ABSTRACT
Objective:
To evaluate the effects that passive cycling exercise, in combination with conventional physical therapy, have on peripheral muscle strength, duration of mechanical ventilation, and length of hospital stay in critically ill patients admitted to the ICU of a tertiary care university hospital.
Methods:
This was a randomized clinical trial involving 38 patients (≥ 18 years of age) on mechanical ventilation who were randomly divided into two groups: control (n = 16), receiving conventional physical therapy; and intervention (n = 22), receiving conventional physical therapy and engaging in passive cycling exercise five days per week. The mean age of the patients was 46.42 ± 16.25 years, and 23 were male. The outcomes studied were peripheral muscle strength, as measured by the Medical Research Council scale, duration of mechanical ventilation, and length of hospital stay.
Results:
There was a significant increase in peripheral muscle strength (baseline vs. final) in both groups (control: 40.81 ± 7.68 vs. 45.00 ± 6.89; and intervention: 38.73 ± 11.11 vs. 47.18 ± 8.75; p < 0.001 for both). However, the range of increase in strength was higher in the intervention group than in the control group (8.45 ± 5.20 vs. 4.18 ± 2.63; p = 0.005). There were no significant differences between the groups in terms of duration of mechanical ventilation or length of hospital stay.
Conclusions:
The results suggest that the performance of continuous passive mobilization on a cyclical basis helps to recover peripheral muscle strength in ICU patients. (ClinicalTrials.gov Identifier: NCT01769846 [http://www.clinicaltrials.gov/])
Descriptors: Physical therapy modalities, Intensive care units, Early ambulation, Muscle strength
RESUMO
Objetivo:
Avaliar os efeitos da realização de exercícios passivos com um cicloergômetro, associada à fisioterapia convencional, na força muscular periférica, no tempo de ventilação mecânica e no tempo de internação hospitalar em pacientes críticos internados em UTI de um hospital universitário terciário.
Métodos:
Ensaio clínico randomizado envolvendo 38 pacientes (idade > 18 anos) em ventilação mecânica e divididos aleatoriamente em grupo controle (n = 16), que realizou fisioterapia convencional, e grupo intervenção (n = 22) submetidos a fisioterapia convencional e exercícios passivos em cicloergômetro cinco vezes por semana. A média de idade dos pacientes foi de 46,42 ± 16,25 anos, e 23 eram homens. Os desfechos analisados foram força muscular periférica, mensurada pela escala Medical Research Council, tempo de ventilação mecânica e tempo de internação hospitalar.
Resultados:
Houve um aumento significativo da força muscular periférica (basal vs. final) tanto no grupo controle (40,81 ± 7,68 vs. 45,00 ± 6,89; p < 0,001) quanto no grupo intervenção (38,73 ± 11,11 vs. 47,18 ± 8,75; p < 0,001). Entretanto, a variação do aumento da força foi maior no grupo intervenção que no controle (8,45 ± 5,20 vs. 4,18 ± 2,63; p = 0,005). Não foram observadas diferenças significativas entre os grupos quanto ao tempo de ventilação mecânica e tempo de internação hospitalar.
