Abstract
Background
Excessive androgen exposure during pregnancy has been suggested to induce diabetic phenotypes in offspring in animal models. The aim of this study was to investigate whether pregestational maternal hyperandrogenism in human influenced the glucose metabolism in offspring via epigenetic memory from mother's oocyte to child's somatic cells.
Methods
Of 1782 reproductive-aged women detected pregestational serum androgen, 1406 were pregnant between 2005 and 2010. Of 1198 women who delivered, 1116 eligible mothers (147 with hyperandrogenism and 969 normal) were recruited. 1216 children (156 children born to mothers with hyperandrogenism and 1060 born to normal mother) were followed up their glycometabolism in mean age of 5 years. Imprinting genes of oocyte from mothers and lymphocytes from children were examined. A pregestational hyperandrogenism rat model was also established.
Findings
Children born to women with hyperandrogenism showed increased serum fasting glucose and insulin levels, and were more prone to prediabetes (adjusted RR: 3.98 (95%CI 1.16–13.58)). Oocytes from women with hyperandrogenism showed increased insulin-like growth factor 2 (IGF2) expression. Lymphocytes from their children also showed increased IGF2 expression and decreased IGF2 methylation. Treatment of human oocytes with dihydrotestosterone upregulated IGF2 and downregulated DNMT3a levels. In rat, pregestational hyperandrogenism induced diabetic phenotypes and impaired insulin secretion in offspring. In consistent with the findings in human, hyperandrogenism also increased Igf2 expression and decreased DNMT3a in rat oocytes. Importantly, the same altered methylation signatures of Igf2 were identified in the offspring pancreatic islets.
Interpretation
Pregestational hyperandrogenism may predispose offspring to glucose metabolism disorder via epigenetic oocyte inheritance.
Clinical trial registry no.: ChiCTR-OCC-14004537; www.chictr.org.
Keywords: Hyperandrogenism, Offspring, Prediabetes, Epigenetics
Highlights
-
•
Maternal hyperandrogenism may increase the risks of glucose metabolism disorder and prediabetes in their children.
-
•
High androgen levels in women may directly increased IGF2 expression and decreased IGF2 methylation in oocytes
-
•
Intergenerational inheritance of epigenetic alteration could be regarded important in determining development of diabetes.
Hyperandrogenemia can be observed in most patients with polycystic ovarian syndrome that is a common endocrine disorder in women of reproductive age, especially in subfertile women. We found that maternal hyperandrogenism may increase the risks of glucose metabolism disorder and prediabetes in their children. Also, Data from human and rat suggest that this glucose metabolism disorder may be mediated by DNA methylation modifications, and this kind of epigenetic modification may be transmitted from oocytes of mothers to somatic cells of offspring. Hence, intergenerational inheritance of epigenetic alteration should be regarded important in determining development of diabetes in the future.
1. Introduction
Hyperandrogenism is a common endocrine disorder among reproductive aged women (Qiao and Feng, 2011). Excessive concentration of androgen in ovarian follicles may alter oocyte developmental competence that consequently decreases the fertilization rate and embryonic development during in vitro fertilization (IVF) (Sen et al., 2014, Murray et al., 2008, Teissier et al., 2000). Animal studies showed that intrauterine testosterone exposure induced metabolic alterations, including reduced glucose stimulated insulin secretion and impaired glucose tolerance, in offspring not only at early age but also in adulthood (Amalfi et al., 2012, Nohara et al., 2013a). However, the long term health implications of children conceived by women with hyperandrogenism remain largely unknown.
Human epidemiological studies have indicated the inheritance of environment-induced phenotype (Huang and Sheng, 2014). Offspring of in utero malnutrition are associated with increased prevalence of type 2 diabetes risk (Petry and Hales, 2000). Famine experiences in grandfathers increased the risk of obesity and cardiovascular diseases in grandchildren (Painter et al., 2008). Although the exact mechanism is unclear, these studies indicate that the environmental factors are associated with the inheritance of disease risk in next generations.
Recently, a series of animal studies have revealed that adverse paternal factors increase the susceptibility to adult metabolic diseases in offspring through gametic epigenetic alterations (Carone et al., 2010, Radford et al., 2014, Wei et al., 2015). Paternal high-fat-diet exposure may program β-cell dysfunction in first-generation (F1) female offspring in rat (Ng et al., 2010). Intrauterine undernutrition impairs lipid metabolism in second-generation (F2) offspring by transmission of DNA methylation alteration via paternal linage (Martinez et al., 2014). Likewise, paternal prediabetes can be transgenerationally inherited through the male germ line depending on methylation changes in mice (Wei et al., 2014). A recent study in rodent, using IVF and embryo transfer (IVF-ET) and foster mother, demonstrated that a parental high-fat diet renders offspring more susceptible to developing obesity and diabetes in a sex- and parent of origin-specific mode (Huypens et al., 2016). Two studies showed that maternal diabetes and obesity resulted in altered oocyte methylation patterns of specific genes (Ge et al., 2013, Ge et al., 2014). Hence, it is very important to investigate whether oocytes, especially human oocytes, from mother with endocrine disorders are associated with increased risks of metabolic disorders in their children, and what mechanisms are involved.
The aim of this study was to investigate whether mothers with pregestational hyperandrogenism could predispose offspring to glucose metabolism disorder through epigenetic oocyte inheritance. Our results demonstrated that children born to mothers with pregestational hyperandrogenism manifested increased serum fasting glucose and insulin levels, and were more prone to prediabetes. High androgen levels significantly upregulated IGF2, an imprinting gene, expression in human oocytes. In parallel, study in rats clearly showed that pregestational hyperandrogenism induced diabetic phenotypes and impaired insulin secretion in offspring. Exposure of rat oocytes with high androgen concentration increased the expression level of Igf2 not only in mothers' oocytes but also in β-cells of F1 pancreatic islets.
