The endocannabinoid system (ECS) is a recently emerging complex regulator of multiple physiological processes. It comprises several endogenous ligands (e.g. N-arachidonoylethanolamine, a.k.a. anandamide [AEA], 2-arachidonoylglycerol [2-AG], palmitoylethanolamide [PEA], etc.), a number of endocannabinoid (eCB)-responsive receptors (e.g. CB1 and CB2, etc.), as well as enzymes and transporters involved in the synthesis and degradation of the eCBs (extensively reviewed in Maccarrone et al., 2015, Ligresti et al., 2016).
Among many other tissues and organs, various members of the ECS were shown to be expressed in the skin as well. Indeed, AEA, 2-AG, CB1 and CB2 together with the major eCB-metabolizing enzymes (e.g. fatty acid amide hydrolase [FAAH], which cleaves AEA to ethanolamine and pro-inflammatory arachidonic acid) were found in various cutaneous cell types. Importantly, the eCB-tone and cannabinoid signaling in general appear to play a key role in regulating several fundamental aspects of cutaneous homeostasis, including proliferation and differentiation of epidermal keratinocytes, hair growth, sebaceous lipid production, melanogenesis, fibroblast activity, etc. (reviewed in Bíró et al., 2009, Maccarrone et al., 2015). Moreover, appropriate eCB-signaling through CB1 and CB2 receptors was found to be crucially important in keeping cutaneous inflammatory processes under control (Karsak et al., 2007). Indeed, by using CB1 and CB2 double KO (DKO) as well as wild-type (WT) mice, Karsak et al. demonstrated that DKO animals, lacking appropriate epidermal cannabinoid signaling, displayed exacerbated allergic skin inflammation, most probably because of the elevated MCP-2/CCL8 chemokine production of the epidermal keratinocytes. On the other hand, FAAH−/− mice (having higher eCB tone due to the impaired AEA degradation) exhibited less severe symptoms, and inflammation was also alleviated by cannabinoid receptor agonists in WT animals (Karsak et al., 2007). Moreover, AEA was recently shown to suppress production and release of key Th1- and Th17-polarizing cytokines (IL-12 and IL-23) via CB1-mediated inhibition of mammalian target of rapamycin (mTOR) in human keratinocytes (Chiurchiù et al., 2016). Collectively, these findings (together with many other recently published data) implied keratinocytes to be “non-classical” immune competent cells, playing a central role in initiation and regulation of cutaneous immune processes, and the “c(ut)annabinoid” system is now proven to be one of their master regulators.
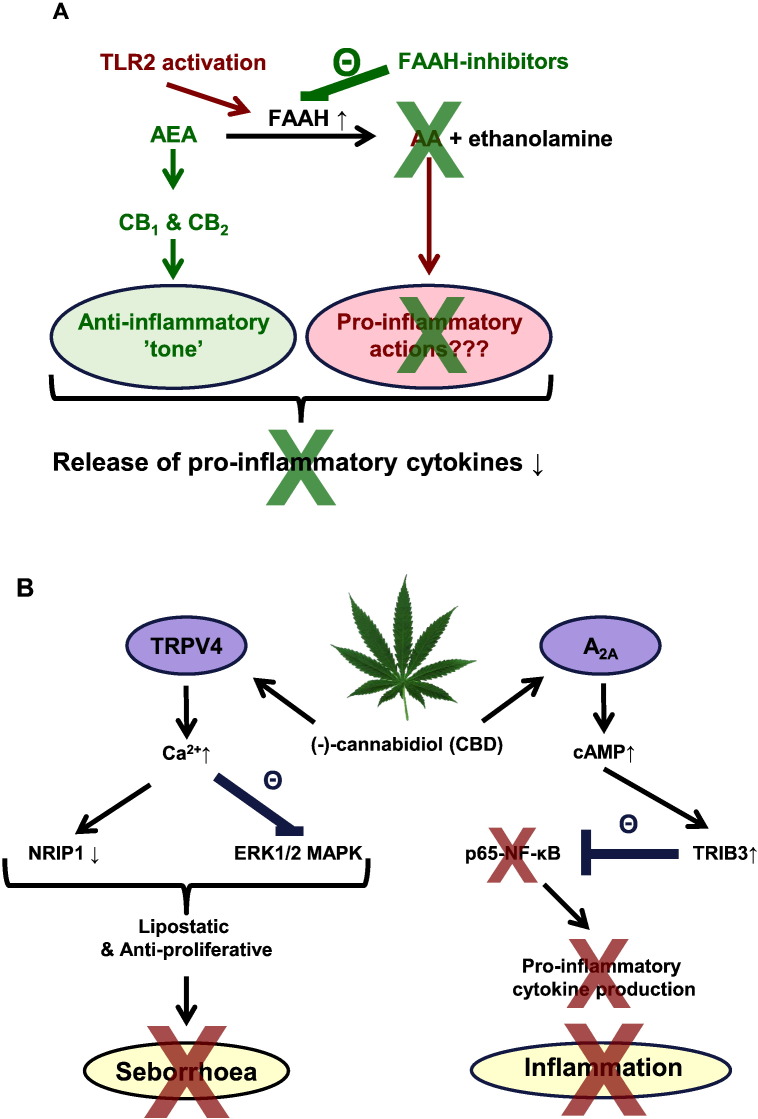
On the footsteps of Karsak et al., we further investigated the immunological role of the eCB-signaling using human epidermal keratinocytes. We found that protein (but, interestingly, not mRNA) expression and activity of the aforementioned eCB-degrading enzyme FAAH was positively regulated by Toll-like receptor (TLR) 2 activation (Oláh et al., 2016a). This quite fascinating result clearly demonstrated how deeply the ECS is involved in the regulation of one of the most fundamental immune processes, i.e. the TLR-activation-induced pro-inflammatory signaling. Indeed, administration of selective FAAH-inhibitors (and thereby restoration of the homeostatic, anti-inflammatory eCB signaling leading to the activation of CB1 and CB2 receptors) was able to almost completely abrogate the TLR2-mediated pro-inflammatory response (Oláh et al., 2016a). Moreover, topically applied FAAH-inhibitors alleviated atopic dermatitis (AD)-like symptoms of Nc/Tnd mice (a.k.a. NC/Nga; a mouse strain developing AD-like skin condition upon meeting certain dust mite antigens; Yamada et al., 2016) too (Oláh et al., 2016a). Based on these data, FAAH appears to be an important “decision maker” in the initiation and maintenance of cutaneous inflammation; thus, increase/restoration of the homeostatic cutaneous eCB tone by topically applied FAAH-inhibitors possesses great potential in alleviating skin inflammation (Fig. 1A).
