Abstract
Major depressive disorder (MDD) and bipolar disorder (BD) are severe psychiatric diseases with overlapping symptomatology. Although previous studies reported abnormal brain structures in MDD or BD patients, the disorder-specific underlying neural mechanisms remain poorly understood. The purpose of this study was to investigate the whole-brain gray matter morphological patterns in unmedicated patients with MDD or BD and to identify the shared and disease-specific brain morphological alterations in these two disorders.
We acquired high-resolution brain structural MRI data from a sample of 36 MDD patients, 32 BD patients, and 30 healthy controls. Using FreeSurfer, we estimated their brain cortical thickness (CT) and compared between-group difference in multiple locations across the continuous cortical surface.
Compared to the healthy controls, both the MDD and BD patient groups showed significantly reduced CT in the left inferior temporal cortex (ITC). However, compared to the MDD patients, the BD patients showed a significantly thinner CT in the left rostral middle frontal region. In addition, compared to the healthy controls, the BD patients displayed thinner CT in the left ITC, left frontal pole (FPO), left superior frontal, right lateral occipital, right pars triangularis (PTRI) and right lateral orbitofrontal regions. Further analysis revealed a significantly positive correlation between the mean CT in the left FPO and the onset age, but a negative correlation between the mean CT in the right PTRI and the number of episodes, in the BD patients.
Our findings revealed that the BD and MDD patients had variations in CT that were in common, but many more that were distinct, suggesting potential differences in their neural mechanisms.
Abbreviations: MDD, major depressive disorder; BD, bipolar disorder; BD-I, bipolar I disorder; BD-II, bipolar II disorder; HC, healthy controls; VBM, voxel-based morphometry; SBM, surface-based morphometry; GM, gray matter; WM, white matter; CSF, cerebrospinal fluid; CT, cortical thickness; HDRS, Hamilton Depression Rating Scale; YMRS, Young Mania Rating Scale; FWHM, full-width half-maximum; ANOVA, analysis of variance; GLM, general linear model; RA, relative alteration; ROI, region of interesting; rMFC, rostral middle frontal cortex; ITC, inferior temporal cortex; FPO, frontal pole; SFC, superior frontal cortex; LOC, lateral occipital cortex; PTRI, pars triangularis; lOFC, lateral orbitofrontal cortex; DLPFC, dorsolateral prefrontal cortex; PFC, prefrontal cortex; VPFC, ventral prefrontal cortex; fNIRS, functional near-infrared spectroscopy; SSRI, selective serotonin reuptake inhibitor
Keywords: Affective disorder, Unmedicated, Gray matter, Surface-based morphometry, Vertex-based morphometry, Relative alteration
Highlights
-
•
We found thinner CT in the left ITC in both MDD and BD groups compared to controls.
-
•
We detected thinner CT in the left rMFC in the BD group compared to the MDD group.
-
•
The BD group had more pronounced abnormality in CT primarily in the PFC than the MDD group.
-
•
Clinical variables of BD group were associated with decreased CT in the left FPO and right PTRI.
This study aims to detect abnormal cortical thickness in patients with major depressive disorder (MDD) or bipolar disorder (BD), and to identify the shared and disease-specific brain morphological alterations in these two disorders. The two patient groups showed several common but more distinct variation patterns in cortical thickness, and the BD patients had lower cortical thickness in widespread brain areas than the MDD and the controls. These findings may have potential clinical implications for distinguishing BD from MDD patients.
1. Introduction
Major depressive disorder (MDD) and bipolar disorder (BD) are two major types of mood disorder. Although mania or hypomania is a defining feature of BD patients, the presence of subthreshold manic symptoms can be observed in both disorders during a depressive episode (de Almeida & Phillips, 2013). This leads to difficulty in distinguishing BD from MDD patients as they have the same diagnostic criteria for a depressive episode (Phillips & Kupfer, 2013). Actually, misdiagnosing BD as MDD has many potentially deleterious consequences because treatment with antidepressants in the absence of a mood stabilizer carries the risk of precipitating mania and may increase rates of cycling between mood states (Baldessarini et al., 2010). In fact, whether MDD and BD have different neural mechanisms or share some in common remains ambiguous (de Almeida and Phillips, 2013, McGuffin et al., 2003). Neuroimaging studies have identified a number of differences between patients with depressive disorders and healthy controls in brain structure and function. Until now, it is still unclear to what extent specific or common morphological alterations occur in MDD and BD given the paucity of direct comparisons.
Based on brain structural images, brain morphologic characteristics have been studied in various brain diseases (de Vos et al., 2016, van Lutterveld et al., 2014). Using the voxel-based morphometry (VBM) method, some studies detected altered brain GM volume or density in patients with MDD or BD (Redlich et al., 2014) and found between-group difference in GM volume primarily in the prefrontal cortex (PFC), anterior cingulate gyrus (ACG), amygdala, and hippocampus (Redlich et al., 2014, Koutsouleris et al., 2015). Actually, the VBM method is susceptible to several potential confounds, including the accuracy of the brain segmentation, degree of smoothing, strategies used in registration, and the choice of a normalization template (Bookstein, 2001). Especially, VBM analysis is a method of measuring MRI signal alteration in brain tissue rather than a directly technique to detect brain structural alteration about the volume size of a region, CT and cortical surface area (Bookstein, 2001). CT analysis is similar to VBM, albeit the analysis is performed at the nodes of a 3D polygonal mesh rather than on a 3D voxel grid. And the CT analysis has the advantage of providing a direct quantitative index (in unit of mm), rather than qualitative index, of cortical morphology. Therefore, the measurement of CT alteration has been suggested as a way to obtain a complementary indication of alterations in brain GM morphology (Ecker et al., 2013).
For depressive disorders, previous studies focused primarily on CT alteration in just one of the depressive disorders, comparing the patients with healthy controls (Redlich et al., 2014, Maller et al., 2014), but ignoring the abnormal CT between the two disorders. Several studies of BD patients reported subtle but widespread CT abnormalities and showed decreased CT in the left anterior cingulate/paracingulate, left superior temporal gyrus and prefrontal regions (Rimol et al., 2010, Rimol et al., 2012, Hanford et al., 2016). And several studies of MDD patients reported reduced CT in the medial orbitofrontal gyrus and pars opercularis (van Eijndhoven et al., 2013, Tu et al., 2012), and a study reported increased CT in similar regions (Qiu et al., 2014). By now, very few studies have directly compared the difference in brain CT between MDD and BD patients (Lan et al., 2014, Fung et al., 2015), and those that did obtained partially inconsistent results. For example, Lan et al. (2014) investigated the difference in CT between 18 BD patients and 56 MDD patients and reported thinner CT in the right caudal middle frontal cortex, left inferior parietal cortex, and right precuneus in a mixed group of BD-I and BD-II patients. However, Fung et al. (2015) failed to find any brain regions with differences in CT between MDD and BD patients. Notably, in these two studies, the analyses were performed on patients who were taking medications, which may have influenced the results.
The CT analysis may be affected by several factors, such as sample sizes, medication status (Lanzenberger et al., 2012, Foland-Ross et al., 2011), or heterogeneity in the patient samples. Most studies published to date have included patients who were taking medications. Although the effects of medication on brain morphology are not yet fully understood, several studies have indicated that the use of psychotropic medications, such as lithium, may cause an increase in GM volume in the cortical and subcortical regions (Foland-Ross et al., 2011, Brooks et al., 2009). Lanzenberger et al. (2012) and Benmansour et al. (1999) reported that the alteration of brain structure may be resulted from the use of selective serotonin reuptake inhibitors (SSRI). The heterogeneity of patient samples included the age of the participants, their mood states at the time of scanning, and mixing the types of BD patients. As for BD patient, BD-II might have a genetic etiology distinguishable from BD-I (Huang et al., 2010). BD-II is especially difficult to diagnose accurately because of the difficulty in differentiating this disorder from recurrent MDD (recurrent depressive episodes) in depressed patients (Phillips & Kupfer, 2013). Unfortunately, no study has yet directly compared differences in CT between MDD patients and BD-II, although a few studies compared brain CT in combined samples of BD-I, BD-II and BD not otherwise specified (BD-NOS) patients with MDD patients (de Almeida and Phillips, 2013, Lan et al., 2014).
