Abstract
Phytochemicals or their derived compounds are being increasingly recognized as potentially potent complementary treatments for cancer. Among them, some phytochemicals are being actively evaluated for use as adjuvants in anticancer therapies. For instance, shikonin and hypericin were found to induce immunogenic cell death of specific cancer cells, and this effect was able to further activate the recognition activity of tumor cells by the host immune system. On the other hand, some derivatives of phytochemicals, such as dihydrobenzofuran lignan (Q2-3) have been found to induce the secretion of an endogenous anticancer factor, namely IL-25, from non-malignant cells. These findings suggest that phytochemicals or their derivatives confer a spectrum of different pharmacological activities, which contrasts with the current cytotoxic anticancer drugs commonly used in clinics. In this review, we have collected together pertinent information from recent studies about the biochemical and cellular mechanisms through which specific phytochemicals regulate target immune systems in defined tumor microenvironments. We have further highlighted the potential application of these immunotherapeutic modifiers in cell-based cancer vaccine systems. This knowledge provides useful technological support and know how for future applications of phytochemicals in cancer immunotherapy.
Keywords: phytochemicals, herbal extract, cancer immunotherapy, tumor microenvironment
Application of Phytochemical-Induced Immunogenic Cell Death in the Development of Cancer Immunotherapy
Immunogenic cell death (ICD) is a kind of cell death that can effectively stimulate specific immune response against dying or dead cell antigens (Kroemer et al., 2013). The molecular features of ICD has been characterized as specific expression changes in some DAMPs associated with the induced immunogenicity, for instance against targeted tumor cells. These immune-modifying molecules include heat shock proteins (HSP), HMGB1, CRT, glucose-related protein (GRP) and others (Garg et al., 2010; Krysko et al., 2012). As a result of these findings, the induction of tumor cell ICD and DAMP expression has been considered to be a useful therapeutic approach to activate the immunogenicity of target tumor cells.
Shikonin, a secondary plant metabolite isolated from Lithospermum erythrorhizon, can confer a broad spectrum of specific cellular and biochemical activities. These include the inhibition of promoter/transcriptional activities of the pro-inflammatory cytokines TNF-a (Staniforth et al., 2004) and GM-CSF (Su et al., 2008), the blockade of splicing of TNF-a pre-mRNA (Chiu and Yang, 2007), the induction of EMT activity in skin wound-healing (Yin et al., 2013a), and a differential effect on the genomic expression of cytokine/chemokine genes in human monocytes (Chiu et al., 2010). In our previous studies, shikonin was also found to strongly stimulate ICD of tumor cells (Chen et al., 2012), which induced a potent immune response through DCs to suppress tumor growth and/or metastasis (Lin et al., 2015a,b). A molecular target of shikonin, namely hnRNPA1, was proved to play an important role in shikonin-induced ICD in mammary tumor cells (Yin et al., 2016a). Moreover, hnRNPA1 was known as an important substrate of GzmA that can disrupt the nuclear export activity of newly synthesized RNA, and this resulted in a specific type of immune-mediated programmed cell death (Rajani et al., 2012). These findings together suggest that shikonin can confer various molecular or intracellular effects on the post-transcriptional processing of RNAs, including nuclear export and splicing activities of pre-mRNA. Importantly, the shikonin-caused dysfunction of hnRNPA1 may further provide a sound pharmacological basis for the potential application of shikonin in cancer immunotherapeutics, for example, in DC-based cancer vaccines.
Immunogenic cell death-associated immunogenicity will also be effective if it is induced by a specific ROS-based ER stress, such as hypericin-based PDT (Krysko et al., 2012), rather than by secondary or collateral ER stress effects. Hypericin, an anthraquinone derivative present in the yellow flower of Hypericum perforatum (Wurglics et al., 2001), has also been used to develop hypericin-based PDT (Zheng et al., 2014a). Recently, the use of DC vaccines in combination with hypericin-based PDP–induced ICD was applied to treat HGG in an animal model. ICD-based DC vaccines improved survival of test animals, and this effect was found to be dependent on cell-associated ROS and the release of DAMPs, including the extra-cellular HMGB1 and the surface CRT (Garg et al., 2016). These findings thus suggest that Hyp-PDT–based anticancer vaccines may also be worth development for clinical use in the future.
Regulation of Immune Cells in the Tumor Microenvironment by Phytochemicals
The tumor microenvironment is a critical determinant of distant cancer metastasis (Mlecnik et al., 2016). Immune cells in the tumor microenvironment, including regulatory T cells, dendritic cells, MDSCs and TAMs, are known to express a low level of MHC class I molecules but high levels of various immunosuppressive factors, such as IL-10, IL-6, and TGF-β. And these factors can contribute to tumor progression and the evasion of systemic immune surveillance (Albini and Sporn, 2007). Currently, most anticancer drugs are based mainly on specific targeted or cytotoxic agents which were discovered by using the “one gene, one target, one disease” approach (Anighoro et al., 2014). Over the last few years, however, the application of some traditional medicines, such as GLPS extracted from Ganoderma lucidum, have been reported to enhance the CD4+/CD8+ T cell ratio in the tumor microenvironment (Liu and Sun, 2012; Sun et al., 2014). In addition, the anoectochilus formosanus and a schisandra polysaccharides isolated from Schisandra chinensis, were found to promote the M1 differentiation of TAM (Kuan et al., 2012). Recently, some plant natural products or phytoagents, including terpenoids, phenolics, plant-derived lipids, alkaloids and PHY906 (a multi-herb formulation), have also highlighted and summarized for their effects on the oxylipin dynamics in a defined tumor microenvironment (Apaya et al., 2016). These studies on natural compounds have brought into clarity the poly-pharmacological nature of phyto agents, which can be thought of as “multiple targets, multiple effect and complex disease”.
