Abstract
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous subtype of non-Hodgkin lymphoma. In addition to clinical and immunophenotypic characteristics, recurrent gene mutations have recently been identified in patients with DLBCL using next-generation sequencing technologies. The aim of this study is to investigate the clinical relevance of B-cell function gene mutations in DLBCL. Clinical analysis was performed on 680 Chinese DLBCL patients (146 non-CR and 534 CR cases) treated with six cycles of 21-day R-CHOP (Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), alone or followed by two additional doses of rituximab consolidation on patients' own intention. Somatic mutations of B-cell function genes were further screened on 275 (71 non-CR and 204 CR) cases with available tumor samples by targeted sequencing, including genes involved in B-cell receptors (BCRs) pathway (CARD11, LYN, CD79A, and CD79B), Toll-like receptors (TLRs) pathway (MYD88), and tumor necrotic factor receptor (TNFR) pathway (TRAF2 and TNFAIP3). B-cell function gene mutations occurred in 44.0% (121/275) of DLBCL patients. The TLRs and TNFR related gene mutations were more frequently observed in non-CR patients (p = 0.019 and p = 0.032, respectively). BCRs related gene mutations, as well as revised IPI (R-IPI) and double BCL-2/MYC expression, were independently related to short progression-free survival in DLBCL after CR. The adverse prognostic effect of BCRs related gene mutations could be overcome by two additional doses of rituximab consolidation. These results highlight the molecular heterogeneity of DLBCL and identify a significant role of B-cell function gene mutations on lymphoma progression and response to rituximab in DLBCL.
Keywords: Diffuse large B-cell lymphoma, B-cell function gene mutations, Rituximab, Prognosis
Highlights
-
•
Next-generation sequencing technologies permit rapid screening of gene mutations.
-
•
TLRs and TNFR related gene mutations indicate poor response to R-CHOP in DLBCL.
-
•
BCRs related gene mutations could be overcome by prolonged rituximab consolidation.
We performed a retrospective study and assessed B-cell function gene mutations in a large cohort of Chinese patients with diffuse large B-cell lymphoma (DLBCL). Patients not achieving complete remission show significant increased TLRs (MYD88) and TNFR related gene mutations (TRAF2, TNFAIP3). Patients with BCRs related gene mutations (CARD11, LYN, CD79A, CD79B) display improved progression-free survival from additional two doses of rituximab, along with those of low-risk revised International Prognostic Index or negative for double BCL-2/MYC expression. Our study highlights the molecular heterogeneity of DLBCL and provides clinical significance of B-cell function gene mutations in guiding risk stratification treatment in DLBCL.
1. Introduction
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous subtype of non-Hodgkin lymphoma with varied clinical, immunophenotypic and genetic features (SH, 2008). Although the outcome of DLBCL patients has been significantly improved by anti-CD20 monoclonal antibody rituximab combined with induction chemotherapy (mainly as cyclophosphamide, doxorubicin, vincristine, and prednisone [R-CHOP]); the lack of remission or early relapse remains a major clinical issue. Therefore, the identification of biomarkers related to therapeutic efficacy, particularly the response to rituximab, may be greatly helpful to conduct risk stratification treatment in DLBCL.
In the era of rituximab, in addition to clinical parameters based on International Prognostic Index (IPI) (Sehn et al., 2007), tumor cell of origin (COO) (Alizadeh et al., 2000), as well as BCL-2 and MYC double translocation/expression (Johnson et al., 2012, Green et al., 2012, Horn et al., 2013), are validated as important prognostic indicators of DLBCL. More recently, recurrent gene mutations have been revealed by next-generation sequencing technologies, including those involved in B-cell function (Compagno et al., 2009, Ngo et al., 2011, Davis et al., 2010, Lenz et al., 2008, Kheirallah et al., 2010). B-cell receptors (BCRs) activate NF-κB by harboring receptor mutations in CD79A or CD79B, along with mutations of kinase LYN or effector CARD11 (Rossi et al., 2013). Toll-like receptors (TLRs) recruit protein kinases mainly via adaptor molecule MYD88, also leading to NF-κB activation (Rossi et al., 2013). As an important member of the tumor necrotic factor receptor (TNFR) associated factor (TRAF) protein family, TRAF2 is responsible for TNF-α-mediated modulation of NF-κB (Rossi et al., 2013). Finally, TNFAIP3 mutations resulted in a loss of NF-κB cascade inhibition (Honma et al., 2009). All these genes are closely associated with B-cell function and frequently mutated in DLBCL (Compagno et al., 2009). However, their relationship with lymphoma progression and treatment response warrants further investigation in DLBCL.
In the present study, we assessed the mutational pattern of key B-cell function genes on a large cohort of Chinese DLBCL patients treated with R-CHOP. The results showed that B-cell function gene mutations occurred in 44.0% of DLBCL patients, with the TLRs and TNFR related gene mutations reflecting non-remission status. Along with revised IPI (R-IPI) and double BCL-2/MYC expression, the presence of BCRs related gene mutations independently correlates with the disease relapse in DLBCL, which could be overcome by two additional doses of rituximab consolidation.
2. Methods
2.1. Patients
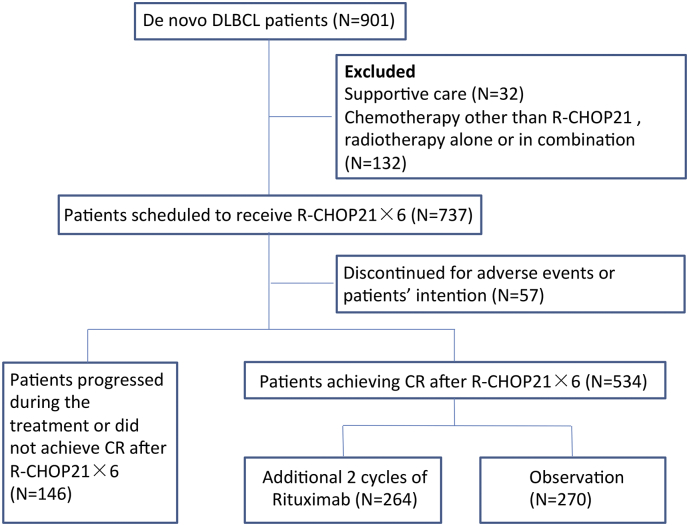
From December 2002 to December 2012, a total of 901 consecutive patients with de novo DLBCL based on registry data were enrolled in this study. The histological diagnosis was established according to World Health Organization (WHO) classification (SH, 2008), with exclusion of mediastinal large B-cell lymphoma or primary central nervous system DLBCL. A flow chart describing the cohort selection was outlined in Fig. 1. IPI (1993), R-IPI (Sehn et al., 2007) and National Comprehensive Cancer Network (NCCN)-IPI (Zhou et al., 2014) were calculated, as previously described. The study was approved by the Shanghai Rui Jin Hospital Review Board with informed consent obtained in accordance with the Declaration of Helsinki.
