Abstract
Respiratory syncytial virus (RSV) is one of the most prevalent causative agents of lower respiratory tract infections worldwide, especially in infants around 3 to 4 months old. Infants at such a young age have maternally-transferred passive antibodies against RSV but do not have active immune systems efficient enough for the control of RSV infection. In order to elucidate age-specific profiles of immune responses against RSV protection, antibody responses were examined by using blood samples in both acute and convalescent phases obtained from child patients and adult patients. In addition to the serum neutralization activity, antibody responses to the RSV fusion protein (F protein) were dissected by analyzing levels of total IgG, IgG subclasses, the binding stability, and the levels of antibody for the neutralization epitopes. It was suggested that children's antibody responses against RSV are matured over months and years in at least 5 stages based on 1) levels of the neutralization titer and IgG3 for F protein in the convalescent phase, 2) geometric mean ratios of the neutralization titers and levels of IgG1 and IgG2 for F protein in the convalescent phase compared to those levels in the acute phase, 3) the affinity maturation of IgG for F protein and the cross reactivity of IgG for RSV glycoproteins of groups A and B, 4) levels of neutralization epitope-specific IgG, and 5) augmentation of overall antibody responses due to repetitive RSV infection.
Keywords: RSV, F protein, IgG3, Palivizumab-like neutralization antibody
Highlights
-
•
Children's antibody responses against RSV are matured over months and years in at least 5 stages.
-
•
Age-specific profiles of antibody responses after RSV infection are proposed.
-
•
The profile of IgG3 specific to F protein reflects infants' own antibody response after RSV infection.
In this study, antibody responses to respiratory syncytial virus (RSV) were evaluated by quantitative and qualitative multivariate analyses of acute and convalescent sera from patients infected with RSV. The results suggested that the profiles of antibody responses after RSV infections are delineated by independent factors such as the development of children's immune system and the natural infection with RSV in the presence or absence of maternal antibodies.
1. Introduction
Respiratory syncytial virus (RSV) causes severe lower respiratory tract infection (LRTI), especially in infants < 6 months old and the elderly, posing public health concerns worldwide. The results of a recent study based on the global mortality surveillance from 1990 to 2010 suggested that the cause of the highest mortality rate in infants is LRTI and that the most frequent causative agent of LRTI in infants is RSV (Lozano et al., 2012). It is also suggested that among contagious pathogens RSV is the second largest cause of mortality in infants next to the Plasmodium parasite (Lozano et al., 2012). As a disease burden associated with RSV infection, high risk for the development of asthma in children who experience RSV bronchiolitis in infancy has been demonstrated (Bacharier et al., 2012). In addition, results of recent clinical research in the UK estimated that the year-to-year mortality rate in the elderly, and the number of elderly outpatients associated with RSV infection are more consistent than those of influenza (Flu), and that the average mortality rate of RSV and the number of RSV patients over the years are comparable with or higher than those of Flu (Fleming et al., 2015). Results of the research also suggested that rates of both the mortality and the hospitalization attributable to RSV infection are higher in the high risk group of the elderly, defined as chronic conditions indicative of risk for severe influenza as per UK recommendations for influenza vaccination, than those in the non-risk group of the elderly (Fleming et al., 2015). Thus, prophylactic measures, i.e., vaccines especially for infants and elderly, are required for controlling RSV infections worldwide, but no approved vaccines are currently available. According to WHO reports and a PATH program, there are a number of different types of RSV vaccine candidates in a variety of development stages, whereas one of the greatest regulatory issues on RSV vaccines is that there are no consolidated immunological biomarkers. Also, an international serum standard is required to normalize data between different tests and different laboratories for the clinical evaluation of vaccine efficacy. The difficulty lies in the fact that infants' active antibody responses are hardly discriminated from pre-existing passively transferred maternal antibodies in infants. It is also suggested that regulatory authorities should harmonize and standardize the evaluation methods of biomarkers by using standard reagents and reference materials with common procedures (Higgins et al., 2016).
Palivizumab, an antibody drug targeting antigenic site II of the RSV fusion protein (F protein), is available for the prevention of severe RSV illness in certain infants and children who are at high risk. The drug can help prevent development of serious RSV diseases, yet with limited efficacy (Groothuis et al., 1993, Parnes et al., 2003, The IMpact-RSV Study Group, 1998). The results of palivizumab's clinical trials in preterm infants showed that 40 μg/mL of serum palivizumab corresponds to 7.3 log2 of the neutralization titer, enabling a 55% reduction of the hospitalization associated with RSV infections (The IMpact-RSV Study Group, 1998). The results of the past clinical research showed that participants with naturally acquired serum neutralization titers of 6.0 log2 or more to RSV group A and 8.0 log2 or more to RSV group B resulted in approximately 70% reduction in not having an RSV related-hospitalization (Piedra et al., 2003). The results of clinical studies on the prophylactic intravenous administration of RSV immune globulin to high risk infants and on serological assessment of RSV patients < 6 months old have suggested that the sera with neutralization titers > 1:200 or 8.0 log2 confer protection against LRTI (Blaser and Valentine, 2008, Groothuis et al., 1993, Wright et al., 2002). Another clinical study focused on the RSV-specific antibody kinetics in mother-infant pairs in Bangladesh, and demonstrated a strong correlation between maternal RSV antibody titers at the third trimester and at birth with efficient trans-placental antibody transfer to the fetus (Chu et al., 2014). Reduced risk of LRTI was associated with higher cord blood neutralization titers above 8.0 log2 (Chu et al., 2014). Taken together, these findings suggest that serum neutralization titers of > 8.0 log2 or serum palivizumab-like neutralization antibodies (PLNA) of > 40 μg/mL correlate with protection against RSV.
Accumulating scientific evidence suggests different profiles of each IgG subclass in protection against pathogens. Human IgG1 is the most dominant subclass in human sera and generally plays an important role in the protection against a variety of viral infections. In addition to inactivation and/or inhibition of the pathogenicity by direct interaction with target molecules, IgG1 and IgG3 have the ability to activate subsequent host immune responses, such as complement pathways, Fcγ receptor-mediated immune responses, and antibody-dependent cellular cytotoxicity. Such arms of responses are shown to significantly contribute to the elimination of pathogens and inactivation of pathogenic factors and some reports suggest that IgG3 has the strongest effector functions among all IgG subclasses (Cao et al., 2013, Hofmeister et al., 2011, Irani et al., 2015, Scharf et al., 2001, Natsume et al., 2008, Stapleton et al., 2011). With regard to the kinetics of the trans-placental transfer of maternal antibodies to the fetus, the efficiency of the IgG transport by neonatal Fc receptor (FcRn) in the placenta seems to marginally depend on its subclasses, i.e., a little higher efficiency for IgG1 and a little lower efficiency for IgG2, but is significantly reduced due to maternal diseases such as placental malaria or hyperglobulinemia, in which the transfer of IgG1 and IgG2 but not that of IgG3 is impaired (Hamilton, 1987, Okoko et al., 2001). A past clinical study focused on the comparison of levels of RSV-specific IgG1 and IgG3 in patients, and its results suggested that the avidity of IgG1 against the whole RSV protein correlates with the protection against RSV infection in infants < 3 months old. The correlation between avidity of IgG3 and protection against RSV infection was hardly characterized because of lower concentrations of IgG3 as compared with IgG1 (Freitas et al., 2011).
Here, in order to examine the dynamics of human immune responses against acute RSV infections, we designed a clinical study targeting 2 different age segments (child patients < 3 years old and adult patients) and obtained blood samples in both the acute and convalescent phases. Based on quantitative and qualitative multivariate analyses of the immune response against RSV, age-specific profiles and maturation of antibody responses were elucidated. Such results will significantly contribute to the design of RSV vaccines, i.e., adding adjuvants in the formulation and/or selection of the optimal route of administration to overcome the immaturity of immune responses in infants, and to their clinical evaluation by harnessing such parameters as biomarker candidates for determining efficacies of vaccines containing F protein antigen.
2. Materials and Methods
2.1. Design of Clinical Research
This clinical research was conducted throughout Japan from October 2014 to April 2016 at 6 regional base hospitals having pediatric departments, and 3 pediatric clinics in Tokyo and Kanagawa prefectures in Japan, and 13 medical clinics belonging to the Influenza Study Group of the Japan Physicians Association. In order to confirm RSV infections in medically attended patients having acute respiratory illness, nasal swab samples were obtained by investigators from those who were suspected of having been infected with RSV, and those consenting to the testing were examined by using an immunochromatographic test kit for RSV (Imunoace RSV Neo®). The exclusion criteria included: 1) any history of palivizumab administration, 2) any infection with HBV, HCV, syphilis, or HIV, 3) any participation in another clinical research or clinical trial, 4) any other conditions that were judged by investigators as inadequate for joining this clinical research. After enrollment, acute phase blood samples were taken from participants on the first day visiting the medical institutions where they were diagnosed as having been infected with RSV for the first time in the RSV season. The data of acute serum samples at < 6 days after onset of fever were analyzed, since 6 days or greater after illness onset will likely have RSV-specific responses from the infection, which was not suggested to represent a true baseline response. For child participants, blood was again taken as samples of the convalescent phase at 2 to 3 weeks after the first visit, whereas for adult participants, blood was again taken at 2 weeks after the first visit. Nasal swab samples collected by flocked swabs were taken on the day of the first visit, the RSV genome was extracted from them, and then RSV group A and B were determined based on cDNA sequences of the G protein gene. This study was approved by the institutional review boards of Yokohama City University, Japan Physicians Association, National Institute of Biomedical Innovation, Health and Nutrition, and Daiichi Sankyo Co., Ltd. The study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all parents of child participants and all adult participants before enrollment.
