Abstract
Neonatal feeding has been traditionally understudied so guidelines and evidence-based support for common feeding practices are limited. A major contributing factor to the paucity of evidence-based practice in this area has been the lack of simple-to-use, low-cost tools for monitoring sucking performance. We describe new methods for quantifying neonatal sucking performance that hold significant clinical and research promise. We present early results from an ongoing study investigating neonatal sucking as a marker of risk for adverse neurodevelopmental outcomes. We include quantitative measures of sucking performance to better understand how movement variability evolves during skill acquisition. Results showed the coefficient of variation of suck duration was significantly different between preterm neonates at high risk for developmental concerns (HRPT) and preterm neonates at low risk for developmental concerns (LRPT). For HRPT, results indicated the coefficient of variation of suck smoothness increased from initial feeding to discharge and remained significantly greater than healthy full-term newborns (FT) at discharge. There was no significant difference in our measures between FT and LRPT at discharge. Our findings highlight the need to include neonatal sucking assessment as part of routine clinical care in order to capture the relative risk of adverse neurodevelopmental outcomes at discharge.
Keywords: Premature neonate, suck behavior, nutritive sucking patterns, oral feeding, feeding skills
Neonatal feeding has been described as the most precocious and complex behavior of the newborn and requires integration of physiologic function and neurobehavioral ability.1–3 Safe and efficient feeding is necessary to provide essential nutrients for neonatal brain development, the importance of which is underscored by the fact that independent oral feeding is a final criteria of hospital discharge for preterm and sick full-term newborns.4 At the same time, neonatal feeding has been traditionally understudied so guidelines and evidence-based support for common feeding practices are limited.5 A major contributing factor to the paucity of evidence-based practice in this area has been the lack of simple-to-use, low-cost tools for monitoring sucking performance.6
In this article, we describe new methods for quantifying neonatal sucking performance that have the potential to support crib-side visual assessment of sucking, help caregivers interpret their neonates’ feeding cues, and serve as valuable resource for studying a myriad of important research questions surrounding neonatal feeding. The approach we describe leverages several significant technological advances to inform and advance neonatal feeding practice. To illustrate the utility of this approach, we present early results from our ongoing study investigating early sucking as a marker for later neurodevelopmental outcomes.
The survival of preterm neonates has increased markedly in the last decade due to advances in technology and neonatal care.7 Despite these medical advances, the risk of poor neurobehavioral functioning is common.8 Consequently, early identification of neonates at risk for adverse neurodevelopmental outcomes is crucial to enable prevention or treatment during infancy, when neuroplasticity mechanisms are thought to be greatest.9,10 Scientists studying the maturational sequence of newborn feeding have theorized that early nutritive sucking skills may be an early marker of overall central nervous system integrity,1 and as such, a potential predictor of neurodevelopmental outcomes.11–13
Indeed, the literature suggests a strong relationship between early nutritive sucking and neurodevelopmental outcomes early on. For example, Medoff-Cooper et al investigated the relationship between early nutritive sucking patterns of preterm neonates and neurodevelopmental outcomes in the first year of life. The study population included early and moderate preterm neonates. Newborns received a 5-minute sucking test at 34 and 40 weeks’ postmenstrual age (PMA) and developmental outcomes were measured at 6 and 12 months’ corrected age using the Bayley Scales of Infant Development (BSID).13 Sucking performance was quantified using a prototype sucking apparatus fitted with an adapted nipple. Results indicated that sucking parameters (number of sucks, sucks per burst, mean sucking pressure peaks, and suck maturity index) at 40 weeks’ PMA were significant predictors of developmental status at 1 year.
Observational scale data also suggest a relationship between early sucking and relative risk of developmental concerns. In a study by Tsai et al, the Neonatal Oral Motor Assessment Scale (NOMAS), a 28-item observational scale, was used to characterize sucking patterns in preterm neonates.14 Participants included early preterm, moderate preterm, and late preterm neonates. The first 2 minutes of nutritive sucking were video-recorded weekly from initiation of oral feeding until discharge and then the videos were reviewed by NOMAS-certified assessors. Based on those results, the neonates were assigned to one of two groups: normal sucking pattern (by 37 weeks) or persistent disorganized sucking pattern (after 37 weeks). At 6 and 12 months’ corrected age, the BSID was administered to determine developmental status. Results suggested that the risk for developmental delay was significantly greater in those newborns classified as having a persistent disorganized sucking pattern. A similar trend was noted at 12 months; however, the results were not statistically significant.
Early sucking has also been shown to relate to neurodevelopmental outcomes at later ages. For example, Mizuno and Ueda investigated whether neonatal feeding performance would predict neurodevelopmental outcomes at 18 months.1 Subjects included a mix of late preterm and term neonates. Nipples were modified to measure the suction and expression components of nutritive sucking. Adverse events during feeding were noted; however, feedings were not stopped following a desaturation or bradycardic event. Visual inspection of compression-expression wave forms was used to classify feeding performance from immature to mature. Neurodevelopmental status at 18 months was determined using the BSID. Results indicated a significant correlation between feeding pattern and neurodevelopmental outcomes; more mature feeding patterns at 2 weeks after initial oral feeding were associated with better neurodevelopmental outcomes.
