Abstract
Schistosomes are intravascular parasitic worms that cause the debilitating disease schistosomiasis. To better understand how these long-lived parasites may subvert host immune and hemostatic capabilities, we examine here the impact of adult Schistosoma mansoni worms on the human serum proteome. Normal human serum (150 μl) was incubated at 37°C for one hour either in the presence or absence of adult worms (~50 pairs). Thereafter parasites were removed, serum samples were labeled and their proteins resolved for comparative analysis by 2D-Differential in-Gel Electrophoresis (2D-DIGE). Several differences were noted between the two samples. Twenty protein spots were recovered and identified following trypsin digestion and mass spectroscopy. Strikingly, most of these (11/20) are associated with the complement system and include complement components C3, C4, factor B, complement factor H related protein 1 and clusterin. Western blot analysis confirms the impact of the worms on C3, C4 and factor B in serum. The data suggest that schistosomes engage complement but can rapidly degrade selected complement proteins which may help explain the worm’s refractoriness towards complement-mediated attack. Other serum proteins identified as being impinged upon by schistosomes are alpha 2 macroglobulin, alpha 1 anti-chymotrypsin, actin cytoplasmic 2, serum amyloid A-4, protein DDX26B, hemoglobin subunit B and serum albumin. While the molecular nature of the interaction between these proteins and schistosomes is not known, possible roles for some of them in hemostasis, immune evasion and in the host response to serum stress are suggested.
Keywords: Schistosoma, serum proteomics, host-parasite interaction, complement
Graphical abstract
1. Introduction
Schistosomes are intravascular parasitic worms that cause the debilitating disease schistosomiasis. Over 200 million people are estimated to be infected with these worms around the world and more than 600 million live at risk of infection [1]. Infection occurs when the free-swimming larval form (the cercaria) penetrates the skin and transforms into a morphologically and biochemically distinct life stage called the schistosomulum. This juvenile parasite then invades a blood vessel, matures and finds a partner to begin its reproductive life. The worms can live for many years in the vasculature of their hosts. How the parasites surmount host immune defenses and negotiate hemostasis is poorly understood. We are interested in understanding how schistosomes interact with and modify their environment so as to overcome immune and hemostatic challenges to promote their survival [2–4]. To better understand these capabilities, in this work we examine the impact of adult Schistosoma mansoni on the human serum proteome.
2. Materials and Methods
2.1. Parasites and Mice
Schistosome-infected snails were provided by the Schistosome Research Reagent Resource Center for distribution by BEI Resources, NIAID, NIH: Schistosoma mansoni, strain NMRI exposed Biomphalaria glabrata snails, strain NMRI, NR-21962. Cercariae were obtained from infected snails and isolated parasite bodies were prepared. Parasites were cultured in complete DMEM/F12 medium supplemented with 10% heat-inactivated fetal bovine serum, 200 U/ml penicillin and 200 μg/ml streptomycin, 0.2 μM Triiodo-L-thyronine, 1.0 μM serotonin and 8 μg/ml human insulin and were maintained at 37 °C, in an atmosphere of 5% CO2. Adult male and female parasites were recovered by perfusion from Swiss Webster mice that were infected with 125 cercariae, 7 weeks previously. All protocols involving animals were approved by the Institutional Animal Care and Use Committees (IACUC) of Tufts University under protocol G2015-113. All animals were fed, housed and handled in strict agreement with the recommendations of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, the Animal Welfare Act and guidelines established by the American Veterinary Medical Association Panel on Euthanasia.
2.2. Treatment of human serum with schistosome parasites
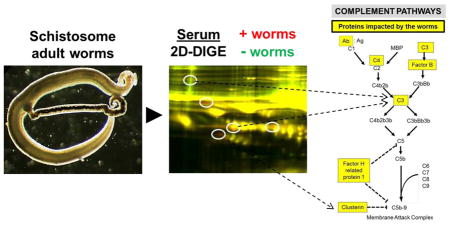
Adult parasites were recovered from culture medium and extensively washed with Hanks Balanced Salt Solution (HBSS). Approximately 50 pairs of adult worms were incubated in 150 μl of normal human serum (Euro Diagnostica) for one hour at 37°C. As a control, normal human serum alone was likewise incubated at 37°C for 1 h. Thereafter parasites were removed and serum samples were submitted to Applied Biomics Inc. (Hayward, CA) for two-dimensional Differential In-Gel Electrophoresis (2D-DIGE) and proteomic analysis. Worms appeared unimpaired by this treatment; after incubation in serum they were returned to culture (in medium, as above) where they exhibited no overtly diminished viability.
2.3. Two-Dimensional Differential In-Gel Electrophoresis (2D-DIGE)
The protein concentration in each serum sample was determined using the bicinchoninic acid (BCA) assay. At Applied Biomics Inc., serum samples were labeled with CyDye fluors: Cy2 (for the control (no parasite) sample) or Cy3 (for the serum sample incubated with parasites for 1 h). Labeled samples were then mixed with rehydration buffer and proteins were resolved first by isoelectric focusing and then (in the second dimension) by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The resulting 2-D gel was scanned using a Typhoon image scanner to reveal each of the CyDye signals (Cy2 and Cy3). The scan image was analyzed using ImageQuant software and comparative analysis of all spots was performed using DeCyder “in-gel” analysis software (Applied Biomics) to generate protein level ratios.
To determine the identities of the selected proteins within the gel, spots of interest were picked using an Ettan Spot Picker. Excised spots were subjected to in-gel trypsin digestion followed by Mass Spectrometry analysis at Applied Biomics, Inc to identify each protein by comparison with human, schistosome and murine databases. The schistosome database was included to look for the presence of parasite proteins released during the experiment and the murine database to look for potential carryover of mouse proteins, since the worms were recovered from infected mice.