Conclusões:
Os resultados sugerem que a realização de mobilização passiva contínua de forma cíclica auxilia na recuperação da força muscular periférica de pacientes internados em UTI. (ClinicalTrials.gov Identifier: NCT01769846 [http://www.clinicaltrials.gov/])
INTRODUCTION
Currently, as a result of increasing technological advances, critically ill patients have prolonged ICU stays, which predisposes to the incidence of complications arising from immobility. 1 Prolonged immobility is harmful, with rapid reduction in muscle mass and bone mineral density, as well as impairment in other body systems; these manifestations are evident within the first week of bed rest, 2 which can contribute to functional decline and reduced quality of life. 3 Among immobility-related complications are malnutrition, increased rates of nosocomial infection, 4 changes in sleep quality, 5 and prolonged hospital stay. 6
Development of generalized muscle weakness is a complication that affects 30% to 60% of ICU patients 6 and can persist for six months to as long as two years after ICU discharge, 7 , 8 consequently impacting physical functioning in such patients. 8 In addition, patients with reduced peripheral muscle strength have a longer duration of mechanical ventilation (MV). 9 However, these deleterious effects of immobility can be reversed or mitigated by physical therapy, 10 , 11 which contributes to decreased length of stay in the ICU and hospital. 12 Studies demonstrate that early mobilization of critically ill patients is considered a safe approach 13 that is aimed at preserving muscle mass and reducing muscle weakness after hospital discharge and that facilitates resumption of activities of daily living. 14 , 15 According to Hodgson et al., 16 early mobilization is defined as the intensification and early application (within the first 2 to 5 days of critical illness) of the physical therapy that is administered to critically ill patients. However, the major reported barrier to performing early mobilization continues to be sedation, which, despite being necessary in some cases, limits the work of the physical therapist in the functional recovery of patients. 17 , 18
In this context, passive mobilization is a strategy that the physical therapist has to prevent functional decline in critically ill patients. Although it has been widely used by professionals, 19 , 20 its effects in terms of the clinical recovery of patients have yet to be well established.
To date, cycle ergometers have been one of the most widely studied adjuvant tools in the treatment provided by physical therapists to ICU patients. 21 - 23 The use of early passive cycling exercise (< 72 h on MV) is safe and is associated with few hemodynamic changes even in more critically ill patients. 21 However, as already stated, little is known about the effects that passive mobilization (performed with a cycle ergometer or with a therapist) have on the clinical recovery of patients. Therefore, the objective of the present study was to evaluate the effects that passive cycling exercise, in combination with conventional physical therapy, have on the recovery of peripheral muscle strength, duration of MV, and length of hospital stay in ICU patients.
METHODS
This was a randomized clinical trial, with blinding of outcome assessment, conducted in the ICU of the Santa Maria University Hospital of the Federal University of Santa Maria, in the city of Santa Maria, Brazil, between January and July of 2015. The study was approved by the local research ethics committee (Protocol no. CAAE 07201712.8.0000.5346). All participants or their family members gave written informed consent before inclusion in the study.
This ICU has 16 beds, of which 10 are general intensive care beds and 6 are cardiac unit beds; it predominantly admits neurological, clinical, and surgical patients. Physical therapy is made available 12 h/day in the cardiac unit and 18 h/day in the general ICU. The physical therapist-to-patient ratio in the ICU as a whole is 1:8, whereas the nurse-to-patient ratio is 1:5 and the nursing technician-to-patient ratio is 1:2.
Our study included 49 patients (≥ 18 years of age; convenience sample) who were on MV; were maintained at a light level of sedation, as assessed by the Richmond Agitation-Sedation Scale 24 (score −2); and were hemodynamically stable. We excluded patients who were receiving palliative care, amputees, patients with leg fractures, and patients with neuromuscular disease, neurological disease, and/or ICU-acquired muscle weakness, as well as patients who were unable to use a cycle ergometer because of preexisting joint and/or musculoskeletal disorders.
The patients recruited for the study were assessed by clinical records, demographic information, clinical history, primary reason for ICU admission, and the Acute Physiology and Chronic Health Evaluation II (APACHE II) score. 25
Patients were allocated from a computer-generated table of random numbers, and a randomization sequence was generated using Random Number Generator Pro 2.00 software (Segobit; Issaquah, WA, USA). All participants received the intervention by two physical therapists. Blinded assessments were performed by a single physical therapist, who was also responsible for the randomization.
Peripheral muscle strength in arms and legs was measured by the score on the Medical Research Council (MRC) scale, 26 before and after the implementation of the study protocol, by a single, previously trained rater. Muscle strength was initially assessed on the first day the patient was cooperative and responsive (Richmond Agitation and Sedation Scale score = −1) and then on the last day of ICU stay.