2.2. Materials
Hep-2 cells (ATCC) were maintained in MEM supplemented with 10% FBS, 1 mM sodium pyruvate, 1% non-essential amino acids solution and 1% penicillin-streptomycin solution at 37 °C with 5% CO2. RSV strains, A2, and 18,537 were purchased from ATCC. Palivizumab (Synagis®) was purchased from AbbVIE Inc. A neutralization mouse monoclonal antibody against antigenic site I (131-2A) was purchased from Millipore, whereas that against antigenic site IV (6H6-2B4) was obtained by selecting hybridoma clones produced from rats immunized with the recombinant F protein. A recombinant neutralization antibody, palivizumab (rat Fc) recognizing antigenic site II, was genetically engineered by fusion of the F(ab′)2 region of palivizumab to the rat Fc region, and expressed in HEK293F cells.
2.3. Microneutralization Assay
Human sera were heat-inactivated at 56 °C for 30 min. Two-fold serial dilutions of the serum were carried out starting at 1:10 dilution in 96-well plates and the diluted serum samples were adjusted to a final volume of 50 μL. The samples were assayed in triplicate. An equal volume (50 μL) of RSV A2 at 1000 PFU/mL was added to the diluted serum samples. After mixing by Voltex at 600 rpm for 15 s, the mixtures were incubated at 37 °C for 2 h. Hep-2 cells were freshly prepared at 1 × 105 cells/mL and 100 μL of cell suspension was added into each well of the 96-well plates. The plates were incubated at 37 °C for 3 d (A2) and 4 d (18537). The cells were fixed with an ice cold 80% acetone/PBS (−) solution for 15 min and the plates were dried after removing the fix solution. After blocking the cells with 25% Block Ace (Yukijirushi) in TBS, HRP-labeled palivizumab was added, and then the plates were incubated at room temperature for an hour. After the coloring with TMB solution, the plates were treated with TMB stop solution (KPL), and finally OD450 was measured to calculate the neutralization titer as follows.
First, RSV replication ratio (%) was calculated as [(OD450 of the sample − OD450 of the background) / (OD450 of the virus control − OD450 of the background)] × 100. The background was cells exposed to neither the virus nor serum. The virus control was cells infected with the virus in the absence of serum. If the mean of RSV proliferation ratio acquired from triplicate wells of the starting dilution is < 50%, EC50 was determined as < 10. If that was greater than or equal to 50%, RSV proliferation ratio (Y-axis) was plotted against serum dilution (X-axis). EC50[(Emin + Emax) / 50] was determined using 4-parameter logistic curve fit. In case the curve failed to converge, EC50 value was calculated by linear curve fit using two or three points closest to the 50%.
2.4. Enzyme-linked ImmunoSorbent Assay (ELISA) of RSV F and G Protein
The 96-well plates were coated with 0.5 μg/mL of F or G protein (SinoBiological Inc.) and serially diluted human IgG protein (Nordic Immunology, Human Standard Serum NOR-01) at 4 °C for 12 h to 18 h. After washing with PBS (-) containing 0.05% Tween 20 (PBST), the plates were treated with the blocking solution (1% BSA in PBST). The blocking solution was removed, and serially-diluted serum samples, i.e., initially diluted at 1:20 and then a series of three-fold serial dilutions, were added and then the plates were incubated at 25 °C for 2 h. Since preliminary analysis showed that samples used in this study contained a wide range of IgG concentration against F protein or G protein, we have set serial three-fold dilutions rather than serial two-fold dilutions in order to determine serum IgG titers of broad concentration ranges. After washing and being treated with a detection antibody labeled with HRP (Cygnus Technologies, cat# IM-40 for total human IgG, cat# IM-50 for human IgG subclasses), the plates were colored with TMB solution following treatment with 1 M H2SO4 stop solution. Finally, OD450 and OD540 were measured for the calculation of antibody concentration. Concentrations of anti-F or G protein IgG were calculated by the standard curve generated by using a human IgG reference. The limitations of detection in ELISA of RSV F are 0.1 μg/mL for total IgG and IgG1, 0.01 μg/mL for IgG2, and 0.001 μg/mL for IgG3. The limitation of detection in ELISA of RSV G is 0.01 μg/mL.
2.5. Serum IgG versus Monoclonal Antibody Competitive Evaluation of Binding Abilities to Neutralize Epitopes, Antigenic Sites I, II, or IV in RSV F Protein
In order to examine the kinetics of antibodies specific to such neutralization epitopes in RSV infections, sera from children and adults were subjected to the competitive ELISA by using a monoclonal antibody specific to either antigenic sites I (131-2A), II (palivizumab [rat Fc]), or IV (6H6-2B4). Ni2+-NTA coated plates (QIAGEN) were coated with 50 ng of F protein per each well at concentration of 0.5 μg/mL at 25 °C for an hour. After washing with PBS (-) containing 0.05% Tween 20 (PBST), the plates were blocked with 1% BSA in PBST at 25 °C for 1 h. After washing with PBST, the plates were incubated with human serum samples, i.e., initially diluted at 1:10 and then a series of two-fold serial dilutions, in the presence of a constant concentration, 0.5 μg/mL of a monoclonal antibody, either 131-2A(site I), palivizumab (rat Fc)(site II), or 6H6-2B4(site IV), at 25 °C for an hour. After washing again with PBST, the plates were incubated with a 1:40,000 dilution of HRP-conjugated goat anti-rat IgG specific to Fcγ fragment (Jackson ImmunoResearch) or a 1:4000 dilution of goat anti-mouse IgG (H + L) (Southern Biotech) at 25 °C for an hour. After washing a final time with PBST, TMB solution (KPL) was added, and then the plates were incubated at 25 °C for 4 min to 10 min. After adding TMB stop solution (KPL), OD450 and OD650 were measured for the calculation of the half maximal inhibitory concentration (IC50). Binding Inhibition ratio (%) for F protein was calculated as 100 − {[(OD450 − OD650 of the sample) − (OD450 − OD650 of the background)] ⁄ [(OD450 − OD650 of the control) − (OD450 − OD650 of the background)] × 100}. The background was wells exposed to neither the virus nor serum. The control was wells exposed to the monoclonal antibody alone. IC50[(ICmin + ICmax) / 50] was determined using sigmoid curve model by SAS System Release 9.2. The value of samples showing the below detection limit of the IC50 was defined as 5. Concentrations of palivizumab-like neutralization antibody (PLNA) in sera were calculated according to the standard curve generated by data points obtained by using three-fold serial dilutions of palivizumab with the initial concentration of 54 μg/mL.
2.6. Evaluation of the Antibody Binding Stability by Surface Plasmon Resonance (SPR)
All experiments were performed using a Biacore T200 (GE Healthcare). Five micrograms per milliliter of F protein was coupled to a CM5 sensor chip (GE Healthcare) by the amine coupling reaction at 5000 response units (RU). The reference chip was prepared by subjecting it to the amine coupling reaction without F protein. Ten-fold dilutions of human serum samples in 1 × HBS (0.01 M HEPES, 0.15 M NaCl, 3 mM EDTA, 0.05% surfactant P20 [GE Healthcare]) containing 1 mg/mL of NSB reducer (GE Healthcare) were injected at 30 μL/min (120-second contact time) and dissociated (180-second dissociation time). To analyze the antibody binding stability, values of the sensorgram were recorded at 2 report points (Stability Point at 17 s after the end of injection and Stability-Late 1 Point at 150 s after the end of injection). Each recorded value was double referenced by subtracting the value recorded using the reference chip and that of a blank injection. An apparent dissociation rate constant (kd) was determined by report point analysis algorithm (GE Healthcare). As a validation of each assay for evaluation of serum kd to F protein, we evaluated kd of palivizumab to F protein in the concentrations of 0.1 to 100 μg/mL since serum samples in this study contained < 1000 μg/mL, and kd was evaluated with diluted serum samples at 1:10. It was shown that the binding stability of palivizumab slightly increased as the concentration of palivizumab decreased.
2.7. Statistical Analysis
For neutralization titer, serum IgG for RSV F protein, geometric mean ratios in the convalescent phase compared to those in the acute phase (GMRs C/A), and other data, the means with corresponding 95% confidence interval (CI) in each age group and phase (acute, convalescents) were estimated using the linear model. To evaluate the relationship between the variables, Pearson correlation coefficient was used. If there is departure for the normality assumption for the distributions of the variable values, an appropriate transformation (e.g., log-transformation) was applied to the variables prior to analysis. If a transformation is used, back-transformed values were used for presentation. The statistical analyses were carried out with SAS version 9.3 software (SAS Institute, Cary, NC).
