Summary
Environmental enrichment (EE) replicates mind-body therapy by providing complex housing to laboratory animals to improve their activity levels, behavior and social interactions. Using a Tcf4Het/+ ApcMin/+-mediated model of colon tumorigenesis, we found that EE vastly improved the survival of tumor-bearing animals, with differential effect on tumor load in male compared to female animals. Analysis of Tcf4Het/+ ApcMin/+ males showed drastically reduced expression of circulating inflammatory cytokines and induced nuclear hormone receptor signaling, both of which are common in the wound repair process. Interestingly, EE provoked tumor wound repair resolution through revascularization, plasma cell recruitment and IgA secretion, replacement of glandular tumor structures with pericytes in a process reminiscent of scarring, and normalization of microbiota. These EE-dependent changes likely underlie the profound improvement in survival of colon-tumor-bearing Tcf4Het/+ ApcMin/+ males. Our studies highlight the exciting promise of EE in the design of future therapeutic strategies for colon cancer patients.
Graphical abstract

Introduction
Colorectal cancer (CRC) is the third leading cause of cancer death in men and the second in women around the world (Ferlay et al., 2015). There are several risk factors associated with CRC including age, deficiencies in adenomatous polyposis coli (APC) and mismatch repair (MMR) proteins, inflammation, smoking, poor diet, sedentary lifestyle, alcohol consumption, and imbalance of intestinal microbiota (Aran et al., 2016). Tumor and stromal cells, pericytes, inflammatory cells, immune cells, and tumor vasculature make up the heterogeneous tumor microenvironment, and tumor cell survival depends on the cells within the stromal microenvironment (Bhowmick and Moses, 2005). However, it is unclear how these factors contribute to CRC development, whether there is cross talk between risk factors, and which cell types are critical for tumorigenesis.
The tumor microenvironment shares many features with defective wound repair. The wound repair process is accomplished through several different phases characterized by vascular permeability, inflammation, formation of a tissue stroma, angiogenesis, tissue remodeling, revascularization, improved oxygen tension, and ultimately the formation of scar tissue (Arnold et al., 2015). Disruption of any phase results in chronic wounds that cannot heal. For instance, inflammation must be resolved before tissue remodeling and scar formation can proceed. The tumor microenvironment is similar to chronic wounding in that hypoxia and reactive oxygen species drive continuous angiogenesis and inflammation that is unable to be resolved, ultimately giving rise to barrier defects and associated tumor expansion (Arnold et al., 2015). In normal tissue, various nuclear hormone receptors (NHR) play an important role in tissue homeostasis and wound repair (Rieger et al., 2015), and are often functionally altered in colorectal cancer (D’Errico and Moschetta, 2008).
A dysfunctional barrier can further reinforce the pro-inflammatory response because normal defenses against pathogenic intestinal microbiota are compromised (Schwabe and Jobin, 2013). Plasma cells produce Immunoglobulin A (IgA), which helps maintain mutualism by fostering commensals while simultaneously neutralizing pathogenic bacteria (Palm et al., 2014). The ability to efficiently discriminate between beneficial commensals and harmful bacteria thereby reduces the generation of pro-inflammatory signals (Macpherson et al., 2015). During barrier dysfunction and infection this mutualism is lost, and the immune response results in dysbiosis, infection, inflammation, and cancer (Hand et al., 2012).
Lifestyle interventions, such as alterations in diet and physical activity, show promise for colon cancer prevention and treatment (Chan and Giovannucci, 2010; Ciombor et al., 2015; Schmid and Leitzmann, 2014). Mind-body medicine, in particular, focuses on reducing the physiological manifestations of stress and anxiety by improving social and cognitive stimulation as well as physical activities and is associated with profound improvements in overall health and disease (Gordon, 2008). Mind-body medicine is replicated in animal models using EE housing conditions and has been shown to elicit anti-tumor effects in several mouse models of cancer, including cancers of the intestine (Cao et al., 2010). Here, we further investigate the mechanisms that account for EE-mediated anti-tumor effects in a mouse genetic model of colon cancer.
We previously developed a unique mouse model system in which heterozygous loss of the Tcf4 gene (TCF7L2) leads to unregulated proliferation of colonic epithelial cells and significantly enhances colon tumorigenesis when combined with an initiating mutation in the Apc gene (Angus-Hill et al., 2011). Here, we show that EE profoundly improves the survival of Tcf4Het/+ ApcMin/+ colon-tumor-bearing animals. While survival is improved in both male and female animals, we find that they respond differently to EE, since only females show significantly decreased tumor growth. We set out to explain the striking result by analyzing systemic changes, and changes within the tumor microenvironment and within the resident microbiome in male Tcf4Het/+ ApcMin/+ animals. Our findings suggest that in the tumors of Tcf4Het/+ ApcMin/+ males exposed to EE, nuclear hormone receptor activation promotes resolution of the wound repair process, which ultimately restores mucosal barrier function and microbial biodiversity. This EE-dependent restoration of barrier integrity likely contributes to the drastic improvement in the lifespan of tumor-bearing animals.
Results
EE improves survival of Tcf4Het/+ ApcMin/+ mice and reduces systemic inflammatory cytokines in male animals
To determine the effects of long-term EE on tumorigenesis and survival of Tcf4Het/+ ApcMin/+ colon cancer mice, separately housed male and female wild-type (WT), Tcf4Het/+ Apc+/+, Tcf4+/+ ApcMin/+, and Tcf4Het/+ ApcMin/+ animals were weaned into either an EE or non-enriched (NE) control environment (Fig. S1A). Animal health was assessed daily, and animals were collected when they began to exhibit symptoms of cachexia, including decreased mobility, weight loss, and anemia. These phenotypes were used to define the end of lifespan for these animals (Supplemental Experimental Procedures, EE1). We found that EE drastically improved survival of male and female colon-tumor-bearing Tcf4Het/+ ApcMin/+ mice compared to NE cohorts (Fig. 1A). Following enrichment, median survival days increased in Tcf4Het/+ ApcMin/+ males from 173 to 228 days (55-day increase) and from 156 to 238 days (82-day increase) in females (Fig. 1A, Fig. S1C). Importantly, we observed no significant increase in survival of EE compared to NE ApcMin/+ animals (Fig. S1B–C), suggesting that this phenotype is Tcf4Het/+-dependent and perhaps colon-tumor specific.
Fig. 1.
EE increases the lifespan of male and female Tcf4Het/+ ApcMin/+ mice without effect on tumor number or weight. Animals were collected when cachexia symptoms became apparent (see text for details). (A–C) Lifespan EE. (A) Survival curves for male and female Tcf4Het/+ ApcMin/+ mice. N= number of animals per sex/genotype. P values by chi-square statistical analysis were calculated using the log-rank Mantel-Cox tests for greater than 3 groups. (B) Tumor count and (C) tumor weight in NE and EE Tcf4+/+ ApcMin/+ and Tcf4Het/+ ApcMin/+ animals. (D–G) Short Term EE, 4 months at collection. (D–E) Colon tumor count and volume. (F) Animal weight comparisons in NE and EE animals. (G) Systemic changes in inflammatory cytokine levels of male animals, as indicated. Lower limit of detection: IL-6 4.5 pg/μl and TNFα 0.85 pg/μl. * p=<0.05, ** p=0.005 using two-sample t-test with Welch correction.
We next questioned whether EE altered tumor load (tumor number and weight) in NE and EE Tcf4Het/+ ApcMin/+ and ApcMin/+ animals. We found that tumor load was not significantly reduced in response to EE (Figs. 1B–C, S1D). Small intestinal tumors from these animals also failed to respond to EE, (Fig. S1E). This suggested that EE improves the survival of Tcf4Het/+ ApcMin/+ animals by a mechanism that does not decrease tumor number or size.
