patients and FCs differed only on gender and marital status. Compared to the patients, a greater proportion of FCs was female (p<.0001) and married/partnered (p<.0001).
Table 1.
Differences in Demographic and Clinical Characteristics Between Patients and Family Caregivers at Enrollment
| Characteristic | Total Sample | Patients | Family Caregivers | Statistic |
|---|---|---|---|---|
| n=253 | n=168 | n=85 | ||
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age (years) | 61.4 (11.3) | 60.9 (11.6) | 62.5 (10.5) | t=−1.03, p=.305 |
| Education (years) | 15.9 (3.0) | 16.0 (2.9) | 15.8 (3.2) | t=0.56, p=.575 |
| Number of comorbid conditions | 4.6 (2.7) | 4.8 (2.6) | 4.2 (2.9) | t=1.52, p=.131 |
| Karnofsky Performance Status score | 92.0 (11.5) | 91.1 (11.9) | 93.7 (10.6) | t=−1.65, p=.100 |
| STAI-T score | 34.1 (9.9) | 33.8 (10.0) | 34.7 (9.7) | t=0.49, p=.484 |
| STAI-S score | 31.0 (10.8) | 30.9 (10.9) | 31.0 (10.7) | t=−0.06, p=.951 |
| CES-D score | 8.8 (8.2) | 9.1 (8.7) | 8.3 (7.2) | t=0.79, p=.429 |
| % | % | % | ||
| Gender Male Female |
46.2 53.8 |
55.4 44.6 |
28.2 71.8 |
FE, p<.0001 |
| Race/Ethnicity White Asian/Pacific Islander Black Hispanic/Mixed Ethnic Background/Other |
74.6 6.3 13.5 5.6 |
71.9 7.2 15.0 6.0 |
80.0 4.7 10.6 4.7 |
Χ2=4.89, p=.429 |
| Marital status Married/partnered Other |
69.3 30.7 |
56.0 44.0 |
95.3 4.7 |
FE, p<.0001 |
| Works for pay (% yes) | 46.4 | 47.0 | 45.2 | FE, p=.89 |
| Children at home (% yes) | 17.0 | 17.0 | 16.9 | FE, p=1.00 |
| Pain (% yes) | 47.8 | 56.0 | 31.8 | FE, p<.0001 |
Abbreviations: CES-D=Center for Epidemiologic Studies-Depression Scale; FE=Fisher’s Exact; SD=standard deviation;
STAI-T=State-Trait Anxiety Inventory-Trait; STAI-S=State-Trait Anxiety Inventory-State
Relationships Between Demographic and Clinical Characteristics and State and Trait Anxiety Scores
As shown in Table 1, no significant differences were found in patients’ and FCs’ trait (p=.484) and state (p=.951) anxiety scores at enrollment. Trait anxiety did not differ significantly between patients who participated with (32.5, SD=10.1) or without a FC (35.5, SD=9.7, p=.06). However, mean state anxiety scores were higher for patients who participated without a FC (33.0, SD=11.7) compared to those of patients who were members of a dyad (29.4, SD=10.0, p=.04).
As shown in Table 2, for the entire sample, both trait and state anxiety scores were negatively correlated with age (both p ≤.001) and KPS score (both p<.001). Both trait and state anxiety scores were positively correlated with the number of comorbid conditions (both p ≤.015). In addition, women reported higher trait and state anxiety scores (both p <.05). Higher levels of trait anxiety were associated with lower weight (p=.043) and caring for children at home (p=.036).
Table 2.
Relationships between Trait and State Anxiety Scores and Demographic and Clinical Characteristics
| Trait Anxiety | State Anxiety | |||
|---|---|---|---|---|
| Characteristic | Correlation | p value | Correlation | p value |
| Age (years) | −.24 | <.0001 | −.21 | .001 |
| Education (years) | −.02 | .756 | −.04 | .551 |
| Number of comorbid conditions | .18 | .005 | .16 | .015 |
| Weight (pounds) | −.13 | .043 | −.13 | .052 |
| Karnofsky Performance Status score | −.27 | <.0001 | −.27 | <.0001 |
| Mean (SD) | Statistic | Mean (SD) | Statistic | |
| Gender Female Male |
35.5 (10.7) 32.5 (8.6) |
t=2.46, p=.015 |
33.0 (12.2) 28.6 (8.4) |
t=3.36, p=.001 |
| Ethnicity White Asian/Pacific Islander Black/African American Hispanic/Mixed Background/Other |
33.4 (10.0) 40.6 (11.6) 34.4 (7.7) 35.8 (8.9) |
F=2.88, p=.036 |
30.6 (11.0) 33.7 (13.3) 30.7 (9.8) 34.0 (8.0) |
F=.80, p=.496 |
| Lives Alone Yes No |
34.5 (9.8) 33.4 (10.2) |
t=.68, p=.500 |
31.9 (10.9) 30.5 (10.9) |
t=.80, p=.424 |
| Married or partnered Yes No |
33.8 (10.3) 34.3 (8.9) |
t=−.32, p=.748 |
30.5 (11.1) 31.9 (10.1) |
t=−.95, p=.343 |
| Work for pay Yes No |
33.5 (8.7) 34.6 (10.8) |
t=−.89, p=.375 |
29.9 (8.9) 31.6 (11.9) |
t=−1.31, p=.193 |
| Children at home Yes No |
37.4 (10.2) 33.6 (9.4) |
t=2.12, p=.036 |
34.1 (12.6) 30.5 (10.1) |
t=1.83, p=.068 |
| Older adult at home Yes No |
38.4 (13.0) 34.1 (9.5) |
t=1.18, p=.238 |
32.9 (15.7) 31.0 (10.5) |
t=.−44, p=.659 |
| Patient/FC Patient Family caregiver |
33.8 (10.0) 34.7 (9.7) |
t=−.70, p=.484 |
30.9 (10.9) 31.0 (10.7) |
t=−.06, p=.951 |
Abbreviation: SD = standard deviation
Associations Between Cytokine Gene Variations and Trait and State Anxiety
In the bivariate analyses, using Independent sample t-tests, five SNPs (i.e., IFNG1 rs2069727, IL1R2 rs4141134, IL17A rs7747909, NFKB2 rs7897947, TNFA rs1800629) and two haplotypes (i.e., IFNG1 Haplotype A5 (HapA5), IL1R2 HapA2) were associated with both trait and state anxiety. Six SNPs in IL1B (i.e., rs3917356, rs1143629, rs1143627, rs16944, rs1143623, rs13032029) were associated only with trait anxiety. Three SNPs (i.e., IL1R2 rs7570441, IL6 rs2069861, IL13 rs2069743) and two haplotypes (i.e., IL1R2 HapA4, IL6 HapA6) were associated only with state anxiety.