In this study, our goal was to detect alteration of CT in the unmedicated adult MDD and BD-II patients who were in a depressive episode, and compare to the healthy controls to assess morphometric differences and similarities that may reflect common and/or distinct brain regions in affective disorders. Considering the similarities and differences in the clinical symptoms and brain alterations revealed in previous studies (de Almeida and Phillips, 2013, Lan et al., 2014), we hypothesized that depressive episode unmedicated adult MDD and BD-II patients would not only have CT abnormalities that they shared in common but also abnormalities in CT that are specific to each disease. Considering the similarities and differences in clinical symptoms and brain alterations revealed in previous studies (de Almeida and Phillips, 2013, Lan et al., 2014), we hypothesized that the depressive unmedicated adult MDD and BD-II patients would not only have CT abnormalities that they shared in common but also abnormalities in CT that are specific to each disorder. And these abnormal regions would likely be the key brain structures involving in depression symptoms, such as the limbic and prefrontal regions. In addition, as BD is considered to have more complexity and severity than MDD, we supposed that the BD patients had more widespread CT abnormalities than the MDD patients.
2. Methods and Materials
2.1. Subjects
A total of 68 currently unmedicated depressed patients and 30 healthy controls were recruited from the Psychiatry Department of the First Affiliated Hospital of Jinan University (FAHJU), Guangzhou, China, during July 2013–August 2015. All the subjects were determined to have no abnormalities on conventional MRI by two experienced radiologists (Y.W. and Y.S.) and were right-handed according to the Edinburgh Handedness Inventory (Oldfield, 1971). The three groups of subjects, MDD (17M/19F, aged 18–43 years old), BD (15M/17F, aged 18–50 years old), healthy controls (17M/13F, aged 19–44 years old), were matched on age and gender. The study was approved by the Institute Review Board of the First Affiliated Hospital of Jinan University, China. Each subject signed a written informed consent form after a full written and verbal explanation of the study. Table 1 lists the detailed demographics for all the subjects in this study.
Table 1.
Demographics and clinical data.a
Demographic characteristics of the adult unmedicated patients with major depressive disorder (MDD) and bipolar depression (BD), as well as the healthy controls (HC) in this study. Abbreviations: HDRS, Hamilton Depression Rating Scale; YMRS, Young Mania Rating Scale; N/A, not applicable.
| Characteristics | MDD | BD | HC | Statisticb | p-Value | Post-hoce |
|---|---|---|---|---|---|---|
| General demographics | ||||||
| Age (years old) | 29.08 ± 7.16 | 28.78 ± 83 | 27.83 ± 6.38 | F = 0.25 | 0.78 | |
| Gender (M/F) | 17/19 | 15/17 | 17/13 | X2 = 0.77 | 0.68 | |
| Handedness (right/left) | 36/0 | 32/0 | 30/0 | |||
| Education (years)c | 13.56 ± 2.76 | 14.72 ± 2.50 | 15.56 ± 2.33 | F = 5.18 | 0.007⁎⁎ | MDD vs. BD; p = 0.064 MDD vs. HC; p = 0.002 BD vs. HC; p = 0.194 |
| Onset age (years old) | 24.92 ± 7.32 | 24.28 ± 9.85 | N/A | t = 0.30 | 0.76 | |
| Total duration (months) | 42.06 ± 61.60 | 33.56 ± 50.14 | N/A | t = 0.62 | 0.54 | |
| Disorder characteristics | ||||||
| No. of episodesd | 1.83 ± 1.25 | 2.37 ± 1.23 | N/A | t = − 1.79 | 0.07 | |
| HDRS scores | 26.67 ± 4.73 | 26.50 ± 4.77 | N/A | t = 0.14 | 0.88 | |
| YMRS scores | 2.75 ± 2.51 | 1.84 ± 1.80 | N/A | t = 1.69 | 0.007⁎⁎ |
Mean and std. are reported unless otherwise specified.
Difference in gender was tested using a χ2-test. Differences in age and years of education across the three groups were tested using a one-way ANOVA. An independent samples t-test was used to determine group differences in the age of onset, illness duration, number of depressive episodes, HDRS scores, and YMRS scores, which were only available for the two patient groups (MDD and BD).
Years of education refers to the total number of years of education which were reported by the participants.
Number of episodes means the total number of depressive episodes and manic episodes.
Bonferroni post-hoc tests.
ANOVA significant (p < 0.01).
The MDD and BD patients were diagnosed according to the DSM-IV criteria and the Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P) (Steinberg, 1994). All patients fulfilled the criteria for either MDD or BD. Diagnosis of patients was determined by two experienced clinical psychiatrists (Y.J. and S.Z., with 20 and 5 years of experience in clinical psychiatry, respectively). Current depressive symptoms were assessed by using the 24-item Hamilton Depression Rating Scale (HDRS) (Williams, 1988) and current manic symptoms were assessed by the Young Mania Rating Scale (YMRS) (Young et al., 1978) during the 7-day period before the imaging session. All patients with BD or MDD were diagnosed with depression (a YMRS score < 7 and a total HDRS score > 21). The exclusion criteria for the patients were other Axis-I psychiatric disorders, a history of organic brain disorder or neurological disorders, mental retardation, cardiovascular diseases, pregnancy, or any physical illness. None of the subjects had lifetime substance use disorders, including alcohol abuse, marijuana use, and cocaine abuse. None of the patients had ever received electroconvulsive therapy prior to participating in this study. The exclusion criteria for the healthy controls were same to the patients, in addition to any history of psychiatric illness in first-degree relatives and current or past significant medical or neurological illness. At the time of the scanning, 37 patients (18 BD and 19 MDD) were medication-naïve; they had never been diagnosed or did not want to take medication. While for the others, the recruited patients generally visited their physicians (psychiatrist/general practitioner) because of depressive relapse after quitting medication. Among them, 14 BD patients had been treated with antidepressants (duloxetine or paroxetine), and/or mood stabilizers (lithium, sodium valproate), and/or atypical antipsychotic medications (olanzapine or risperidone), and 17 MDD patients had been treated with antidepressants (duloxetine or paroxetine). For all of the patients, they had been off medication for at least 6 months prior to the scan.
2.2. Image Acquisition
All the MRI data were acquired on a 3 T GE MR750 scanner with an eight-channel phased-array head coil in the Medical Imaging Department, the First Affiliated Hospital of Jinan University, Guangzhou. For each subject, we obtained high resolution brain structural images by using a T1-weighted 3D Ax FSPGR BRAVO sequence. The sequence parameters were as follows: repetition time (TR) = 8.2 ms, echo time (TE) = 3.2 ms, flip angle (FA) = 12°, data matrix = 256 × 256, field of view (FOV) = 256 mm × 256 mm, slice thickness = 1.0 mm, and 136 axial slices covering the whole brain. In addition, two routine scans using axial T1-weighted fluid attenuation inversion recovery (FLAIR) and fast spin-echo T2-weighted MR sequences were also applied to obtain brain images to confirm the absence of any brain structural abnormalities.
2.3. Image Processing
We first visually inspected all the T1-weighted brain structural data to exclude poor quality images. Then we inputted them into a FreeSurfer software package (version 5.3.0, http://surfer.nmr.mgh.harvard.edu), a widely documented and automated program for reconstructing brain cortical surfaces and calculating CT (Fischl, 2012). In brief, the reconstruction processes involved the following steps: 1) correcting for small head motions and signal intensity non-uniformity in the structural images, 2) removing the non-brain tissue, 3) segmenting the brain into the GM and WM, 4) labeling the subcortical structures and computing statistics on the segmented subcortical structures, 5) performing a surface tessellation to generate triangular cortical meshes in the GM/WM boundary and GM/CSF (cerebrospinal fluid) boundary, 6) smoothing the fold surface and inflating it, and 7) finding topological defects and removing or fixing them automatically or manually (Dale et al., 1999, Fischl et al., 1999, Fischl et al., 2004, Fischl and Dale, 2000). After these preliminary reconstruction processes, we measured two shortest distances, from the GM/WM boundary to the GM/CSF boundary and from the GM/CSF boundary to the GM/WM boundary at each vertex and averaged these two values as the CT at the given vertex. For each subject, we morphed and used a spherical transform to register the reconstructed brain to an average spherical surface and mapped the thickness measurement at each vertex on a common spherical coordinate system. In the calculations, we adopted a smoothing Gaussian kernel with a full-width half-maximum (FWHM) of 10 mm.