2.4. Western blot analysis
Normal human serum (Euro Diagnostica) samples were taken at the start of an experiment (time 0) or following 1h incubation at 37°C in the presence or absence of parasites (~50 pairs in 150 μl). Aliquots of these sera (0.5 μl) were subjected to SDS-PAGE, blotted to polyvinylidene difluoride (PVDF) membrane and blocked using 5% skim milk in phosphate buffered saline (PBS) containing 0.1% Tween 20 (PBST) for 1 h at room temperature. The membrane was then probed overnight at 4°C with one of the following polyclonal antisera: rabbit anti-human C3 (Abcam, ab117244) or goat anti-human C4 (AbD Serotec (BioRad) AHP1753) or goat anti-human factor B (Quidel A311) antiserum, all at 1:1000 dilution. Blots were washed 3 times with PBST and incubated with either donkey anti-rabbit IgG or donkey anti-goat IgG (as appropriate), conjugated to horse radish peroxidase (GE Healthcare, Pittsburgh, PA USA), at 1:5,000 dilution for 1 hour at 37°C. Following a further 3 washes in PBST, protein bands were visualized using ECL Western Blotting Detection Reagents (GE Healthcare) in a ChemiDoc Touch Imaging System (BioRad).
3. Results
3.1. Changes in the human serum proteome
Normal human serum that contained adult schistosomes for 1 h at 37°C displays a different proteomic profile, as assessed by 2D-DIGE, compared to serum incubated without schistosomes. An image of the 2D electrophoresis pattern of both sera is shown in figure 1 where spots colored green depict proteins that are in greater relative abundance in control (no schistosome) serum while spots colored red are in greater abundance in the serum sample that contained worms for 1 hour. The vast majority of proteins display no detectable difference between the two samples and are colored yellow in figure 1.
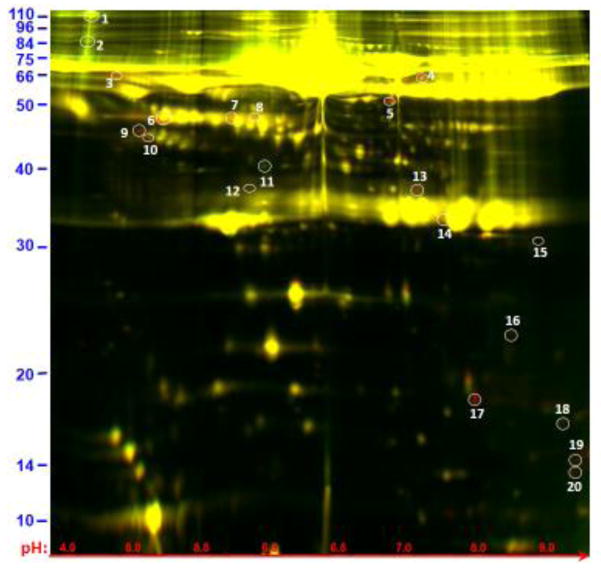
Figure 1.
Image showing 2D gel resolution of samples obtained following normal human serum incubation for 1 hour at 37°C either in the presence of schistosome parasites (red) or without parasites (green). Most proteins are the same in both samples and appear yellow. The protein spots selected for analysis here are circled and numbered. Three exhibit greater relative abundance in the absence of parasites (green spots 1, 2, 11); the remaining proteins are more abundant in the presence of parasites. Spots 1–20 were recovered and analyzed using mass spectroscopy and their identities are listed in table 1. The numbers in blue (left) represent molecular mass (kDa) and in red (bottom) represent pH value.
Examination using ImageQuant software and comparative analysis of all spots using DeCyder software resulted in the identification of several proteins that exhibited notably different abundance in the two samples. Figure 2 depicts an example of gel spot analysis using the DeCyder software in which the identified spot is barely detectable in the control sample (left panels) but is strikingly revealed in the presence of the worms (right panels). We focused on 20 protein spots that exhibited a >1.5 (absolute value) fold-change between samples. The twenty proteins circled in figure 1 were recovered and mass spectroscopy analysis was conducted on their trypsin digests to reveal their identities. All proteins were of human origin; no schistosome proteins were detected. The twenty identified proteins are listed in table 1. Three of the twenty are found in greater abundance in the control sample and these are numbered 1, 2 and 11. The remaining proteins are detected in greater abundance in the serum sample that contained schistosomes.
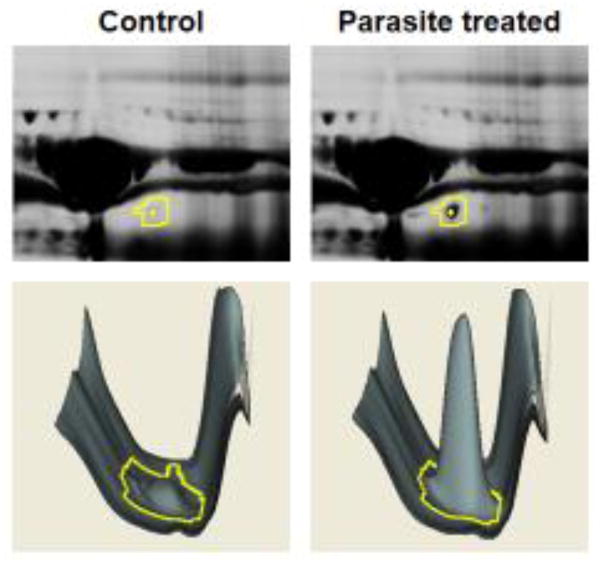
Figure 2.
Example of gel spot analysis by DeCyder software. Top, magnified view of a section of the 2D-DIGE gel with spot number 4 indicated (yellow boundary). This spot is identified as complement factor Bb and is more abundant in the parasite-treated serum sample (upper right) compared with control serum (upper left). Bottom, DeCyder representation of spot 4 (yellow boundary) and surrounding proteins. The software calculates spot peak volume to measure comparative abundance.