One possible explanation for the lack of tumor load reduction following EE is that tumors develop more slowly with EE, but ultimately animals reach a tumor-load threshold and succumb to the disease. To test this directly, we conducted a shorter-term experiment, where at 4 months of age, NE and EE Tcf4Het/+ ApcMin/+ and ApcMin/+ animals were collected and tumor load was determined for each animal (EE2, Supplemental Experimental Procedures, Fig. S1F, Fig. 1D–E, Table S1). Interestingly, we found that under NE conditions, there were sex-specific differences in tumor initiation, but not necessarily tumor growth, in Tcf4Het/+ ApcMin/+ animals (8.5 tumors in males compared to 4 tumors in females; p=0.03, charted in Fig. S1G, Table S1C). However, when comparing NE and EE tumors within female Tcf4Het/+ ApcMin/+ animals, we found that while the two groups develop similar numbers of colon tumors, colon tumor volume is significantly lower in response to EE (42% reduction in size; p=0.016; Fig. 1D–E, Table S1B), demonstrating that EE specifically limits tumor growth but not initiation in female Tcf4Het/+ ApcMin/+ mice. On the other hand, males show tumor count and volume (reduced by 27%; p=0.299 and 32%; p=0.413, respectively) that are widely variable and not significantly different between NE and EE (Fig. 1D–E, Table S1A). This indicates that a reduction in tumor load may underlie the EE-mediated improvement in survival of female, but not necessarily in male, Tcf4Het/+ ApcMin/+ animals, and suggests that the lifespan extension in males and females in response to EE may arise from different mechanisms.
To assess the general health of the animals in the short-term study, animal weights were compared. Overall female health was stable, with relatively no weight change in NE or EE animals when compared to WT (Fig. 1F, Table S1B). Interestingly, the NE male Tcf4Het/+ ApcMin/+ animal weights were reduced by over 25% when compared to NE WT animals, but this reduction was mitigated by EE (Fig. 1F, Table S1A). However, EE was not sufficient to alleviate anemia and both the absolute number and composition of leukocytes in peripheral blood remained unchanged (Table S1A–B). These findings indicate that EE improves the health of male tumor bearing Tcf4Het/+ ApcMin/+ EE animals without hematological effects.
Severe inflammation is often associated with barrier dysfunction and colon cancer progression (Kraus and Arber, 2009). Both male and female Tcf4Het/+ ApcMin/+ animals have elevated serum levels of the inflammatory cytokines IL-6 and TNFα (Table S1). Interestingly, we found that EE reduced serum IL-6 protein levels in male Tcf4Het/+ ApcMin/+ animals by 45% and TNFα levels by 70% (Fig. 1G, Table S1A–B). This is an important finding since injection of IL-6 is sufficient to cause cancer cachexia in ApcMin/+ mice (Baltgalvis et al., 2008). However, EE had no significant effect on IL-6 and TNFα levels in female Tcf4Het/+ ApcMin/+ animals (Table S1B). These results suggest that EE improves the health and survival of male Tcf4Het/+ ApcMin/+ animals, at least in part, through reduction of IL-6 and TNFα-dependent cachexia symptoms. However, in female Tcf4Het/+ ApcMin/+ animals, the effects of EE on improved health and survival are likely due to reduced tumorigenesis. Though it is unclear whether female specific factors play a role, since estrogen has a protective role in tumorigenesis (Guo et al., 2004). We therefore focus on addressing the mechanisms that underlie the EE-dependent alterations, including systemic inflammatory cytokine reduction, in the colons of male animals.
EE stimulates the re-epithelialization of colon tumors from Tcf4Het/+ ApcMin/+ males
Adenomas can cause a loss of barrier function, promoting microbial infiltration and inflammatory cytokine secretion, including IL-6 (Grivennikov et al., 2012). The EE-induced systemic reduction of IL-6 and TNFα levels therefore suggests that Tcf4Het/+ ApcMin/+ male animals may have restored barrier function. To define possible changes within the tumor microenvironment that could promote improved barrier function, we conducted RNA-Seq analysis of distal colon samples isolated from male EE and NE Tcf4Het/+ ApcMin/+ animals. We found many significant gene expression changes, including 205 up- and 105 downregulated genes in EE compared to NE animals (Table S2A–B). Notably, when EE animals are compared to NE, several genes that are normally upregulated in adenomas are downregulated by EE, and those that are downregulated in adenomas are upregulated by EE, demonstrating that gene expression profiles in tumors are altered towards a normalized expression pattern in response to EE (Table S2C–D).
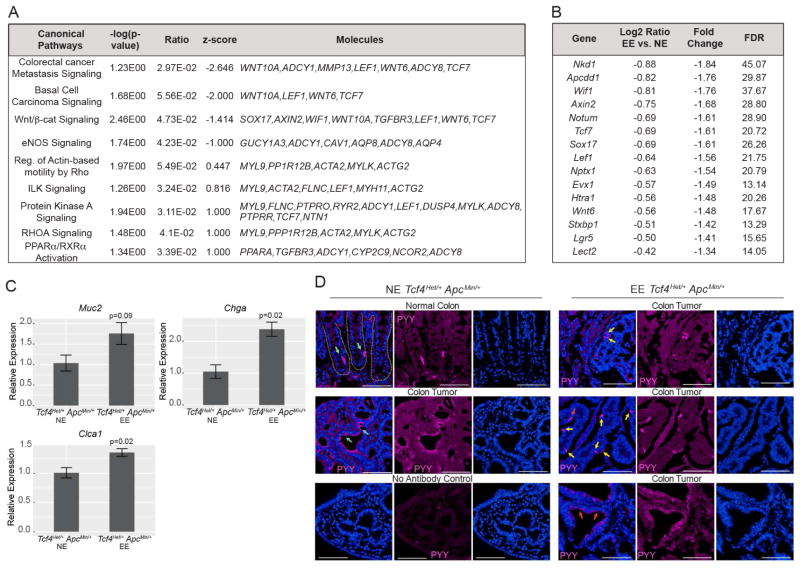
Barrier defects may arise through aberrant activation of the Wnt/β-catenin signaling pathway, which induces proliferation and prevents differentiation of epithelial cell lineages (Grivennikov et al., 2012). Many members of this pathway are more highly expressed in the colons of Tcf4Het/+ ApcMin/+ animals compared to ApcMin/+ animals (Angus-Hill et al., 2011). The canonical pathway of Wnt/β-catenin signaling was identified with a negative Z score in Tcf4Het/+ ApcMin/+ animals in response to EE, suggesting reduced activation of this pathway (Fig. 2A, Table S3). Indeed, several critical activating factors in this pathway were significantly reduced following EE of these animals (including Wif1, Axin2, Tcf7, and Lef1, and Nkd1; Fig. 2B) by both Ingenuity Pathway Analysis (IPA) and functional analysis (Fig. 2A and Tables S2–S3). Importantly, this data demonstrates that EE reduces the expression of many genes involved in Wnt/β-catenin-mediated tumor cell proliferation.
Fig. 2.
EE reduces expression of tumor growth genes and improves tumor cell differentiation in Tcf4Het/+ ApcMin/+ colons. (A) IPA canonical pathway analysis of RNA-Seq data. (B) RNA-Seq analysis revealed significantly decreased Wnt-signaling components and downstream targets. (C) QPCR analysis of genes involved in differentiation. (D) Differentiation of enteroendocrine L cells (PYY) in EE and NE animals. Green and yellow arrows mark enteroendocrine cells in normal and tumor tissue, respectively. Cyan and red arrows mark PYY bound to NPY2R receptor in NE and EE, respectively, with internalization of PYY following EE. Dashed lines delineate crypts in normal colon tissue. Scale bar: 100 μM.
Next, we set out to define whether decreased expression of Wnt/β-catenin signaling pathway following EE promotes the differentiation of tumor cells, thereby improving epithelial barrier function. Indeed, we found by RNA-Seq and QPCR that several markers for secretory and absorptive differentiated epithelial cell lineages were upregulated in response to EE in colons isolated from male Tcf4Het/+ ApcMin/+ animals, including Sucrase Isomaltase (Sis, enterocytes), Chromogranin A (Chga, enteroendocrine), Chloride Channel Accessory 1 (Clca1, goblet) and Mucin 2 (Muc2, goblet) (Fig. 2C, Table S2A). We also examined colon tissue isolated from NE and EE Tcf4Het/+ ApcMin/+ animals for specific signs of differentiation. Peptide YY (PYY) can be used to directly visualize differentiated colonic enteroendocrine L cells, which have a distinctive size and shape (Fig. 2D, “normal tissue”, green arrows). Importantly, these cells are not visible in tumors from NE Tcf4Het/+ ApcMin/+ animals, and instead PYY binds to the NPY2R receptor on the cell surface of tumor cells (Fig. 2D, cyan arrows). In response to EE, tumor cells internalize PYY (Fig. 2D, red arrows), or L cells become visible throughout the tumor (Fig. 2D, yellow arrows). Thus, tumors appear to have reduced proliferation and improved differentiation in male EE Tcf4Het/+ ApcMin/+ animals. These findings demonstrate that EE promotes tumor re-epithelialization, which may define the first step towards resolution of the tumor wound repair process.