2.4. Statistical Analysis
2.4.1. Demographics and Questionnaires
One-way analyses of variance (ANOVA) with Bonferroni post hoc tests were performed to detect group differences in age and education level. A χ2-test was used to assess the gender composition across the three groups. An independent two-sample t-test was used to determine differences in the onset age, illness duration, number of episodes, HDRS scores, and YMRS scores between the MDD and BD patients. All these statistical analyses were performed using SPSS 17.0 (http://www.spss.com).
2.4.2. Vertex-based Analysis
A vertex-based analysis was conducted using the FreeSurfer Qdec application by fitting a general linear model (GLM) at each vertex to compare the CT between the different groups. Specifically, the measurement of CT (yij) and the main effect of group (Gi) were estimated by regression using a GLM at each vertex i for subject j, with the gender and age as covariates:
| (1) |
where β1, β2 and β3 correspond to coefficients of various factors, and ε to the residual error. Between-group differences in the CT were estimated from the fixed-effect coefficient β1 normalized by the corresponding standard error. Corrections for multiple comparisons across the whole brain were performed using a Monte Carlo permutation cluster analysis (5000 iterations) with a cluster-based threshold of p < 0.05. Once a significant between-group difference was observed for a parameter, we estimated its effect size (Cohen d) and statistical power according to the Cohen's definition (1992). The clusters with significant between-group difference in CT were preserved for subsequent analysis.
2.4.3. Cluster-based Analysis
For each of the clusters showing a significant between-group difference, we mapped the cluster to each individual brain, extracted the thickness at each vertex, and estimated the mean thickness in this cluster for each subject. Then, we calculated the average CT for each group. Subsequently, we conducted between-group comparisons for each cluster by taking age and gender as covariates.
2.4.3.1. Rank Analysis
A non-parametric analysis (van Lutterveld et al., 2014) was used to assess the rank of the average CT in each cluster among the three groups. There were six possible rankings of the CT, which are as follows: (i) CTHC > CTMDD > CTBD, (ii) CTHC > CTBD > CTMDD, (iii) CTMDD > CTHC > CTBD, (iv) CTMDD > CTBD > CTHC, (v) CTBD > CTMDD > CTHC, and (vi) CTBD > CTHC > CTMDD, where CTMDD, CTBD, and CTHC represents the values of the average CT at a given cluster for the MDD patients, BD patients, and healthy controls, respectively. In the end, we determined the rank order of the average CT in each cluster.
2.4.3.2. Relative Alterations
For each cluster showing a significant between-group difference in the CT, we estimated the relative alteration (RA) in the CT by using the following equations:
| (2) |
| (3) |
| (4) |
where RAMDD-HC (RABD-HC) represents the alteration of the CT in the MDD (BD) patients relative to the controls, and RABD-MDD represents the alteration of the CT in the BD patients relative to the MDD patients, for a given cluster.
2.4.3.3. Relationship Between CT and Clinical Variables
We also calculated the partial Pearson's correlation coefficients between the average CT in each cluster and each of the clinical variables (onset age, illness duration, number of depressive episodes, and HDRS scores) for the MDD and BD patients separately. In the calculations, we regressed out the confounding factors of age and gender (Sowell et al., 2007, Luders et al., 2006). After calculated the partial Pearson's correlation, we applied a false discovery rate (FDR) correction.
3. Results
3.1. Demographics and Questionnaires
No significant between-group differences were found for age, gender, or handedness. Additionally, no significant differences between the MDD and BD groups were found in depression severity (HDRS score), illness duration, illness onset age, or number of episodes. Also, the MDD patients showed significantly higher YMRS scores than the BD patients (p = 0.007).
3.2. Vertex-based Analysis
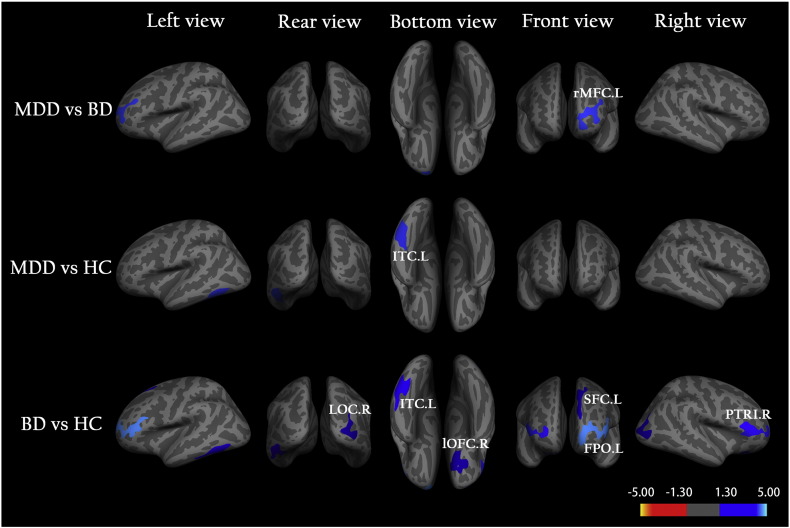
Fig. 1 shows the clusters with a significant between-group difference in the CT. For each of three between-group comparisons, MDD vs. BD, MDD vs. HC, and BD vs. HC, in the CT, the detailed information is described as follows.
MDD vs. BD: There is only one cluster that was thinner in patients with BD compared to MDD (referred to as C1), which is located in the left rostral middle frontal cortex (rMFC), (Fig. 1 and Table 2). According to Eq. (2), we obtained RABD-MDD = − 3.5%; that is, the CT of the BD group was 3.5% thinner than that of the MDD group in the left rMFC (Table 2).
MDD vs. HC: Compared to the controls, there is only one cluster that was significantly thinner in the MDD group (referred to as C2), which is located in the left inferior temporal cortex (ITC) (Fig. 1 and Table 2). We obtained its RAMDD-HC = − 4.3% according to Eq. (3), indicating a thinner CT in the left ITC for the MDD group compared to the controls (Table 2).
BD vs. HC: We detected a significantly thinner CT in the BD group compared to the control group in six clusters (referred as C3, C4, C5, C6, C7, and C8, respectively), which are located in the left frontal pole (FPO), left superior frontal cortex (SFC), left ITC, right lateral occipital cortex (LOC), right pars triangularis (PTRI), and right lateral orbitofrontal cortex (lOFC) (Fig. 1 and Table 2). According to Eq. (4), we estimated the relative alteration in the CT in these six clusters and found that they were in the range of RABD = − 3.9% to − 5.7%, in the BD group compared to the control group (Table 2).
Fig. 1.
Vertex-based analysis of cortical thickness in the three groups, the unmedicated adult patients with major depressive disorder (MDD) and bipolar depression (BD) as well as the healthy controls (HC). Clusters were obtained from an independent samples t-test to show statistically significant between-group differences in cortical thickness (p < 0.05). Clusters color-coded in blue indicate significantly decreased cortical thickness in the BD group compared to either the MDD or HC or in the MDD group compared to the HC. Clusters are overlaid on average inflated images with sulci displayed as dark relative to gyri. Abbreviations: L (R), left (right) hemisphere; rMFC, rostral middle frontal cortex; ITC, inferior temporal cortex; LOC, lateral occipital cortex; lOFC, lateral orbitofrontal cortex; FPO, frontal pole; SFC, superior frontal cortex; PTRI, pars triangularis.
Table 2.
Clusters with significant differences in cortical thickness across the three groups after correction for multiple comparisons.
