Abstract
Despite advances in cardiovascular biology and medical therapy, heart disorders are the leading cause of death worldwide. Cell-based regenerative therapies become a promising treatment for patients affected by heart failure, but also underline the need for reproducible results in preclinical and clinical studies for safety and efficacy. Enthusiasm has been tempered by poor engraftment, survival and differentiation of the injected adult stem cells. The crucial challenge is identification and selection of the most suitable stem cell type for cardiac regenerative medicine. Human pluripotent stem cells (PSCs) have emerged as attractive cell source to obtain cardiomyocytes (CMs), with potential applications, including drug discovery and toxicity screening, disease modelling and innovative cell therapies. Lessons from embryology offered important insights into the development of stem cell-derived CMs. However, the generation of a CM population, uniform in cardiac subtype, adult maturation and functional properties, is highly recommended. Moreover, hurdles regarding tumorigenesis, graft cell death, immune rejection and arrhythmogenesis need to be overcome in clinical practice. Here we highlight the recent progression in PSC technologies for the regeneration of injured heart. We review novel strategies that might overcome current obstacles in heart regenerative medicine, aiming at improving cell survival and functional integration after cell transplantation.
Keywords: Embryonic cardiomyogenesis, Heart regeneration, Stem cell-based therapy, Human pluripotent stem cell, Stem cell-derived exosome
Highlights
-
•
Human pluripotent stem cells emerge as attractive tool for cardiac regeneration approaches.
-
•
Plasticity of human pluripotent stem cells towards cardiac-related cell types guarantees repopulation of injured heart.
-
•
Combination of stem cell and gene editing therapies has potential to become next generation treatment for cardiac diseases.
Data for this Review were identified by searches of MEDLINE and PubMed, and references from relevant articles using the search terms “cardiomyogenesis”, “adult stem cells”, “pluripotent stem cells” and “cardiac regeneration”. Only articles published in English between 1976 and 2017 were included. The majority of the articles reported were published after 2000.
1. Introduction
Cardiovascular disorders remain the major cause of morbidity and mortality throughout the world. Over the last decades, death rates have even increased significantly (Nichols et al., 2014, Go et al., 2014). The heart, previously considered as a terminally differentiated organ with no regenerative capacity in post-natal life, has been documented to exhibit a limited degree of regeneration. As a consequence, the human heart regeneration capacity is unable to counteract the severe loss of heart muscle tissue during myocardial infarction (MI) or other myocardial disorders. Heart transplantation is the standard therapy to replace the injured heart. Several experimental studies and recent clinical trials assumed that stem cell-based transplantation might be indicated as an alternative and promising therapeutic strategy for heart failure. Multiple candidate stem cell types, including pluripotent stem cells (PSCs), have been evaluated in animal models and in clinical human studies for their ability to regenerate cardiac damage and reconstitute the cardiomyocyte (CM) loss.
This review describes the current research status on cell transplantation in animal models and humans. We discuss various stem cell types with cardiac regenerative potential in a clinically relevant setting, such as human embryonic (ESCs) and induced pluripotent stem cells (iPSCs), as well as adult stem cells derived from the bone marrow, mesenchymal tissues and the heart. In addition, we summarise some challenges that need to be overcome, and the future directions of stem cell-based therapies for in vivo cardiac regeneration.
2. Lessons From Embryonic Cardiac Development: Translating Embryology to PSCs
The formation of the three developmental germ layers, known as ectoderm, mesoderm and endoderm, is one of the most important hallmarks in embryogenesis. In the mouse, the early phase of gastrulation is characterised by the generation of the primitive streak (PS) in the epiblast that ultimately will form the posterior end of the embryo (Tam and Behringer, 1997). Uncommitted epiblast cells undergo epithelial-mesenchymal transition (EMT) and migrate through the PS to contribute to the embryonic structures and, finally, egress either as mesoderm or definitive endoderm derivatives (Fig. 1). Patterning in the PS is defined as anterior, mid and posterior regions with differential gene expression profiles and developmental potential. The heart originates from the cardiac mesoderm, which arises from the anterior PS. Brachyury (T) (Herrmann and Kispert, 1994) and Mixl1 (Hart et al., 2002) are expressed throughout the PS, while Foxa2 and Goosecoid are expressed mainly in the anterior regions (Kinder et al., 2001) and HoxB1 and Evx1 posterior (Forlani et al., 2003, Dush and Martin, 1992). The patterning of distinct subpopulations of mesoderm and endoderm is not random but seems to be a regulated temporal and spatial process. Mobilised epiblast cells diffuse through the anterior parts of the PS and generate cranial and cardiac mesoderm, and subsequently paraxial and axial mesoderm. Epiblast cells, which cross the most anterior region of the PS, derive definitive endoderm. Ectoderm develops also from the epiblast anterior region, although without entering the PS.
Fig. 1.
Mouse gastrulation.
Early primitive streak (PS) formation at 6.5 days after fertilisation. The posterior region of the PS coexpresses Brachyury and HoxB1/Evx1. The anterior region coexpresses Brachyury and Foxa2/Goosecoid. Epiblast cells enter the anterior PS (black arrows on top of the embryo) and generate cardiac mesoderm.
During gastrulation, the temporal and spatial determination of cell fates in different PS regions towards specific developmental lineages depends on the signalling cues in the surrounding environment. Members of the Transforming Growth Factor Beta (TGFβ) family (including BMP4 and Nodal) (Hogan, 1996, Conlon et al., 1994) and Wnt family members (Yamaguchi, 2001) play an essential role. Moreover, germ layer formation is a dynamic process that is tightly regulated by the coordinated activation and inhibition of BMP4, Activin/Nodal and Wnt signalling pathways (Gadue et al., 2005).
The BMP4, Activin/Nodal and Wnt signalling pathways are required in establishing the cardiovascular system. Mouse and human PSCs represent distinct development stages, although the signalling pathways regulating human PSC differentiation are comparable to pathways controlling differentiation in mice. Knowledge, obtained from mouse embryonic development studies, has been translated to in vitro differentiation of human PSCs to improve their differentiation efficiency towards CMs (Sumi et al., 2008). The Wnt/β-catenin pathway has a stage-specific biphasic role in cardiomyogenesis. It is required for mesoderm induction, whereas inhibition occurs during the specification of the cardiac progenitor phase (Naito et al., 2006). Stimulating mouse and human PSCs with BMP4 alone or in combination with Activin/Nodal induces BRACHYURY and MIXL1 expression and the subsequent formation of KDR+ and PDGFR+ cardiac mesoderm (Laflamme et al., 2007, Kattman et al., 2011). The heart originates from the lateral plate mesoderm and develops in two distinct cardiomyogenesis waves from the primary (PHF) and secondary heart field (SHF). Both heart fields express KDR and the transcription factor NKX2.5, whereas the SHF is marked selectively by the transcription factor ISL1. These markers are useful to identify cardiac progenitor cells (CPCs) from PSCs. Finally, the PHF gives rise to the left ventricle and both atria, while the SHF develops into the right ventricle and outflow tract (Wu et al., 2006).
3. Candidate Stem Cell Sources in Heart Regeneration
Adult stem cells have cardiogenic differentiation capacity, and therefore present cardiac regenerative research focuses on developing stem cell sources to repair injured heart muscles. Several preclinical and clinical studies have been conducted, providing the pros and cons of various adult stem cell types for therapeutic approaches, including skeletal myoblasts, bone marrow-derived mononuclear cells (BMMNCs), mesenchymal stem cells (MSCs), haematopoietic stem cells (HSCs), endothelial progenitor cells (EPCs) and CPCs (Fig. 2). However, the ideal stem cell has not yet emerged and a limited number of studies have compared different stem cell types (Wollert and Drexler, 2005). Until now, all of these cell types have been thoroughly tested in clinical trials in the heart, except human ESCs and iPSCs.
Fig. 2.
Potential cell sources for heart regeneration therapy.
Embryonic (ESC) and induced pluripotent stem cell (iPSC) populations as well as adult stem cell types have been shown to improve cardiac morphological and functional characteristics via differentiation towards cardiomyocytes (CMs), smooth muscle cells (SMCs) and endothelial cells (ECs) or through paracrine effects.
3.1. Human PSCs: ESCs and iPSCs
In 1998, Thomson and colleagues succeeded to isolate human ESCs from the inner cell mass of blastocysts (Thomson et al., 1998). ESCs are considered as a promising cell source to achieve cardiac regeneration through CM replacement. They exhibit unlimited self-renewal and can differentiate into any cell type present in the adult organism, including CMs or CPCs (Hartman et al., 2016). The first transplantation reports of human ESC-derived CMs (ESC-CMs) into pigs and guinea pigs have shown their potential to function as biological pacemakers in electrophysiologically silenced or atrioventricular (AV) blocked hearts (Kehat et al., 2004). One of the initial technical challenges in ESC differentiation towards the cardiovascular lineages was to obtain a high purity and large yield of differentiated cells. However, as knowledge of the mouse embryonic heart development increased, mouse and human ESC-CM differentiation became more efficient and reproducible by manipulating the cardiac specific signalling pathways (Sumi et al., 2008). Various strategies, like specialised culturing methods, genetic modifications or treatments with biological and chemical factors, have been conducted to enrich and purify homogeneous and functional ESC-CMs (Schwach and Passier, 2016). Recently, human ESC-CMs, successfully generated on a large scale, were able to engraft and repair damaged heart tissue in a primate MI model (Chong et al., 2014). These results seemed hopeful since only a few different cell types showed efficacy in large animal models. However, the clinical use of human ESC-CMs has been hampered by crucial limitations, like potential tumorigenic and immunogenic properties, genetic instability, as well as ethical issues (Robertson, 2001).
