Abstract
Although multiple sclerosis (MS) is considered to be a CD4, Th17-mediated autoimmune disease, supportive evidence is perhaps circumstantial, often based on animal studies, and is questioned by the perceived failure of CD4-depleting antibodies to control relapsing MS. Therefore, it was interestingly to find that current MS-treatments, believed to act via T cell inhibition, including: beta-interferons, glatiramer acetate, cytostatic agents, dimethyl fumarate, fingolimod, cladribine, daclizumab, rituximab/ocrelizumab physically, or functionally in the case of natalizumab, also depleted CD19 +, CD27 + memory B cells. This depletion was substantial and long-term following CD52 and CD20-depletion, and both also induced long-term inhibition of MS with few treatment cycles, indicating induction-therapy activity. Importantly, memory B cells were augmented by B cell activating factor (atacicept) and tumor necrosis factor (infliximab) blockade that are known to worsen MS. This creates a unifying concept centered on memory B cells that is consistent with therapeutic, histopathological and etiological aspects of MS.
Keywords: Autoimmunity, Disease modifying treatment, Immunotherapy, Multiple sclerosis, Memory B cell
Abbreviations: AIDS, acquired immunodeficiency syndrome; APRIL, a proliferation-inducing ligand; BAFF, B cell activating factor; (BLyS), B lymphocyte stimulator; CNS, central nervous system; DMD, disease modifying drug; EAE, experimental autoimmune encephalomyelitis; EBV, Epstein Barr virus; Gd +, gadolinium-enhancing; HIV, human immunodeficiency virus; HSCT, hematopoietic stem cell therapy; IL, interleukin; mAb, monoclonal antibodies; MRI, magnetic resonance imaging; MS, multiple sclerosis; TNF, tumor necrosis factor
Highlights
-
•
Memory B cells activity is consistent with the etiology, pathology and therapy of MS, thought to be T cell-mediated.
-
•
Deletion of memory B cells occurs with all effective MS treatments and is more marked with high-efficacy treatments.
-
•
Drugs that worsen MS, can also increase memory B cell production.
1. Introduction
Multiple sclerosis (MS) is the major inflammatory demyelinating disease of the grey and white matter of the central nervous system (CNS), leading to neurodegeneration and the accumulation of disability (Compston and Coles, 2002, Compston and Coles, 2008). It is clear that MS is a complex disease influenced by a large number of immune- associated genes, notably major histocompatibility complex class II alleles and sex chromosomes (Compston and Coles, 2008, Sawcer et al., 2014). However, the discordance between identical twins clearly indicates that any genetic susceptibility is heavily influenced by environmental influences (Compston and Coles, 2008). These include: a geographical/latitude effect relating to sunlight exposure; lifestyle effects including diet, education and smoking and an infection effect; virtually all people with MS have been infected with Epstein Barr Virus (EBV), which may be a key trigger in susceptibility to MS (Compston and Coles, 2002, Compston and Coles, 2008, Giovannoni and Ebers, 2007). Whilst pathology helps elucidate disease mechanisms (Compston and Coles, 2002, Compston and Coles, 2008) perhaps the most informative method is via the analysis of the response or lack of response to disease modifying drugs (DMD), with consideration to the trial design and implementation (Baker and Amor, 2014), and the adverse responses to DMD (Deiß et al., 2013, Marta and Giovannoni, 2012).
2. Inflammatory and Neurodegenerative Disease in MS
This approach to disease mechanisms often defines a two immune-compartmental model of MS (Fig. 1): (a) A peripheral compartment that drives relapsing disease and is associated with entry of mononuclear cells and plasma proteins into the CNS and (b) an intrathecal/CNS compartment that supports further white matter and grey matter demyelination and the loss of nerve circuitry that drives the neurodegeneration associated with progressive MS (showing deterioration without obvious relapses) (Lublin et al., 2014), and accumulating disability (Compston and Coles, 2002, Compston and Coles, 2008, Lublin et al., 2014). As such MS has been viewed as both an autoimmune and neurodegenerative disease requiring different treatments (Compston and Coles, 2002, Compston and Coles, 2008). However, these events are inter-related and occur concurrently from disease onset (Giovannoni et al., 2017) and it is clear that immunomodulation/suppression may be sufficient to control both relapsing and active progressive elements of MS (Steinman and Zamvil, 2016), which may slow deterioration to systems with sufficient neural reserve (Giovannoni et al., 2017, Steinman and Zamvil, 2016). However, pathology and responses to therapy indicate that targeting the peripheral component without change in the central compartment, is often insufficient to control more advanced worsening MS (Fig. 1) (Compston and Coles, 2002, Compston and Coles, 2008, Giovannoni et al., 2017). Thus, optimal disease control is likely to require neuroprotection and repair strategies in addition to immunomodulation to the limit the accumulation of disability (Compston and Coles, 2002, Compston and Coles, 2008, Giovannoni et al., 2017). Current DMD, largely target the peripheral immune component with the view of terminating focal inflammatory-relapse and/or magnetic resonance imaging (MRI) activity (Fig. 1) (Marta and Giovannoni, 2012). Although there is an increasing number of agents available to treat relapsing MS (Marta and Giovannoni, 2012, Martin et al., 2016), failure of trials by immunosuppressive agents was a common problem, until the methods to perform and monitor phase II (based on accumulation of gadolinium-enhancing (Gd +) T1 and new T2 lesions in MRI, respectively, and phase III trials (outcomes based on relapses) were improved and implemented (Compston and Coles, 2002, Compston and Coles, 2008, Marta and Giovannoni, 2012). For this reason many drugs failed, as they were tested in people with advanced progressive MS who respond poorly or too slowly to immunosuppressive agents that control inflammatory relapsing MS (Coles et al., 1999, Compston and Coles, 2002, Giovannoni et al., 2017). This is best seen with hematopoietic stem cell therapy (HSCT) where treatment is most effective in people with active inflammatory disease with Gd + lesions and clinical relapses (Atkins et al., 2016, Burt et al., 2015). This suggests that once neurodegeneration is triggered within a neural circuit, probably through innate immune activation, it may no longer respond to the therapies that halt the relapses that trigger the damage (Compston and Coles, 2002, Giovannoni et al., 2017, Hampton et al., 2013). This neurodegenerative process is detectable from the initial attacks (De Stefano et al., 2010, Giovannoni et al., 2017), but clinical progressive deterioration may only become noticed once the compensating neural reserve within affected pathways become exhausted (Giovannoni et al., 2016a, Giovannoni et al., 2017). This can occur early as in primary progressive MS or following a number of attacks in secondary progressive MS (progressive worsening following a period of relapsing attacks) (Compston and Coles, 2002, Giovannoni et al., 2016a, Lublin et al., 2014). Importantly, this argues for early and effectively treatment to maintain brain health (Giovannoni et al., 2016a).
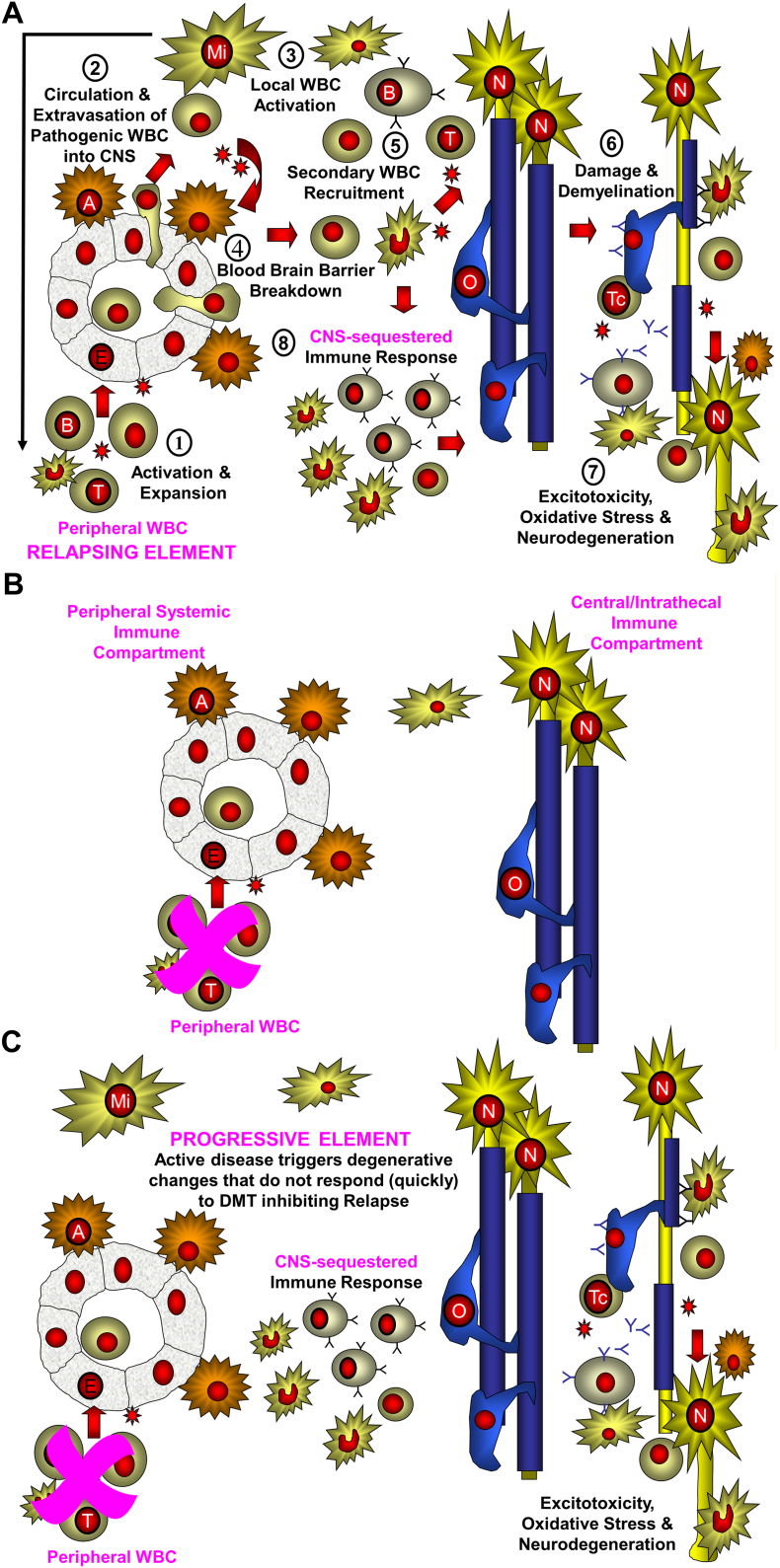
Fig. 1.