Cortical clusters showing significant differences in cortical thickness between the unmedicated adult patients with major depressive disorder (MDD) and the healthy controls (HC) as well as between the patients with bipolar depression (BD) and the HC. The cluster-based p-value corresponds to the peak vertex that showed the greatest statistical difference within a cluster. Abbreviations: L (R), left (right) hemisphere; rMFC, rostral middle frontal cortex; FPO, frontal pole; SFC, superior frontal cortex; ITC, inferior temporal cortex; FG, fusiform gyrus; PTRI, pars triangularis; LOC, lateral occipital cortex; IPC, inferior parietal cortex; lOFC, lateral orbitofrontal cortex; mOFC, medial orbitofrontal cortex. The Cohen d indicates the magnitude of the effect size.
| Location | Peak Talairach Coordinates | p-Value | Cohen d (statistical power) | Cluster size (mm2) | CTMDD (mm) | CTBD (mm) | CTHC (mm) | RAMDD-HC | RABD-HC | RABD-MDD | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BD < MDD | C1 | rMFC.L, FPO.L, SFC.L | (− 23.1, 52.2, 7.0) | 5.00E − 04 | 0.62 (0.72) | 1353.0 | 2.494 | 2.398 | 2.484 | 0.38% | − 3.48% | − 3.84% |
| MDD < HC | C2 | ITC.L, FG.L | (− 50.6, − 53.3, − 9.7) | 1.05E − 02 | 0.61 (0.68) | 934.4 | 2.362 | 2.310 | 2.468 | − 4.27% | − 6.40% | − 2.23% |
| BD < HC | C3 | FPO.L, PTRI.L, rMFC.L, SFC.L | (− 8.7, 62.5, − 6.6) | 1.00E − 04 | 0.99 (0.97) | 1846.8 | 2.439 | 2.326 | 2.436 | 0.10% | − 4.54% | − 4.64% |
| C4 | SFC.L | (− 6.8, 32.7, 46.6) | 1.07E − 02 | 0.64 (0.70) | 928.9 | 3.090 | 3.012 | 3.159 | − 2.19% | − 4.67% | − 2.54% | |
| C5 | ITC.L, FG.L, LOC.L | (− 50.5, − 53.7, − 9.2) | 3.70E − 03 | 0.62 (0.72) | 1077.4 | 2.427 | 2.379 | 2.521 | − 3.75% | − 5.65% | − 1.98% | |
| C6 | LOC.R, IPC.L | (39.2, − 81.0, 5.6) | 2.42E − 02 | 0.62 (0.72) | 859.95 | 2.257 | 2.167 | 2.291 | − 1.48% | − 5.41% | − 3.99% | |
| C7 | PTRI.R, rMFC.R | (47.7, 27.6, 0.6) | 1.00E − 03 | 0.62 (0.72) | 1228.5 | 2.323 | 2.238 | 2.331 | − 0.32% | − 3.98% | − 3.67% | |
| C8 | lOFC.R, mOFC.R | (24.1, 24.9, − 12.7) | 2.46E − 02 | 0.62 (0.72) | 857.7 | 2.432 | 2.395 | 2.495 | − 2.53% | − 4.02% | − 1.53% | |
3.3. Cluster-based Analysis
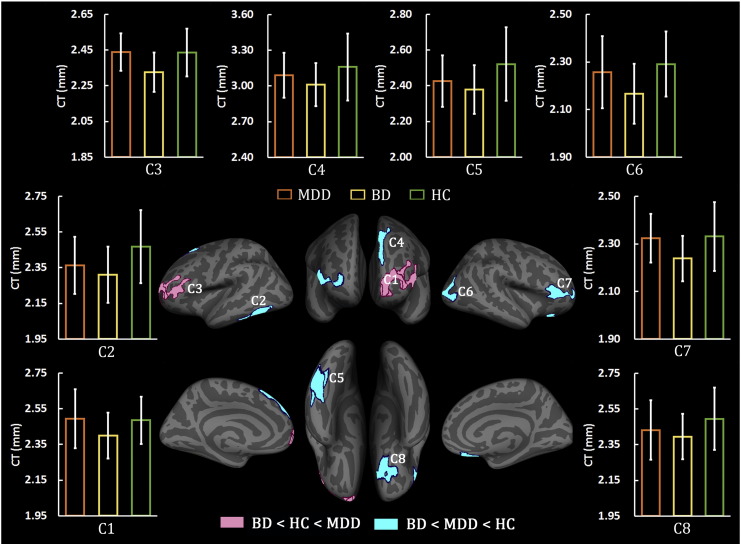
3.3.1. Rank of CT in the Clusters
Fig. 2 shows an overview of the results of the rank analysis. For the eight clusters listed in Table 2, six of them (C2, C4, C5, C6, C7, and C8) had the rank of CTBD < CTMDD < CTHC; that is, in these six clusters, the BD patients had the lowest CT, the controls had the highest CT, and the MDD patients were in between. For the other two clusters, C1 and C3, we obtained the rank of CTBD < CTHC < CTMDD; that is, the BD group showed the lowest CT, the MDD patients had the highest CT, and the controls were in between in the clusters of C1 and C3.
Fig. 2.
Rank of the average cortical thickness in cluster-based analyses. On six clusters, which are located in the left inferior temporal (C2, C5), left superior frontal (C4), right lateral occipital (C6), right pars triangularis (C7), and right lateral orbitofrontal (C8), the patients with bipolar depression (BD) had the lowest while the healthy controls (HC) had the highest average cortical thickness. On the other two clusters, the left rostral middle frontal (C1) and the left frontal pole (C3), the BD patients showed the lowest while the MDD patients had the highest average cortical thickness. Bars and error bars correspond to the average cortical thickness and the standard deviation for a given subject group of MDD (orange), or BD (yellow), or HC (green).
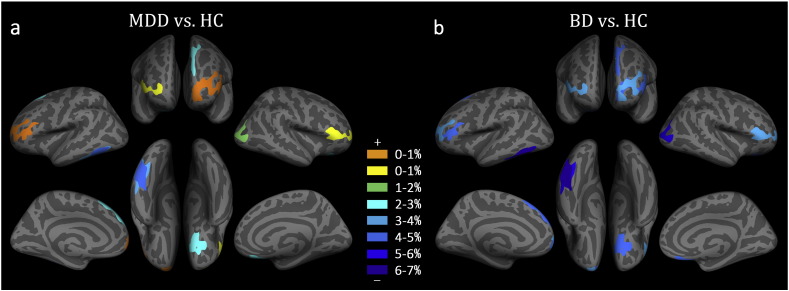
3.3.2. Relative Alterations
Fig. 3 shows the relative alterations in the CT in the eight clusters listed in Table 2. Of these, six clusters (C2, C4, C5, C6, C7, and C8) showed a thinner CT in the two types of disorder patients compared to the controls. In addition, we found | RABD-HC | > | RAMDD-HC | in these six clusters; that is, the BD patients had a more severe reduction in the CT than the MDD patients. Compared to the control group, the MDD patients showed a relatively thinner CT (RAMDD-HC < 0, ranging from − 0.3% to − 4.3%) in six clusters (C2, C4, C5, C6, C7 and C8), but a relatively thicker CT (RAMDD-HC > 0, ranging from 0.1% to 0.2%) in the other two clusters (C1 and C3). Compared to the control group, the BD patients showed a relatively thinner CT in all eight clusters (RABD-HC < 0, ranging from − 3.2% to − 6.1%).
Fig. 3.
Relative alteration in average cortical thickness derived from the cluster-based analysis. (a) Patients with major depressive disorder (MDD) compared to healthy controls (HC). (b) Patients with bipolar depression (BD) compared to HC. The clusters coded in warm (cold) color indicate significantly increased (decreased) cortical thickness in the two types of patients compared to the HC.