Elevated levels of inflammatory cytokines IL-6 and TNFα help to initiate re-epithelialization, but levels decrease following re-epithelialization (Kondo and Ohshima, 1996). Therefore, this re-epithelialization in Tcf4Het/+ ApcMin/+ animals may underlie the reduction in systemic IL-6 and TNFα levels, and may be representative of an anti-inflammatory response necessary for the late wound repair response. Indeed, analysis of RNA-Seq data for upstream regulators revealed that IL10RA, dexamethasone, and glucocorticoid (GC) anti-inflammatory pathways are highly active in response to EE in Tcf4Het/+ ApcMin/+ animals, while the pro-inflammatory upstream regulators TNFα and STAT3 are inhibited (Table S3A).
EE promotes activation of nuclear hormone receptor signaling pathways often involved in wound repair
GCs are involved in wound repair and exert an anti-inflammatory effect in the late wound repair process by binding to the glucocorticoid receptor (GR) that in turn elicits an anti-inflammatory transcriptional response. GR is a member of a large family of nuclear hormone receptors (NHR), and cross-talk between family members is commonly observed in an array of diverse processes, including tissue development and wound repair (Contreras-Jurado et al., 2014; Rieger et al., 2015). To further define the contribution of the GR and other NHRs, we utilized IPA causal analysis (Kramer et al., 2014) of RNA-Seq data from NE and EE Tcf4Het/+ ApcMin/+ colons. As for the glucocorticoid pathway, causal analysis revealed the activation of both the glucocorticoid receptor (NR3C1) and mineral corticoid receptor (NR3C2; MC; Figure 3A; Table S3B) signaling pathways. In addition to activation of these NHRs, common inhibitors of the GC pathway including breakpoint cluster region (BCR), 11-beta-dehydrogenase isozyme 2 (HSD11β2), and gamma secretase complex are inhibited (Figure 3A; Table S3B), an activity that would further increase GC signaling. Interestingly, a subset of NHRs also involved in wound repair (Rieger et al., 2015) are activated by EE in the colons of Tcf4Het/+ ApcMin/+ mice (Figure 3A; Tables S3B and S3D) including; peroxisome proliferator-activated receptor (PPARα; NR1C1; Figure 2A; (Michalik and Wahli, 2006)), thyroid hormone receptor (THRA; NR1A1; (Bloise et al., 2014; Contreras-Jurado et al., 2014)), oxysterol receptors (Liver X receptor; LXR; NR1H2, NR1H3, NR1H4; (Beyer et al., 2015)) retinoic acid receptors (RARA and RXRA; (Hunt et al., 1969; Rieger et al., 2015)) as well as the nuclear receptor coactivator (NCOA1) involved in co-activation of GR, RXR, THRA and PPAR nuclear hormone receptors (Walsh et al., 2012). This finding suggests that NHRs commonly involved in the wound repair process may play a role in improving the lifespan of EE Tcf4Het/+ ApcMin/+ male animals.
Fig. 3.
(A) IPA Causal Network analysis depicting significant changes in NHRs and NHR signaling pathways. See text for references regarding NHR in wound repair. (B–E) THRA, DIO1, ME1 or ME2 staining in NE or EE tumors. Yellow arrows mark some positive stromal cells, magenta arrows mark some positive tumor glandular cells. Scale bar: 100 μM.
The NHR THRA is essential for re-epithelialization in the wound repair process in mice (Contreras-Jurado et al., 2014), suggesting that activation of THRA signaling may contribute to the improvement in barrier function observed in male EE Tcf4Het/+ ApcMin/+ animals. To define whether EE animals have active thyroid hormone signaling within mucosal or stromal compartments of the tumor microenvironment, we stained tumors isolated from NE and EE animals for THRA, and downstream THRA transcriptional targets, and co-stained with the myofibroblast and pericyte marker smooth muscle actin (SMA) to delineate the stromal compartment. Immunohistochemical analysis of THRA revealed that staining was largely absent in NE tumors (Figure 3B), while THRA expression was found across mucosal and stromal compartments in EE tumors (Figure 3B). Transcription of iodothyronine deiodinase 1 (DIO1) is stimulated by T3 hormone and both THRA and THRB (Toyoda et al., 1995). By RNA-Seq, DIO1 is significantly upregulated in EE compared to NE Tcf4Het/+ ApcMin/+ animals (Table S2A). By immunohistochemistry, we found that DIO1 expression is absent in NE Tcf4Het/+ ApcMin/+ tumors, and localized to membranes of both mucosal and stromal cell types in EE Tcf4Het/+ ApcMin/+ tumors (Figure 3C). These results suggest that THRA is expressed in tumors isolated from EE Tcf4Het/+ ApcMin/+ animals, and that active T3 hormone is present and able to transcriptionally activate DIO1. Therefore, thyroid hormone signaling may restore the re-epithelialization process essential for wound repair.
A second direct THRA transcriptional target is malic enzyme (ME1) (Kaneshige et al., 2001). Malic enzymes (cytoplasmic ME1 and mitochondrial ME2) catalyze the oxidative decarboxylation of malate to pyruvate and are essential for maintaining cellular NADPH levels (Jiang et al., 2013). Both ME1 and ME2 increase migration and invasion in wound repair assays, without effect on metastatic potential or animal survival (Chang et al., 2015; Zheng et al., 2012). Given the role of ME1 and ME2 in wound repair, we tested their expression patterns by immunohistochemical staining of EE and NE Tcf4Het/+ ApcMin/+ tumors. ME1 is absent in tumors of NE Tcf4Het/+ ApcMin/+ animals, and is expressed primarily in stromal cells within the tumors of EE Tcf4Het/+ ApcMin/+ animals (Figure 3D). Different from ME1 expression, ME2+ cells are largely found within the stroma of NE Tcf4Het/+ ApcMin/+ tumors (Figure 3E, yellow arrows), while in EE Tcf4Het/+ ApcMin/+ tumors, ME2+ cells are primarily localized to the tumor mucosal structures (Figure 3E, magenta arrows). Taken together, cell-type specific ME1 and ME2 expression is altered following EE, and suggests a metabolic switch within the tumor mucosal cells and stromal cells of EE animals at least partially dependent on T3, and that this change in metabolism may be involved in cell migration, a requirement for wound repair.