2. Materials & Methods
2.1. Participants and Prospective Cohort Study Design
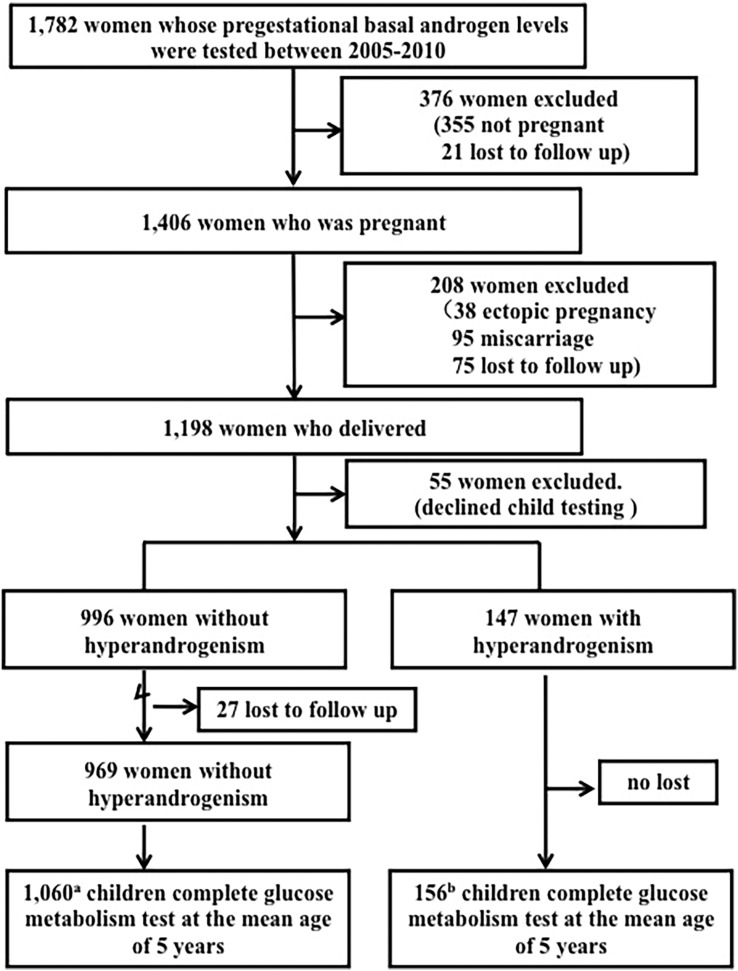
The study was approved by the ethical committee of School of Medicine, Zhejiang University, Hangzhou, China. Total of 1782 women, who registered in Reproductive Center, Women's Hospital, School of Medicine, Zhejiang University for IVF between 2005 and 2010, voluntarily participated in this study. All participants provided informed written consents. Their basal hormones including testosterone (TTE), dehydroepiandrosterone (DHEAS) levels were examined at menstrual cycle day-3. Women were excluded if they were not pregnant till December 2010 (n = 355) or withdrew consents (n = 21). Among the remaining 1406 pregnant women, 38 were with ectopic pregnancy, 95 went through miscarriage and 75 women were lost to follow up before delivery. After delivery, 55 women declined child testing. The inclusion criteria for hyperandrogenism group were basal TTE level higher than 2.4 nmol/L and/or basal DHEAS level higher than 8.8 μmol/L. During the follow up period, 27 women without hyperandrogenism were lost to follow up. Finally, 156 children born to women with hyperandrogenism and 1060 children born to women without hyperandrogenism attended our face to face follow up. Twins were handled as 2 separate children. None of the children had significant medical conditions which might affect their development. Children were followed up for 2–7 years after they were born (mean ages about 5 years).
2.2. Anthropometrics and Glycometabolism of Children
Children were required to attend the hospital at 8 am after an overnight fasting. Blood pressure measurements were performed after 20 min of quiet rest. Prediabetes was diagnosed if fasting glucose was 5.6–6.9 mmol/L. Oral glucose tolerance test (OGTT) was administrated in 132 singleton children born to women without hyperandrogenism and 80 singleton children born to women with hyperandrogenism after taking glucose at a dose of 1.75 g/kg body weight.
2.3. Human Oocytes and Lymphocytes Collection
Human MII oocytes were donated by the patients undergoing IVF/intra-cytoplasmic sperm injection (ICSI) procedure. All patients signed consent forms. Oocytes (n = 10) from women with hyperandrogenism and oocytes (n = 12) from control women were directly used for immunofluorescence analysis. Additional 20 oocytes from control women were cultured with 0 M (n = 10) and 10− 9 M (n = 10) DHT for 30 h at 37 °C in a humidified atmosphere of 5% CO2 in air. Lymphocytes from children were separated using density gradient and stored at − 80 °C for further experiments.
2.4. Pregestational Hyperandrogenism Rat Model
Rat experiments were approved by the Animal Care and Use Committee, Zhejiang University. Hyperandrogenism female rat model was established using testosterone propionate injection at age of 9 days as previously described (Fan et al., 2012). At 6 weeks old, serum testosterone/sex hormone-binding globulin (SHBG) levels were examined using ELISA kit (R&D Systems, Minneapolis, MN, USA). Ovarian CYP17a was examined by immunohistochemical analysis (anti-CYP17a antibody, 1:200, Bioss, MA, USA; and CWBIO DAB kit, Guangzhou, China).
Mature oocytes were collected from 6-week-old rats. Meanwhile, hyperandrogenism female rats were mated with normal male rats, and pregnancy was determined by the presence of copulation plug.
2.5. Rat Offspring Glycometabolism and Islet Isolation
Glucose tolerance tests (GTT) of offspring were performed at 3-week-old and 8-week-old rats (male and female numbers were even in each groups). Offspring pancreatic islets were isolated at embryonic day 20 (E20), 3 weeks and 8 weeks old, respectively, as previously reported (Ding et al., 2012).