2.8. Abbreviations
Technical abbreviations in the Materials and Methods section are listed in Supplemental Table 1
3. Results
3.1. Age-specific Profiles of the Serum Neutralization Activity against RSV
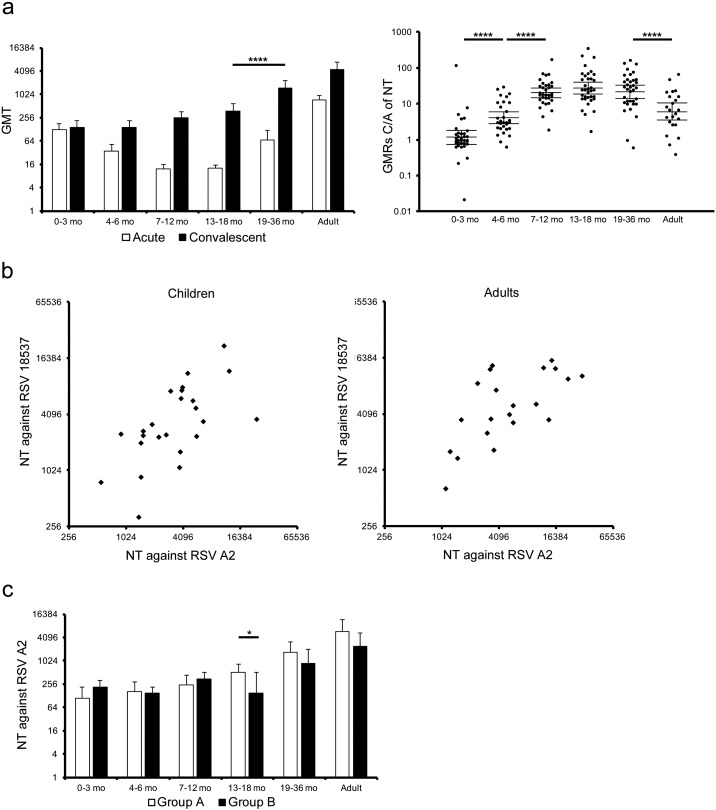
In order to examine antibody responses against RSV infection, the serum neutralization activity against RSV A2 strain, an experimental strain of RSV group A, was compared between acute and convalescent phases. Serum samples were divided into 6 different age groups: 1) 0 to 3 months old, 2) 4 to 6 months old, 3) 7 to 12 months old, 4) 13 to 18 months old, 5) 19 to 36 months old, and 6) adults as shown in Table 1. The results of the neutralization activity in the acute phase showed higher titers in infants < 6 months old, children 19 months and older, and adults as compared with children at 7 to 18 months old (Fig. 1a). Such a U-shaped distribution pattern was suggested to reflect an immunological background due to the presence of maternal antibodies transferred in infants, especially < 6 months old, and past exposures to RSV in children 19 months and older and adults, which was consistent with the results of previous clinical studies (Meurman et al., 1992, Sande et al., 2014). In contrast, the neutralization titers in the convalescent phase were higher in children 19 months and older than in children < 19 months old [mean values of NT: 1516 (19–36 mo) versus 371 (13–18 mo), p < 0.0001]. In accordance with the timing of RSV exposure in early life (nearly 60% of infants are infected during the first year of life and almost 100% of children are infected by the second year of life (Hall et al., 2013, Hall et al., 2009, Resch, 2012)), it was suggested that repetitive RSV infections boost the neutralization activity. GMRs of neutralization titers in the convalescent phase compared to those in the acute phase (GMRs C/A of NT) were > 20 in children at 7 to 36 months old, while GMRs C/A of NT were > 4 in children at 4 to 6 months old and adults, suggesting that the serum neutralization activity was inducted in response to RSV infection. In contrast, titers in the convalescent phase were comparable with those in the acute phase in infants < 6 months old, suggesting that serum neutralization titers induced in response to RSV infection were too low to be distinguished from the pre-existing neutralization titers transferred from mothers (Fig. 1a). With regard to GMRs C/A of NT, the lower limits of 95% CI were > 14 in infants at 7 to 12 months old, > 18 in children at 13 to 18 months old, > 14 in children at 19 to 36 months old, and > 3.4 in adults (Table 2). In addition, percentages of significant higher levels, as determined by > 4-fold increase of GMRs C/A of NT, were 97.0% in infants at 7 to 12 months old, 97.1% in children at 13 to 18 months old, 94.3% in children at 19 to 36 months old, and 65.2% in adults, suggesting that such GMRs C/A of NT reflect antibody responses specific to RSV infection (Table 2). Such profiles of antibody responses against RSV infection in adults are consistent with the results of a past clinical cohort study showing that preseason serum neutralization titers in elderly who had not been infected with RSV during the observation period were approximately 4-fold higher than those who had been infected with RSV during the period (Falsey and Walsh, 1998). In addition, past retrospective clinical studies for RSV infant patients and clinical trials for the RSV vaccine also set “4-fold” as a criterion for the ratio of protective level of the serum neutralization activity against RSV (Esposito et al., 2016, Karron et al., 2015, Sande et al., 2014).
Table 1.
Demographic characteristics of children and adults with RSV infection in this study. Among 169 child patients, 7 subjects had respiratory diseases, 1 subject had renal dysfunction, and 1 subject had West syndrome. Among 23 adult patients, 3 subjects had respiratory diseases, 2 subjects had heart diseases, 4 subjects had diabetes, and 1 subject had autoimmune disease.
| Age group | N | Mean age (min–max) | Male/female |
|---|---|---|---|
| All children | 169 | 11.6 mo (0.4–34.7 mo) | 92/77 |
| 0–3 mo | 35 | 2.3 mo (0.4–3.9 mo) | 18/17 |
| 4–6 mo | 30 | 5.3 mo (4.0–6.8 mo) | 16/14 |
| 7–12 mo | 34 | 9.1 mo (7.0–12.3 mo) | 21/13 |
| 13–18 mo | 35 | 15.7 mo (13.1–18.6 mo) | 21/14 |
| 19–36 mo | 35 | 24.6 mo (19.3–34.7 mo) | 16/19 |
| Adults < 65 yo | 11 | 43 yo (24–63 yo) | 6/5 |
| ≥ 65 yo | 12 | 79 yo (65–89 yo) | 4/8 |
Fig. 1.
Serum neutralization activity upon natural RSV infection.
(a) Sera were subjected to a microneutralization assay to determine neutralization titers against RSV A2 strain. A left panel represented neutralization titer in acute phase or convalescent phase, and a right panel did the GMRs C/A of NT. The mean ± 95% CI was estimated by linear model, and was shown in the graphs. Neutralization titer in acute phase: 0–3 mo, n = 34; 4–6 mo, n = 30; 7–12 mo, n = 33; 13–18 mo, n = 35; 19–36 mo, n = 35; adults, n = 23. Neutralization titer in convalescent phase: 0–3 mo, n = 35; 4–6 mo, n = 30; 7–12 mo, n = 34; 13–18 mo, n = 35; 19–36 mo, n = 35; adults, n = 23. GMRs C/A of NT: 0–3 mo, n = 34; 4–6 mo, n = 30; 7–12 mo, n = 33; 13–18 mo, n = 35; 19–36 mo, n = 35; adults, n = 23. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. (b) Sera, which showed high neutralization activity against RSV A2 in panel (a), were subjected to a microneutralization assay to evaluate NT against RSV 18537 strain. Children: r = 0.705, p < 0.0001 (n = 24), adults: r = 0.657, p = 0.0012 (n = 21). (c) Neutralization titers were also analyzed in two distinct groups according to RSV groups infected during the research period to evaluate the cross neutralization activity at convalescent phase. Group A: 0–3 mo, n = 20; 4–6 mo, n = 16; 7–12 mo, n = 17; 13–18 mo, n = 25; 19–36 mo, n = 21; adults, n = 12. Group B: 0–3 mo, n = 14; 4–6 mo, n = 11; 7–12 mo, n = 13; 13–18 mo, n = 6; 19–36 mo, n = 8; adults, n = 5. The mean ± 95% CI was estimated by the linear model, and were shown in the graphs. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Table 2.
Summary of lower limits of 95% CI of GMRs C/A of NT, IgGs for F protein and IgG for neutralization epitopes, and positive rates of patients who had > 4-fold GMRs C/A of NT, IgGs for F protein and IgG for neutralization epitopes.