Fig. 1.
Classical and “non-classical” cannabinoid pathways in cutaneous inflammation.
A) TLR2-activation increases FAAH expression and activity in human epidermal keratinocytes, leading to the loss of homeostatic, anti-inflammatory eCB signaling and therefore results in elevated pro-inflammatory cytokine expression and release. Thus, FAAH-inhibitors can selectively target the very first step of the cutaneous inflammatory processes by restoring the epidermal anti-inflammatory eCB-tone, and thereby normalizing the cytokine expression and release of the keratinocytes (Oláh et al., 2016a). B) Schematic overview of the mechanism of CBD's complex anti-acne effects (Oláh et al., 2014).
A2A: adenosine receptor 2A; AA: arachidonic acid; AEA: anandamide; FAAH: fatty acid amide hydrolase; ERK1/2: extracellular signal-regulated kinase 1 and 2; MAPK: mitogen activated protein kinase; NF-κB: nuclear factor kappa-light-chain enhancer of activated B cells; NRIP1: nuclear receptor interacting protein 1; TLR: Toll-like receptor; TRIB3: tribbles homolog 3; TRPV4: transient receptor potential vanilloid 4.
Another recently emerging, fascinating possibility to manage cutaneous inflammation through the cannabinoid signaling is the administration of phytocannabinoids (pCB). Cannabis sativa contains over 100 different pCBs, the vast majority of which have no psychotropic activity, and usually possess a “favorable” side-effect profile, which makes these substances particularly interesting drug candidates in treating several inflammation-accompanied diseases (Maccarrone et al., 2015, Ligresti et al., 2016). With respect to the skin, we have recently shown that one of the best studied pCBs, (−)-cannabidiol (CBD), may have great potential in managing acne, an inflammation-accompanied, extremely prevalent cutaneous disease. Briefly, we found that without decreasing either the viability or basal sebaceous lipid production, CBD normalized acne-mimicking, seborrheic lipid synthesis, decreased proliferation of sebocytes, and exerted strong anti-inflammatory actions via “non-classical” cannabinoid pathways (Fig. 1B). Indeed, sebostatic (lipid lowering and anti-proliferative) actions were found to be coupled to the activation of transient receptor potential vanilloid 4 (TRPV4) ion channels followed by the subsequent inhibition of the ERK1/2 MAPK pathway and down-regulation of nuclear receptor interacting protein 1 (NRIP1, a.k.a. RIP140). On the other hand, anti-inflammatory effects were realized by the (most probably indirect) activation of A2A adenosine receptors followed by up-regulation of tribbles homolog 3 (TRIB3) and inhibition of the pro-inflammatory p65 NF-κB pathway (Oláh et al., 2014). Moreover, although they exhibited important functional heterogeneity in influencing sebaceous lipogenesis, several other pCBs (namely (−)-cannabichromene [CBC], (−)-cannabidivarin [CBDV], (−)-cannabigerol [CBG], (−)-cannabigerovarin [CBGV] and (−)-Δ9-tetrahydrocannabidivarin [THCV]) were proven to exert potent anti-inflammatory actions in human sebocytes (Oláh et al., 2016b).