2.6. Quantitative Real Time-PCR and Bisulfite Genomic Sequencing PCR (BSP)
Total RNA was extracted from human peripheral lymphocytes and rat islets using RNAiso™ Reagent (TAKARA, Dalian, China), and cDNA was reverse-transcribed using the PrimeScript™ RT Reagent Kit (TAKARA). The methylation status of human IGF2, GRB10 genes and rat Igf2 gene were analyzed by sequencing of bisulfite-treated DNA using the EpiTect bisulfite kit (Qiagen). The schematic diagrams of CpG sites in human IGF2 and rat Igf2 were shown in Supplementary Fig. 1.
2.7. Immunofluorescence
Human oocytes were incubated with rabbit primary anti-IGF2 antibody (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or rabbit primary anti-DNMT3a antibody (1:200; Santa Cruz Biotechnology) at 4 °C overnight, and rat oocytes were incubated with rabbit primary anti-DNMT3a antibody (1:200; Santa Cruz Biotechnology) under the same condition. Alexa Fluor 594 goat anti-rabbit IgG (1:400; Invitrogen, Carlsbad, CA, USA) and Alexa Fluor 488 goat anti-rabbit IgG secondary antibody (1:400; Invitrogen) were used, respectively.
2.8. Statistical Analysis
Data were analyzed using SPSS 16.0 for Windows (SPSS, Inc., Chicago, USA). We estimated crude and adjusted relative risks (RRs) with 95% confidence intervals using logistic regression. The independent-samples t-test was used to evaluate statistical significance between two groups. Chi-square test was used to compare quantitative data between two groups. P value < 0.05 was considered statistically significant.
3. Results
3.1. Children Born to Mothers with Pregestational Hyperandrogenism Were More Susceptible to Prediabetes
The flow chart of our prospective cohort study was presented in Fig. 1. Of 1782 reproductive-aged women detected androgen levels in pregestation, 1406 were pregnant (accumulating pregnancy rate: 78.90%) between 2005 and 2010. Of 1198 women who delivered, 1116 eligible mothers (147 women with hyperandrogenism and 969 normal women) were recruited (93.16%). Information about their 1216 children (1060 from normal mothers and 156 from women with hyperandrogenism) was available. Perinatal data showed that birth weight and gestational weeks were comparable (Supplementary Table 1). Offspring ages at follow-up were 59.85 ± 13.05 and 60.42 ± 12.40 months born to women with and without hyperandrogenism, respectively. As presented in Table 1, there were no significant differences in baseline index of children's anthropometry, blood pressures and fasting blood lipid levels. Fasting glucose, insulin and HOMA-IR levels were significantly higher in the children born to women with hyperandrogenism, although the BMI was comparable. There were no significant differences in metabolic phenotypes between male and female (Supplementary Table 2). Furthermore, OGTT demonstrated that both glucose and insulin levels at fasting and 120 min after glucose intake were significantly elevated in children born to women with hyperandrogenism. Importantly, we found that 2.56% (4/156) children born to mothers with hyperandrogenism and 0.66% (7/1060) children born to mothers without hyperandrogenism manifested prediabetes (crude RR = 3.88 (95%CI 1.15–13.20), adjusted RR = 3.98 (95%CI 1.16–13.58), Table 2), indicating that offspring born to mothers with hyperandrogenism was more susceptible to prediabetes.
Fig. 1.
Flow chart of the clinic study and follow-up of the study participants.
a, 878 singletons and 182 twins. b, 138 singletons and 18 twins.
Table 1.
Follow-up characteristics of children born to mothers without or with hyperandrogenism.
| Without hyperandrogenism | With hyperandrogenism | p-Value | |
|---|---|---|---|
| No. of subjects | 1060 | 156 | |
| Age (months) | 59.9 ± 13.1 | 60.4 ± 12.5 | NS |
| BMI (kg/m2) | 15.3 ± 1.1 | 15.6 ± 1.6 | NS |
| Blood pressure (mmHg) | |||
| Systolic blood pressure | 98.1 ± 13.9 | 98.1 ± 13.9 | NS |
| Diastolic blood pressure | 55.8 ± 10.1 | 55.7 ± 10.2 | NS |
| Blood lipids (mmol/L) | |||
| Triglyceride | 0.72 ± 0.20 | 0.71 ± 0.27 | NS |
| Cholesterol | 4.29 ± 0.70 | 4.33 ± 0.89 | NS |
| HDL | 1.49 ± 0.27 | 1.45 ± 0.24 | NS |
| LDL | 2.29 ± 0.56 | 2.41 ± 0.71 | NS |
| Fasting glucose (mmol/L) | 4.73 ± 0.43 | 4.82 ± 0.43 | < 0.05 |
| Fasting insulin (μU/mL) | 3.52 ± 1.62 | 4.13 ± 2.69 | < 0.01 |
| HOMA-IR | 0.80 ± 0.45 | 0.89 ± 0.72 | < 0.05 |
| OGTTa | |||
| Fasting glucose (mmol/L) | 4.60 ± 0.41 | 4.72 ± 0.37 | < 0.05 |
| 60 min glucose (mmol/L) | 6.08 ± 0.81 | 6.05 ± 0.42 | NS |
| 120 min glucose (mmol/L) | 4.85 ± 0.70 | 5.13 ± 0.73 | < 0.05 |
| Fasting insulin (μU/mL) | 3.12 ± 1.88 | 3.58 ± 1.62 | < 0.05 |
| 60 min insulin (μU/mL) | 13.51 ± 7.38 | 15.76 ± 8.67 | NS |
| 120 min insulin (μU/mL) | 4.72 ± 4.09 | 10.53 ± 5.25 | < 0.01 |
Data = mean ± SD, P values were calculated by t-test.
n = 132 and 80 for singleton born to mother without and with hyperandrogenism, respectively.
Table 2.
Odds ratios of prediabetes in children born to women without and with hyperandrogenism.
| Prediabetes | Without hyperandrogenism (n, %) |
With hyperandrogenism (n, %) |
Crude RR (95%CI) |
Adjusted RRa (95%CI) |
|---|---|---|---|---|
| Yes | 7 (0.66%) | 4 (2.56%) | 3.88 (1.15,13.20) | 3.98 (1.16,13.58) |
| No | 1053 (99.34%) | 152 (97.44) | 1 | 1 |
Adjusted for children's age, BMI, maternal preeclampsia and gestational diabetes.