Myeloid-derived suppressor cells (MDSCs) have been found to be largely responsible for the inhibition of host antitumor immunities, consequently impairing the efficacy of anticancer immunotherapeutic approaches (Gabrilovich and Nagaraj, 2009). Some phytochemicals or herbal extracts have been shown to confer suppressive activity in controlling MDSC expansion. Silibinin, a flavanone from the seeds of Silybum marianum, is a dietary supplement which is widely used for hepato- and chemo-preventive activities (Agarwal et al., 2006). Among several effects of silibinin in the tumor microenvironment (Deep and Agarwal, 2013), the supplement was found to strongly suppress the formation of 5-LOX metabolites in human macrophages, mast cells and granulocytes, and inhibit COX-2 expression in vivo (Tager et al., 2001; Bannwart et al., 2010). In a murine mammary tumor model, silibinin treatment also inhibited the accumulation of MDSCs, increased the quantity of T cells in the tumor-associated microenvironment and increased the survival rate of test mice (Forghani et al., 2014). Recently, an ethanol extract of Bidens pilosa (BP-E) was found to effectively suppress the G-CSF-induced differentiation of gMDSCs from mouse myeloid precursor cells. Consistently, in vivo studies further demonstrated that oral delivery of BP-E can effectively suppress 4T1 tumor metastasis in a tumor-resection model (Wei et al., 2016). Our bio-organic chemical analysis also showed that a specific group of polyacetylenic glycosides may act as the principle phytochemicals responsible for the detected MDSC activities, ex vivo and in vivo (Wei et al., 2016). These findings clearly indicate that specific types of polyacetylene may have therapeutic potential against the metastasis of mammary tumor cells.
In our previous studies, plant extracts of different plant species were shown to actively enhance the immunity or efficacy of a DC-based vaccine against tumor metastasis in animal models (Chang et al., 2013, 2015). A specific, ethanol-precipitated fraction of Dioscorea alata var. purpurea Tainung No. 5 extract, designated as DsII-TN5, effectively enhanced the DC-mediated activation of T-cell proliferation and suppressed the growth of melanoma tumor cells in vivo (Chang et al., 2013). Mechanistically, other studies showed that immunological activities of specific polysaccharides and/or glycoproteins in Dioscorea tubers were in part due to their TLR4-signaling pathway-mediated immunomodulatory and cytokine-regulation activities (Fu et al., 2006; Liu et al., 2008). Moreover, our previous study further showed that Dioscorea phytoextracts can enhance the proliferation of murine splenocytes ex vivo and promote regeneration of specific myeloid-derived progenitor cells in chemotherapy-damaged bone marrow tissues (Su et al., 2011). These findings suggest that certain plant polysaccharides and/or glycoproteins in Dioscorea extracts may serve as potential candidates for acting as non-toxic TLR4 agonists that can activate TLR4 signaling to induce anti-tumor immune responses. Other traditional Chinese medicine preparations, including root extracts of Radix Astragalus (Astragalus membranaceus) and Radix Codonopsis (Codonopsis pilosulae), were also shown to modulate the functions of specific immune cells. In particular, the highly purified Astragalus polysaccharides can activate mouse macrophages and B cells (Shao et al., 2004), restore depressed mitogen response and inhibit the growth of leukemia and lymphoma tumor cells in tumor-bearing mice (Cho and Leung, 2007). In addition, other studies also indicated that the Codonopsis polysaccharides caused a change in T-cell polarization from Th2 to Th1 responses and further suppressed Treg cells (Zheng et al., 2014b). Clinically, these polysaccharides may be responsible for conferring pharmacological bioactivities for repair or restoration of immunosuppressive activities in treated patients (Wang et al., 2012). To similarly address this issue, in our previous study, we further evaluated two highly purified but still mixed polysaccharide fractions from the root of A. membranaceus (Am) and Codonopsis pilosulae (Cp), for their use in stimulating a highly anti-metastatic efficiency of a DC-based cancer vaccine against 4T1 mammary carcinoma in vivo (Chang et al., 2015). Our findings suggest that these plant polysaccharides in Am and Cp can also be effectively employed as a potent adjuvant for development of DC-based cancer immunotherapies.
Suppression of Oxidative Stress in the Tumor Microenvironment by Phytochemicals
Reactive oxygen species (ROS), including superoxide, hydroxyl radicals or non-reactive oxygen, are a group of molecules that contain reactive oxygen and can be readily converted into a reactive species, such as H2O2 (Son et al., 2013). In the tumor microenvironment, ROS have been observed to play multiple roles in the hallmarks of carcinogenesis, such as genome instability and mutation, angiogenesis, invasion and metastasis (Grivennikov et al., 2010). Several phytochemicals or herbal extracts have been evaluated in clinical trials as well as in many studies for their anti-cancer activities through the modulation of oxidative stress and the tumor microenvironment. Among them, the proposed pharmacological mechanisms of selected phytochemicals or herbal extracts, such as curcumin, resveratrol, artemisinin, berberine, paclitaxel, broccoli isothiocyanate, green tea extract, mistletoe extract, noscapine and its derivatives and piperine have been evaluated in clinical trials, as well as intensively studied in preclinical mouse tumor models for their anti-cancer activities via the modulation of oxidative stress in the tumor-associated microenvironment (Cheng et al., 2016). These pleiotropic functions of phytomedicines or phytoagents have been considered to be a novel adjuvant approach that may be useful in combination with chemotherapeutics for overcoming drug resistance or tumor metastasis in cancer therapy (Cheng et al., 2016).