Fig. 1.
Flow chart of the study.
DLBCL: diffuse large B-cell lymphoma; R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone; CR: complete remission.
2.2. Response Criteria
The treatment response was evaluated according to the International Workshop Criteria (Cheson et al., 1999, Cheson et al., 2007). Patients with complete remission (CR) and unconfirmed complete remission (CRu) were defined as CR group, while patients with partial response or no response were defined as non-CR group.
2.3. Immunohistochemistry
Immunohistochemistry was performed on 5 μm-paraffin sections with an indirect immunoperoxidase method using antibodies against CD10, BCL-6, MUM-1, Ki-67, BCL-2, MYC, NF-κB1 (p105/p50, 1:250, Cell Signaling Technology) and NF-κB2 (p100/p52, 1:300, Cell Signaling Technology). Germinal center B-cell (GCB) or non-GCB subgroups were determined using Hans classification (Hans et al., 2004), with 30% cut-off value of CD10, BCL-6, and MUM-1. As for BCL-2/MYC double expression, cut-off value of BCL-2 and MYC were 70% and 40% respectively, as previously described (Hu et al., 2013). Nuclear NF-κB localization for > 30% of tumor cells was considered positive for NF-κB activity (Compagno et al., 2009).
2.4. Targeted Sequencing
Genomic DNA was extracted from formalin-fixed paraffin-embedded tumor tissue and matched peripheral blood from patients with DLBCL, using a QIAamp DNA FFPE Tissue Kit (Qiagen) and a QuickGene DNA Whole Blood Kit L (Kurabo), respectively. Sequences for B-cell function genes, including CD79A, CD79B, LYN, CARD11, MYD88, TRAF2 and TNFAIP3 were obtained from the UCSC Human Genome database, using the corresponding mRNA accession number as a reference. PCR primers were designed by iPLEX Assay Design software (Sequenom), adding universal sequence tags (CS1 and CS2) to the targeted sequencing forward and reverse primers, which produce amplicons about 200 bp at the coding regions of the genes of interest. Microfluidic PCR reactions were run in a 48 × 48 Access array system (Fluidigm) with FastStart High Fidelity PCR system (Roche) and high-throughput DNA sequencing was performed on Illumina Genome Analyzer IIx (GAIIx) and HiSeq2000 systems, according to the manufacturer's instructions. SAMtools version 0.1.19 was used to generate chromosomal coordinate-sorted bam files and to remove PCR duplications. Cases with identified mutations were sent for Sanger sequencing for verification. Primer sequences and polymerase chain reaction (PCR) conditions for each gene are available upon request. PCR reactions were run in a total volume of 25 μl containing 1UGoTaq polymerase (Promega), 0.4 μM of forward and reverse primers, 1.5 mM MgCl2, 200 μM dNTPs and 10 ng DNA.
2.5. Statistical Analysis
Baseline characteristics of patients were analyzed using two-sided χ2 test. Progression-free survival (PFS) was calculated from the date when treatment began to the date when the disease progression was recognized or the date of the last follow-up. Overall survival (OS) time was measured from the date of diagnosis to the date of death or the last follow-up. Survival functions were estimated using the Kaplan-Meier method and compared by log-rank test. Univariate hazard estimates were generated with unadjusted Cox proportional hazards models. Covariates demonstrating significance with p < 0.100 on univariate analysis were included in the multivariate model. Statistical significance was defined as p < 0.050. All statistical analysis was carried out using Statistical Package for the Social Sciences (SPSS) 20.0 software (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Clinical and Pathological Characteristics
As shown in Fig. 1, 737 of 901 patients with DLBCL were scheduled to receive six cycles of 21-day R-CHOP as induction chemotherapy. Excluding 57 cases who discontinued treatment due to adverse events or patients' intention, and 146 cases who failed to achieve CR; 534 CR patients were either given additional two cycles of rituximab on a 21-day basis (AR, n = 264) or under observation (OBS, n = 270), on the principle of the patients' intention.
The main characteristics of the non-CR and CR patients were summarized in Table 1. Non-CR patients had multiple adverse prognostic factors of IPI, including age > 60 years (47.9% vs 33.0%, p = 0.001), poor performance status (25.3% vs 7.3%, p < 0.001), advanced Ann Arbor stage (74.0% vs 45.3%, p < 0.001), elevated serum lactic dehydrogenase (LDH) level (74.7% vs 36.0%, p < 0.001) and multiple extranodal involvement (34.2% vs 15.9%, p < 0.001), as compared to those of the CR patients. Consequently, in terms of IPI, R-IPI and NCCN-IPI, 20.6%, 6.2% and 9.6% of the patients were categorized as low-risk or very good in the non-CR group, significantly lower than those of the CR group (58.4%, 29.2% and 26.2%, p all < 0.001). In the pathological setting, with similar distribution of GCB and non-GCB subtype, the non-CR patients showed significantly higher percentage of double BCL-2/MYC expression than the CR patients (45.4% vs 34.0%, p = 0.040).
Table 1.
Clinical and pathological characteristics of the patients with DLBCL.