Regression Analyses for Trait Anxiety
After controlling for age, functional status, number of comorbidities, and genomic estimates of and self-reported race/ethnicity, the only genotypic predictors of trait anxiety that remained significant were: IL1B rs1143629 (Figure 1A); IL1R2 HapA2 (Figure 2); and NFKB2 rs7897947 (Figure 1B; Table 3).
Figure 1.
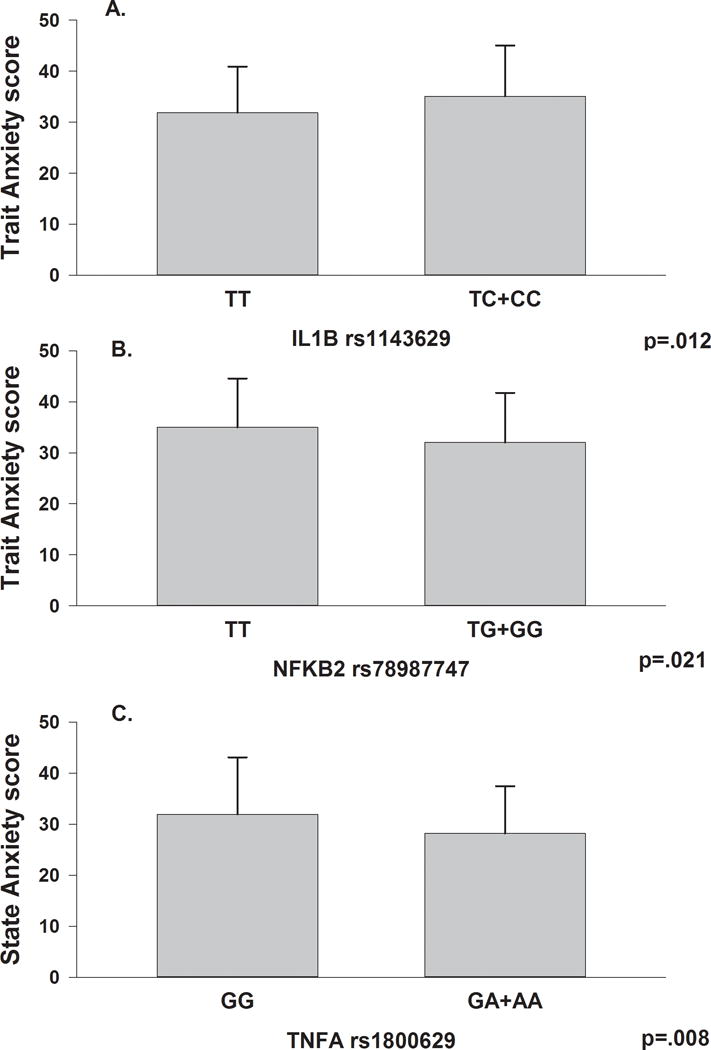
Panel A – Differences in Trait Anxiety scores, not adjusting for covariates, between participants who were homozygous for the common T allele in Interleukin 1 beta (IL1B) rs1143629 and participants who were homozygous or heterozygous for the rare C allele.
Panel B - Differences in Trait Anxiety, not adjusting for covariates, scores between participants who were homozygous for the common T allele in Nuclear Factor Kappa Beta 2 (NFKB2) rs78987747 and participants who were homozygous or heterozygous for the rare G allele.
Panel C - Differences in State Anxiety scores, not adjusting for covariates, between participants who were homozygous for the common G allele in Tumor Necrosis Factor Alpha (TNFA) rs1800629 and participants who were homozygous or heterozygous for the rare A allele. All analyses were done using Independent Student’s t-tests. All values are plotted as means ± standard deviations.
Figure 2.
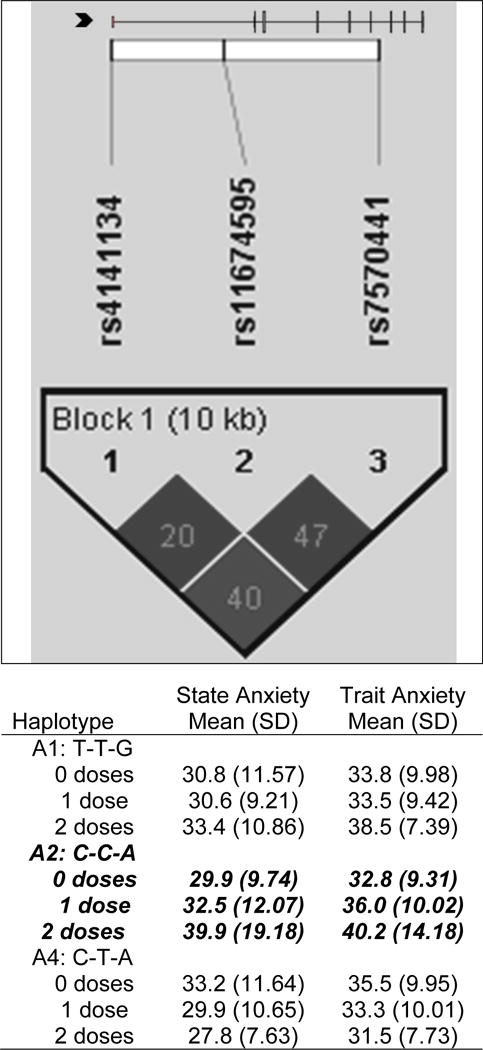
IL1R2 linkage disequilibrium-based heatmap and haplotype analysis. In the figure embedded in the top row of the table, an ideogram of interleukin 1 receptor 2 (IL1R2) is presented above the white bar that represents the physical distance along human chromosome 2 (position 31, 96,370,336 to 96,380,807; genome build 36.3, contig NT_022171.14). Exons are represented as tick marks. Gray lines connecting the exons represent introns. The black chevron indicates the direction of gene transcription. Reference sequence identifiers (rsID) for each single nucleotide polymorphism (SNP) are plotted both in terms of their physical distance (i.e., the white bar at the top of the figure) and also equidistantly to render the pairwise linkage disequilibrium (LD) estimates that were calculated and visualized with Haploview 4.2. The gene structure for IL1R2 (i.e., reference sequence NM_004633) was rendered with FancyGene 1.4. The correlation statistics (r2 and D’) are provided in the heatmap. LD-based haplotype block definition was based on the D’ confidence interval method. The haploblock is indicated in a bolded triangle and its component SNPs are rendered in bold font. Pairwise D’ values (range: 0-1, inclusive) were rendered in grey, with darker grey diamonds representing D’ values approaching 1.0. When the r2 values (range of 0–100, inclusive) are not equal to 0 or 100, they are provided in a given diamond. The haplotypes observed in the haploblock are listed in each row, starting with the nucleotide composition across the two SNPs that compose the haplotype (i.e., rs4141134, rs11674595, rs7570441) and both the mean and standard deviation (SD) for trait and state anxiety for each of the three subgroups for a given haplotype (i.e., zero doses of the haplotype, one dose of the haplotype, two doses of the haplotype). The C-C-A haplotype (i.e., IL1R2 HapA2) identified in the bivariate analyses (Supplemental Table 1) that remained significant after controlling for relevant confounders is rendered in bold and italicized.