Potential Pharmacological Activities of Phytochemicals in Activating the Expression/Secretion of Specific Endogenous Anticancer Factors in the Tumor Microenvironment
Tumor suppressor p53 is a transcription factor that can play important roles in the regulation of the DNA repair, cell-cycle, apoptosis, angiogenesis and senescence (Shangary and Wang, 2008). Through activation of p53, many herbal extracts, including the extracts of Hibiscus syriacus (Cheng et al., 2008), Bing De Ling (Xu et al., 2005), Suillus collinitus (Vaz et al., 2012), Emilia sonchifolia (Lan et al., 2012), Cochinchina momordica (Liu H.R. et al., 2012), Zeng Sheng Ping (Zhang et al., 2004), and JP-1 (Yao et al., 2016), have been shown to induce growth arrest or metastatic activity of test cancer cells. These herbal medicines or their derived compounds have been contemplated as potential resources in the search for proper agents to restore the p53 function in cancer cells. Mechanistically, p53 can also further stimulate the expression of specific sets of target microRNAs, such as miR-34a (He et al., 2007), and this is known to play an essential role in the anti-proliferative activities of specific phytochemicals, such as artemisinin and artesunate (Hargraves et al., 2016).
Mutations or deletions in the human p53 gene (TP53) are present in nearly 50% of human cancers (Wang et al., 2017). One p53-inhibitory protein, namely murine double minute 2 (MDM2), tightly controls the expression levels of p53 protein (Klein and Vassilev, 2004). Mechanistically, MDM2 induces degradation of p53 via the ubiquitin-proteasome machinery through the binding and blocks to the N-terminal transactivation domain of p53 (Klein and Vassilev, 2004). The crystal structures of MDM2 and p53 further indicate their interaction is mediated by a surface pocket and three hydrophobic key residues: Phe19, Trp23, and Leu26 (Wiman, 2010). Because of the antagonistic role of MDM2 in controlling p53 expression, many small molecules have been designed or developed to mimic p53-binding residues (Okabe et al., 2008). Among them, some herbal compounds, such as α-mangostin and gambogic acid, were indicated to inhibit the p53-MDM2 interaction by binding to MDM2 (Parks et al., 2005; Hientz et al., 2017). These two compounds exhibited high binding affinities with hydrophobic MDM2 through the residues Gly58, Asp68, Val75, and Cys77 (Parks et al., 2005). These findings suggest that specific phytochemicals may also be valuable contenders in cancer therapeutics by targeting the MDM2-p53 interaction.
Specific tumor microenvironment systems are also associated with different dynamic feedback systems for secretion of some cytokines, endogenous second messengers or other soluble factors between cancer and the surrounding stromal cells (Swartz and Lund, 2012). Some tumor-associated stromal cells can produce tumor suppressor factors, such as nucleoside NME1 (Dooley et al., 1994), Kangai 1 (KAI1/CD82) (Dong et al., 1997) and IL-25 (Furuta et al., 2011), in the tumor microenvironment, and these activities can restrict the development or metastasis of surrounding tumor cells. The investigation of specific molecular agents that can confer stimulatory effect on the expression or secretion of these endogenous anticancer factors is hence a new direction that may lead to new cancer therapies (Bykov et al., 2002). Among these endogenous anticancer factors, IL-25 (IL-17E) secreted by normal mammary epithelial calls has been reported to confer a specific anticancer effect on breast cancer cells, however, it has little or no cytotoxic effect on the non-malignant counterpart cells (Furuta et al., 2011). This apoptosis-promoting activity of IL-25 may be due to the differential expression of its receptor, IL-25RB. The expression level IL-25RB was found to be much higher in tumors from patients with poor prognoses, as comparing to that in non-malignant breast tissues (Furuta et al., 2011). Recently, our study showed that a dimerization product of plant caffeic acid methyl ester, the dihydrobenzofuran lignan (Q2-3), can effectively suppress the metastasis of mammary tumor cells (Yin et al., 2016b). By using a tumor resection model, we demonstrated that the in vivo treatment of Q2-3 efficiently induced the TAFs to secrete IL-25 at high levels. This IL-25 secretion was shown to play a key role in the Q2-3-mediated anti-metastatic activity in mouse mammary tumor models. In addition, our in vivo experiments further showed that the anti-metastatic effects of Q2-3 on both mouse (4T1) and human (MDA-MD-231) tumor cells are additive, when it was administrated in combination with the clinically used anticancer drug, docetaxel (Yin et al., 2016b). Together, our findings revealed that the secretion of IL-25 from TAFs could serve as a highly inducible therapeutic strategy for the control of mammary tumor metastasis. Moreover, our study again indicated that the potential pharmacological activity of specific phytochemicals or their synthetic compounds may effectively activate the secretion/expression of specific endogenous anticancer factors in the tumor microenvironment, resulting in strong anti-metastatic effect.
Summary and Future Prospects
Although different pharmacological activities of phytochemicals or herbal extracts have been indicated for the development of cancer immunotherapies (Table 1), the exact molecular/cellular targets and hierarchical mechanisms for many of them still need to be evaluated. To address issues related to immune regulation or non-malignant cells in the tumor-associated microenvironment, we consider that new strategies that combine specific in vivo tumor model systems with various omics tools/approaches, as shown in Figure 1, may provide new directions for phytomedicinal research. Conventional phytomedicines are thought to involve multiple active components and interact with multiple targeted molecules related to various cellular or physiological effects (e.g., immunomodulation). Through considering different responsive elements for their observed medicinal efficacy, including the expressional change of genes, proteins, microRNA and other metabolites, we propose a hypothesis or a prediction for specific pharmacological activity of phytochemicals after systematic analysis (Figure 1). Although many herbal medicines are used worldwide, their efficacies for disease control or health care, have unfortunately only been studied in a sporadic way, with few or no RCTs (Yin et al., 2013b). In 2004 an international group of pharmacologists, methodologists, pharmacognosists, and clinical trial researchers met for a consensus-forming meeting, which develop the recommendations for the reporting of herbal medicine trials in Toronto, Canada (Gagnier et al., 2006b). These recommendations and CONSORT statements aimed to assist researchers to accurately assess the reproducibility of herbal medicine trials and internal/external validity, to allow the safety and efficacy of specific herbal medicines be assessed in a more accurate way (Gagnier et al., 2006a). The 22 CONSORT checklist items including title and abstract, background, participants, outcomes, baseline data, interpretation, generalizability, detailed recommendations for interventions, and the overall evidence (Gagnier et al., 2006b). For future clinical applications of specific herbal extracts or phytochemicals in cancer immunotherapy, we expect more stringent RCTs to be performed after systematic and modern biology-defined preclinical studies have determined particular formulas/phytochemicals to be promising.