The patients who met the inclusion criteria were allocated to the intervention group (IG) or the control group (CG). The CG patients received conventional physical therapy, whereas the IG patients received conventional physical therapy and engaged in passive exercise on a leg cycle ergometer (MOTOmed letto 2; RECK-Technik GmbH & Co.KG, Betzenweiler, Germany). This device offers the possibility of performing the exercise passively, in the supine position, even if the patient is under sedation. Therefore, 20-min sessions of passive cycling exercise at a fixed rate of 20 cycles/min were performed 5 days per week, until the last day of ICU stay. In order to ensure that true passive exercise was performed, the device screen, which allows the visualization/analysis of the training session and detects active movements, was constantly monitored during the protocol.
Conventional physical and respiratory therapy were provided by the ICU physical therapists twice daily, for approximately 30 min, 7 days per week. The protocol included vibrocompression maneuvers; lung hyperinflation by the mechanical ventilator; and tracheal aspiration, when necessary; as well as passive and active-assisted motor exercises for arms and legs, depending on the clinical course of patients.
In order to ensure the clinical stability of the patients during and after the protocol, cardiovascular parameters, such as SpO2, HR, mean arterial pressure, systolic blood pressure, and diastolic blood pressure, were continuously monitored noninvasively with a multiparameter monitor (DX 2022; Dixtal Biomédica, Manaus, Brazil).
The following parameters were used as the criteria for discontinuing the protocol: hemodynamic instability (mean arterial pressure < 60 or > 125 mmHg); SpO2 < 88%; HR > 130 bpm or < 40 bpm; and signs of respiratory distress.
The statistical analysis was performed with the IBM SPSS Statistics software package, version 20.0 (IBM Corporation, Armonk, NY, USA). Variables were tested for normality with the Shapiro-Wilk test. Continuous variables were expressed as mean and standard deviation or as median and interquartile range, whereas categorical variables were expressed as absolute and relative frequencies. The paired Student's t-test or the Wilcoxon test was used for intragroup comparisons between the pre- and post-implementation periods. The unpaired Student's t-test or the Mann-Whitney U test was used for intergroup comparisons. The level of statistical significance was set at p < 0.05.
RESULTS
In the study period, 58 patients were admitted to the adult ICU of the institution. Of those, 49 met the inclusion criteria and were randomized to the CG (n = 23) or the IG (n = 26). Subsequently, 7 and 4 patients in the CG and the IG, respectively, died. Therefore, the final sample consisted of 38 patients, of whom 16 were in the CG and 22 were in the IG (Figure 1). Table 1 shows the general characteristics of the sample. We found that the primary reason for ICU admission was respiratory-related. Duration of sedation, time to first treatment, and time to first muscle strength assessment were similar in the two groups. The median number of sessions (interquartile range) in the CG and the IG was 30.0 (26.5-53.0) and 36.0 (30.0-59.0), respectively. During the study period, there was no need to discontinue the protocol, nor were there any adverse events during or after its administration.
Figure 1. Flowchart of the study.
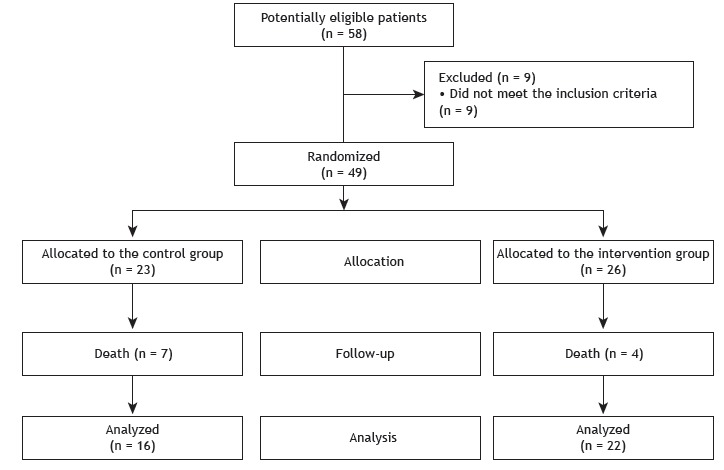
Table 1. Patient clinical and demographic characteristics.a .