Two immune-compartment model of multiple sclerosis.
The initial trigger of the lesions is due to: (a) peripheral sensitization due to molecular mimicry or another event in the lymph node (outside-in) or (b) oligodendrocyte damage leading to liberation of antigen proteins or peptides that exit via the glymphatics to draining lymph nodes (inside-out) where autoreactive lymphocytes are sensitized. A. 1. Primed T and B cells are generated and travel round the body. 2. Immune cells enter into the CNS. 3. Following recognition of a target presented by a perivascular microglial (Mi) cell there is local activation of the infiltrating lymphocytes. 4. Cytokine release occurs to activate the blood brain barrier to express adhesion molecules. 5. A second wave of influx of T cells, B cells and monocytes enters the CNS. 6. These cause damage to the oligodendrocyte (O) via release of antibodies, and soluble products and possibly by direct killing by cytotoxic T cells (Tc). 7. Demyelinated nerves (N) have an elevated energy requirement to maintain neurotransmission. These are vulnerable to excitotoxic and other damage elements such as by activated microglial cells, and B cell products. 8. Microglial and B cells are sequestered into CNS compartment. B. Current DMD prevent entry of the peripheral adaptive immune cells into the CNS. This will block relapsing disease allowing natural repair mechanisms to act and induce a long-term status of no evidence of disease activity. C. These events produce an innate inflammatory environment formed from glial cells and adaptive immune niches, such as B cell infiltrates are created within the CNS. These may not responsive to peripheral immune control and may allow neurodegeneration and accumulation of disability to continue in the absence of active lesion formation.
3. T Cell-specific Immunotherapies Have Proved Ineffective at Blocking Relapsing MS
The question remains about the nature of the peripheral target for immunotherapy. There is abundant evidence to suggest that MS is a mainly CD4 Th1/Th17 T-cell mediated disease (Martin et al., 2016). This concept is largely based on autoimmune experimental encephalomyelitis (EAE) studies in animals (Martin et al., 2016, Rostami and Ciric, 2013, Volpe et al., 2015). Surprisingly whilst all treatments that affect MS can influence T cell function and T cell subset distribution (Martin et al., 2016), clinical trial data with specific CD4, Th1/Th17 immunotherapies have all largely failed to exhibit more than marginal impact on relapsing MS (Deiß et al., 2013, Segal et al., 2008, van Oosten et al., 1997). This may argue against a significant role for CD4 T cells in the control of MS. However, CD4-depletion studies were undertaken when HIV/AIDS mechanisms uncovered the risks of CD4 lymphopenia, therefore deletion was targeted to maintain CD4 T cell numbers above 250 cells/μL. Although there was some effect on relapse rate, the trials failed to show an effect in reducing new MRI lesion formation, with about a 60–70% CD4 T cell depletion (van Oosten et al., 1997). In the animal model, > 85% CD4 T cell depletion inhibits EAE and depletion of 30% exhibits essentially no effect, whereas about a 60% depletion exhibits a marginal effect in an optimized system (von Kutzleben et al., 2016). This therefore creates a concern that the human studies failed to deplete sufficiently to control disease.
Likewise, blockade of interleukin (IL)-12 and IL-23 with ustekinumab to inhibit Th1 and Th17 did not significantly affect the MRI lesion load in MS (Segal et al., 2008). Again, whilst blockade of IL-12/23-P40 inhibits the induction of EAE, it fails to inhibit spontaneous relapses, which is more relevant to use in MS (Heremans et al., 1999). Blockade of IL-17 in EAE usually exhibits a modest inhibitory effect (Kap et al., 2011, Mardiguian et al., 2013) and perhaps not surprisingly blockade of IL-17 with secukinumab only inhibited new MRI lesions by 49% and Gd + lesions by 67% compared to placebo from 4 to 24 weeks (Deiß et al., 2013), which is similar or worse to that achieved with other low-moderately effective DMD (Arnold et al., 2014). Highly effective DMD inhibit MRI lesion formation by over 85–90% (Kappos et al., 2011). Alemtuzumab has high efficacy in active relapsing MS and depletes 70–95% CD4 T cells during the whole course of the pivotal clinical trials (Cohen et al., 2012, Kasper et al., 2013). This suggests that, based on response to treatments, one should not completely dismiss the possibility that MS is a T cell-mediated disease as active immunotherapeutics all influence T cell activity (Martin et al., 2016). However, upon dissection of the impact of other effective DMD, a mechanism of action on B lymphocytes (Fig. 2) is perhaps more compelling (Fig. 2) (Disanto et al., 2012). CD20-specific B cell depleting agents were clearly active at inhibiting, not only new MRI lesion formation, but relapses and the accumulation of disability (Hauser et al., 2008, Kappos et al., 2011, Sorensen et al., 2014).
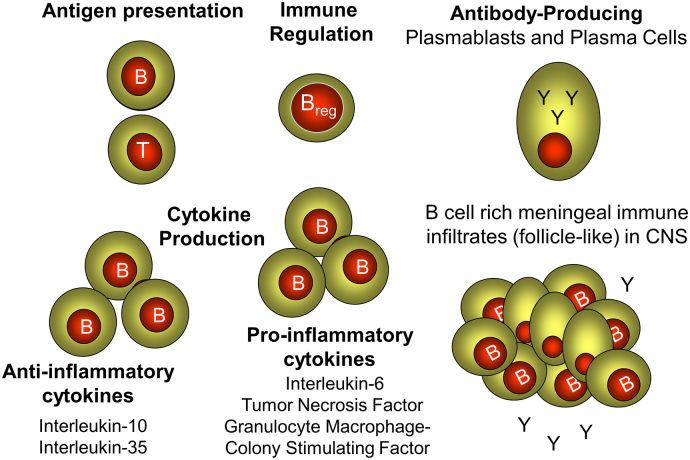
Fig. 2.
Potential B cell functions in multiple sclerosis.
B cells can exhibit a variety of different functions (bold) that may influence MS, both in the periphery and in the CNS where follicle-like structures accumulate during MS.
4. Some MS DMD Exhibit Pleiotropic Effects, but Anti-proliferative Drugs Preferentially Target B Cells Because of Their Enhanced Proliferative Activity
Some DMD exert such pleiotropic effects that their putative mechanisms of action have often followed changes in scientific dogma. As such, their actions have been ascribed to mechanisms such as: CD8 T suppressor cells activity, anergy, Th1 to Th2 switching, T regulatory cell induction (Compston and Coles, 2002, Marta and Giovannoni, 2012, Martin et al., 2016). Whilst many putative mechanisms have their origins in T cell biology (Deiß et al., 2013, Marta and Giovannoni, 2012, Martin et al., 2016), since the efficacy exhibited by CD20-specific monoclonal antibodies (mAb) (Hauser et al., 2008, Kappos et al., 2011, Sorensen et al., 2014), many mechanisms of action have been re-evaluated in the context of B lymphocyte function (Ireland et al., 2014, Rizzo et al., 2016, Schubert et al., 2015). Teriflunomide inhibits both proliferating T and B cells (Li et al., 2013) and the vast majority of cytostatic agents, such as mitoxantrone and cyclophosphamide, described to target activated T cells, actually preferentially inhibit B cells, including memory B cells (Fig. 3), by virtue of their more rapid proliferation kinetics compared to T cells (Duddy et al., 2007, Tangye et al., 2003).