3.2. Hyperandrogenism Upregulated IGF2 Expression in Human Oocytes and Offspring Lymphocyte
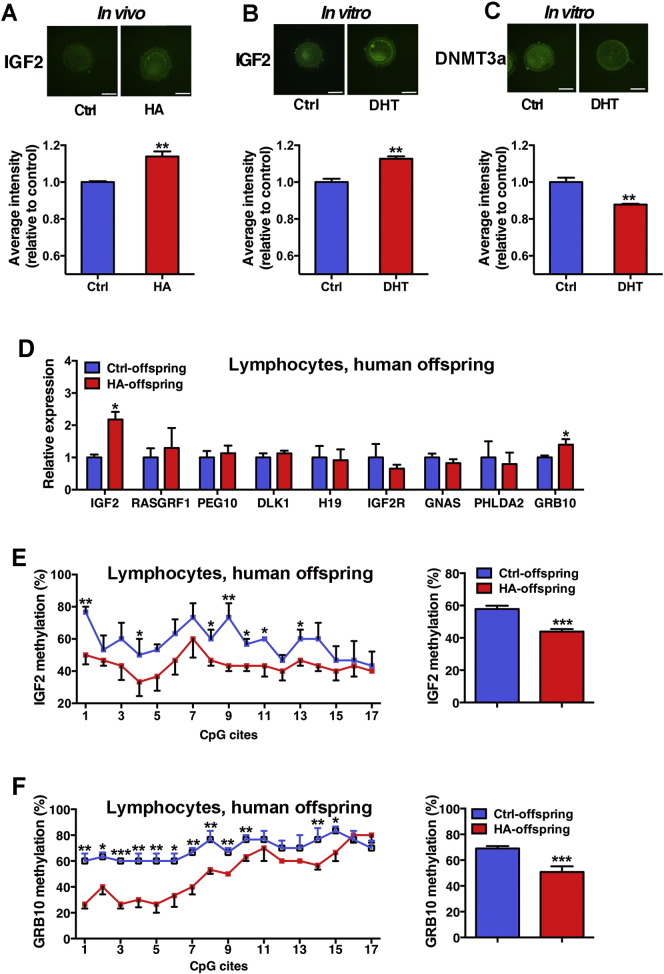
Oocytes from women with hyperandrogenism demonstrated an increased expression level of IGF2 (Fig. 2A). In vitro experiment verified that treatment of human oocytes with DHT significantly upregulated IGF2 expression (Fig. 2B) while downregulated DNMT3a expression (Fig. 2C). Real-time PCR analysis showed that among 9 imprinting genes related to glucose metabolism, IGF2, a maternal imprinting gene, and GRB10, an equal biallelic expressed imprinting gene, levels were significantly upregulated in offspring born to women with hyperandrogenism (Fig. 2D). We conducted BSP to check the methylation rates of IGF2 and GRB10 genes. The overall methylation rate in DMR2 of IGF2 in offspring born to women with hyperandrogenism was significantly decreased (Fig. 2E). Meanwhile, the methylation rate in DMR of GRB10 was also decreased (Fig. 2F).
Fig. 2.
Imprinting genes in human oocytes (mother) and lymphocytes (offspring). A: IGF2 expression in oocytes donated by women with hyperandrogenism (HA, n = 10) or without hyperandrogenism (control, n = 12) was detected by immunofluorescence. B and C: Effects of 10− 9 M DHT on IGF2 and DNMT3a expression in human oocytes donated by women without hyperandrogenism were examined by immunofluorescence (n = 5 for each group). D: Expression of imprinting genes in lymphocyte of offspring conceived by women with (HA, n = 80) or without hyperandrogenism (control, n = 146). E and F: Methylation status of IGF2 and GRB10 in lymphocyte of offspring. Methylation status of the IGF2 DMR2 and GRB10 DMR was analyzed by BSP (n = 3 per group). The methylation rates of individual CpG sites within the IGF2 DMR2 and GRB10 DMR were presented as a line graph. *p < 0.05, vs. Ctrl, **p < 0.01, vs. Ctrl, ***p < 0.001, vs. Ctrl.
3.3. Pregestational Hyperandrogenism Induced Early-onset Diabetes Due to Impaired Insulin Secretion in Rat Offspring
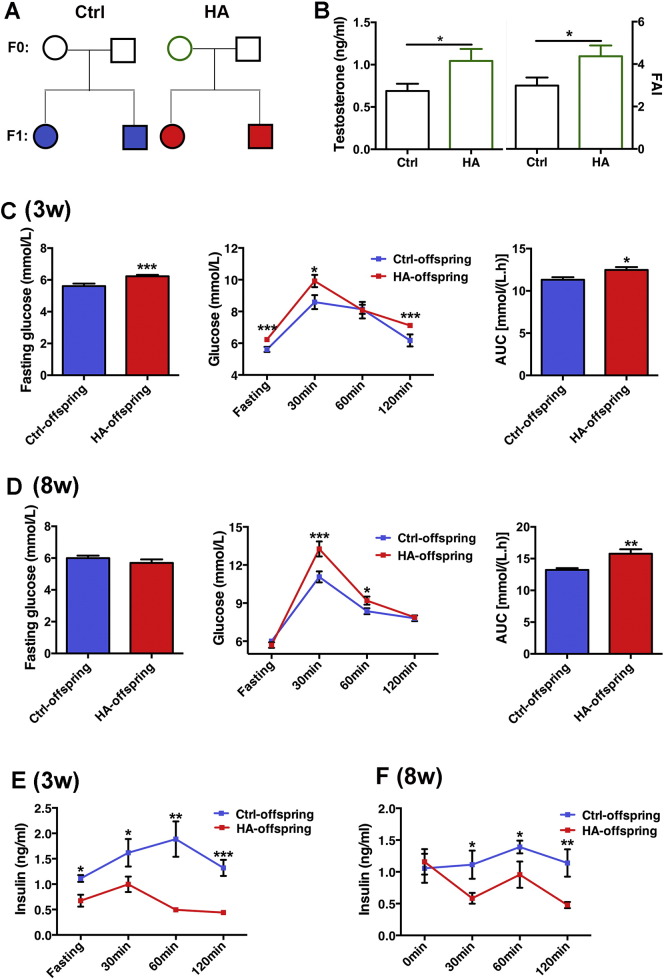
Female rats with hyperandrogenism (F0) were mated to normal males to obtain offspring (F1) (Fig. 3A). Hyperandrogenism female founders displayed increased testosterone levels and free androgen index before mating (Fig. 3B). Ovary immunohistochemistry showed increased expression of CYP17a in theca and granulosa cells, indicating that there were higher androgen levels in follicles (Supplementary Fig. 2). F1 born to hyperandrogenism F0 and normal F0 showed comparable birth weight (Supplementary Fig. 3A) and body weight at 3 and 8 weeks old (Supplementary Fig. 3B), but F1 born to hyperandrogenism F0 showed increased energy and water intakes (Supplementary Fig. 3C and D), and significantly increased fasting glucose level at as early as 3 weeks old (Fig. 3C, left). In addition, data from GTT showed that offspring of hyperandrogenism at both 3 and 8 weeks old had higher glucose levels (Fig. 3C and D, middle) and increased under curve area (Fig. 3C and D, right). Meanwhile, decreased insulin secretion was detected in 3- and 8-week-old offspring born to hyperandrogenism (Fig. 3E and F), indicating pancreatic islet dysfunction. There were no significant differences in glucose and insulin levels between male and female offspring (Supplementary Table 3).