EE recruits pericytes, reduces angiogenesis and normalizes tumor vasculature similar to later stages of revascularization in the wound repair process
Typically, angiogenesis within the tumor microenvironment results in tortuous and nonfunctional vessels, and ischemic and hypoxic tissue (Fukumura et al., 2010), effectively blocking completion of the wound repair process. In wound repair, revascularization must have both an angiogenic component to form new vessels that are then stabilized by pericytes, and also the inhibition of additional angiogenesis (King et al., 2014). IPA analysis of RNA-Seq expression data from EE versus NE Tcf4Het/+ ApcMin/+ males revealed decreased expression of genes involved in endothelial cell proliferation and movement, hypertension, vasculogenesis, and angiogenesis, and increased expression of genes involved in the regulation of blood pressure (Fig. 4A; Table S3C). Pericytes are multipotent cells that surround and stabilize blood vessels which is important for wound repair (Bodnar et al., 2016). Tortuous tumor vessels are highly angiogenic and lack pericytes. Interestingly, most of the top upregulated genes in the colons of EE Tcf4Het/+ ApcMin/+ males are specific markers of the pericyte lineage (Fig. 4B; Table S2A), including Sma, desmin (Des) and regulator of G-protein signaling 5 (Rgs5). Consistent with an EE-mediated increase in pericyte recruitment, functional analysis also revealed signs of improved vascular integrity through upregulation of several genes involved in smooth muscle contraction, circulatory system process, and blood circulation (Table S2C). We therefore hypothesized that improved vascular integrity through recruitment of pericytes reduces angiogenesis and increases normalized tumor vasculature. To test this hypothesis, we examined colon tumor tissue isolated from NE and EE Tcf4Het/+ ApcMin/+ animals and stained with the blood vessel marker platelet endothelial cell adhesion molecule (PECAM) and SMA (a pericyte marker of vascular maturity). We found fewer PECAM-positive cells within the tumor stroma (outlined by yellow dotted lines) following EE (Fig. 4C). We also examined the expression of a second pericyte marker, RGS5, which is expressed on activated pericytes (pericytes with mesenchymal potential) in tumors (Hamzah et al., 2008) and upregulated 2.5-fold in Tcf4Het/+ ApcMin/+ colons in response to EE (Fig. 4B). Interestingly, RGS5+ pericytes are commonly SMA− in both NE and EE colons of Tcf4Het/+ ApcMin/+ animals, while there are more RGS5+ SMA− pericytes in response to EE (Fig. 4D). The lack of RGS5/SMA co-staining is not unexpected as pericytes represent a heterogeneous population that does not uniformly express SMA (Wang et al., 2006). As such, the RGS5+/SMA− subset may represent a less mature pericyte that promotes a repair response, while the RGS5+/SMA+ subset may stabilize vessels in the wound repair process.
Fig. 4.
EE reduces angiogenesis, improves pericyte recruitment, and normalizes vessels in Tcf4Het/+ ApcMin/+ colons. (A) IPA diseases and functional analysis of RNA-Seq data in EE compared to NE animals. (B) QPCR analysis of pericyte markers in EE compared to NE mice. (C) PECAM and SMA immunofluorescence staining of tumor vessels. Dashed yellow lines outline stromal cells. (D) RGS5 and SMA immunofluorescence staining of tumor pericytes. (E) QPCR of Npy and Npy2r. (F) NPY2R immunofluorescence staining of tumor vessels. Dashed yellow lines outline stromal cells. Yellow arrows mark endothelial cells. Green arrows mark blood cells, including red blood cells. P-values calculated using two-sample t-test with Welch correction. Scale bar: 100 μM.
The NPY peptide and its receptor NPY2R are important drivers of angiogenesis under ischemic conditions (Zukowska et al., 2003). The NPY2R receptor is primarily expressed on endothelial cells in ischemic tissue or on pericytes when vessels are injured. By QPCR, we examined Npy and Npy2r expression in colon tumors from NE Tcf4Het/+ ApcMin/+ compared to NE Tcf4Het/+ Apc+/+ males. We found that under NE conditions, the presence of the ApcMin allele in a Tcf4Het/+ background upregulated colon expression of both Npy (25-fold) and Npy2r (5-fold) (Fig. 4E, top panel). Interestingly, EE reduced these increases in expression in EE Tcf4Het/+ ApcMin/+ compared to EE Tcf4Het/+ Apc+/+ males (Fig. 4E, middle panel) and for Npy in EE compared to NE Tcf4Het/+ ApcMin/+ animals. We next examined colons from EE versus NE Tcf4Het/+ ApcMin/+ animals and found a similar EE-mediated reduction in Npy and Npy2r expression (Fig. 4E, bottom panel). Endothelial cells from NE, but not EE, Tcf4Het/+ ApcMin/+ animals showed protein expression of NPY2R by immunohistochemistry (Fig. 4F; yellow arrows). Instead, EE Tcf4Het/+ ApcMin/+ males showed NPY2R+ blood cells (Fig. 4F; cyan arrows) and differentiated enterocytes (Fig. 4F, double yellow asterisks), at the tumor surface, indicating that blood cells are recruited to the microenvironment and further validating EE-dependent differentiation of colonic mucosal cells. These results suggest that EE reduces angiogenesis, normalizes vessels and improves circulation within the colon tumor microenvironment, and supports the role of EE in resolving the tumor-specific block of revascularization in the wound repair process.
EE increases IgA levels and promotes a pericyte-mediated wound repair-like response
Antibodies (IgA) secreted from plasma cells are actively engaged in wound repair in many tissue types (Nishio et al., 2009). By RNA-Seq, we found that 20% of upregulated genes in the distal colon of EE compared to NE Tcf4Het/+ ApcMin/+ animals are components of immunoglobulin (Ig; see Table S2), including; immunoglobulin (Ig) constant heavy chain (IGHA), and many light chains (kappa, lambda). J Chain is a component of the secreted IgA polymer and is significantly upregulated in EE Tcf4Het/+ ApcMin/+ animals (Table S2). This data suggests that EE stimulates IgA-secretion from colonic plasma cells in male Tcf4Het/+ ApcMin/+ animals. We next examined normal and tumor tissue isolated from EE and NE Tcf4Het/+ ApcMin/+ animals by immunohistochemistry for plasma cell localization (anti-CD138) and IgA secretion (anti-IgA). Surprisingly, we found that IgA staining intensity and localization differed between NE and EE sections of normal tissue (Fig. 5A). EE sections showed more intense IgA staining as well as localization to the luminal surface, in contrast to IgA+ cells from NE sections that were mainly concentrated at the crypt base. Similarly, in colon tumor sections, IgA+ staining was mainly localized to the tumor periphery in EE animals (Fig. 5Bb, b1′ b2′, red arrows), compared to a general lack of IgA staining in NE tumors, with the exception of nonspecific staining of necrotic debris in the glandular lumina found in both NE and EE animals (Fig. 5B a′, black arrows). To determine whether the IgA+ cells at the periphery of EE Tcf4Het/+ ApcMin/+ tumors represented plasma cells, we analyzed expression of CD138. Indeed, many IgA+ cells in EE-tumor periphery (Fig. 5B b2′) were plasma cells based on anti-CD138 staining (Fig. 5C b2′). Taken together, our data suggests that EE increases migratory plasma cells within normal and tumor tissue, and these cells secrete higher levels of IgA than plasma cells in NE animals. The presence of EE plasma cells in this environment likely provides continuing immunity to barrier challenges.
Fig. 5.
EE increases IgA levels, plasma cell recruitment, and pericyte activation and migration into glandular structures in Tcf4Het/+ ApcMin/+ males. Colon-specific IgA immunohistochemical staining in NE and EE Tcf4Het/+ ApcMin/+ male normal (A) and tumor (B) tissue. a′ and b′ denote higher magnification views of boxed regions. Red arrows mark IgA+ tumor regions while black arrows mark necrotic debris. (C) Immunohistochemical staining of tumor tissue for the glandular epithelial and plasma cell marker CD138. Black asterisks denote the presence of CD138+ glandular structures, while red asterisks denote the replacement of glandular structures with CD138− cells. (D) Blue arrows mark some SMA+ RGS5+ IgA-low vascular pericytes, while yellow arrows mark SMA− RGS5+ IgA-high cells within the tumor glandular structures. (E) Blue Arrows mark cells with high FCAMR and IgA expression, while yellow arrows mark cells with low FCAMR and IgA expression. Red arrows denote necrotic debris. Scale bar: 100 μM.
CD138 is also a marker for epithelium within the glandular lumina (McCluggage, 2006). When we analyzed glandular lumina from EE and NE Tcf4Het/+ ApcMin/+ tumor sections, we found that NE tumors showed the expected epithelial expression of CD138 in glandular structures (Fig. 5C a and a′, black asterisks); however, EE tumors failed to show glandular CD138 expression (Fig. 5C b1′, red asterisks) and instead cells that were present were bound by IgA (Fig. 5B-b1′, red arrows). IgA expression was not found in glandular cells from NE tumors (Fig. 5B a′). This suggested that in EE tumors, IgA-bound CD138− cells replace glandular epithelia structures.