Table 1.
Different pharmacological activities of phytochemicals or medicinal herb extracts that are promising candidates for the development of cancer immunotherapeutics.
| Strategies for cancer immunotherapy | Specific phytochemicals or herbal extracts | Specific effect on targeted cancer or in vivo tumor models | Reference |
|---|---|---|---|
| (1) Induction of immunogenic cell death for enhancing efficacy of tumor vaccines | Shikonin | B16 melanoma or 4T1 mammary carcinoma mice tumor model | Chen et al., 2012; Lin et al., 2015b; Yin et al., 2016a |
| Hypericin | Orthotopic high grade glioma (HGG) mice tumor model | Krysko et al., 2012 | |
| (2) Activation of specific immune cell types or immunity for cell-based vaccine potency in the tumor microenvironment | Polysaccharides (GLPS) from Ganoderma lucidum Anoectochilus formosanus and schisandra polysaccharide isolated from Schisandra chinensis |
Plasma-induced suppression of lymphocyte activation in lung cancer patient |
Liu Q. et al., 2012; Sun et al., 2014 Kuan et al., 2012 |
| Dioscorea alata var. purpurea extract (DsII-TN5) | B16 melanoma mice tumor model | Chang et al., 2013 | |
| Extracts of Astragalus membranaceus and Codonopsis pilosulae | 4T1 mammary carcinoma mice tumor model |
Shao et al., 2004; Chang et al., 2015 |
|
| (3) Suppression of gMDSC activity | Silibinin | The suppressive effects of silibinin on progressive brain metastases in non-small cell lung cancer patients 4T1 mammary carcinoma mice tumor model |
Bosch-Barrera et al., 2016 Agarwal et al., 2006 |
| Extract of Bidens pilosa (BP-E) | 4T1 mammary carcinoma mice tumor model | Wei et al., 2016 | |
| (4) Regulation of oxylipin dynamics | Monogalactosyl-diacyl-glycerols (MGDG) | B16 melanoma mice tumor model | Hou et al., 2007;Apaya et al., 2016 |
| (5) Suppression of oxidative stress | Curcumin, resveratrol, artemisinin, berberine, paclitaxel, broccoli isothiocyanate, green tea extract, mistletoe extract, noscapine and its derivatives, and piperine | Related references as summarized, (Cheng et al., 2016) | |
| (6) Induction of endogenous anticancer factors (p53 or IL-25) | Extracts of Hibiscus syriacus | A549-xenograft (human lung cancer) mice tumor model | Cheng et al., 2008 |
| Bing De Ling | CT26-xenograft (human cancer cell line) mice tumor model | Xu et al., 2005; Zhang et al., 2010 | |
| Extracts of Suillus collinitus | Vaz et al., 2012 | ||
| Extracts of Emilia sonchifolia | B16 melanoma mice tumor model | Lan et al., 2012 | |
| Extracts of Cochinchina momordica | Liu H.R. et al., 2012 | ||
| Zeng Sheng Ping | A/J mice (dominant-negative p53 mutation and/or a heterozygous deletion of Ink4a/Arf) model for human lung adenocarcinoma cells | Zhang et al., 2004 | |
| JP-1 | Yao et al., 2016 | ||
| Artemisinin and artesunate | The phase I trial defines a well-tolerated dose of oral artesunate (200 mg/d) in patients with metastatic breast cancer | Hargraves et al., 2016; von Hagens et al., 2017 | |
| Dihydrobenzofuran lignan (Q2-3) | 4T1 mammary carcinoma tumor model | Yin et al., 2016b |
FIGURE 1.
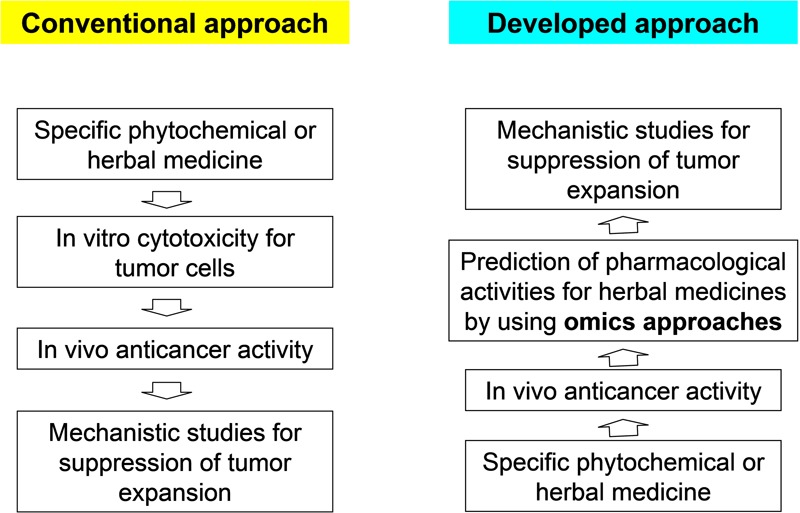
Omics approaches to reveal the in vivo anticancer activity of herbal edicines. According to the conventional approach, studies are initiated by screening the in vitro cytotoxicity of tumor cells. In this way, it can be efficiently determined which compound or active component in an herbal medicine can confer a cytotoxic effect on cancer cells. To overcome the limitation of this approach when studying issues related to immune regulation or other cellular activities in the tumor microenvironment, using the developed approach, studies can be initiated through a combination of omics approaches and different in vivo tumor model systems. Other mechanistic studies for suppression of tumor expansion can be used to confirm the hypothesis formulated through the omics analyses.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Ms. Miranda Loney of Academia Sinica for professional manuscript editing.