Table 3.
Multiple Linear Regression Analyses for Interleukin 1B (IL1B) rs1143629, Interleukin 1R2 (IL1R2) HapA2, and Nuclear Factor Kappa, Beta 2 Subunit (NFKB2) rs7897947 to Predict Low to High Trait Anxiety (n=234)
| Predictor of Low to High Trait Anxiety | β Coefficient | Standard Error | 95% CI | t | p-value |
|---|---|---|---|---|---|
| IL1B rs1143629 | 2.980 | 1.248 | .520, 5.440 | 2.39 | .018 |
| Age | −.875 | .283 | −1.433, −.318 | −3.10 | .002 |
| Functional status | −1.843 | .529 | −2.884, −.801 | −3.49 | .001 |
| Number of comorbidities | .588 | .231 | .132, 1.044 | 2.54 | .012 |
| Overall model fit: F(10,223) = 5.31, p <.0001 R2 = .1923 | |||||
| IL1R2 HapA2 genotype | 2.744 | 1.236 | .308, 5.180 | 2.22 | .027 |
| Age | −.875 | .283 | −1.434, −.317 | −3.09 | .002 |
| Functional status | −1.766 | .528 | −2.808, −.725 | −3.34 | .001 |
| Number of comorbidities | .563 | .232 | .105, 1.020 | 2.42 | .016 |
| Overall model fit: F(10,223) = 5.22, p <.0001 R2 = .1895 | |||||
| NFKB2 rs78979947 | −2.698 | 1.217 | −5.096, −.301 | −2.22 | .028 |
| Age | −.897 | .283 | −1.454, −.340 | −3.17 | .002 |
| Functional status | −1.826 | .529 | −2.869, −.783 | −3.45 | .001 |
| Number of comorbidities | .534 | .233 | .074, .994 | 2.29 | .023 |
| Overall model fit: F(10,223) = 5.21, p<.0001 R2 = .1895 | |||||
For this model, the first three principle components identified from the analysis of ancestry informative markers as well as self-reported race/ethnicity (White, Asian/Pacific Islander, Black, Hispanic/Mixed Ethnic Background/Other) were retained to adjust for potential confounding due to race or ethnicity (data not shown). Predictors evaluated in the model included genotype (IL1B rs1143629: TT versus CT+CC; IL1R2 HapA2: composed of IL1R2 rs4141134 [rare “C” allele], rs11674595 [common “T” allele], and rs7570441 [common “G” allele]; and NFKB2 rs7897947: TT versus TG+GG), age (in 5-year increments), functional status (Karnofsky Performance Status score, in 10-point increments), and number of comorbidities.
For IL1B, rs1143629, individuals who carried one or two doses of the rare “C” allele (i.e., TC+CC) had a mean trait anxiety score that was 2.98 points higher than common “T” allele carriers (95% confidence interval [CI]: .52, 5.44, p=.018). The overall model explained 19.2% of the variance in trait anxiety.
Each dose of the haplotype of IL1R2 HapA2 (composed of IL1R2 rs4141134 [rare “C” allele], rs11674595 [common “T” allele], and rs7570441 [common “G” allele]) was associated with a 2.74-point increase in trait anxiety score (95% CI: .31, 5.18, p = .027). The overall model explained 19.0% of the variance in trait anxiety.
For NFKB2 rs78979947, individuals who carried one or two doses of the rare “G” allele (TG+GG) had a mean trait anxiety score that was 2.70 points lower than common “T” allele carriers (95% CI: −5.10, −.30, p=.028). The overall model explained 19.0% of the variance in trait anxiety.
Regression Analyses for State Anxiety
After controlling for age, functional status, and genomic estimates of and self-reported race/ethnicity, the only genotypic predictors that remained significant for state anxiety were IL1R2 HapA2 (Figure 2) and TNFA rs1800629 (Figure 1C, Table 4).
Table 4.
Multiple Linear Regression Analyses for Interleukin 1R2 (IL1R2) Haplotype A2 (HapA2) and Tumor Necrosis Factor Alpha (TNFA) rs1800629 to Predict Low to High State Anxiety (n=231)
| Predictor of Low to High State Anxiety | β Coefficient | Standard Error | 95% CI | t | p-value |
|---|---|---|---|---|---|
| IL1R2 HapA2 genotype | 3.014 | 1.380 | .294, 5.735 | 2.18 | .030 |
| Age | −.968 | .314 | −1.587, −.349 | −3.08 | .002 |
| Functional status | −2.210 | .573 | −3.338, −1.081 | −3.86 | <.001 |
| Overall model fit: F(9, 221) = 4.45, p <.0001 R2 = .1534 | |||||
| TNFA rs1800629 | −3.679 | 1.462 | −6.560, −.797 | −2.52 | .013 |
| Age | −1.031 | .312 | −1.647, −.415 | −3.30 | .001 |
| Functional status | −2.070 | .574 | −3.202, −.939 | −3.61 | <.001 |
| Overall model fit: F(9, 221) = 4.65, p <.0001 R2 = .1592 | |||||
For this model, the first three principle components identified from the analysis of ancestry informative markers as well as self-reported race/ethnicity (White, Asian/Pacific Islander, Black, Hispanic/Mixed Ethnic Background/Other) were retained to adjust for potential confounding due to race or ethnicity (data not shown). Predictors evaluated in the model included genotype (IL1R2 HapA2: composed of IL1R2 rs4141134 [rare “C” allele], rs11674595 [common “T” allele], and rs7570441 [common “G” allele]; and TNFA rs1800629: GG versus GA+AA), age (in 5-year increments), and functional status (Karnofsky Performance Status score, in 10-point increments).
Each dose of IL1R2 HapA2 haplotype was associated with a 3.01 point increase in state anxiety scores (95% CI: .29, 5.74, p=.030). The overall model explained 15.3% of the variance in state anxiety.
For TNFA rs1800629, individuals who carried of one or two doses of the rare “A” allele (GA+AA) had a mean state anxiety score that was 3.68 points lower than carriers of the common “G” allele (95% CI: −6.56, −.80, p=.013). The overall model explained 15.9% of the variance in state anxiety.