Because human ESCs are derived from the inner cell mass of pre-implantation stage blastocysts, the ethical and legislative debate arose surrounding the use of ESCs for research purposes (Thomson et al., 1998). As a consequence, in 2006, Yamanaka and Takahashi developed a novel method to induce a pluripotent state in mouse somatic cells, referred as iPSCs, by introducing a defined set of transcription factors through retroviral transduction. The combination of transcription factors used for cellular reprogramming is Krüppel-Like Factor 4 (Klf4), Sex Determining Region Y-Box 2 (Sox2), V-Myc Avian Myelocytomatosis Viral Oncogene Homolog (cMyc) and POU Class 5 Homeobox 1 (Oct3/4) (Takahashi and Yamanaka, 2006). In 2007, iPSCs were generated from human origin, using the same cocktail of transcription factors (KLF4, SOX2, cMYC and OCT3/4) (Takahashi et al., 2007) or mediated via SOX2, OCT3/4, Lin-28 Homolog A (LIN28) and Nanog Homeobox (NANOG) (Yu et al., 2007). Like ESCs, iPSCs have wide differentiation ability, giving rise to all the cell types of the three germ layers (Lee et al., 2014). The cardiogenic potential of the iPSC population has been extensively studied with iPSCs derived from mice (Mauritz et al., 2008) and human (Zhang et al., 2009). IPSCs have been found to differentiate in vitro in CMs and other cardiovascular cells, such as smooth muscle cells and endothelial cells. More interestingly, iPSCs administered to the injured heart of mice differentiated and resembled a cardiac phenotype (Mauritz et al., 2011). Although iPSCs have several characteristics in common with ESCs (Gherghiceanu et al., 2011), genome-wide analyses revealed significant differences between both cell types regarding gene expression profiles, methylation signatures and microRNA patterns (Chin et al., 2009). Moreover, iPSCs circumvent the ethical and allogeneic transplantation problems in cardiac regenerative therapy (Table 1) (Takahashi et al., 2007, Yu et al., 2007).
Table 1.
Characteristics of human adult and pluripotent stem cells.
| Characteristics | Adult stem cells | Embryonic stem cells | Induced pluripotent stem cells |
|---|---|---|---|
| Origin | Found in postnatal tissues and organs | Derived from embryos (inner cell mass of blastocysts) | Derived from adult somatic cells |
| Ethics | No ethical issues | Ethical and legislative issues | No ethical issues |
| Teratoma formation propensity | No teratoma risk | Potential tumorigenic properties; Purification required before clinical use |
Potential tumorigenic properties; Purification required before clinical use |
| Genetics and immunogenicity | Genetic identity to patient; Genetic stability and mild immune rejection; Accumulation of mutations due to ageing |
No genetic identity to patient; Genetic instability and immune rejection |
Genetic identity to patient; Genetic instability and mild immune rejection; Viral-based reprogramming may trigger antiviral and anti-DNA antibody-mediated immunity |
| Cell availability | Hard to access and advanced purification strategies needed; Depends on differentiation efficiency |
Depends on ethical issues; Depends on differentiation efficiency |
Depends on reprogramming efficiency; Depends on differentiation efficiency |
| Differentiation potential | Pluripotent or multipotent (depending of source tissue); Have restricted differentiation capacity |
Pluripotent; Give rise to derivatives of all three germ layers |
Pluripotent; Give rise to derivatives of all three germ layers; “Epigenetic memory” |
| Cardiac maturation | More mature ultrastructural phenotype, electrophysiological functionality and sarcoplasmic reticulum | Immature ultrastructural phenotype, immature electrophysiological functionality and sarcoplasmic reticulum | Immature ultrastructural phenotype and electrophysiological functionality; Evidence of more mature sarcoplasmic reticulum |
| Cardiac subtype heterogeneity | Heterogeneous population of cardiomyocytes with unclear paracrine or direct effects | Heterogeneous population of cardiomyocytes with nodal-, atrial- and ventricular-like action potentials | Heterogeneous population of cardiomyocytes with nodal-, atrial- and ventricular-like action potentials |
3.2. Mesodermal iPSC-derived Progenitors
Several potential therapeutic applications of stem cells have been advised or are already under investigation in clinical trials. Stem cells, including PSCs, could be used to generate in vitro tissues or artificial organs for transplantation and to regenerate damaged tissue in vivo per se. However, stem cell-derived artificial and functional organs are still far from being realistic, the stem cell-based therapeutic approaches in regenerative medicine and in hereditary diseases have already reached a more advanced stage (Kimbrel and Lanza, 2015). Nowadays, several groups are focusing on the development of strategies to target simultaneously multiple tissues to improve the treatment of hereditary disorders. For example, muscular dystrophies (MDs) are monogenetic disorders, in which chronic degeneration of both the skeletal and cardiac muscles could occur (Emery, 2002). Therefore, strategies to target at the same time both striated muscle types would be a highly appreciated and much-needed improvement for the treatment of MDs. In this content, a novel iPSC-derived cell pool from mouse and human origin was described, called mesodermal iPSC-derived progenitors (MiPs), which were able to engraft into both skeletal and cardiac muscles (Quattrocelli et al., 2015). MiPs were generated from fibroblast- and mesoangioblast (MAB)-derived iPSCs, showing a significant higher myogenic propensity compared to their fibroblast-derived counterpart due to their “epigenetic memory”. MiPs were amenable to gene corrections and could restore muscle function in murine dystrophic models. The in vivo regenerative potential of human MiPs and their translational applications for striated muscle repair were not yet fully addressed. However, these preliminary results could pave the way for a combined therapy for skeletal and cardiac muscles to treat MD patients.
4. Current Obstacles Towards Successful Therapeutic Applications
For optimal potential applications of human ESCs and iPSCs in cardiovascular medicine, it is crucial to transplant highly pure human PSC-CM populations, lacking undifferentiated PSCs. This circumvents the PSC tumorigenicity and guarantees seamless integration of transplanted cells for the uninterrupted cardiac function. It is also mandatory to recapitulate the molecular, ultrastructural and electrophysiological characteristics of these PSC-CMs in respect to CMs exhibiting an adult phenotype (Table 1).
4.1. Teratomas and Karyotypic Abnormalities
A major concern that has to be taken into account in every clinical therapy based on PSCs is their tendency to form tumours or teratomas. Inefficient differentiation or purification techniques of undifferentiated PSCs can cause undesirable tumours or teratomas after transplantation. Still, several cardiac differentiation protocols deal with an unacceptable amount of residual PSCs. Methods have been developed in which highly purified CMs were generated from human PSCs (Xu et al., 2008). However, those methods are less clinically relevant because they introduce genetic modifications in the cells. Last decades, various nongenetic CM enrichment and isolation methods have been developed. Fluorescent-activated cell sorting (FACS) based techniques, including mitochondria-specific fluorescent dyes (Hattori et al., 2010) or cardiac-specific cell surface markers (like signal-regulatory protein alpha, SIRPA) (Dubois et al., 2011), are widely applied. Another nongenetic purification method for CMs derived from PSCs is based on a differential glucose and lactate metabolism among CMs and other cells, including undifferentiated cells (Tohyama et al., 2013). These approaches yielded CMs of 99% purity and, more importantly, did not induce tumorigenesis after transplantation. Nevertheless, none of these techniques is ideal for therapeutic applications of human PSC-CMs because of insufficient purity and large-scale production for clinical use.
PSCs can show karyotypic instability with long-term culturing in vitro, raising concerns about potential neoplastic transformation and dysregulation of gene expression. Chromosomal abnormalities are highly variable and depending on the cell line and culturing conditions (Taapken et al., 2011).
4.2. Genetics and Immune Rejection
Autologous stem cell populations have a major advantage in cardiovascular medicine. Transplantation of (allogeneic) human ESC-CMs can be identified as foreign, triggering an immune response. Therefore in preclinical models, immunodeficient or immunosuppressed animals are used. Human iPSCs could have an important clinical advantage over human ESCs because they can be created from the patient, leading to genome matching iPSC-CMs. Surprisingly, studies reported that iPSCs can still trigger an immune response, ascribed to the pluripotency reprogramming procedure that induces both genetic and epigenetic defects in iPSCs (Zhao et al., 2011, Lister et al., 2011). Most reprogramming methods use viral components, which increase the risk of altering the genome of the target cells, and triggering antiviral and anti-DNA antibody-mediated immunity (Kaneko and Yamanaka, 2013). Innovative iPSC reprogramming techniques using nonviral vectors have been developed, such as microRNAs (Anokye-Danso et al., 2011, Miyoshi et al., 2011) and small molecules accompanied with chemical compounds (Zhu et al., 2010). In conclusion, nonviral and non-integrating reprogramming techniques offer a better option for PSC-based therapy, although mild immunogenicity can still occur due to culturing and differentiation conditions, as well as epigenetic modifications.
4.3. Immature Phenotype
Molecular, ultrastructural and functional analyses of human PSC-CMs revealed features of an early and immature phenotype. In terms of morphology, spontaneous pacemaker activity, contractile apparatus elements, gap junction expression, electrophysiology and calcium handling properties, human PSCs have characteristics resembling fetal rather than adult CMs (Karakikes et al., 2015). Before their clinical application in cell-based therapies, the maturation phenotype of human PSC-CMs must be addressed, since these de novo generated CMs should replace the damaged heart tissue. In case the CM maturation issue cannot be solved using the current available cardiac differentiation protocols, alternative methods should be developed to enhance maturation. Research groups are exploring the beneficial effect on the maturation of CMs mediated by electrical stimulation (Heidi Au et al., 2009), cyclic stretch-induced mechanisms (Tulloch et al., 2011), chemical manipulation (Pillekamp et al., 2012) and three-dimensional (3D) tissue engineering techniques (Yang et al., 2014). Nowadays, robust protocols to increase the maturation of human PSC-CMs are still lacking and therefore, functional maturation is one of the critical obstacles to overcome before PSC-CMs can be used for clinical applications.