Collectively, in light of the above results, both increase/restoration of the homeostatic cutaneous eCB-tone by FAAH-inhibitors and topical administration of non-psychotropic pCBs hold out the promise to exert remarkable anti-inflammatory actions, making them very exciting drug candidates, deserving full clinical exploration as potent, yet safe (Maccarrone et al., 2015, Ligresti et al., 2016) novel class of anti-inflammatory agents.
Conflict of Interest
TB is the director of applied research of Phytecs Inc. (LA, CA).
Acknowledgements
Writing of this manuscript was supported by Hungarian research grants (NKFIH K_120552 and NKFIH PD_121360). The authors are grateful to Attila G. Szöllősi for his expert contribution.
References
- Bíró T., Tóth B.I., Haskó G., Paus R., Pacher P. The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol. Sci. 2009;30:411–420. doi: 10.1016/j.tips.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiurchiù V., Rapino C., Talamonti E., Leuti A., Lanuti M., Gueniche A., Jourdain R., Breton L., Maccarrone M. Anandamide suppresses proinflammatory T cell responses in vitro through type-1 cannabinoid receptor-mediated mTOR inhibition in human keratinocytes. J. Immunol. 2016;197:3545–3553. doi: 10.4049/jimmunol.1500546. [DOI] [PubMed] [Google Scholar]
- Karsak M., Gaffal E., Date R., Wang-Eckhardt L., Rehnelt J., Petrosino S., Starowicz K., Steuder R., Schlicker E., Cravatt B., Mechoulam R., Buettner R., Werner S., Di Marzo V., Tüting T., Zimmer A. Attenuation of allergic contact dermatitis through the endocannabinoid system. Science. 2007;316:1494–1497. doi: 10.1126/science.1142265. [DOI] [PubMed] [Google Scholar]
- Ligresti A., De Petrocellis L., Di Marzo V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: pleiotropic physiological and pathological roles through complex pharmacology. Physiol. Rev. 2016;96:1593–1659. doi: 10.1152/physrev.00002.2016. [DOI] [PubMed] [Google Scholar]
- Maccarrone M., Bab I., Bíró T., Cabral G.A., Dey S.K., Di Marzo V., Konje J.C., Kunos G., Mechoulam R., Pacher P., Sharkey K.A., Zimmer A. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol. Sci. 2015;36:277–296. doi: 10.1016/j.tips.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oláh A., Tóth B.I., Borbíró I., Sugawara K., Szöllõsi A.G., Czifra G., Pál B., Ambrus L., Kloepper J., Camera E., Ludovici M., Picardo M., Voets T., Zouboulis C.C., Paus R., Bíró T. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J. Clin. Invest. 2014;124:3713–3724. doi: 10.1172/JCI64628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oláh A., Ambrus L., Nicolussi S., Gertsch J., Tubak V., Kemény L., Soeberdt M., Abels C., Bíró T. Inhibition of fatty acid amide hydrolase exerts cutaneous anti-inflammatory effects both in vitro and in vivo. Exp. Dermatol. 2016;25:328–330. doi: 10.1111/exd.12930. [DOI] [PubMed] [Google Scholar]
- Oláh A., Markovics A., Szabó-Papp J., Szabó P.T., Stott C., Zouboulis C.C., Bíró T. Differential effectiveness of selected non-psychotropic phytocannabinoids on human sebocyte functions implicates their introduction in dry/seborrhoeic skin and acne treatment. Exp. Dermatol. 2016;25:701–707. doi: 10.1111/exd.13042. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Ueda Y., Nakamura A., Kanayama S., Tamura R., Hashimoto K., Kido H., Matsumoto T., Ishii R. Biphasic increase in scratching behaviour induced by topical application of Dermatophagoides farinae extract in NC/Nga mice. Exp. Dermatol. 2016;25:611–617. doi: 10.1111/exd.12999. [DOI] [PubMed] [Google Scholar]