| Variable | Control group (n = 16) | Intervention group (n = 22) |
|---|---|---|
| Age, years | 45.13 ± 18.91 | 44.64 ± 19.23 |
| Male gender, n (%) | 7 (43) | 16 (72) |
| Glasgow Coma Scale score | 13. 33 ± 2.91 | 14.21± 3.62 |
| APACHE II score | 18.12 ± 6.36 | 17.34 ± 6.72 |
| Duration of sedation, days | 4 (3-7) | 4 (2-7) |
| Primary reason for ICU admission, n (%)* | ||
| Neurological | 3 (18.75) | 2 (9.09) |
| Respiratory | 5 (31.25) | 6 (27.27) |
| Abdominal | 3 (18.75) | 3 (13.64) |
| Cardiac | 0 (0.00) | 4 (18.18) |
| Other | 5 (31.25) | 7 (31.82) |
| Time to first session, days | 2 (1-3) | 3 (2-5) |
| Time to first assessment by the MRC scale, days | 2.5 (2.5-2.5) | 2.5 (2.0-2.5) |
| Duration of the protocol, days | 15 (12-30) | 12 (7-25) |
APACHE II: Acute Physiology and Chronic Health Evaluation II; and MRC: Medical Research Council (scale). aValues expressed as mean ± SD or as median (interquartile range), except where otherwise indicated. *All comparisons were performed by using the Student's t-test, except where otherwise indicated (Mann-Whitney U test).
Table 2 presents the duration of MV, length of ICU stay, and length of hospital stay in the two groups studied. There were no significant differences between the groups in terms of length of ICU stay (p = 0.824), duration of MV (p = 0.715), or length of hospital stay (p = 0.794).
Table 2. Length of hospital stay, length of ICU stay, and duration of mechanical ventilation in the control and intervention groups.a .
| Variable | Control group | Intervention group | p |
|---|---|---|---|
| (n = 16) | (n = 22) | ||
| Length of hospital stay, days | 46 (25-75) | 38 (17-73) | 0.794* |
| Duration of mechanical ventilation, days | 15 (10-44) | 18 (8.5-37) | 0.824* |
| Length of ICU stay, days | 18 (12-35) | 22 (10-28) | 0.715** |
Values expressed as median (interquartile range). *Student's t-test. **Mann-Whitney U test.
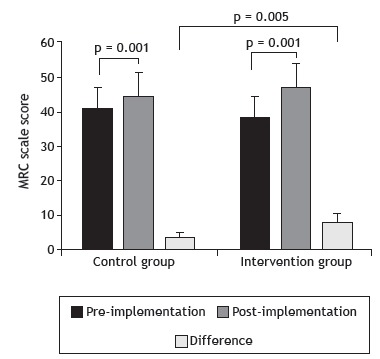
Figure 2 shows the MRC scale scores obtained before and after the implementation of the study protocol. There was a significant increase in peripheral muscle strength, as assessed by the MRC scale, in both groups (control: 40.81 ± 7.68 vs. 45.00 ± 6.89; and intervention: 38.73 ± 11.11 vs. 47.18 ± 8.75; p < 0.001 for both) after the implementation of the protocol. In the comparison of the differences shown by the groups between the pre- and post-implementation periods, the IG showed a significantly greater increase in the MRC scale scores than did the CG (8.45 ± 5.20 vs. 4.18 ± 2.63; p = 0.005).
Figure 2. Peripheral muscle strength, as measured by the Medical Research Council (MRC) scale, before and after the implementation of the study protocol. Student's t-test.
DISCUSSION
The present study is the first randomized clinical trial to analyze the effects that a passive cycling exercise program, in combination with conventional physical therapy, have on peripheral muscle strength, duration of MV, and length of hospital stay in critically ill ICU patients. An increase in peripheral muscle strength, as assessed by the MRC scale, occurred in both groups (CG and IG); however, the increase in strength was greater in the IG. There were no differences between the groups in terms of length of ICU stay, duration of MV, or length of hospital stay.
