Abstract
Genetic and environmental factors, i.e. infections, have been proposed to contribute to disease induction and relapsing events in multiple sclerosis (MS), an autoimmune demyelinating disease of the central nervous system (CNS). While research has mainly focused on virus associated autoimmune activation, less is known about prevention of autoimmunity, especially following resolving infections associated with CNS tissue damage. This review discusses novel insights on control of self-reactive (SR) T cells activated during neurotropic coronavirus-induced demyelination. A new concept is introduced that SR T cells can be dampened by distinct regulatory mechanisms in the periphery and the CNS, thereby preventing autoimmune disease.
Abbreviations: APC, antigen presenting cell; CLN, cervical lymph node; CNS, central nervous system; EAE, experimental autoimmune encephalomyelitis; IFN, interferon; MHV, mouse hepatitis virus; MOG, myelin oligodendrocyte glycoprotein; MS, multiple sclerosis; PLP, proteolipid protein; SR, self reactive; TMEV, Theiler's Murine Encephalomyelitis Virus; Tr-1, type 1 regulatory T cells; Tregs, regulatory T cells; WT, wild type
Keywords: Central nervous system (CNS), Multiple sclerosis, Virus, Self-reactive (SR) CD4 T cells, Regulatory T cells
Highlights
-
•
Virus-induced demyelination activates myelin specific T cells.
-
•
Virus-induced regulatory mechanisms limit pathogenic self-reactive R CD4 T cells.
-
•
Self-reactive CD4 T cells are controlled by distinct mechanisms in the CLN and CNS.
1. Introduction
Multiple Sclerosis (MS) is a chronic autoimmune disease of the central nervous system (CNS) characterized by demyelination and axonal damage (Dutta and Trapp, 2014, Frohman et al., 2006). More than one million people worldwide suffer from MS, which is the leading cause of disabilities in young adults. The complexity and diversity of clinical disease manifestations has proven challenging in the diagnosis and understanding of MS pathogenesis. Nevertheless, combinatorial studies of MS patients, MS autopsy material and murine models of MS have provided extensive insights into potential disease mechanisms. It is now accepted that inflammatory cells, including activated auto-reactive T cells, infiltrate the CNS and contribute to tissue damage characterized by multi-focal demyelinating lesions and axonal injury (Steinman and Zamvil, 2016). However, initiating factors driving and sustaining the activation of peripheral self-reactive (SR) T cells remain unclear, as the etiology of MS has not been resolved and appears multi-factorial. A combination of genetic and environmental factors, including diet and viral infections, have been implicated in breaching self-tolerance and leading to pathogenic SR T cell responses (Ascherio and Munger, 2007a, Ascherio and Munger, 2007b, Rothhammer and Quintana, 2016, Sawcer et al., 2014).
2. Multiple sclerosis and viral infections
2.1. Viruses in MS: culprit or protective?
A role of environmental factor(s) in disease etiology was first noted by epidemiological studies revealing a North-South gradient of MS incidence (Ebers and Sadovnick, 1993, Simpson et al., 2011). Migration studies also indicated that exposure to an infectious agent at a young age may increase the risk of developing MS (Gale and Martyn, 1995). Among several viruses linked to MS, the most prominent are Epstein Barr Virus and Human Herpes Virus type 6 (Moore and Wolfson, 2002, Salvetti et al., 2009). Although viral antigens and anti-viral antibodies have been detected within brain tissue and cerebrospinal fluid of MS patients, detection of active virus replication has been elusive (Libbey et al., 2014). A specific virus as a cause of MS initiation and/or progression has also not been found. Irrespective of the uncertain causal link of a specific infection to MS pathogenesis, mechanisms proposed to explain how viral infections can trigger autoimmunity include molecular mimicry, epitope spreading, bystander activation and cryptic antigen (Kivity et al., 2009, Vanderlugt and Miller, 2002). Depending on the infectious insult, autoimmune responses may thus manifest themselves temporally remote from acute infection, making it difficult to retrospectively pinpoint a single agent. Furthermore, as stated in this comprehensive “point of view” (Brahic, 2010), association does not necessarily mean causation. In this context it is important to note that a decreased incidence of infectious diseases inversely correlates with increased incidence of autoimmune diseases, including MS (Bach, 2002). The hygiene hypothesis thus challenges the causal function of viruses in autoimmunity and rather implies a protective role. While much interest has focused on the detrimental role of infection in autoimmunity, the opposing link to protection has not been extensively explored.