Table 1.
Identities of the protein spots 1–20 depicted in figure 1, representing major differences in the normal human serum proteome following exposure to adult schistosomes for 1 hour at 37°C versus control. Yellow highlights proteins known or implicated as being involved in the complement system. The theoretical molecular weight (MW) of each protein is listed as is the observed MW (kDa); pI, theoretical isoelectric point. The Accession Designation for each identified protein is given. The protein “Identification Score” is calculated by Mascot software and the value in parentheses, the “Peptide Count”, indicates the number of matching peptides identified by mass spectrometry in each case. The Protein Score Confidence Interval (CI) is listed (%); scores above 95% are significant, a lower score may still represent the correct identification, but requires corroboration. The Ratio represents the difference in spot volume between schistosome-exposed serum (S) and control serum (C) as determined by DeCyder software (see Figure 2 for an example).
| Spot No. | Protein Name | MW Theoretical | MW Observed | Accession Designation | pI | Identification Score (Peptide Count) | Protein Score (CI %) | Ratio (S/C) |
|---|---|---|---|---|---|---|---|---|
| 1 | Alpha-2-macroglobulin | 163.2 | >110 | A2MG_HUMAN | 6.03 | 439 (38) | 100 | −1.54 |
| 2 | Complement C3 (α chain) | 113.0 | 85 | CO3_HUMAN | 5.55 | 456 (36) | 100 | −1.73 |
| 3 | Alpha-1-antichymotrypsin | 47.6 | 64 | AACT_HUMAN | 5.33 | 261 (17) | 100 | 1.68 |
| 4 | Complement factor B (b fragment) | 57.0 | 64 | CFAB_HUMAN | 7.27 | 589 (25) | 100 | 4.5 |
| 5 | Protein DDX26B | 96.6 | 50 | DX26B_HUMAN | 8.89 | 47 (8) | 62 | 2.39 |
| 6 | Complement C3 (α′2 fragment) | 39.5 | 47 | CO3_HUMAN | 4.79 | 611 (25) | 100 | 2.7 |
| 7 | Actin, cytoplasmic 2 | 41.8 | 47 | ACTG_HUMAN | 5.31 | 299 (9) | 100 | 1.79 |
| 8 | Actin, cytoplasmic 2 | 41.8 | 47 | ACTG_HUMAN | 5.31 | 243 (9) | 100 | 1.67 |
| 9 | Clusterin | 52.5 | 46 | CLUS_HUMAN | 5.89 | 116 (11) | 100 | 2.63 |
| 10 | Clusterin | 52.5 | 45 | CLUS_HUMAN | 5.89 | 101 (12) | 100 | 2.15 |
| 11 | Complement factor H-related protein 1 | 30.6 | 40 | FHR2_HUMAN | 6.00 | 73 (6) | 100 | −1.92 |
| 12 | Complement C4 (α3 fragment) | 27 | 36 | CO4B_HUMAN | 6.89 | 196 (16) | 100 | 1.69 |
| 13 | Complement C3 (α′3 fragment) | 24 | 35 | CO3_HUMAN | 6.89 | 437 (22) | 100 | 1.81 |
| 14 | Ig kappa chain C region | 11.6 | 32 | IGKC_HUMAN | 5.58 | 338 (5) | 100 | 1.54 |
| 15 | Serum albumin | 69.3 | 31 | ALBU_HUMAN | 5.92 | 364 (16) | 100 | 2.13 |
| 16 | Serum amyloid A-4 protein | 14.7 | 23 | SAA4_HUMAN | 9.17 | 99 (7) | 100 | 4.61 |
| 17 | Hemoglobin subunit beta | 15.98 | 17 | HBB_HUMAN | 6.75 | 688 (13) | 100 | 19.96 |
| 18 | Serum amyloid A-4 protein | 14.74 | 15.5 | SAA4_HUMAN | 9.17 | 193 (7) | 100 | 1.54 |
| 19 | Complement C3 (α fragment) | 9 | 14 | CO3_HUMAN | 9.69 | 161 (13) | 100 | 3.63 |
| 20 | Complement C3 (α fragment) | 9 | 13 | CO3_HUMAN | 9.69 | 227 (15) | 100 | 4.74 |
The most striking finding from this analysis is that most of the analyzed proteins (eleven of twenty) are associated with, or are implicated as having a role in, the complement system. These proteins are indicated in table 1 by yellow shading. These include two of the three proteins identified as being more abundant in the control sample: complement component C3 (spot 2) and complement factor H related protein 1 (spot 11). The remaining complement-associated proteins are more abundant in the serum sample that contained worms. These proteins have been identified as fragments of complement component C3 (spots 6, 13, 19 and 20), complement component C4 (spot 12), complement factor B (spot 4), clusterin (spots 9 and 10) and the C region of Immunoglobulin (Ig) kappa chain (spot 14).
Regarding complement component C3: spot 2 is identified as the C3 α chain; Spot 6 is the α′2 fragment of this protein and spot 13 is the α′3 fragment. Spots 19 and 20 are both identified as the small “a” fragment of C3α. The molecular basis for the differences in size and charge of spot 19 versus spot 20 (both C3a) are not known.
Spot 5 is identified as the b chain of complement factor B and spot 14 is the α3 fragment of complement component C4α chain. Two forms of clusterin differing slightly in molecular weight and charge are identified as spots 10 and 11.