Wolthuis-Stigter and colleagues reported evidence that the status of sucking behavior as late as 6 weeks after birth is associated with developmental concerns at 2 years of age. The researchers investigated the association between specific elements of sucking and neurodevelopmental outcomes. The study population included very early, early, moderate, and late preterm neonates. Nutritive sucking behavior was assessed using the NOMAS. NOMAS scores were collected at two times: weekly between 37 and 40 weeks’ PMA and every 2 weeks between 40 and 50 weeks’ PMA.15 The Dutch version of the BSID was completed at 27 months’ corrected age. Results showed that atypical sucking performance at 4 to 6 weeks post-term was positively correlated with poor neurodevelopmental outcomes in preterm neonates at 2 years of age.
The relationship between early sucking and neurodevelopment has also been documented in children as old as 3 years. In a study reported by Hiramoto and colleagues, a questionnaire focused on sucking behavior was administered to mothers of children during routine 18-month and/or 3-year-old child health checkups. No demographic data detailing the characteristics of the sample were provided. Public health nurses completed an assessment of age-appropriate developmental milestones adapted from the Enjoji Scale of Infant Analytical Development.16 Based on screening results, subjects were categorized as either typically developing (pass) or possible developmental delay (fail). Odds ratios were used to calculate the likelihood for questionnaire items to associate with developmental delay. Results indicated that the lack of a smooth suck-and-rest pattern during feeding, as reported by parents, was associated with significantly higher likelihood of being labeled developmental delayed at both 18 months and 3 years.
Taken together, these studies provide strong evidence that early sucking is a reflection of the overall integrity of the central nervous system, and as such, may be a behavioral marker for risk of poor neurodevelopment outcomes from as early as 6 months to 3 years. However, the methods reported have some important limitations. First, the use of the NOMAS as the sole measures of sucking integrity is limiting,14,15 because it requires specialty certification, it is subjective, and the interrater reliability has been shown to vary significantly from rater to rater.17 The use of modified nipples to quantify sucking characteristics is also considered a limitation.1 Modifying a nipple could in fact alter critical sensory aspects of sucking for neonates and potentially confound findings. Also of importance is the fact that several reported studies have analyzed sucking performance using a specific time frame in the feeding (e.g., first 5 minutes). Yet the feeding experience of premature neonates varies from moment to moment so summary statistics from a limited time in the feeding could potentially miss important clinical and predictive information.18 Moreover, in most cases, studies did not include healthy full-term newborns as a comparison population, which we believe is clinically valuable to provide context for results and interpretation of findings. Finally, multiple observations of sucking performance are necessary to consider the ways in which the behavior changes as a function of maturation and experience.
The purpose of the current study was twofold: (1) compare metrics of sucking performance between preterm neonates and healthy term neonates at initiation of feeding and at discharge and (2) investigate changes in sucking performance longitudinally among preterm neonates from initiation of oral feeding through post discharge. Our methods for collecting sucking data address the limitations of previous approaches. Here, we relied on automated analyses of elements of sucking to provide a set of summary statistics. Moreover, our present methodology uses a scalable and noninvasive tool to measure important sucking parameters throughout the course of a feeding so no modification of nipples was required and the moment to moment variability characterizing neonatal sucking was captured.
METHODS
Participants
Two groups of newborns were recruited for participation: healthy full-term neonates (FT; n = 15) defined as >37 weeks’ gestational age, with appropriate weight for gestational age, and healthy preterm neonates (PT; n = 40) with appropriate weight for gestational age (Table 1). Neonates in the FT group met the inclusion criteria for no anomalies or diseases known to interfere with feeding (e.g., cleft lip and/or palate). PT neonates met the following inclusion criteria: no anomalies or diseases known to interfere with feeding, no congenital disorders, chromosomal abnormalities, or major congenital anomalies, no disorders secondary to known perinatal exposure to toxic substances, and no history of intraventricular hemorrhage greater than grade II. Preterm neonates could have a diagnosis of respiratory distress syndrome but could not be ventilated for a prolonged period of time. Participants were recruited from Kentucky Children’s Hospital, a 70-bed level IV NICU. The study was approved by the Institutional Review Board of the University of Kentucky where the study was performed.
Table 1.
Mean (Standard Deviation) of Variables of Interest for the Full Study Population
| Variable | Preterm (n = 40) |
Full Term (n = 40) |
|---|---|---|
| F:M | 24:16 | 4:11 |
| Gestational age (wk) | 30.7 (2.8) | 38.6 (1.0) |
| Birth weight (g) | 1,496 (575) | 3,158 (343) |
| FRLOS (d) | 25.6 (11) | 2.5 (2) |
Abbreviation: FRLOS, feeding-related length of stay calculated from date of initial oral feeding to discharge.