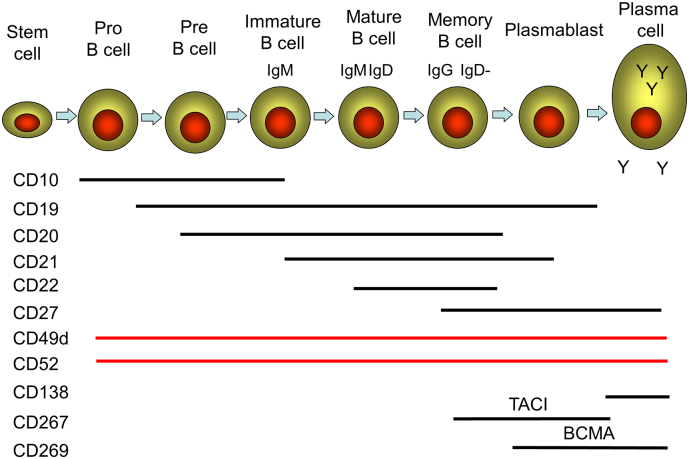
Fig. 3.
B cell lineage and surface marker expression.
A diagram of a simplified development and differentiation pathway of the B cell lineage showing some distinguishing surface markers including ATCI (transmembrane activator and CAML interactor) protein and BCMA (B cell maturation antigen). The memory B cell subset is heterogeneous and includes both unswitched (CD19 +, CD27 +, IgM +, IgD +) and class switched (CD19 +, CD27 +, IgM −, IgD −) memory B cells, which were not individually reported in some of the studies analysed.
Spingosine-1-phosphate receptors, which mediate the action of fingolimod and the alpha-4 integrin (CD49d), blocked by natalizumab, have broad expression not only on lymphocytes but also in other mononuclear cells (Marta and Giovannoni, 2012, Martin et al., 2016). Lymphocyte depleting agents could shed light on the mechanism of highly active disease, but apparent confusion emerges as cladribine and alemtuzumab deplete both T and B cells (Cohen et al., 2012, Kasper et al., 2013, Marta and Giovannoni, 2012, Rieckmann et al., 2009), rituximab/ocrelizumab deplete primarily B cells (Hauser et al., 2008, Kappos et al., 2011, Marta and Giovannoni, 2012), whilst daclizumab inhibits activated T cells and augments natural killer cell function (Kappos et al., 2015, Marta and Giovannoni, 2012, Martin et al., 2016).
5. Inhibiting Memory B Cell Function Blocks Relapsing MS
Alemtuzumab is one of the most effective drugs and can induce long-term no evident disease activity following a 5 then 3 day course of 12 mg/day, one year apart (Cohen et al., 2012, Deiß et al., 2013, Marta and Giovannoni, 2012). Based on lymphocyte effects of alemtuzumab, T cells are most affected and CD19 B cell numbers are normal by 6 months post-treatment (Cohen et al., 2012, Kasper et al., 2013). However, it is evident that the CD19 + B cell response is a composite of different B cell subsets (Fig. 3) and there is early and marked hyper-repopulation of immature B cells followed by a later mature B cell response, with a continued marked depletion of CD19 +, CD27 + B memory cells (Fig. 4) (Thompson et al., 2010). Consequently this raises the question whether T cells or memory B cell depletion mediated the therapeutic effect, especially as it is reported that disease activity is unrelated to CD4/CD8 levels (Kousin-Ezewu et al., 2014). Whilst, the rapid repopulation of immature B cells, during a period of marked depletion of absolute numbers of regulatory T cells (Cox et al., 2005, Thompson et al., 2010), may account for the secondary B cell autoimmunities following alemtuzumab (Cohen et al., 2012, Cox et al., 2005, Krupica et al., 2006), inhibition of MS could relate to the depletion of the memory B cell subsets (Fig. 4) (Thompson et al., 2010). Phenotypic analysis from the oral cladribine studies indicated that CD4 T cells were only depleted in the range of 40–45% and CD8 T cells were depleted by about 15–20% from baseline, over the first year of treatment with effective doses of oral cladribine (Duddy et al., 2007, Giovannoni et al., 2010). In contrast depletion of CD19 B cells was most marked (Duddy et al., 2007).In the oral cladribine studies there was a clear dose-effect between the 5.25 mg/kg and 3.5 mg/kg dose arms in terms of both CD4 and CD8 T cell populations, but no dose-effect in relation to the B-cell population (Duddy et al., 2007). As the 5.25 mg/kg and 3.5 mg/kg dose were equally effective (Giovannoni et al., 2010), this would argue that the effect of cladribine is via B cell depletion.
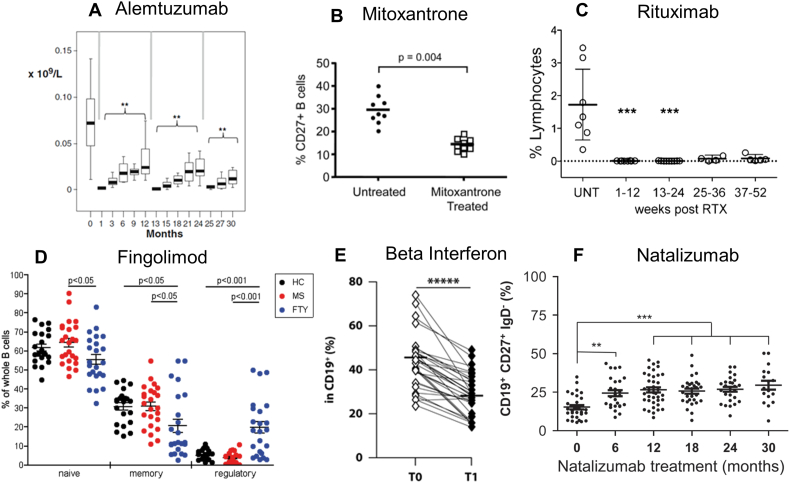
Fig. 4.
Active DMD in MS physically or functionally deplete memory B cell activity.
Depletion of CD19 +, CD27 + memory B cells following treatment of MS with: (A) alemtuzumab (Thompson et al., 2010). The results show the depletion of the absolute numbers of memory B cells over three treatment cycles. **P < 0.01. Reproduced by permission from Springer Science: J. Clinical Immunology. DOI: 10.1007/s10875-009-9327-3. (B) Mitoxantrone (Duddy et al., 2007). The results show percentage reduction in the individual levels of CD19, CD27 memory B cell before (untreated) and one month after treatment. Reproduced by permission American Association of Immunology: Journal of Immunology. DOI: 10.4049/jimmunol.140011845. (C) Rituximab (Palanichamy et al., 2014). The results show the percentage of memory B cells (CD19, CD27, IgD −) in blood before, in untreated (UNT) individuals, and at different time points after rituximab (RTX). ***P < 0.0001. Reproduced by permission American Association of Immunology: Journal of Immunology. DOI: 10.4049/jimmunol.140011845. (D) Fingolimod (Grützke et al., 2015). The results are individual percentage of CD38, CD27 −, CD24 − mature/naïve cells (black); CD38 −, CD24 +, CD27 + memory B cells, CD38 +, CD27 −, CD24 +, CD5 + regulatory B cells in healthy controls (HC. Black dots) and people with multiple sclerosis who were untreated (MS. Red dots) or treated with oral fingolimod (FTY. Blue dots). *P < 0.05. Reproduced under creative commons license CC BY-NC-ND. DOI: 10.1002/acn3.155. (E) Beta interferon (Rizzo et al., 2016). The results show a reduction in the individual percentage of CD19 +, CD27 + B memory cells before (T0) and one month (T1) after weekly treatment. *****P ≤ 0.00001. Reproduced by permission by the Nature publishing group and Macmillan Publishers Ltd.: Immunology & Cell Biology. DOI: 10.1038/icb.2016.55. (Epub 2016 Jun 6). (F) Natalizumab (Planas et al., 2012) induced augmentation of circulating CD19 +, CD27, IgD − memory B cells. The result show individual values at baseline and at various time after natalizumab infusion.**P < 0.01, ***P < 0.001. Reproduced by permission from Wiley-VCH Verlag GmbH: European Journal of Immunology. DOI: 10.1002/eji.201142108.
It has been found that B cell depletion with CD20-specific mAb is effective at inhibiting relapsing MS (Hauser et al., 2008, Kappos et al., 2011, Sorensen et al., 2014) thereby arguing against the importance of targeting T cells to control MS. To reconcile this difference, it has been suggested either these B cell depleting reagents block antigen presentation to T cells to limit their disease-inducing activity (Fig. 2) or that the therapeutic antibodies target T cells (Graves et al., 2014, Martin et al., 2016, Palanichamy et al., 2014). However, whilst CD20 + B cells are markedly depleted following rituximab treatment, CD4 and CD8 T cells populations are depleted only by about 10–25% in the blood (Graves et al., 2014, Palanichamy et al., 2014, Piccio and Naismith, 2010). Unless the cells responsible for driving MS activity consist of a very selective subpopulation, this level of depletion would be insufficient, given the fact that 60–70% depletion of CD4 T cells by CD4-depleting antibody had a marginal impact on relapses (van Oosten et al., 1997). In contrast to the blood levels, analysis of the T and B cell levels within the cerebrospinal fluid has reported a marked (over 50%) reduction of T cells that can occur following rituximab treatment (Piccio and Naismith, 2010). Whilst this could be a reason for effective disease control, we feel this is more likely a consequence of effective disease control, causing both a reduction in T and B cell levels in the CNS (Piccio and Naismith, 2010).