DISCUSSION
This study is the first to evaluate the relationships between trait and state anxiety and cytokine gene variations in oncology patients and their FCs. Findings from this study suggest that trait and state anxiety are related both phenotypically and genotypically. However, consistent with two previous reports [17,58], unique phenotypic and genotypic predictors of trait and state anxiety were identified that suggest that these two symptoms are distinct.
Comparisons of Phenotypic Characteristics of Anxiety
In this sample, trait and state anxiety scores were highly correlated (r=.78, p=.001). While the bivariate analyses revealed common and distinct demographic and clinical characteristics associated with trait and state anxiety, in the multivariate analyses, only age and functional status were retained in the final regression models for both trait and state anxiety. For each 5-year increase in age, both trait and state anxiety scores decreased by approximately one point. Consistent with previous reports [59], younger patients reported higher trait and state anxiety scores. The mean scores for trait and state anxiety (i.e., 34.1 and 31.0, respectively) reported by study participants are comparable to two previous reports in oncology patients at the initiation of RT [60,61].
In addition, consistent with previous reports [62,63], participants who reported poorer functional status scores reported higher levels of trait and state anxiety scores. For each 10-point decrease in KPS scores (which equates with a clinically meaningful decrement in functional status), both trait and state anxiety scores increased by approximately 2 points.
In terms of trait anxiety, only number of comorbid conditions was a unique predictor. On average, each additional comorbid condition was associated with a half-point increase in mean trait anxiety scores (p<.05). In previous studies [24,64], higher levels of trait anxiety at the initiation of cancer treatment predicted decreases in health status and QOL measured one to five years later.
Some of the findings from the bivariate analyses warrant additional consideration. Both patients and FCs reported similar levels of trait and state anxiety, which suggests that both groups experience psychological distress at the initiation of a new cancer treatment. In addition, state anxiety, but not trait anxiety, was significantly higher in patients who did not participate with a FC. This finding is consistent with previous reports that suggest that social support can reduce psychological distress in oncology patients [65–68]. Taken together, these phenotypic findings suggest that clinicians need to perform assessments of patients’ and FCs’ levels of anxiety prior to the initiation of a new cancer treatment and utilize appropriate interventions to reduce distress.
Comparisons of Genotypic Characteristics of Anxiety
Only one genomic marker, an IL1R2 haplotype, composed of rs4141134, rs11674595, and 7570441, was associated with a 3-point increase in ratings of both trait and state anxiety. While no published associations were found between this haplotype and anxiety, in another study from the same sample, this haplotype was associated with a 2-fold increase in the odds of belonging to the group with higher levels of depressive symptoms [41]. In addition, in a sample of patients who were followed for six months after breast cancer surgery, a haplotype in IL1R2 that contained two of the same SNPs identified in this study (i.e., rs11674595 and rs7570441) was associated with 2-fold increase in the odds of belonging to the group with higher levels of sleep disturbance [69]. Given the fact that inflammation is one of the proposed mechanisms for anxiety [43,70] depression [71–73], and sleep disturbance [39,74,75,76], our findings across two independent samples suggest that IL1R2 may be a common mediator of these three common symptoms that are associated with cytokine-induced “sickness behavior” [77–80].
IL1R2 is an anti-inflammatory cytokine that inhibits inflammatory signalling by binding to IL1β and preventing its binding to IL1R1 [81]. Therefore, one can hypothesize that the activity of the SNPs in this haplotype, or an unmeasured SNP(s) in linkage disequilibrium, decreases IL1R2 expression, which would increase the amount of pro-inflammatory IL1-β bound to IL1R1 and result in higher levels of trait and state anxiety. While the functionality of each SNP in the haplotype is unknown, rs4141134 is located in the promoter region of the IL1R2 gene and may impact its expression. The other two SNPs in the haplotype are located in intronic regions of the gene that are evolutionarily conserved. Given the associations between the SNPs in this haplotype and higher levels of trait anxiety, state anxiety, depressive symptoms [41], and sleep disturbance [69], the role that IL1R2 plays in the regulation of inflammation and common symptoms experienced by individuals with chronic medical conditions warrants investigation in future studies. In addition, functional studies are needed to confirm the hypothesized mechanism proposed above for IL1R2 rs4141134.
IL1B rs1143629 and NFKB2 rs78989947 were associated with higher levels of trait anxiety. A growing body of evidence suggests that increased levels of IL1-β are associated with increased levels of anxiety in animals [82,83] and humans [38,84,85]. While the exact mechanisms by which inflammation results in anxiety are not completely understood, several lines of evidence suggest that IL1β may induce: alterations in serotonin metabolism [86–88]; changes in the activity of the hypothalamic-pituitary-adrenal axis [89]; and/or changes in the sensitivity of cannabinoid receptors [90]. Although the SNP in IL1B is intronic and has no function, it may be a surrogate marker in LD with other functional SNPs.
While in the bivariate analyses, NFKB2 rs7897947 was associated with increased levels of both trait and state anxiety, this relationship remained significant in the multivariate analyses only for trait anxiety. This SNP is located in an intronic region of the gene and has no known function. Of note, this SNP, in this same sample of patients and FCs, was associated with a 74% reduction in the odds of belonging to a group with higher levels of sleep disturbance [42]. In addition, another polymorphism in NFKB2 (i.e., rs1056890) was associated with a 47% reduction in the odds of belonging to a group with higher levels of sleep disturbance in a sample of patients who underwent surgery for breast cancer [69]. NFKB2 is a gene that belongs to the nuclear factor-kappa beta family. This family is made up of transcription factors that regulate a variety of biological processes (e.g., immunity, stress responses, apoptosis, cellular differentiation) [91]. Given the fact that anxious individuals often report sleep disturbance, additional research is warranted on the mechanisms by which genetic variations in NFKB2 may result in decreased levels of anxiety and sleep disturbance.
While in the bivariate analyses, TNFA rs1800629 was associated with both trait and state anxiety, this relationship remained significant in the multivariate analyses only for state anxiety. In this same sample, this SNP was associated with a 57% reduction in the odds of belonging to a group with higher levels of depressive symptoms [41]. In addition, in this same sample, individuals who were heterozygous or homozygous for the rare A allele in rs1800629 reported lower levels of sleep disturbance and morning fatigue at the initiation of RT [92]. This SNP is a common functional promoter polymorphism (i.e., c.G-308A). However, investigations on the direction and magnitude of the gene’s expression because of the minor allele have yielded conflicting results [93–95]. The findings on the association between this functional polymorphism and state anxiety warrant additional investigation given recent reports of associations between TNFα and its receptors and rodent models of anxiety [96,97].