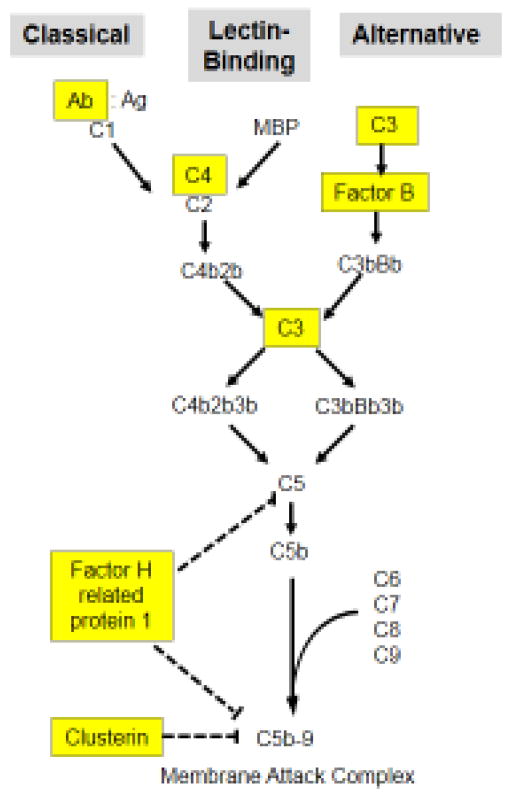
Figure 3 is a simplified schematic illustrating the three pathways of complement – the classical pathway (left), the lectin binding pathway (middle) and the alternative pathway (right). Highlighted in yellow in boxes are complement serum components identified in this work as being impacted by schistosomes.
Figure 3.
Depiction of the major protein components comprising the three complement pathways, the classical (left), lectin binding (middle) and alternative (right) pathways. Highlighted in yellow boxes are proteins involved in these pathways that were identified in this study as being impinged upon by schistosomes. C1–C9: complement proteins 1–9; C2b-C5b: large fragments of complement proteins 2–5; MBP, mannose binding protein; Ab:Ag, antibody:antigen complex. Dashed lines indicate points of pathway inhibition.
The worms exert additional changes on the human serum proteome beyond their impact on complement cascades. Of the non-complement proteins identified as being impinged upon by schistosomes, one (spot 1, alpha 2 macroglobulin) is found in greater abundance in the sample lacking schistosomes. The remaining proteins are found in greater abundance in the sample containing worms. These are: alpha 1 anti-chymotrypsin (spot 3); two forms of actin cytoplasmic 2 that differ slightly in their isoelectric points (spots 7 and 8); two forms of serum amyloid A-4 protein (spots 16 and 18) that differ markedly in both molecular weight and charge. Other serum proteins impacted by the presence of schistosomes include protein DDX26B (spot 5), hemoglobin subunit B (spot 17) and serum albumin (spot 15). While the size of serum albumin is ~69 kDa, spot 16 runs at ~31kDa, peptide identification indicates that the ~31kDa fragment derives from the carboxyl end of the protein.
3.2. Western blot analysis of selected complement proteins
To test the validity of our proteomic data, samples of serum that either contained schistosomes for 1 hour or did not contain worms were resolved by SDS-PAGE and blotted to PVDF membrane. As an additional control, samples of serum taken at the zero time point were similarly treated. Blots were probed with either anti-human complement component C3 antiserum (results shown in Figure 4) or anti-human complement component C4 antiserum (figure 5) or anti-human factor B antiserum (figure 6). In agreement with proteomic data, in each western blot analyzed the presence of the worms for 1 hour (center “+” lanes) led to the generation of additional bands not seen in the absence of worms (right “−“ lanes) or in control serum collected at the start of the experiment (0 hour lanes, left).
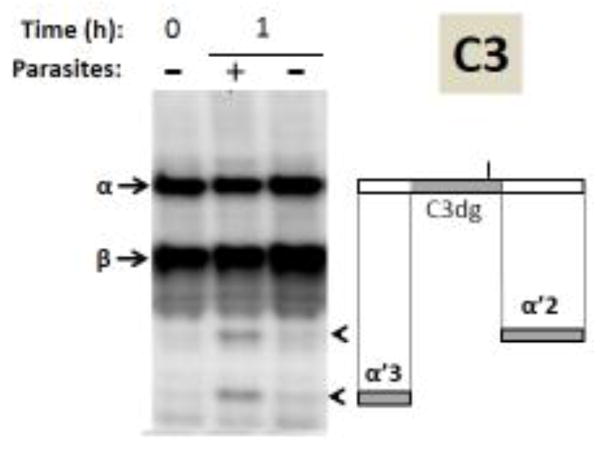
Figure 4.
Analysis of complement protein C3 by western blotting. Samples obtained following serum incubation for 1 hour at 37°C either in the presence of schistosome parasites (+) or without parasites (−) taken at 0 or 1 hour (as indicated) were resolved by SDS-PAGE, blotted to PVDF and probed with anti-human C3 antiserum. The arrows indicate the α and β chains of C3 and the arrowheads indicate the positions of migration of the C3α breakdown products α′2 and α′3. At right is a depiction of the C3α chain (large rectangle) and beneath this the α′2 and α′3 fragments are indicated (small grey rectangles). The central region of C3α represents the C3dg fragment (colored grey in upper rectangle) and the short vertical bar above C3dg indicates the position of molecular attachment of C3 to pathogens once complement is engaged.
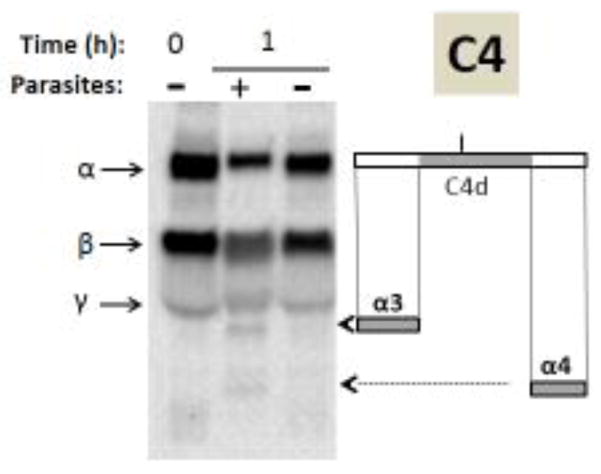
Figure 5.