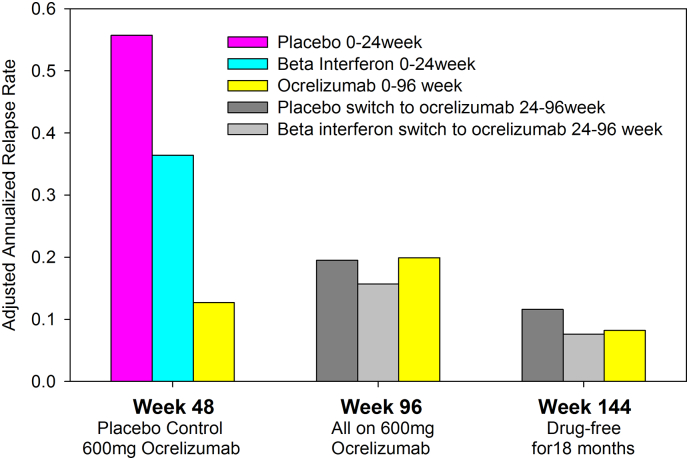
The recommendation for a 6 monthly dosing schedule with rituximab is based on the analysis of CD19 levels that are suppressed for 6 months followed by recovery around 9–12 months after infusion (Palanichamy et al., 2014). However, analysis of immunoglobulin non-class switched (CD19 +, CD27 +, IgD +) and class-switched (CD19 +, CD27 +, IgD −) memory B cell populations confirmed both of these subpopulations are markedly suppressed for over 12 months (Fig. 4) (Palanichamy et al., 2014). This is consistent with observation that relapses were largely suppressed for at least 12 months after the last dose of rituximab (Bar-Or et al., 2008). Furthermore, at clinical trial doses (Kappos et al., 2011), 600 mg ocrelizumab may generate even more marked depletion of CD19 B cells than rituximab (Genovese et al., 2008). Six monthly dosing may thus be too frequent for some people. Indeed based on the phase II ocrelizumab in relapsing-remitting MS trial extension studies (Hauser et al., 2013, Kappos et al., 2011, Kappos et al., 2012), where the initial placebo and interferon beta comparator groups started ocrelizumab at 24 weeks, were treated at 24, 48 and 72 weeks and were followed until week 144 (Hauser et al., 2013, Hughes, 2013, Kappos et al., 2012), the relapse rate (Fig. 5) (Hughes, 2013) and progression of disability (detected in only 6/160 people in the study) remained low for the 18 months after the last dose (Hauser et al., 2013, Kappos et al., 2012). This indicates either a long-term depletion effect or, more likely, an induction effect with long term-efficacy following only a short series of treatments, given the similarity of the putative mechanisms of action with cladribine and alemtuzumab. Nevertheless, repeated dosing may be necessary to maintain control of MS relapses. Indeed, a personalized, repeat-treatment depending on levels of circulating memory B cells rather than total CD19 + B cells may be sufficient to control relapsing disease. This strategy has been successfully applied to rituximab treatment of neuromyelitis optica, a demyelinating disease associated with optic neuritis and myelitis and the presence of pathogenic aquaporin-4-specific antibody (Kim et al., 2011).
Fig. 5.
Long term relapse inhibition following a short course of ocrelizumab.
People with MS were treated on day 1 and day 15 with placebo or 600 mg ocrelizumab and repeated at week 24 as a double injection; at week 48 all participants were switched to a single injection of 600 mg ocrelizumab that was repeated at week 72 Hauser et al., 2013. The results represent the reported adjusted annualized relapse rate at week 48 (placebo-controlled), week 96 (cross-over and extension) and week 144, following an 18 month drug free period (Hughes, 2013).
Daclizumab blocks the high affinity interleukin-2 (IL-2) receptor (CD25), which will inhibit T cell activation, and controls relapsing multiple sclerosis (Gold et al., 2016, Kappos et al., 2015). This was thought to be due to the expansion of CD56high natural killer cells that express the intermediate affinity IL-2 receptor containing the IL-2beta (CD122) and IL-2 gamma (CD132) chains (Gold et al., 2016, Kappos et al., 2015, Marta and Giovannoni, 2012). However, it is interesting that 10-65% of peripheral B cells can express CD25, in addition to CD122 and CD132 (Brisslert et al., 2006). Furthermore, only the memory B cell population expresses CD25, in particular, 60% of CD19 +, CD27 + B cell express CD25 and blockade of IL-2 activity can inhibit B cell activity (Brisslert et al., 2006). As such, it is evident that daclizumab not only depletes cells in the CD4 T cell pool but also significantly affects CD19 + B cells (Lin et al., 2015, Toso et al., 2009). It is therefore possible that the effect of daclizumab may be via a reduction in the activity of B cell memory in MS. Indeed in the transplantation setting, daclizumab decreased the absolute number of B memory cells, but consistent with its lower efficacy, this was less than that observed with alemtuzumab (Toso et al., 2009). In addition, whilst fingolimod is known for sequestration of central memory T cell within lymph nodes, B cells are likewise reduced within a few weeks of treatment onset, with a significant decrease in activated memory B cells (Fig. 4) (Grützke et al., 2015, Nakamura et al., 2014). Likewise, mitoxantrone, causes a drop in the number of peripheral memory B cells (Fig. 4) (Duddy et al., 2007), as does dimethyl fumarate (Lundy et al., 2016). Interestingly the effect was, again, not as marked with alemtuzumab or rituximab (Palanichamy et al., 2014, Thompson et al., 2010) and may account for dimethyl fumarate exhibiting a lower level of activity at inhibiting relapses and MRI lesions, compared to CD20 and CD52 depletion via mAb (Deiß et al., 2013, Martin et al., 2016). Teriflunomide is an anti-proliferative agent that blocks pyrimidine synthesis by inhibition of dihydroorotate dehydrogenase (Marta and Giovannoni, 2012). Teriflunomide is the active metabolite of leflunomide, which produces a modest decrease of memory B cells in rheumatoid arthritis (McComish et al., 2015). In contrast, whilst natalizumab has some influence on circulating T cells and monocytes, B cells and notably circulating memory B cells were increased (Fig. 4) (Planas et al., 2012). This would be consistent with blockade of alpha 4 integrin (CD49d) binding to vascular cell adhesion molecule one (CD105) on inflamed CNS blood vessels (Marta and Giovannoni, 2012, Martin et al., 2016) blocking entry of activated cells into the brain and sequestering them in the periphery (Fig. 4). Whilst these current MS treatments have complex mechanisms of action involving different leukocyte subsets (Marta and Giovannoni, 2012, Martin et al., 2016), this insight provides a unifying hypothesis that all current DMD are active through depletion, or functional inhibition, of cells within the CD19 +, CD27 + B cell memory lineage (Table 1).
Table 1.
MS treatments limit the capacity of memory B cells to enter the CNS.
| MS treatment | Memory B cells in the blood | Availability of B cells to enter CNS | Relapse rate/MRI lesions |
|---|---|---|---|
| Glatiramer acetate | Reduced (Ireland et al., 2014) | Decreased | Decreased |
| Beta Interferon | Reduced (Rizzo et al., 2016) | Decreased | Decreased |
| Dimethyl fumarate | Reduced (Lundy et al., 2016) | Decreased | Decreased |
| Mitoxantrone | Reduced (Duddy et al., 2007) | Decreased | Decreased |
| Fingolimod | Reduced (Grützke et al., 2015) | Decreased | Decreased |
| Natalizumab | Increased (Planas et al., 2012) | Decreased | Decreased |
| Alemtuzumab | Reduced (Thompson et al., 2010) | Decreased | Decreased |
| Daclizumab | Reduced (Gold et al., 2016)* | Decreased | Decreased |
| Rituximab | Reduced (Palanichamy et al., 2014) | Decreased | Decreased |
| Atacicept | Increased (Sergott et al., 2015)* | Increased | Increased |
| Infliximab | Increased (Souto-Carneiro et al., 2009)* | Increased | Increased |
| HSCT | Reduced (Burns et al., 2015)* | Decreased | Decreased |
The known effects of various DMD on the relapse rate and MRI lesion formation in MS and their reported effects, with citation indicated, on the levels of memory cells within the blood in MS, or other conditions where data in MS is lacking*, and the likely impact on such cells entering the CNS.
6. Augmenting B Memory Cell Function can Worsen MS
Following the apparent efficacy of CD20-depleting agents in controlling MS (Hauser et al., 2008, Kappos et al., 2011, Sorensen et al., 2014), alternative methods of depleting B cells have been developed. One approach was the use of CD19 depleting antibodies (MEDI-551), which have a broader range of B cell depletion than CD20-targeting to include plasmablasts (Ward et al., 2011). MEDI-551 effectively blocked the formation of new MRI lesions in a phase II trial (NCT01585766) in MS (Aquis et al., 2015).