Limitations
Limitations of this investigation must be acknowledged. While our sample size was adequate, these findings warrant replication in independent samples. During recruitment, the most common reasons for refusal were being too overwhelmed or too busy to participate. Therefore, the anxiety scores reported by study participants may be an underestimation. Larger samples with more heterogeneity in anxiety scores may identify additional genetic associations. Although valid and reliable self-report measures of trait and state anxiety were used in this investigation, a clinical diagnostic interview should be done to obtain a more comprehensive evaluation of the nature, severity, and time course of the patients’ and FCs’ level of both trait and state anxiety, as well as specific anxiety disorders. Furthermore, serum cytokine levels could be collected to support the genetic associations. Studies of genes that encode for proteins involved in other pathways (e.g., dopaminergic, serotonergic) will provide additional insights into the mechanisms that underlie anxiety in both patients and their FCs.
Conclusions
Despite these limitations, these findings suggest that an assessment of both trait and state anxiety in oncology patients and their FCs may more fully characterize these individuals’ specific needs for psychosocial interventions. In addition, the genomic analyses suggest that inflammatory mechanisms are involved in both forms of anxiety, as well as in the development of other common symptoms (e.g., depression, fatigue, sleep disturbance) in both oncology patients and their FCs. An increased understanding of the common mechanisms that underlie the most frequently occurring symptoms in oncology patients and their FCs may lead to the identification of new therapeutic targets to reduce symptom burden in these individuals.
Supplementary Material
Acknowledgments
This research was supported by a grant from the National Institute of Nursing Research (NINR, NR04835) and partially supported by an Oncology Nursing Society Research Fellowship Award to JKC, an American Cancer Society (ACS) Mentored Research Scholar Grant (MRSG-12-01-PCSM) to CRB, and a University of California San Francisco (UCSF) Academic Senate Grant to LBD and BEA. CM is funded by the ACS as a Clinical Research Professor and a K05 award from the National Cancer Institute (CA168960). AD is funded through NIH Mentored Patient-Oriented Research Career Development Award (K23 AT 005340). DJL is supported by a Department of Defense Breast Cancer Research Program Postdoctoral Fellowship. JDM is supported by a NINR postdoctoral fellowship in Cancer Survivorship Research (T32NR011972). The funders have no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of interest: None of the authors have a financial relationship with the organization that sponsored the research. The authors have full control of all of the primary data and we agree to allow the journal to review it if requested.
References
- 1.Cliff A, MacDonagh R. Psychosocial morbidity in prostate cancer: II. A comparison of patients and partners. BJU Int. 2000;86:834–839. doi: 10.1046/j.1464-410x.2000.00914.x. [DOI] [PubMed] [Google Scholar]
- 2.Clavarino AM, Lowe JB, Carmont SA, Balanda K. The needs of cancer patients and their families from rural and remote areas of Queensland. Aust J Rural Health. 2002;10:188–195. doi: 10.1046/j.1440-1584.2002.00436.x. [DOI] [PubMed] [Google Scholar]
- 3.Linden W, Vodermaier A, Mackenzie R, Greig D. Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord. 2012;141:343–351. doi: 10.1016/j.jad.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 4.Janda M, Steginga S, Dunn J, Langbecker D, Walker D, Eakin E. Unmet supportive care needs and interest in services among patients with a brain tumour and their carers. Patient Educ Couns. 2008;71:251–258. doi: 10.1016/j.pec.2008.01.020. doi:S0738–3991(08)00070–0. [DOI] [PubMed] [Google Scholar]
- 5.Brintzenhofe-Szoc KM, Levin TT, Li Y, Kissane DW, Zabora JR. Mixed anxiety/depression symptoms in a large cancer cohort: prevalence by cancer type. Psychosomatics. 2009;50:383–391. doi: 10.1176/appi.psy.50.4.383. doi:50/4/383. [DOI] [PubMed] [Google Scholar]
- 6.Bruera E, Schmitz B, Pither J, Neumann CM, Hanson J. The frequency and correlates of dyspnea in patients with advanced cancer. J Pain Symptom Manage. 2000;19:357–362. doi: 10.1016/s0885-3924(00)00126-3. [DOI] [PubMed] [Google Scholar]
- 7.Tchekmedyian NS, Kallich J, McDermott A, Fayers P, Erder MH. The relationship between psychologic distress and cancer-related fatigue. Cancer. 2003;98:198–203. doi: 10.1002/cncr.11463. [DOI] [PubMed] [Google Scholar]
- 8.Andrykowski MA. The role of anxiety in the development of anticipatory nausea in cancer chemotherapy: a review and synthesis. Psychosom Med. 1990;52:458–475. doi: 10.1097/00006842-199007000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Delgado-Guay M, Parsons HA, Li Z, Palmer JL, Bruera E. Symptom distress in advanced cancer patients with anxiety and depression in the palliative care setting. Support Care Cancer. 2009;17:573–579. doi: 10.1007/s00520-008-0529-7. [DOI] [PubMed] [Google Scholar]
- 10.Smith EM, Gomm SA, Dickens CM. Assessing the independent contribution to quality of life from anxiety and depression in patients with advanced cancer. Palliat Med. 2003;17:509–513. doi: 10.1191/0269216303pm781oa. [DOI] [PubMed] [Google Scholar]
- 11.Spencer R, Nilsson M, Wright A, Pirl W, Prigerson H. Anxiety disorders in advanced cancer patients. Cancer. 2010;116:1810–1819. doi: 10.1002/cncr.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greer JA, Pirl WF, Park ER, Lynch TJ, Temel JS. Behavioral and psychological predictors of chemotherapy adherence in patients with advanced non-small cell lung cancer. J Psychosom Res. 2008;65:549–552. doi: 10.1016/j.jpsychores.2008.03.005. doi:S0022-3999(08)00110-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prieto JM, Blanch J, Atala J, Carreras E, Rovira M, Cirera E, Gasto C. Psychiatric morbidity and impact on hospital length of stay among hematologic cancer patients receiving stem-cell transplantation. J Clin Oncol. 2002;20:1907–1917. doi: 10.1200/JCO.2002.07.101. [DOI] [PubMed] [Google Scholar]
- 14.Swore Fletcher BA, Dodd MJ, Schumacher KL, Miaskowski C. Symptom experience of family caregivers of patients with cancer. Oncol Nurs Forum. 2008;35:E23–44. doi: 10.1188/08.ONF.E23-E44. [DOI] [PubMed] [Google Scholar]