Measures
nfant Feeding Solution (NFANT Labs, Atlanta, Georgia, United States) was used to collect nutritive sucking data and determine our sucking variables of interest (Fig. 1). nfant Feeding Solution consists of a disposable nfant Coupling that connects a bottle to a standard nipple or pacifier. The nfant SSB Sensor connects to the coupling and noninvasively measures nipple movement. Data are streamed from the sensor to a mobile tablet, and nipple movement is displayed in real time on the nfant Mobile App. Following a feeding, data were stored in the nfant Cloud Database for later analyses. Signal processing of nipple movement included a 1-second rolling minimum baseline shift and outlier suck peak removal to account for any movement artifact. Amplitudes were then normalized to the maximum peak observed (0 to 100%).
Figure 1.
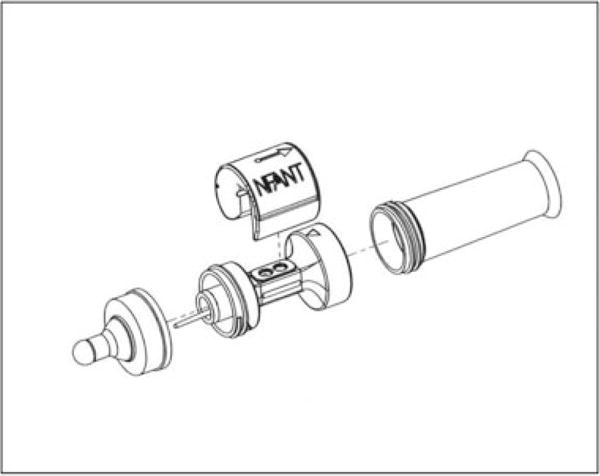
Diagram of nfant Feeding Solution (NFANT Labs, Atlanta, Georgia).
Recently, Tamilia and colleagues described the importance of including analyses of quantitative measures of sucking performance more characteristic of sucking in the context of dynamical systems theory.19,20 For example, previous researchers have limited findings to sucking parameters such as number of sucks, sucks per burst, and mean sucking pressure peaks, among others.13 In contrast, Tamilia and colleagues introduced variables calculated across each sucking burst to reflect sucking stability versus variability. Because variability contains important information about human movement in general,21 a shift in focus to metrics of coordinated movement could be particularly important in investigations of early sucking as a potential marker of later neurodevelopment. Therefore, for this study, algorithms to identify key features and metrics describing the suck pattern, consistent with Tamilia et al,20 were developed, and all analyses were performed using custom software (Matlab 2013a, Mathworks, Natick, Massachusetts, United States).
Our primary measures of interest focused on movement variability of the nipple during sucking (Fig. 2). Variability was assessed using the coefficient of variation (COV; i.e., standard deviation divided by the mean) for four distinct suck metrics: suck peak amplitude (COVPKA), duration (COVD), frequency (COVF i.e., 1/suck period), and smoothness (COVSM). Summarized here but best described in previous literature,19,22 smoothness is a measure of motor performance that is derived from the speed profile of the movement signal and indicates the amount of oscillations within a suck where the fewer the oscillations, the “smoother” the movement.
Figure 2.
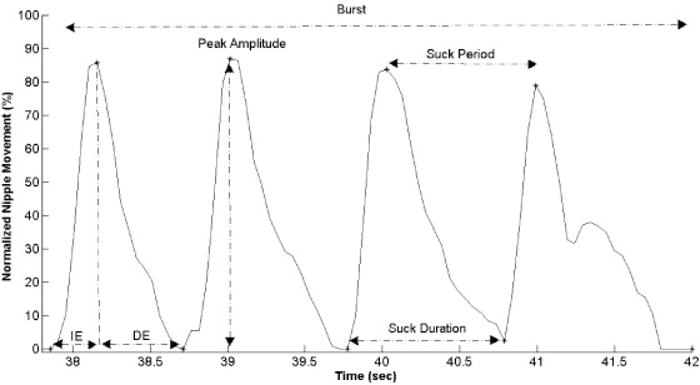
Suck pattern excerpt from a representative full-term subject. Points indicate the start, end, and peak of the suck events, generated from the custom suck algorithm employed in this study. Indicated suck features are used to generate metrics for analyses.