The final step in wound repair is the formation of the scar, and this process involves invasion by activated pericytes and myofibroblasts and subsequent scar formation (Bodnar et al., 2016). In IgA nephropathy, a disease characterized by fibrosis, kidney pericytes (mesangial cells) are activated by IgA polymer binding (Papista et al., 2011). Because EE promotes pericyte recruitment to colon tumor cells in Tcf4Het/+ ApcMin/+ animals, we questioned whether the IgA-bound cells in glandular structures from these animals (Fig. 5B–C) were indeed activated pericytes that migrate and contribute to wound repair. To test this directly, we stained tumor tissue isolated from EE and NE animals for IgA and the pericyte markers SMA and RGS5. We found RGS5+ SMA− IgA+ intratumoral pericytes (Fig. 5D; yellow arrows) in close contact with RGS5+ SMA+ IgA+ perivascular pericytes (Fig. 5D; blue arrows), an observation that is consistent with invasion of glandular structures by pericytes that have migrated from the perivascular space. Moreover, the RGS5+ SMA− pericytes exhibited high levels of IgA staining (Fig. 5D). This suggests that IgA-mediated pericyte invasion acts to restructure glandular tissue in a wound repair-like response.
IgA exerts its action through IgA Fc receptors located on target cells, and activity of these receptors is important for the activation of mesangial cells (Papista et al., 2011). In IgA nephropathy, IgA binds the Fc receptor Cd89 on mesangial cells (Berthelot et al., 2012), which also express the Fc receptor Fcα/μR (FCAMR; (McDonald et al., 2002)). Mice lack the Fc receptor CD89 but express FCAMR (Mestas and Hughes, 2004). To define whether colonic IgA binds FCAMR on pericytes, we co-stained tumor tissue from NE and EE animals for FCAMR, IgA and the pericyte marker RGS5. In tumors isolated from EE mice, many pericytes that are RGS5+ and IgA− have lower expression levels of FCAMR (Figure 5E, yellow arrows). Surprisingly, RGS5+ pericytes that are IgA+ highly express FCAMR (Figure 5E, cyan arrows). This result demonstrates that high FCAMR expression in RGS5+ pericytes correlates with IgA binding, and that FCAMR is the IgA receptor expressed on RGS5+ pericytes.
EE-dependent wound repair response restores microbiota mutualism within the colon barrier
Epithelial barrier dysfunction in colon tumor tissue can lead to microbial invasion and associated inflammation (Grivennikov et al., 2012), and decreased microbial species biodiversity has been associated with inflamed gut tissues commonly found in patients with inflammatory bowel disease (Manichanh et al., 2006). Since EE restores barrier function through the wound repair process and leads to an overall decrease in inflammatory signaling in colon tumor bearing mice, we questioned whether EE normalizes gut microbiota composition. To test this, we collected stool from the distal colons of both Tcf4Het/+ ApcMin/+ and WT animals exposed to NE and EE conditions, and examined the composition of the microbiome using 16S rRNA sequencing. Surprisingly, NE WT and Tcf4Het/+ ApcMin/+ animals have similar biodiversity as demonstrated by similar number of unique sequences identified (Fig. 6A). However, EE increased the biodiversity of Tcf4Het/+ ApcMin/+ tumor-bearing animals beyond that observed in NE Tcf4Het/+ ApcMin/+ animals and both NE and EE WT animals (Fig. 6A). These results demonstrate an EE-dependent increase of biodiversity in Tcf4Het/+ ApcMin/+ tumor-bearing males.
Fig. 6.
EE-mediated microbiota changes in Tcf4Het/+ ApcMin/+ mice. (A) Alpha diversity of NE and EE of WT (20,000 reads p=0.64) and NE and EE Tcf4Het/+ ApcMin/+ (20,000 reads p=0.03) animals. Specific contributions to the proteome within the (B) phylum Proteobacteria and the classes (C) Alphaproteobacteria (D) Betaproteobacteria, the (E) genus Sutterella, and (F) the phylum Gammaproteobacteria in response to EE in WT, Tcf4Het/+ Apc+/+, Tcf4+/+ ApcMin/+ and Tcf4Het/+ ApcMin/+ animals. ** p=0.005 using two-sample t-test with Welch correction.
Studies of colorectal cancer models have illuminated phyla level shifts in microbial populations that are apparent in diseased compared to normal tissue (Borges-Canha et al., 2015). We therefore questioned whether EE alters the composition of the microbiome, but did not observe significant differences at the phyla level between NE WT and NE Tcf4Het/+ ApcMin/+ animals. Surprisingly, however, we found drastic changes at the phyla level in tumor-bearing Tcf4Het/+ ApcMin/+ males in response to EE, including an increase in relative abundance of proteobacteria (Table S4A). The EE–dependent increases in the phylum proteobacteria are highly significant, with a 2-fold increase in average relative abundance following EE (5.8% in NE Tcf4Het/+ ApcMin/+ and 11.3% in EE Tcf4Het/+ ApcMin/+ animals, Fig. 6B, Table S4). Given the pathogenic role of proteobacteria in health (Shin et al., 2015), it is surprising that an increase in proteobacteria was associated with improved survival of EE Tcf4Het/+ ApcMin/+ animals. We found that the proteobacteria subclasses Alphaproteobacteria (2.8%) and Betaproteobacteria (6.7%) constituted the majority of proteobacteria that were significantly increased in EE Tcf4Het/+ ApcMin/+ animals (Fig. 6C–D, Table S4B). Of the isolated Alphaproteobacteria in enriched conditions, 50% were classified at the genus level as RF32 (1.4%) (Table S4E). Isolated Betaproteobacteria were further classified as primarily the genus Sutterella (Fig. 6E, Table S4E). It has been suggested that Sutterella is likely a commensal (Miller et al., 2015) and may actually serve a protective role in inflammatory bowel syndrome (Mukhopadhya et al., 2011). The proteobacteria subclass Gammaproteobacteria includes the pathogenic Klebsiella, Enterobacter, and Escherichia, which are well known LPS-membrane-containing microbes that can trigger inflammation (Spencer et al., 2011). While levels of the subclass Gammaproteobacteria are highly variable in NE Tcf4Het/+ ApcMin/+ animals, they are severely diminished in the majority of EE Tcf4Het/+ ApcMin/+ animals (Fig. 6F, Table S4B). The diverse phylum Proteobacteria may have either a protective or pathogenic role within the colons of Tcf4Het/+ ApcMin/+ animals, yet the improved phenotype of EE Tcf4Het/+ ApcMin/+ animals suggests that EE fosters colonization of commensal members and neutralizes colonization of pathogenic members of this phylum.
Model of EE-dependent effects on wound repair in the tumor microenvironment in male Tcf4Het/+ ApcMin/+ animals
Our study suggests that EE promotes a resolution of the wound repair response in tumors of Tcf4Het/+ ApcMin/+ animals (Fig. 7A–B). Here, environmental enrichment provokes the activation of NHRs involved in the following wound repair process (Figure 3A) in tumors: (1–2) similar to revascularization in the wound repair process, pericyte recruitment to the perivascular region of tumors results in vessel normalization and reduced angiogenesis. (3) Re-epithelialization occurs within tumors through differentiation of proliferative cells into distinct and functional epithelial cell types. (4–5) Plasma cells are recruited to the tumor periphery and secrete IgA. (6) Inflammatory signaling is reduced, further supporting the final steps of a repair process. (1, 7) Glandular structures at the tumor periphery are replaced by IgA-bound pericytes, an effect that may be intended to “seal the wound” to prevent entry by pathogens. (8) EE drastically increases the prevalence of the symbiotic bacteria Sutterella within the colon.
Fig. 7.
Model depicting the mechanism of EE-dependent improvements in survival of Tcf4Het/+ ApcMin/+ males. See text for details.
Discussion
Our studies reveal a remarkable, beneficial effect on the survival of both male and female mice with colon cancer in response to EE, a finding with profound clinical implications. Curiously, we observed clear phenotypic differences between male and female colon-tumor-bearing animals. The marked reduction in tumor growth in females alone would be expected to contribute to the extension of survival in females, but it clearly is not responsible for this EE-mediated health benefit in males. Moreover, it is possible that EE promotes health in each sex by different mechanisms. Sex-specific differences have previously been noted in epidemiological studies of psychosocial risk factors for colon cancer. Specifically, aberrant crypt foci (ACF), a marker of colon cancer risk, are found to be specifically increased in women with depression and men with reduced social structure (O’Leary et al., 2011). Importantly, our studies suggest that EE can reduce the adverse symptoms associated with colon cancer similarly in both sexes, to extend overall health and survival, albeit by different mechanisms.