Fig. 3.
Experimental design and GTT in rat offspring. A: Experiment design. Circles designate females and squares designate males. Hyperandrogenism and normal female rats (HA-F0 and Ctrl-F0) were mated with normal female to generate offspring (F1). B: Testosterone and Free Androgen Index (FAI) levels in F0 ovarian follicles. C: Fasting glucose levels of 3-week-old offspring (left panel), GTT and AUC of 3-week-old offspring (middle and right panel). n = 8 and 22 for control and hyperandrogenism, respectively. D: Fasting glucose levels of 8-week-old offspring (left panel), GTT and AUC of 8-week-old offspring (middle and right panel). n = 10 and 16 for control and hyperandrogenic, respectively. E and F: Insulin levels of 3- and 8-week-old offspring in GTT. Data are presented as means ± SE. *p < 0.05, vs. Ctrl, **p < 0.01, vs. Ctrl, ***p < 0.001, vs. Ctrl.
3.4. Hyperandrogenism-induced IGF2 Hypomethylation in Rat Offspring Pancreatic Islet was Inherited from Oocyte
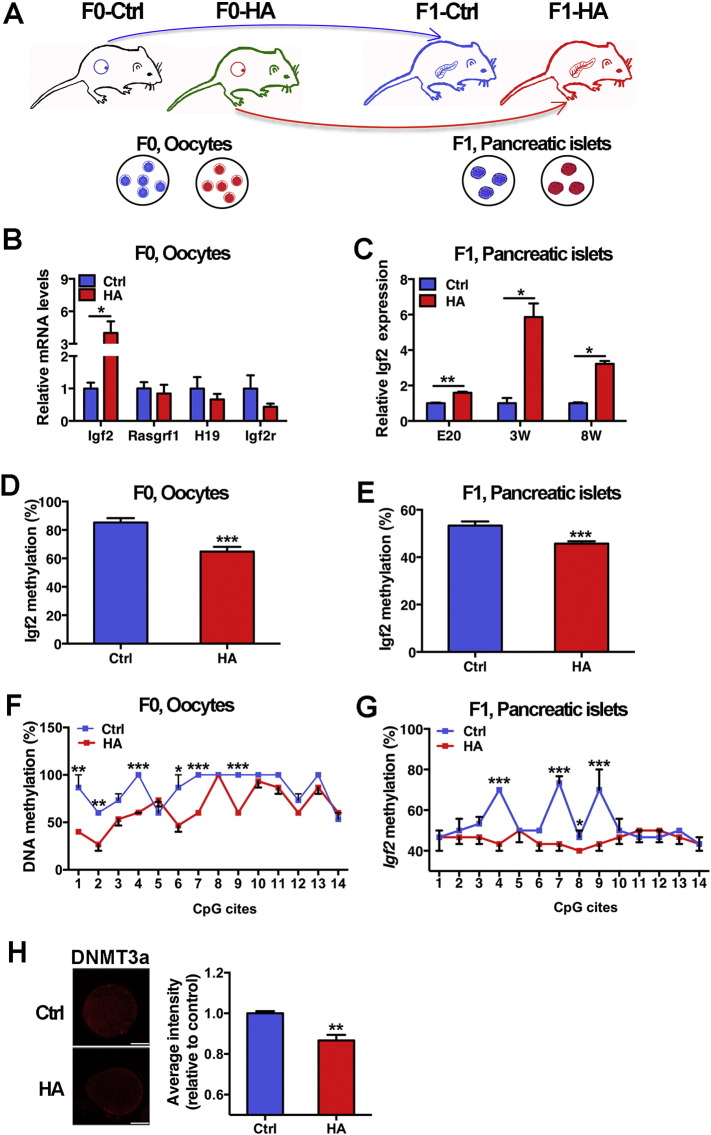
To directly investigate the inheritance of epigenetic marks, we examined four imprinting genes related to glucose metabolism in F0 oocytes and F1 pancreatic islets (Fig. 4A). Significantly upregulated Igf2 expression was found in both the F0 oocytes (Fig. 4B) and F1 pancreatic islets (Fig. 4C). The methylation patterns of Igf2 DMR in both F0 oocytes (gametic cells) and F1 embryonic islets (somatic cells) were analyzed. The overall methylation level of Igf2 gene was significantly decreased in F0 oocytes (Fig. 4D) as well as in F1 islets (Fig. 4E) from hyperandrogenism group. In particular, the same signatures of hypomethylation in sites 4, 7 and 9 from both oocytes (Fig. 4F) and fetal islets (Fig. 4G) were identified, suggesting that the methylation alteration in offspring islets might be inherited from mother's oocytes. On the other hand, immunofluorescence analysis showed that the intensity of DNMT3a in oocytes from hyperandrogenism F0 was significantly higher than that in oocytes from control group (Fig. 4H).