Procedures
Once subjects were declared medically stable and ready to begin oral feeding, nurse researchers checked daily notes to determine readiness to begin data collection. Data collection for FT neonates took place within 2 weeks of birth. PT neonates entered the protocol when they showed evidence of sustained bottle-feeding (1 to 2 feedings per day). Measures of interest were collected at three times during the hospital stay: when newborns were taking 1 to 2 feedings per day (evidence of sustained bottle-feeding), when they were taking 4 to 6 oral feedings per day (established bottle-feeding) and at discharge when they were taking all feedings orally.23 Measures were collected within 24 to 36 hours of being notified that a neonate had met the respective criteria for each time period. Specific feeding instructions as ordered by the attending physician were maintained and recorded. Neonatal cue-based feeding procedures were followed so that the feeding was stopped according to the cues of the neonate and/or after 30 minutes.24 In all cases, data collection was scheduled so as not to interfere with breast-feeding. Each session began with 1 minute of nonnutritive sucking followed by nutritive sucking in their typical feeding position (e.g., held by caregiver). Data were collected for the entire feeding. For the purposes of this article, only the nutritive sucking results are reported and used in analyses.
To meet the aims of the study, neonates were stratified into three groups (Table 2). Neonates in group 1 were preterm but were not considered high risk for developmental concerns (LRPT subjects) and so were released to their primary care physician and/or pediatrician after discharge. Neonates in group 2 were also preterm but considered at risk for developmental concerns (HRPT subjects) based on criteria established by our outpatient follow-up clinic, including but not limited to gestational age and birth weight. These newborns were released to a multidisciplinary follow-up team with expertise in preterm neonatal care. Group 3 was comprised of healthy, full-term (FT) newborns. HRPT neonates were seen at critical developmental times including 1, 3, and 6 months’ corrected age. The BSID is administered at 1 and 2 years’ corrected age and then again at 3 years’ chronological age. (Note: At the time of this reporting, only five of our study neonates had reached the age for administration of their 1-year BSID so these data are not reported.)
Table 2.
Mean (Standard Deviation) of Variables of Interest for Our Study Population Subcategories, Outlier Removed
| Variable | LRPT (n = 12) | HRPT (n = 28) | FT (n = 14) | Post hoc p value* |
|---|---|---|---|---|
| F:M | 4:8 | 20:8 | 4:10 | NA |
| GA | 34.1 (1.3) | 29.2 (2) | 39.1 (1) | FT > LRPT > HRPT |
| BW | 2139 (610) | 1220 (253) | 3203 (309) | FT > LRPT > HRPT |
| GA at initial exam | 35.6 (1.5) | 34.2 (1.3) | 39.3 (1.3) | FT > LRPT > HRPT |
| GA at discharge exam | 36.4 (1.4) | 37 (1.6) | 39 (1) | HRPT > FT, LRPT |
| FRLOS | 16 (4) | 30 (10) | 2 (2) | FT > LRPT > HRPT |
Abbreviations: BW, birth weight; FRLOS, feeding-related length of stay in days; FT, full term infants; GA, gestational age; HRPT, preterm infants considered high risk for developmental concerns; LRPT, preterm infants considered low risk for developmental concerns.
All analyses of variance were significant at p < 0.0001; post hoc direction of significant findings.
Statistical Analyses
Sample population characteristics were analyzed using univariate analyses of variance. A least squares difference post hoc analysis was performed for metrics with α < 0.05. Measures of interest were calculated for the initial exam and discharge exam for HRPT and LRPT. Additionally, for the HRPT, measures were calculated for their follow-up exams. For FT subjects, only the discharge exam was used in analyses because there was only one feeding session for term babies prior to discharge. A mixed analysis of variance was performed between groups comparing exam times. Paired t-tests were used within HRPT and LRPT to determine changes in metrics within each group between exam times and unpaired t-tests between groups. Significance for all analyses was set a priori at p < 0.05. One FT subject was removed from analysis due to low-quality signal, leaving 14 FT, 28 HRPT, and 12 LRPT subjects for analysis (Table 2).
RESULTS
Results from analyses of sample population characteristics were as expected. Preliminary analyses indicated significant differences in mean gestational age, birth weight, age at first feeding, age at discharge feeding, and feeding-related length of stay between the three groups (Table 2). Probably the most interesting finding was the fact that, for our study population, there was no significant difference in age at discharge feeding between our LRPT subjects and FT subjects. Yet, the time from initial feeding to discharge feeding was significantly greater for LRPT subjects as compared to FT subjects, reinforcing the notion that even in healthy preterm neonates, gestational age may not be the best predictor of readiness to feed or expectations relative to feeding. Moreover, these results reinforce the suggestion that moderate and late preterm neonates may be at higher risk for subtle feeding problems than previously reported,25,26 and so warrant more careful observation by feeding specialists and health care teams.
COV measures for HRPT, LRPT, and FT groups for all respective exam times are shown in Fig. 3. These results suggest that LRPTs have comparable variability in suck amplitudes, frequencies, durations, and smoothness values from the time they begin oral feeding to the time they are discharged from the hospital. Moreover, only smoothness variability at the time they begin oral feeding was statistically distinguishable between our population of LRPT and FT neonates. Findings suggest that the nature of sucking behavior for these two groups is, at least initially, very similar. These findings are in contrast to those of our HRPT subjects. In comparison with full-term babies, HRPT babies actually start out with comparable variability in suck frequencies, durations, and smoothness values from the time they begin oral feeding to the time they are discharged from the hospital. However, at follow-up exams as the HRPT neonates matured, the value for these metrics, with the exception of suck amplitude and duration variability, were statistically different compared with FT at hospital discharge. When comparing our variability measures between HRPT and LRPT neonates, results showed that only the variability of suck duration at discharge feeding was statistically different between these two groups; the LRPT groups had lower variability relative to suck duration as compared with the HRPT group.