- 15.Spielberger CG, Gorsuch RL, Suchene R, Vagg PR, Jacobs GA. Manual for the State-Anxiety (Form Y): Self Evaluation Questionnaire. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- 16.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 17.Lau JY, Eley TC, Stevenson J. Examining the state-trait anxiety relationship: a behavioural genetic approach. J Abnorm Child Psychol. 2006;34:19–27. doi: 10.1007/s10802-005-9006-7. [DOI] [PubMed] [Google Scholar]
- 18.Stiegelis HE, Ranchor AV, Sanderman R. Psychological functioning in cancer patients treated with radiotherapy. Patient Educ Couns. 2004;52:131–141. doi: 10.1016/s0738-3991(03)00021-1. [DOI] [PubMed] [Google Scholar]
- 19.De Vries J, Van der Steeg AF, Roukema JA. Trait anxiety determines depressive symptoms and fatigue in women with an abnormality in the breast. Br J Health Psychol. 2009;14:143–157. doi: 10.1348/135910708X310200. [DOI] [PubMed] [Google Scholar]
- 20.D’Angelo C, Mirijello A, Leggio L, Ferrulli A, Carotenuto V, Icolaro N, Miceli A, D’Angelo V, Gasbarrini G, Addolorato G. State and trait anxiety and depression in patients with primary brain tumors before and after surgery: 1-year longitudinal study. Journal of neurosurgery. 2008;108:281–286. doi: 10.3171/JNS/2008/108/2/0281. [DOI] [PubMed] [Google Scholar]
- 21.Ando N, Iwamitsu Y, Kuranami M, Okazaki S, Nakatani Y, Yamamoto K, Watanabe M, Miyaoka H. Predictors of psychological distress after diagnosis in breast cancer patients and patients with benign breast problems. Psychosomatics. 2011;52:56–64. doi: 10.1016/j.psym.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Aerts P, De Vries J, Van der Steeg A, Roukema J. The relationship between morbidity after axillary surgery and long-term quality of life in breast cancer patients: The role of anxiety. Eur J Surg Oncol. 2011;37:344–349. doi: 10.1016/j.ejso.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Van Esch L, Roukema JA, Van der Steeg AFW, De Vries J. Trait anxiety predicts disease-specific health status in early-stage breast cancer patients. Qual Life Res. 2011:1–9. doi: 10.1007/s11136-010-9830-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ristvedt SL, Trinkaus KM. Trait anxiety as an independent predictor of poor health-related quality of life and post-traumatic stress symptoms in rectal cancer. Br J Health Psycho. 2009;14:701–715. doi: 10.1348/135910708X400462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunn LB, Aouizerat BE, Cooper BA, Dodd M, Lee K, West C, Paul SM, Wara W, Swift P, Miaskowski C. Trajectories of anxiety in oncology patients and family caregivers during and after radiation therapy. Eur J Oncol Nurs. 2012;16:1–9. doi: 10.1016/j.ejon.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luciano M, Huffman JE, Arias-Vasquez A, Vinkhuyzen AA, Middeldorp CM, Giegling I, Payton A, Davies G, Zgaga L, Janzing J, Ke X, Galesloot T, Hartmann AM, Ollier W, Tenesa A, Hayward C, Verhagen M, Montgomery GW, Hottenga JJ, Konte B, Starr JM, Vitart V, Vos PE, Madden PA, Willemsen G, Konnerth H, Horan MA, Porteous DJ, Campbell H, Vermeulen SH, Heath AC, Wright A, Polasek O, Kovacevic SB, Hastie ND, Franke B, Boomsma DI, Martin NG, Rujescu D, Wilson JF, Buitelaar J, Pendleton N, Rudan I, Deary IJ. Genome-wide association uncovers shared genetic effects among personality traits and mood states. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:684–695. doi: 10.1002/ajmg.b.32072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webb BT, Guo AY, Maher BS, Zhao Z, van den Oord EJ, Kendler KS, Riley BP, Gillespie NA, Prescott CA, Middeldorp CM. Meta-analyses of genome-wide linkage scans of anxiety-related phenotypes. Eur J Hum Genet. 2012;20:1078–1084. doi: 10.1038/ejhg.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Domschke K, Deckert J. Genetics of anxiety disorders - status quo and quo vadis. Curr Pharm Des. 2012;18:5691–5698. doi: 10.2174/138161212803530781. doi:CPD-EPUB-20120524-2. [DOI] [PubMed] [Google Scholar]
- 29.McGrath LM, Weill S, Robinson EB, Macrae R, Smoller JW. Bringing a developmental perspective to anxiety genetics. Dev Psychopathol. 2012;24(4):1179–1193. doi: 10.1017/S0954579412000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Binder EB. The genetic basis of mood and anxiety disorders - changing paradigms. Biol Mood Anxiety Disord. 2012;2:17. doi: 10.1186/2045-5380-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sokolowska E, Hovatta I. Anxiety genetics - findings from cross-species genome-wide approaches. Biol Mood Anxiety Disord. 2013;3:9. doi: 10.1186/2045-5380-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou R, Baldwin DS. A neuroimmunological perspective on anxiety disorders. Hum Psychopharmacol. 2012;27:6–14. doi: 10.1002/hup.1259. [DOI] [PubMed] [Google Scholar]
- 33.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK. A metaanalysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 34.Kustova Y, Sei Y, Morse HC, Jr, Basile AS. The influence of a targeted deletion of the IFN gamma gene on emotional behaviors. Brain Behav Immun. 1998;12:308–324. doi: 10.1006/brbi.1998.0546. doi:S0889-1591(98)90546-3. [DOI] [PubMed] [Google Scholar]
- 35.Lesch KP. Mouse anxiety: the power of knockout. Pharmacogenomics J. 2001;1:187–192. doi: 10.1038/sj.tpj.6500016. [DOI] [PubMed] [Google Scholar]
- 36.Connor TJ, Leonard BE. Depression, stress and immunological activation: the role of cytokines in depressive disorders. Life Sci. 1998;62:583–606. doi: 10.1016/s0024-3205(97)00990-9. doi:S0024320597009909. [DOI] [PubMed] [Google Scholar]
- 37.Fiore M, Alleva E, Probert L, Kollias G, Angelucci F, Aloe L. Exploratory and displacement behavior in transgenic mice expressing high levels of brain TNF-alpha. Physiol Behav. 1998;63:571–576. doi: 10.1016/s0031-9384(97)00514-3. doi:S0031938497005143. [DOI] [PubMed] [Google Scholar]