2.2. Lessons learned from viral-induced murine models of MS
Various murine models of MS have been used to elucidate distinct aspects of MS disease (Kipp et al., 2016, Martin et al., 2016). The toxin based demyelination models (cuprizone, lysolecithin) focus on oligodendrocyte and axonal injury largely independent of leukocyte infiltration, while myelin reactive T cell induced experimental autoimmune encephalomyelitis (EAE) and viral-induced demyelination models allow exploration of various T cell functions and immune-glia interactions in contributing to demyelination (Lassmann and Bradl, 2016). While the murine models all rely on distinct manipulations to trigger demyelinating disease, thereby circumventing natural events dictating interactions between the CNS and immune system during disease initiation and progression, each model has helped understanding specific aspects of MS pathogenesis. This review specifically focuses on the dichotomy of protective and deleterious roles of virus infections in autoimmune MS pathogenesis by discussing two prominent viral-induced demyelination models, i.e. Theiler's Murine Encephalomyelitis Virus (TMEV) and neurotropic murine hepatitis virus (MHV).
2.2.1. Theiler's Murine Encephalomyelitis Virus (TMEV)
TMEV is a non-enveloped single stranded RNA virus that belongs to the family of Picornaviridae. Pathogenesis associated with TMEV infection varies depending on the viral strain and genetic background of the mouse (Monteyne et al., 1997, Oleszak et al., 2004). The highly neurovirulent strains GDVII and FA induce an acute encephalitis from which only very few mice recover. Another TMEV subgroup includes the attenuated variants BeAn and DA. CNS infection of C57BL/6 mice with these attenuated neurovirulent strains results in an acute infection that is rapidly cleared without neurological sequelae, whereas a biphasic CNS disease is observed in susceptible SJL mice (Lipton, 1975). Acute BeAn and DA infections in SJL mice are characterized by viral replication in the CNS grey matter with prominent initial neuronal infection. This mild and subclinical acute phase is characterized by neuronal loss with little or no demyelination. Although anti-viral immune responses reduce viral replication, thus it fails to completely clear infectious virus resulting in a persistent infection. During the chronic phase, also termed TMEV-induced demyelinating disease, low virus titers predominantly persist in macrophages and microglia, with few astrocytes and oligodendrocytes infected (Lipton et al., 1995). This chronic phase is characterized by a progressively abnormal gait and spastic hind limb paralysis correlating with ongoing inflammation, demyelination and axonal damage. Progressive disease associated with myelin loss in SJL mice correlates with induction of SR T cell responses within the inflammatory milieu of the persistent CNS infection. In this well characterized model, reactivity to myelin antigens only emerges after the onset of viral-induced clinical symptoms and is due to epitope spreading occurring after initial virus-specific Th1 and macrophage-mediated bystander demyelination (Karpus et al., 1995, Miller et al., 2001). The spectrum of self-reactivity starts with recognition of the immuno-dominant myelin proteolipid protein PLP139–151 epitope one to two months post infection and progresses to an expanding number of sub-dominant myelin epitopes in a hierarchical manner (Miller et al., 1997). Thus, the TMEV model supports the hypothesis that viral infection can trigger autoimmune disease and has been instrumental in deciphering possible mechanisms of autoimmune activation following viral infection. In addition, distinct Foxp3 Treg expansion during TMEV infection marks resistant C57BL/6 and susceptible SJL mice (Richards et al., 2011). Treg depletion in SJL mice correlated with enhanced anti-viral response and control of TMEV replication within the CNS, thus preventing development of autoimmunity. Altogether, these data demonstrated an essential role of Foxp3 regulatory T cells (Tregs) in limiting anti-viral T cell response thus promoting virus persistence and subsequent autoimmunity.