| Characteristics |
Total (n = 680) |
Non-CR (n = 146) |
CR (N = 534) |
p valueb | ||
|---|---|---|---|---|---|---|
| Additional rituximab (n = 264) |
Observation (n = 270) |
p valuea | ||||
| Gender | ||||||
| Male | 384/680 | 85/146 | 155/264 | 144/270 | 0.223 | 0.639 |
| (56.5%) | (58.2%) | (58.7%) | (53.3%) | |||
| Female | 296/680 | 61/146 | 109/264 | 126/270 | ||
| (43.5%) | (41.8%) | (41.3%) | (46.7%) | |||
| Age (years) | ||||||
| ≤ 60 | 434/680 | 76/146 | 184/264 | 174/270 | 0.199 | 0.001 |
| (63.8%) | (52.1%) | (69.7%) | (64.4%) | |||
| > 60 | 246/680 | 70/146 | 80/264 | 96/270 | ||
| (36.2%) | (47.9%) | (30.3%) | (35.6%) | |||
| Performance status (ECOG) | ||||||
| 0–1 | 604/680 | 109/146 | 246/264 | 249/270 | 0.741 | < 0.001 |
| (88.8%) | (74.7%) | (93.2%) | (92.2%) | |||
| ≥ 2 | 76/680 | 37/146 | 18/264 | 21/270 | ||
| (11.2%) | (25.3%) | (6.8%) | (7.8%) | |||
| Ann Arbor Stage | ||||||
| I-II | 330/680 | 38/146 | 141/264 | 151/270 | 0.602 | < 0.001 |
| (48.5%) | (26.0%) | (53.4%) | (55.9%) | |||
| III-IV | 350/680 | 108/146 | 123/264 | 119/270 | ||
| (51.5%) | (74.0%) | (46.6%) | (44.1%) | |||
| Lactic dehydrogenase | ||||||
| Normal | 379/680 | 37/146 | 168/264 | 174/270 | 0.857 | < 0.001 |
| (55.7%) | (25.3%) | (63.6%) | (64.4%) | |||
| Elevated | 301/680 | 109/146 | 96/264 | 96/270 | ||
| (44.3%) | (74.7%) | (36.4%) | (35.6%) | |||
| Extranodal involvement | ||||||
| 0–1 | 545/680 | 96/146 | 219/264 | 230/270 | 0.554 | < 0.001 |
| (80.1%) | (65.8%) | (83.0%) | (85.2%) | |||
| > 1 | 135/680 | 50/146 | 45/264 | 40/270 | ||
| (19.9%) | (34.2%) | (17.0%) | (14.8%) | |||
| International Prognostic Index (IPI) | ||||||
| Low | 342/680 | 30/146 | 149/264 | 163/270 | 0.394 | < 0.001 |
| (50.3%) | (20.6%) | (56.4%) | (60.4%) | |||
| Low-intermediate | 167/680 | 38/146 | 63/264 | 66/270 | ||
| (24.6%) | (26.0%) | (23.9%) | (24.4%) | |||
| Intermediate-high | 106/680 | 44/146 | 37/264 | 25/270 | ||
| (15.6%) | (30.1%) | (14.0%) | (9.3%) | |||
| High | 65/680 | 34/146 | 15/264 | 16/270 | ||
| (9.6%) | (23.3%) | (5.7%) | (5.9%) | |||
| Revised International Prognostic Index (R-IPI) | ||||||
| Very good | 165/680 | 9/146 | 83/264 | 73/270 | 0.109 | < 0.001 |
| (24.3%) | (6.2%) | (31.4%) | (27.0%) | |||
| Good | 342/680 | 57/146 | 129/264 | 156/270 | ||
| (50.3%) | (30.9%) | (48.9%) | (57.8%) | |||
| Poor | 173/680 | 80/146 | 52/264 | 41/270 | ||
| (25.4%) | (54.8%) | (19.7%) | (15.2%) | |||
| National Comprehensive Cancer Network (NCCN)-IPI | ||||||
| Low | 154/680 | 14/146 | 74/264 | 66/270 | 0.306 | < 0.001 |
| (22.6%) | (9.6%) | (28.0%) | (24.4%) | |||
| Low-intermediate | 325/680 | 47/146 | 128/264 | 150/270 | ||
| (47.8%) | (32.2%) | (48.5%) | (55.6%) | |||
| Intermediate-high | 173/680 | 69/146 | 54/264 | 50/270 | ||
| (25.4%) | (47.3%) | (20.5%) | (18.5%) | |||
| High | 28/680 | 16/146 | 8/264 | 4/270 | ||
| (4.1%) | (11.0%) | (3.0%) | (1.5%) | |||
| Cell of origin | ||||||
| GCB | 192/581 | 36/123 | 77/228 | 79/230 | 0.992 | 0.333 |
| (33.0%) | (29.3%) | (33.8%) | (34.3%) | |||
| non-GCB | 389/581 | 87/123 | 151/228 | 151/230 | ||
| (67.0%) | (70.7%) | (66.2%) | (65.7%) | |||
| Double BCL-2/MYC expression | ||||||
| Positive | 172/470 | 49/108 | 67/191 | 56/171 | 0.658 | 0.040 |
| (36.6%) | (45.4%) | (35.1%) | (32.7%) | |||
| Negative | 298/470 | 59/108 | 124/191 | 115/171 | ||
| (63.4%) | (54.6%) | (64.9%) | (67.3%) | |||
| Ki-67 > 80% | ||||||
| Yes | 263/517 | 58/110 | 113/223 | 92/184 | 0.921 | 0.669 |
| (50.9%) | (52.7%) | (50.7%) | (50.0%) | |||
| No | 254/517 | 52/110 | 110/223 | 92/184 | ||
| (49.1%) | (47.3%) | (49.3%) | (50.0%) | |||
p value indicated difference between additional rituximab and observation.
p value indicated difference between non-CR and CR.
Of note, according to the clinical and pathological characteristics, no obvious difference was observed between the AR and the OBS group of the CR patients (Table 1).
3.2. Mutational Pattern of B-cell Function Genes
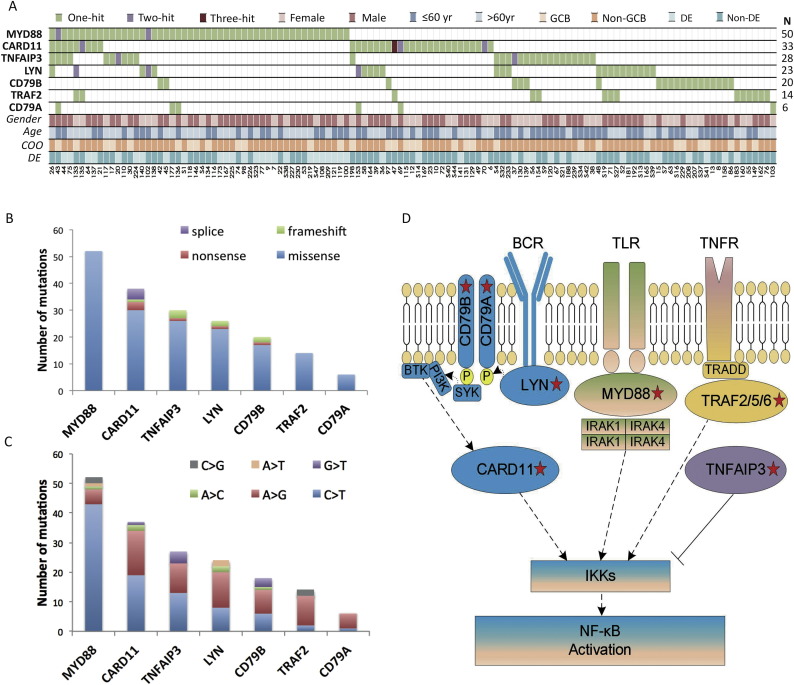
Mutations of B-cell function genes were screened in 71 of the 146 non-CR patients and 204 of the 534 CR patients with available tumor samples (Fig. 2A). Overall, a total of 186 non-silent somatic mutations were identified in 121 patients, including 168 missense, 8 insertion or deletion, 6 nonsense, and 4 splice-site mutations, and a preference for C > T/A > G alterations analogous to the somatic single nucleotide variation (SNV) spectrum in other cancers (Fig. 2B–C and Supplementary Table 1).