- 38.Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 39.Illi J, Miaskowski C, Cooper B, Levine JD, Dunn L, West C, Dodd M, Dhruva A, Paul SM, Baggott C, Cataldo J, Langford D, Schmidt B, Aouizerat BE. Association between pro- and anti-inflammatory cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression. Cytokine. 2012;58:437–447. doi: 10.1016/j.cyto.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCann B, Miaskowski C, Koetters T, Baggott C, West C, Levine JD, Elboim C, Abrams G, Hamolsky D, Dunn L, Rugo H, Dodd M, Paul SM, Neuhaus J, Cooper B, Schmidt B, Langford D, Cataldo J, Aouizerat BE. Associations between pro- and anti-inflammatory cytokine genes and breast pain in women prior to breast cancer surgery. J Pain. 2012;13:425–437. doi: 10.1016/j.jpain.2011.02.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dunn LB, Aouizerat BE, Langford DJ, Cooper BA, Dhruva A, Cataldo JK, Baggott CR, Merriman JD, Dodd M, West C, Paul SM, Miaskowski C. Cytokine gene variation is associated with depressive symptom trajectories in oncology patients and family caregivers. Eur J Oncol Nurs. 2013;17:346–353. doi: 10.1016/j.ejon.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miaskowski C, Cooper BA, Dhruva A, Dunn LB, Langford DJ, Cataldo JK, Baggott CR, Merriman JD, Dodd M, Lee K. Evidence of associations between cytokine genes and subjective reports of sleep disturbance in oncology patients and their family caregivers. PLoS ONE. 2012;7:e40560. doi: 10.1371/journal.pone.0040560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vogelzangs N, Beekman AT, de Jonge P, Penninx BW. Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry. 2013;3:e249. doi: 10.1038/tp.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bieling PJ, Antony MM, Swinson RP. The State-Trait Anxiety Inventory, Trait version: structure and content re-examined. Behav Res Ther. 1998;36:777–788. doi: 10.1016/s0005-7967(98)00023-0. doi:S0005-7967(98)00023-0. [DOI] [PubMed] [Google Scholar]
- 45.Kennedy BL, Schwab JJ, Morris RL, Beldia G. Assessment of state and trait anxiety in subjects with anxiety and depressive disorders. Psychiatr Q. 2001;72:263–276. doi: 10.1023/a:1010305200087. [DOI] [PubMed] [Google Scholar]
- 46.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measur. 1977;1:385–401. [Google Scholar]
- 47.Sheehan TJ, Fifield J, Reisine S, Tennen H. The measurement structure of the Center for Epidemiologic Studies Depression Scale. J Pers Assess. 1995;64:507–521. doi: 10.1207/s15327752jpa6403_9. [DOI] [PubMed] [Google Scholar]
- 48.Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8:887–899. doi: 10.1038/nrc2507. doi:nrc2507. [DOI] [PubMed] [Google Scholar]
- 49.Conde L, Vaquerizas JM, Dopazo H, Arbiza L, Reumers J, Rousseau F, Schymkowitz J, Dopazo J. PupaSuite: finding functional single nucleotide polymorphisms for large-scale genotyping purposes. Nucleic Acids Res. 2006;34:W621–625. doi: 10.1093/nar/gkl071. doi:34/suppl_2/W621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 51.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Human Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tian C, Kosoy R, Lee A, Ransom M, Belmont JW, Gregersen PK, Seldin MF. Analysis of East Asia genetic substructure using genome-wide SNP arrays. PLoS ONE. 2008;3:e3862. doi: 10.1371/journal.pone.0003862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoggart CJ, Parra EJ, Shriver MD, Bonilla C, Kittles RA, Clayton DG, McKeigue PM. Control of confounding of genetic associations in stratified populations. Am J Human Genet. 2003;72:1492. doi: 10.1086/375613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Halder I, Shriver M, Thomas M, Fernandez JR, Frudakis T. A panel of ancestry informative markers for estimating individual biogeographical ancestry and admixture from four continents: utility and applications. Human Mutat. 2008;29:648–658. doi: 10.1002/humu.20695. [DOI] [PubMed] [Google Scholar]
- 55.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nature Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 56.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 57.Hattersley AT, McCarthy MI. What makes a good genetic association study? Lancet. 2005;366:1315–1323. doi: 10.1016/S0140-6736(05)67531-9. [DOI] [PubMed] [Google Scholar]
- 58.Legrand LN, McGue M, Iacono WG. A twin study of state and trait anxiety in childhood and adolescence. J Child Psychol Psychiatry. 1999;40:953–958. [PubMed] [Google Scholar]
- 59.Wolitzky-Taylor KB, Castriotta N, Lenze EJ, Stanley MA, Craske MG. Anxiety disorders in older adults: A comprehensive review. Depress Anxiety. 2010;27:190–211. doi: 10.1002/da.20653. [DOI] [PubMed] [Google Scholar]
- 60.Wallace LM, Priestman SG, Dunn JA, Priestman TJ. The quality of life of early breast cancer patients treated by two different radiotherapy regimens. Clin Oncol. 1993;5:228–233. doi: 10.1016/s0936-6555(05)80234-1. [DOI] [PubMed] [Google Scholar]
- 61.Andersen BL, Tewfik HH. Psychological reactions to radiation therapy: reconsideration of the adaptive aspects of anxiety. J Pers Soc Psychol. 1985;48:1024–1032. doi: 10.1037//0022-3514.48.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valdes-Stauber J, Vietz E, Kilian R. The impact of clinical conditions and social factors on the psychological distress of cancer patients: an explorative study at a consultation and liaison service in a rural general hospital. BMC Psychiatry. 2013;13:226. doi: 10.1186/1471-244X-13-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reilly CM, Bruner DW, Mitchell SA, Minasian LM, Basch E, Dueck AC, Cella D, Reeve BB. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013 doi: 10.1007/s00520-012-1688-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schreier AM, Williams SA. Anxiety and quality of life of women who receive radiation or chemotherapy for breast cancer. Oncol Nurs Forum. 2004;31:127–130. doi: 10.1188/04.ONF.127-130. [DOI] [PubMed] [Google Scholar]
- 65.Michael YL, Kawachi I, Berkman LF, Holmes MD, Colditz GA. The persistent impact of breast carcinoma on functional health status: prospective evidence from the Nurses’ Health Study. Cancer. 2000;89:2176–2186. doi: 10.1002/1097-0142(20001201)89:11<2176::AID-CNCR5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 66.Ganz PA, Guadagnoli E, Landrum MB, Lash TL, Rakowski W, Silliman RA. Breast cancer in older women: quality of life and psychosocial adjustment in the 15 months after diagnosis. J Clin Oncol. 2003;21:4027–4033. doi: 10.1200/JCO.2003.08.097. [DOI] [PubMed] [Google Scholar]