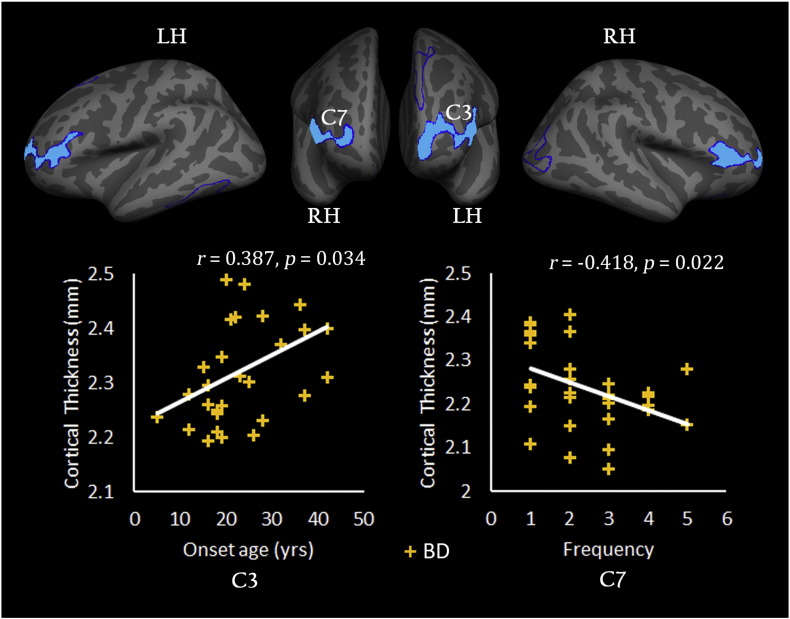
3.3.3. Relationship Between CT and Clinical Variables
Fig. 4 shows the correlations between the CT and clinical variables in the BD group. The statistical analysis revealed a significantly positive correlation between the mean CT in the left FPO (C3) and the onset age (r = 0.387, p = 0.034) but a negative correlation between the mean CT in the right PTRI (C7) and the number of episodes (r = − 0.418, p = 0.022). However, for the MDD group, no significant correlation was found between the CT and any of the clinical variables. Unfortunately, the significant result can't survive after the FDR correction.
Fig. 4.
Relationship between the cortical thickness and clinical variables. The scatter plot shows that the mean cortical thickness values for the clusters in the left frontal pole (C3) and the left pars triangularis (C7) changed with the onset age or number of depressive episodes. The correlation analysis was only performed for the patients with bipolar depression (BD). The symbol ‘+’ in yellow color represents a subject in the BD group. LH (RH), left (right) hemisphere.
4. Discussion
Using an SBM analysis, we compared the CT between the currently unmedicated depressed adult MDD patients, BD-II patients, and the healthy controls. We found that both the MDD and BD groups shared common CT abnormalities and the BD group had its specific CT abnormalities. Specifically, both the BD and MDD groups showed a thinner CT in the left ITC compared to the controls. Additionally, the BD group had a significantly thinner CT in the left rMFC compared to the MDD group. Finally, the BD group showed additional alterations primarily in the frontal lobe compared to the control group and had a tendency toward a thinner CT in most areas of the whole brain compared to either the MDD group or the control group.
4.1. Common Alteration in Cortical Thickness in MDD and BD
In this study, both the MDD and BD groups showed a significantly thinner CT in the left ITC compared to the controls (Fig. 1). This result is consistent with several previous studies (Phillips et al., 2015, Elvsåshagen et al., 2013). For example, Elvsåshagen et al. (2013) analyzed the CT difference between BD-II patients and healthy controls and found that the BD-II group showed a significantly thinner CT in the left temporal region involving the superior, middle, and inferior temporal cortex. Phillips et al. (2015) revealed that patients with treatment-resistant MDD had a thinner CT in the ITC compared to healthy controls. Meanwhile, several studies also reported GM tissue losses in the ITC in patients with MDD and BD (Grieve et al., 2013).
Previous study (Kolb et al., 2014) indicated that the ITC belongs to the ventral stream of visual processing, and is associated with the representation of complex object features, such as face perception (Haxby et al., 2000) and the recognition of numbers (Dehaene, 2011). And several other studies also indicated that it may be involved in o emotional processing (Phan et al., 2002, Liotti et al., 2000). Liotti et al. (2002) reported decreased activation in the ITC when MDD patients responded to sadness stimuli. Similarly, Gotlib et al. (2005) found that the MDD patients had significantly reduced responses to the happy facial expressions in the right ITC compared to the healthy controls. In addition, Liotti et al. (2000) found both memory-driven sadness and anxiety caused a decrease in the cerebral blood flow in the bilateral posterior ITC in BD patients. Taken together, our finding provides an evidence for the ITC may contribute to these two disorders.
4.2. Specific Alteration in Cortical Thickness Between MDD and BD
Compared to the MDD patients and controls, the BD patients showed a significantly thinner CT in the left rMFC (Fig. 1), which is the gyral-based representative of the dorsolateral prefrontal cortex (DLPFC, a functionally defined area) (Desikan et al., 2006). This result is consistent with a previous study, in which Lan et al. (2014) found less CT in the right DLPFC in BD patients compared to either MDD patients or healthy controls. Savitz and Drevets reviewed past MRI and metabolic PET studies in MDD and BD patients and suggested that the BD patients tended to display evidence of DLPFC GM volume loss (Savitz & Drevets, 2009). Such changes are consistent with aberrant DLPFC gene expression in patients with BD (Pennington et al., 2008, Shao and Vawter, 2008). Using a stereological 3D cell counting method, Rajkowska et al. (2001) demonstrated that the DLPFC from BD patients is characterized by significant reductions in glial density and changes in the shape of the glial nuclei and that their alterations are accompanied by reductions in neuronal density. This histopathological evidence from neurons and glial cells may be related to the reduction of the CT in BD patients.
The DLPFC is widely believed involving in the executive function (Miller & Cummings, 2007), such as working memory (Belger et al., 1998, Curtis, 2006), planning (Rosenbloom et al., 2012), and attentional control (Hopfinger et al., 2000). Actually, a previous study indicated that the deficit in executive function in BD is an important prognostic factor (Harvey et al., 2010). As a part of the cortico-thalamic-insular circuit, the DLPFC is also involved in the pathophysiology of mood disorders. In this neural circuitry model, a deficit in DLPFC would result in decreased cortical regulation of emotional processing. For example, Goldin et al. (2008) and Vizueta et al. (2012) revealed that re-evaluation of negative stimuli is positively correlated with activity in the DLPFC in healthy volunteers. And several task-fMRI studies also found increased activity in the DLPFC during suppression of negative emotions (Lévesque et al., 2003, Phan et al., 2005). Rive et al. (2015) investigated emotion regulation in patients with MDD and BD using task-fMRI and found that the BD patients had more seriously impaired emotion regulation. Additionally, they showed increased DLPFC activity in the BD patients compared to the MDD patients and healthy controls in an emotion regulation task, particularly in the sad emotion regulation task. Our findings indicate that the structural differences in the DLPFC in BD and MDD may be related to different clinical symptoms in these two disorders.
4.3. Pronounced Thinner Cortical Thickness in BD
In this study, the thinner CT in the BD patients versus the controls were observed in widespread areas, mainly located in the frontal lobe but also in the LOC (Fig. 1). Our results indicate that these findings are a BD-specific phenomenon, as we did not find them in the MDD patients. We found less CT in the dorsal PFC, including the SFC, PTRI, and rMFC, in the BD patients. Less CT of the left SFC was also observed in the BD patients compared to the controls in our study. The same finding of GM loss in the SFC was reported not only in previous SBM analyses (Maller et al., 2014, Hartberg et al., 2011) but also in VBM analyses (Lisy et al., 2011) in BD patients. In addition, a resting state fMRI study found significantly decreased functional connectivity in DLPFC networks in BD patients versus control subjects (Dickstein et al., 2010), and a ROI-based DTI study reported reduced fractional anisotropy in the superior frontal WM in BD patients (Benedetti et al., 2011). Moreover, we found that the BD patients showed a thinner CT in the left PTRI compared to the control group. This result is also consistent with several previous VBM studies. For example, Serafini et al. (2014) reported reduced GM density in the left PTRI in BD patients, and Maller et al. (2014) found two clusters with abnormal GM density in the PTRI which were correlated with HAMD scores in BD patients. Our finding provides evidence that the CT of the PTRI is correlated inversely with the number of depressive episodes in BD patients. This means the more number of episodes may be associated with more pronounced alteration in cortical morphology in the PTRI. This finding may suggest that the CT defect in the PTRI in BD is an indicator of the number of episodes in recurrent BD.