Fig. 2.
B-cell function gene mutations in diffuse large B-cell lymphoma (DLBCL).
(A) Gene mutations identified by targeted sequencing in 275 patients with DLBCL. (B) Number and type of non-silent somatic mutations. (C) Number and percentage of non-silent somatic SNVs (6 most frequent SNVs were listed on the Figure). (D) Schematic description of B-cell function genes. The key mutated genes are indicated, including MYD88, CARD11, TNFAIP3, LYN, CD79A, CD79B and TRAF2. COO: cell of origin; DE: double BCL-2/MYC expression; GCB: germinal center B-cell.
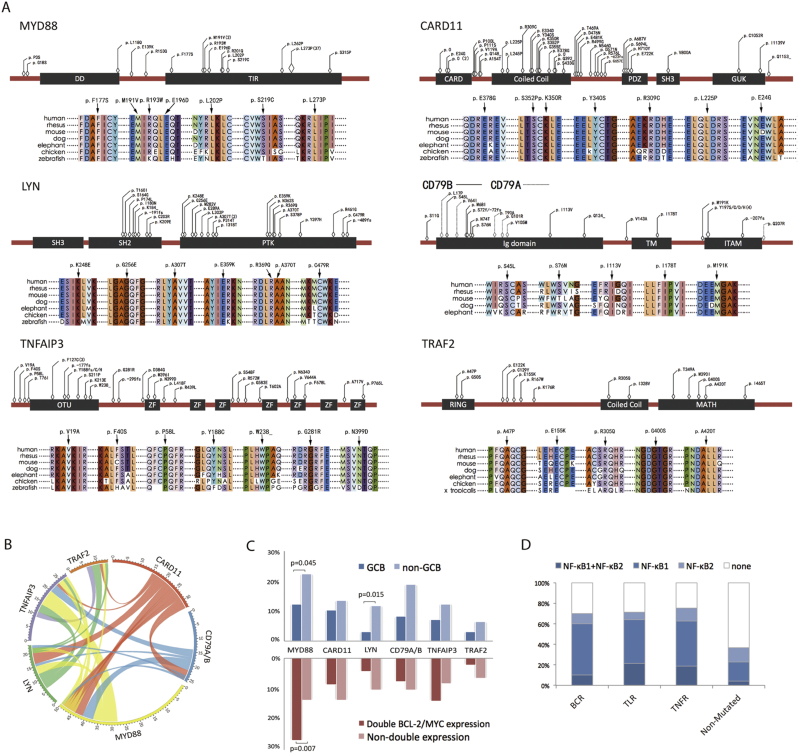
As schematically summarized in Fig. 2D, these mutations were involved in BCRs pathway (BCRs related gene, CARD11, LYN, CD79A and CD79B), TLRs pathway (TLRs related gene, MYD88) and TNFR pathway (TNFR related gene, TRAF2 and TNFAIP3). The most frequent mutations observed were somatic mutations in MYD88, which occurred in 50 out of 275 cases (18.2%). The MYD88 gene encodes an adaptor protein and is composed of an N-terminal Death domain (DD) and a C-terminal Toll-interleukin 1 receptor (TIR) domain (Ngo et al., 2011). All MYD88 mutations were single nucleotide substitutions, mostly situated in the TIR domain, with the prevalent mutation (an L273P substitution, 37 cases) targeting the conserved B-B loop of the TIR domain. The CARD11 mutations (33/275, 12.0%) often affected amino acids within or adjacent to the coiled-coil domain of the protein, which were required for BCR-induced NF-κB activation (Lenz et al., 2008). The LYN mutations (23/275, 8.4%) were located mostly in PTK, as well as the SH2 domain (Scapini et al., 2009, de Miranda et al., 2014). Mutations of the BCRs proximal adaptors CD79B/CD79A occurred in 26 of the 275 patients (9.5%) and targeted both inside and outside the intracellular immunoreceptor tyrosine-based activation motif (ITAM) (Davis et al., 2010). As for TNFR related gene mutations, TNFAIP3 (28/275, 10.2%) and TRAF2 (14/275, 5.1%) mutations were relatively disseminated (Fig. 3A).
Fig. 3.
Mutational patterns of B-cell function genes in diffuse large B-cell lymphoma (DLBCL).
A: Schematic location of B-cell function gene mutations, and sequence alignment of protein across distinct species. B: Circos diagram of the correlation between genes, representing the combinations of mutations in different genes. C: Distribution of gene mutations in patients with germinal center B-cell (GCB)/non-GCB subtype and patients with and without double BCL-2/MYC expression. D: Distribution of NF-κB activation in patients with and without B-cell function gene mutations.
The most frequently concurred pairs of genes were MYD88 and TNFAIP3 (10 concurrence out of 68 cases, 14.7%), CARD11 and LYN (7 concurrence out of 49 cases, 14.2%), LYN and TRAF2 (4 concurrence out of 33 cases, 12.1%), and CARD11 and MYD88 (9 concurrence out of 74 cases, 12.1%, Fig. 3B). Regarding pathological features, 92/175 (52.6%) of the non-GCB patients harbored at least one mutation, significantly higher than those of the GCB patients (29/100, 29.0%, p < 0.001). In the non-GCB group, significantly increased proportion of MYD88 (38/175 vs 12/100, p = 0.045) and LYN (20/175 vs 3/100, p = 0.015) mutations were observed, as compared to the GCB group (Fig. 3C). As for double BCL-2/MYC expression group, MYD88 mutations happened more frequently than those without double expression (26.9% vs 13.7%, p = 0.007, Fig. 3C).