- 67.Zhou ES, Penedo FJ, Bustillo NE, Benedict C, Rasheed M, Lechner S, Soloway M, Kava BR, Schneiderman N, Antoni MH. Longitudinal effects of social support and adaptive coping on the emotional well-being of survivors of localized prostate cancer. J Support Oncol. 2010;8:196–201. doi: 10.1016/j.suponc.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mallinckrodt B, Armer JM, Heppner PP. A threshold model of social support, adjustment, and distress after breast cancer treatment. J Couns Psychol. 2012;59:150–160. doi: 10.1037/a0026549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alfaro E, Dhruva A, Langford DJ, Koetters T, Merriman JD, West C, Dunn LB, Paul SM, Cooper B, Cataldo J, Hamolsky D, Elboim C, Kober K, Aouizerat BE, Miaskowski C. Associations between cytokine gene variations and self-reported sleep disturbance in women following breast cancer surgery. Eur J Oncol Nurs. 2014;18:85–93. doi: 10.1016/j.ejon.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. 2013;30:297–306. doi: 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Courtier N, Gambling T, Enright S, Barrett-Lee P, Abraham J, Mason MD. Psychological and immunological characteristics of fatigued women undergoing radiotherapy for early-stage breast cancer. Support Care Cancer. 2013;21:173–181. doi: 10.1007/s00520-012-1508-6. [DOI] [PubMed] [Google Scholar]
- 72.Dantzer R. Depression and inflammation: an intricate relationship. Biol Psychiatry. 2012;71:4–5. doi: 10.1016/j.biopsych.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 73.Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep. 2011;13:467–475. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lyon D, Elmore L, Aboalela N, Merrill-Schools J, McCain N, Starkweather A, Elswick RK, Jr, Jackson-Cook C. Potential epigenetic mechanism(s) associated with the persistence of psychoneurological symptoms in women receiving chemotherapy for breast cancer: A hypothesis. Biol Res Nurs. 2013 doi: 10.1177/1099800413483545. doi:1099800413483545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomas KS, Motivala S, Olmstead R, Irwin MR. Sleep depth and fatigue: role of cellular inflammatory activation. Brain Behav Immun. 2011;25:53–58. doi: 10.1016/j.bbi.2010.07.245. doi:S0889-1591(10)00419-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12:255–269. doi: 10.1159/000087104. doi:NIM2005012005255. [DOI] [PubMed] [Google Scholar]
- 77.Bay-Richter C, Janelidze S, Hallberg L, Brundin L. Changes in behaviour and cytokine expression upon a peripheral immune challenge. Behav Brain Res. 2011;222:193–199. doi: 10.1016/j.bbr.2011.03.060. [DOI] [PubMed] [Google Scholar]
- 78.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ, Critchley HD. Neural origins of human sickness in interoceptive responses to inflammation. Biol Psychiatry. 2009;66:415–422. doi: 10.1016/j.biopsych.2009.03.007. doi:S0006-3223(09)00323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tizard I. Sickness behavior, its mechanisms and significance. Anim Health Res Rev. 2008;9:87–99. doi: 10.1017/S1466252308001448. doi:S1466252308001448. [DOI] [PubMed] [Google Scholar]
- 80.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. doi:S0889-1591(06)00300-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Colotta F, Re F, Muzio M, Bertini R, Polentarutti N, Sironi M, Giri JG, Dower SK, Sims JE, Mantovani A. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993;261:472–475. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- 82.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. doi:nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. doi:26/6/971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. S0006-3223(08)01532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Henje Blom E, Lekander M, Ingvar M, Asberg M, Mobarrez F, Serlachius E. Pro-inflammatory cytokines are elevated in adolescent females with emotional disorders not treated with SSRIs. J Affect Disord. 2012;136:716–723. doi: 10.1016/j.jad.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 86.Moreau M, Lestage J, Verrier D, Mormede C, Kelley KW, Dantzer R, Castanon N. Bacille Calmette-Guerin inoculation induces chronic activation of peripheral and brain indoleamine 2,3-dioxygenase in mice. J Infect Dis. 2005;192:537–544. doi: 10.1086/431603. doi:JID34079. [DOI] [PubMed] [Google Scholar]
- 87.Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31:2121–2131. doi: 10.1038/sj.npp.1301029. doi:1301029. [DOI] [PubMed] [Google Scholar]
- 88.Snyder SH. Serotonin, cytokines, p11, and depression. Proc Natl Acad Sci U S A. 2011;108(22):8923–8924. doi: 10.1073/pnas.1106103108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. doi:S0889-1591(06)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rossi S, Sacchetti L, Napolitano F, De Chiara V, Motta C, Studer V, Musella A, Barbieri F, Bari M, Bernardi G, Maccarrone M, Usiello A, Centonze D. Interleukin-1beta causes anxiety by interacting with the endocannabinoid system. J Neurosci. 2012;32:13896–13905. doi: 10.1523/JNEUROSCI.1515-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 92.Aouizerat BE, Dodd M, Lee K, West C, Paul SM, Cooper BA, Wara W, Swift P, Dunn LB, Miaskowski C. Preliminary evidence of a genetic association between tumor necrosis factor alpha and the severity of sleep disturbance and morning fatigue. Biol Res Nurs. 2009;11:27–41. doi: 10.1177/1099800409333871. doi:1099800409333871. [DOI] [PubMed] [Google Scholar]
- 93.Abraham LJ, Kroeger KM. Impact of the -308 TNF promoter polymorphism on the transcriptional regulation of the TNF gene: relevance to disease. J Leukoc Biol. 1999;66:562–566. doi: 10.1002/jlb.66.4.562. [DOI] [PubMed] [Google Scholar]
- 94.Kroeger KM, Carville KS, Abraham LJ. The -308 tumor necrosis factor-alpha promoter polymorphism effects transcription. Mol Immunol. 1997;34:391–399. doi: 10.1016/s0161-5890(97)00052-7. doi:S0161589097000527. [DOI] [PubMed] [Google Scholar]
- 95.Kroeger KM, Steer JH, Joyce DA, Abraham LJ. Effects of stimulus and cell type on the expression of the -308 tumour necrosis factor promoter polymorphism. Cytokine. 2000;12:110–119. doi: 10.1006/cyto.1999.0529. [DOI] [PubMed] [Google Scholar]
- 96.Camara ML, Corrigan F, Jaehne EJ, Jawahar MC, Anscomb H, Koerner H, Baune BT. TNF-alpha and its receptors modulate complex behaviours and neurotrophins in transgenic mice. Psychoneuroendocrinology. 2013;38:3102–3114. doi: 10.1016/j.psyneuen.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 97.Naude PJ, Dobos N, van der Meer D, Mulder C, Pawironadi KG, den Boer JA, van der Zee EA, Luiten PG, Eisel UL. Analysis of cognition, motor performance and anxiety in young and aged tumor necrosis factor alpha receptor 1 and 2 deficient mice. Behav Brain Res. 2014;258:43–51. doi: 10.1016/j.bbr.2013.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