Figure 3.
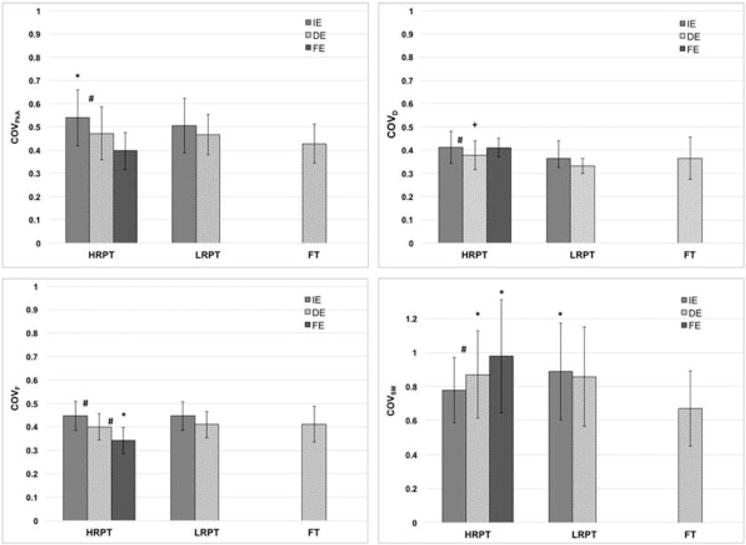
Comparison of coefficient of variation for peak amplitude (COVPkA), duration (COVD), frequency (COVF), and smoothness (COVSM) at initial exam (IE) and discharge exam (DE) for the high-risk preterm (HRPT) versus full-term (FT) group. *Significant differences in mean values for each exam in comparison to FT; +significant differences in mean values between HRPT and low-risk preterm (LRPT) groups; #changes in exams values within groups.
To examine changes in variability over time, we conducted within-group comparisons for the HRPT neonates in the study. Variability in amplitude, duration, and frequency decreased significantly from initiation of feeding, to feeding at hospital discharge, and then again, for suck frequency, to postdischarge final feeding. In contrast, variability of smoothness actually increased significantly from the time of the initial exam to the time of the discharge exam. Variability of smoothness of the suck did not change substantially from values obtained at hospital discharge and those collected at the final follow-up exam. What follows is an interpretation of these results in the context of what is known about movement variability in general and the complexity of nutritive sucking.
DISCUSSION
Variability is an intrinsic property of human movement, and it has been suggested that there may be a degree of “healthy” movement variability associated with the optimal state for producing a given movement.21 In an attempt to capture that optimal state, we collected metrics of movement variability longitudinally for a group of preterm neonates who were determined a priori to be at risk for poor developmental outcomes. The duration, amplitude, and frequency of a suck are fundamental characteristics that newborns must learn to control as they master the complex skill of nutritive sucking. Variations in these suck parameters, as described by COV metrics, may be an indication of a neonate’s skill at any point in time. Therefore, the aims of the current study were to (1) compare metrics of sucking performance between preterm neonates and healthy term neonates at initiation of feeding and at discharge and (2) investigate changes in sucking performance longitudinally among preterm neonates at high risk for adverse outcomes from initiation of oral feeding through postdischarge.
Our results indicated that variability in suck duration (COVD) was a distinguishing feature when comparing inpatient suck performance; values were similar for LRPT and FT subjects as compared with HRPT subjects, suggesting that LRPT and FT subjects had greater control of this suck feature at discharge. At the same time, the mean COVD for our high-risk babies at the final exam indicated advancement to a more stable state, comparable to that of FT neonates, even while all other COV features remained significantly different between the two groups.