To determine the role of B-cell function gene mutations on NF-κB pathway, immunostaining of nuclear NF-κB1 (p105/p50, classical pathway) and NF-κB2 (p100/p52, alternative pathway) were performed on 98 patients, including 49 mutated and 49 non-mutated cases with matched clinical and pathological characteristics (Supplementary table 2). Significantly higher fraction of nuclear NF-κB-positive cells (> 30%) was observed in tumors of patients with mutation (34/49, 69.4%) than those without mutation (18/49, 36.7%, p = 0.002). The distribution of NF-κB positivity among the three pathways was also similar (Fig. 3D). These results indicated that B-cell function gene mutations are biologically functional and contribute to NF-κB activation.
3.3. Treatment Response
In the univariate analysis, the clinical and pathological factors significantly associated with a lower probability of achieving CR were age > 60 years [odds ratio (OR) = 1.455, 95% confidence interval (CI) 1.182–1.791, p = 0.001], poor performance status (OR = 3.470, 95%CI 2.301–5.233, p < 0.001), advanced Ann Arbor stage (OR = 1.632, 95%CI 1.428–1.866, p < 0.001), elevated serum LDH level (OR = 2.076, 95%CI 1.792–2.406, p < 0.001), multiple extranodal involvement (OR = 2.151, 95%CI 1.598–2.897, p < 0.001) and double BCL-2/MYC expression (OR = 1.335, 95%CI 1.038–1.718, p = 0.040). In addition, IPI, R-IPI and NCCN-IPI correlated with remission status (p all < 0.001, Table 1). Regarding IPI, the CR rate for low, low-intermediate, intermediate-high and high-risk patient were 91.5%, 77.2%, 58.5% and 47.7%, respectively. Similarly, CR rate for very good, good and poor R-IPI were 94.5%, 83.3% and 53.8%, respectively. CR rate for low, low-intermediate, intermediate-high and high-risk NCCN-IPI were 90.9%, 85.5%, 60.1% and 42.9%, respectively.
Of note, the TLRs and TNFR related gene mutations were more frequently detected in non-CR than in CR patients (OR = 2.275, 95%CI 1.192–4.340, p = 0.019 and OR = 2.182, 95%CI 1.082–4.398, p = 0.032, respectively, Table 2). In the CR group, 17 of 204 patients presented early relapse within 6 months. BCRs, TLRs and TNFR related genes were mutated in 5 (29.4%), 3 (17.6%) and 1 (5.9%) cases, respectively. Although the mutation incidence was higher than the CR group and lower than the non-CR group, no statistical difference was observed, probably due to the limited number of early relapsed patients.
Table 2.
Mutational profile of B-cell function genes in the patients with DLBCL.
| Mutation | Total (N = 275) | Non-CR (N = 71) | CR (N = 204) |
p valueb | ||
|---|---|---|---|---|---|---|
| Additional rituximab (N = 98) |
Observation (N = 106) | p valuea | ||||
| BCRs related mutations | ||||||
| Positive | 70/275 | 22/71 | 26/98 | 22/106 | 0.409 | 0.268 |
| (25.5%) | (31.0%) | (26.5%) | (20.8%) | |||
| Negative | 205/275 | 49/71 | 72/98 | 84/106 | ||
| (74.5%) | (69.0%) | (73.5%) | (79.2%) | |||
| TLRs related mutation | ||||||
| Positive | 50/275 | 20/71 | 13/98 | 17/106 | 0.693 | 0.019 |
| (18.2%) | (28.2%) | (13.3%) | (16.0%) | |||
| Negative | 225/275 | 51/71 | 85/98 | 89/106 | ||
| (81.8%) | (71.8%) | (86.7%) | (84.0%) | |||
| TNFR related mutations | ||||||
| Positive | 40/275 | 16/71 | 13/98 | 11/106 | 0.664 | 0.032 |
| (14.5%) | (22.5%) | (13.3%) | (10.4%) | |||
| Negative | 235/275 | 55/71 | 85/98 | 95/106 | ||
| (85.5%) | (77.5%) | (86.7%) | (89.6%) | |||
p value indicated difference between additional rituximab and observation.
p value indicated difference between non-CR and CR.
3.4. Survival Analysis
The median follow-up time was 40.5 months (0.6–154.2 months). The 3-year OS of the non-CR patients and the CR patients were 18.4% and 83.3%, respectively.
Among the CR patients, in the univariate analysis, IPI, R-IPI, NCCN-IPI and double BCL-2/MYC expression were significant prognostic factors for both PFS and OS, while COO and BCRs related gene mutations were only for PFS (Table 3). In the multivariate analysis, when R-IPI, IPI, or NCCN-IPI was controlled, double BCL-2/MYC expression and BCRs related gene mutations were independent prognostic factors for PFS (Table 4, Supplementary table 3 and Supplementary table 4). The 3-year PFS rate was 70.9% and 85.8% for patients in remission with and without double BCL-2/MYC expression and 3-year PFS rate was 68.6% and 79.5% for patients positive or negative for BCRs related gene mutations, respectively.
Table 3.
Univariate analysis of predictors of progression-free survival (PFS) and overall survival (OS) in CR patients with DLBCL.
| Variable | PFS |
OS |
||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |||
| Gender | ||||||||
| Male vs female | 0.893 | 0.618 | 1.289 | 0.545 | 1.028 | 0.651 | 1.623 | 0.905 |
| International Prognostic Index (IPI) | ||||||||
| Low/low-intermediate/intermediate-high/high | 1.930 | 1.628 | 2.287 | < 0.001 | 2.210 | 1.790 | 2.728 | < 0.001 |
| Revised International Prognostic Index (R-IPI) | ||||||||
| Very good/good/poor | 2.467 | 1.861 | 3.272 | < 0.001 | 3.139 | 2.179 | 4.524 | < 0.001 |
| National Comprehensive Cancer Network (NCCN)-IPI | ||||||||
| Low/low-intermediate/ Intermediate-high/high |
2.081 | 1.631 | 2.656 | < 0.001 | 2.397 | 1.784 | 3.221 | < 0.001 |
| Cell of origin | ||||||||
| GCB vs non-GCB | 0.557 | 0.346 | 0.896 | 0.016 | 0.701 | 0.402 | 1.225 | 0.212 |
| Double BCL-2/MYC expression | ||||||||
| Positive vs negative | 1.971 | 1.260 | 3.082 | 0.003 | 2.284 | 1.302 | 4.007 | 0.004 |
| Ki-67 > 80% | ||||||||
| Yes vs no | 0.875 | 0.569 | 1.343 | 0.541 | 1.366 | 0.792 | 2.356 | 0.262 |
| BCRs related mutations | ||||||||
| Positive vs negative | 2.239 | 1.251 | 4.008 | 0.007 | 1.651 | 0.818 | 3.329 | 0.162 |
| TLRs related mutations | ||||||||
| Positive vs negative | 1.712 | 0.874 | 3.352 | 0.117 | 1.581 | 0.688 | 3.638 | 0.281 |
| TNFR related mutations | ||||||||
| Positive vs negative | 1.337 | 0.649 | 2.753 | 0.431 | 1.662 | 0.753 | 3.669 | 0.209 |
| Additional Rituximab | ||||||||
| Yes vs no | 0.811 | 0.564 | 1.166 | 0.259 | 0.836 | 0.533 | 1.312 | 0.436 |
Table 4.