ApcMin animals are known to develop primarily small intestinal tumors, and our current study suggests that EE does not affect lifespan or colon or small intestinal tumor load in this model. However, Cao et al. found an EE-mediated reduction in small intestinal tumor growth in male ApcMin mice with no effect on colon tumorigenesis (Cao et al., 2010). It is likely that this is a result of the drastic differences in the way the two studies were conducted (Supplemental Experimental Procedures). It is unclear whether variables such as food, breeding, animal genotypes, housing, coprophagia, or facilities contribute to the differences between our studies.
NHRs are ligand-regulated transcription factors that have key roles in normal tissue development and homeostasis. As transcription factors, they often form heterodimers with other NHRs and also act synergistically to modulate target gene expression during wound repair. In EE colons, several different NHR signaling pathways are activated, suggesting that each may play a role in the tumor wound repair response. For example in EE tumor bearing animals, GC and MC signaling may play a role in reduced inflammation and revascularization (Ullian, 1999), while the reactivation of thyroid hormone signaling may effect both re-epithelialization and B cell recruitment and migration to the tumor periphery (Bloise et al., 2014; Contreras-Jurado et al., 2014). In our study, we have further characterized thyroid hormone signaling within the stromal and tumor cell compartments in EE tumor bearing mice, and found activation of both hormone metabolism and the hormone receptor. However, the molecular mechanism that improves NHR signaling in EE colon tumor bearing mice is unclear. At least for thyroid hormone, it is possible that reduced IL-6 levels in EE animals play a role in the restoration of T3 metabolism, since local thyroid hormone metabolism is often inactivated as a direct effect of illness, and IL-6 cytokine levels are inversely correlated with the levels of activated thyroid hormone T3 (Bartalena et al., 1994). It remains to be tested whether IL-6 inhibitors alone or in combination with NHR agonists mimic the wound repair effects of EE in tumor bearing animals.
Our data supports a function for RGS5+ SMA+ and RGS5+ SMA− pericyte subtypes both in revascularization and wound repair, respectively. It is possible that EE-dependent NHR ligands recruit and stabilize pericytes in the tumor microenvironment (Hung et al., 2013), as the nuclear hormone receptor PPARα has been shown to protect pericytes in a mouse model of diabetic retinopathy (Ding et al., 2014). Further studies will be required to define the EE-dependent mechanisms driving increased pericyte localization to the perivascular and intratumoral regions in EE Tcf4Het/+ ApcMin/+ animals.
Based on the well-known protective effect of IgA secretion in colon tissue (Mantis et al., 2011), it is likely that the comparative abundance of colonic IgA in EE Tcf4Het/+ ApcMin/+ animals at least partially drives the EE-mediated increase in their health and survival. A review of the relatively limited mind-body-therapy studies available revealed that secretion of salivary IgA was the only significant outcome involving the immune system (Wahbeh et al., 2009). Our studies support and extend these findings to the colon. Recently, Moon et. al., found a correlation between mice with low levels of Secretory IgA (SIgA) on the luminal surface and the presence of Sutterella bacteria (Moon et al., 2015), suggesting that Sutterella may function in SIgA degradation. However, in our study, IgA levels increase in response to EE concomitantly with accumulation of Sutterella, suggesting that Sutterella may actually serve a protective function, possibly by normalizing SIgA levels at the mucosal surface.
In conclusion, we have described an NHR-, pericyte-, and IgA-dependent mechanism that supports the improvement of lifespan following EE in Tcf4Het/+ ApcMin/+ males. Our study indicates that EE delivers a tumor wound repair-centric therapeutic effect that improves the overall health of tumor bearing animals. The reactivation of the NHR pathways in wound repair following EE has revealed potential therapeutic strategies for pharmaceutical interventions that mimic the beneficial effects of EE in wound repair in colon tumors.
Experimental Procedures
Environmental Enrichment
Four genotypes were used in the enrichment experiments: WT, Tcf4Het/+ Apc+/+, Tcf4+/+ ApcMin/+, and Tcf4Het/+ ApcMin/+. NE animals were housed 5 mice per cage, while EE cages had at least 20 animals per cage. Animal behavior was observed and recorded using infrared cameras (Movies S1–S4). See Supplemental Experimental Procedures for in depth details regarding NE and EE housing conditions for the EE Lifespan (EE1) and EE Short-term (EE2) experimental setup. All experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Utah and conform to NIH requirements. All mice are in the inbred C57BL/6J standard genetic background.
Mouse Tissue Immunofluorescence and Immunohistochemistry
Frozen tissue sections were prepared and stained using conventional methods. Primary antibodies were anti-PYY (Abcam), anti-NPY2R (Abcam), anti-SMA (Sigma, Dako), anti-PECAM (Pharmingen), anti-IgA (Novus), anti-RGS5 (Santa Cruz Biotech), and biotin conjugated anti-IgA (Biolegend), anti-CD138 (Biolegend), anti-THRA (ThermoFisher), FITC conjugated anti-ME1 (Aviva Systems Biology), anti-ME2 (ThermoFisher), anti-FCAMR (Biolegend), anti-DIO1 (Proteintech). All secondary antibodies used were Alexa Fluor conjugates (Molecular Probes). Phalloidin 647 was used to stain Actin (Thermofisher). Biotin conjugated primaries were used along with an ABC immunoperoxidase system and DAB chromagen (Vector Laboratories). Confocal imaging was performed using a Leica TCS SP5 laser-scanning or Olympus FV1200 confocal microscope.
RNA Isolation and Sequencing
Distal colons were isolated from NE and EE animals, four of each condition. Colon RNA was isolated using Ambion RNAqueous Kit (LifeTechnologies) and utilized for both RNA-Seq and QPCR. DNAse treatment was performed using Ambion DNA Free DNase Kit (LifeTechnologies), and RNA quality was assessed using an Agilent RNA 6000 Nano Chip. RNA was sequenced and data analyzed as described in Supplemental Experimental Procedures.
Quantitative PCR
cDNA was generated from mouse distal colon RNA using qScript Flex cDNA kit (Quanta Biosciences) QPCR was performed using Power SYBR Green PCR Master Mix (LifeTechnologies). Data was analyzed as described in Supplemental Experimental Procedures. QPCR primers are listed in Table S5.
Blood Hematology and Chemistries
Blood hematological analysis was done using Hemagen MS-4 5-part differential cell analyzer and counter (Hemagen Diagnostics). Serum was analyzed on Hemagen Analyst III Chemistry Analyzer using Vet16 chemistry panel (Hemagen Diagnostics).
Other Serum Measurements
Insulin (Meso Scale Discovery (MSD) Mouse/Rat Insulin Kit), Adiponectin (MSD mouse 4 spot plate), and IL-6 and TNFα (MSD 4-spot custom plate) were measured following the manufacturer’s instructions. Both Albumin and Triglycerides were measured using Albumin Reagent Set (Pointe Scientific) and Triglyceride Reagent Set (Pointe Scientific) following manufacturer’s instructions.
Microbiota Isolation
Stool was collected and microbial DNA was isolated and characterized as described in Supplemental Experimental Procedures. Primers are listed in Table S5.
Statistics
Analysis of taxa was performed in R, version 3.2.3, “Wooden Christmas Tree” [R Core Team (2013), R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/.). P-values were calculated using R’s independent 2-group t-test with the assumption of unequal variances and Welsh’s correction applied. All graphs were created using ggplot2 package (http://ggplot2.org) with barplot and boxplot objects applied.
Supplementary Material
Long-term Survival (EE1) and Short-term (EE2) Environmental Enrichment Studies, Related to Fig. 1.
Short-term Environmental Enrichment (EE2) Studies of EE and NE Male and Female Mice, Related to Fig. 1.