Fig. 4.
Expression and methylation status of Igf2 in rat oocytes (F0) and pancreatic islets (F1). A: Schematic diagram showing transmission of epigenetic marks from germ cells (F0, oocytes) into somatic cells (F1, pancreatic islets cells). B: Expression of Igf2 mRNA in oocytes. C: Expression of Igf2 mRNA in offspring islets (embryonic day 20 (E20), 3 and 8 weeks old). D and E: Overall methylation status of Igf2 in F0 oocytes and F1 pancreatic islets. F and G: Methylation status in individual CpG sites of Igf2 in F0 oocytes and F1 pancreatic islets. Methylation status of the Igf2 DMR2 was analyzed by bisulfite sequencing (n = 3 per group). The methylation rates of individual CpG sites within the Igf2 DMR2 are presented as a line graph. H: Expression of Dnmt3a in rat oocyte treated with in-vitro DHT (10− 9 M) by immunofluorescence. Data are presented as means ± SE. *p < 0.05, vs. Ctrl, **p < 0.01, vs. Ctrl, ***p < 0.001, vs. Ctrl.
4. Discussion
In this clinical cohort study, we found that hyperandrogenism in mothers might increase the risks of abnormal glucose metabolism in their children. According to the Barker's Theory “fetal origins of adult diseases”, adverse intrauterine environment during pregnancy can predispose offspring to diabetes, cardiovascular diseases and obesity in later life. Recently, animal studies demonstrated that high-fat-diet exposure in fathers (Ng et al., 2010) or mothers (Huypens et al., 2016) before conception might also induce metabolic disorders in their offspring via gametic epigenetic alterations. The present study is an investigation in humans to examine whether hyperandrogenism in mothers may induce the alteration of glucose metabolism in their offspring via epigenetic inheritance from mother's oocyte to offspring's somatic cells.
Hyperandrogenemia is a common disorder in women of reproductive age, especially in subfertile women (Qiao and Feng, 2011). In this investigation, 147 women manifesting hyperandrogenism among the 1116 women were recruited (13.17%). Some clinic study indicated that in vivo fertilization rates women with hyperandrogenism were normal (Franks et al., 2003) meanwhile some showed that they might have poor oocyte quality leading to decreased fertilization and implantation rates (Qiao and Feng, 2011, Murray et al., 2008, Teissier et al., 2000). The long term health of their children is well worth studying. Our previous work reported that maternal excessive estrogen exposure before pregnancy might predispose offspring to cardiovascular dysfunction (Xu et al., 2014). Here, we provide evidence that pregestational hyperandrogenism may induce alteration of glucose metabolism in offspring. We found that children born to mothers with pregestational hyperandrogenism had the increased fasting glucose, insulin and HOMA-IR levels. Importantly, they were more susceptible to prediabetes at as early as 5 years old. The risk for prediabetes was still significant after adjusting for gestational complications, suggesting a relationship between pregestational hyperandrogenism in mothers and prediabetes in their children.
In the present study, we took advantage of the IVF procedure to focus on the imprinting alteration in oocyte before fertilization. Analysis in human showed that expression of IGF2, an acknowledged maternal imprinted gene responsible for β-cell function, was increased in oocytes from patients with hyperandrogenism. In vitro DHT treatment also increased IGF2 expression in human oocytes. In female germ line, methylation establishment of imprinting genes is catalyzed by DNA methyltransferase (DNMT) 3a. So we also evaluated the effect of high androgen on DNMT3a expression. We found that in vitro DHT treatment decreased DNMT3a expression in human oocytes. It has been reported that disturbance before conception was associated with widespread epigenetic modifications in offspring even though the phenotype was normal (Batcheller et al., 2011). In agreement with this research, our data showed that the upregulation of IGF2 was also detected in children's somatic cells (lymphocytes) as found in oocytes, which might be due to hypomethylation in IGF2 DMR2. These results suggested that hyperandrogenism-induced imprinting marks in female germline might be passed down to the next generation. Because of the limitation of human tissue samples collected, we don't know whether the epigenetic alterations which we detected in lymphocytes might occur in other somatic cells including pancreatic islet cells. Further study is needed to clarify it.
In order to clarify the epigenetic inheritance from oocyte to pancreatic islets, we established a rat model. We found that offspring born to pregestational hyperandrogenism rat developed diabetes at young age, which was probably attributed to impaired pancreatic islet function because of decreased response of insulin secretion to glucose stimulation. This result was similar to a previous study in mice, in which male offspring born to excessive testosterone showed lower fasting and fed insulin levels (Nohara et al., 2013). Moreover, the decreased insulin levels to glucose stimulation suggest that the disruption in pancreatic islets might be more obvious in the rat offspring of hyperandrogenism mothers than human being.
In mice, when fertilized oocytes of diabetic mothers were transferred to non-diabetic pseudo-pregnant recipients, specific phenotypes such as growth retardation and congenital malformation were observed in offspring (Wyman et al., 2008). Another report showed that ovarian stimulation alone impaired fetal growth in IVF mice (Chen et al., 2014). These studies suggested that the health problem in offspring might be initiated from oocyte. In this study, we collected the oocytes from F0 rats and pancreatic islets from F1 offspring to directly investigate the intergenerational transmission of gametic epigenetic changes. Our data showed that pregestational high androgen upregulated Igf2 mRNA level, meanwhile downregulated Igf2 DMR2 methylation rate in the pancreatic islets of offspring from prenatal period to adulthood. Similarly to our results, BPA (an endocrine disruptor which can bind to androgen receptor) induced β-cell dysfunction in adult offspring was attributed to the transmission of methylation changes in igf2 genes from parents (Mao et al., 2015).