2.2.2. Neurotropic mouse hepatitis virus (MHV)
MHV is an enveloped single-stranded RNA virus that belongs to the family of coronaviridae. Two neurotropic strains of MHV are generally used to examine neuropathogenesis, namely MHV-A59 and a non-lethal glial tropic variant of MHV-JHM designated JHM v2.2-1. Following intracerebral infection, the dual liver and CNS tropic MHV-A59 strain infects neurons and glial cells and induces a mild encephalitis associated with demyelination after initial viral control. JHM v2.2-1 infection is limited to the CNS and induces a clinical episode of severe encephalitis followed by flaccid hind limb paralysis from which the majority of mice nevertheless recover (Bender and Weiss, 2010, Bergmann et al., 2006). The neutralizing monoclonal antibody derived v2.2-1 variant is highly oligodendroglia tropic, with very sparse neuronal infection. CNS infection of 6–7 week old mice by JHM v2.2-1 results in an influx of innate and adaptive immune cells, whose functions have been characterized extensively (Bergmann et al., 2006, Templeton and Perlman, 2007). CD4 and CD8 T cells are essential to control infectious virus via cell type specific effector functions (Bergmann et al., 2004, Stohlman et al., 2008). Infection of astrocytes and microglia/macrophages is controlled via perforin, while replication in oligodendrocytes is limited by a non-lytic mechanism dependent upon IFN-γ. Despite effective control of infectious virus, the anti-viral T cell response is unable to completely eliminate virus from the CNS resulting in a persistent infection of spinal cord white matter. In contrast to TMEV, infectious virus cannot be detected and JHMV persistence is characterized by viral antigen and viral RNA associated with T cell and antibody secreting cell retention. The pathological hallmark of JHMV infection is focal myelin loss associated with axonal damage, which can be detected as early as day 7 post-infection (p.i.) coincident with T cell mediated viral control (Wu et al., 2001). However, myelin destruction is not maximal until 2 to 3 weeks p.i., indicating that the majority of tissue destruction occurs well after infectious virus has been cleared (Stohlman and Hinton, 2001). The paucity of demyelination in infected immunodeficient Rag−/− and SCID mice demonstrated that myelin loss is not the consequence of direct virus destruction of oligodendrocytes, but rather mediated by adaptive immunity (Pewe et al., 2002, Pewe and Perlman, 2002, Savarin et al., 2008). Indeed, Rag−/− and SCID recipients of virus-induced activated or memory T cells develop demyelination accompanied by recruitment of activated macrophages/microglia to sites of myelin loss. Macrophages/microglia are responsible for primary myelin destruction, but how T cell effector mechanisms can influence demyelination is still incompletely understood. Persistence is characterized by gradually declining levels of viral RNA in oligodendrocytes and resolution of hind limb paralysis, leaving only minimal residual dysfunction by day 30 p.i. Clinical recovery has been associated with remyelination, but persisting viral RNA for out to one year p.i. is nevertheless associated with new foci of myelin loss (Stohlman and Hinton, 2001). Thus, despite decreased but sustained demyelination and T cell retention, clinical recovery initially indicated no apparent autoimmune syndrome during viral persistence. Nevertheless, the striking similarities between the lesion pathology in mice, which have recovered from acute JHMV 2.2v-1 infection with lesions noted in MS make this an attractive model to examine why virus induced tissue damage in the form of demyelination, can cause induction of SR adaptive responses with benign rather than pathogenic consequences.
3. Self reactive CD4 T cell response during neurotropic MHV infection
3.1. Indirect evidence suggesting virus-mediated induction of SR T cells
The absence of clinically apparent autoimmunity consistent with the slowly diminishing extent of demyelination and active remyelination suggested that SR T cells are not induced during persistent JHMV infection. However, indirect evidence from several studies disputed this hypothesis. The initial indication that MHV infection may induce autoimmunity was indicated by development of lesions resembling those in EAE in naïve recipients of T cells derived from JHMV infected rats and expanded in vitro prior to transfer (Watanabe et al., 1983). In addition, peripheral MHV infection correlated with induction of SR T cells detected within the spleen of chronically infected mice (Kyuwa et al., 1991). More recently, it was demonstrated that acute MHV-A59 infection supports peripheral proliferation and CNS recruitment of transferred T-cell receptor transgenic myelin-specific CD4 T cells (Cervantes-Barragan et al., 2012). Similarly, we showed that naïve myelin oligodendrocyte glycoprotein (MOG)-specific CD4 T cells transferred prior to JHMV infection proliferated in cervical lymph nodes (CLN) and migrate to the CNS (Savarin et al., 2015). However, in contrast to MHV-A59 infection, SR CD4 T cell activation and CNS recruitment was not apparent during acute infection, but was specifically observed during chronic infection correlating with demyelination kinetics. The absence of peripheral proliferation and CNS access of CD4 T cells specific for an irrelevant antigen further confirmed that both activation and CNS recruitment of transferred SR CD4 T cells is myelin-driven and not a bystander effect of JHMV-induced inflammation.