RNA-Seq Data of Distal Colon Isolated from NE and EE 4-month-old Male Tcf4Het/+ ApcMin/+ Mice, Related to Figs. 2–4.
EE Male Voluntary Wheel Running, Related to Experimental Procedures.
EE Male Food and Social Interactions, Related to Experimental Procedures.
EE Female Nesting, Related to Experimental Procedures.
Female Non-Enriched (NE) Conditions, Related to Experimental Procedures.
Ingenuity Pathway Analysis of RNA-Seq Data of Distal Colon Isolated from 4-month-old Male EE and NE Tcf4Het/+ ApcMin/+ Mice, Related to Figs. 2–4.
Microbiota Classification of Bacteria Isolated from Stool Collected from EE and NE mice, Related to Fig. 6.
QPCR and Sequencing Primers Utilized Throughout the Study, Related to Experimental Procedures.
Acknowledgments
We would like to thank D. Lim for the graphical abstract and her illustration shown in Fig. 7. We thank C. Jette, A. Welm, T. Tuohy, D. Stafforini and B. Cairns for helpful discussions and critical reading of the manuscript. We acknowledge technical assistance from B. Dalley and T. Mosbruger in the University of Utah Genomics and Bioinformatics cores. The project described was supported by National Cancer Institute grants CA073992 and CA128891. This work was supported by access to technical cores supported by the Cancer Center Support Grant CA042014.
Footnotes
ACCESSION NUMBERS:
The RNA-Seq and Microbiome data in this manuscript have been deposited in NCBI’s GEO and are accessible through GEO: GSE90802.
AUTHOR CONTRIBUTIONS:
Conceptualization, Writing – Original Draft, Visualization, Validation, Supervision, B.D.B. and M.L.A-H.; Investigation, B.D.B, M.R.S., S.J.G., A.R.V., P.C.B., A.V.W., K.E.A., J.R.H. M.L.A-H.; Formal Analysis, M.L.A-H, B.D.B., A.R.V.; Writing – Review & Editing M.L.A-H, A.R.V., P.C.B.; Funding Acquisition, Project Administration, Resources, M.L.A-H.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angus-Hill ML, Elbert KM, Hidalgo J, Capecchi MR. T-cell factor 4 functions as a tumor suppressor whose disruption modulates colon cell proliferation and tumorigenesis. Proc Natl Acad Sci U S A. 2011;108:4914–4919. doi: 10.1073/pnas.1102300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aran V, Victorino AP, Thuler LC, Ferreira CG. Colorectal Cancer: Epidemiology, Disease Mechanisms and Interventions to Reduce Onset and Mortality. Clin Colorectal Cancer. 2016 doi: 10.1016/j.clcc.2016.02.008. [DOI] [PubMed] [Google Scholar]
- Arnold KM, Opdenaker LM, Flynn D, Sims-Mourtada J. Wound healing and cancer stem cells: inflammation as a driver of treatment resistance in breast cancer. Cancer Growth Metastasis. 2015;8:1–13. doi: 10.4137/CGM.S11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltgalvis KA, Berger FG, Pena MM, Davis JM, Muga SJ, Carson JA. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R393–401. doi: 10.1152/ajpregu.00716.2007. [DOI] [PubMed] [Google Scholar]
- Bartalena L, Brogioni S, Grasso L, Velluzzi F, Martino E. Relationship of the increased serum interleukin-6 concentration to changes of thyroid function in nonthyroidal illness. J Endocrinol Invest. 1994;17:269–274. doi: 10.1007/BF03348974. [DOI] [PubMed] [Google Scholar]
- Berthelot L, Papista C, Maciel TT, Biarnes-Pelicot M, Tissandie E, Wang PH, Tamouza H, Jamin A, Bex-Coudrat J, Gestin A, et al. Transglutaminase is essential for IgA nephropathy development acting through IgA receptors. J Exp Med. 2012;209:793–806. doi: 10.1084/jem.20112005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer C, Huang J, Beer J, Zhang Y, Palumbo-Zerr K, Zerr P, Distler A, Dees C, Maier C, Munoz L, et al. Activation of liver X receptors inhibits experimental fibrosis by interfering with interleukin-6 release from macrophages. Ann Rheum Dis. 2015;74:1317–1324. doi: 10.1136/annrheumdis-2013-204401. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Moses HL. Tumor-stroma interactions. Curr Opin Genet Dev. 2005;15:97–101. doi: 10.1016/j.gde.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloise FF, Oliveira FL, Nobrega AF, Vasconcellos R, Cordeiro A, Paiva LS, Taub DD, Borojevic R, Pazos-Moura CC, Mello-Coelho V. High levels of circulating triiodothyronine induce plasma cell differentiation. J Endocrinol. 2014;220:305–317. doi: 10.1530/JOE-13-0315. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Satish L, Yates CC, Wells A. Pericytes: A newly recognized player in wound healing. Wound Repair Regen. 2016;24:204–214. doi: 10.1111/wrr.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges-Canha M, Portela-Cidade JP, Dinis-Ribeiro M, Leite-Moreira AF, Pimentel-Nunes P. Role of colonic microbiota in colorectal carcinogenesis: a systematic review. Rev Esp Enferm Dig. 2015;107:659–671. doi: 10.17235/reed.2015.3830/2015. [DOI] [PubMed] [Google Scholar]
- Cao L, Liu X, Lin EJ, Wang C, Choi EY, Riban V, Lin B, During MJ. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell. 2010;142:52–64. doi: 10.1016/j.cell.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010;138:2029–2043. e2010. doi: 10.1053/j.gastro.2010.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YL, Gao HW, Chiang CP, Wang WM, Huang SM, Ku CF, Liu GY, Hung HC. Human mitochondrial NAD(P)(+)-dependent malic enzyme participates in cutaneous melanoma progression and invasion. J Invest Dermatol. 2015;135:807–815. doi: 10.1038/jid.2014.385. [DOI] [PubMed] [Google Scholar]
- Ciombor KK, Wu C, Goldberg RM. Recent therapeutic advances in the treatment of colorectal cancer. Annu Rev Med. 2015;66:83–95. doi: 10.1146/annurev-med-051513-102539. [DOI] [PubMed] [Google Scholar]
- Contreras-Jurado C, Garcia-Serrano L, Martinez-Fernandez M, Ruiz-Llorente L, Paramio JM, Aranda A. Impaired hair growth and wound healing in mice lacking thyroid hormone receptors. PLoS One. 2014;9:e108137. doi: 10.1371/journal.pone.0108137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Errico I, Moschetta A. Nuclear receptors, intestinal architecture and colon cancer: an intriguing link. Cell Mol Life Sci. 2008;65:1523–1543. doi: 10.1007/s00018-008-7552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Cheng R, Hu Y, Takahashi Y, Jenkins AJ, Keech AC, Humphries KM, Gu X, Elliott MH, Xia X, et al. Peroxisome proliferator-activated receptor alpha protects capillary pericytes in the retina. Am J Pathol. 2014;184:2709–2720. doi: 10.1016/j.ajpath.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Duda DG, Munn LL, Jain RK. Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models. Microcirculation. 2010;17:206–225. doi: 10.1111/j.1549-8719.2010.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JS. Mind-body medicine and cancer. Hematol Oncol Clin North Am. 2008;22:683–708. ix. doi: 10.1016/j.hoc.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JY, Li X, Browning JD, Jr, Rottinghaus GE, Lubahn DB, Constantinou A, Bennink M, MacDonald RS. Dietary soy isoflavones and estrone protect ovariectomized ERalphaKO and wild-type mice from carcinogen-induced colon cancer. J Nutr. 2004;134:179–182. doi: 10.1093/jn/134.1.179. [DOI] [PubMed] [Google Scholar]
- Hamzah J, Jugold M, Kiessling F, Rigby P, Manzur M, Marti HH, Rabie T, Kaden S, Grone HJ, Hammerling GJ, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453:410–414. doi: 10.1038/nature06868. [DOI] [PubMed] [Google Scholar]