Variation in suck duration cannot, however, be contextualized independently from other suck features or from maturation. The noted decrease in COV of suck peak amplitude and trend toward decreasing COV for frequency for the HRPT group and the simultaneous stabilization of COVD (i.e., comparable to FT) at follow-up exams could reflect greater adaptability of the neonate to control sucking characteristics from suck duration to peak amplitude and frequency with experience and maturation. From a dynamical systems perspective, these measures could be a reflection of the newborn’s ability to decrease the number of degrees of freedom of one suck characteristic in favor of others in response to a shift in attractor states, resulting from maturity level or underlying pathology. It is hard to know the causative nature of the differences observed across these measures considering that nutritive sucking comes under volitional control during the period covered by the follow-up exam. It should be mentioned that a major limitation in this comparison is that we do not know how these metrics change for FT in response to experience and maturation, because we did not follow them after discharge. However, these results add support to the idea that a decrease in variability over time is consistent with skill acquisition (motor learning) and how human movement in general evolves over time is important and should be explored longitudinally for all of these populations.21
A surprising finding of this study was the amount and direction of variation in smoothness between HRPT and FT. Mastery of a skill is typically associated with improved smoothness (decrease in value/oscillations) and a concomitant decrease in movement variability. Our results show COVSM increasing for the HRPT group over time. There are two main factors that could influence the suck characteristic of smoothness and thus COVSM for a population: (1) perturbations within a suck indicative of neuromuscular instability or (2) increased oscillations due to the move toward a more complex, coordinated sucking pattern. We think that the latter contributed to the increased amount of COVSM observed for the HRPT group. Theoretically, we would expect variability in smoothness to decrease in response to maturation and skill acquisition. Consistent with Lau,7 we observed several types of sucking patterns seemingly influenced by maturation. For example, our HRPT neonates started with a “simple” suck pattern during the initial stages of data collection (inpatient) characterized by small peak amplitudes of short duration. We would have expected that by the follow-up exam, HRPT neonates would have progressed to a more complex, coordinated sucking pattern with defined oscillations characterized by larger amplitudes, even shorter durations, and greater frequencies—all of which suggest greater control and coordination of these suck characteristics.
The natural tendency for smoothness values to become larger with an increased amount of oscillations within the suck, coupled with the possibility of the newborn’s control pattern switching between simple suck patterns and more coordinated patterns in response to experience and learning, could have led to the increased COVSM values noted for HRPT. To explore this idea, we conducted a post hoc analysis of mean smoothness values, and results supported this notion. Smoothness values for HRPT neonates decreased from initial exam to discharge exam and values at discharge exam remained consistent through to the final exam. However, the final exam values remained substantially less as compared with term babies. The fact that mean smoothness values at final exam did not increase to the level of term newborns at hospital discharge could be an indication that this group continues to shift between simple and complex sucking patterns even after discharge and that HRPT neonates are challenged in their ability to develop rhythmic, successive sucks across an entire feeding.27 With more data, we may find that relative smoothness may be indicative of neurologic dysfunction and a response variable similar to that observed by Nieuwenhuis and colleagues, who equated “fidgety” movements to dysfunctional or disorganized sucking patterns using the NOMAS.28
Finally, the procedures described for collecting these data take into account the suggestions of researchers who have previously reported on neonatal sucking. For example, as suggested by Medoff-Cooper et al,13 we have collected data beyond the more common single 5-minute sucking test to capture the natural variability in sucking performance as a function of arousal, temperament, and so on. We collected data consecutively for our population of preterm neonates, completing feedings on average once a week from initiation of feeding to discharge for an average of three feedings. As a result, we were able to distinguish the HRPT and the LRPT groups, validating the potential use of the variability as an early marker for newborns at risk for adverse neurodevelopmental outcomes.
LIMITATIONS AND FUTURE DIRECTIONS
Early identification of newborns at risk for adverse neurodevelopmental outcomes is necessary if we are to positively impact the rates of disability and poor neurodevelopmental sequelae in this population. This is especially the case for neonates who survive preterm birth and whose long-term outcomes have remained a challenge despite the significant advances in medicine over the past decade. The noninvasive technology used here to capture and report sucking metrics enables greater insight into what researchers have postulated for quite some time: that sucking behavior is a manifestation of the overall integrity of the neuromuscular system in this fragile, developing population. However, given the complexity of feeding and the dynamical systems that may drive sucking skill development, there are important feeding parameters we need to capture and investigate beyond those reported here.
For the current study, we focused solely on capturing nipple movement and COV metrics as descriptors. Capturing additional sucking metrics (e.g., sucks per burst and intersuck interval), feeding parameters (e.g., negative intraoral pressure), and measures influenced by feeding would allow us to use more advanced nonlinear statistical analyses to better estimate the coordinative structure of sucking dynamics. Such an approach might yield additional distinguishing suck characteristics among high-risk population cohorts. Moreover, factors that influence the newborn’s feeding environment (e.g., nipple type/flow rate, position during feeding, time of day) were captured but not analyzed in this study. Such information, combined with traditional comorbidity indicators (gestational age, birth weight) and measures of physiologic stability during feeding (i.e., oxygen saturation, respiration rate) will allow us to gain full insight into the overall neonatal feeding construct and the ability of the neonate to adapt to conditions that could vary across feedings.
Simultaneous capture of all of these variables may seem difficult, but in reality, it is not. The technology and algorithms described here easily capture all of the feeding parameters and metrics mentioned. The single limiting factor is simultaneous noninvasive integration of additional physiologic signals into everyday NICU workflow. We believe that technology and the enhancement of predictive algorithms coupled with near real-time statistical modeling techniques are no longer a limiting factor to fully understanding the end effect of the complex dynamical system of feeding. Our methods and results are a critically important addition to the emerging body of evidence that neonatal sucking is a measurable behavior that has the potential to alert us to the risk of poor neurodevelopmental outcomes.