3.2. Characterization of endogenous SR CD4 T cells during gliatropic infection
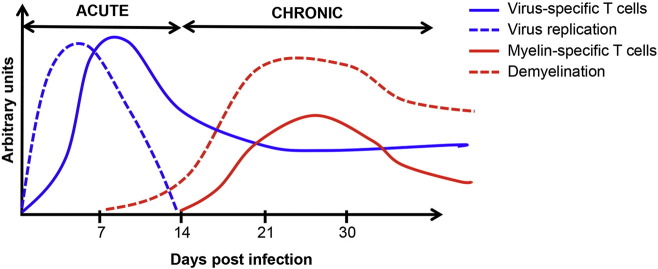
The absence of clinically apparent autoimmunity during chronic JHM v2.2-1 infection despite antigen-driven activation and CNS recruitment of transferred myelin-specific CD4 T cells prompted us to investigate whether endogenous SR CD4 T cells are actually induced following onset of demyelination. Indeed, we were the first to detect endogenous CD4 T cells specific for myelin-derived MOG35–55 and MBP60–80 encephalitogenic epitopes in both the CLN and CNS of JHM v2.2-1-infected wild type (WT) mice (Savarin et al., 2015). Moreover, peak frequencies of SR T cells correlated with peak demyelination after control of infectious virus (Fig. 1 ). While SR CD4 T cells could not be detected within the CNS during acute infection, their frequencies increased as myelin damage worsened. Importantly, although SR CD4 T cells were sustained within the CNS later during chronic infection, their frequencies and total numbers dropped along with virus specific CD4 T cells (Fig. 1). Declining percentages of SR CD4 T cells despite sustained demyelination was intriguing; especially as both CLN- and CNS-derived CD11b+ antigen presenting cells (APC) sustained their ability to support SR CD4 T cell proliferation ex vivo (Savarin et al., 2015). The lack of preferential expansion or retention of SR CD4 T cells within the CNS, despite sufficient endogenous myelin antigen presentation by APC, thus suggested that persistent infection establishes regulatory mechanisms to limit SR T cells and thereby prevent development of CNS autoimmune disease.
Fig. 1.
Kinetics of virus- and SR T cell responses during JHM v2.2-1 infection. Following intracerebral inoculation, JHM v2.2-1 replicates in the brain and spinal cord. Viral titers peak around d5 post infection (p.i.). CNS infiltration of virus-specific T cells is maximal between days 7–10 p.i. and essential to control infectious virus, which is no longer detectable after d14 p.i. Anti-viral T cell effector function triggers demyelination. Release of myelin antigen leads to activation of myelin-specific T cells. SR T cells peak around days 21–30 p.i., coincident with peak demyelination. At later time points during chronic infection, sustained demyelination is balanced by remyelination and associated with retention of both virus- and SR T cells at declining levels.
4. Regulations of SR CD4 T cells during chronic JHMV infection
Immune regulation associated with viral infections is vital to the host to balance effective pathogen clearance while limiting tissue damage. It is especially critical during infections of the CNS, which has limited regenerative capacity. Mechanisms limiting tissue damage mediated by virus-specific effector T cells may also benefit the host via suppression of SR T cells and autoimmunity. However, this concept is poorly explored. Both Foxp3 Tregs and type 1 regulatory T (Tr-1) cells, known to limit autoimmune diseases, are induced following JHM v2.2-1 infection (de Aquino et al., 2014, Puntambekar et al., 2011). Furthermore, they are the major producers of the anti-inflammatory cytokine IL-10, which is essential in limiting expansion of demyelinating lesions during chronic infection (Puntambekar et al., 2015). Interestingly, both T regulatory populations displayed limited functions in altering viral clearance and level of persistence, suggesting they may play a more prominent role in regulating JHMV-associated SR T cells. We were particularly interested in evaluating the role of Foxp3 Tregs, which were shown to limit clinical disease severity and the extent of tissue damage without affecting virus clearance following transfer into acutely infected mice (Trandem et al., 2010). We therefore assessed Foxp3 Treg functions by specifically depleting them during the persistent phase of infection. These studies showed that Foxp3 Treg ablation during persistence correlated with increased frequencies of SR CD4 T cell within CLN. CNS inflammation including SR CD4 T cells were not affected, consistent with no alteration in demyelination severity (Savarin et al., 2016). The apparent absence of Foxp3 Treg mediated regulation of CNS autoimmunity implied a potential protective function of Tr-1 cells, the major IL-10 producers during chronic JHM v2.2-1 infection (Puntambekar et al., 2011). SR CD4 T cells were therefore analyzed in infected Il-27R deficient (IL-27R−/−) mice, which were previously demonstrated to have a drastically reduced Tr1 response (de Aquino et al., 2014). Contrasting Foxp3 Treg function, SR T cells were similar within the CLN, but specifically increased within the CNS of IL-27R−/− mice compared to WT mice (Savarin et al., 2016). Altogether, our data demonstrated that JHM v2.2-1 infection induced differential regulatory mechanisms to control SR CD4 T cells in the CLN and CNS. Foxp3+ Tregs are important to limit SR T cells within the CLN, consistent with previous observations indicating Foxp3 Treg regulatory functions primarily in CLN (Korn et al., 2007, Trandem et al., 2010). In contrast, Tr-1 cells appear essential in controlling SR T cells within the CNS during chronic infection.