Another approach was the neutralization of B cell growth and differentiation factors which are altered in people with MS in particular during relapses (Kannel et al., 2015). Atacicept is a fusion protein composed by the transmembrane activator and calcium modulator and cyclophilin ligand interactor protein (TACI) and the constant region of human IgG1 (Gatto, 2008). Atacicept neutralizes a proliferation-inducing ligand (APRIL) and B lymphocyte stimulator (BLyS)/B cell activating Factor (BAFF), that stimulate B cell numbers, activation and antibody synthesis (Gatto, 2008). However, in trials to control optic neuritis, atacicept precipitated CNS inflammation and augmented the conversion of people with optic neuritis to clinically definite MS (Sergott et al., 2015). Although, the effect on B cell subsets in this trial was not reported (Sergott et al., 2015), it is evident from other studies in humans and mice that whilst atacicept results in a loss of mature B cells, it upregulates interleukin-15 (IL-15) and stimulates memory B cells (Table 1) (Ma et al., 2014, Tak et al., 2008). Therefore, in contrast to memory B cell reduction and disease inhibition seen with other MS treatments, atacicept caused disease worsening in some people with optic neuritis, an effect probably associated with enhanced memory B cell function. Furthermore, a trial (NCT00882999) of tabulumab (Lys2127399), which is a human IgG4 antibody that inhibits both membrane and soluble BAFF/BLyS and augments memory B cell function (Genovese et al., 2013), may have failed or was terminated (Deiß et al., 2013). In addition, tumor necrosis factor (TNF) inhibitors can worsen MS (Lenercept Multiple Sclerosis Study Group and the University, van Oosten et al., 1996). Interestingly, TNF inhibition is associated with augmentation of memory B cells numbers, when used in some people with rheumatoid arthritis or Crohn's disease, who sometimes develop central nervous system demyelinating disease after TNF inhibition (Hyrich et al., 2004, Roll et al., 2012, Souto-Carneiro et al., 2009). In conclusion, evidence shows that drugs that reduce the CNS activity of the subset of CD19 +, CD27 + memory B cell are beneficial for MS and drugs that promote it are not effective and can actually make MS worse or trigger the disease (Table 1). A strategy that specifically targets the memory B cell function but avoids the immature, mature non-memory B cell subsets may improve the benefit:risk ratio (Krupica et al., 2006).
Natalizumab induced circulating memory B cell elevation (Fig. 4) (Planas et al., 2012) is not associated with worsening of MS, whilst it was the case for atacicept (Table 1). This is presumably because memory B cells are prevented from entering the CNS to trigger relapse with natalizumab (Michel et al., 2015), but not atacicept. Alternatively natalizumab could be disturbing critical niches within the bone marrow and lymphoid organs and/or inhibiting an important peripheral immune function (Martin et al., 2016). However, the enhanced levels of memory B cells in the blood may contribute to rapid disease rebound that can occur following cessation of natalizumab (Rasenack and Derfuss, 2016). This would support CD20-depleting agents as the switching agent of choice post-natalizumab to deplete the increased numbers of circulating memory B cells (Giovannoni et al., 2016b). Indeed post-natalizumab treatment with rituximab has been associated with lower relapse levels than with fingolimod switching and is arguably safer than fingolimod as it does affect cytotoxic T cells that may be required to clear subclinical progressive multifocal leukoencephalopathy (Alping et al., 2016, Asztely et al., 2015).
7. Mechanisms of B Cell Activity in MS
In MS, higher BAFF levels are associated with less active disease (Kannel et al., 2015). Conversely, there is disease worsening when BAFF is neutralized with atacicept, which will increase memory B cell numbers (Sergott et al., 2015).Whilst B cell subpopulations fluctuate in MS (Teniente-Serra et al., 2016), it is interesting that people with pediatric MS have generated a substantial pool of memory B cells compared to healthy children and adolescents (Schwarz et al., 2016). Cells within the CD19 +, CD27 + pool may decrease in the blood during relapse and they accumulate within the CNS (Schwarz et al., 2016). Furthermore, increased circulating memory B cell numbers were associated with accumulation of neurodegeneration in relapsing MS (Comabella et al., 2016) and they can be detected in MS post-mortem lesions (Serafini et al., 2010). However, it will be important to determine how this is achieved, as memory B cells can exhibit many different functions (Fig. 2) and the CD19 +, CD27 + B cell population is heterogeneous (Michel et al., 2015). It is possible that these B cells act key antigen-presenting cells to drive T cells to cause the central problems of MS; they do express co-stimulatory molecules and major histocompatibility complex and can present myelin antigens (Harp et al., 2010, Michel et al., 2015, Toso et al., 2009). As such it is of interest that soluble CD27, produced by memory T and B cells, has been proposed as a biomarker of inflammation in MS and is associated with the immunoglobulin index in the cerebrospinal fluid/serum (Hintzen et al., 1991, Komori et al., 2015). Whilst this was associated with T cell activity, it may in fact also relate to memory B cell activity within the CNS tissue (Hintzen et al., 1991). Loss of memory cells may alter the balance in favor of mature cells or regulatory B cells that produce IL-10 and create a more regulatory environment (Duddy et al., 2007, Michel et al., 2015). Alternatively these cells could be forerunner to the formation of plasma cells responsible for the production of pathogenic intra-thecal antibodies. It is clear in MS that autoantibodies exist and are part of the pathology (Agahozo et al., 2016, Michel et al., 2015, Pedotti et al., 2013). Pathogenic antibodies specifically target oligodendrocytes or neurons through antigen binding via the antibody Fab regions or even through activation of innate immune cells via signaling through Fc receptors (Agahozo et al., 2016, Michel et al., 2015, Pedotti et al., 2013). It appears as if the pathogenic memory B cells have been identified (Li et al., 2015). They are the pro-inflammatory granulocyte macrophage-colony stimulating factor producing B cells expressing: CD22, CD24, CD25, CD27, CD49d, CD83, CD180, CD38low, HLA-DR, CCR2, CCR6 (Li et al., 2015), which are increased in MS (9.3 ± 1.4% untreated MS verses 4.4 ± 0.5%) compared to healthy matched controls (Li et al., 2015). Further work, will be needed to determine that this subset is indeed affected by the different MS treatments.
8. Memory B Cells in the Etiology of MS
The data strongly support that the memory B cell population is a key component of the pathological features in MS (Table 1). Although the etiology of MS is thought to be autoimmune, no consistent T or B cell autoantigen has been described, even after analysis of sequences of infiltrating B cells (Blauth et al., 2015, van Nierop et al., 2016). Whilst T and B cell autoimmunity is clearly present in people with MS (Agahozo et al., 2016, Blauth et al., 2015, van Noort et al., 2010), this may be secondary to damage rather than being the primary driver of the disease. As such, the complement C2 receptor that is expressed by CD27 + memory B cells is the co-receptor used by Epstein Barr Virus (EBV) to infect and subsequently immortalize B cells (Ascherio and Munger, 2015, Burns et al., 2015, Fernández-Menéndez et al., 2016). It has been observed that the BAFF levels, which are elevated in MS, are related to EBV immunity that depletes, or is associated with a reduction in B memory cells (Mameli et al., 2016, Panikkar et al., 2015). Furthermore, as latent EBV proteins mimic B cell receptor signaling, if the BAFF signaling pathway is blocked the memory-EBV infected B cell population is likely to expand (Vrazo et al., 2012). Importantly EBV reactivation can drive the expansion of latently-infected CD19 +, CD27 + B cells (Burns et al., 2015) and possibly contribute to disease worsening in MS. Memory B cells thus form the reservoir for latent EBV infection and therefore their destruction will remove the viral load and potential etiological triggers that could precipitate relapse (Ascherio and Munger, 2015, Fernández-Menéndez et al., 2016). Furthermore most B cells in MS lesions, meninges and ectopic B cell aggregates are CD27 antigen positive and are the population of cells that have been shown to co-express latent EBV proteins and support a role for EBV infection in B-cell activation in the MS brain (Serafini et al., 2010). This will need to be elucidated further.
However, irrespective of the data implicating EBV-infected B cells, the data presented here suggests memory B cells are of central importance in for the development of MS. Developing agents that are more specific for these CD19 +, CD27 + B memory cells may improve the risk-benefit ratio compared to current therapies, which target multiple subsets of cells.
Contributions
Freedom of Information requests KS. Concepts: DB, GG, MM, GP, KS; Literature Search: DB, MM; Figures: DB; Writing: DB, GG, MM, GP, KS; Final Editing: DB, GG, KS.
Source of Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Declaration of Interests
None considered to be relevant. However, DB is a founder and consultant to Canbex therapeutics and has received research funds from Canbex therapeutics, Sanofi-Genzyme and Takeda in the past 3 years. MM has received honoraria or meeting support from Novartis, Genzyme and AbbVie; GP is a shareholder of Canbex therapeutics. GG has received fees for participation in advisory board for AbbVie Biotherapeutics, Biogen, Canbex, Ironwood, Novartis, Merck, Merck Serono, Roche, Sanofi Genzyme, Synthon, Teva and Vertex; speaker fees from AbbVie, Biogen, Bayer HealthCare, Genzyme, Merck Serono, Sanofi-Aventis and Teva. Research support from Biogen, Genzyme, Ironwood, Merck, Merck Serono and Novartis; KS has been a PI of trials sponsored by Novartis, Roche and Teva and involved in trials sponsored by Biogen, Sanofi-Genzyme, BIAL, Cytokinetics, and Canbex and has received honoraria and meeting support from Biogen, Merck, Novartis, Teva, Merck.