Multivariate analysis of predictors of progression-free survival (PFS) and overall survival (OS) in CR patients with DLBCL controlled by Revised International Prognostic Index (R-IPI).
| Variable | PFS |
OS |
||||||
|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | p value | RR | 95% CI | p value | |||
| R-IPI | ||||||||
| Very good/good/poor | 2.289 | 1.486 | 3.526 | < 0.001 | 2.723 | 1.617 | 4.585 | < 0.001 |
| Double BCL-2/MYC expression | ||||||||
| Positive vs negative | 2.266 | 1.293 | 3.973 | 0.004 | 2.140 | 1.098 | 4.172 | 0.025 |
| BCRs related mutations | ||||||||
| Positive vs negative | 2.192 | 1.209 | 3.973 | 0.010 | ||||
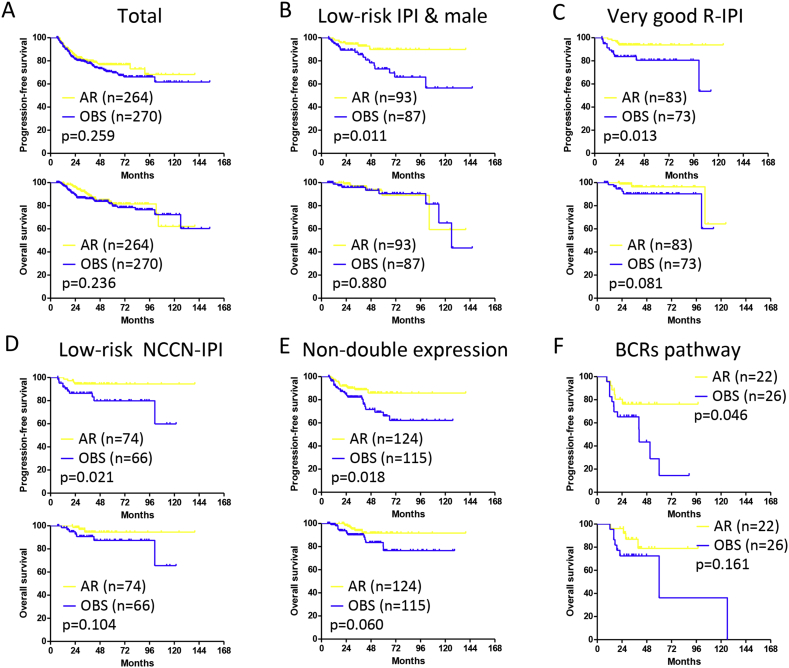
In terms of treatment, the 3-year PFS and OS rate were 79.6% and 89.7% in the AR group, 77.8% and 86.2% in the OBS group, respectively (Fig. 4A). In male patients with low-risk IPI, as well as patients with very good R-IPI and low-risk NCCN-IPI, remarkable improvement of 3-year PFS were observed in the AR group, as compared to those of the OBS group (p = 0.011, p = 0.013, and p = 0.021, respectively), while 3-year OS remained similar (Fig. 4B–D). Moreover, in the subgroup negative for double BCL-2/MYC expression, 3-year PFS was significantly higher in the AR group than in the OBS group (p = 0.018, Fig. 4E). According to B-cell function genes, 3-year PFS of the patients with BCRs related gene mutations was also improved in the AR arm (p = 0.046, Fig. 4F).
Fig. 4.
Progression-free survival and overall survival curves of patients with diffuse large B-cell lymphoma receiving additional rituximab (AR) or observation (OBS).
A: Total patients. B: Male patients with low-risk International Prognostic Index (IPI). C: Patients with very good revised IPI (R-IPI). D: Patients with low-risk National Comprehensive Cancer Network (NCCN)-IPI. E: Patients without double BCL-2 and MYC expression. F: Patients with B-cell receptors (BCRs) related gene mutations.
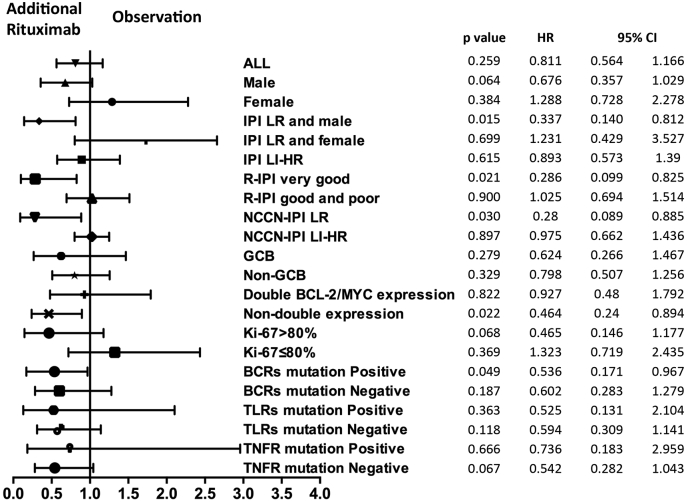
In a Forest plot of univariate analysis on PFS, a favorable response to AR was noted in male patients with low-risk IPI, in patients with very good R-IPI and low-risk NCCN-IPI, subgroup negative for double BCL-2/MYC expression, and with BCRs related gene mutations (p = 0.015, p = 0.021, p = 0.030, p = 0.022, and p = 0.049, respectively, Fig. 5).
Fig. 5.
Forest plot of univariate analysis on progression-free survival of selected subgroups.