4.4. Cardiomyocyte Subtype Heterogeneity
Currently available cardiac differentiation protocols from human PSCs result usually in a heterogeneous pool of atrial-, ventricular- and nodal-like CMs (Mummery et al., 2012). Recently published papers have reported the importance of the exogenous addition of retinoic acid (RA) (Zhang et al., 2011) and Wnt during human PSC differentiation for cardiac chamber cell specification. The administration of a high percentage of nodal cells to a damaged heart increases the risk of graft-associated arrhythmias. Treating differentiating human PSCs with the Wnt inhibitors, IWP and IWR-1, resulted in CMs with divergent atrial and ventricular expression levels of respectively MLC-2a and MLC-2v (Parikh et al., 2015). Inhibition of the Wnt signalling pathway by using IWR-1 has been shown to differentiate human PSC-CMs towards CM populations with mainly an atrial-like phenotype (Hudson et al., 2012). However, conflicting data exists (Karakikes et al., 2014). Indeed, human PSCs, subjected to an embryoid body (EB) based cardiac differentiation protocol mediated by the Wnt inhibitor IWR-1, generated more than 60% atrial-like CMs. By modulating the Activin signalling pathway, the differentiation of human PSC-CMs was potentially enriched in a ventricular-like adult phenotype (Duelen et al., 2017).
4.5. Scalability and Clinical Grade Production
The human adult heart consists of approximately 4 billion CMs, and cardiovascular insults can lead to loss of cardiac tissue. For example, after a typical MI up to 25% of CMs are lost, initiating progressive heart failure (Caulfield et al., 1976). Human PSC-CMs proliferate in vitro and are highly expandable, making it reasonable to reconstitute the damaged heart tissue, although significantly high cell amounts are needed for therapeutic goals. Recently, the feasibility to produce CMs and transplant them on a large-scale has been demonstrated in a preclinical non-human primate model (Chong et al., 2014). However, in this proof-of-concept study the CM differentiation from PSCs was very labor-intensive, expensive and clinically irrelevant (one-dozen 150 cm2 flasks of human PSC-CMs for each primate). Therefore, several groups use suspension bioreactor-based culture systems to expand human PSCs and differentiate towards very high quantities of CMs at purities up to 85% (Kempf et al., 2014). Noteworthy, a decade ago it seemed unrealistic to obtain such high quantities of human PSC-CMs.
5. Novel Strategies for Stem Cell-Based Therapies
Since the aforementioned obstacles are not yet completely overcome, the development of novel strategies for stem cell therapies in cardiac regeneration purposes remains the main focus. A successful stem cell therapy for cardiac diseases depends on cell delivery routes. Derivatives of PSCs can be systemically (intravenously or intracoronary) or locally (intramyocardial injection) administered. Intravenous cell delivery is particularly appealing because of the ease of administration. However, the major problem is the entrapment of the delivered cells in the pulmonary microcirculation. Intravenous cell injections require appropriate homing to the site of heart damage (Garbern and Lee, 2013). In 2003, a pioneer study, in which radioactively labelled EPCs were intravenously delivered into a rat MI model, showed a small portion of radioactivity (about 2%) in the heart after 24–96 h (Aicher et al., 2003). Afterwards, these results have been confirmed with the use of other cells (Brenner et al., 2004, Nagaya et al., 2004). Intracoronary and intramyocardial routes are relatively more complex compared to intravenous injections. The most common delivery route for cell therapy after acute MI is the intracoronary route (Table 2). Main advantages include selective administration in the infarcted region and better uniform cell distribution in the target region. Intramyocardial injections have been shown to be superior to intravenous and intracoronary injections regarding cell retention. There is no need for homing signals because cells are administered directly in the myocardium. Both delivery routes have their limitations. The intracoronary injection demands a transient ischemic period, detrimental for the heart, to get the optimal distribution of the injected cells. While in an acute MI, the intramyocardial injection could increase the risk for perforation due to ischemia and necrosis (Hou et al., 2005). A comparative study of these three delivery routes in a porcine MI model demonstrated that the engraftment of the transplanted cells within the infarct zone 14 days after delivery was significantly higher after intracoronary and intramyocardial injections than intravenous administration, respectively 106,000 ± 43,000 and 51,000 ± 24,000 cells/g in respect to the absence of MSCs in the infarct zone after intravenous injection (Freyman et al., 2006). In the near future, the advance of transplantation techniques might provide a more efficient method for injected stem cells to retain and survive in the damaged heart.
Table 2.
Clinical trials of cell-based therapy after acute myocardial infarction.
| Study name | Design | Cell type/dose (× 108) | Route of injection | Imaging modalities | Cell delivery after MI (days) | Time follow-up/results | Reference(s) |
|---|---|---|---|---|---|---|---|
| BOOST | RCT | Nucleated BM cells 24.6 ± 0.94 |
IC | CMR, Echo | 4–6 (STEMI) |
6 months: improved EF 18 months: no improved EF, LV volumes and RWM 5 years: no improved EF, LV volumes, infarct size and RWM |
Wollert et al. (2004) Meyer et al., 2006, Meyer et al., 2009 |
| / | RDBCT | Nucleated BM cells 3 ± 1.28 (containing 1.72 ± 0.72 BMMNCs) |
IC | CMR | 1–2 | 4 months: no improved EF, decreased infarct size, increased RWM 12 months: no improved EF, LV volumes and infarct size, increased RWM |
Janssens et al. (2006) |
| ASTAMI | RCT | BMMNCs 0.7 (0.54–1.3) |
IC | SPECT, CMR, Echo | 4–8 | 6 months: no improved EF, LV volumes and infarct size 12 months: no improved EF, LV volumes and RWM 3 years: no improved EF, LV volumes, LV mass, infarct zone and RWM |
Lunde et al. (2006),Lunde, and Aakhus (2008) Beitnes et al. (2009) |
| REPAIR-AMI | RDBCT | BMMNCs 2.36 ± 1.74 |
IC | CMR, LV angiography | 3–6 (STEMI) |
4 months: improved EF, ESV and RWM 1 year: decreased MACE, increased RWM 2 years: no improved EF and LV volumes, decreased MACE and infarct size, increased RWM 5 years: decreased MACE |
Schächinger et al. (2006) Assmus et al., 2010, Assmus et al., 2014 |
| FINCELL | RDBCT | BMMNCs 0.04 ± 0.02 |
IC | Echo, LV angiography | 2–6 | 6 months: no improved EF, improvement in ΔEF | Huikuri et al. (2008) |
| REGENT | RCT | BMMNCs 1.78 CD34+ CXCR4+ 0.019 |
IC | CMR, LV angiography | 3–12 | 6 months: no improved EF and LV volumes | Tendera et al. (2009) |
| / | RDBCT | Allogeneic BM MSCs 0.005, 0.016, 0.05/kg |
IV | CMR, Echo | 1–10 | 6 months: no improved EF | Hare et al. (2009) |
| TOPCARE-AMI | RT | Circulating progenitors BMMNCs |
IC | CMR | 3–7 (STEMI) |
5 years: improved EF and decreased infarct size | Leistner et al. (2011) |
| SCIPIO | RCT | CDCs 0.005–0. 01 |
IC | CMR | 113 (after CABG) |
4 months: improved EF and decreased infarct size 12 months: improved EF and decreased infarct size |
Bolli et al. (2011) |
| TIME | RDBCT | BMMNCs 1.5 |
IC | CMR | 3–7 | 6 months: no improved EF, LV volumes, infarct size and RWM | Traverse et al. (2012) |
| LateTIME | RDBCT | BMMNCs 1.5 |
IC | CMR | 15–20 | 6 months: no improved EF, LV volumes, infarct size and RWM | Traverse et al. (2011) |
| APOLLO | RDBCT | ADRCs 0.17 ± 0.04 |
IC | SPECT, CMR, Echo | STEMI | 6 months: no improved EF | Houtgraaf et al. (2012) |
| CADUCEUS | RCT | CDCs 0.125–0.25 |
IC | CMR | 45–90 | 6 months: no improved EF, decreased scar mass and increased RWM 12 months: no improved EF |
Makkar et al. (2012) |
| SWISS-AMI | RCT | BMMNCs 1.4–1.6 |
IC | CMR | 5–7 21–28 |
4 months: no improved EF, LV volumes and scar mass | Surder et al. (2013) |
| CELLWAVE | RCT | Shock wave Pretreatment + BMMNCs |
IC | CMR, Echo, LV angiography |
NA | 4 months: improved EF, decreased infarct size and increased RWM | Assmus et al. (2013) |
ADRCs, autologous adipose tissue-derived regenerative cells; BM, bone marrow; BMMNCs, bone marrow mononuclear cells; CABG, coronary artery bypass grafting; CDCs, cardiosphere-derived cells; CMR, cardiac magnetic resonance imaging; echo, echocardiography; EF, ejection fraction; ESV, end-systolic volume; IC, intracoronary; IV, intravenous; LV, left ventricular; MACE, major adverse cardiovascular events, MSCs, mesenchymal stromal/stem cells; RT, randomized trial; RCT, randomized controlled trial; RDBCT, randomized double-blind controlled trial; RWM, regional wall motion; SPECT, single-photon emission computed tomography; STEMI, ST-segment elevation acute myocardial infarction.
5.1. Combined Gene- and Cell-based Therapeutic Approaches
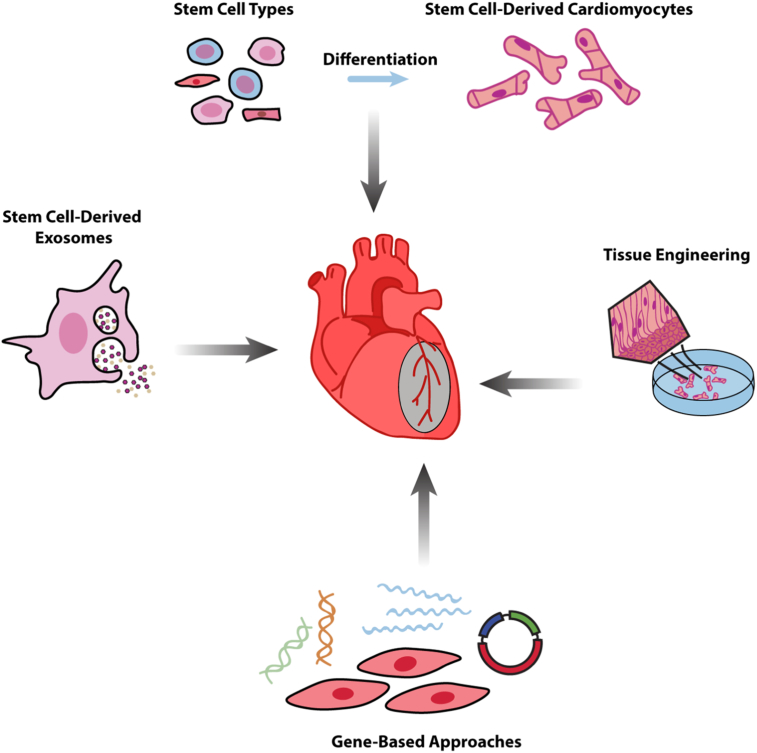
Cell therapy in combination with gene therapy raises the hope to increase survival of transplanted cells by providing a continuous local delivery of targeted bioactive mediators. The growth of new blood vessels, called angiogenesis, has become the main target of promoting long-standing survival of injected cells. In addition, induced angiogenesis could lead towards a better cardiac repair since functional regeneration of the heart tissue requires a high metabolic activity, demanding sufficient blood supply (Fig. 3).
Fig. 3.
Stem cell-based strategies for cardiac regeneration after heart disease.
Multiple techniques to improve morphological and electromechanical properties of the diseased heart: (1) In vitro cardiac differentiation of different stem cell types. (2) Tissue engineering approaches combining cells with biomaterials to design in vitro cardiac patches or injectable scaffolds for transplantation into the infarcted heart area. (3) Cell- and gene-based strategies secreting cytokines, growth factors and microRNAs to promote cardiac regeneration. (4) Stem-cell derived exosomes as an innovative cell-free therapy in heart regenerative medicine.
Cytokines and growth factors, known to induce angiogenesis, were co-delivered into stem cells to increase the possibility for successful and long-term engraftment in the heart. HGF- and VEGF-overexpressing MSCs have been shown to promote revascularization and support cardiac recovery after MI (Deuse et al., 2009). Furthermore, human CD34+ haematopoietic stem cells genetically modified for the angiogenic morphogen sonic hedgehog (Shh) protein showed preservation of cardiac function after MI (Mackie et al., 2012). Recently, the oxygen-sensitive transcription factor hypoxia-inducible factor-1 (HIF1) co-delivered with injected CPCs into the ischemic myocardium has been demonstrated to improve their survival (Ong and Hausenloy, 2012). Although the precise therapeutic mechanism of the co-delivered angiogenic genes and proteins are not yet completely elucidated, it is believed that they might improve survival or in vivo reprogramming and proliferation of transplanted cells.
MicroRNAs, small (20–25 nucleotides) non-coding RNAs that regulate post-transcriptional gene expression, have become a new treatment strategy with therapeutic evidence for MI. Several microRNAs have been identified to improve CM survival. MicroRNA-126 overexpression in MSCs had beneficial effects on their survival and migration, and enhanced angiogenesis in the infarcted area, possibly due to stimulation of the AKT/ERK-related pathway (Chen and Zhou, 2011), paracrine effects (Huang et al., 2013) or either due to a combination. In addition, overexpressed microRNA-155 prevented necrotic cell death in CPCs via targeting receptor interacting protein 1 (RIP1) (Liu et al., 2011). In contrast, several microRNAs, such as microRNA-15, -34, -140 and -320, negatively influenced CM survival and proliferation. As a consequence, inhibiting these microRNAs promoted heart regeneration (Boon and Dimmeler, 2015). Downregulation of microRNA-34 has been determined to increase cell survival both in vitro and in vivo (Xu et al., 2012). Overexpression of the cardiac enriched microRNA-133a protected CPCs after MI by interfering with caspase 3 and proapoptotic genes Bim and Bmf (Izarra et al., 2014). Interestingly, in vivo manipulation of the microRNA machinery resulted in CM dedifferentiation and improved heart functionality after injury (Eulalio et al., 2012, Aguirre et al., 2014).
Despite a positive progression in the last years, it is still unclear whether the myocardium regenerative ability of cytokines and growth factors, and microRNAs could be translated to a clinical practice with a comparable efficiency in human being as observed in experimental animal models.
5.2. Combined Cell- and Tissue-based Therapeutic Approaches
To counteract poor cell engraftment and survival of transplanted cells, diverse biomaterials and tissue engineering techniques have revealed promising results over the past decade. Two major strategies are described in cardiac regeneration, namely cardiac patches and injectable scaffolds, which support either endogenous repair and regeneration or act as vehicles to encourage cell delivery (Fig. 3).
Cardiac patches are in vitro engineered scaffolds of biomaterials to deliver cells into the myocardium and to attract cells for endogenous healing or to support the ventricle wall, maintaining its geometry during remodelling. Approaches, in which biomaterials deliver stem cells into the myocardium, have been reported to improve therapeutic effects after MI. Embedding cardiac stem cells (CSCs) within matrix-enriched hydrogel capsules positively affected long-term cell survival, retention and cardiac function in post-ischemic events (Mayfield et al., 2014). Natural biomaterials improve the mechanical strength of the ventricular wall and promote cell survival, in the presence or absence of growth factors. In a recent study, an IGF-1 microsphere-loaded fibrin patch, administered onto the epicardial surface of a porcine heart post-MI, in combination with human iPSC-CMs, significantly improved survival and retention of the transplanted cells, and reduced ventricular wall stress (Ye et al., 2014).
Engineered heart tissue (EHT) exhibits a potential cardiac restoration function, demonstrating the integration of the implanted EHT with the host heart and improving post-ischemic heart function. Zimmerman and colleagues successfully developed large force-generating EHTs from neonatal rat heart cells and, for the first time, proved that these large contractile EHT grafts could survive after implantation and resulted in a better functional outcome after MI (Zimmermann et al., 2002, Zimmermann et al., 2006). Despite these promising results, the optimal maturation status of EHTs before transplantation is still undetermined, and obtaining sufficient oxygen and nutrients supply through vascularization for CMs in the EHTs is another remaining challenge.
Injectable scaffolds, another strategy combining biomaterials with cells for transplantation, were examined in a rat MI model by delivering skeletal myoblasts with injectable fibrin glue (Christman et al., 2004). Biomaterials are produced from protein-based materials (Matrigel) to synthetic materials (polyethylene glycol; PEG). Recently, injectable scaffolds are used as drug-releasing systems for localised and sustained secretion of growth factors, genes or small RNAs to promote cardiac function and cell survival (Radisic and Christman, 2013). To date, diverse growth factors, like bFGF, HGF, IGF-1, PDGF, SDF-1, TGFβ1 and VEGF, have been delivered. An innovative vector for tissue engineering, called pharmacologically active microcarriers (PAMs), combines stem cell therapy with drug-releasing systems. PAMs consist of growth factors and non-cytotoxic poly(lactic-co-glycolic acid) (PLGA) microspheres covered with extracellular matrix (ECM) molecules (Giteau et al., 2008). Injecting stem cells with VEGF-releasing PAMs provide a biomimetic 3D microenvironment for the transplanted cells and enhance their survival, differentiation and angiogenesis (Madonna et al., 2015).
Tissue engineering methods, combining stem cells with biomaterials, show optimistic perspectives for heart repair. However, advanced progression in the field demands a more multidisciplinary cooperation.
5.3. Stem Cell-derived Exosomes as Cell-free Alternative for Stem Cell-based Cardiac Regeneration Therapy
Stem cell-conditioned medium has been documented to have beneficial effects on the injured heart, supporting the paracrine hypothesis that formulates a chemical and physical signal mediated mode-of-action. Indeed, different studies have proved that stem cells produce and release a wide range of cytokines, growth factors and chemokines involved in heart repair (Gnecchi et al., 2005, Takahashi et al., 2006). Furthermore, stem cells have also the capacity to secrete membranous vesicles (like microparticles, microvesicles and exosomes) into the extracellular space, contributing to intercellular communication (Chen et al., 2010, Ratajczak et al., 2006, Sahoo et al., 2011) (Fig. 3).
Exosomes, extracellular membrane-bound vesicles with a diameter typically ranging between 30 and 100 nm and released through the fusion of multivesicular bodies with the plasma membrane (Thery et al., 2002b), have been documented for the first time in 2002 in dendritic cells to be able to activate naïve CD4+ T cells (Thery et al., 2002a). Another landmark study validated exosomes as natural carriers of mRNA, microRNA and proteins among cells (Valadi et al., 2007). Exosomes have been demonstrated to exhibit cardioprotective functions by enhancing angiogenesis, decreasing fibrotic tissue formation or reducing apoptosis (Ibrahim et al., 2014). Therefore, exosomes could be used as cell-free therapeutic candidates for heart regeneration. Mouse ESC-derived exosomes (ESC-Exos) could enhance endogenous repair and preserved heart function in a mouse MI model mediated at least partially by microRNA-294. Preserved heart function was associated with increased angiogenesis, decreased apoptosis and enhanced CM proliferation. In addition, mouse ESC-Exos increased the number of proliferating CPCs up to 4 weeks post-MI (Khan et al., 2015). The Phase I completed CADUCEUS (CArdiosphere-Derived aUtologous Stem CElls to Reverse ventricUlar dySfunction; NCT00893360) trial have shown that it might be possible that cardiosphere-derived cells (CDCs) regenerate damaged heart muscles. The exact mechanism is unknown, although seemed to be indirectly. Exosomes produced by CDCs were identified as key mediators of the CDC-induced regeneration. Moreover, CDC-derived exosomes contained high amounts of microRNA-146a, and selective administration of microRNA-146a mimics some of the beneficial effects of CDC exosomes (Ibrahim et al., 2014). Based on these studies, a currently recruiting Phase I/II clinical trial called HOPE-Duchenne (Halt cardiomyOPathy progrEssion in Duchenne; NCT02485938) will assess the safety and efficacy of a multi-vessel intracoronary delivery of allogeneic CDCs in MD patients with cardiomyopathy secondary to Duchenne MD. These and other studies provided innovative insights for exosomes as cell-free therapeutic candidates in heart regeneration instead of injected stem cells, which were hampered by poor cell survival, teratomas formation, as well as electric and mechanical coupling issues. Importantly, immunogenicity concerns have to be addressed before exosomes will be used in stem cell therapies. Although stem cell-derived exosomes are less immunogenic than their parental cells, an inherent risk for an exosome-triggered immune response in the infarcted myocardium still exists. Exosome-mediated cardiac repair mechanisms are not yet fully explored, but so far obtained results are encouraging.
6. Conclusions
Stem cell technology is undoubtedly the most attractive approach for the generation of cardiac tissue and has been moved towards the use of PSCs in the last few years. Adult stem cells, including skeletal myoblasts, MSCs and cardiac stem cells were the most tested cell source for cardiac regeneration in clinical trials as they have no ethical obstacle and are non-tumorigenic. However, they exhibit limited self-renewal and differentiation potential to become functional CMs. Experiments in vitro but also in small and large animal models proved the cardiomyogenic potential of PSC derivatives. Specific microRNAs control muscle progenitor proliferation and promote the differentiation of PSC-derived muscle progenitors into CMs, where metabolic activity, survival and remodelling process in response to stress are also fine-tuned by miRNAs. In this context, microRNA cargo within exosome-like vesicle transfer is an intriguing possibility to burst the cardiomyogenic potential of progenitor cells in the diseased heart. It is an emerging fact that exosome-microRNA technologies combined to stem cells could build up unexplored therapeutic strategies for cardiac disorders. However, it is crucial to better understand the basic principles of cardiac differentiation of PSCs, including transcription factors, microRNA networks and molecular pathways that contribute to CM maturation. Several challenges, including tumour formation, ethical and immunological concerns, the optimal cardiac differentiation protocol, timing and delivery route of stem cells remain to be overcome.
In addition, leading laboratories are investing in gene editing, mainly in CRISPR/Cas9 technology, to successfully correct disease-causing alleles and PSCs are preferred since they can be easily expanded. These novel approaches are raising hope for therapeutic genome editing of cardiac genetic diseases in the clinics. Consequently, it is reasonable to expect more and more combinations of PSC- and gene editing-based therapies for cardiac genetic diseases.
The actual enormous costs of GLP-GMP (good laboratory and manufacture practice) in PSC technology limit the feasibility of cell therapy treatment. Strategies to lower costs are desirable to make PSC-based treatments available for large numbers of patients. Despite a decade of numerous clinical trials based on adult stem cell treatments with limited positive outcomes and encouraging results with PSC derivatives in a preclinical setting, we are still in the infancy of a propitious cruise in the field of regenerative cardiac medicine.
Acknowledgements
We would like to apologise to all authors whose work has not been reported here due to space limitations. This work has been supported with the contribution of “Opening The Future” Campaign [EJJ-OPTFUT-02010] CARIPLO 2015_0634, FWO (#G088715N, #G060612N, #G0A8813N), GOA (EJJ-C2161-GOA/11/012), IUAP-VII/07 (EJJ-C4851-17/07-P), OT#09-053 (EJJ-C0420-OT/09/053) and Project Financiering Stem Cells (PFO3 10/019) grants. We would also like to thank Rondoufonds voor Duchenne Onderzoek (EQQ-FODUCH-O2010) for kind donations.
References
- Aguirre A., Montserrat N., Zacchigna S., Nivet E., Hishida T., Krause M.N., Kurian L., Ocampo A., Vazquez-Ferrer E., Rodriguez-Esteban C., Kumar S., Moresco J.J., Yates J.R., 3rd, Campistol J.M., Sancho-Martinez I., Giacca M., Izpisua Belmonte J.C. In vivo activation of a conserved microRNA program induces mammalian heart regeneration. Cell Stem Cell. 2014;15:589–604. doi: 10.1016/j.stem.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicher A., Brenner W., Zuhayra M., Badorff C., Massoudi S., Assmus B., Eckey T., Henze E., Zeiher A.M., Dimmeler S. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation. 2003;107:2134–2139. doi: 10.1161/01.CIR.0000062649.63838.C9. [DOI] [PubMed] [Google Scholar]
- Anokye-Danso F., Trivedi C.M., Juhr D., Gupta M., Cui Z., Tian Y., Zhang Y., Yang W., Gruber P.J., Epstein J.A., Morrisey E.E. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8:376–388. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmus B., Rolf A., Erbs S., Elsasser A., Haberbosch W., Hambrecht R., Tillmanns H., Yu J., Corti R., Mathey D.G., Hamm C.W., Suselbeck T., Tonn T., Dimmeler S., Dill T., Zeiher A.M., Schachinger V., Investigators, R.-A. Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circ. Heart Fail. 2010;3:89–96. doi: 10.1161/CIRCHEARTFAILURE.108.843243. [DOI] [PubMed] [Google Scholar]
- Assmus B., Walter D.H., Seeger F.H., Leistner D.M., Steiner J., Ziegler I., Lutz A., Khaled W., Klotsche J., Tonn T., Dimmeler S., Zeiher A.M. Effect of shock wave-facilitated intracoronary cell therapy on LVEF in patients with chronic heart failure: the CELLWAVE randomized clinical trial. JAMA. 2013;309:1622–1631. doi: 10.1001/jama.2013.3527. [DOI] [PubMed] [Google Scholar]
- Assmus B., Leistner D.M., Schachinger V., Erbs S., Elsasser A., Haberbosch W., Hambrecht R., Sedding D., Yu J., Corti R., Mathey D.G., Barth C., Mayer-Wehrstein C., Burck I., Sueselbeck T., Dill T., Hamm C.W., Tonn T., Dimmeler S., Zeiher A.M., Group, R.A.S. Long-term clinical outcome after intracoronary application of bone marrow-derived mononuclear cells for acute myocardial infarction: migratory capacity of administered cells determines event-free survival. Eur. Heart J. 2014;35:1275–1283. doi: 10.1093/eurheartj/ehu062. [DOI] [PubMed] [Google Scholar]
- Beitnes J.O., Hopp E., Lunde K., Solheim S., Arnesen H., Brinchmann J.E., Forfang K., Aakhus S. Long-term results after intracoronary injection of autologous mononuclear bone marrow cells in acute myocardial infarction: the ASTAMI randomised, controlled study. Heart. 2009;95:1983–1989. doi: 10.1136/hrt.2009.178913. [DOI] [PubMed] [Google Scholar]
- Bolli R., Chugh A.R., D'amario D., Loughran J.H., Stoddard M.F., Ikram S., Beache G.M., Wagner S.G., Leri A., Hosoda T., Sanada F., Elmore J.B., Goichberg P., Cappetta D., Solankhi N.K., Fahsah I., Rokosh D.G., Slaughter M.S., Kajstura J., Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Boon R.A., Dimmeler S. MicroRNAs in myocardial infarction. Nat. Rev. Cardiol. 2015;12:135–142. doi: 10.1038/nrcardio.2014.207. [DOI] [PubMed] [Google Scholar]
- Brenner W., Aicher A., Eckey T., Massoudi S., Zuhayra M., Koehl U., Heeschen C., Kampen W.U., Zeiher A.M., Dimmeler S., Henze E. 111In-labeled CD34 + hematopoietic progenitor cells in a rat myocardial infarction model. J. Nucl. Med. 2004;45:512–518. [PubMed] [Google Scholar]
- Caulfield J.B., Leinbach R., Gold H. The relationship of myocardial infarct size and prognosis. Circulation. 1976;53:I141–I144. [PubMed] [Google Scholar]
- Chen J.J., Zhou S.H. Mesenchymal stem cells overexpressing MiR-126 enhance ischemic angiogenesis via the AKT/ERK-related pathway. Cartogr. J. 2011;18:675–681. doi: 10.5603/cj.2011.0032. [DOI] [PubMed] [Google Scholar]
- Chen T.S., Lai R.C., Lee M.M., Choo A.B., Lee C.N., Lim S.K. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38:215–224. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin M.H., Mason M.J., Xie W., Volinia S., Singer M., Peterson C., Ambartsumyan G., Aimiuwu O., Richter L., Zhang J., Khvorostov I., Ott V., Grunstein M., Lavon N., Benvenisty N., Croce C.M., Clark A.T., Baxter T., Pyle A.D., Teitell M.A., Pelegrini M., Plath K., Lowry W.E. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell. 2009;5:111–123. doi: 10.1016/j.stem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J.J., Yang X., Don C.W., Minami E., Liu Y.W., Weyers J.J., Mahoney W.M., Van Biber B., Cook S.M., Palpant N.J., Gantz J.A., Fugate J.A., Muskheli V., Gough G.M., Vogel K.W., Astley C.A., Hotchkiss C.E., Baldessari A., Pabon L., Reinecke H., Gill E.A., Nelson V., Kiem H.P., Laflamme M.A., Murry C.E. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman K.L., Vardanian A.J., Fang Q., Sievers R.E., Fok H.H., Lee R.J. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J. Am. Coll. Cardiol. 2004;44:654–660. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- Conlon F.L., Lyons K.M., Takaesu N., Barth K.S., Kispert A., Herrmann B., Robertson E.J. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120:1919–1928. doi: 10.1242/dev.120.7.1919. [DOI] [PubMed] [Google Scholar]
- Deuse T., Peter C., Fedak P.W., Doyle T., Reichenspurner H., Zimmermann W.H., Eschenhagen T., Stein W., Wu J.C., Robbins R.C., Schrepfer S. Hepatocyte growth factor or vascular endothelial growth factor gene transfer maximizes mesenchymal stem cell-based myocardial salvage after acute myocardial infarction. Circulation. 2009;120:S247–S254. doi: 10.1161/CIRCULATIONAHA.108.843680. [DOI] [PubMed] [Google Scholar]
- Dubois N.C., Craft A.M., Sharma P., Elliott D.A., Stanley E.G., Elefanty A.G., Gramolini A., Keller G. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat. Biotechnol. 2011;29:1011–1018. doi: 10.1038/nbt.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duelen R., Gilbert G., Patel A., de Schaetzen N., De Waele L., Roderick L., Sipido K.R., Verfaillie C.M., Buyse G.M., Thorrez L., Sampaolesi M. Activin A modulates CRIPTO-1/HNF4α + cells to guide cardiac differentiation from human embryonic stem cells. Stem Cells Int. 2017;2017:17. doi: 10.1155/2017/4651238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dush M.K., Martin G.R. Analysis of mouse Evx genes: Evx-1 displays graded expression in the primitive streak. Dev. Biol. 1992;151:273–287. doi: 10.1016/0012-1606(92)90232-6. [DOI] [PubMed] [Google Scholar]
- Emery A.E. The muscular dystrophies. Lancet. 2002;359:687–695. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- Eulalio A., Mano M., Dal Ferro M., Zentilin L., Sinagra G., Zacchigna S., Giacca M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492:376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- Forlani S., Lawson K.A., Deschamps J. Acquisition of Hox codes during gastrulation and axial elongation in the mouse embryo. Development. 2003;130:3807–3819. doi: 10.1242/dev.00573. [DOI] [PubMed] [Google Scholar]
- Freyman T., Polin G., Osman H., Crary J., Lu M., Cheng L., Palasis M., Wilensky R.L. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur. Heart J. 2006;27:1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- Gadue P., Huber T.L., Nostro M.C., Kattman S., Keller G.M. Germ layer induction from embryonic stem cells. Exp. Hematol. 2005;33:955–964. doi: 10.1016/j.exphem.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Garbern J.C., Lee R.T. Cardiac stem cell therapy and the promise of heart regeneration. Cell Stem Cell. 2013;12:689–698. doi: 10.1016/j.stem.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherghiceanu M., Barad L., Novak A., Reiter I., Itskovitz-Eldor J., Binah O., Popescu L.M. Cardiomyocytes derived from human embryonic and induced pluripotent stem cells: comparative ultrastructure. J. Cell. Mol. Med. 2011;15:2539–2551. doi: 10.1111/j.1582-4934.2011.01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giteau A., Venier-Julienne M.C., Marchal S., Courthaudon J.L., Sergent M., Montero-Menei C., Verdier J.M., Benoit J.P. Reversible protein precipitation to ensure stability during encapsulation within PLGA microspheres. Eur. J. Pharm. Biopharm. 2008;70:127–136. doi: 10.1016/j.ejpb.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Gnecchi M., He H., Liang O.D., Melo L.G., Morello F., Mu H., Noiseux N., Zhang L., Pratt R.E., Ingwall J.S., Dzau V.J. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat. Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- Go A.S., Mozaffarian D., Roger V.L., Benjamin E.J., Berry J.D., Blaha M.J., Dai S., Ford E.S., Fox C.S., Franco S., Fullerton H.J., Gillespie C., Hailpern S.M., Heit J.A., Howard V.J., Huffman M.D., Judd S.E., Kissela B.M., Kittner S.J., Lackland D.T., Lichtman J.H., Lisabeth L.D., Mackey R.H., Magid D.J., Marcus G.M., Marelli A., Matchar D.B., Mcguire D.K., Mohler E.R., 3rd, Moy C.S., Mussolino M.E., Neumar R.W., Nichol G., Pandey D.K., Paynter N.P., Reeves M.J., Sorlie P.D., Stein J., Towfighi A., Turan T.N., Virani S.S., Wong N.D., Woo D., Turner M.B., American Heart Association Statistics Committee, Stroke Statistics Subcommittee Heart disease and stroke statistics-2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare J.M., Traverse J.H., Henry T.D., Dib N., Strumpf R.K., Schulman S.P., Gerstenblith G., Demaria A.N., Denktas A.E., Gammon R.S., Hermiller J.B., Jr., Reisman M.A., Schaer G.L., Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J. Am. Coll. Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart A.H., Hartley L., Sourris K., Stadler E.S., Li R., Stanley E.G., Tam P.P., Elefanty A.G., Robb L. Mixl1 is required for axial mesendoderm morphogenesis and patterning in the murine embryo. Development. 2002;129:3597–3608. doi: 10.1242/dev.129.15.3597. [DOI] [PubMed] [Google Scholar]
- Hartman M.E., Dai D.F., Laflamme M.A. Human pluripotent stem cells: prospects and challenges as a source of cardiomyocytes for in vitro modeling and cell-based cardiac repair. Adv. Drug Deliv. Rev. 2016;96:3–17. doi: 10.1016/j.addr.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori F., Chen H., Yamashita H., Tohyama S., Satoh Y.S., Yuasa S., Li W., Yamakawa H., Tanaka T., Onitsuka T., Shimoji K., Ohno Y., Egashira T., Kaneda R., Murata M., Hidaka K., Morisaki T., Sasaki E., Suzuki T., Sano M., Makino S., Oikawa S., Fukuda K. Nongenetic method for purifying stem cell-derived cardiomyocytes. Nat. Methods. 2010;7:61–66. doi: 10.1038/nmeth.1403. [DOI] [PubMed] [Google Scholar]
- Heidi AU H.T., Cui B., Chu Z.E., Veres T., Radisic M. Cell culture chips for simultaneous application of topographical and electrical cues enhance phenotype of cardiomyocytes. Lab Chip. 2009;9:564–575. doi: 10.1039/b810034a. [DOI] [PubMed] [Google Scholar]
- Herrmann B.G., Kispert A. The T genes in embryogenesis. Trends Genet. 1994;10:280–286. doi: 10.1016/0168-9525(90)90011-t. [DOI] [PubMed] [Google Scholar]
- Hogan B.L. Bone morphogenetic proteins in development. Curr. Opin. Genet. Dev. 1996;6:432–438. doi: 10.1016/s0959-437x(96)80064-5. [DOI] [PubMed] [Google Scholar]
- Hou D., Youssef E.A., Brinton T.J., Zhang P., Rogers P., Price E.T., Yeung A.C., Johnstone B.H., Yock P.G., March K.L. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112:I150–I156. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- Houtgraaf J.H., Den Dekker W.K., Van Dalen B.M., Springeling T., De Jong R., Van Geuns R.J., Geleijnse M.L., Fernandez-Aviles F., Zijlsta F., Serruys P.W., Duckers H.J. First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2012;59:539–540. doi: 10.1016/j.jacc.2011.09.065. [DOI] [PubMed] [Google Scholar]
- Huang F., Zhu X., Hu X.Q., Fang Z.F., Tang L., Lu X.L., Zhou S.H. Mesenchymal stem cells modified with miR-126 release angiogenic factors and activate Notch ligand Delta-like-4, enhancing ischemic angiogenesis and cell survival. Int. J. Mol. Med. 2013;31:484–492. doi: 10.3892/ijmm.2012.1200. [DOI] [PubMed] [Google Scholar]
- Hudson J., Titmarsh D., Hidalgo A., Wolvetang E., Cooper-White J. Primitive cardiac cells from human embryonic stem cells. Stem Cells Dev. 2012;21:1513–1523. doi: 10.1089/scd.2011.0254. [DOI] [PubMed] [Google Scholar]
- Huikuri H.V., Kervinen K., Niemela M., Ylitalo K., Saily M., Koistinen P., Savolainen E.R., Ukkonen H., Pietila M., Airaksinen J.K., Knuuti J., Makikallio T.H., Investigators, F. Effects of intracoronary injection of mononuclear bone marrow cells on left ventricular function, arrhythmia risk profile, and restenosis after thrombolytic therapy of acute myocardial infarction. Eur. Heart J. 2008;29:2723–2732. doi: 10.1093/eurheartj/ehn436. [DOI] [PubMed] [Google Scholar]
- Ibrahim A.G., Cheng K., Marban E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep. 2014;2:606–619. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izarra A., Moscoso I., Levent E., Canon S., Cerrada I., Diez-Juan A., Blanca V., Nunez-Gil I.J., Valiente I., Ruiz-Sauri A., Sepulveda P., Tiburcy M., Zimmermann W.H., Bernad A. miR-133a enhances the protective capacity of cardiac progenitors cells after myocardial infarction. Stem Cell Rep. 2014;3:1029–1042. doi: 10.1016/j.stemcr.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens S., Dubois C., Bogaert J., Theunissen K., Deroose C., Desmet W., Kalantzi M., Herbots L., Sinnaeve P., Dens J., Maertens J., Rademakers F., Dymarkowski S., Gheysens O., Van Cleemput J., Bormans G., Nuyts J., Belmans A., Mortelmans L., Boogaerts M., Van De Werf F. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- Kaneko S., Yamanaka S. To be immunogenic, or not to be: that's the iPSC question. Cell Stem Cell. 2013;12:385–386. doi: 10.1016/j.stem.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Karakikes I., Senyei G.D., Hansen J., Kong C.W., Azeloglu E.U., Stillitano F., Lieu D.K., Wang J., Ren L., Hulot J.S., Iyengar R., Li R.A., Hajjar R.J. Small molecule-mediated directed differentiation of human embryonic stem cells toward ventricular cardiomyocytes. Stem Cells Transl. Med. 2014;3:18–31. doi: 10.5966/sctm.2013-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakikes I., Ameen M., Termglinchan V., Wu J.C. Human induced pluripotent stem cell-derived cardiomyocytes: insights into molecular, cellular, and functional phenotypes. Circ. Res. 2015;117:80–88. doi: 10.1161/CIRCRESAHA.117.305365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattman S.J., Witty A.D., Gagliardi M., Dubois N.C., Niapour M., Hotta A., Ellis J., Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Kehat I., Khimovich L., Caspi O., Gepstein A., Shofti R., Arbel G., Huber I., Satin J., Itskovitz-Eldor J., Gepstein L. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat. Biotechnol. 2004;22:1282–1289. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- Kempf H., Olmer R., Kropp C., Ruckert M., Jara-Avaca M., Robles-Diaz D., Franke A., Elliott D.A., Wojciechowski D., Fischer M., Roa Lara A., Kensah G., Gruh I., Haverich A., Martin U., Zweigerdt R. Controlling expansion and cardiomyogenic differentiation of human pluripotent stem cells in scalable suspension culture. Stem Cell Rep. 2014;3:1132–1146. doi: 10.1016/j.stemcr.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M., Nickoloff E., Abramova T., Johnson J., Verma S.K., Krishnamurthy P., Mackie A.R., Vaughan E., Garikipati V.N., Benedict C., Ramirez V., Lambers E., Ito A., Gao E., Misener S., Luongo T., Elrod J., Qin G., Houser S.R., Koch W.J., Kishore R. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ. Res. 2015;117:52–64. doi: 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrel E.A., Lanza R. Current status of pluripotent stem cells: moving the first therapies to the clinic. Nat. Rev. Drug Discov. 2015;14:681–692. doi: 10.1038/nrd4738. [DOI] [PubMed] [Google Scholar]
- Kinder S.J., Tsang T.E., Wakamiya M., Sasaki H., Behringer R.R., Nagy A., Tam P.P. The organizer of the mouse gastrula is composed of a dynamic population of progenitor cells for the axial mesoderm. Development. 2001;128:3623–3634. doi: 10.1242/dev.128.18.3623. [DOI] [PubMed] [Google Scholar]
- Laflamme M.A., Chen K.Y., Naumova A.V., Muskheli V., Fugate J.A., Dupras S.K., Reinecke H., Xu C., Hassanipour M., Police S., O'sullivan C., Collins L., Chen Y., Minami E., Gill E.A., Ueno S., Yuan C., Gold J., Murry C.E. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Lee J.B., Shapovalova Z., Fiebig-Comyn A., Mitchell R.R., Laronde S., Szabo E., Benoit Y.D., Bhatia M. Somatic transcriptome priming gates lineage-specific differentiation potential of human-induced pluripotent stem cell states. Nat. Commun. 2014;5:5605. doi: 10.1038/ncomms6605. [DOI] [PubMed] [Google Scholar]
- Leistner D.M., Fischer-Rasokat U., Honold J., Seeger F.H., Schachinger V., Lehmann R., Martin H., Burck I., Urbich C., Dimmeler S., Zeiher A.M., Assmus B. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI): final 5-year results suggest long-term safety and efficacy. Clin. Res. Cardiol. 2011;100:925–934. doi: 10.1007/s00392-011-0327-y. [DOI] [PubMed] [Google Scholar]
- Lister R., Pelizzola M., Kida Y.S., Hawkins R.D., Nery J.R., Hon G., Antosiewicz-Bourget J., O'malley R., Castanon R., Klugman S., Downes M., Yu R., Stewart R., Ren B., Thomson J.A., Evans R.M., Ecker J.R. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature. 2011;471:68–73. doi: 10.1038/nature09798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Van Mil A., Vrijsen K., Zhao J., Gao L., Metz C.H., Goumans M.J., Doevendans P.A., Sluijter J.P. MicroRNA-155 prevents necrotic cell death in human cardiomyocyte progenitor cells via targeting RIP1. J. Cell. Mol. Med. 2011;15:1474–1482. doi: 10.1111/j.1582-4934.2010.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde K., Solheim S., Aakhus S., Arnesen H., Abdelnoor M., Egeland T., Endresen K., Ilebekk a., Mangschau A., Fjeld J.G., Smith H.J., Taraldsrud E., Grogaard H.K., Bjornerheim R., Brekke M., Muller C., Hopp E., Ragnarsson A., Brinchmann J.E., Forfang K. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N. Engl. J. Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- Lunde K., Aakhus S. Cell therapy in acute myocardial infarction: measures of efficacy. Heart. 2008;94:969–970. doi: 10.1136/hrt.2007.138479. [DOI] [PubMed] [Google Scholar]
- Mackie A.R., Klyachko E., Thorne T., Schultz K.M., Millay M., Ito A., Kamide C.E., Liu T., Gupta R., Sahoo S., Misener S., Kishore R., Losordo D.W. Sonic hedgehog-modified human CD34 + cells preserve cardiac function after acute myocardial infarction. Circ. Res. 2012;111:312–321. doi: 10.1161/CIRCRESAHA.112.266015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madonna R., Petrov L., Teberino M.A., Manzoli L., Karam J.P., Renna F.V., Ferdinandy P., Montero-Menei C.N., Yla-Herttuala S., De Caterina R. Transplantation of adipose tissue mesenchymal cells conjugated with VEGF-releasing microcarriers promotes repair in murine myocardial infarction. Cardiovasc. Res. 2015;108:39–49. doi: 10.1093/cvr/cvv197. [DOI] [PubMed] [Google Scholar]
- Makkar R.R., Smith R.R., Cheng K., Malliaras K., Thomson L.E., Berman D., Czer L.S., Marban L., Mendizabal A., Johnston P.V., Russell S.D., Schuleri K.H., Lardo A.C., Gerstenblith G., Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauritz C., Schwanke K., Reppel M., Neef S., Katsirntaki K., Maier L.S., Nguemo F., Menke S., Haustein M., Hescheler J., Hasenfuss G., Martin U. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008;118:507–517. doi: 10.1161/CIRCULATIONAHA.108.778795. [DOI] [PubMed] [Google Scholar]
- Mauritz C., Martens A., Rojas S.V., Schnick T., Rathert C., Schecker N., Menke S., Glage S., Zweigerdt R., Haverich A., Martin U., Kutschka I. Induced pluripotent stem cell (iPSC)-derived Flk-1 progenitor cells engraft, differentiate, and improve heart function in a mouse model of acute myocardial infarction. Eur. Heart J. 2011;32:2634–2641. doi: 10.1093/eurheartj/ehr166. [DOI] [PubMed] [Google Scholar]
- Mayfield A.E., Tilokee E.L., Latham N., Mcneill B., Lam B.K., Ruel M., Suuronen E.J., Courtman D.W., Stewart D.J., Davis D.R. The effect of encapsulation of cardiac stem cells within matrix-enriched hydrogel capsules on cell survival, post-ischemic cell retention and cardiac function. Biomaterials. 2014;35:133–142. doi: 10.1016/j.biomaterials.2013.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G.P., Wollert K.C., Lotz J., Steffens J., Lippolt P., Fichtner S., Hecker H., Schaefer A., Arseniev L., Hertenstein B., Ganser A., Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- Meyer G.P., Wollert K.C., Lotz J., Pirr J., Rager U., Lippolt P., Hahn A., Fichtner S., Schaefer A., Arseniev L., Ganser A., Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: 5-year follow-up from the randomized-controlled BOOST trial. Eur. Heart J. 2009;30:2978–2984. doi: 10.1093/eurheartj/ehp374. [DOI] [PubMed] [Google Scholar]
- Miyoshi N., Ishii H., Nagano H., Haraguchi N., Dewi D.L., Kano Y., Nishikawa S., Tanemura M., Mimori K., Tanaka F., Saito T., Nishimura J., Takemasa I., Mizushima T., Ikeda M., Yamamoto H., Sekimoto M., Doki Y., Mori M. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell. 2011;8:633–638. doi: 10.1016/j.stem.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Mummery C.L., Zhang J., Ng E.S., Elliott D.A., Elefanty A.G., Kamp T.J. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ. Res. 2012;111:344–358. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaya N., Fujii T., Iwase T., Ohgushi H., Itoh T., Uematsu M., Yamagishi M., Mori H., Kangawa K., Kitamura S. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H2670–H2676. doi: 10.1152/ajpheart.01071.2003. [DOI] [PubMed] [Google Scholar]
- Naito A.T., Shiojima I., Akazawa H., Hidaka K., Morisaki T., Kikuchi A., Komuro I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc. Natl. Acad. Sci. U. S. A. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols M., Townsend N., Scarborough P., Rayner M. Cardiovascular disease in Europe 2014: epidemiological update. Eur. Heart J. 2014;35:2929. doi: 10.1093/eurheartj/ehu378. [DOI] [PubMed] [Google Scholar]
- Ong S.G., Hausenloy D.J. Hypoxia-inducible factor as a therapeutic target for cardioprotection. Pharmacol. Ther. 2012;136:69–81. doi: 10.1016/j.pharmthera.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Parikh A., Wu J., Blanton R.M., Tzanakakis E.S. Signaling pathways and gene regulatory networks in cardiomyocyte differentiation. Tissue Eng. Part B Rev. 2015;21:377–392. doi: 10.1089/ten.teb.2014.0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillekamp F., Haustein M., Khalil M., Emmelheinz M., Nazzal R., Adelmann R., Nguemo F., Rubenchyk O., Pfannkuche K., Matzkies M., Reppel M., Bloch W., Brockmeier K., Hescheler J. Contractile properties of early human embryonic stem cell-derived cardiomyocytes: beta-adrenergic stimulation induces positive chronotropy and lusitropy but not inotropy. Stem Cells Dev. 2012;21:2111–2121. doi: 10.1089/scd.2011.0312. [DOI] [PubMed] [Google Scholar]
- Quattrocelli M., Swinnen M., Giacomazzi G., Camps J., Barthelemy I., Ceccarelli G., Caluwe E., Grosemans H., Thorrez L., Pelizzo G., Muijtjens M., Verfaillie C.M., Blot S., Janssens S., Sampaolesi M. Mesodermal iPSC-derived progenitor cells functionally regenerate cardiac and skeletal muscle. J. Clin. Invest. 2015;125:4463–4482. doi: 10.1172/JCI82735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisic M., Christman K.L. Materials science and tissue engineering: repairing the heart. Mayo Clin. Proc. 2013;88:884–898. doi: 10.1016/j.mayocp.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J., Miekus K., Kucia M., Zhang J., Reca R., Dvorak P., Ratajczak M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- Robertson J.A. Human embryonic stem cell research: ethical and legal issues. Nat. Rev. Genet. 2001;2:74–78. doi: 10.1038/35047594. [DOI] [PubMed] [Google Scholar]
