Abstract
Sun and colleagues (2017) find that individual Grp+ spinal interneurons can respond to and distinguish between stimuli that provoke itch or pain. The nociceptive response is limited by enkaphalin-expressing interneurons that are connected synaptically to the Grp+ neurons.
What is the first thing you do when you have an itch? You scratch to get relief. And what do you do when you experience pain from bumping your head? Usually you rub the area to alleviate the pain. These are examples of behavioral responses that follow the deconvolution and interpretation of itch and pain sensations by complex neurocircuitry in the spinal cord. Overlapping theories have been advanced to explain the differential interpretation of itch versus pain. The selectivity theory posits that itchy stimuli activate itch-selective primary afferents to generate this sensation while painful stimuli activate primary afferents and a larger nociceptive population, the activation of which inhibits itch so as to produce only pain. In contrast, the gate control theory of pain (which must somehow include itch) asserts that large myelinated fibers, Aβ fibers, associated with touch, pressure, and vibration, but not pain, can activate interneurons that modulate, or gate, firing of secondary spinal neurons associated with primary afferent pain fibers (nociceptors) (Handwerker, 1992; Melzack and Wall, 1965). In this issue of Neuron, Sun et al. (2017) propose a “leaky gate” model to refine the gate control theory. They deployed state-of-the art genetic approaches in combination with pruritic and nociceptive stimuli to identify a subset of spinal interneurons in mice that transmits both pain and itch. As expected, the response to increasing concentrations of itch stimuli reaches a plateau. Surprisingly, the concentration-effect response to a painful stimulus, capsaicin, while intensity dependent, is U-shaped. The higher concentrations alter a gate in which “leaking” of an endogenous opiate, enkephalin, limits the nociceptive response (Figure 1).
Figure 1.
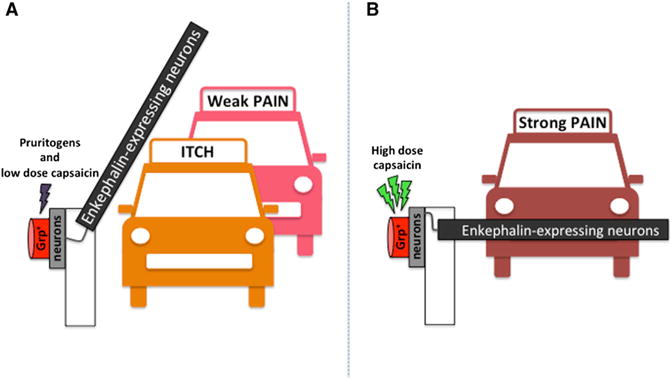
Enkephalin Modulates the Sensing of a Strong Pain Stimulus
(A) Stimulation by pruritogens and low levels of capsaicin leads to itch and weak pain.
(B) Increased neuronal firing associated with high levels of capsaicin leads to the activation of neurons that release enkaphalin, which modulates the degree of pain.
Pain and itch protect against potentially noxious agents and are effective by being unpleasant. They are intimately related although they are distinct sensations and trigger different behavioral responses (Ross, 2011). It is easy to see the itch-pain interaction as antagonistic: itch is alleviated by scratching (painful stimulus), while μ-opioids produce analgesia yet induce itch. However, the fibers that transmit itch also respond to pain-producing substance, also known as algogens, demonstrating that the relationship between itch and pain is not simple (Bautista et al., 2014). In fact, psychophysical studies in humans reveal that most noxious chemicals are not wholly specific to itch over pain. Rather, subjects describe chemical-induced itch as being accompanied by nociceptive sensations including burning, pricking, and stinging. Stated simply, what the subjects feel is not necessarily “pure” itch (Sikand et al., 2009, 2011). The “leaky gate” model of Sun and colleagues provides a tweak that can better explain these observations.
The authors found previously that primary sensory neurons that express MrgprA3 responded selectively to itch rather than pain stimuli and that the axons of these DRG neurons terminate in lamina II of the spinal cord (Han et al., 2013). In the current study, they find that a subset of excitatory interneurons that express gastrin-releasing peptide (Grp+) in inner lamina II receives monosynaptic input from MrgprA3+ sensory neurons. This result was obtained using an optogenetic approach in which MrgprA3+ neurons expressing channelrhodopsin were exposed to blue light, revealing that 100% of Grp+ neurons received input from itch-selective MrgprA3+ neurons. But could those Grp+ interneurons also receive monosynaptic input from nociceptors? To address this question, mono-synaptic rabies tracing was used to target Grp+ spinal neurons, and thus transynaptically labeling DRG neurons. By correlating the percentage of DRG neurons detected with an in-house-generated antibody to MrgprC11, and thus most MrgA3+ itch neurons, and those expressing peptidergic or non-peptidergic markers, together with the viral tracer, it could be deduced that the Grp+ neurons also receive monosynaptic input from nociceptive populations. If the Grp+ neurons receive inputs from both itch and pain sensory neurons, can they distinguish between these stimuli? To answer this question, the authors employed an ex vivo approach to record from spinal slices still attached to their DRG ganglia. It was observed that both algogens (capsaicin) and pruritogens (SLIGRL, chloroquine, and histamine) triggered action potentials in Grp+ neurons. The difference was that algogens produced high-frequency firing while pruritogens caused weak firing, indicating that Grp+ neurons can distinguish between painful and itchy stimuli.