Abbreviations
- CRT
calreticulin
- DAMPs
damage-associated molecular patterns
- DCs
dendritic cells
- EMT
epithelial-to-mesenchymal transition
- ER
endoplasmic reticulum
- GLPS
Ganoderma lucidum polysaccharides
- H2O2
hydrogen peroxide
- HGG
high-grade glioma
- HMGB1
high-mobility group box 1 protein
- hnRNPA1
heterogeneous nuclear ribonucleoprotein A1
- ICD
immunogenic cell death
- ITC
isothermal titration calorimetry
- KAI1/CD82
Kangai 1
- MDSC
myeloid derived suppressor cell
- NME1
diphosphate kinase A
- PKM2
pyruvate kinase-M2
- PDT
photodynamic therapy
- RCTs
randomized and controlled trials
- ROS
reactive oxygen species
- SK
shikonin
- TAF
tumor-associated fibroblast
- TAM
tumor-associated macrophage
- TCL
tumor cell lysate
- TNF-α
tumor necrosis factor-α
Footnotes
Funding. This work was supported by grants from the National Science Council (NSC 105-2320-B-001-008) and the Innovative Translational Agricultural Research Program (2017HM02), Taiwan.
References
- Agarwal R., Agarwal C., Ichikawa H., Singh R. P., Aggarwal B. B. (2006). Anticancer potential of silymarin: from bench to bed side. Anticancer. Res. 26 4457–4498. [PubMed] [Google Scholar]
- Albini A., Sporn M. B. (2007). The tumour microenvironment as a target for chemoprevention. Nat. Rev. Cancer 7 139–147. 10.1038/nrc2067 [DOI] [PubMed] [Google Scholar]
- Anighoro A., Bajorath J., Rastelli G. (2014). Polypharmacology: challenges and opportunities in drug discovery. J. Med. Chem. 57 7874–7887. 10.1021/jm5006463 [DOI] [PubMed] [Google Scholar]
- Apaya M. K., Chang M. T., Shyur L. F. (2016). Phytomedicine polypharmacology: cancer therapy through modulating the tumor microenvironment and oxylipin dynamics. Pharmacol. Ther. 162 58–68. 10.1016/j.pharmthera.2016.03.001 [DOI] [PubMed] [Google Scholar]
- Bannwart C. F., Peracoli J. C., Nakaira-Takahagi E., Peracoli M. T. (2010). Inhibitory effect of silibinin on tumour necrosis factor-alpha and hydrogen peroxide production by human monocytes. Nat. Prod. Res. 24 1747–1757. 10.1080/14786410903314492 [DOI] [PubMed] [Google Scholar]
- Bosch-Barrera J., Sais E., Canete N., Marruecos J., Cuyas E., Izquierdo A., et al. (2016). Response of brain metastasis from lung cancer patients to an oral nutraceutical product containing silibinin. Oncotarget 7 32006–32014. 10.18632/oncotarget.7900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bykov V. J., Issaeva N., Shilov A., Hultcrantz M., Pugacheva E., Chumakov P., et al. (2002). Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat. Med. 8 282–288. 10.1038/nm0302-282 [DOI] [PubMed] [Google Scholar]
- Chang W. T., Chen H. M., Yin S. Y., Chen Y. H., Wen C. C., Wei W. C., et al. (2013). Specific Dioscorea phytoextracts enhance potency of TCL-loaded DC-based cancer vaccines. Evid. Based Complement. Alternat. Med. 2013:932040 10.1155/2013/932040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W. T., Lai T. H., Chyan Y. J., Yin S. Y., Chen Y. H., Wei W. C., et al. (2015). Specific medicinal plant polysaccharides effectively enhance the potency of a DC-based vaccine against mouse mammary tumor metastasis. PLoS ONE 10:e0122374 10.1371/journal.pone.0122374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. M., Wang P. H., Chen S. S., Wen C. C., Chen Y. H., Yang W. C., et al. (2012). Shikonin induces immunogenic cell death in tumor cells and enhances dendritic cell-based cancer vaccine. Cancer Immunol. Immunother. 61 1989–2002. 10.1007/s00262-012-1258-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. L., Lee S. C., Harn H. J., Huang H. C., Chang W. L. (2008). The extract of Hibiscus syriacus inducing apoptosis by activating p53 and AIF in human lung cancer cells. Am. J. Chin. Med. 36 171–184. 10.1142/S0192415X08005680 [DOI] [PubMed] [Google Scholar]
- Cheng Y. T., Yang C. C., Shyur L. F. (2016). Phytomedicine-Modulating oxidative stress and the tumor microenvironment for cancer therapy. Pharmacol. Res. 114 128–143. 10.1016/j.phrs.2016.10.022 [DOI] [PubMed] [Google Scholar]
- Chiu S. C., Tsao S. W., Hwang P. I., Vanisree S., Chen Y. A., Yang N. S. (2010). Differential functional genomic effects of anti-inflammatory phytocompounds on immune signaling. BMC Genomics 11:513 10.1186/1471-2164-11-513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. C., Yang N. S. (2007). Inhibition of tumor necrosis factor-alpha through selective blockade of Pre-mRNA splicing by shikonin. Mol. Pharmacol. 71 1640–1645. 10.1124/mol.106.032821 [DOI] [PubMed] [Google Scholar]
- Cho W. C., Leung K. N. (2007). In vitro and in vivo anti-tumor effects of Astragalus membranaceus. Cancer Lett. 252 43–54. 10.1016/j.canlet.2006.12.001 [DOI] [PubMed] [Google Scholar]
- Deep G., Agarwal R. (2013). Targeting tumor microenvironment with silibinin: promise and potential for a translational cancer chemopreventive strategy. Curr. Cancer Drug. Targets 13 486–499. 10.2174/15680096113139990041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J. T., Isaacs W. B., Barrett J. C., Isaacs J. T. (1997). Genomic organization of the human KAI1 metastasis-suppressor gene. Genomics 41 25–32. 10.1006/geno.1997.4618 [DOI] [PubMed] [Google Scholar]