The next important step, which has not yet been reported, is to assess the complex brain network that controls feeding and sucking in neonates via advanced neuroimaging technologies and to correlate deficits in the feeding brain network with early abnormalities identified in patterns of sucking performance during bottle-feeding. Such an approach is a necessary way to provide clear evidence of the association between early abnormalities in feeding performance and underlying brain injury. Combining anatomical or dysfunctional neurologic features with sucking parameters and other physiologic signals will allow us to definitively categorize HRPT newborns and develop predictive algorithms to help guide clinicians in the assessment and treatment of neurologic disorders.
Learning Outcomes.
As a result of this activity, the reader will be able to (1) summarize the literature suggesting early sucking as a predictor of neurodevelopmental outcomes in preterm neonates; (2) discuss the limitations of previous findings and how they are overcome in the current study; and (3) explain neonatal sucking in the context of dynamical systems theory.
Acknowledgments
Collection of data was made possible with a grant from the University of Kentucky NIH Center for Clinical and Translational Science (NIH CTSA UL1TR000117) and the University of Kentucky College of Health Sciences Office of Research (Grant 1012003440). Grantors had no role in study design, collection, analyses and/or interpretation of data, writing of the manuscript, or the decision to submit the manuscript. The authors thank Dr. Lu Dong and Mr. Jeff Rowberg for their technical expertise in development of instrumentation and signal analyses. The authors are also grateful to nurse researchers Deb Grider, Vicki Whitehead, Holly Nieves, and Kimberly Walker for their assistance with recruitment and inpatient data collection and graduate student Christina Eimers for her help with data input. Finally, we thank the participants and their families for taking part in this research.
Footnotes
DISCLOSURES
Gilson J. Capilouto receives a salary from the University of Kentucky where the work was performed. She has a financial interest in NFANT Labs, LLC. Dr. Capilouto is serving as the Guest Editor for the 20th Anniversary Issue on “Pediatric Feeding and Swallowing” for Thieme’s Seminars in Speech and Language.
Tommy J. Cunningham receives a salary from NFANT Labs, LLC, serves as a Board Member, and has a financial interest in the company. He is an adjunct assistant professor at the University of Kentucky where the work was performed.
David R. Mullineaux receives a salary from the University of Lincoln, England.
Eleonora Tamilia receives a salary as a Postdoctoral Research Fellow of Boston Children’s Hospital, the primary pediatric teaching hospital of Harvard Medical School.
Christos Papadelis receives a salary as Assistant Professor of Pediatrics from the Division of Newborn Medicine at Harvard Medical School, Boston Children’s Hospital.
Peter J. Giannone receives a salary from the University of Kentucky where the work was performed.
References
- 1.Mizuno K, Ueda A. Neonatal feeding performance as a predictor of neurodevelopmental outcome at 18 months. Dev Med Child Neurol. 2005;47(5):299–304. doi: 10.1017/s0012162205000587. [DOI] [PubMed] [Google Scholar]
- 2.Holloway EM. The dynamic process of assessing infant feeding readiness. Newborn Infant Nurs Rev. 2014;14(3):119–123. [Google Scholar]
- 3.Browne JV, Ross ES. Eating as a neurodevelopmental process for high-risk newborns. Clin Perinatol. 2011;38(4):731–743. doi: 10.1016/j.clp.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Bertoncelli N, Cuomo G, Cattani S, et al. Oral feeding competences of healthy preterm infants: a review. Int J Pediatr. 2012;2012:896257. doi: 10.1155/2012/896257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lau C. Interventions to improve oral feeding performance of preterm infants. Perspectives on Swallowing and Swallowing Disorders. 2014;23(2):23–45. [Google Scholar]