5. Conclusions and perspectives
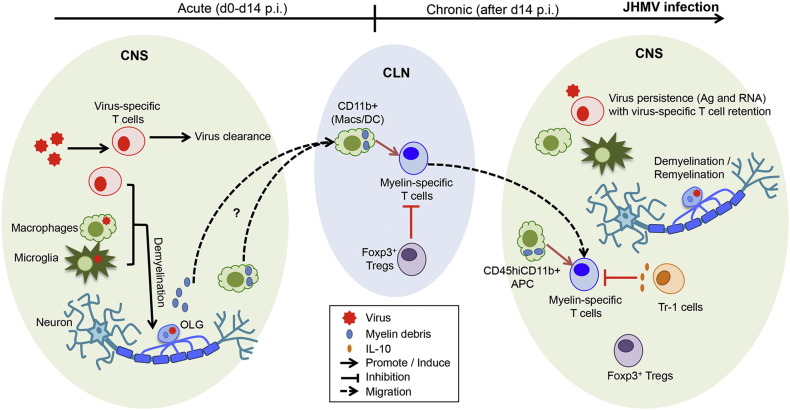
Neurotropic MHV induced demyelination has proven to be a unique and valuable model to investigate how a post infectious immune suppressive environment limits expansion and/or pathogenic functions of SR T cells during viral persistence. Research during the past few years has revealed activation of endogenous SR CD4 T cells, which nevertheless appear not to propagate chronic autoimmunity. Mechanistic insights to date can be summarized in the following schematic (Fig. 2 ): CD4 and CD8 T cell mediated control of viral replication leads to collateral damage manifested by immune-mediated demyelination and axonal damage. Myelin debris is either directly or cell-associated drained to the CLN, where CD11b+ APC can activate SR CD4 T cells specifically during chronic JHMV infection. Auto-reactive CD4 T cells then migrate to the CNS, where they can be restimulated by local CD11b+ APC, but not microglia (Savarin et al., 2015). Although peak SR CD4 T cells correlate with peak demyelination, they progressively decline in the CNS and do not affect clinical recovery. Regulatory mechanisms are provided by both Foxp3 Tregs and Tr-1 cells, which specifically control SR CD4 T cells within the CLN and within the CNS, respectively. Importantly, the finding that differential site-specific regulation of SR CD4 T cells during chronic infection did not affect viral persistence opens up new avenues for immune based therapies for autoimmunity, while limiting the risk for endogenous virus reactivation.
Fig. 2.
Regulation of SR CD4 T cells during chronic JHM v2.2-1 infection. CD4 and CD8 T cell-dependent virus control leads to immune-mediated demyelination. Myelin debris is drained to the CLN (as a soluble form or cell-associated) where CD11b+ myeloid cells present myelin antigen. Activation of SR CD4 T cells is temporally restricted to chronic infection associated with primary demyelination. Following peripheral activation, SR CD4 T cells migrate to the CNS where CD45hiCD11b+ myeloid cells can present myelin antigen to reactive SR CD4 T cells for access to the CNS parenchyma. Nevertheless, Foxp3 Tregs and Tr-1 cells regulate proliferation of SR CD4 T cells in the CLN and CNS respectively, thereby counteracting autoimmune tissue destruction. Altogether, JHM v2.2-1-associated regulatory mechanisms prevent development of autoimmune disease during chronic infection without affecting viral control. OLG: oligodendrocyte.