An observation that was key to unlocking the “gate” so as to identify its “leakiness” was the response to different concentrations of intrathecal capsaicin. Mice in which TrpV1 was globally knocked out were re-engineered so that TrpV1 expression was restricted to Grp+ spinal neurons. Behavioral assays were performed to determine which sensations were induced by Grp neurons. Surprisingly, while robust itch and pain responses were observed, the profiles were different. While itch responses increased until reaching a plateau, the pain responses showed an inverted U. Might the inverted U profile be related to a pain inhibition circuit? If so, the authors reasoned, then the endogenous δ-opioid agonist enkephalin might be a plausible candidate. This reasoning was supported by the finding that naltrindole, a δ-opioid antagonist, could “rescue” pain responses without affecting itch. Further support came from patch-clamp analysis, which revealed that the firing of a single Grp+ neuron triggered activation of enkephalin-expressing neurons. In addition, enkephalin was released after treatment with high dose of capsaicin (pain), but not after low dose (itch) or no capsaicin, demonstrating that only strong activation of Grp+ neurons leads to an inhibitory response from a painful stimulus. To demonstrate specificity to the enkephalin system, a series of other chemicals were found to be without effect. Thus, bicuculline (a GABAA antagonist), cyclo-somatostatin (a somatostatin receptor antagonist), CTAP, and CTOP (μ-opioid antagonists) were assessed and not found to have an effect on pain or itch responses in these mice.
Sun et al. (2017) relate their findings to the gate control theory. They propose that Grp+ neurons, akin to Aβ fibers, use the type I incoherent feedforward loop (I1-FFL) as a “gate” to control pain transmission. The enkephalin-expressing neurons would have a role similar to that of the inhibitory interneurons from the theory of Melzak and Wall, except that as proposed here, strong activation of Grp+ neurons triggers enkephalin release to “close the gate” to painful signals from these cells and potentially other neurons in the spinal cord. However, there are some differences between the two models. In the gate control theory, the Aβ fibers do not allow any signal to pass “through the gate,” while the Grp+ neurons let weak pain signals “through the gate” but suppress strong pain signals. It is for this reason that Sun et al. have termed the model a “leaky gate.” The authors suggest that the advantage of the “leaky gate” is to guarantee sensitivity to weak painful stimuli by letting them pass and to block the strong signals that would otherwise result in overwhelming pain sensations. Might a defect in the leaky gate contribute to the intensity of neuropathic pain and itch, such as that often associated with shingles, and thus the reactivation of herpes zoster or non-cutaneous itches?
As the Grp I1-FFL is proposed to work as a “break” in the “leaky gate” model, then ablation of Grp+ neurons would be predicted to lead to augmented pain. Indeed, the loss of these neurons was associated with an increase in chemical and thermal pain responses. On the other hand, itch responses to histamine, chloroquine, SLIGRL, and serotonin were reduced, supporting the pivotal role of Grp+ neurons in itch coding. Thus, the report from Sun et al. (2017) is consistent with the Grp I1-FFL modulating the coding of pain and itch.
The findings in this paper may begin to shed light on the mechanisms underlying the events described by subjects in psychophysical studies. Itch is typically accompanied by weaker sensations of pricking and burning in such studies. The authors suggest that perhaps subjects feel itch induced by a pruritogen and also pricking and burning as weak painful sensations because the itchy stimuli are not sufficient to strongly fire the Grp+ neurons. As a result, enkephalin-expressing neurons are not activated, enkephalin is not released, and weak pain is not blocked. In conclusion, spinal Grp+ neurons indeed code for itch while limiting pain via the “leaky gate.”
References
- Bautista DM, Wilson SR, Hoon MA. Nat Neurosci. 2014;17:175–182. doi: 10.1038/nn.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, et al. Nat Neurosci. 2013;16:174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker HO. APSJ. 1992;1:135–138. [Google Scholar]
- Melzack R, Wall PD. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Ross SE. Curr Opin Neurobiol. 2011;21:880–887. doi: 10.1016/j.conb.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Sikand P, Shimada SG, Green BG, LaMotte RH. Pain. 2009;144:66–75. doi: 10.1016/j.pain.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikand P, Dong X, LaMotte RH. J Neurosci. 2011;31:7563–7567. doi: 10.1523/JNEUROSCI.1192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Xu Q, Guo C, Guan Y, Lin Q, Dong X. Neuron. 2017;93(this issue):840–853. doi: 10.1016/j.neuron.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