Our results corroborate those of a study by Dantas et al., 27 who also found no differences in duration of MV or length of hospital stay between a control group receiving conventional physical therapy and a group receiving early mobilization, which was based on a systematic mobility protocol but did not include leg cycling exercise.
Recently, Witcher et al., 28 in a retrospective study conducted in a neurological ICU, demonstrated that the implementation of an early mobilization program did not change duration of MV, length of ICU stay, or length of hospital stay, a finding that is similar to that of the present study. Schweickert et al., 14 in a pioneering, prospective randomized controlled trial, submitted 49 ICU patients on MV to an early active and passive mobilization program for 28 days. Those authors demonstrated that, at hospital discharge, the intervention group had achieved earlier functional independence, as well as having required 2.4 fewer days of ventilatory support, than had the control group, an outcome that did not occur in our study. However, it is important to emphasize that, in the study by Schweickert et al., 14 the control group did not receive any physical therapy intervention, whereas, in our study, the CG engaged in respiratory and motor exercises, and passive cycling exercise was the only difference between the CG and the IG.
A recent systematic review 29 concluded that there is evidence that ICU early mobilization programs are safe and improve the clinical outcomes of ICU patients. Our findings are consistent with those of this review, in which no significant adverse effects were found during physical therapy in the critically ill patients, and an increase in muscle strength was found in both groups.
Regarding our finding of a significantly greater increase in peripheral muscle strength in the IG than in the CG, it is important to mention a pioneering randomized study by Burtin et al., 23 who also found increased quadriceps strength after active and passive mobilization combined with early cycling exercise. However, that study differs from ours in the following aspects: eligibility criteria (those authors included patients who had been on MV for more than 7 days, whereas, in our study, we included patients with a mean duration of MV of 2.5 days); and isometric strength assessment of a single muscle group (quadriceps) using a dynamometer.
Patients exposed to a period of immobilization in the ICU are predisposed to morphological muscle changes, which can lead to reduced muscle strength and hypotrophy. 30 Koukourikos et al. 31 mentioned that muscle atrophy is one the most common and most important problems observed in critically ill patients, its prevention being the major focus in the ICU. The risk factors for this outcome include corticosteroid use, immobility, sepsis, and inadequate glycemic control. In this sense, those authors point to early mobilization as one of the strategies adopted to reduce the incidence of muscle weakness in the ICU. Increased peripheral muscle strength, which was demonstrated in our CG at ICU discharge, can be explained by the effectiveness of conventional physical therapy. One possible explanation for this finding is that, in our study, all patients received physical therapy during their ICU stay, which does not occur in centers in the United States. 12 The only difference between the groups in our study was the performance of passive cycling exercise. Regarding our finding of a significantly greater muscle strength increase in the IG, which was demonstrated by higher scores on the MRC scale, Llano-Diez et al. 32 and Renaud et al. 33 state that passive exercise has a positive effect on the ability to generate muscle force, because it mitigates the deleterious effects of immobility by maintaining the architecture and intrinsic contractility properties of the muscle.
There are some limitations to our study that should be mentioned. First, our assessment was restricted to peripheral muscle strength in the ICU. Therefore, we cannot state whether the difference in muscle strength observed between our two groups would remain at hospital discharge. Second, although the cycle ergometer was used for passive exercise, it was impossible to ensure the total absence of muscle contraction by the patient (at the end of sedation) during the cycle ergometer protocol. In fact, in approximately 20% of the sessions in the IG, the visual resource available on the cycle ergometer detected active contraction. In these cases, the physical therapist immediately instructed the patients neither to contract their muscles nor to perform any effort with their legs. Therefore, we believe that such contractions were small in magnitude and may have had minimal influence on our results. We also emphasize that, although this active activity was caught by the equipment, most of the session occurred passively. Finally, we did not use objective measures of muscle strength, such as handgrip strength testing, in our assessment. However, it is important to emphasize that previous studies have demonstrated a good correlation between objective measures of muscle strength and manual muscle testing. 34
In conclusion, early mobilization in the ICU, by implementing a passive cycling exercise protocol in patients on MV, can significantly increase peripheral muscle strength in such patients; however, it does not change duration of MV or length of hospital stay. Future studies of larger populations are needed to arrive at conclusions that are more definitive about this issue.