- Hand TW, Dos Santos LM, Bouladoux N, Molloy MJ, Pagan AJ, Pepper M, Maynard CL, Elson CO, 3rd, Belkaid Y. Acute gastrointestinal infection induces long-lived microbiota-specific T cell responses. Science. 2012;337:1553–1556. doi: 10.1126/science.1220961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung C, Linn G, Chow YH, Kobayashi A, Mittelsteadt K, Altemeier WA, Gharib SA, Schnapp LM, Duffield JS. Role of Lung Pericytes and Resident Fibroblasts in the Pathogenesis of Pulmonary Fibrosis. American Journal of Respiratory and Critical Care Medicine. 2013;188:820–830. doi: 10.1164/rccm.201212-2297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt TK, Ehrlich HP, Garcia JA, Dunphy JE. Effect of vitamin A on reversing the inhibitory effect of cortisone on healing of open wounds in animals and man. Ann Surg. 1969;170:633–641. doi: 10.1097/00000658-196910000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Du W, Mancuso A, Wellen KE, Yang X. Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence. Nature. 2013;493:689–693. doi: 10.1038/nature11776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneshige M, Suzuki H, Kaneshige K, Cheng J, Wimbrow H, Barlow C, Willingham MC, Cheng S. A targeted dominant negative mutation of the thyroid hormone alpha 1 receptor causes increased mortality, infertility, and dwarfism in mice. Proc Natl Acad Sci U S A. 2001;98:15095–15100. doi: 10.1073/pnas.261565798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, Balaji S, Keswani SG, Crombleholme TM. The Role of Stem Cells in Wound Angiogenesis. Advances in wound care. 2014;3:614–625. doi: 10.1089/wound.2013.0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Ohshima T. The dynamics of inflammatory cytokines in the healing process of mouse skin wound: a preliminary study for possible wound age determination. Int J Legal Med. 1996;108:231–236. doi: 10.1007/BF01369816. [DOI] [PubMed] [Google Scholar]
- Kramer A, Green J, Pollard J, Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014;30:523–530. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus S, Arber N. Inflammation and colorectal cancer. Current Opinion in Pharmacology. 2009;9:405–410. doi: 10.1016/j.coph.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Koller Y, McCoy KD. The bilateral responsiveness between intestinal microbes and IgA. Trends Immunol. 2015;36:460–470. doi: 10.1016/j.it.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, Jarrin C, Chardon P, Marteau P, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55:205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantis NJ, Rol N, Corthesy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal immunology. 2011;4:603–611. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluggage WG. My approach to the interpretation of endometrial biopsies and curettings. J Clin Pathol. 2006;59:801–812. doi: 10.1136/jcp.2005.029702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald KJ, Cameron AJ, Allen JM, Jardine AG. Expression of Fc alpha/mu receptor by human mesangial cells: a candidate receptor for immune complex deposition in IgA nephropathy. Biochem Biophys Res Commun. 2002;290:438–442. doi: 10.1006/bbrc.2001.6218. [DOI] [PubMed] [Google Scholar]
- Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Michalik L, Wahli W. Involvement of PPAR nuclear receptors in tissue injury and wound repair. J Clin Invest. 2006;116:598–606. doi: 10.1172/JCI27958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PG, Bonn MB, Franklin CL, Ericsson AC, McKarns SC. TNFR2 Deficiency Acts in Concert with Gut Microbiota To Precipitate Spontaneous Sex-Biased Central Nervous System Demyelinating Autoimmune Disease. J Immunol. 2015;195:4668–4684. doi: 10.4049/jimmunol.1501664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon C, Baldridge MT, Wallace MA, Burnham CA, Virgin HW, Stappenbeck TS. Vertically transmitted faecal IgA levels determine extra-chromosomal phenotypic variation. Nature. 2015;521:90–93. doi: 10.1038/nature14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhya I, Hansen R, Nicholl CE, Alhaidan YA, Thomson JM, Berry SH, Pattinson C, Stead DA, Russell RK, El-Omar EM, et al. A comprehensive evaluation of colonic mucosal isolates of Sutterella wadsworthensis from inflammatory bowel disease. PLoS One. 2011;6:e27076. doi: 10.1371/journal.pone.0027076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio N, Ito S, Suzuki H, Isobe K. Antibodies to wounded tissue enhance cutaneous wound healing. Immunology. 2009;128:369–380. doi: 10.1111/j.1365-2567.2009.03119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary KE, Cruess DG, Pleau D, Swede H, Anderson J, Rosenberg D. Sex differences in associations between psychosocial factors and aberrant crypt foci among patients at risk for colon cancer. Gender medicine. 2011;8:165–171. doi: 10.1016/j.genm.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, Degnan PH, Hu J, Peter I, Zhang W, et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158:1000–1010. doi: 10.1016/j.cell.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papista C, Berthelot L, Monteiro RC. Dysfunctions of the Iga system: a common link between intestinal and renal diseases. Cell Mol Immunol. 2011;8:126–134. doi: 10.1038/cmi.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger S, Zhao H, Martin P, Abe K, Lisse TS. The role of nuclear hormone receptors in cutaneous wound repair. Cell Biochem Funct. 2015;33:1–13. doi: 10.1002/cbf.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. 2014;25:1293–1311. doi: 10.1093/annonc/mdu012. [DOI] [PubMed] [Google Scholar]
- Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13:800–812. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends in biotechnology. 2015;33:496–503. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Spencer MD, Hamp TJ, Reid RW, Fischer LM, Zeisel SH, Fodor AA. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology. 2011;140:976–986. doi: 10.1053/j.gastro.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda N, Zavacki AM, Maia AL, Harney JW, Larsen PR. A novel retinoid X receptor-independent thyroid hormone response element is present in the human type 1 deiodinase gene. Mol Cell Biol. 1995;15:5100–5112. doi: 10.1128/mcb.15.9.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian ME. The role of corticosteriods in the regulation of vascular tone. Cardiovasc Res. 1999;41:55–64. doi: 10.1016/s0008-6363(98)00230-2. [DOI] [PubMed] [Google Scholar]
- Wahbeh H, Haywood A, Kaufman K, Zwickey H. Mind-Body Medicine and Immune System Outcomes: A Systematic Review. The open complementary medicine journal. 2009;1:25–34. doi: 10.2174/1876391X00901010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh CA, Qin L, Tien JC, Young LS, Xu J. The function of steroid receptor coactivator-1 in normal tissues and cancer. International journal of biological sciences. 2012;8:470–485. doi: 10.7150/ijbs.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Voisin MB, Larbi KY, Dangerfield J, Scheiermann C, Tran M, Maxwell PH, Sorokin L, Nourshargh S. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med. 2006;203:1519–1532. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng FJ, Ye HB, Wu MS, Lian YF, Qian CN, Zeng YX. Repressing malic enzyme 1 redirects glucose metabolism, unbalances the redox state, and attenuates migratory and invasive abilities in nasopharyngeal carcinoma cell lines. Chin J Cancer. 2012;31:519–531. doi: 10.5732/cjc.012.10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukowska Z, Grant DS, Lee EW. Neuropeptide Y: a novel mechanism for ischemic angiogenesis. Trends Cardiovasc Med. 2003;13:86–92. doi: 10.1016/s1050-1738(02)00232-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Long-term Survival (EE1) and Short-term (EE2) Environmental Enrichment Studies, Related to Fig. 1.
Short-term Environmental Enrichment (EE2) Studies of EE and NE Male and Female Mice, Related to Fig. 1.
RNA-Seq Data of Distal Colon Isolated from NE and EE 4-month-old Male Tcf4Het/+ ApcMin/+ Mice, Related to Figs. 2–4.
EE Male Voluntary Wheel Running, Related to Experimental Procedures.
EE Male Food and Social Interactions, Related to Experimental Procedures.
EE Female Nesting, Related to Experimental Procedures.
Female Non-Enriched (NE) Conditions, Related to Experimental Procedures.
Ingenuity Pathway Analysis of RNA-Seq Data of Distal Colon Isolated from 4-month-old Male EE and NE Tcf4Het/+ ApcMin/+ Mice, Related to Figs. 2–4.
Microbiota Classification of Bacteria Isolated from Stool Collected from EE and NE mice, Related to Fig. 6.
QPCR and Sequencing Primers Utilized Throughout the Study, Related to Experimental Procedures.