- Dooley S., Seib T., Engel M., Theisinger B., Janz H., Piontek K., et al. (1994). Isolation and characterization of the human genomic locus coding for the putative metastasis control gene nm23-H1. Hum. Genet. 93 63–66. 10.1007/BF00218915 [DOI] [PubMed] [Google Scholar]
- Forghani P., Khorramizadeh M. R., Waller E. K. (2014). Silibinin inhibits accumulation of myeloid-derived suppressor cells and tumor growth of murine breast cancer. Cancer Med. 3 215–224. 10.1002/cam4.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S. L., Hsu Y. H., Lee P. Y., Hou W. C., Hung L. C., Lin C. H., et al. (2006). Dioscorin isolated from Dioscorea alata activates TLR4-signaling pathways and induces cytokine expression in macrophages. Biochem. Biophys. Res. Commun. 339 137–144. 10.1016/j.bbrc.2005.11.005 [DOI] [PubMed] [Google Scholar]
- Furuta S., Jeng Y. M., Zhou L., Huang L., Kuhn I., Bissell M. J., et al. (2011). IL-25 causes apoptosis of IL-25R-expressing breast cancer cells without toxicity to nonmalignant cells. Sci. Transl. Med. 3:78ra31 10.1126/scitranslmed.3001374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich D. I., Nagaraj S. (2009). Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9 162–174. 10.1038/nri2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnier J. J., Boon H., Rochon P., Moher D., Barnes J., Bombardier C., et al. (2006a). Recommendations for reporting randomized controlled trials of herbal interventions: explanation and elaboration. J. Clin. Epidemiol. 59 1134–1149. 10.1016/j.jclinepi.2005.12.020 [DOI] [PubMed] [Google Scholar]
- Gagnier J. J., Boon H., Rochon P., Moher D., Barnes J., Bombardier C., et al. (2006b). Reporting randomized, controlled trials of herbal interventions: an elaborated CONSORT statement. Ann. Intern. Med. 144 364–367. [DOI] [PubMed] [Google Scholar]
- Garg A. D., Nowis D., Golab J., Vandenabeele P., Krysko D. V., Agostinis P. (2010). Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim. Biophys. Acta 1805 53–71. 10.1016/j.bbcan.2009.08.003 [DOI] [PubMed] [Google Scholar]
- Garg A. D., Vandenberk L., Koks C., Verschuere T., Boon L., Van Gool S. W., et al. (2016). Dendritic cell vaccines based on immunogenic cell death elicit danger signals and T cell-driven rejection of high-grade glioma. Sci. Transl. Med. 8 328ra327 10.1126/scitranslmed.aae0105 [DOI] [PubMed] [Google Scholar]
- Grivennikov S. I., Greten F. R., Karin M. (2010). Immunity, inflammation, and cancer. Cell 140 883–899. 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargraves K. G., He L., Firestone G. L. (2016). Phytochemical regulation of the tumor suppressive microRNA, miR-34a, by p53-dependent and independent responses in human breast cancer cells. Mol. Carcinog. 55 486–498. 10.1002/mc.22296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., He X., Lowe S. W., Hannon G. J. (2007). microRNAs join the p53 network–another piece in the tumour-suppression puzzle. Nat. Rev. Cancer 7 819–822. 10.1038/nrc2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hientz K., Mohr A., Bhakta-Guha D., Efferth T. (2017). The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget 8 8921–8946. 10.18632/oncotarget.13475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C. C., Chen Y. P., Wu J. H., Huang C. C., Wang S. Y., Yang N. S., et al. (2007). A galactolipid possesses novel cancer chemopreventive effects by suppressing inflammatory mediators and mouse B16 melanoma. Cancer Res. 67 6907–6915. 10.1158/0008-5472.CAN-07-0158 [DOI] [PubMed] [Google Scholar]
- Klein C., Vassilev L. T. (2004). Targeting the p53-MDM2 interaction to treat cancer. Br. J. Cancer 91 1415–1419. 10.1038/sj.bjc.6602164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G., Galluzzi L., Kepp O., Zitvogel L. (2013). Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 31 51–72. 10.1146/annurev-immunol-032712-100008 [DOI] [PubMed] [Google Scholar]
- Krysko D. V., Garg A. D., Kaczmarek A., Krysko O., Agostinis P., Vandenabeele P. (2012). Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 12 860–875. 10.1038/nrc3380 [DOI] [PubMed] [Google Scholar]
- Kuan Y. C., Lee W. T., Hung C. L., Yang C., Sheu F. (2012). Investigating the function of a novel protein from Anoectochilus formosanus which induced macrophage differentiation through TLR4-mediated NF-kappaB activation. Int. Immunopharmacol. 14 114–120. 10.1016/j.intimp.2012.06.014 [DOI] [PubMed] [Google Scholar]