Analysis of complement protein C4 by western blotting. Samples obtained following human serum incubation for 1 hour at 37°C either in the presence of schistosome parasites (+) or without parasites (−) taken at 0 or 1 hour (as indicated) were resolved by SDS-PAGE, blotted to PVDF and probed with anti-human C4 antiserum. The arrows indicate the α, β and γ chains of C4 and the arrowheads indicate the positions of migration of the C4α breakdown products α3 and α4. At right is a depiction of the C4α chain (large rectangle) and beneath this the α3 and α4 fragments are indicated (small grey rectangles). The central region of C4α represents the C4d fragment (colored grey in upper rectangle) and the short vertical bar above C4d indicates the position of molecular attachment of C4 to pathogens once complement is engaged.
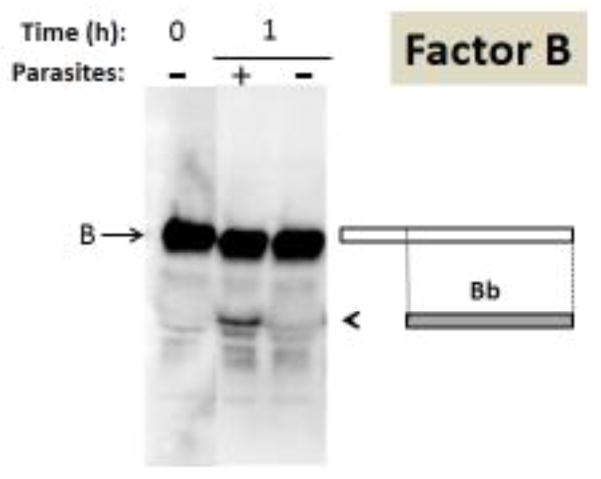
Figure 6.
Analysis of complement protein factor B by western blotting. Samples obtained following human serum incubation for 1 hour at 37°C either in the presence of schistosome parasites (+) or without parasites (−) taken at 0 or 1 hour (as indicated) were resolved by SDS-PAGE, blotted to PVDF and probed with anti-human factor B antiserum. The arrow indicates factor B and the arrowhead indicates the position of migration of the large fragment of factor B designated Bb. At right is a depiction of factor B (large rectangle) and beneath this the Bb fragment is indicated (smaller grey rectangle).
In the case of C3 (figure 4), both the α chain and the β chain are seen in all lanes (arrows). The molecular weights of the fragments generated in the presence of the worms (“+” lane) are consistent with their being the following fragments of the C3α chain: α′2 (43kDa) and α′3 (27kDa) (arrowheads, figure 4). The rectangles to the right of the blot shown in Figure 4 represent the intact C3α protein (top) and that the α′2 fragment derives from the carboxyl terminus of C3α (grey rectangle, right) while the α′3 fragment derives from the amino terminus (grey rectangle, left). The central portion of C3α - the 41kDa C3dg fragment - is depicted by the grey rectangle within full length C3α (top, figure 4 right). This fragment is not detected on the blot. This C3 western blot analysis confirms the proteomic data presented above: the presence of worms leads to the generation of C3 protein fragments not seen in their absence.
In the case of our analysis of C4 by western blotting, the α, β and γ C4 chains are detected in all lanes (arrows, figure 5). As for C3, here too it is apparent that the presence of schistosomes leads to the generation of two additional complement protein fragments not seen in the absence of the worms (arrowheads, figure 5). The molecular weights of these two proteins are consistent with their being the α3 (27kDa) and α4 (16kDa) fragments of C4α. The rectangles to the right of the blot shown in Figure 5 represent the intact C4α protein (top) and that the α3 fragment derives from the amino terminus of C4α (grey rectangle, left) while the α4 fragment derives from the carboxyl terminus (grey rectangle, right). The central portion of C4α - the 45kDa C4d fragment - is depicted by the grey rectangle within full length C4α (top, Figure 5 right). This fragment is not detected on the blot. Again, western blot analysis confirms the proteomic data in that the α3 fragment of C4 is detected in serum containing worms by both approaches. The α4 fragment, detected by western blotting, was not detected in our limited serum proteomic analysis. Thus, as for C3, the presence of schistosomes leads also to the generation of C4 protein fragments not seen in the absence of worms.
Finally, in the case of factor B (whose position of migration by SDS-PAGE is indicated by an arrow in figure 6), a fragment is clearly detected in the serum sample that contained worms (“+” lane) whose molecular weight is consistent with it being Bb (running at 65kDa, arrowhead, figure 6), the large enzymatically active fragment of complement factor B. The rectangles to the right of the blot shown in figure 6 represent the intact factor B protein (white rectangle, top) and that the Bb fragment derives from the carboxyl end of factor B (grey rectangle, right). Here again, western blot analysis confirms the proteomic data in that the Bb fragment of factor B is detected in serum that contained worms by both approaches. As for C3 and C4: the presence of schistosomes leads to the generation of a new protein fragment (here Bb) not seen in the absence of worms.