- 6.Tamilia E, Taffoni F, Formica D, et al. Technological solutions and main indices for the assessment of newborns’ nutritive sucking: a review. Sensors (Basel) 2014;14(1):634–658. doi: 10.3390/s140100634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lau C. Development of suck and swallow mechanisms in infants. Ann Nutr Metab. 2015;66(Suppl 5):7–14. doi: 10.1159/000381361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silberstein D, Geva R, Feldman R, et al. The transition to oral feeding in low-risk premature infants: relation to infant neurobehavioral functioning and mother-infant feeding interaction. Early Hum Dev. 2009;85(3):157–162. doi: 10.1016/j.earlhumdev.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Biarge M, Diez-Sebastian J, Rutherford MA, Cowan FM. Outcomes after central grey matter injury in term perinatal hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86(11):675–682. doi: 10.1016/j.earlhumdev.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Fiori S, Guzzetta A. Plasticity following early-life brain injury: insights from quantitative MRI. Semin Perinatol. 2015;39(2):141–146. doi: 10.1053/j.semperi.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Gewolb IH, Vice FL. Maturational changes in the rhythms, patterning, and coordination of respiration and swallow during feeding in preterm and term infants. Dev Med Child Neurol. 2006;48(7):589–594. doi: 10.1017/S001216220600123X. [DOI] [PubMed] [Google Scholar]
- 12.Gewolb IH, Vice FL, Schwietzer-Kenney EL, Taciak VL, Bosma JF. Developmental patterns of rhythmic suck and swallow in preterm infants. Dev Med Child Neurol. 2001;43(1):22–27. doi: 10.1017/s0012162201000044. [DOI] [PubMed] [Google Scholar]
- 13.Medoff-Cooper B, Shults J, Kaplan J. Sucking behavior of preterm neonates as a predictor of developmental outcomes. J Dev Behav Pediatr. 2009;30(1):16–22. doi: 10.1097/DBP.0b013e318196b0a8. [DOI] [PubMed] [Google Scholar]
- 14.Tsai SW, Chen CH, Lin MC. Prediction for developmental delay on Neonatal Oral Motor Assessment Scale in preterm infants without brain lesion. Pediatr Int. 2010;52(1):65–68. doi: 10.1111/j.1442-200X.2009.02882.x. [DOI] [PubMed] [Google Scholar]
- 15.Wolthuis-Stigter MI, Luinge MR, da Costa SP, Krijnen WP, van der Schans CP, Bos AF. The association between sucking behavior in preterm infants and neurodevelopmental outcomes at 2 years of age. J Pediatr. 2015;166(1):26–30. doi: 10.1016/j.jpeds.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Hiramoto A, Takagai S, Tsuchiya K, et al. Abnormal sucking behavior in infants as a predictor of developmental delay in 18-month or 3-year of age. Journal of Brain Science. 2014;43:5–23. [Google Scholar]
- 17.da Costa SP, van der Schans CP. The reliability of the Neonatal Oral-Motor Assessment Scale. Acta Paediatr. 2008;97(1):21–26. doi: 10.1111/j.1651-2227.2007.00577.x. [DOI] [PubMed] [Google Scholar]
- 18.Shaker C. Cue-based co-regulated feeding in the neonatal intensive care unit: Supporting parents in learning to feed their preterm infant. Newborn Infant Nurs Rev. 2013;13:51–55. [Google Scholar]
- 19.Tamilia E, Delafield J, Fiore S, Taffoni F. An automatized system for the assessment of nutritive sucking behavior in infants: a preliminary analysis on term neonates. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:5752–5755. doi: 10.1109/EMBC.2014.6944934. [DOI] [PubMed] [Google Scholar]
- 20.Tamilia E, Formica D, Scaini A, Taffoni F. An automated system for the analysis of newborns’ oral-motor behavior. IEEE Trans Neural Syst Rehabil Eng. 2015 doi: 10.1109/TNSRE.2015.2496150. [DOI] [PubMed] [Google Scholar]
- 21.Stergiou N, Decker LM. Human movement variability, nonlinear dynamics, and pathology: is there a connection? Hum Mov Sci. 2011;30(5):869–888. doi: 10.1016/j.humov.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rohrer B, Fasoli S, Krebs HI, et al. Movement smoothness changes during stroke recovery. J Neurosci. 2002;22(18):8297–8304. doi: 10.1523/JNEUROSCI.22-18-08297.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheel CE, Schanler RJ, Lau C. Does the choice of bottle nipple affect the oral feeding performance of very-low-birthweight (VLBW) infants? Acta Paediatr. 2005;94(9):1266–1272. doi: 10.1080/08035250510027255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaker CS. Cue-based feeding in the NICU: using the infant’s communication as a guide. Neonatal Netw. 2013;32(6):404–408. doi: 10.1891/0730-0832.32.6.404. [DOI] [PubMed] [Google Scholar]
- 25.Natarajan G, Shankaran S. Short- and long-term outcomes of moderate and late preterm infants. Am J Perinatol. 2016;33(3):305–317. doi: 10.1055/s-0035-1571150. [DOI] [PubMed] [Google Scholar]
- 26.Horgan MJ. Management of the late preterm infant: not quite ready for prime time. Pediatr Clin North Am. 2015;62(2):439–451. doi: 10.1016/j.pcl.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Pandit AS, Robinson E, Aljabar P, et al. Whole-brain mapping of structural connectivity in infants reveals altered connection strength associated with growth and preterm birth. Cereb Cortex. 2014;24(9):2324–2333. doi: 10.1093/cercor/bht086. [DOI] [PubMed] [Google Scholar]
- 28.Nieuwenhuis T, da Costa SP, Bilderbeek E, Geven WB, van der Schans CP, Bos AF. Uncoordinated sucking patterns in preterm infants are associated with abnormal general movements. J Pediatr. 2012;161(5):792–798. doi: 10.1016/j.jpeds.2012.04.032. [DOI] [PubMed] [Google Scholar]