Cortical thinning in the BD patients was not only observed in the dorsal PFC but also in the ventral prefrontal cortex (VPFC) which includes the FPO and lOFC. In a previous postmortem study, Cotter et al. (2005) found evidence of reduced glial cell density and neuronal size in the caudal OFC of BD patients. This finding indicated that tissue loss in the OFC seems to be a characteristic of BD patients. Several DTI studies also found disrupted structural integrity of WM tracts in the OFC in adult (Beyer et al., 2005, Haznedar et al., 2005) and pediatric (Frazier et al., 2007) BD patients. We also observed that the BD patients had a thinner CT in the left FPO and that the mean CT in this region was significantly positively correlated with the onset age in the BD patients (Fig. 4). This means that early disorder-onset may be associated with more pronounced changes in cortical morphology in the FPO relative to late disorder-onset. Generally, the VPFC is associated with emotion regulation and evaluation during emotion processing (Rygula et al., 2010, Phillips and Swartz, 2014). Several studies have documented that BD patients manifested abnormal functional connectivity between the VPFC and other task-related brain areas during emotional-processing and regulation tasks (Townsend et al., 2013, Strakowski et al., 2011, Delvecchio et al., 2012). Thus, we infer that the thinning of the VPFC may be related to abnormal emotion processing in BD patients.
In this study, more pronounced thinner CT was found in the BD patients than in the MDD patients. Specifically, we found that the BD patients had the lowest CT in all the clusters listed in Table 2 that showed differences between any two groups (Fig. 2) and that the BD patients showed a higher reduction rate in CT than the MDD patients (Fig. 3). Alterations in CT in severe mental disorders, such as schizophrenia and BD, have been suggested as being due to a reduced number of synaptic contacts in affected areas (Harrison, 1999) or to neuronal apoptosis (Glantz et al., 2006). Our findings are in accord with several previous studies, which revealed that BD patients had more widespread WM abnormalities, GM volume reductions, and different aberrant functional connectivity in the neural circuits responsible for emotion regulation, attentional control, and reward-processing compared to MDD patients (Fung et al., 2015, Serafini et al., 2014). This is not surprising, given that BD is considered to be a more chronic illness and is associated with an earlier age of onset and more episodes of major depression compared with MDD (Merikangas et al., 2007). In addition, we found lower cortical thickness in the left hemisphere in the BD patients than the MDD patients and the controls. Actually, the issue of lateralized hemispheric imbalance in BD has not been systematically explored and several hypothesis-driven morphometric studies in BD have reported right-lateralized findings in BD (Selvaraj et al., 2012, Arnone et al., 2008). Surely, the significance of lateralized findings is needed to be well studied in the future.
We also noticed some discrepancy between this study and previous studies. Although several studies reported aberrant GM in the PFC and anterior cingulate cortex (ACC) in MDD patients (Phillips et al., 2015, Bora et al., 2012), we did not find decreased CT in these regions in the MDD patients. This discrepancy may have resulted from a number of factors. A possible factor is the relative small sample size of our study. In addition, the patients in our sample were unmedicated at the time of their scan; most of them were medication-naïve, which could partly account for some of our findings that are discrepant from previously published works which included medicated patients. In fact, the effect of medication on GM has been well documented in MDD and BD patients (Lanzenberger et al., 2012, Boldrini et al., 2013). Furthermore, most studies compared combined samples of BD-I and BD-II patients with MDD patients and controls (Lan et al., 2014, Fung et al., 2015). Maller et al. (2014) reported brain volumetric, thickness, and WM integrity differences between BD-I and BD-II and suggested that abnormal regional brain volume may underlie BD-I but not BD-II. This issue needs to be considered in a future study.
5. Limitations
There are several limitations to be addressed in this study. First, the samples of patients may not fully represent the MDD and BD populations. A previous study indicated that, due to the potential instability of the diagnosis, based on an epidemiological study, approximately 1–5% of MDD subjects will develop BD more than one year after their initial identification (Whiteford et al., 2013). Second, multiple recurrent episodes of conditions in the BD and MDD patients may have impacted the observed morphometric abnormalities. Third, the MDD group showed not only significantly lower in the years of education than the controls, but also significantly higher YMRS score than the BD patients. These may potentially have biased the observed morphometric abnormalities. Therefore, we assessed if the cortical thickness difference was associated with YMRS score or years of education, and found that cortical thickness in these clusters had no significant correlations with years of education or YMRS score. In addition, changes to cortical thickness by some confounders cannot be considered in this study, such as IQ, medication history and rapid cycling in BD patients. Furthermore, the significant results of correlation didn't survive after the FDR correction. A future replication study that examines the CT using FreeSurfer by separating first-episode and recurrent episodes of BD or MDD patients in a larger matched sample would be beneficial for elucidating the underlying neurobiological differences between BD and MDD patients. Last but not least, because we carried out a cross-sectional study, we cannot infer whether the abnormal CT in the patients was caused by the brain disorders or whether the patients had innate abnormal brain structures (e.g., neural disposition, genetic origins). A longitudinal study is needed to find evidence for disorder-induced CT changes.
6. Conclusion
In summary, we estimated the cortical thickness in unmedicated adult patients with MDD or BD and controls by using a surface-based morphometric analysis and revealed the alteration patterns in the MDD and BD patients that were either in common between the disease groups or specific to one of the groups. Relative to the healthy controls, the two patient groups showed reduced cortical thickness in the left ITC in common. However, compared with the MDD patients, the BD patients displayed widespread reduction in cortical thickness in brain regions primarily located in the frontal lobe. Our results also showed that the BD patients had the lowest cortical thickness in widespread areas of the whole brain relative to the other two groups. These findings seem to imply that BD and MDD have different underlying pathophysiological mechanisms and may be useful for classifying individual patients with either MDD or BD.
Funding Sources
The study was supported by grants from the National Natural Science Foundation of China (81501456, 81471650, 81428013, 81471654, and 81371535); Natural Science Foundation of Guangdong Province, China (2014A030313375); Planned Science and Technology Project of Guangdong Province, China (2014B020212022); Planned Science and Technology Project of Guangzhou, China (1563000653); Fundamental Research Funds for the Central Universities, China (21615476). The funding organizations play no further role in study design, data collection, analysis and interpretation and paper writing.
Conflicts of Interest
The authors declare that they have no competing financial interests.
Author Contributions
Guarantors of integrity of entire study, M.N., Y.W., L.H., R.H.; literature search, M.N., Y.W., R.H.; study concepts/study design, M.N., Y.W., J.W., R.H.; data acquisition, Y.W., Y.J., S.Z., Y.S., L.H.; data analysis, M.N., J.W., J.L., L.Z., X.L., R.H.; manuscript drafting or manuscript revision for important intellectual content, all authors; approval of final version of submitted manuscript, all authors; agrees to ensure any questions related to the work are appropriately resolved, all authors; clinical studies, Y.W., Y.J., S.Z., Y.S., L.H.; figures, M.N., L.Z., X.L., R.H.; statistical analysis, M.N., J.W., J.L., L.Z., X.L., R.H.; and manuscript editing, M.N., Y.W., L.H., R.H.
Acknowledgments
The authors express appreciation to Drs. Rhoda E. and Edmund F. Perozzi for editing assistance.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.01.010.
Contributor Information
Li Huang, Email: cjr.huangli@vip.163.com.
Ruiwang Huang, Email: ruiwang.huang@gmail.com.