4. Discussion
In this work we examine the impact of schistosomes on the human serum proteome. It is clear that in a short time the parasites exert a considerable effect on the composition of serum. Using 2D-DIGE, we identified twenty protein spots that differed markedly in serum that contained worms compared to serum lacking worms. Our most striking finding is that many of the spots contained proteins that play a role in the complement system (and these are highlighted in Figure 3) [5, 6]. Of particular interest is that five of the twenty analyzed spots contained key complement component C3 or fragments of this protein. Notably less of the intact C3 protein is detected in the serum sample containing worms while multiple C3 fragments are revealed in serum containing worms. These fragments are C3a, C3α′2 and C3α′3. These data show that the worms do not appear to avoid interaction with complement; rather they engage C3. C3 activation begins with its cleavage into an amino terminal C3a fragment and a larger C3b component [5]. The detection of the C3a fragment in serum containing worms is most easily explained by assuming that C3 activation occurs readily in the presence of the worms. The resulting C3b fragment would be expected to attach to the pathogen and drive further complement activation. Indeed, some studies have detected the C3 protein associated with schistosomes by immunofluorescence localization [7, 8]. Efficient complement activation in this manner should lead to damage being visited upon the parasites and yet schistosomes seem refractory to the adverse impact of complement. Our detection of not just the C3a fragment but the C3α′2 and C3α′3 fragments too in the presence of the worms provides one explanation for this apparent conundrum. These fragments are detected not only by proteomics but by western blot analysis and suggest that rapid association of C3b with the parasites is paired with the rapid degradation of the C3α chain (as revealed by the appearance of the C3α′2 and C3α′3 fragments) thereby preventing functional, full length C3b from amplifying complement pathway progression to effectively debilitate the worms. This scheme suggests that the central portion of C3α – a fragment designated C3dg (and illustrated in figure 3) largely remains bound to the worms and this binding may be one reason we do not detect a fragment of its size in our serum western blots. In support of this, proteomic analysis of the S. mansoni tegument confirms the presence of the C3dg fragment associated with the worm [9, 10].
In a similar fashion to C3, C4 may also be engaged by schistosomes but be rapidly degraded. This is suggested by the prompt appearance of C4 fragments in serum containing schistosomes. The C4 α3 fragment is detected both by proteomics and by western blotting and the C4 α4 fragment is additionally detected by western blotting. In this scheme we assume that the C4α chain attaches to schistosomes; subsequent cleavage of C4α yielding the α3 and α4 fragments is predicted to leave the central C4α fragment (designated C4d and illustrated in Figure 5) attached to the worms. In support of this, proteomic analysis of the S. mansoni tegument shows the presence of the C4d fragment associated with the parasites [10]. This rapid breakdown of C4 in the presence of the worms (just like the breakdown of C3) would impede the generation of efficient schistosome-damaging complement effector functions.
The fragments of C3 and C4 described here can be generated by the serum protease factor I and we hypothesize that schistosomes recruit this host protease so as to degrade C3 and C4 and generate the fragments noted. However, we cannot rule out the possibility that the worms themselves possess a protease that acts like factor I to produce the fragments reported here.
Further evidence that schistosomes engage complement arises by the detection of the large fragment of complement protein factor B (called Bb) in serum containing worms by both proteomics and western blotting analysis. This is indicative of alternative pathway activation and, unlike the case for proteins C3 and C4, no factor B degradation product is detected in serum that contained worms. Rapid activation of factor B in the presence of the worms would be expected to work against the parasites but perhaps the concurrent rapid degradation of C3 (which, in conjunction with factor B, plays a key role in initiating and driving the alternative complement pathway, see Figure 3 [5, 6]) negates the impact of rapid factor B activation. For C3, C4 and factor B the changes detected by proteomics were confirmed by follow-up western blotting analysis.
Other complement–related proteins impacted by schistosomes include the complement-activating Ig kappa chain whose molecular weight is 12 kDa but which appears here (perhaps in a multimeric form) at ~32 kDa. In addition, we detect two forms of the A chain of clusterin as being more abundant in serum that contained worms compared to control serum. Among other things, clusterin has been shown to be a potent inhibitor of complement membrane attack complex assembly [11] and it may be that schistosomes engage clusterin towards this end. Similarly, factor H–related protein 1 – whose level decreases in serum in the presence of the worms - has been reported to inhibit both complement C5 convertase activity as well as membrane attack complex formation [12]. Further work will be required to uncover precisely if/how schistosomes employ these proteins to mediate the suggested complement inhibitory effects.
This proteomic work indicates new molecular mechanisms that may be employed by schistosomes to subvert complement. The points in the complement cascades at which the various proteins identified in this work might act are highlighted in Figure 3. Additional schistosome anti-complement strategies are noted in the literature [13] including an ability of the worms to acquire host complement regulatory proteins such as delay accelerating factor (DAF) or complement receptor 1-related protein (Crry) [14–16]. These could act to disrupt complement complex formation (e.g. impeding the generation of the C3 convertases C4b2b3b and C3bBb3b) and thereby obstruct complement cascade progression. The host protein CD59 is able to impede membrane attach complex formation and the schistosome tegument contains CD59 homologs which could play a similar role [17], although recent experiments do not support this suggestion [18].
It should be highlighted that the molecular nature of the interaction between host proteins and parasites documented here is, for the most part, unknown. In general, we do not know how (or if) the interaction between the worms and serum molecules alters target protein activity. For instance, alpha-2-macroglobulin (α2M) is found in greater abundance in serum lacking worms suggesting that the worms act upon this enzyme. The nature of the interaction and how it might assist the worms is unclear. Alpha-2-macroglobulin (α2M) is a member of the serine protease inhibitor (serpin) family that can inhibit the blood clot-degrading enzyme plasmin. It could clearly benefit schistosomes to impede this so as to permit unrestrained plasmin activity and promote blood clot breakdown, and allow greater mobility, in their vicinity. On the other hand, α2M can also inhibit the blood clot-promoting enzyme thrombin, an activity that schistosomes would likely not wish to impede. It is worth pointing out that α2M also acts as a chaperone; the protein plays an important role in extracellular proteostasis by sequestering misfolded proteins that are found in “stressed” extracellular fluids [19]. Clusterin too can act as an extracellular chaperone [20] and, as revealed in this work, schistosomes also impact this protein. These observations suggest a heretofore unrecognized impact of intravascular schistosomes as stressors of blood, damaging proteins that are then engaged by extracellular chaperones like clusterin and α2M.