It is known that environmental changes can alter the overall methylation patterns in gametes, and a number of these methylation are found to be heritable (Park et al., 2008, Sandovici et al., 2011). In the present study, we found that the same Igf2 epigenetic signature, the hypomethylation in CpG sites 4, 7 and 9, identified in offspring pancreatic islets was already present in mature oocyte from their mothers. The upregulation and hypomethylation of Igf2 was present in pancreatic islets of F1 fetuses before born (E20), and this happened before the postnatal epigenetic modification. These results suggest that mothers with hyperandrogenism may induce the intergenerational inheritance of epigenetic alteration via oocytes. Our previous study reported that gestational diabetes predisposed F2 offspring to diabetes, and the transmission of Igf2 methylation alteration via F1 sperm could be an epigenetic mechanism (Fan et al., 2012). Here, we provide an evidence for intergenerational transmission of epigenetic alteration via oocyte.
Taken together, this study is the evidence that maternal hyperandrogenism, a common endocrine disorder in reproductive aged women, may increase the risks of glucose metabolism disorder and prediabetes in their children. Data from human and hyperandrogenism rat model suggest that this glucose metabolism disorder may be mediated by DNA methylation modifications, and the epigenetic modification induced by hyperandrogenism may be transmitted from oocytes of mothers to somatic cells of offspring. So intergenerational inheritance of epigenetic alteration should be regarded important in determining development of diabetes in the future.
Funding
This work is funded by Natural Science Foundation of China (8161101434, 31471405, 81490742, 81270708, 31671569 and 81501340).
Conflict of Interest Disclosures
We have no conflict of interest to disclose.
Author contributions
H.-F.H. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. H.-F.H. coordinated the study. H.-F.H., S.T., X.-H.L., Y.-M.X. co-designed the studies. S.T., X.-H.L., Y.-M.X. contributed equally to this study. S.T., X.-H.L., M.-E.L., T.-T.Y., M.L., W.Z., G.-F.X., G.-L.D., C.-M.X. were responsible for screening and enrolment of participants and arranged informed consent from the participants. S.T., X.-H.L., M.J., C.F., Y.-T.W., Y.-J.T., Q.G., J.Z., C.L., J.R., L.-Y.J., B.C., H.Z., X.-Y.Z., S.-C.C., X.-M.L., Y.L., J.-Y.Z., L.W. provided patient care and/or took samples. S.T., X.-H.L., P.Z., X.-J.C., L.J., X.C., Y.-C.M., D.-D.W., H.L., Q.Y., C.-L.Z, X.-Z.L., Y.-Y.W., Y.-Q.X, Z.-W.L., G.L., L.-T.C., H.-J.P., R.L., L.-F.X., Y.-M.Z carried out or supported data analysis, including the statistical analyses. C.K., P.C.L, J.-X.L., F.S., J.-Z.S. helped with the manuscript writing. All authors critically reviewed the report. No writing assistance was provided.
Acknowledgement
This work is supported by the Natural Science Foundation of China (No. 31471405 and 81490742 to H.F.H., No. 81270708 and 31671569 to J.Z.S., and No. 81501340 to L.X.H), the National Basic Research Program of China (2013CB967404 to H.F.H.), the NSFC-CIHR Joint Health Research Program (No. 8161101434 to H.F.H., No. 81361128007 to J.Z.S.), Key project of the Shanghai Committee of Science and Technology (No. 14DJ1400100 to H.F.H.).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.01.011.
Appendix A. Supplementary data
Supplementary Fig. 1. The schematic diagrams of CpG sites in human IGF2 and rat Igf2.
Supplementary Fig. 2. Expression of CYP17a by immunohistochemistry in rat ovary. Scale bar, 100μm.
Supplementary Fig. 3. Phenotype in rat offspring. A: Birth weight of F1. B: Body weight of F1 at 3-week and 8-week old. C and D: Daily energy and water intake of F1 at 3- and 8-week old. Data are presented as means ± SE (n = 10 for both groups). *p < 0.05, vs. Ctrl, ***p < 0.001, vs. Ctrl.
Supplementary tables.