Footnotes
Study carried out in the Unidade de Terapia Intensiva Adulto do Hospital Universitário de Santa Maria - HUSM - Universidade Federal de Santa Maria - UFSM - Santa Maria (RS) Brasil.
Financial support: This study received financial support from the Research Grant Program for Recent PhD Graduates of the Centro de Ciências da Saúde da Universidade Federal de Santa Maria (CCS-UFSM, Federal University of Santa Maria Health Sciences Center).
REFERENCES
- 1.Lee H, Ko YJ, Suh GY, Yang JH, Park CM, Jeon K. Safety profile and feasibility of early physical therapy and mobility for critically ill patients in the medical intensive care unit Beginning experiences in Korea. J Crit Care. 2015;30(4):673–677. doi: 10.1016/j.jcrc.2015.04.012. https://doi.org/10.1016/j.jcrc.2015.04.012 [DOI] [PubMed] [Google Scholar]
- 2.Parry SM, Puthucheary ZA. The impact of extended bed rest on the musculoskeletal system in the critical care environment. Extrem Physiol Med. 2015;4:16–16. doi: 10.1186/s13728-015-0036-7. https://doi.org/10.1186/s13728-015-0036-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehrholz J, Pohl M, Kugler J, Burridge J, Mückel S, Elsner B. Physical rehabilitation for critical illness myopathy and neuropathy an abridged version of Cochrane Systematic Review. Eur J Phys Rehabil Med. 2015;51(5):655–661. https://doi.org/10.1002/14651858.cd010942.pub2 [PubMed] [Google Scholar]
- 4.Shpata V, Ohri I, Nurka T, Prendushi X. The prevalence and consequences of malnutrition risk in elderly Albanian intensive care unit patients. Clin Interv Aging. 2015;10:481–486. doi: 10.2147/CIA.S77042. https://doi.org/10.2147/CIA.S77042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sterniczuk R, Rusak B, Rockwood K. Sleep disturbance in older ICU patients. Clin Interv Aging. 2014;9:969–977. doi: 10.2147/CIA.S59927. https://doi.org/10.2147/CIA.S59927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puthucheary ZA, Hart N. Skeletal muscle mass and mortality - but what about functional outcome. Crit Care. 2014;18(1):110–110. doi: 10.1186/cc13729. https://doi.org/10.1186/cc13729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hermans G, Van den Berghe G. Clinical review intensive care unit acquired weakness. Crit Care. 2015;19:274–274. doi: 10.1186/s13054-015-0993-7. https://doi.org/10.1186/s13054-015-0993-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wieske L, Dettling-Ihnenfeldt DS, Verhamme C, Nollet F, van Schaik IN, Schulz MJ. Impact of ICU-acquired weakness on post-ICU physical functioning a follow-up study. Crit Care. 2015;19:196–196. doi: 10.1186/s13054-015-0937-2. https://doi.org/10.1186/s13054-015-0937-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JJ, Waak K, Grosse-Sundrup M, Xue F, Lee J, Chipman D. Global muscle strength but not grip strength predicts mortality and length of stay in a general population in a surgical intensive care unit. Phys Ther. 2012;92(12):1546–1555. doi: 10.2522/ptj.20110403. https://doi.org/10.2522/ptj.20110403 [DOI] [PubMed] [Google Scholar]
- 10.Kayambu G, Boots R, Paratz J. Physical therapy for the critically ill in the ICU a systematic review and meta-analysis. Crit Care Med. 2013;41(6):1543–1554. doi: 10.1097/CCM.0b013e31827ca637. https://doi.org/10.1097/CCM.0b013e31827ca637 [DOI] [PubMed] [Google Scholar]