Beyond α2M, additional serum proteins identified here may have a bearing on what must be a major challenge for schistosomes - dealing with the host’s blood clotting apparatus [21, 22]. The worms have been suggested to possess several molecular mechanisms to impede blood clot formation around them [23]. Here we document an impact of schistosomes on actin, cytoplasmic 2 which may have relevance regarding hemostasis control given that this protein is an important component of blood microparticles that engage in platelet aggregation [24]. Serum albumin is also impacted by schistosomes and albumin too has been reported to be able to modulate platelet activation and aggregation [25, 26].
Beyond blood clotting concerns, schistosomes must contend with the gamut of host immune effectors (and not just complement). Some of the serum proteins that schistosomes interact with as revealed here may be relevant in this regard. For instance, serum amyloid A proteins are a family of plasma apolipoproteins that have, among other things, been associated with the recruitment of immune cells to sites of inflammation [27]. Here we show that schistosomes impact one member of this family: serum amyloid A-4 protein. In vivo this action may help deflect the migration of immune cells towards the worms and it is clear that in the vasculature of infected mice, the worms do appear unmolested by host immune effector cells [28]. We similarly speculate that since the serpin alpha 1-antichymotrypsin (ACT) can act as an immunoregulator [29, 30], this may be one reason for schistosome interaction with the protein. Finally, protein DDX26B (DEAD/H (Asp-Glu-Ala-Asp/His) box polypeptide 26B, also known as INTS6L (integrator complex subunit 6 like)) is also identified as altered in serum that contained schistosomes. While the molecular function of this protein has not been determined, it is known to possess a ~200 amino acid Von Willebrand factor type A amino terminal domain. This domain was originally described in the blood coagulation protein von Willebrand factor but is now known to be widespread and to be involved in a variety of cell functions including immune defense [31]. However, the identity of this protein needs to be confirmed using other methods since the protein confidence index for this spot was relatively low, 62%.
The impact of schistosomes on hemoglobin β chain shown here is of interest since this protein has been reported in some proteomic studies to be associated with the schistosome surface [10] and since hemoglobin β-derived peptides can play roles in regulating blood pressure, pain and inflammation [32] – all subjects of relevance to schistosomes.
One limitation of this proteomic study is its focus on major differences between the samples; less obvious, though important, differences may have been overlooked. In addition, even sizable differences may have gone unrecognized due to the fact that highly abundant serum proteins that are unchanged in the presence of schistosomes may mask their detection.
Finally, whether all the serum proteomic changes documented here point to the action of evolutionary selective pressures acting upon schistosomes to target the proteins identified (as we suggest) or are mere bystander effects with little or no biological relevance remains to be determined.
Highlights.
Adult Schistosoma mansoni, incubated in human serum for 1h, change the serum proteome.
Comparative 2D-DIGE and mass spectroscopy were used to identify 20 altered proteins.
Most (11/20) are associated with the complement system.
The data suggest that schistosomes rapidly degrade selected complement proteins.
Roles for other altered proteins in hemostasis and immune evasion are suggested.
Acknowledgments
This work was funded with support from NIH-NIAID grant AI056273 and NIH training grant T35 OD 010963. Infected snails were provided by BRI via the NIAID schistosomiasis resource center under NIH-NIAID Contract No. HHSN272201000005I.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383(9936):2253–64. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhardwaj R, Skelly PJ. Purinergic signaling and immune modulation at the schistosome surface? Trends Parasitol. 2009;25(6):256–60. doi: 10.1016/j.pt.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Da’dara AA, Bhardwaj R, Ali YB, Skelly PJ. Schistosome tegumental ecto-apyrase (SmATPDase1) degrades exogenous pro-inflammatory and pro-thrombotic nucleotides. PeerJ. 2014;2:e316. doi: 10.7717/peerj.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Figueiredo BC, Da’dara AA, Oliveira SC, Skelly PJ. Schistosomes Enhance Plasminogen Activation: The Role of Tegumental Enolase. PLoS Pathog. 2015;11(12):e1005335. doi: 10.1371/journal.ppat.1005335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785–97. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarma JV, Ward PA. The complement system. Cell Tissue Res. 2011;343(1):227–35. doi: 10.1007/s00441-010-1034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruppel A, Rother U, Vongerichten H, Diesfeld HJ. Schistosoma mansoni: complement activation in human and rodent sera by living parasites of various developmental stages. Parasitology. 1983;87(Pt 1):75–86. doi: 10.1017/s0031182000052434. [DOI] [PubMed] [Google Scholar]
- 8.Rasmussen KR, Kemp WM. Schistosoma mansoni: interactions of adult parasites with the complement system. Parasite Immunol. 1987;9(2):235–48. doi: 10.1111/j.1365-3024.1987.tb00503.x. [DOI] [PubMed] [Google Scholar]
- 9.Braschi S, Wilson RA. Proteins exposed at the adult schistosome surface revealed by biotinylation. Mol Cell Proteomics. 2006;5(2):347–56. doi: 10.1074/mcp.M500287-MCP200. [DOI] [PubMed] [Google Scholar]