References
- Amalfi S., Velez L.M., Heber M.F. Prenatal hyperandrogenization induces metabolic and endocrine alterations which depend on the levels of testosterone exposure. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0037658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batcheller A., Cardozo E., Maguire M. Are there subtle, genome-wide epigenetic alterations in normal offspring conceived from assisted reproductive technologies? Fertil. Steril. 2011;96(6):1306–1311. doi: 10.1016/j.fertnstert.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carone B.R., Fauquier L., Habib N. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143:1084–1096. doi: 10.1016/j.cell.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.X., Wu L., Zhao J.L. Altered glucose metabolism in mouse and humans conceived by in-vitro fertilization (IVF) Diabetes. 2014;63(10):3189–3198. doi: 10.2337/db14-0103. [DOI] [PubMed] [Google Scholar]
- Ding G.L., Wang F.F., Shu J. Transgenerational glucose intolerance with Igf2/H19 epigenetic alterations in mouse islet induced by intrauterine hyperglycemia. Diabetes. 2012;61(5):1133–1142. doi: 10.2337/db11-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q., Wang F.F., Yin R. A molecular mechanism underlying ovarian dysfunction of polycystic ovary syndrome: hyperandrogenism induces epigenetic alterations in the granulosa cells. J. Mol. Med. (Berl.) 2012;90(8):911–923. doi: 10.1007/s00109-012-0881-4. [DOI] [PubMed] [Google Scholar]
- Franks S., Roberts R., Hardy K. Gonadotrophin regimens and oocyte quality in women with polycystic ovaries. Reprod. BioMed. Online. 2003;6(2):181–184. doi: 10.1016/s1472-6483(10)61708-7. [DOI] [PubMed] [Google Scholar]
- Ge Z.J., Liang X.W., Guo L. Maternal diabetes causes alterations of DNA methylation statuses of some imprinted genes in murine oocytes. Biol. Reprod. 2013;88:117. doi: 10.1095/biolreprod.112.105981. [DOI] [PubMed] [Google Scholar]
- Ge Z.J., Luo S.M., Lin F. DNA methylation in oocytes and liver of female mice and their offspring: effects of high-fat-diet-induced obesity. Environ. Health Perspect. 2014;122:159–164. doi: 10.1289/ehp.1307047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.F., Sheng J.Z. Springer; New York, NY: 2014. Gamete and Embryo-Fetal Origins of Adult Diseases. [Google Scholar]
- Huypens P., Sass S., Wu M. Epigenetic germline inheritance of diet-induced obesity and insulin resistance. Nat. Genet. 2016;48(5):497–499. doi: 10.1038/ng.3527. [DOI] [PubMed] [Google Scholar]
- Mao Z., Xia W., Chang H., Huo W., Li Y., Xu S. Paternal BPA exposure in early life alters Igf2 epigenetic status in sperm and induces pancreatic impairment in rat offspring. Toxicol. Lett. 2015;238(3):30–38. doi: 10.1016/j.toxlet.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Martinez D., Pentinat T., Ribo S. In utero undernutrition in male mice programs liver lipid metabolism in the second-generation offspring involving altered Lxra DNA methylation. Cell Metab. 2014;19:1–11. doi: 10.1016/j.cmet.2014.03.026. [DOI] [PubMed] [Google Scholar]
- Murray A.A., Swales A.K., Smith R.E., Molinek M.D., Hillier S.G., Spears N. Follicular growth and oocyte competence in the in vitro cultured mouse follicle: effects of gonadotropins and steroids. Mol. Hum. Reprod. 2008;14(2):75–83. doi: 10.1093/molehr/gam092. [DOI] [PubMed] [Google Scholar]
- Ng S.F., Lin R.C., Laybutt D.R., Barres R., Owens J.A., Morris M.J. Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature. 2010;467(7318):963–966. doi: 10.1038/nature09491. [DOI] [PubMed] [Google Scholar]
- Nohara K., Liu S., Meyers M.S. Developmental androgen excess disrupts reproduction and energy homeostasis in adult male mice. J. Endocrinol. 2013;219(3):259–268. doi: 10.1530/JOE-13-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R.C., Osmond C., Gluckman P., Hanson M., Phillips D.I., Roseboom T.J. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. Br. J. Obstet. Gynaecol. 2008;115(10):1243–1249. doi: 10.1111/j.1471-0528.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- Park J.H., Stoffers D.A., Nicholls R.D., Simmons R.A. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J. Clin. Invest. 2008;118(6):2316–2324. doi: 10.1172/JCI33655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry C.J., Hales C.N. Long-term effects on offspring of intrauterine exposure to deficits in nutrition. Hum. Reprod. Update. 2000;6(6):578–586. doi: 10.1093/humupd/6.6.578. [DOI] [PubMed] [Google Scholar]
- Qiao J., Feng H.L. Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum. Reprod. Update. 2011;17(1):13–17. doi: 10.1093/humupd/dmq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford E.J., Ito M., Shi H. In utero undernourishment perturbs the adult sperm methylome and intergenerational metabolism. Science. 2014;345(6198):1255903. doi: 10.1126/science.1255903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandovici I., Smith N.H., Nitert M.D. Maternal diet and aging alter the epigenetic control of a promoter–enhancer interaction at the Hnf4a gene in rat pancreatic islets. Proc. Natl. Acad. Sci. U. S. A. 2011;108(13):5449–5454. doi: 10.1073/pnas.1019007108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A., Kushnir V.A., Barad D.H., Gleicher N. Endocrine autoimmune diseases and female infertility. Nature. 2014;10(1):37–50. doi: 10.1038/nrendo.2013.212. [DOI] [PubMed] [Google Scholar]
- Teissier M.P., Chable H., Paulhac S., Aubard Y. Comparison of follicle steroidogenesis from normal and polycystic ovaries in women undergoing IVF: relationship between steroid concentrations, follicle size, oocyte quality and fecundability. Hum. Reprod. 2000;15:2471–2477. doi: 10.1093/humrep/15.12.2471. [DOI] [PubMed] [Google Scholar]
- Wei Y.C., Yang C.R., Wei Y.P. Paternally induced transgenerational inheritance of susceptibility to diabetes in mammals. Proc. Natl. Acad. Sci. U. S. A. 2014;111(5):1873–1878. doi: 10.1073/pnas.1321195111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y.C., Schatten H., Sun Q.Y. Environmental epigenetic inheritance through gametes and implications for human reproduction. Hum. Reprod. Update. 2015;21(2):194–208. doi: 10.1093/humupd/dmu061. [DOI] [PubMed] [Google Scholar]
- Wyman A., Pinto A.B., Sheridan R., Moley K.H. One-cell zygote transfer from diabetic to nondiabetic mouse results in congenital malformations and growth retardation in offspring. Endocrinology. 2008;149:466–469. doi: 10.1210/en.2007-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G.F., Zhang J.Y., Pan H.T. Cardiovascular dysfunction in offspring of ovarian hyperstimulated women and effects of estradiol and progesterone: a retrospective cohort study and proteomics analysis. J. Clin. Endocrinol. Metab. 2014;99(12):E2494–E2503. doi: 10.1210/jc.2014-2349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. The schematic diagrams of CpG sites in human IGF2 and rat Igf2.
Supplementary Fig. 2. Expression of CYP17a by immunohistochemistry in rat ovary. Scale bar, 100μm.
Supplementary Fig. 3. Phenotype in rat offspring. A: Birth weight of F1. B: Body weight of F1 at 3-week and 8-week old. C and D: Daily energy and water intake of F1 at 3- and 8-week old. Data are presented as means ± SE (n = 10 for both groups). *p < 0.05, vs. Ctrl, ***p < 0.001, vs. Ctrl.
Supplementary tables.