Search Strategy and Selection Criteria
The review was based on initial analysis of leukocyte phenotyping data from clinical trials and was used to formulate the hypothesis for the content of the review. References were then identified by searches of Pubmed and Google.co.uk. These used the search terms relating to the “non-proprietary drug name” and/or “class of drug type” and “memory B cell” or “CD27” with or without “multiple sclerosis”. Preference was given to work conducted in multiple sclerosis versus other autoimmune diseases. The final reference list was generated on the basis of relevance to the topic covered in the review.
Outstanding Questions
This review shifts focus from T cell biology towards B cell memory function and opens a series of new questions concerning the pathogenesis of multiple sclerosis. How does memory B cell function actually drive disease? Is this via immune activity to cause direct pathology or do they stimulate T to cause damage? Importantly, do they act as a reservoir for the tentative etiological viral trigger of multiple sclerosis? Does the benefit relate to a central action of B cells or is peripheral targeting sufficient? What is the precise phenotype of the pathogenic cells within the memory B cell pool? Can monitoring of memory B cell numbers be used as a prognostic feature and biomarker that allows one to personalize treatment and retreatment? Importantly, can more specific targeting of memory B cells be achieved to exhibit comparable benefit to more global B cell depletion, whilst de-risking side effect issues of current treatments?
Acknowledgements
We would like to thank the European Medicines agency for supplying the pivotal phase III trial data that helped formulate the concept.
References
- Agahozo M.C., Peferoen L., Baker D., Amor S. CD20 therapies in multiple sclerosis and experimental autoimmune encephalomyelitis - targeting T or B cells? Mult. Scler. Relat. Disord. 2016;9:110–117. doi: 10.1016/j.msard.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Alping P., Frisell T., Novakova L. Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann. Neurol. 2016;79:950–958. doi: 10.1002/ana.24651. [DOI] [PubMed] [Google Scholar]
- Aquis M., Klodowska-Dida G. Safety and tolerability of MEDI-551 in patients with relapsing forms of multiple sclerosis: results from a phase 1 randomised, placebo-controlled, escalating intravenous and subcutaneous dose study. Mult. Scler. J. 2015;21(S11):235–236. doi: 10.1177/1352458517740641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold D.L., Calabresi P.A., Kieseier B.C. Effect of peginterferon beta-1a on MRI measures and achieving no evidence of disease activity: results from a randomized controlled trial in relapsing-remitting multiple sclerosis. BMC Neurol. 2014;14:24. doi: 10.1186/s12883-014-0240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascherio A., Munger K.L. EBV and autoimmunity. Curr. Top. Microbiol. Immunol. 2015;390:365–385. doi: 10.1007/978-3-319-22822-8_15. [DOI] [PubMed] [Google Scholar]
- Asztely F., Gilland E., Watties M.P., Lycke J. Rituximab treatment did not aggravate ongoing progressive multifocal leukoencephalopathy in a patient with multiple sclerosis. J. Neurol. Sci. 2015;353:155–157. doi: 10.1016/j.jns.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Atkins H.L., Bowman M., Allan D. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: a multicentre single-group phase 2 trial. Lancet. 2016;388:576–585. doi: 10.1016/S0140-6736(16)30169-6. [DOI] [PubMed] [Google Scholar]
- Baker D., Amor S. Experimental autoimmune encephalomyelitis is a good model of multiple sclerosis if used wisely. Mult. Scler. Relat. Disord. 2014;3:555–564. doi: 10.1016/j.msard.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Bar-Or A., Calabresi P.A., Arnold D. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann. Neurol. 2008;63:395–400. doi: 10.1002/ana.21363. [DOI] [PubMed] [Google Scholar]
- Blauth K., Soltys J., Matschulat A. Antibodies produced by clonally expanded plasma cells in multiple sclerosis cerebrospinal fluid cause demyelination of spinal cord explants. Acta Neuropathol. 2015;130:765–781. doi: 10.1007/s00401-015-1500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisslert M., Bokarewa M., Larsson P. Phenotypic and functional characterization of human CD25 + B cells. Imunology. 2006;117:548–557. doi: 10.1111/j.1365-2567.2006.02331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns D.M., Tierney R., Shannon-Lowe C. Memory B-cell reconstitution following allogeneic hematopoietic stem cell transplantation is an EBV-associated transformation event. Blood. 2015;126:2665–2675. doi: 10.1182/blood-2015-08-665000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt R.K., Balabanov R., Xetal H. Association of nonmyeloablative hematopoietic stem cell transplantation with neurological disability in patients with relapsing-remitting multiple sclerosis. JAMA. 2015;313:275–284. doi: 10.1001/jama.2014.17986. [DOI] [PubMed] [Google Scholar]
- Cohen J.A., Coles A.J., Arnold D.L. CARE-MS I investigators. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380:1819–1828. doi: 10.1016/S0140-6736(12)61769-3. [DOI] [PubMed] [Google Scholar]
- Coles A.J., Wing M.G., Molyneux P. Monoclonal antibody treatment exposes three mechanisms underlying the clinical course of multiple sclerosis. Ann. Neurol. 1999;46:296–304. doi: 10.1002/1531-8249(199909)46:3<296::aid-ana4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Comabella M., Cantó E., Nurtdinov R. MRI phenotypes with high neurodegeneration are associated with peripheral blood B-cell changes. Hum. Mol. Genet. 2016;25:308–316. doi: 10.1093/hmg/ddv473. [DOI] [PubMed] [Google Scholar]
- Compston A., Coles A. Multiple sclerosis. Lancet. 2002;359:1221–1231. doi: 10.1016/S0140-6736(02)08220-X. [DOI] [PubMed] [Google Scholar]
- Compston A., Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Cox A.L., Thompson S.A., Jones J.L. Lymphocyte homeostasis following therapeutic lymphocyte depletion in multiple sclerosis. Eur. J. Immunol. 2005;35:3332–3342. doi: 10.1002/eji.200535075. [DOI] [PubMed] [Google Scholar]
- De Stefano N., Giorgio A., Battaglini M. Assessing brain atrophy rates in a large population of untreated multiple sclerosis subtypes. Neurology. 2010;74:1868–1876. doi: 10.1212/WNL.0b013e3181e24136. [DOI] [PubMed] [Google Scholar]
- Deiß A., Brecht I., Haarmann A., Buttmann M. Treating multiple sclerosis with monoclonal antibodies: a 2013 update. Expert. Rev. Neurother. 2013;13:313–335. doi: 10.1586/ern.13.17. [DOI] [PubMed] [Google Scholar]
- Disanto G., Morahan J.M., Barnett M.H., Giovannoni G., Ramagopalan S.V. The evidence for a role of B cells in multiple sclerosis. Neurology. 2012;78:823–832. doi: 10.1212/WNL.0b013e318249f6f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duddy M., Niino M., Adatia F. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J. Immunol. 2007;178:6092–6099. doi: 10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- Fernández-Menéndez S., Fernández-Morán M., Fernández-Vega I., Pérez-Álvarez A., Villafani-Echazú J. Epstein-Barr virus and multiple sclerosis. From evidence to therapeutic strategies. J. Neurol. Sci. 2016;361:213–219. doi: 10.1016/j.jns.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Gatto B. Atacicept, a homodimeric fusion protein for the potential treatment of diseases triggered by plasma cells. Curr. Opin. Investig. Drugs. 2008;9:1216–1227. [PubMed] [Google Scholar]
- Genovese M.C., Kaine J.L., Lowenstein M.B. Ocrelizumab, a humanized anti-CD20 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I/II randomized, blinded, placebo-controlled, dose-ranging study. Arthritis Rheum. 2008;58:2652–2661. doi: 10.1002/art.23732. [DOI] [PubMed] [Google Scholar]
- Genovese M.C., Bojin S., Biagini I.M. Tabalumab in rheumatoid arthritis patients with an inadequate response to methotrexate and naive to biologic therapy: a phase II, randomized, placebo-controlled trial. Arthritis Rheum. 2013;65:880–889. doi: 10.1002/art.37820. [DOI] [PubMed] [Google Scholar]
- Giovannoni G., Ebers G. Multiple sclerosis: the environment and causation. Curr. Opin. Neurol. 2007;20:261–268. doi: 10.1097/WCO.0b013e32815610c2. [DOI] [PubMed] [Google Scholar]
- Giovannoni G., Comi G., Cook S. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N. Engl. J. Med. 2010;362:416–426. doi: 10.1056/NEJMoa0902533. [DOI] [PubMed] [Google Scholar]
- Giovannoni G., Butzkueven H., Dhib-Jalbut S. Brain health: time matters in multiple sclerosis. Mult. Scler. Relat. Disord. 2016;9(Suppl. 1):S5–S48. doi: 10.1016/j.msard.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Giovannoni G., Marta M., Davis A., Turner B., Gnanapavan S., Schmierer K. Switching patients at high risk of PML from natalizumab to another disease-modifying therapy. Pract. Neurol. 2016;16:389–393. doi: 10.1136/practneurol-2015-001355. [DOI] [PubMed] [Google Scholar]