- 11.Mehrholz J, Mückel S, Oehmichen F, Pohl M. First results about recovery of walking function in patients with intensive care unit-acquired muscle weakness from the General Weakness Syndrome Therapy (GymNAST) cohort study. BMJ Open. 2015;5(12):e008828. doi: 10.1136/bmjopen-2015-008828. https://doi.org/10.1136/bmjopen-2015-008828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris PE, Goad A, Thompson C, Taylor K, Harry B, Passmore L. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36(8):2238–2243. doi: 10.1097/CCM.0b013e318180b90e. https://doi.org/10.1097/CCM.0b013e318180b90e [DOI] [PubMed] [Google Scholar]
- 13.Lima NP, Silva GM, Park M, Pires-Neto RC. Mobility therapy and central or peripheral catheter-related adverse events in an ICU in Brazil. J Bras Pneumol. 2015;41(3):225–230. doi: 10.1590/S1806-37132015000004338. https://doi.org/10.1590/S1806-37132015000004338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL. Early physical and occupational therapy in mechanically ventilated, critically ill patients a randomized controlled trial. Lancet. 2009;373(9678):1874–1882. doi: 10.1016/S0140-6736(09)60658-9. https://doi.org/10.1016/S0140-6736(09)60658-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brahmbhatt N, Murugan R, Milbrandt EB. Early mobilization improves functional outcomes in critically ill patients. Crit Care. 2010;14(5):321–321. doi: 10.1186/cc9262. https://doi.org/10.1186/cc9262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodgson CL, Berney S, Harrold M, Saxena M, Bellomo R. Clinical review early patient mobilization in the ICU. Crit Care. 2013;17(1):207–207. doi: 10.1186/cc11820. https://doi.org/10.1186/cc11820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barber EA, Everard T, Holland AE, Tipping C, Bradlley SJ, Hodgson CL. Barriers and facilitators to early mobilisation in Intensive Care A qualitative study. Aust Crit Care. 2015;28(4):177–182. doi: 10.1016/j.aucc.2014.11.001. https://doi.org/10.1016/j.aucc.2014.11.001 [DOI] [PubMed] [Google Scholar]
- 18.Dafoe S, Chapman MJ, Edwards S, Stiller K. Overcoming barriers to the mobilisation of patients in an intensive care unit. Anaesth Intensive Care. 2015;43(6):719–727. doi: 10.1177/0310057X1504300609. [DOI] [PubMed] [Google Scholar]
- 19.Stockley RC, Hughes J, Morrison J, Rooney J. An investigation of the use of passive movements in intensive care by UK physiotherapists. Physiotherapy. 2010;96(3):228–233. doi: 10.1016/j.physio.2009.11.014. https://doi.org/10.1016/j.physio.2009.11.014 [DOI] [PubMed] [Google Scholar]
- 20.Stockley RC, Morrison J, Rooney J, Hughes J. Move it or lose it A survey of the aims of treatment when using passive movements in intensive care. Intensive Crit Care Nurs. 2012;28(2):82–87. doi: 10.1016/j.iccn.2011.10.010. https://doi.org/10.1016/j.iccn.2011.10.010 [DOI] [PubMed] [Google Scholar]
- 21.Camargo Pires-Neto R, Fogaça Kawaguchi YM, Sayuri Hirota A, Fu C, Tanaka C, Caruso P. Very early passive cycling exercise in mechanically ventilated critically ill patients physiological and safety aspects--a case series. PLoS One. 2013;8(9):e74182. doi: 10.1371/journal.pone.0074182. https://doi.org/10.1371/journal.pone.0074182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kho ME, Martin RA, Toonstra AL, Zanni JM, Manthey EC, Nelliot A. Feasibility and safety of in-bed cycling for physical rehabilitation in the intensive care unit. J Crit Care. 2015;30(6):1419–1419. doi: 10.1016/j.jcrc.2015.07.025. https://doi.org/10.1016/j.jcrc.2015.07.025 [DOI] [PubMed] [Google Scholar]