Chronic JHMV infection depicts a dynamic delicate balance between protecting the host against viral recrudescence while inhibiting autoimmunity. One of the critical challenges of therapeutic strategies to treat MS is to specifically eliminate the autoimmune response without compromising protective immunity against new or latent infections. The CNS is the target of several viral latent infections that can cause life-threatening diseases in immune suppressed individuals (McGavern and Kang, 2011). As an example, MS patients treated with anti-alpha4 integrin (Natalizumab) to prevent immune cell CNS entry are at risk of developing progressive multi-focal leukoencephalopathy due to JC virus reactivation (Stuve et al., 2007). The chronic JHMV infection model thus provides a unique opportunity to decipher how regulatory mechanisms can control SR T cells without altering viral persistence. Several critical questions beckon to be addressed: Are the SR CD4 T cells truly inert or do they contribute to smoldering tissue damage independent of persisting virus? How does the local lymph node environment established post infection influence priming and expansion of SR T cells or T cells with irrelevant specificity? Are Foxp3 Tregs controlling SR CD4 T cells during chronic infection virus- or myelin-specific? What is the role of Foxp3 Tregs retained within the CNS? What are the mechanisms underlying Foxp3 Treg- and Tr-1-dependent regulation of SR CD4 T cells within the CLN and CNS respectively? Addressing these questions will provide critical insights into tissue environments and cellular mechanisms naturally limiting autoimmunity without losing protective anti-viral immunity. Insights gained may lead to safer and more efficient immune modulation based treatment against autoimmune diseases.
Acknowledgements
This work was supported by the National Institute of Health grant numbers NS069690 and NS091183 (to CCB) originally awarded to Dr. Stephen A. Stohlman. We are deeply indebted to his scientific acumen and dedicate this review in his memory.
References
- Ascherio A., Munger K.L. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann. Neurol. 2007;61:288–299. doi: 10.1002/ana.21117. [DOI] [PubMed] [Google Scholar]
- Ascherio A., Munger K.L. Environmental risk factors for multiple sclerosis. Part II: noninfectious factors. Ann. Neurol. 2007;61:504–513. doi: 10.1002/ana.21141. [DOI] [PubMed] [Google Scholar]
- Bach J.F. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- Bender S.J., Weiss S.R. Pathogenesis of murine coronavirus in the central nervous system. J. NeuroImmune Pharmacol. 2010;5:336–354. doi: 10.1007/s11481-010-9202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann C.C., Parra B., Hinton D.R., Ramakrishna C., Dowdell K.C., Stohlman S.A. Perforin and gamma interferon-mediated control of coronavirus central nervous system infection by CD8 T cells in the absence of CD4 T cells. J. Virol. 2004;78:1739–1750. doi: 10.1128/JVI.78.4.1739-1750.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann C.C., Lane T.E., Stohlman S.A. Coronavirus infection of the central nervous system: host-virus stand-off. Nat. Rev. Microbiol. 2006;4:121–132. doi: 10.1038/nrmicro1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahic M. Multiple sclerosis and viruses. Ann. Neurol. 2010;68:6–8. doi: 10.1002/ana.22057. [DOI] [PubMed] [Google Scholar]
- Cervantes-Barragan L., Firner S., Bechmann I., Waisman A., Lahl K., Sparwasser T. Regulatory T cells selectively preserve immune privilege of self-antigens during viral central nervous system infection. J. Immunol. 2012;188:3678–3685. doi: 10.4049/jimmunol.1102422. [DOI] [PubMed] [Google Scholar]
- de Aquino M.T., Kapil P., Hinton D.R., Phares T.W., Puntambekar S.S., Savarin C. IL-27 limits central nervous system viral clearance by promoting IL-10 and enhances demyelination. J. Immunol. 2014;193:285–294. doi: 10.4049/jimmunol.1400058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta R., Trapp B.D. Relapsing and progressive forms of multiple sclerosis: insights from pathology. Curr. Opin. Neurol. 2014;27:271–278. doi: 10.1097/WCO.0000000000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebers G.C., Sadovnick A.D. The geographic distribution of multiple sclerosis: a review. Neuroepidemiology. 1993;12:1–5. doi: 10.1159/000110293. [DOI] [PubMed] [Google Scholar]