Appendix A. Supplementary data
Supplementary material
References
- Arnone D., McIntosh A., Chandra P., Ebmeier K. Meta-analysis of magnetic resonance imaging studies of the corpus callosum in bipolar disorder. Acta Psychiatr. Scand. 2008;118(5):357–362. doi: 10.1111/j.1600-0447.2008.01229.x. [DOI] [PubMed] [Google Scholar]
- Baldessarini R.J., Vieta E., Calabrese J.R., Tohen M., Bowden C.L. Bipolar depression: overview and commentary. Harv. Rev. Psychiatry. 2010;18(3):143–157. doi: 10.3109/10673221003747955. [DOI] [PubMed] [Google Scholar]
- Belger A., Puce A., Krystal J.H., Gore J.C., Goldman-Rakic P., McCarthy G. Dissociation of mnemonic and perceptual processes during spatial and nonspatial working memory using fMRI. Hum. Brain Mapp. 1998;6(1):14–32. doi: 10.1002/(SICI)1097-0193(1998)6:1<14::AID-HBM2>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F., Yeh P.-H., Bellani M. Disruption of white matter integrity in bipolar depression as a possible structural marker of illness. Biol. Psychiatry. 2011;69(4):309–317. doi: 10.1016/j.biopsych.2010.07.028. [DOI] [PubMed] [Google Scholar]
- Benmansour S., Cecchi M., Morilak D.A. Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level. J. Neurosci. 1999;19(23):10494–10501. doi: 10.1523/JNEUROSCI.19-23-10494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer J.L., Taylor W.D., MacFall J.R. Cortical white matter microstructural abnormalities in bipolar disorder. Neuropsychopharmacology. 2005;30(12):2225–2229. doi: 10.1038/sj.npp.1300802. [DOI] [PubMed] [Google Scholar]
- Boldrini M., Santiago A.N., Hen R. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology. 2013;38(6):1068–1077. doi: 10.1038/npp.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein F.L. “Voxel-based morphometry” should not be used with imperfectly registered images. NeuroImage. 2001;14(6):1454–1462. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- Bora E., Fornito A., Pantelis C., Yücel M. Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. J. Affect. Disord. 2012;138(1):9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- Brooks J.O., Bonner J.C., Rosen A.C. Dorsolateral and dorsomedial prefrontal gray matter density changes associated with bipolar depression. Psychiatry Res. 2009;172(3):200–204. doi: 10.1016/j.pscychresns.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter D., Hudson L., Landau S. Evidence for orbitofrontal pathology in bipolar disorder and major depression, but not in schizophrenia. Bipolar Disord. 2005;7(4):358–369. doi: 10.1111/j.1399-5618.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- Curtis C. Prefrontal and parietal contributions to spatial working memory. Neuroscience. 2006;139(1):173–180. doi: 10.1016/j.neuroscience.2005.04.070. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- de Almeida J.R.C., Phillips M.L. Distinguishing between unipolar depression and bipolar depression: current and future clinical and neuroimaging perspectives. Biol. Psychiatry. 2013;73(2):111–118. doi: 10.1016/j.biopsych.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos F., Schouten T.M., Hafkemeijer A. Combining multiple anatomical MRI measures improves Alzheimer's disease classification. Hum. Brain Mapp. 2016;37(5):1920–1929. doi: 10.1002/hbm.23147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S. OUP; USA: 2011. The Number Sense: How the Mind Creates Mathematics. [Google Scholar]
- Delvecchio G., Fossati P., Boyer P. Common and distinct neural correlates of emotional processing in bipolar disorder and major depressive disorder: a voxel-based meta-analysis of functional magnetic resonance imaging studies. Eur. Neuropsychopharmacol. 2012;22(2):100–113. doi: 10.1016/j.euroneuro.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dickstein D.P., Gorrostieta C., Ombao H. Fronto-temporal spontaneous resting state functional connectivity in pediatric bipolar disorder. Biol. Psychiatry. 2010;68(9):839–846. doi: 10.1016/j.biopsych.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker C., Ginestet C., Feng Y. Brain surface anatomy in adults with autism: the relationship between surface area, cortical thickness, and autistic symptoms. JAMA Psychiat. 2013;70(1):59–70. doi: 10.1001/jamapsychiatry.2013.265. [DOI] [PubMed] [Google Scholar]
- Elvsåshagen T., Westlye L.T., Bøen E. Bipolar II disorder is associated with thinning of prefrontal and temporal cortices involved in affect regulation. Bipolar Disord. 2013;15(8):855–864. doi: 10.1111/bdi.12117. [DOI] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U. S. A. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B., van der Kouwe A., Destrieux C. Automatically parcellating the human cerebral cortex. Cereb. Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. NeuroImage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross L.C., Thompson P.M., Sugar C.A. Investigation of cortical thickness abnormalities in lithium-free adults with bipolar I disorder using cortical pattern matching. Am. J. Psychiatry. 2011;168(5):530–539. doi: 10.1176/appi.ajp.2010.10060896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier J.A., Breeze J.L., Papadimitriou G. White matter abnormalities in children with and at risk for bipolar disorder. Bipolar Disord. 2007;9(8):799–809. doi: 10.1111/j.1399-5618.2007.00482.x. [DOI] [PubMed] [Google Scholar]
- Fung G., Deng Y., Zhao Q. Distinguishing bipolar and major depressive disorders by brain structural morphometry: a pilot study. BMC Psychiatry. 2015;15(1):1–12. doi: 10.1186/s12888-015-0685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz L.A., Gilmore J.H., Lieberman J.A., Jarskog L.F. Apoptotic mechanisms and the synaptic pathology of schizophrenia. Schizophr. Res. 2006;81(1):47–63. doi: 10.1016/j.schres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Goldin P.R., McRae K., Ramel W., Gross J.J. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol. Psychiatry. 2008;63(6):577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib I.H., Sivers H., Gabrieli J.D. Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport. 2005;16(16):1731–1734. doi: 10.1097/01.wnr.0000183901.70030.82. [DOI] [PubMed] [Google Scholar]
- Grieve S.M., Korgaonkar M.S., Koslow S.H., Gordon E., Williams L.M. Widespread reductions in gray matter volume in depression. Neuroimage Clin. 2013;3:332–339. doi: 10.1016/j.nicl.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanford L.C., Nazarov A., Hall G.B., Sassi R.B. Cortical thickness in bipolar disorder: a systematic review. Bipolar Disord. 2016;18(1):4–18. doi: 10.1111/bdi.12362. [DOI] [PubMed] [Google Scholar]
- Harrison P.J. The neuropathology of schizophrenia. Brain. 1999;122(4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Hartberg C., Sundet K., Rimol L. Brain cortical thickness and surface area correlates of neurocognitive performance in patients with schizophrenia, bipolar disorder, and healthy adults. J. Int. Neuropsychol. Soc. 2011;17(6):1080–1093. doi: 10.1017/S1355617711001081. [DOI] [PubMed] [Google Scholar]
- Harvey P.D., Wingo A.P., Burdick K.E., Baldessarini R.J. Cognition and disability in bipolar disorder: lessons from schizophrenia research. Bipolar Disord. 2010;12(4):364–375. doi: 10.1111/j.1399-5618.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- Haxby J.V., Hoffman E.A., Gobbini M.I. The distributed human neural system for face perception. Trends Cogn. Sci. 2000;4(6):223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Haznedar M.M., Roversi F., Pallanti S. Fronto-thalamo-striatal gray and white matter volumes and anisotropy of their connections in bipolar spectrum illnesses. Biol. Psychiatry. 2005;57(7):733–742. doi: 10.1016/j.biopsych.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Hopfinger J.B., Buonocore M.H., Mangun G.R. The neural mechanisms of top-down attentional control. Nat. Neurosci. 2000;3(3):284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Huang J., Perlis R.H., Lee P.H. Cross-disorder genomewide analysis of schizophrenia, bipolar disorder, and depression. Am. J. Psychiatry. 2010;167(10):1254–1263. doi: 10.1176/appi.ajp.2010.09091335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B., Whishaw I.Q., Teskey G.C. 2014. An Introduction to Brain and Behavior. [Google Scholar]
- Koutsouleris N., Meisenzahl E.M., Borgwardt S. Individualized differential diagnosis of schizophrenia and mood disorders using neuroanatomical biomarkers. Brain. 2015;138(7):2059–2073. doi: 10.1093/brain/awv111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan M.J., Chhetry B.T., Oquendo M.A. Cortical thickness differences between bipolar depression and major depressive disorder. Bipolar Disord. 2014;16(4):378–388. doi: 10.1111/bdi.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzenberger R., Kranz G.S., Haeusler D. Prediction of SSRI treatment response in major depression based on serotonin transporter interplay between median raphe nucleus and projection areas. NeuroImage. 2012;63(2):874–881. doi: 10.1016/j.neuroimage.2012.07.023. [DOI] [PubMed] [Google Scholar]