- Lan Y. H., Chiang J. H., Huang W. W., Lu C. C., Chung J. G., Wu T. S., et al. (2012). Activations of both extrinsic and intrinsic pathways in HCT 116 human colorectal cancer cells contribute to apoptosis through p53-mediated ATM/Fas signaling by Emilia sonchifolia extract, a folklore medicinal plant. Evid. Based Complement. Alternat. Med. 2012:178178 10.1155/2012/178178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. J., Liang W. M., Hsiao P. W., M S. P., Wei W. C., Lin H. T., et al. (2015a). Rapamycin promotes mouse 4T1 tumor metastasis that can be reversed by a dendritic cell-based vaccine. PLoS ONE 10:e0138335 10.1371/journal.pone.0138335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. J., Lin H. T., Chang W. T., Mitapalli S. P., Hsiao P. W., Yin S. Y., et al. (2015b). Shikonin-enhanced cell immunogenicity of tumor vaccine is mediated by the differential effects of DAMP components. Mol. Cancer 14 174 10.1186/s12943-015-0435-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. R., Meng L. Y., Lin Z. Y., Shen Y., Yu Y. Q., Zhu Y. Z. (2012). Cochinchina momordica seed extract induces apoptosis and cell cycle arrest in human gastric cancer cells via PARP and p53 signal pathways. Nutr. Cancer 64 1070–1077. 10.1080/01635581.2012.712737 [DOI] [PubMed] [Google Scholar]
- Liu J. Y., Yang F. L., Lu C. P., Yang Y. L., Wen C. L., Hua K. F., et al. (2008). Polysaccharides from Dioscorea batatas induce tumor necrosis factor-alpha secretion via Toll-like receptor 4-mediated protein kinase signaling pathways. J. Agric. Food Chem. 56 9892–9898. 10.1021/jf8018722 [DOI] [PubMed] [Google Scholar]
- Liu Q., Zhang Z., Fang L., Jiang Y., Yin X. (2012). [Application of network pharmacology and high through-put technology on active compounds screening from traditional Chinese medicine]. Zhongguo Zhong Yao Za Zhi 37 134–137. [PubMed] [Google Scholar]
- Liu Z. H., Sun X. B. (2012). [Network pharmacology: new opportunity for the modernization of traditional Chinese medicine]. Yao Xue Xue Bao 47 696–703. [PubMed] [Google Scholar]
- Mlecnik B., Bindea G., Kirilovsky A., Angell H. K., Obenauf A. C., Tosolini M., et al. (2016). The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci. Transl. Med. 8:327ra326 10.1126/scitranslmed.aad6352 [DOI] [PubMed] [Google Scholar]
- Okabe M., Szakacs G., Reimers M. A., Suzuki T., Hall M. D., Abe T., et al. (2008). Profiling SLCO and SLC22 genes in the NCI-60 cancer cell lines to identify drug uptake transporters. Mol. Cancer Ther. 7 3081–3091. 10.1158/1535-7163.MCT-08-0539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks D. J., Lafrance L. V., Calvo R. R., Milkiewicz K. L., Gupta V., Lattanze J., et al. (2005). 1,4-Benzodiazepine-2,5-diones as small molecule antagonists of the HDM2-p53 interaction: discovery and SAR. Bioorg. Med. Chem. Lett. 15 765–770. 10.1016/j.bmcl.2004.11.009 [DOI] [PubMed] [Google Scholar]
- Rajani D. K., Walch M., Martinvalet D., Thomas M. P., Lieberman J. (2012). Alterations in RNA processing during immune-mediated programmed cell death. Proc. Natl. Acad. Sci. U.S.A. 109 8688–8693. 10.1073/pnas.1201327109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shangary S., Wang S. (2008). Targeting the MDM2-p53 interaction for cancer therapy. Clin. Cancer Res. 14 5318–5324. 10.1158/1078-0432.CCR-07-5136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao B. M., Xu W., Dai H., Tu P., Li Z., Gao X. M. (2004). A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem. Biophys. Res. Commun. 320 1103–1111. 10.1016/j.bbrc.2004.06.065 [DOI] [PubMed] [Google Scholar]
- Son Y., Kim S., Chung H. T., Pae H. O. (2013). Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. 528 27–48. 10.1016/B978-0-12-405881-1.00002-1 [DOI] [PubMed] [Google Scholar]
- Staniforth V., Wang S. Y., Shyur L. F., Yang N. S. (2004). Shikonins, phytocompounds from Lithospermum erythrorhizon, inhibit the transcriptional activation of human tumor necrosis factor alpha promoter in vivo. J. Biol. Chem. 279 5877–5885. 10.1074/jbc.M309185200 [DOI] [PubMed] [Google Scholar]
- Su P. F., Li C. J., Hsu C. C., Benson S., Wang S. Y., Aravindaram K., et al. (2011). Dioscorea phytocompounds enhance murine splenocyte proliferation ex vivo and improve regeneration of bone marrow cells in vivo. Evid. Based Complement. Alternat. Med. 2011:731308 10.1093/ecam/neq032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su P. F., Staniforth V., Li C. J., Wang C. Y., Chiao M. T., Wang S. Y., et al. (2008). Immunomodulatory effects of phytocompounds characterized by in vivo transgenic human GM-CSF promoter activity in skin tissues. J. Biomed. Sci. 15 813–822. 10.1007/s11373-008-9266-7 [DOI] [PubMed] [Google Scholar]
- Sun L. X., Li W. D., Lin Z. B., Duan X. S., Li X. F., Yang N., et al. (2014). Protection against lung cancer patient plasma-induced lymphocyte suppression by Ganoderma lucidum polysaccharides. Cell. Physiol. Biochem. 33 289–299. 10.1159/000356669 [DOI] [PubMed] [Google Scholar]
- Swartz M. A., Lund A. W. (2012). Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nat. Rev. Cancer 12 210–219. 10.1038/nrc3186 [DOI] [PubMed] [Google Scholar]
- Tager M., Dietzmann J., Thiel U., Hinrich Neumann K., Ansorge S. (2001). Restoration of the cellular thiol status of peritoneal macrophages from CAPD patients by the flavonoids silibinin and silymarin. Free Radic. Res. 34 137–151. 10.1080/10715760100300131 [DOI] [PubMed] [Google Scholar]
- Vaz J. A., Ferreira I. C., Tavares C., Almeida G. M., Martins A., Helena Vasconcelos M. (2012). Suillus collinitus methanolic extract increases p53 expression and causes cell cycle arrest and apoptosis in a breast cancer cell line. Food Chem. 135 596–602. 10.1016/j.foodchem.2012.04.127 [DOI] [PubMed] [Google Scholar]
- von Hagens C., Walter-Sack I., Goeckenjan M., Osburg J., Storch-Hagenlocher B., Sertel S., et al. (2017). Prospective open uncontrolled phase I study to define a well-tolerated dose of oral artesunate as add-on therapy in patients with metastatic breast cancer (ARTIC M33/2). Breast Cancer Res. Treat. 10.1007/s10549-017-4261-1 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Wang J., Tong X., Li P., Cao H., Su W. (2012). Immuno-enhancement effects of Shenqi Fuzheng Injection on cyclophosphamide-induced immunosuppression in Balb/c mice. J. Ethnopharmacol. 139 788–795. 10.1016/j.jep.2011.12.019 [DOI] [PubMed] [Google Scholar]
- Wang S., Zhao Y., Aguilar A., Bernard D., Yang C. Y. (2017). Targeting the MDM2-p53 protein–protein interaction for new cancer therapy: progress and challenges. Cold Spring Harb. Perspect. Med. 7:a026245 10.1101/cshperspect.a026245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W. C., Lin S. Y., Lan C. W., Huang Y. C., Lin C. Y., Hsiao P. W., et al. (2016). Inhibiting MDSC differentiation from bone marrow with phytochemical polyacetylenes drastically impairs tumor metastasis. Sci. Rep. 6:36663 10.1038/srep36663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiman K. G. (2010). Pharmacological reactivation of mutant p53: from protein structure to the cancer patient. Oncogene 29 4245–4252. 10.1038/onc.2010.188 [DOI] [PubMed] [Google Scholar]
- Wurglics M., Westerhoff K., Kaunzinger A., Wilke A., Baumeister A., Dressman J., et al. (2001). Comparison of German St. John’s wort products according to hyperforin and total hypericin content. J. Am. Pharm. Assoc. 41 560–566. 10.1016/S1086-5802(16)31280-3 [DOI] [PubMed] [Google Scholar]
- Xu Q., Brabham J. G., Zhang S., Munster P., Fields K., Zhao R. J., et al. (2005). Chinese herbal formula, Bing De Ling, enhances antitumor effects and ameliorates weight loss induced by 5-fluorouracil in the mouse CT26 tumor model. DNA Cell Biol. 24 470–475. 10.1089/dna.2005.24.470 [DOI] [PubMed] [Google Scholar]
- Yao C. J., Chow J. M., Lin P. C., Hu T. S., Kuo H. C., Huang J. S., et al. (2016). Activation of p53/miR-34a tumor suppressor axis by Chinese herbal formula JP-1 in A549 lung adenocarcinoma cells. Evid. Based Complement. Alternat. Med. 2016:5989681 10.1155/2016/5989681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S. Y., Efferth T., Jian F. Y., Chen Y. H., Liu C. I, Wang A. H., et al. (2016a). Immunogenicity of mammary tumor cells can be induced by shikonin via direct binding-interference with hnRNPA1. Oncotarget 7 43629–43653. 10.18632/oncotarget.9660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S. Y., Jian F. Y., Chen Y. H., Chien S. C., Hsieh M. C., Hsiao P. W., et al. (2016b). Induction of IL-25 secretion from tumour-associated fibroblasts suppresses mammary tumour metastasis. Nat. Commun. 7 11311 10.1038/ncomms11311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S. Y., Peng A. P., Huang L. T., Wang Y. T., Lan C. W., Yang N. S. (2013a). The phytochemical shikonin stimulates epithelial-mesenchymal transition (EMT) in skin wound healing. Evid. Based Complement. Alternat. Med. 2013:262796 10.1155/2013/262796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S. Y., Wei W. C., Jian F. Y., Yang N. S. (2013b). Therapeutic applications of herbal medicines for cancer patients. Evid. Based Complement. Alternat. Med. 2013:302426 10.1155/2013/302426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Dong H., Li Z., Xiang S., Zhu Y., Zhang M., et al. (2010). Bing De Ling, a Chinese herbal formula, inhibits cancer cells growth via p53. Front. Biosci. 2 221–230. 10.2741/e84 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Wang Y., Yao R., Li J., Yan Y., La Regina M., et al. (2004). Cancer chemopreventive activity of a mixture of Chinese herbs (antitumor B) in mouse lung tumor models. Oncogene 23 3841–3850. 10.1038/sj.onc.1207496 [DOI] [PubMed] [Google Scholar]
- Zheng Y., Yin G., Le V., Zhang A., Lu Y., Yang M., et al. (2014a). Hypericin-based photodynamic therapy induces a tumor-specific immune response and an effective DC-based cancer immunotherapy. Biochem. Pharmacol. 10.1016/j.bcp.2014.01.036 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Zheng Y. S., Wu Z. S., Ni H. B., Ke L., Tong Z. H., Li W. Q., et al. (2014b). Codonopsis pilosula polysaccharide attenuates cecal ligation and puncture sepsis via circuiting regulatory T cells in mice. Shock 41 250–255. 10.1097/SHK.0000000000000091 [DOI] [PubMed] [Google Scholar]