- 23.Burtin C, Clerckx B, Robbeets C, Ferdinande P, Langer D, Troosters T. Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med. 2009;37(9):2499–2505. doi: 10.1097/CCM.0b013e3181a38937. https://doi.org/10.1097/CCM.0b013e3181a38937 [DOI] [PubMed] [Google Scholar]
- 24.Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S. Monitoring sedation status over time in ICU patients reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289(22):2983–2991. doi: 10.1001/jama.289.22.2983. https://doi.org/10.1001/jama.289.22.2983 [DOI] [PubMed] [Google Scholar]
- 25.Knaus WA, Zimmermann JE, Wagner DP, Draper EA, Lawrence DE. APACHE-acute physiology and chronic health evaluation a physiologically based classification system. Crit Care Med. 1981;9(8):591–597. doi: 10.1097/00003246-198108000-00008. https://doi.org/10.1097/00003246-198108000-00008 [DOI] [PubMed] [Google Scholar]
- 26.Kleyweg RP, van der Meché FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve. 1991;14(11):1103–1109. doi: 10.1002/mus.880141111. https://doi.org/10.1002/mus.880141111 [DOI] [PubMed] [Google Scholar]
- 27.Dantas CM, Silva PF, Siqueira FH, Pinto RM, Matias S, Maciel C. nfluence of early mobilization on respiratory and peripheral muscle strength in critically ill patients. Rev Bras Ter Intensiva. 2012;24(2):173–178. https://doi.org/10.1590/S0103-507X2012000200013 [PubMed] [Google Scholar]
- 28.Witcher R, Stoerger L, Dzierba AL, Silverstein A, Roserngart, Brodie D. Effect of early mobilization on sedation practices in the neurosciences intensive care unit a preimplementation and postimplementation evaluation. J Crit Care. 2015;30(2):344–347. doi: 10.1016/j.jcrc.2014.12.003. https://doi.org/10.1016/j.jcrc.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 29.Albuquerque IM, Machado AS, Carvalho MT, Soares JC. Impacto da mobilização precoce em pacientes de terapia intensiva. Salud Ciencia. 2015;21(4):403–408. [Google Scholar]
- 30.Patel BK, Pohlamn AS, Hall JB, Kress JP. Impact of early mobilization on glycemic control and ICU-acquired weakness in critically ill patients who are mechanically ventilated. Chest. 2014;146(3):583–589. doi: 10.1378/chest.13-2046. https://doi.org/10.1378/chest.13-2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.k Koukourikos, Tsaloglidou A, Kourkouta L. Muscle atrophy in intensive care unit patients. Acta Inform Med. 2014;22(6):406–410. doi: 10.5455/aim.2014.22.406-410. https://doi.org/10.5455/aim.2014.22.406-410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Llano-Diez M, Renaud G, Andersson M, Marrero HG, Cacciani N, Engquist H. Mechanisms underlying ICU muscle wasting and effects of passive mechanical loading. Crit Care. 2012;16(5):R209–R209. doi: 10.1186/cc11841. https://doi.org/10.1186/cc11841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renaud G, Llano-Diez M, Ravar B, Gorza L, Feng HZ, Jin JP. Sparing of muscle mass and function by passive loading in an experimental intensive care unit model. J Physiol. 2013;591(5):1385–1402. doi: 10.1113/jphysiol.2012.248724. https://doi.org/10.1113/jphysiol.2012.248724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan E, Dowdy DW, Colantuoni E, Mendez-Tellez PA, Sevransky JE, Shanholtz C. Physical complications in acute lung injury survivors a two-year longitudinal prospective study. Crit Care Med. 2014;42(4):849–859. doi: 10.1097/CCM.0000000000000040. https://doi.org/10.1097/CCM.0000000000000040 [DOI] [PMC free article] [PubMed] [Google Scholar]