- Giovannoni G., Cutter G., Pia-Sormani M. Is multiple sclerosis a length-dependent central axonopathy? The case for therapeutic lag and the asynchronous progressive MS hypotheses. Mult. Scler. Relat. Disord. 2017 doi: 10.1016/j.msard.2017.01.007. (In press) [DOI] [PubMed] [Google Scholar]
- Gold R., Radue E.W., Giovannoni G. Safety and efficacy of daclizumab in relapsing-remitting multiple sclerosis: 3-year results from the SELECTED open-label extension study. BMC Neurol. 2016;16:117. doi: 10.1186/s12883-016-0635-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves J., Vinayagasundaram U., Mowry E.M. Effects of rituximab on lymphocytes in multiple sclerosis and neuromyelitis optica. Mult. Scler. Relat. Disord. 2014;3:244–252. doi: 10.1016/j.msard.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Grützke B., Hucke S., Gross C.C. Fingolimod treatment promotes regulatory phenotype and function of B cells. Ann. Clin. Transl. Neurol. 2015;2:119–130. doi: 10.1002/acn3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton D.W., Serio A., Pryce G. Neurodegeneration progresses despite complete elimination of clinical relapses in a mouse model of multiple sclerosis. Acta Neuropathol. Commun. 2013;1:84. doi: 10.1186/2051-5960-1-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harp C.T., Ireland S., Davis L.S. Memory B cells from a subset of treatment-naïve relapsing-remitting multiple sclerosis patients elicit CD4 (+) T cell proliferation and IFN-γ production in response to myelin basic protein and myelin oligodendrocyte glycoprotein. Eur. J. Immunol. 2010;40:2942–2956. doi: 10.1002/eji.201040516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser S.L., Waubant E., Arnold D.L. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N. Engl. J. Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- Hauser S., Li D., Calabresi P., O'Connor P. Week 144 results of a phase II, randomized, multicenter trial assessing the safety and efficacy of ocrelizumab in patients with relapsing-remitting multiple sclerosis (RRMS) Neurology. 2013;80(7) (suppl) S31.004. [Google Scholar]
- Heremans H., Dillen C., Groenen M., Matthys P., Billiau A. Role of endogenous interleukin-12 (IL-12) in induced and spontaneous relapses of experimental autoimmune encephalomyelitis in mice. Eur. Cytokine Netw. 1999;10:171–180. [PubMed] [Google Scholar]
- Hintzen R.Q., van Lier R.A., Kuijpers K.C. Elevated levels of a soluble form of the T cell activation antigen CD27 in cerebrospinal fluid of multiple sclerosis patients. J. Neuroimmunol. 1991;35:211–217. doi: 10.1016/0165-5728(91)90175-7. [DOI] [PubMed] [Google Scholar]
- Hughes S. Ocrelizumab in MS: encouraging long-term data. 2013. http://www.medscape.com/viewarticle/781671 (accessed 5 Jan 2017)
- Hyrich K.L., Silman A.J., Watson K.D., Symmons D.P. Anti-tumour necrosis factoral phatherapy in rheumatoid arthritis: an update on safety. Ann. Rheum. Dis. 2004;63:1538–1543. doi: 10.1136/ard.2004.024737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland S.J., Guzman A.A., O'Brien D.E. The effect of glatiramer acetate therapy on functional properties of B cells from patients with relapsing-remitting multiple sclerosis. JAMA Neurol. 2014;71:1421–1428. doi: 10.1001/jamaneurol.2014.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel K., Alnek K., Vahter L. Changes in blood B cell-activating factor (BAFF) levels in multiple sclerosis: a sign of treatment outcome. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0143393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kap Y.S., Jagessar S.A., van Driel N. Effects of early IL-17A neutralization on disease induction in a primate model of experimental autoimmune encephalomyelitis. J. Neuroimmune Pharmacol. 2011;6:341–353. doi: 10.1007/s11481-010-9238-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappos L., Li D., Calabresi P.A., O'Connor P. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet. 2011;378:1779–1787. doi: 10.1016/S0140-6736(11)61649-8. [DOI] [PubMed] [Google Scholar]
- Kappos L., Li D., Calabresi P., O'Connor P. Long-term safety and efficacy of ocrelizumab in patients with relapsing-remitting multiple sclerosis: week 144 results of a phase II, randomised, multicentre trial. Mult. Scler. J. 2012;18(S):140–141. [Google Scholar]
- Kappos L., Wiendl H., Selmaj K. Daclizumab HYP versus interferon Beta-1a in relapsing multiple sclerosis. N. Engl. J. Med. 2015;373:1418–1428. doi: 10.1056/NEJMoa1501481. [DOI] [PubMed] [Google Scholar]
- Kasper L.H., Arnold D.L., Cohen J.A. Lymphocyte subset dynamics following alemtuzumab treatment in the CARE-MS II study. Mult. Scler. J. 2013;18(S1):2717–2718. [Google Scholar]
- Kim S.H., Kim W., Li X.F., Jung I.J., Kim H.J. Repeated treatment with rituximab based on the assessment of peripheral circulating memory B cells in patients with relapsing neuromyelitis optica over 2 years. Arch. Neurol. 2011;68:1412–1420. doi: 10.1001/archneurol.2011.154. [DOI] [PubMed] [Google Scholar]
- Komori M., Blake A., Greenwood M. Cerebrospinal fluid markers reveal intrathecal inflammation in progressive multiple sclerosis. Ann. Neurol. 2015;78:3–20. doi: 10.1002/ana.24408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousin-Ezewu O., Azzopardi L., Parker R.A. Accelerated lymphocyte recovery after alemtuzumab does not predict multiple sclerosis activity. Neurology. 2014;82:2158–2164. doi: 10.1212/WNL.0000000000000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupica T., Fry T.J., Mackall C.L. Autoimmunity during lymphopenia: a two-hit model. Clin. Immunol. 2006;120:121–128. doi: 10.1016/j.clim.2006.04.569. [DOI] [PubMed] [Google Scholar]
- von Kutzleben S., Pryce G., Giovannoni G., Baker D. Depletion of CD52 positive cells inhibits the development of CNS autoimmune disease, but deletes an immune-tolerance promoting CD8 T cell population. Implications for secondary autoimmunity of alemtuzumab in multiple sclerosis. Immunology. 2016 Dec 7 doi: 10.1111/imm.12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenercept Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. Neurology. 1999;53:457–465. [PubMed] [Google Scholar]
- Li L., Liu J., Delohery T. The effects of teriflunomide on lymphocyte subpopulations in human peripheral blood mononuclear cells in vitro. J. Neuroimmunol. 2013;265:82–90. doi: 10.1016/j.jneuroim.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Li R., Rezk A., Miyazaki Y. Proinflammatory GM-CSF-producing B cells in multiple sclerosis and B cell depletion therapy. Sci. Transl. Med. 2015;7(310):310ra166. doi: 10.1126/scitranslmed.aab4176. [DOI] [PubMed] [Google Scholar]
- Lin Y.C., Winokur P., Blake A. Daclizumab reverses intrathecal immune cell abnormalities in multiple sclerosis. Ann. Clin. Transl. Neurol. 2015;2:445–455. doi: 10.1002/acn3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lublin F.D., Reingold S.C., Cohen J.A. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83:278–286. doi: 10.1212/WNL.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy S.K., Wu Q., Wang Q. Dimethyl fumarate treatment of relapsing-remitting multiple sclerosis influences B-cell subsets. Neurol. Neuroimmunol. Neuroinflamm. 2016 Mar 3;3:e211. doi: 10.1212/NXI.0000000000000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N., Xing C., Xiao H. Combination of TACI-IgG and anti-IL-15 treats murine lupus by reducing mature and memory B cells. Cell. Immunol. 2014;289:140–144. doi: 10.1016/j.cellimm.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Mameli G., Cocco E., Frau J. BAFF levels, Methypredsinolone therapy, Epstein-Barr Virus and Mycobacterium avium subsp. paratuberculosis infection in Multiple Sclerosis patients. Sci. Rep. 2016;6:29268. doi: 10.1038/srep29268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardiguian S., Serres S., Ladds E. Anti-IL-17A treatment reduces clinical score and VCAM-1 expression detected by in vivo magnetic resonance imaging in chronic relapsing EAE ABH mice. Am. J. Pathol. 2013;182:2071–2081. doi: 10.1016/j.ajpath.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marta M., Giovannoni G. Disease modifying drugs in multiple sclerosis: mechanisms of action and new drugs in the horizon. CNS Neurol. Disord. Drug Targets. 2012;11:610–623. doi: 10.2174/187152712801661301. [DOI] [PubMed] [Google Scholar]
- Martin R., Sopedra M., Rosito M., Engelhardt B. Current multiple sclerosis treatments have improved our understanding of MS autoimmune pathogenesis. Eur. J. Immunol. 2016;46:2078–2090. doi: 10.1002/eji.201646485. [DOI] [PubMed] [Google Scholar]