- 10.Castro-Borges W, Dowle A, Curwen RS, Thomas-Oates J, Wilson RA. Enzymatic shaving of the tegument surface of live schistosomes for proteomic analysis: a rational approach to select vaccine candidates. PLoS Negl Trop Dis. 2011;5(3):e993. doi: 10.1371/journal.pntd.0000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDonald JF, Nelsestuen GL. Potent inhibition of terminal complement assembly by clusterin: characterization of its impact on C9 polymerization. Biochemistry. 1997;36(24):7464–73. doi: 10.1021/bi962895r. [DOI] [PubMed] [Google Scholar]
- 12.Heinen S, Hartmann A, Lauer N, Wiehl U, Dahse HM, Schirmer S, Gropp K, Enghardt T, Wallich R, Halbich S, Mihlan M, Schlotzer-Schrehardt U, Zipfel PF, Skerka C. Factor H-related protein 1 (CFHR-1) inhibits complement C5 convertase activity and terminal complex formation. Blood. 2009;114(12):2439–47. doi: 10.1182/blood-2009-02-205641. [DOI] [PubMed] [Google Scholar]
- 13.Skelly PJ. Intravascular schistosomes and complement. Trends Parasitol. 2004;20(8):370–4. doi: 10.1016/j.pt.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Pearce EJ, Hall BF, Sher A. Host-specific evasion of the alternative complement pathway by schistosomes correlates with the presence of a phospholipase C-sensitive surface molecule resembling human decay accelerating factor. J Immunol. 1990;144(7):2751–6. [PubMed] [Google Scholar]
- 15.Horta MF, Ramalho-Pinto FJ. Role of human decay-accelerating factor in the evasion of Schistosoma mansoni from the complement-mediated killing in vitro. J Exp Med. 1991;174(6):1399–406. doi: 10.1084/jem.174.6.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braschi S, Borges WC, Wilson RA. Proteomic analysis of the schistosome tegument and its surface membranes. Mem Inst Oswaldo Cruz. 2006;101(Suppl 1):205–12. doi: 10.1590/s0074-02762006000900032. [DOI] [PubMed] [Google Scholar]
- 17.Wilson RA. Proteomics at the schistosome-mammalian host interface: any prospects for diagnostics or vaccines? Parasitology. 2012;139(9):1178–94. doi: 10.1017/S0031182012000339. [DOI] [PubMed] [Google Scholar]
- 18.Farias LP, Krautz-Peterson G, Tararam CA, Araujo-Montoya BO, Fraga TR, Rofatto HK, Silva FP, Jr, Isaac L, Da’dara AA, Wilson RA, Shoemaker CB, Leite LC. On the three-finger protein domain fold and CD59-like proteins in Schistosoma mansoni. PLoS Negl Trop Dis. 2013;7(10):e2482. doi: 10.1371/journal.pntd.0002482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyatt AR, Zammit NW, Wilson MR. Acute phase proteins are major clients for the chaperone action of alpha(2)-macroglobulin in human plasma. Cell Stress Chaperones. 2013;18(2):161–70. doi: 10.1007/s12192-012-0365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wyatt AR, Wilson MR. Identification of human plasma proteins as major clients for the extracellular chaperone clusterin. J Biol Chem. 2010;285(6):3532–9. doi: 10.1074/jbc.M109.079566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Da’dara AA, Skelly PJ. Schistosomes versus platelets. Thromb Res. 2014;134(6):1176–81. doi: 10.1016/j.thromres.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 22.Da’dara AA, de Laforcade AM, Skelly PJ. The impact of schistosomes and schistosomiasis on murine blood coagulation and fibrinolysis as determined by thromboelastography (TEG) J Thromb Thrombolysis. 2016;41(4):671–7. doi: 10.1007/s11239-015-1298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mebius MM, van Genderen PJ, Urbanus RT, Tielens AG, de Groot PG, van Hellemond JJ. Interference with the host haemostatic system by schistosomes. PLoS Pathog. 2013;9(12):e1003781. doi: 10.1371/journal.ppat.1003781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cauwenberghs S, Feijge MA, Harper AG, Sage SO, Curvers J, Heemskerk JW. Shedding of procoagulant microparticles from unstimulated platelets by integrin-mediated destabilization of actin cytoskeleton. FEBS Lett. 2006;580(22):5313–20. doi: 10.1016/j.febslet.2006.08.082. [DOI] [PubMed] [Google Scholar]
- 25.Rubenstein DA, Yin W. Glycated albumin modulates platelet susceptibility to flow induced activation and aggregation. Platelets. 2009;20(3):206–15. doi: 10.1080/09537100902795492. [DOI] [PubMed] [Google Scholar]
- 26.Lam FW, Cruz MA, Leung HC, Parikh KS, Smith CW, Rumbaut RE. Histone induced platelet aggregation is inhibited by normal albumin. Thromb Res. 2013;132(1):69–76. doi: 10.1016/j.thromres.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 27.Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem. 1999;265(2):501–23. doi: 10.1046/j.1432-1327.1999.00657.x. [DOI] [PubMed] [Google Scholar]
- 28.Keating JH, Wilson RA, Skelly PJ. No overt cellular inflammation around intravascular schistosomes in vivo. J Parasitol. 2006;92(6):1365–9. doi: 10.1645/GE-864R.1. [DOI] [PubMed] [Google Scholar]
- 29.YM, Kurahashi K1, Nakamura K, Kato K, Katsunuma T. Alpha-1-antichymotrypsin (ACT) as an immune regulatory agent. Tokai J Exp Clin Med. 1988;133(6):37–44. [PubMed] [Google Scholar]
- 30.Kalsheker NA. Alpha 1-antichymotrypsin. Int J Biochem Cell Biol. 1996;28(9):961–4. doi: 10.1016/1357-2725(96)00032-5. [DOI] [PubMed] [Google Scholar]
- 31. [accessed 20 Sept 2016]; http://www.ncbi.nlm.nih.gov/gene/203522.
- 32.Zhao Q, Piot JM. Investigation of inhibition angiotensin-converting enzyme (ACE) activity and opioid activity of two hemorphins, LVV-hemorphin-5 and VV-hemorphin-5, isolated from a defined peptic hydrolysate of bovine hemoglobin. Neuropeptides. 1997;31(2):147–53. doi: 10.1016/s0143-4179(97)90084-6. [DOI] [PubMed] [Google Scholar]