- Frohman E.M., Racke M.K., Raine C.S. Multiple sclerosis—the plaque and its pathogenesis. N. Engl. J. Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- Gale C.R., Martyn C.N. Migrant studies in multiple sclerosis. Prog. Neurobiol. 1995;47:425–448. [PubMed] [Google Scholar]
- Karpus W.J., Pope J.G., Peterson J.D., Dal Canto M.C., Miller S.D. Inhibition of Theiler's virus-mediated demyelination by peripheral immune tolerance induction. J. Immunol. 1995;155:947–957. [PubMed] [Google Scholar]
- Kipp M., Nyamoya S., Hochstrasser T., Amor S. Multiple sclerosis animal models: a clinical and histopathological perspective. Brain Pathol. 2016 doi: 10.1111/bpa.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivity S., Agmon-Levin N., Blank M., Shoenfeld Y. Infections and autoimmunity—friends or foes? Trends Immunol. 2009;30:409–414. doi: 10.1016/j.it.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Korn T., Reddy J., Gao W., Bettelli E., Awasthi A., Petersen T.R. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat. Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyuwa S., Yamaguchi K., Toyoda Y., Fujiwara K. Induction of self-reactive T cells after murine coronavirus infection. J. Virol. 1991;65:1789–1795. doi: 10.1128/jvi.65.4.1789-1795.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H., Bradl M. Multiple sclerosis: experimental models and reality. Acta Neuropathol. 2016 doi: 10.1007/s00401-016-1631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey J.E., Cusick M.F., Fujinami R.S. Role of pathogens in multiple sclerosis. Int. Rev. Immunol. 2014;33:266–283. doi: 10.3109/08830185.2013.823422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H.L. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect. Immun. 1975;11:1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H.L., Twaddle G., Jelachich M.L. The predominant virus antigen burden is present in macrophages in Theiler's murine encephalomyelitis virus-induced demyelinating disease. J. Virol. 1995;69:2525–2533. doi: 10.1128/jvi.69.4.2525-2533.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R., Sospedra M., Rosito M., Engelhardt B. Current multiple sclerosis treatments have improved our understanding of MS autoimmune pathogenesis. Eur. J. Immunol. 2016;46:2078–2090. doi: 10.1002/eji.201646485. [DOI] [PubMed] [Google Scholar]
- McGavern D.B., Kang S.S. Illuminating viral infections in the nervous system. Nat. Rev. Immunol. 2011;11:318–329. doi: 10.1038/nri2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S.D., Vanderlugt C.L., Begolka W.S., Pao W., Yauch R.L., Neville K.L. Persistent infection with Theiler's virus leads to CNS autoimmunity via epitope spreading. Nat. Med. 1997;3:1133–1136. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- Miller S.D., Olson J.K., Croxford J.L. Multiple pathways to induction of virus-induced autoimmune demyelination: lessons from Theiler's virus infection. J. Autoimmun. 2001;16:219–227. doi: 10.1006/jaut.2000.0489. [DOI] [PubMed] [Google Scholar]
- Monteyne P., Bureau J.F., Brahic M. The infection of mouse by Theiler's virus: from genetics to immunology. Immunol. Rev. 1997;159:163–176. doi: 10.1111/j.1600-065x.1997.tb01014.x. [DOI] [PubMed] [Google Scholar]
- Moore F.G., Wolfson C. Human herpes virus 6 and multiple sclerosis. Acta Neurol. Scand. 2002;106:63–83. doi: 10.1034/j.1600-0404.2002.01251.x. [DOI] [PubMed] [Google Scholar]
- Oleszak E.L., Chang J.R., Friedman H., Katsetos C.D., Platsoucas C.D. Theiler's virus infection: a model for multiple sclerosis. Clin. Microbiol. Rev. 2004;17:174–207. doi: 10.1128/CMR.17.1.174-207.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pewe L., Perlman S. Cutting edge: CD8 T cell-mediated demyelination is IFN-gamma dependent in mice infected with a neurotropic coronavirus. J. Immunol. 2002;168:1547–1551. doi: 10.4049/jimmunol.168.4.1547. [DOI] [PubMed] [Google Scholar]