- Lévesque J., Eugene F., Joanette Y. Neural circuitry underlying voluntary suppression of sadness. Biol. Psychiatry. 2003;53(6):502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Liotti M., Mayberg H.S., Brannan S.K., McGinnis S., Jerabek P., Fox P.T. Differential limbic–cortical correlates of sadness and anxiety in healthy subjects: implications for affective disorders. Biol. Psychiatry. 2000;48(1):30–42. doi: 10.1016/s0006-3223(00)00874-x. [DOI] [PubMed] [Google Scholar]
- Liotti M., Mayberg H.S., McGinnis S., Brannan S.L., Jerabek P. Unmasking disease-specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depression. Am. J. Psychiatry. 2002;159(11):1830–1840. doi: 10.1176/appi.ajp.159.11.1830. [DOI] [PubMed] [Google Scholar]
- Lisy M.E., Jarvis K.B., DelBello M.P. Progressive neurostructural changes in adolescent and adult patients with bipolar disorder. Bipolar Disord. 2011;13(4):396–405. doi: 10.1111/j.1399-5618.2011.00927.x. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Kupfer D.J. Bipolar disorder diagnosis: challenges and future directions. Lancet. 2013;381(9878):1663–1671. doi: 10.1016/S0140-6736(13)60989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuffin P., Rijsdijk F., Andrew M., Sham P., Katz R., Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch. Gen. Psychiatry. 2003;60(5):497–502. doi: 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- van Lutterveld R., van den Heuvel M.P., Diederen K.M. Cortical thickness in individuals with non-clinical and clinical psychotic symptoms. Brain. 2014;137(10):2664–2669. doi: 10.1093/brain/awu167. [DOI] [PubMed] [Google Scholar]
- Redlich R., Almeida J.R., Grotegerd D. Brain morphometric biomarkers distinguishing unipolar and bipolar depression: a voxel-based morphometry–pattern classification approach. JAMA Psychiat. 2014;71(11):1222–1230. doi: 10.1001/jamapsychiatry.2014.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller J.J., Thaveenthiran P., Thomson R.H., McQueen S., Fitzgerald P.B. Volumetric, cortical thickness and white matter integrity alterations in bipolar disorder type I and II. J. Affect. Disord. 2014;169:118–127. doi: 10.1016/j.jad.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Rimol L.M., Nesvåg R., Hagler D.J. Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol. Psychiatry. 2012;71(6):552–560. doi: 10.1016/j.biopsych.2011.11.026. [DOI] [PubMed] [Google Scholar]
- Rimol L.M., Hartberg C.B., Nesvåg R. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol. Psychiatry. 2010;68(1):41–50. doi: 10.1016/j.biopsych.2010.03.036. [DOI] [PubMed] [Google Scholar]
- van Eijndhoven P., van Wingen G., Katzenbauer M. Paralimbic cortical thickness in first-episode depression: evidence for trait-related differences in mood regulation. Am. J. Psychiatry. 2013;170(12):1477–1486. doi: 10.1176/appi.ajp.2013.12121504. [DOI] [PubMed] [Google Scholar]
- Tu P., Chen L., Hsieh J., Bai Y., Li C., Su T. Regional cortical thinning in patients with major depressive disorder: a surface-based morphometry study. Psychiatry Res. Neuroimaging. 2012;202(3):206–213. doi: 10.1016/j.pscychresns.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Qiu L., Lui S., Kuang W. Regional increases of cortical thickness in untreated, first-episode major depressive disorder. Transl. Psychiatry. 2014;4(4) doi: 10.1038/tp.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Steinberg M. American Psychiatric Pub; 1994. Interviewer's Guide to the Structured Clinical Interview for DSM-IV Dissociative Disorders (SCID-D) [Google Scholar]
- Williams J.B. A structured interview guide for the Hamilton depression rating scale. Arch. Gen. Psychiatry. 1988;45(8):742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- Young R., Biggs J., Ziegler V., Meyer D. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry. 1978;133(5):429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Peterson B.S., Kan E. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb. Cortex. 2007;17(7):1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E., Narr K.L., Thompson P.M. Gender effects on cortical thickness and the influence of scaling. Hum. Brain Mapp. 2006;27(4):314–324. doi: 10.1002/hbm.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J.L., Batten L.A., Tremblay P., Aldosary F., Blier P. A prospective, longitudinal study of the effect of remission on cortical thickness and hippocampal volume in patients with treatment-resistant depression. Int. J. Neuropsychopharmacol. 2015;18(8):pyv037. doi: 10.1093/ijnp/pyv037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K.L., Wager T., Taylor S.F., Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Savitz J., Drevets W.C. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci. Biobehav. Rev. 2009;33(5):699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington K., Beasley C., Dicker P. Prominent synaptic and metabolic abnormalities revealed by proteomic analysis of the dorsolateral prefrontal cortex in schizophrenia and bipolar disorder. Mol. Psychiatry. 2008;13(12):1102–1117. doi: 10.1038/sj.mp.4002098. [DOI] [PubMed] [Google Scholar]
- Shao L., Vawter M.P. Shared gene expression alterations in schizophrenia and bipolar disorder. Biol. Psychiatry. 2008;64(2):89–97. doi: 10.1016/j.biopsych.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G., Halaris A., Selemon L.D. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol. Psychiatry. 2001;49(9):741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- Miller B.L., Cummings J.L. Guilford Press; 2007. The Human Frontal Lobes: Functions and Disorders. [Google Scholar]
- Rosenbloom M.H., Schmahmann J.D., Price B.H. The functional neuroanatomy of decision-making. J. Neuropsychiatr. Clin. Neurosci. 2012;24(3):266–277. doi: 10.1176/appi.neuropsych.11060139. [DOI] [PubMed] [Google Scholar]
- Vizueta N., Rudie J.D., Townsend J.D. Regional fMRI hypoactivation and altered functional connectivity during emotion processing in nonmedicated depressed patients with bipolar II disorder. Am. J. Psychiatry. 2012;169(8):831–840. doi: 10.1176/appi.ajp.2012.11030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K.L., Fitzgerald D.A., Nathan P.J., Moore G.J., Uhde T.W., Tancer M.E. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol. Psychiatry. 2005;57(3):210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Rive M.M., Mocking R.J., Koeter M.W. State-dependent differences in emotion regulation between unmedicated bipolar disorder and major depressive disorder. JAMA Psychiatry. 2015;72(7):687–696. doi: 10.1001/jamapsychiatry.2015.0161. [DOI] [PubMed] [Google Scholar]
- Serafini G., Pompili M., Borgwardt S. Brain changes in early-onset bipolar and unipolar depressive disorders: a systematic review in children and adolescents. Eur. Child Adolesc. Psychiatry. 2014;23(11):1023–1041. doi: 10.1007/s00787-014-0614-z. [DOI] [PubMed] [Google Scholar]
- Rygula R., Walker S.C., Clarke H.F., Robbins T.W., Roberts A.C. Differential contributions of the primate ventrolateral prefrontal and orbitofrontal cortex to serial reversal learning. J. Neurosci. 2010;30(43):14552–14559. doi: 10.1523/JNEUROSCI.2631-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Swartz H.A. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am. J. Psychiatry. 2014;171(8):829–843. doi: 10.1176/appi.ajp.2014.13081008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J.D., Torrisi S.J., Lieberman M.D., Sugar C.A., Bookheimer S.Y., Altshuler L.L. Frontal-amygdala connectivity alterations during emotion downregulation in bipolar I disorder. Biol. Psychiatry. 2013;73(2):127–135. doi: 10.1016/j.biopsych.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski S.M., Eliassen J.C., Lamy M. Functional magnetic resonance imaging brain activation in bipolar mania: evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biol. Psychiatry. 2011;69(4):381–388. doi: 10.1016/j.biopsych.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas K.R., Akiskal H.S., Angst J. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch. Gen. Psychiatry. 2007;64(5):543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj S., Arnone D., Job D. Grey matter differences in bipolar disorder: a meta-analysis of voxel-based morphometry studies. Bipolar Disord. 2012;14(2):135–145. doi: 10.1111/j.1399-5618.2012.01000.x. [DOI] [PubMed] [Google Scholar]
- Whiteford H.A., Degenhardt L., Rehm J. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material