- McComish J., Mundy J., Sullivan T. Changes in peripheral blood B cell subsets at diagnosis and after treatment with disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis: correlation with clinical and laboratory parameters. Int. J. Rheum. Dis. 2015;18:421–432. doi: 10.1111/1756-185X.12325. [DOI] [PubMed] [Google Scholar]
- Michel L., Touil H., Pikor N.B., Gommerman J.L., Prat A., Bar-Or A. B cells in the multiple sclerosis central nervous system: trafficking and contribution to CNS-compartmentalized inflammation. Front. Immunol. 2015;6:636. doi: 10.3389/fimmu.2015.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Matsuoka T., Chilhara N. Differential effects of fingolimod on B-cell populations in multiple sclerosis. Mult. Scler. J. 2014;20:1371–1380. doi: 10.1177/1352458514523496. [DOI] [PubMed] [Google Scholar]
- van Nierop G.P., Janssen M., Mitterreiter J.G. Intrathecal CD4 + and CD8 + T cell responses to endogenously synthesized candidate disease-associated human autoantigens in multiple sclerosis patients. Eur. J. Immunol. 2016;46:347–353. doi: 10.1002/eji.201545921. [DOI] [PubMed] [Google Scholar]
- van Noort J.M., Bsibsi M., Gerritsen W.H. Alpha B-crystallin is a target for adaptive immune responses and a trigger of innate responses in preactive multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. 2010;69:694–703. doi: 10.1097/NEN.0b013e3181e4939c. [DOI] [PubMed] [Google Scholar]
- van Oosten B.W., Barkhof F., Truyen L. Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology. 1996;47:1531–1534. doi: 10.1212/wnl.47.6.1531. [DOI] [PubMed] [Google Scholar]
- van Oosten B.W., Lai M., Hodgkinson S. Treatment of multiple sclerosis with the monoclonal anti-CD4 antibody cM-T412:results of a randomized, double-blind, placebo-controlled, MR-monitored phase II trial. Neurology. 1997;49:351–357. doi: 10.1212/wnl.49.2.351. [DOI] [PubMed] [Google Scholar]
- Palanichamy A., Jahn S., Nickles D. Rituximab efficiently depletes increased CD20-expressing T cells in multiple sclerosis patients. J. Immunol. 2014;193:580–586. doi: 10.4049/jimmunol.1400118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panikkar A., Smith C., Hislop A. Impaired Epstein-Barr virus-specific neutralizing antibody response during acute infectious mononucleosis is coincident with global B-cell dysfunction. J. Virol. 2015;89:9137–9141. doi: 10.1128/JVI.01293-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedotti R., Musio S., Scabeni S. Exacerbation of experimental autoimmune encephalomyelitis by passive transfer of IgG antibodies from a multiple sclerosis patient responsive to immunoadsorption. J. Neuroimmunol. 2013;262:19–26. doi: 10.1016/j.jneuroim.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Piccio L., Naismith R., Trinkaus Changes in B and T lymphocytes and chemokines with rituximab treatment in multiple sclerosis. Arch. Neurol. 2010;67:7070-714. doi: 10.1001/archneurol.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas R., Jelčić I., Schippling S. Natalizumab treatment perturbs memory and marginal zone-like B-cell homing in secondary lymphoid organs in multiple sclerosis. Eur. J. Immunol. 2012;42:790–798. doi: 10.1002/eji.201142108. [DOI] [PubMed] [Google Scholar]
- Rasenack M., Derfuss T. Disease activity return after natalizumab cessation in multiple sclerosis. Expert. Rev. Neurother. 2016;16:587–594. doi: 10.1586/14737175.2016.1168295. [DOI] [PubMed] [Google Scholar]
- Rieckmann P., Comi G., Cook S. Effects of cladribine tablets on peripheral lymphocyte subtypes implicated in multiple sclerosis immunopathogenesis: surface marker analysis for a subset of patients from the 96-week, phase III, double-blind, placebo controlled CLARITY study. Mult. Scler. J. 2009;15(S9):S248–S249. [Google Scholar]
- Rizzo F., Giacomini E., Mechelli R. Interferon-β specifically reduced pathogenic memory B cells in multiple sclerosis by inducing a FAS-mediated apoptosis. Immunol. Cell Biol. 2016;94:886–894. doi: 10.1038/icb.2016.55. [DOI] [PubMed] [Google Scholar]
- Roll P., Muhammad K., Schumann M., Kleinert S., Tony H.P. RF positivity has substantial influence on the peripheral memory B-cell compartment and its modulation by TNF inhibition. Scand. J. Rheumatol. 2012;41:180–185. doi: 10.3109/03009742.2011.645056. [DOI] [PubMed] [Google Scholar]
- Rostami A., Ciric B. Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination. J. Neurol. Sci. 2013;333:76–87. doi: 10.1016/j.jns.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawcer S., Franklin R.J., Ban M. Multiple sclerosis genetics. Lancet Neurol. 2014;13:700–709. doi: 10.1016/S1474-4422(14)70041-9. [DOI] [PubMed] [Google Scholar]
- Schubert R.D., Hu Y., Kumar G. IFN-β treatment requires B cells for efficacy in neuroautoimmunity. J. Immunol. 2015;194:2110–2116. doi: 10.4049/jimmunol.1402029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz A., Balint B., Korporal-Kuhnke M. B-cell populations discriminate between pediatric- and adult-onset multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2016;4(1) doi: 10.1212/NXI.0000000000000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal B.M., Constantinescu C.S., Raychaudhuri A. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 2008;7:796–804. doi: 10.1016/S1474-4422(08)70173-X. [DOI] [PubMed] [Google Scholar]
- Serafini B., Severa M., Columba-Cabezas S. Epstein-Barr virus latent infection and BAFF expression in B cells in the multiple sclerosis brain: implications for viral persistence and intrathecal B-cell activation. J. Neuropathol. Exp. Neurol. 2010;69:677–693. doi: 10.1097/NEN.0b013e3181e332ec. [DOI] [PubMed] [Google Scholar]
- Sergott R.C., Bennett J.L., Rieckmann P. ATON: results from a phase II randomized trial of the B-cell-targeting agent atacicept in patients with optic neuritis. J. Neurol. Sci. 2015;15:174–178. doi: 10.1016/j.jns.2015.02.019. [DOI] [PubMed] [Google Scholar]
- Sorensen P.S., Lisby S., Grove R. Safety and efficacy of ofatumumab in relapsing-remitting multiple sclerosis: a phase 2 study. Neurology. 2014;82:573–581. doi: 10.1212/WNL.0000000000000125. [DOI] [PubMed] [Google Scholar]
- Souto-Carneiro M.M., Mahadevan V., Takada K. Alterations in peripheral blood memory B cells in patients with active rheumatoid arthritis are dependent on the action of tumour necrosis factor. Arthritis Res. Ther. 2009;11:R84. doi: 10.1186/ar2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L., Zamvil S.S. Beginning of the end of two-stage theory purporting that inflammation then degeneration explains pathogenesis of progressive multiple sclerosis. Curr. Opin. Neurol. 2016;29:340–344. doi: 10.1097/WCO.0000000000000317. [DOI] [PubMed] [Google Scholar]
- Tak P.P., Thurlings R.M., Rossier C. Atacicept in patients with rheumatoid arthritis: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating, single- and repeated-dose study. Arthritis Rheum. 2008;58:61–72. doi: 10.1002/art.23178. [DOI] [PubMed] [Google Scholar]
- Tangye S.G., Avery D.T., Deenick E.K., Hodgkin P.D. Intrinsic differences in the proliferation of naive and memory human B cells as a mechanism for enhanced secondary immune responses. J. Immunol. 2003;170:686–694. doi: 10.4049/jimmunol.170.2.686. [DOI] [PubMed] [Google Scholar]
- Teniente-Serra A., Grau-López L., Mansilla M.J. Multiparametric flow cytometric analysis of whole blood reveals changes in minor lymphocyte subpopulations of multiple sclerosis patients. Autoimmunity. 2016;49:219–228. doi: 10.3109/08916934.2016.1138271. [DOI] [PubMed] [Google Scholar]
- Thompson S.A., Jones J.L., Cox A.L. B-cell reconstitution and BAFF after alemtuzumab (CAMPATH-1H) treatment of multiple sclerosis. J. Clin. Immunol. 2010;30:99–105. doi: 10.1007/s10875-009-9327-3. [DOI] [PubMed] [Google Scholar]
- Toso C., Edgar R., Pawlick R. Effect of different induction strategies on effector, regulatory and memory lymphocyte sub-populations in clinical islet transplantation. Transpl. Int. 2009;22:182–191. doi: 10.1111/j.1432-2277.2008.00746.x. [DOI] [PubMed] [Google Scholar]
- Volpe E., Battistini L., Borsellino G. Advances in T helper 17 cell biology: pathogenic role and potential therapy in multiple sclerosis. Mediat. Inflamm. 2015;2015:475158. doi: 10.1155/2015/475158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrazo A.C., Chauchard M., Raab-Traub N., Longnecker R. Epstein-Barr virus LMP2A reduces hyperactivation induced by LMP1 to restore normal B cell phenotype in transgenic mice. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E., Mittereder N., Kuta E. A glycoengineered anti-CD19 antibody with potent antibody- dependent cellular cytotoxicity activity in vitro and lymphoma growth inhibition in vivo. Br. J. Haematol. 2011;155:426–437. doi: 10.1111/j.1365-2141.2011.08857.x. [DOI] [PubMed] [Google Scholar]