| Age group | Lower limit of 95% CI of GMRs C/A of NT | Positive rate of patients who had > 4-fold GMRs C/A of NT (%) | Lower limit of 95% CI of GMRs C/A of IgGs for F protein |
Positive rate of patients who had > 4-fold GMRs C/A of IgGs for F protein (%) |
Lower limit of 95% CI of GMRs C/A of IgG for neutralization epitopes (antigenic sites) |
Positive rate of patients who had > 4-fold GMRs C/A of IgG for neutralization epitopes (antigenic sites) (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total IgG | IgG1 | IgG2 | IgG3 | Total IgG | IgG1 | IgG2 | IgG3 | I | II | IV | I | II | IV | |||
| 0–3 mo | 0.7 | 8.8 | 0.8 | 0.8 | 0.6 | 9.3 | 0.0 | 0.0 | 0.0 | 78.3 | – | 0.9 | 0.9 | 0.0 | 0.0 | 0.0 |
| 4–6 mo | 2.7 | 36.7 | 2.3 | 2.3 | 1.1 | 29 | 35.7 | 35.7 | 17.9 | 96.4 | – | – | – | 0.0 | 0.0 | 0.0 |
| 7–12 mo | 14 | 97.0 | 58 | 48 | 5.3 | 142 | 96.4 | 96.4 | 64.3 | 100 | – | 0.9 | 0.8 | 0.0 | 3.8 | 3.8 |
| 13–18 mo | 18 | 97.1 | 255 | 227 | 12 | 207 | 100 | 100 | 79.4 | 100 | 0.9 | 0.9 | 1.0 | 0.0 | 11.8 | 11.8 |
| 19–36 mo | 14 | 94.3 | 23 | 22 | 11 | 18 | 85.3 | 88.2 | 85.3 | 85.3 | 1.9 | 3.3 | 5.1 | 35.8 | 56.3 | 71.0 |
| Adult | 3.4 | 65.2 | 5.0 | 4.7 | 3.9 | 1.9 | 69.6 | 69.6 | 69.6 | 39.1 | 2.2 | 4.5 | 5.8 | 42.8 | 69.6 | 73.9 |
| Age group | Upper limit of 95% CI of F IgG3 in the acute phase (μg/mL) | Lower limit of 95% CI of F IgG3 in the convalescent phase (μg/mL) | Lower limit of 95% CI of GMRs C/A of F IgG3 |
|---|---|---|---|
| 0–3 mo | 0.003 | 0.056 | 9.3 |
| 4–6 mo | 0.003 | 0.236 | 29 |
| 7–12 mo | 0.005 | 1.499 | 142 |
| 13–18 mo | 0.003 | 1.655 | 207 |
| 19–36 mo | 0.021 | 1.037 | 18 |
| Adult | 0.008 | 0.029 | 1.9 |
Next, serum neutralization antibody titers to 18,537, an experimental strain of RSV group B, were evaluated in serum samples with high neutralization titers to A2 strain, i.e., > 512 in children or 1024 in adults. As shown in Fig. 1b, at least in samples with high neutralization titers, it was shown that neutralization titers against RSV A2 strain correlated with those against 18,537, suggesting that cross reactive neutralization titers were induced regardless of RSV strains infected in this research period [children: Pearson correlation coefficient (r) = 0.624, p = 0.0011, adults: r = 0.657, p = 0.0012]. The cross reactivity was further analyzed based on RSV groups infected in this research period. Group A corresponds to patients that had been infected with RSV group A, while Group B to patients that had been infected with RSV group B, and their neutralization titers against RSV A2 strain (group A) were compared (Fig. 1c). Convalescent sera from different age groups infected with group B showed levels of neutralization titers against RSV A2 comparable with or marginally lower than those infected with group A (Fig. 1c, 13 to18 months old, p = 0.0184; other age groups, p > 0.05). Taken together, these results suggested an age-specific background, dynamics, and quality of the serum neutralization activity against RSV.
3.2. Age-specific Profiles of the Serum Antibody Response to RSV F Protein
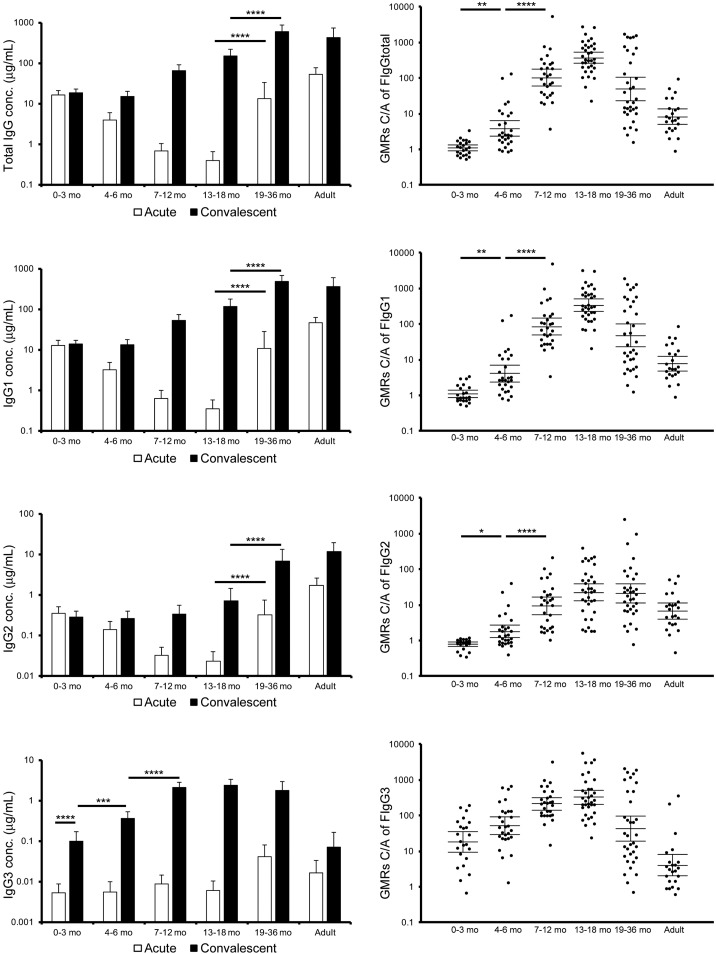
One of the major antigens known to induce neutralization antibody responses is F protein expressed on the surface of the RSV virion. GMRs C/A of total IgG, IgG1 and IgG2 specific for F protein (GMRs C/A of F IgGtotal, IgG1, and IgG2) were higher in children 4 months and older and adults than in infants < 3 months old (Fig. 2). The age-specific patterns of the kinetics of total IgG, IgG1 and IgG2 for F protein were similar to that of the neutralization activity. The levels of anti-F IgG in acute phase were significantly higher in children aged at 19 to 36 months old than those at 13 to 18 months old, and the levels of IgG1 and IgG2 for F protein in the convalescent phase were higher in children 19 months and older than in children < 19 months old [mean values of IgG1: 472 (19–36 mo) versus 118 (13–18 mo), p < 0.0001, that of IgG2: 6.63 (19–36 mo) versus 0.70 (13–18 mo), p < 0.0001], suggesting that repetitive RSV infections boost the responses of IgG1 and IgG2 to F protein. Recent epidemiological surveillance data suggested that the annual RSV circulation boosts basic immune responses against RSV especially in healthy children aged 2 years and older and healthy adults, resulting in few symptomatic RSV patients in such population (Kutsaya et al., 2016). With regard to GMRs C/A of F IgGtotal, IgG1, and IgG2, the lower limits of 95% CI were > 58, 48, and 5.3 in infants at 7 to 12 months old, > 255, 227, and 12 in children at 13 to 18 months old, > 23, 22, and 11 in children at 19 to 36 months old, and > 5.0, 4.7, and 3.9 in adults (Table 2). In addition, percentages of significant higher levels, as determined by > 4-fold increase of GMRs C/A of F IgGtotal, IgG1, and IgG2, were 96.4%, 96.4%, and 64.3% in infants at 7 to 12 months old, 100%, 100%, and 79.4% in children at 13 to 18 months old, 85.3%, 88.2%, and 85.3% in children at 19 to 36 months old, and 69.6%, 69.6%, and 69.6% in adults, suggesting that such ratios reflect antibody responses specific to RSV infection (Table 2). Of interest, the average level of IgG3 for F protein in acute sera in infants < 13 months old was below 0.01 μg/mL, whereas those in convalescent sera were 0.10 μg/mL (95% CI, 0.06–0.17) in infants < 4 months old, 0.35 μg/mL (95% CI, 0.24–0.53) in infants at 4 to 6 months old, and 2.0 μg/mL (95% CI, 1.5–2.8) in infants 7 to 12 months old. GMRs C/A of F IgG3 were 18.3 (95% CI, 9.40–35.7) in infants < 4 months old, 52.0 (95% CI, 29.8–90.8) in infants at 4 to 6 months old, and 213 (95% CI, 142–320) in infants 7 to 12 months old, whereas only 4.02 (95% CI, 1.99–8.11) in adults, suggesting that IgG3 specific to F protein can be used as a good biomarker for RSV infection in infants and as an exclusive marker in especially those infants < 6 months old (Fig. 2 and Table 2). With regard to F IgG3 C and GMRs of F IgG3, the lower limits of 95% CI were > 0.056 μg/mL and 9.3 in infants at 0 to 3 months old, > 0.236 μg/mL and 29 in infants at 4 to 6 months old, > 1.499 μg/mL and 142 in infants at 7 to 12 months old, > 1.655 μg/mL and 207 in children at 13 to 18 months old, and > 1.037 μg/mL and 18 in children 19 to 36 months old, suggesting that such ratios reflect antibody responses specific to RSV infection (Table 2).
Fig. 2.
Serum antibody response to RSV F protein.
The sera were subjected to ELISA coating RSV F protein. Series of detection antibody labeled with HRP were used to acquire the profile of IgG subclasses specific to RSV F protein. A left panel represents concentration of total IgG, IgG1, IgG2 and IgG3 for RSV F in acute phase or convalescent phase, and a right panel does the GMRs C/A of F IgGtotal, IgG1, IgG2, and IgG3. The mean ± 95% CI was estimated by the linear model, and were shown in the graphs. Concentration of antibody in acute phase: 0–3 mo, n = 29; 4–6 mo, n = 28; 7–12 mo, n = 29; 13–18 mo, n = 34; 19–36 mo, n = 35; adults, n = 23. Concentration of antibody in convalescent phase: 0–3 mo, n = 28; 4–6 mo, n = 30; 7–12 mo, n = 33; 13–18 mo, n = 35; 19–36 mo, n = 34; adults, n = 23. GMRs C/A: 0–3 mo, n = 23; 4–6 mo, n = 28; 7–12 mo, n = 28; 13–18 mo, n = 34; 19–36 mo, n = 34; adults, n = 23. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
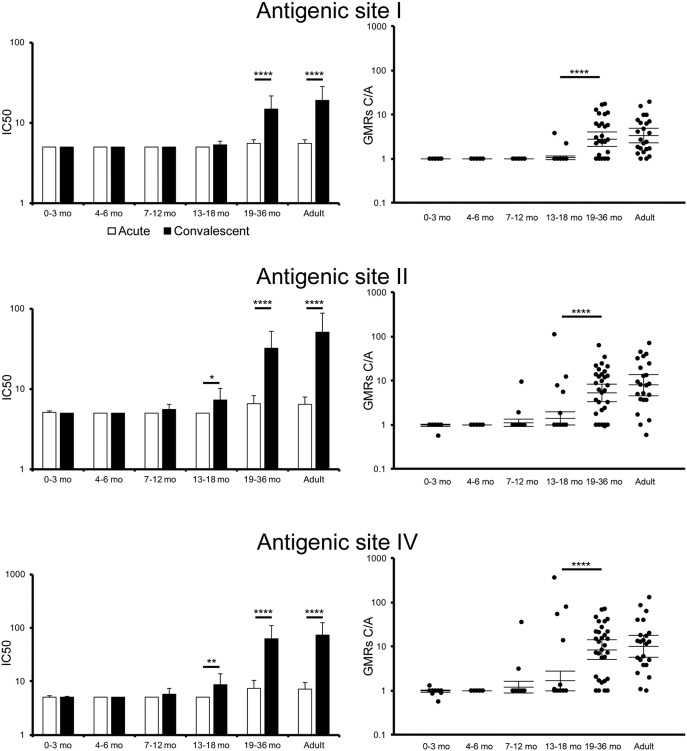
3.3. Antibody Responses to Neutralization Epitopes, Antigenic Sites I, II, and IV, in F Protein
Palivizumab is a prophylactic antibody drug indicated for the protection against RSV infections and known to bind to antigenic site II in F protein. In addition, early studies have elucidated other neutralization epitopes in F protein, called antigenic sites I and IV (Lopez et al., 1998). In order to examine the kinetics of antibodies specific to such neutralization epitopes in RSV infections, sera from children and adults were subjected to the competitive ELISA. As shown in Fig. 3, levels of all types of neutralization epitope-specific IgG, i.e., competitiveness for antigenic sites I, II, or IV, was higher in convalescent sera than in acute sera from children at 19 to 36 months old and adults, whereas levels of neutralization epitope II or IV-specific IgG were marginally induced in convalescent sera from children at 13 to 18 months old (Fig. 3). According to previous clinical studies, the trough level of palivizumab concentration in blood required for protection from severe RSV illness was demonstrated to be 40 μg/mL. In the case of adult patients, the serum concentration of calculated palivizumab-like neutralization antibody (PLNA) was 52.3 μg/mL (95% CI, 30.4–90.2) in convalescent sera, whereas in acute sera it was 6.52 μg/mL (95% CI, 5.19–8.20) (Table 3).
Fig. 3.
Evaluation of serum IgG to recognize antigenic sites I, II, and IV in F protein.
Sera were subjected to competitive ELISA by using monoclonal antibody specific to antigenic sites I, II, or IV. The amounts of serum IgG specific for each antigenic epitopes are represented by IC50 resulting in 50% reduction of biding RSV F with competitive monoclonal antibody. The limitation of the IC50 detection was defined as 5. The concentration of serum IgG specific to antigenic site II was calculated based on the result of palivizumab on competitive ELISA. The mean ± 95% CI was estimated by the linear model, and were shown in the graphs. IC50 of antigenic site I-specific IgG in acute phase: 0–3 mo, n = 29; 4–6 mo, n = 26; 7–12 mo, n = 28; 13–18 mo, n = 34; 19–36 mo, n = 32; adults, n = 23. IC50 of antigenic site I-specific IgG in convalescent phase: 0–3 mo, n = 24; 4–6 mo, n = 27; 7–12 mo, n = 32; 13–18 mo, n = 35; 19–36 mo, n = 32; adults, n = 23. GMRs C/A of antigenic site I-specific IgG: 0–3 mo, n = 21; 4–6 mo, n = 25; 7–12 mo, n = 26; 13–18 mo, n = 34; 19–36 mo, n = 31; adults, n = 23. IC50 of antigenic site II-specific IgG in acute phase: 0–3 mo, n = 29; 4–6 mo, n = 28; 7–12 mo, n = 28; 13–18 mo, n = 34; 19–36 mo, n = 33; adults, n = 23. IC50 of antigenic site II-specific IgG in convalescent phase: 0–3 mo, n = 27; 4–6 mo, n = 29; 7–12 mo, n = 32; 13–18 mo, n = 35; 19–36 mo, n = 33; adults, n = 23. GMRs C/A of antigenic site II-specific IgG: 0–3 mo, n = 24; 4–6 mo, n = 27; 7–12 mo, n = 26; 13–18 mo, n = 34; 19–36 mo, n = 32; adults, n = 23. IC50 of antigenic site IV-specific IgG in acute phase: 0–3 mo, n = 29; 4–6 mo, n = 28; 7–12 mo, n = 28; 13–18 mo, n = 34; 19–36 mo, n = 31; adults, n = 23. IC50 of antigenic site IV-specific IgG in convalescent phase: 0–3 mo, n = 27; 4–6 mo, n = 29; 7–12 mo, n = 32; 13–18 mo, n = 35; 19–36 mo, n = 33; adults, n = 23. GMRs C/A of antigenic site IV-specific IgG: 0–3 mo, n = 24; 4–6 mo, n = 27; 7–12 mo, n = 26; 13–18 mo, n = 34; 19–36 mo, n = 31; adults, n = 23. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Table 3.
Concentration and GMRs C/A of PLNA.
| Age group | PLNAa (95% CI) |
GMRs C/A of PLNA | p value⁎⁎ | |
|---|---|---|---|---|
| Acute | Convalescent | |||
| 0–3 mo | 5.17 (4.97–5.38) | 5.07 | 0.98 (0.93–1.03) | 0.8962 |
| 4–6 mo | 5.07 | 5.07 | 1.00 | 1.0000 |
| 7–12 mo | 5.07 | 5.56 (4.79–6.46) | 1.12 (0.93–1.35) | 0.5139 |
| 13–18 mo | 5.07 | 7.29 (5.20–10.2) | 1.41 (1.00–1.99) | 0.0241 |
| 19–36 mo | 6.82 (5.40–8.62) | 33.3 (20.3–54.8) | 5.30 (3.36–8.36) | < 0.0001 |
| Adult | 6.52 (5.19–8.20) | 52.3 (30.4–90.2) | 8.02 (4.60–14.0) | < 0.0001 |
The sample size in the acute phase: 0–3 mo, n = 29; 4–6 mo, n = 28; 7–12 mo, n = 28; 13–18 mo, n = 34; 19–36 mo, n = 33; adults, n = 23. The sample size in the convalescent phase: 0–3 mo, n = 27; 4–6 mo, n = 29; 7–12 mo, n = 32; 13–18 mo, n = 35; 19–36 mo, n = 33; adults, n = 23. The sample size in GMRs C/A of antigenic site II-specific IgG: 0–3 mo, n = 24; 4–6 mo, n = 27; 7–12 mo, n = 26; 13–18 mo, n = 34; 19–36 mo, n = 32; adults, n = 23.
Concentration of PLNA (μg/mL).
Statistical analysis was evaluated in concentrations of PLNA between the acute phase and the convalescent phase.
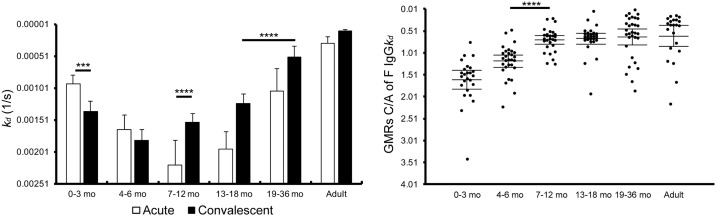
3.4. Age-specific Levels of the Antibody Affinity to F Protein
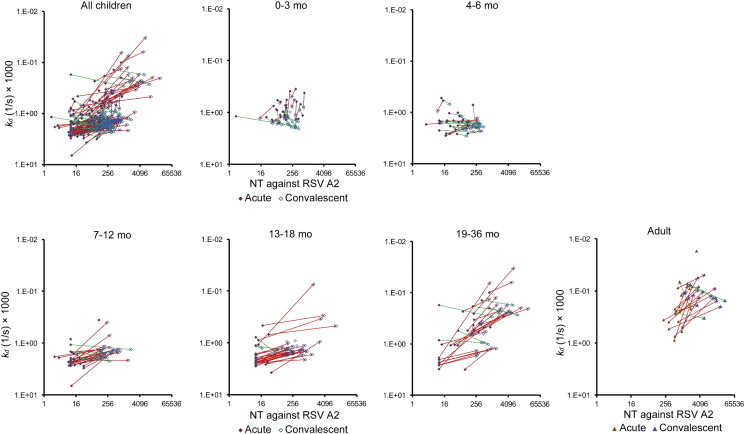
Previous studies have demonstrated that levels of the antibody affinity maturation to RSV antigens correlate well with the protection against RSV infections (Delgado et al., 2009, Khurana et al., 2011, Meurman et al., 1992). In order to evaluate the kinetics of the antibody affinity to F protein, levels of the binding stability of IgG specific to F protein were examined by surface plasmon resonance (SPR) assay. Since the binding stability was evaluated by values of apparent dissociation rate constant (kd), lower values of kd were considered as higher binding stability. As shown in Fig. 4, GMRs of kd of IgG specific to F protein (GMRs C/A of F IgGkd) were < 0.72 in children 7 months and older and adults, suggesting that the affinity maturation of IgG specific to F protein was induced. In contrast, GMRs C/A of F IgGkd were > 1.19 in infants < 7 months old, suggesting that in addition to amounts of maternally-derived antibodies to F protein decrease in the circulation of infants over time, the binding stability of maternal IgG to F protein also decrease in the circulation of infants. Taken together, it was suggested that maternal IgG to F protein decays in the circulation of infants over time in view of amount and quality (Fig. 4).
Fig. 4.
Antibody affinity to RSV F protein.
Sera were subjected to a surface plasmon resonance (SPR) assay. Apparent dissociation rate constants (kd) were calculated and shown in different age groups. The mean ± 95% CI was estimated by linear model, and were shown in the graphs. kd in acute phase: 0–3 mo, n = 30; 4–6 mo, n = 29; 7–12 mo, n = 30; 13–18 mo, n = 30; 19–36 mo, n = 32; adults, n = 22. kd in convalescent phase: 0–3 mo, n = 29; 4–6 mo, n = 30; 7–12 mo, n = 32; 13–18 mo, n = 35; 19–36 mo, n = 33; adults, n = 23. GMRs C/A of F IgG kd: 0–3 mo, n = 25; 4–6 mo, n = 29; 7–12 mo, n = 28; 13–18 mo, n = 30; 19–36 mo, n = 31; adults, n = 22. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
The kinetics and correlation between the serum neutralization activity and the antibody affinity maturation to F protein were further examined. The correlation was evaluated with all the data points derived from both acute and convalescent samples. As a result, the serum neutralization activity correlated with the kd of IgG specific to F protein [r = − 0.68, the estimated slope (b) = − 0.213]. The estimated slope between NT and F IgG kd is lower in children 7 months and older and adults (b = − 0.026 with p = 0.6961 in infants at 0 to 3 months old; b = 0.066 with p = 0.1954 in infants at 4 to 6 months old, b = − 0.116 with p = 0.0017 in infants at 7 to 12 months old; b = − 0.146 with p < 0.0001 in children at 13 to 18 months old; b = − 0.300 with p < 0.0001 in children at 19 to 36 months old; b = − 0.330 with p < 0.0001 in adults) (Fig. 5). In addition, the estimated slope between kd and NT was significantly different from 4 to 6 months old group to 7–12 months old group (p = 0.0042), and from 13 to 18 months old group to 19–36 months old group (p = 0.0006). These findings are well consistent with results of previous studies showing that the age-dependent antibody affinity maturation plays an important role in protection from viral infections (Delgado et al., 2009, Freitas et al., 2011, Meurman et al., 1992).
Fig. 5.
The correlation between the serum neutralization activity and the antibody affinity maturation to F protein.
Two-dimensional plots were prepared to investigate the correlation between serum neutralization titer (NT) and kd. Red arrows represents the changes from acute phase to convalescent phase to show an apparent positive correlation between IgG binding stability and NT, i.e., increase of kd accompanied with decrease of NT or decrease of kd with increase of NT. Green arrows represents the changes to show an apparent negative correlation between IgG binding stability and NT. In each age group, regression coefficient of NT on kd was calculated. 0–3 mo, n = 34; 4–6 mo, n = 30; 7–12 mo, n = 34; 13–18 mo, n = 35; 19–36 mo, n = 34; adults, n = 23.
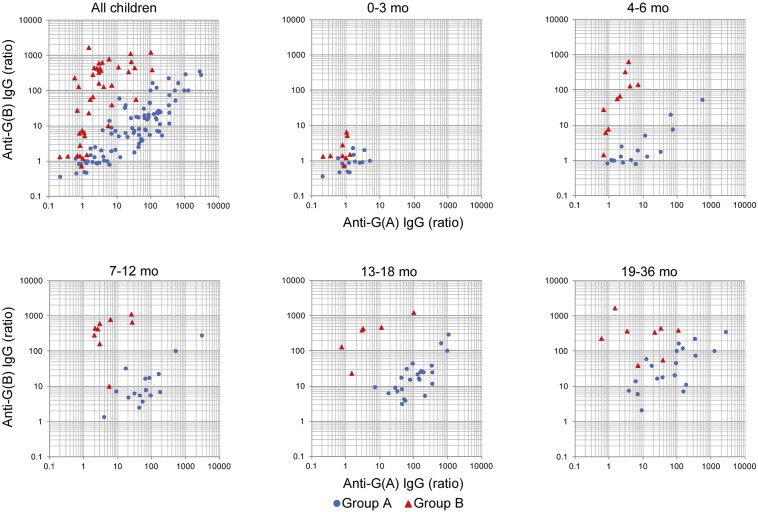
3.5. Age-specific Profiles of Antibody Responses to RSV G Proteins
Results of earlier studies suggested that G protein is also a target for neutralization antibody responses against RSV (Anderson et al., 1988, Miao et al., 2009). Because it has been reported that the homology of G protein sequence at the amino acid level is around 50% among circulating RSV strains, we examined the levels of IgG specific to G proteins of group A (G (A)) and group B (G (B)) separately, and analyzed the correlation between the induction of group-specific IgG for G protein and the RSV group infected during this research period (Supplemental Table 2 and Fig. 6). Similar to the kinetics of IgG for F protein, levels of IgG for G protein were not significantly increased in convalescent sera as compared with in acute sera in infants < 3 months old (Supplemental Table 2 and Fig. 6). GMRs C/A of G (A) IgG in children at 7 to 12 months old with RSV group A infection were 8.8-fold higher than that in children at 4 to 6 months old with RSV group A infection [64.3 (95% CI, 32.2–128), p < 0.0001 and 7.32 (95% CI, 3.66–14.6), p < 0.0001, respectively] (Fig. 6). Of interest, GMRs C/A of G (A) IgG in children at 7 to 12 months old with RSV group B infection were 5.05 (95% CI, 2.01–12.7), while those in children at 4 to 6 months old with RSV group B infection were 1.80 (95% CI, 0.75–4.33), suggesting that cross reactive antibody responses against G proteins are matured after 7 months old. Consistently, GMRs C/A of G (B) IgG in children at 7 to 12 months old with RSV A infection were 10.1 (95% CI, 5.57–18.5), but those in children at 4 to 6 months old were 2.12 (95% CI, 1.16–3.85) (Fig. 6). In addition, GMRs C/A of G (B) IgG in children aged at 7 to 12 and 13 to 18 months old with RSV A infection was significantly higher than that in children aged at 4 to 6 months old with RSV group A infection (7–12 mo vs 4–6 mo; p = 0.0004, 13–18 mo vs 4–6 mo; p < 0.0001), while GMRs C/A of G (A) IgG in children with RSV B infection were not significant among age groups at 4 to 6, 7 to 12 and 13 to 18 months old (7–12 mo vs 4–6 mo; p = 0.1118, 13–18 mo vs 4–6 mo; p = 0.1778, 7–12 mo vs 13–18 mo; p = 0.9459), suggesting that the primary RSV A infection induces better cross-reactive G responses than primary RSV B infection. Unfortunately, the sample size of adult subjects was not sufficient for evaluation of the cross reactivity. Taken together with results of the binding stability of IgG specific to F protein shown in Fig. 4, it was suggested that the cross reactive IgG for G proteins of both groups is significantly induced in accordance with the maturation of immune system after 7 months old. These findings also indicate that the evaluation of antibody responses specific to G protein are useful for the serological assessment of recent RSV infections in children 4 months and older and for assessment of infected RSV groups in children at 4 to 6 months old.
Fig. 6.
Serum antibody response to RSV G protein.
The sera were subjected to ELISA coating RSV G protein of group A or group B. Serum IgG concentrations against G of group A and group B in acute phase and convalescent phase were measured, and then GMRs C/A of total IgG specific for G protein were calculated. Data were analyzed in different age groups and in different RSV groups infected during the research period. Plots represent the GMRs C/A of G IgG. 0–3 mo: n = 16 (group A), n = 9 (group B); 4–6 mo: n = 16 (group A), n = 10 (group B); 7–12 mo: n = 16 (group A), n = 9 (group B); 13–18 mo: n = 25 (group A), n = 6 (group B); 19–36 mo: n = 19 (group A), n = 8 (group B).
4. Discussion
This is the study to perform quantitative and qualitative multivariate analyses of antibody responses to RSV and F protein. The results suggested that children's antibody responses to RSV are matured over months and years in at least 5 stages: 1st stage responses where IgG3 for F protein is significantly induced but levels of IgG3 for F protein in the convalescent phase (F IgG3 C) are low in infants at 0 to 3 months old; 2nd stage responses where F IgG3 C levels are average and GMRs C/A of NT, F IgG1, and F IgG2 levels are low in infants at 4 to 6 months old; 3rd stage responses where F IgG3 C levels are high, GMRs C/A of NT, F IgG1, and F IgG2 levels are high, and the antibody affinity maturation and the cross reactivity of IgG for G proteins of groups A and B are induced in infants at 7 months and older; 4th stage responses where low levels of neutralization epitope-specific IgG are induced in children at 13 to 18 months old; 5th stage responses where levels of NT, F IgG1, F IgG2, F neutralization epitope-specific IgG, and F IgGkd in the convalescent phase are high after repetitive RSV infections in children 19 months and older (Table 4).
Table 4.
Summary of children's antibody responses to RSV.
| Age group | Maternal antibody | Acquired antibody responses |
||||
|---|---|---|---|---|---|---|
| NT | IgG3 | Antibody affinity maturation | Cross reactivity to G proteins | Neutralization epitope-specific IgG | ||
| 0–3 mo | ++ | ± | + | − | − | − |
| 4–6 mo | + | + | ++ | − | − | − |
| 7–12 mo | − | ++ | +++ | + | + | − |
| 13–18 mo | − | ++ | +++ | + | + | + |
| 19–36 mo | − | +++ | +++ | ++ | + | ++ |
In accordance with the results of other previous clinical studies, in this study the presence of maternal antibody was also suggested in infants < 6 months old. Although the infants' own antibody responses to neutralize RSV were actually induced since all child participants recovered from RSV infection in the convalescent phase, it was suggested that serum neutralization titers induced by the infants' own immunity were too low to be discriminated in the presence of the maternal antibody.
In spite of different concentrations of IgG subclasses specific to F protein, the kinetics of IgG1 and IgG2 for F protein was similar to that of the serum neutralization activity. In contrast, although concentrations of IgG3 for F protein were almost 1/20 of IgG1, GMRs C/A of F IgG3 were higher even in newborns and young infants < 4 months old. The results suggested that levels of maternal IgG3 for F protein were low enough to discriminate infants' own IgG3 for F protein. Such evidence enables us to use it as one of the best biomarkers of infants' own immune responses to RSV infection, although biological roles of IgG3 for F protein in protection against RSV have not yet been elucidated. Since levels of IgG1 and IgG2 but not IgG3 are increased in accordance with the maturation of the immune system after birth, it was suggested that the relative levels of IgG3 to other subclasses decrease accordingly in life. In contrast, it has been proposed that a finite number of FcRn, an essential transporter of maternal systemic IgG to the fetal circulation, determines levels of total IgG transfer and ratios of IgG subclass transfer to the fetus. In addition, a previous research revealed that FcRn-mediated transport of IgG3 is inhibited in the presence of IgG1 (Stapleton et al., 2011). It was therefore suggested that a lower amount of IgG3 relative to IgG1 or IgG2 is transferred into the fetus.
Levels of serum IgG competitiveness with neutralization monoclonal antibodies also correlated with serum neutralization titers. The kinetics of PLNA in samples of children aged at 25 to 36 months old and adults reasonably correlated with the trough level of palivizumab, 40 μg/mL, in view of viral clearance in the course of the clinical stage. A recent study, however, has demonstrated that palivizumab has the potential to compete with both neutralization antibodies and non-neutralization antibodies, suggesting that PLNA evaluated in this study may contain non-neutralization antibodies. With regard to GMRs of PLNA titers, antigenic site I neutralization antibody titers, and antigenic site IV neutralization antibody titers, the lower limits of 95% CI were > 4.5, 2.2, and 5.8 in adults. In addition, percentages of significant higher levels, as determined by > 4-fold increase of GMRs of PLNA titers, antigenic site I neutralization antibody titers, and antigenic site IV neutralization antibody titers, were 69.6%, 43.5%, and 73.9% in adults, suggesting that such ratios reflect antibody responses specific to RSV infection.
In this study, RSV infections have been specified by using a rapid immunochromatographic diagnosis kit licensed in Japan. It is therefore suggested that the data is biased to small subset of RSV infected patients identified by real-time PCR due to such a kit may be less sensitive, although it is unlikely to have a significant effect on the overall findings.
Recent findings on the pre-fusion type of F protein have demonstrated that antigenic sites I, II, and IV are shared between the pre-fusion and post-fusion type of F protein, while antigenic site 0 is unique to the pre-fusion type of F protein (McLellan et al., 2013). In addition, other publications indicate that neutralization titers measure responses primarily to site 0 and other epitopes that are specific only to the pre-fusion F protein (Gilman et al., 2016, Ngwuta et al., 2015). Thus, correlations between neutralization titers and ELISA titers examined in this study may be limited.
Our study is the exploratory study for age-specific immune response against RSV infection without adjustments of multiple comparisons, and has a limitation that the results of statistical test are referential but not confirmatory. In addition, the other limitation in our study includes the small cohort size. Due to the limited cohort size of this study, immunological factors significantly induced following RSV infection might have been underestimated. Following up the results of this study, in-depth studies by following a larger cohort especially examining the correlation between clinical severities and immunological factors would elucidate true correlates of protein against RSV infections. Thus, age-specific profiles of antibody responses against RSV infection in this study are considered as hypothesis, and should be confirmed by further clinical data in future study.
In summary, this study elucidated levels and qualities of immune responses against RSV which are modulated by independent factors such as the development of children's immune system and the natural infection with RSV in the presence or absence of maternal antibodies. The kinetics of such responses could be harnessed for the setting of the vaccination regimen and the estimation of the protective efficacy in clinical development of RSV vaccines.
The following are the supplementary data related to this article.
List of abbreviations used in the text.
Serum IgG concentrations against G of group A and group B in acute phase and convalescent phase.
The sera were subjected to ELISA coating RSV G protein of group A or group B. Serum IgG concentrations against G of group A and group B in acute phase and convalescent phase were measured. Data were analyzed in different age groups and in different RSV groups infected during the research period. The sample size in acute phase: 0 − 3 mo: n = 18 (group A), n = 11 (group B); 4 − 6 mo: n = 16 (group A), n = 10 (group B); 7 − 12 mo: n = 16 (group A), n = 10 (group B); 13 − 18 mo: n = 25 (group A), n = 6 (group B); 19 − 36 mo: n = 21 (group A), n = 8 (group B). The sample size in convalescent phase: 0 − 3 mo: n = 18 (group A), n = 11 (group B); 4 − 6 mo: n = 16 (group A), n = 11 (group B); 7 − 12 mo: n = 17 (group A), n = 12 (group B); 13 − 18 mo: n = 25 (group A), n = 6 (group B); 19 − 36 mo: n = 19 (group A), n = 8 (group B). The data represented the geometric means with corresponding 95%CI.
Funding Sources
This research was supported by NexTEP grant (#J13-09) from Japan Science and Technology.
Conflicts of Interest
Masaaki Mori received the consulting fee from Daiichi Sankyo Co., Ltd.
Ken J Ishii received the grant from Daiichi Sankyo Co., Ltd.
Nao Jounai, Megumi Yoshioka, Miyuki Tozuka, Kazue Inoue, Tatsuya Oka, and Kazuki Miyaji are employees of Kitasato Daiichi Sankyo Vaccine Co., Ltd.
Katsuyasu Ishida and Fumihiko Takeshita are employees of Daiichi Sankyo Co., Ltd.
Author Contributions
N.J., M.Y., M.T., K·Inoue, T.O., K.M., K.J.I., and F.T. designed and performed the research and analyzed the data. K·Ishida, N.K., H.I., C.K., H.S., and M.M. designed and performed the recruitment of patients with RSV infection, and prepared the blood and flocked swab samples. N.J., M.T., T.O., and K.M. performed ELISA of RSV F and G protein. M.Y. performed competitive ELISA and SPR assay. K. Inoue performed Microneutralization assay. N.J., M.Y., M.T., K·Inoue, T.O., K.M., K·Ishida, N.K., H.I., C.K., H.S., M.M., K.J.I., and F.T. wrote and revised the manuscript throughout.
Acknowledgements
We thank the members of K.J.I.’s laboratory and Vaccine Research Laboratories of Kitasato Daiichi Sankyo Vaccine Co., Ltd. for valuable comments and help.
References
- Anderson L.J., Bingham P., Hierholzer J.C. Neutralization of respiratory syncytial virus by individual and mixtures of F and G protein monoclonal antibodies. J. Virol. 1988;62:4232–4238. doi: 10.1128/jvi.62.11.4232-4238.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacharier L.B., Cohen R., Schweiger T., Yin-Declue H., Christie C., Zheng J., Schechtman K.B., Strunk R.C., Castro M. Determinants of asthma after severe respiratory syncytial virus bronchiolitis. J. Allergy Clin. Immunol. 2012;130:91–100. doi: 10.1016/j.jaci.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M.J., Valentine F.T. Viral commensalism in humans? J. Infect. Dis. 2008;198:1–3. doi: 10.1086/588705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R.Y., Dong D.Y., Liu R.J., Han J.F., Wang G.C., Zhao H., Li X.F., Deng Y.Q., Zhu S.Y., Wang X.Y. Human IgG subclasses against enterovirus Type 71: neutralization versus antibody dependent enhancement of infection. PLoS One. 2013;8 doi: 10.1371/journal.pone.0064024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H.Y., Steinhoff M.C., Magaret A., Zaman K., Roy E., Langdon G., Formica M.A., Walsh E.E., Englund J.A. Respiratory syncytial virus transplacental antibody transfer and kinetics in mother-infant pairs in Bangladesh. J. Infect. Dis. 2014;210:1582–1589. doi: 10.1093/infdis/jiu316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M.F., Coviello S., Monsalvo A.C., Melendi G.A., Hernandez J.Z., Batalle J.P., Diaz L., Trento A., Chang H.Y., Mitzner W. Lack of antibody affinity maturation due to poor toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat. Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito S., Scarselli E., Lelii M., Scala A., Vitelli A., Capone S., Fornili M., Biganzoli E., Orenti A., Nicosia A. Antibody response to respiratory syncytial virus infection in children < 18 months old. Hum. Vaccin. Immunother. 2016;12:1700–1706. doi: 10.1080/21645515.2016.1145847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey A.R., Walsh E.E. Relationship of serum antibody to risk of respiratory syncytial virus infection in elderly adults. J. Infect. Dis. 1998;177:463–466. doi: 10.1086/517376. [DOI] [PubMed] [Google Scholar]
- Fleming D.M., Taylor R.J., Lustig R.L., Schuck-Paim C., Haguinet F., Webb D.J., Logie J., Matias G., Taylor S. Modelling estimates of the burden of respiratory syncytial virus infection in adults and the elderly in the United Kingdom. BMC Infect. Dis. 2015;15:443. doi: 10.1186/s12879-015-1218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas G.R., Silva D.A., Yokosawa J., Paula N.T., Costa L.F., Carneiro B.M., Ribeiro L.Z., Oliveira T.F., Mineo J.R., Queiroz D.A. Antibody response and avidity of respiratory syncytial virus-specific total IgG, IgG1, and IgG3 in young children. J. Med. Virol. 2011;83:1826–1833. doi: 10.1002/jmv.22134. [DOI] [PubMed] [Google Scholar]
- Gilman M.S., Castellanos C.A., Chen M., Ngwuta J.O., Goodwin E., Moin S.M., Mas V., Melero J.A., Wright P.F., Graham B.S. Rapid profiling of RSV antibody repertoires from the memory B cells of naturally infected adult donors. Sci. Immunol. 2016;1 doi: 10.1126/sciimmunol.aaj1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groothuis J.R., Simoes E.A., Levin M.J., Hall C.B., Long C.E., Rodriguez W.J., Arrobio J., Meissner H.C., Fulton D.R., Welliver R.C. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. N. Engl. J. Med. 1993;329:1524–1530. doi: 10.1056/NEJM199311183292102. [DOI] [PubMed] [Google Scholar]
- Hall C.B., Weinberg G.A., Blumkin A.K., Edwards K.M., Staat M.A., Schultz A.F., Poehling K.A., Szilagyi P.G., Griffin M.R., Williams J.V. Respiratory syncytial virus-associated hospitalizations among children < 24 months of age. Pediatrics. 2013;132:e341–e348. doi: 10.1542/peds.2013-0303. [DOI] [PubMed] [Google Scholar]
- Hall C.B., Weinberg G.A., Iwane M.K., Blumkin A.K., Edwards K.M., Staat M.A., Auinger P., Griffin M.R., Poehling K.A., Erdman D. The burden of respiratory syncytial virus infection in young children. N. Engl. J. Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R.G. Human IgG subclass measurements in the clinical laboratory. Clin. Chem. 1987;33:1707–1725. [PubMed] [Google Scholar]
- Higgins D., Trujillo C., Keech C. Advances in RSV vaccine research and development - a global agenda. Vaccine. 2016;34:2870–2875. doi: 10.1016/j.vaccine.2016.03.109. [DOI] [PubMed] [Google Scholar]
- Hofmeister Y., Planitzer C.B., Farcet M.R., Teschner W., Butterweck H.A., Weber A., Holzer G.W., Kreil T.R. Human IgG subclasses: in vitro neutralization of and in vivo protection against West Nile virus. J. Virol. 2011;85:1896–1899. doi: 10.1128/JVI.02155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani V., Guy A.J., Andrew D., Beeson J.G., Ramsland P.A., Richards J.S. Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Mol. Immunol. 2015;67:171–182. doi: 10.1016/j.molimm.2015.03.255. [DOI] [PubMed] [Google Scholar]
- Karron R.A., Luongo C., Thumar B., Loehr K.M., Englund J.A., Collins P.L., Buchholz U.J. A gene deletion that up-regulates viral gene expression yields an attenuated RSV vaccine with improved antibody responses in children. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.aac8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana S., Verma N., Yewdell J.W., Hilbert A.K., Castellino F., Lattanzi M., Del Giudice G., Rappuoli R., Golding H. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsaya A., Teros-Jaakkola T., Kakkola L., Toivonen L., Peltola V., Waris M., Julkunen I. Prospective clinical and serological follow-up in early childhood reveals a high rate of subclinical RSV infection and a relatively high reinfection rate within the first 3 years of life. Epidemiol. Infect. 2016;144:1622–1633. doi: 10.1017/S0950268815003143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J.A., Bustos R., Orvell C., Berois M., Arbiza J., Garcia-Barreno B., Melero J.A. Antigenic structure of human respiratory syncytial virus fusion glycoprotein. J. Virol. 1998;72:6922–6928. doi: 10.1128/jvi.72.8.6922-6928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V., Abraham J., Adair T., Aggarwal R., Ahn S.Y. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan J.S., Chen M., Leung S., Graepel K.W., Du X., Yang Y., Zhou T., Baxa U., Yasuda E., Beaumont T. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340:1113–1117. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurman O., Waris M., Hedman K. Immunoglobulin G antibody avidity in patients with respiratory syncytial virus infection. J. Clin. Microbiol. 1992;30:1479–1484. doi: 10.1128/jcm.30.6.1479-1484.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao C., Radu G.U., Caidi H., Tripp R.A., Anderson L.J., Haynes L.M. Treatment with respiratory syncytial virus G glycoprotein monoclonal antibody or F(ab')2 components mediates reduced pulmonary inflammation in mice. J. Gen. Virol. 2009;90:1119–1123. doi: 10.1099/vir.0.009308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsume A., In M., Takamura H., Nakagawa T., Shimizu Y., Kitajima K., Wakitani M., Ohta S., Satoh M., Shitara K., Niwa R. Engineered antibodies of IgG1/IgG3 mixed isotype with enhanced cytotoxic activities. Cancer Res. 2008;68:3863–3872. doi: 10.1158/0008-5472.CAN-07-6297. [DOI] [PubMed] [Google Scholar]
- Ngwuta J.O., Chen M., Modjarrad K., Joyce M.G., Kanekiyo M., Kumar A., Yassine H.M., Moin S.M., Killikelly A.M., Chuang G.Y. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.aac4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoko B.J., Wesumperuma L.H., Ota M.O., Pinder M., Banya W., Gomez S.F., McAdam K.P., Hart A.C. The influence of placental malaria infection and maternal hypergammaglobulinemia on transplacental transfer of antibodies and IgG subclasses in a rural West African population. J. Infect. Dis. 2001;184:627–632. doi: 10.1086/322808. [DOI] [PubMed] [Google Scholar]
- Parnes C., Guillermin J., Habersang R., Nicholes P., Chawla V., Kelly T., Fishbein J., McRae P., Goessler M., Gatti A. Palivizumab prophylaxis of respiratory syncytial virus disease in 2000-2001: results from The Palivizumab Outcomes Registry. Pediatr. Pulmonol. 2003;35:484–489. doi: 10.1002/ppul.10288. [DOI] [PubMed] [Google Scholar]
- Piedra P.A., Jewell A.M., Cron S.G., Atmar R.L., Glezen W.P. Correlates of immunity to respiratory syncytial virus (RSV) associated-hospitalization: establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine. 2003;21:3479–3482. doi: 10.1016/s0264-410x(03)00355-4. [DOI] [PubMed] [Google Scholar]
- Resch B. Burden of respiratory syncytial virus infection in young children. World J. Clin. Pediatr. 2012;1:8–12. doi: 10.5409/wjcp.v1.i3.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sande C.J., Cane P.A., Nokes D.J. The association between age and the development of respiratory syncytial virus neutralising antibody responses following natural infection in infants. Vaccine. 2014;32:4726–4729. doi: 10.1016/j.vaccine.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf O., Golding H., King L.R., Eller N., Frazier D., Golding B., Scott D.E. Immunoglobulin G3 from polyclonal human immunodeficiency virus (HIV) immune globulin is more potent than other subclasses in neutralizing HIV type 1. J. Virol. 2001;75:6558–6565. doi: 10.1128/JVI.75.14.6558-6565.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton N.M., Andersen J.T., Stemerding A.M., Bjarnarson S.P., Verheul R.C., Gerritsen J., Zhao Y., Kleijer M., Sandlie I., de Haas M. Competition for FcRn-mediated transport gives rise to short half-life of human IgG3 and offers therapeutic potential. Nat. Commun. 2011;2:599. doi: 10.1038/ncomms1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The IMpact-RSV Study Group Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- Wright P.F., Gruber W.C., Peters M., Reed G., Zhu Y., Robinson F., Coleman-Dockery S., Graham B.S. Illness severity, viral shedding, and antibody responses in infants hospitalized with bronchiolitis caused by respiratory syncytial virus. J. Infect. Dis. 2002;185:1011–1018. doi: 10.1086/339822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of abbreviations used in the text.
Serum IgG concentrations against G of group A and group B in acute phase and convalescent phase.
The sera were subjected to ELISA coating RSV G protein of group A or group B. Serum IgG concentrations against G of group A and group B in acute phase and convalescent phase were measured. Data were analyzed in different age groups and in different RSV groups infected during the research period. The sample size in acute phase: 0 − 3 mo: n = 18 (group A), n = 11 (group B); 4 − 6 mo: n = 16 (group A), n = 10 (group B); 7 − 12 mo: n = 16 (group A), n = 10 (group B); 13 − 18 mo: n = 25 (group A), n = 6 (group B); 19 − 36 mo: n = 21 (group A), n = 8 (group B). The sample size in convalescent phase: 0 − 3 mo: n = 18 (group A), n = 11 (group B); 4 − 6 mo: n = 16 (group A), n = 11 (group B); 7 − 12 mo: n = 17 (group A), n = 12 (group B); 13 − 18 mo: n = 25 (group A), n = 6 (group B); 19 − 36 mo: n = 19 (group A), n = 8 (group B). The data represented the geometric means with corresponding 95%CI.