- Pewe L., Haring J., Perlman S. CD4 T-cell-mediated demyelination is increased in the absence of gamma interferon in mice infected with mouse hepatitis virus. J. Virol. 2002;76:7329–7333. doi: 10.1128/JVI.76.14.7329-7333.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntambekar S.S., Bergmann C.C., Savarin C., Karp C.L., Phares T.W., Parra G.I. Shifting hierarchies of interleukin-10-producing T cell populations in the central nervous system during acute and persistent viral encephalomyelitis. J. Virol. 2011;85:6702–6713. doi: 10.1128/JVI.00200-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntambekar S.S., Hinton D.R., Yin X., Savarin C., Bergmann C.C., Trapp B.D. Interleukin-10 is a critical regulator of white matter lesion containment following viral induced demyelination. Glia. 2015 doi: 10.1002/glia.22880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M.H., Getts M.T., Podojil J.R., Jin Y.H., Kim B.S., Miller S.D. Virus expanded regulatory T cells control disease severity in the Theiler's virus mouse model of MS. J. Autoimmun. 2011;36:142–154. doi: 10.1016/j.jaut.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V., Quintana F.J. Environmental control of autoimmune inflammation in the central nervous system. Curr. Opin. Immunol. 2016;43:46–53. doi: 10.1016/j.coi.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvetti M., Giovannoni G., Aloisi F. Epstein-Barr virus and multiple sclerosis. Curr. Opin. Neurol. 2009;22:201–206. doi: 10.1097/WCO.0b013e32832b4c8d. [DOI] [PubMed] [Google Scholar]
- Savarin C., Bergmann C.C., Hinton D.R., Ransohoff R.M., Stohlman S.A. Memory CD4 + T-cell-mediated protection from lethal coronavirus encephalomyelitis. J. Virol. 2008;82:12432–12440. doi: 10.1128/JVI.01267-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarin C., Bergmann C.C., Gaignage M., Stohlman S.A. Self-reactive CD4(+) T cells activated during viral-induced demyelination do not prevent clinical recovery. J. Neuroinflammation. 2015;12:207. doi: 10.1186/s12974-015-0426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarin C., Bergmann C.C., Hinton D.R., Stohlman S.A. Differential regulation of self-reactive CD4 + T cells in cervical lymph nodes and central nervous system during viral encephalomyelitis. Front. Immunol. 2016;7:370. doi: 10.3389/fimmu.2016.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawcer S., Franklin R.J., Ban M. Multiple sclerosis genetics. Lancet Neurol. 2014;13:700–709. doi: 10.1016/S1474-4422(14)70041-9. [DOI] [PubMed] [Google Scholar]
- Simpson S., Jr., Blizzard L., Otahal P., Van der Mei I., Taylor B. Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2011;82:1132–1141. doi: 10.1136/jnnp.2011.240432. [DOI] [PubMed] [Google Scholar]
- Steinman L., Zamvil S.S. Beginning of the end of two-stage theory purporting that inflammation then degeneration explains pathogenesis of progressive multiple sclerosis. Curr. Opin. Neurol. 2016;29:340–344. doi: 10.1097/WCO.0000000000000317. [DOI] [PubMed] [Google Scholar]
- Stohlman S.A., Hinton D.R. Viral induced demyelination. Brain Pathol. 2001;11:92–106. doi: 10.1111/j.1750-3639.2001.tb00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohlman S.A., Hinton D.R., Parra B., Atkinson R., Bergmann C.C. CD4 T cells contribute to virus control and pathology following central nervous system infection with neurotropic mouse hepatitis virus. J. Virol. 2008;82:2130–2139. doi: 10.1128/JVI.01762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuve O., Marra C.M., Cravens P.D., Singh M.P., Hu W., Lovett-Racke A. Potential risk of progressive multifocal leukoencephalopathy with natalizumab therapy: possible interventions. Arch. Neurol. 2007;64:169–176. doi: 10.1001/archneur.64.2.169. [DOI] [PubMed] [Google Scholar]
- Templeton S.P., Perlman S. Pathogenesis of acute and chronic central nervous system infection with variants of mouse hepatitis virus, strain JHM. Immunol. Res. 2007;39:160–172. doi: 10.1007/s12026-007-0079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trandem K., Anghelina D., Zhao J., Perlman S. Regulatory T cells inhibit T cell proliferation and decrease demyelination in mice chronically infected with a coronavirus. J. Immunol. 2010;184:4391–4400. doi: 10.4049/jimmunol.0903918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderlugt C.L., Miller S.D. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat. Rev. Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- Watanabe R., Wege H., ter Meulen V. Adoptive transfer of EAE-like lesions from rats with coronavirus-induced demyelinating encephalomyelitis. Nature. 1983;305:150–153. doi: 10.1038/305150a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G.F., Dandekar A.A., Pewe L., Perlman S. The role of CD4 and CD8 T cells in MHV-JHM-induced demyelination. Adv. Exp. Med. Biol. 2001;494:341–347. doi: 10.1007/978-1-4615-1325-4_51. [DOI] [PubMed] [Google Scholar]