A shift to the left favored additional rituximab. X-axis: Hazard ratio. IPI: International Prognostic Index; LR: low-risk; HR: high-risk; R-IPI: Revised IPI; NCCN-IPI: National Comprehensive Cancer Network-IPI GCB: germinal center B-cell; BCRs: B-cell receptors; TLRs: Toll-like receptors; TNFR: tumor necrotic factor receptor.
4. Discussion
Prolonged rituximab administration was adopted by several studies in de novo DLBCL patients in the first remission (reviewed in Table 5) (Jaeger et al., 2015, Witzens-Harig et al., 2015, Huang et al., 2012, Habermann et al., 2006). Instead of rituximab maintenance up to three years, we applied rituximab consolidation with two additional doses on patients' intention when CR was achieved. Consistent with the results of rituximab maintenance in the NHL13 trial (Jaeger et al., 2015), the PFS of male DLBCL patients with low-risk IPI was improved by rituximab consolidation in our study. Moreover, irrespective of gender, very good R-IPI or low-risk NCCN-IPI patients also achieved the best outcome with rituximab consolidation. Although prospective study is needed for further confirmation, we proposed that low-risk DLBCL may benefit from short-term rituximab consolidation, without long-term rituximab maintenance.
Table 5.
Comparison of our study and previous studies on prolonged administration of rituximab in DLBCL.
|
Jaeger et al., 2015 Prospective |
Witzens-Harig et al., 2015 Prospective |
Huang et al., 2012 Retrospective |
Habermann et al., 2006 Prospective |
Xu et al. Retrospective |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Patients | De novo DLBCL and FL3B |
B-cell lymphoma, primary and relapsed |
De novo DLBCL | De novo DLBCL, 60 years or older |
De novo DLBCL | ||||
| N | 683 | 321 | 207 | 415 | 534 | ||||
| Rituximab | Every 2 months × 12 doses | Every 3 months × 12 doses | Every month × 12 doses | 16 doses in 6 months | Every month × 2 doses | ||||
| End point | EFS | PFS | RFS | OS | PFS | OS | FFS | PFS | OS |
| All | − | + | − | + | + | − | − | − | − |
| Male | + | + | + (in DLBCL) | − | NA | NA | NA | − | − |
| Male & IPI ≤ 1 | − | + | NA | NA | NA | NA | NA | + | − |
| IPI ≥ 3 | NA | NA | NA | NA | − | + | NA | − | − |
| CHOP as induction therapy | NA | NA | NA | NA | NA | NA | + | NA | NA |
| Negative for double BCL-2/MYC expression | NA | NA | NA | NA | NA | NA | NA | + | − |
| BCRs related gene mutations | NA | NA | NA | NA | NA | NA | NA | + | − |
NA: not available.
Besides the clinical prognostic indexes, no subgroup analysis of immunophenotypic and genetic characteristics has been performed on response to rituximab. Double BCL-2/MYC expression has recently been recognized as an adverse prognostic factor in DLBCL patients, presenting with 5-year PFS and OS only as 30%, irrespective of CR status (Hu et al., 2013). Our study further revealed that even after CR is achieved, double BCL-2/MYC expression still correlated with poor disease outcome. Interestingly, MYD88 mutation was related to double BCL-2/MYC expression in our study, which could be explained by MYD88-mediated upregulation of BCL-2 (Knittel et al., 2016) and MYC (Rousseau and Martel, 2016). In terms of treatment, the improvement of PFS was observed in DLBCL patients negative for double BCL-2/MYC expression, suggesting another indication for short-term rituximab consolidation. Conversely, prolonged rituximab treatment failed to alter the aggressive clinical course of DLBCL with double BCL-2/MYC expression, which may induce marked stroma- and proliferation-associated genes (Hu et al., 2013) like SPARC (Meyer et al., 2011) and LMO2 (Green et al., 2016) and might confer resistance to rituximab-containing therapy.
Meanwhile, to our knowledge, this is the first report of B-cell function gene mutation profile in a large cohort of Chinese DLBCL patients treated with R-CHOP. Presenting with similar incidence as Western population, B-cell function gene mutations were closely related to DLBCL progression, particularly those involved in TLRs and TNFR pathways, resulting in aberrant activation of NF-κB cascade and resistance to immunochemotherapy in DLBCL (Davis et al., 2010). Clinical trials simply targeting NF-κB have been attempted recent years in patients but most of the results were disappointing (Offner et al., 2015, Offner et al., 2016). Here we provided evidence that, among mutations involving NF-κB activation, the BCRs pathway, rather than the TLRs and TNFR pathway, was inhibited by rituximab, in consistence with previous basic study (Kheirallah et al., 2010). Thus, the presence of BCR/NF-κB mutations may be more precise in guiding the response to rituximab and referred as a major consideration on rituximab consolidation. As for the TLRs and TNFR related gene mutations, they reflected poor therapeutic response and represented actionable targets for new therapeutic approaches like the BTK inhibitor ibrutinib (Wilson et al., 2015) and IRAK1/4 inhibitor for MYD88 (Li et al., 2015), as well as proteasome inhibitors bortezomib and carfilzomib for TNFAIP3 (Shembade et al., 2010).
In conclusion, the identification of B-cell function gene mutations helped to elucidate molecular heterogeneity in DLBCL. Rituximab consolidation may decrease the risk of relapse in patients with BCRs related gene mutations, as well as those with low-risk and subgroup negative for double BCL-2/MYC expression, providing clues for risk stratification treatment of DLBCL.
Funding
This study was supported, in part, by research funding from the National Natural Science Foundation of China (81325003, 81520108003, 81670716 and 81201863), the Shanghai Commission of Science and Technology (14430723400, 14140903100 and 16JC1405800), Shanghai Municipal Education Commission Gaofeng Clinical Medicine Grant Support (20152206 and 20152208), Multi-center clinical research project by Shanghai Jiao Tong University School of Medicine (DLY201601), SMC-Chen Xing Scholars Program, Chang Jiang Scholars Program, Collaborative Innovation Center of Systems Biomedicine and the Samuel Waxman Cancer Research Foundation.
Authorship
P-PX performed the study, collected and analyzed data, and wrote the article. H-JZ performed the experiment and collected clinical data. Y-HH performed the experiment and analyzed the sequencing data. XD-G performed the experiment. XZ collected the tumor samples and related information. YS and SC supervised the clinical data. J-YH analyzed the sequencing data. S-JC and LW supervised the study. W-LZ designed and supervised the study, and wrote the article.
Disclosures
All authors declare no competing interests.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.01.027.