- Sahoo S., Klychko E., Thorne T., Misener S., Schultz K.M., Millay M., Ito A., Liu T., Kamide C., Agrawal H., Perlman H., Qin G., Kishore R., Losordo D.W. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ. Res. 2011;109:724–728. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachinger V., Tonn T., Dimmeler S., Zeiher A.M. Bone-marrow-derived progenitor cell therapy in need of proof of concept: design of the REPAIR-AMI trial. Nat. Clin. Pract. Cardiovasc. Med. 2006;3(Suppl 1):S23–S28. doi: 10.1038/ncpcardio0441. [DOI] [PubMed] [Google Scholar]
- Schwach V., Passier R. Generation and purification of human stem cell-derived cardiomyocytes. Differentiation. 2016;91:126–138. doi: 10.1016/j.diff.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Sumi T., Tsuneyoshi N., Nakatsuji N., Suemori H. Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/beta-catenin, Activin/Nodal and BMP signaling. Development. 2008;135:2969–2979. doi: 10.1242/dev.021121. [DOI] [PubMed] [Google Scholar]
- Surder D., Manka R., Lo Cicero V., Moccetti T., Rufibach K., Soncin S., Turchetto L., Radrizzani M., Astori G., Schwitter J., Erne P., Zuber M., Auf Der Maur C., Jamshidi P., Gaemperli O., Windecker S., Moschovitis A., Wahl A., Buhler I., Wyss C., Kozerke S., Landmesser U., Luscher T.F., Corti R. Intracoronary injection of bone marrow-derived mononuclear cells early or late after acute myocardial infarction: effects on global left ventricular function. Circulation. 2013;127:1968–1979. doi: 10.1161/CIRCULATIONAHA.112.001035. [DOI] [PubMed] [Google Scholar]
- Taapken S.M., Nisler B.S., Newton M.A., Sampsell-Barron T.L., Leonhard K.A., Mcintire E.M., Montgomery K.D. Karotypic abnormalities in human induced pluripotent stem cells and embryonic stem cells. Nat. Biotechnol. 2011;29:313–314. doi: 10.1038/nbt.1835. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Li T.S., Suzuki R., Kobayashi T., Ito H., Ikeda Y., Matsuzaki M., Hamano K. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H886–H893. doi: 10.1152/ajpheart.00142.2006. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tam P.P., Behringer R.R. Mouse gastrulation: the formation of a mammalian body plan. Mech. Dev. 1997;68:3–25. doi: 10.1016/s0925-4773(97)00123-8. [DOI] [PubMed] [Google Scholar]
- Tendera M., Wojakowski W., Ruzyllo W., Chojnowska L., Kepka C., Tracz W., Musialek P., Piwowarska W., Nessler J., Buszman P., Grajek S., Breborowicz P., Majka M., Ratajczak M.Z., Investigators, R. Intracoronary infusion of bone marrow-derived selected CD34 + CXCR4 + cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur. Heart J. 2009;30:1313–1321. doi: 10.1093/eurheartj/ehp073. [DOI] [PubMed] [Google Scholar]
- Thery C., Duban L., Segura E., Veron P., Lantz O., Amigorena S. Indirect activation of naive CD4 + T cells by dendritic cell-derived exosomes. Nat. Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- Thery C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tohyama S., Hattori F., Sano M., Hishiki T., Nagahata Y., Matsuura T., Hashimoto H., Suzuki T., Yamashita H., Satoh Y., Egashira T., Seki T., Muraoka N., Yamakawa H., Ohgino Y., Tanaka T., Yoichi M., Yuasa S., Murata M., Suematsu M., Fukuda K. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell. 2013;12:127–137. doi: 10.1016/j.stem.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Traverse J.H., Henry T.D., Ellis S.G., Pepine C.J., Willerson J.T., Zhao D.X., Forder J.R., Byrne B.J., Hatzopoulos A.K., Penn M.S., Perin E.C., Baran K.W., Chambers J., Lambert C., Raveendran G., Simon D.I., Vaughan D.E., Simpson L.M., Gee A.P., Taylor D.A., Cogle C.R., Thomas J.D., Silva G.V., Jorgenson B.C., Olson R.E., Bowman S., Francescon J., Geither C., Handberg E., Smith D.X., Baraniuk S., Piller L.B., Loghin C., Aguilar D., Richman S., Zierold C., Bettencourt J., Sayre S.L., Vojvodic R.W., Skarlatos S.I., Gordon D.J., Ebert R.F., Kwak M., Moye L.A., Simari R.D., Cardiovascular Cell Therapy, R. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA. 2011;306:2110–2119. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse J.H., Henry T.D., Pepine C.J., Willerson J.T., Zhao D.X., Ellis S.G., Forder J.R., Anderson R.D., Hatzopoulos A.K., Penn M.S., Perin E.C., Chambers J., Baran K.W., Raveendran G., Lambert C., Lerman A., Simon D.I., Vaughan D.E., Lai D., Gee A.P., Taylor D.A., Cogle C.R., Thomas J.D., Olson R.E., Bowman S., Francescon J., Geither C., Handberg E., Kappenman C., Westbrook L., Piller L.B., Simpson L.M., Baraniuk S., Loghin C., Aguilar D., Richman S., Zierold C., Spoon D.B., Bettencourt J., Sayre S.L., Vojvodic R.W., Skarlatos S.I., Gordon D.J., Ebert R.F., Kwak M., Moye L.A., Simari R.D., Cardiovascular Cell Therapy Research, N. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. JAMA. 2012;308:2380–2389. doi: 10.1001/jama.2012.28726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulloch N.L., Muskheli V., Razumova M.V., Korte F.S., Regnier M., Hauch K.D., Pabon L., Reinecke H., Murry C.E. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ. Res. 2011;109:47–59. doi: 10.1161/CIRCRESAHA.110.237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H., Ekstrom K., Bossios A., Sjostrand M., Lee J.J., Lotvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Wollert K.C., Meyer G.P., Lotz J., Ringes-Lichtenberg S., Lippolt P., Breidenbach C., Fichtner S., Korte T., Hornig B., Messinger D., Arseniev L., Hertenstein B., Ganser A., Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- Wollert K.C., Drexler H. Clinical applications of stem cells for the heart. Circ. Res. 2005;96:151–163. doi: 10.1161/01.RES.0000155333.69009.63. [DOI] [PubMed] [Google Scholar]
- Wu S.M., Fujiwara Y., Cibulsky S.M., Clapham D.E., Lien C.L., Schultheiss T.M., Orkin S.H. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–1150. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Xu X.Q., Zweigerdt R., Soo S.Y., Ngoh Z.X., Tham S.C., Wang S.T., Graichen R., Davidson B., Colman A., Sun W. Highly enriched cardiomyocytes from human embryonic stem cells. Cytotherapy. 2008;10:376–389. doi: 10.1080/14653240802105307. [DOI] [PubMed] [Google Scholar]
- Xu Q., Seeger F.H., Castillo J., Iekushi K., Boon R.A., Farcas R., Manavski Y., Li Y.G., Assmus B., Zeiher A.M., Dimmeler S. Micro-RNA-34a contributes to the impaired function of bone marrow-derived mononuclear cells from patients with cardiovascular disease. J. Am. Coll. Cardiol. 2012;59:2107–2117. doi: 10.1016/j.jacc.2012.02.033. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T.P. Heads or tails: Wnts and anterior-posterior patterning. Curr. Biol. 2001;11:R713–R724. doi: 10.1016/s0960-9822(01)00417-1. [DOI] [PubMed] [Google Scholar]
- Yang X., Pabon L., Murry C.E. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ. Res. 2014;114:511–523. doi: 10.1161/CIRCRESAHA.114.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L., Chang Y.H., Xiong Q., Zhang P., Zhang L., Somasundaram P., Lepley M., Swingen C., Su L., Wendel J.S., Guo J., Jang A., Rosenbush D., Greder L., Dutton J.R., Zhang J., Kamp T.J., Kaufman D.S., Ge Y., Zhang J. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 2014;15:750–761. doi: 10.1016/j.stem.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R., Slukvin I., Thomson J.A. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zhang J., Wilson G.F., Soerens A.G., Koonce C.H., Yu J., Palecek S.P., Thomson J.A., Kamp T.J. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 2009;104:e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Jiang J., Han P., Yuan Q., Zhang J., Zhang X., Xu Y., Cao H., Meng Q., Chen L., Tian T., Wang X., Li P., Hescheler J., Ji G., Ma Y. Direct differentiation of atrial and ventricular myocytes from human embryonic stem cells by alternating retinoid signals. Cell Res. 2011;21:579–587. doi: 10.1038/cr.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T., Zhang Z.N., Rong Z., Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- Zhu S., Li W., Zhou H., Wei W., Ambasudhan R., Lin T., Kim J., Zhang K., Ding S. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell. 2010;7:651–655. doi: 10.1016/j.stem.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W.H., Didie M., Wasmeier G.H., Nixdorff U., Hess A., Melnychenko I., Boy O., Neuhuber W.L., Weyand M., Eschenhagen T. Cardiac grafting of engineered heart tissue in syngenic rats. Circulation. 2002;106:I151–I157. [PubMed] [Google Scholar]
- Zimmermann W.H., Melnychenko I., Wasmeier G., Didie M., Naito H., Nixdorff U., Hess A., Budinsky L., Brune K., Michaelis B., Dhein S., Schwoerer A., Ehmke H., Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat. Med. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]