Appendix A. Supplementary Data
Supplementary tables
References
- Alizadeh A.A., Eisen M.B., Davis R.E. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- Cheson B.D., Horning S.J., Coiffier B. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J. Clin. Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- Cheson B.D., Pfistner B., Juweid M.E. Revised response criteria for malignant lymphoma. J. Clin. Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- Compagno M., Lim W.K., Grunn A. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature. 2009;459:717–721. doi: 10.1038/nature07968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R.E., Ngo V.N., Lenz G. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Miranda N.F., Georgiou K., Chen L. Exome sequencing reveals novel mutation targets in diffuse large B-cell lymphomas derived from Chinese patients. Blood. 2014;124:2544–2553. doi: 10.1182/blood-2013-12-546309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T.M., Jensen A.K., Holst R. Multiplex polymerase chain reaction-based prognostic models in diffuse large B-cell lymphoma patients treated with R-CHOP. Br. J. Haematol. 2016;174:876–886. doi: 10.1111/bjh.14138. [DOI] [PubMed] [Google Scholar]
- Green T.M., Young K.H., Visco C. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J. Clin. Oncol. 2012;30:3460–3467. doi: 10.1200/JCO.2011.41.4342. [DOI] [PubMed] [Google Scholar]
- Habermann T.M., Weller E.A., Morrison V.A. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J. Clin. Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- Hans C.P., Weisenburger D.D., Greiner T.C. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- Honma K., Tsuzuki S., Nakagawa M. TNFAIP3/A20 functions as a novel tumor suppressor gene in several subtypes of non-Hodgkin lymphomas. Blood. 2009;114:2467–2475. doi: 10.1182/blood-2008-12-194852. [DOI] [PubMed] [Google Scholar]
- Horn H., Ziepert M., Becher C. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood. 2013;121:2253–2263. doi: 10.1182/blood-2012-06-435842. [DOI] [PubMed] [Google Scholar]
- Hu S., Xu-Monette Z.Y., Tzankov A. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood. 2013;121:4021–4031. doi: 10.1182/blood-2012-10-460063. (quiz 4250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B.T., Zeng Q.C., Yu J. How to determine post-RCHOP therapy for risk-tailored adult patients with diffuse large B-cell lymphoma, addition of maintenance rituximab or observation: multicenter experience. J. Cancer Res. Clin. Oncol. 2012;138:125–132. doi: 10.1007/s00432-011-1074-1. [DOI] [PubMed] [Google Scholar]
- IPI A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N. Engl. J. Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- Jaeger U., Trneny M., Melzer H. Rituximab maintenance for patients with aggressive B-cell lymphoma in first remission: results of the randomized NHL13 trial. Haematologica. 2015;100:955–963. doi: 10.3324/haematol.2015.125344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson N.A., Slack G.W., Savage K.J. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J. Clin. Oncol. 2012;30:3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirallah S., Caron P., Gross E. Rituximab inhibits B-cell receptor signaling. Blood. 2010;115:985–994. doi: 10.1182/blood-2009-08-237537. [DOI] [PubMed] [Google Scholar]
- Knittel G., Liedgens P., Korovkina D. B-cell-specific conditional expression of Myd88p.L252P leads to the development of diffuse large B-cell lymphoma in mice. Blood. 2016;127:2732–2741. doi: 10.1182/blood-2015-11-684183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz G., Davis R.E., Ngo V.N. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 2008;319:1676–1679. doi: 10.1126/science.1153629. [DOI] [PubMed] [Google Scholar]
- Leonard J.P., Kolibaba K., Reeves J.A. Randomized phase 2 open-label study of R-CHOP+/− bortezomib in patients (Pts) with untreated non-germinal center B-cell-like (non-GCB) subtype diffuse large cell lymphoma (DLBCL): results from the pyramid trial ( NCT00931918) [ASH abstract 811]. Blood. 2016;126(suppl 23.) [PubMed] [Google Scholar]
- Li Z., Younger K., Gartenhaus R. Inhibition of IRAK1/4 sensitizes T cell acute lymphoblastic leukemia to chemotherapies. J. Clin. Invest. 2015;125:1081–1097. doi: 10.1172/JCI75821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer P.N., Fu K., Greiner T. The stromal cell marker SPARC predicts for survival in patients with diffuse large B-cell lymphoma treated with rituximab. Am. J. Clin. Pathol. 2011;135:54–61. doi: 10.1309/AJCPJX4BJV9NLQHY. [DOI] [PubMed] [Google Scholar]
- Ngo V.N., Young R.M., Schmitz R. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner F., Samoilova O., Osmanov E. Frontline rituximab, cyclophosphamide, doxorubicin, and prednisone with bortezomib (VR-CAP) or vincristine (R-CHOP) for non-GCB DLBCL. Blood. 2015;126:1893–1901. doi: 10.1182/blood-2015-03-632430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau S., Martel G. Gain-of-function mutations in the toll-like receptor pathway: TPL2-mediated ERK1/ERK2 MAPK activation, a path to tumorigenesis in lymphoid neoplasms? Front. Cell Dev. Biol. 2016;4:50. doi: 10.3389/fcell.2016.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi D., Ciardullo C., Gaidano G. Genetic aberrations of signaling pathways in lymphomagenesis: revelations from next generation sequencing studies. Semin. Cancer Biol. 2013;23:422–430. doi: 10.1016/j.semcancer.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Scapini P., Pereira S., Zhang H. Multiple roles of Lyn kinase in myeloid cell signaling and function. Immunol. Rev. 2009;228:23–40. doi: 10.1111/j.1600-065X.2008.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehn L.H., Berry B., Chhanabhai M. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857–1861. doi: 10.1182/blood-2006-08-038257. [DOI] [PubMed] [Google Scholar]
- Shembade N., Ma A., Harhaj E.W. Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science. 2010;327:1135–1139. doi: 10.1126/science.1182364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SH S., editor. International Agency for Research on Cancer. fourth ed. World Health Organization classification of tumors; Lyon: 2008. WHO classificaiton of tumors of haematopoietic and lymphoid tissues. [Google Scholar]
- Wilson W.H., Young R.M., Schmitz R. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat. Med. 2015;21:922–926. doi: 10.1038/nm.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzens-Harig M., Benner A., McClanahan F. Rituximab maintenance improves survival in male patients with diffuse large B-cell lymphoma. Results of the HD2002 prospective multicentre randomized phase III trial. Br. J. Haematol. 2015;171:710–719. doi: 10.1111/bjh.13652. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Sehn L.H., Rademaker A.W. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123:837–842. doi: 10.1182/blood-2013-09-524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables