Abstract
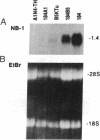
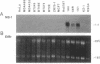
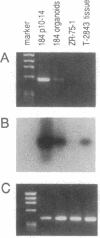
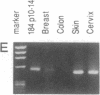
A human cDNA library obtained from cultured normal mammary epithelial cells (HMECs) was searched by subtractive hybridization for genes whose decrease in expression might be relevant to epithelial transformation. One clone identified by this procedure corresponded to a 1.4-kilobase mRNA, designated NB-1, whose expression was decreased greater than 50-fold in HMECs tumorigenically transformed in vitro after exposure to benzo[a]pyrene and Kirsten sarcoma virus. Sequence analysis of NB-1 cDNA revealed an open reading frame with a high degree of homology to calmodulin. NB-1 expression could be demonstrated by polymerase chain reaction amplification in normal breast, prostate, cervix, and epidermal tissues. The presence of NB-1 transcripts was variable in primary breast carcinoma tissues and undetectable in tumor-derived cell lines of breast, prostate, or other origins. NB-1 mRNA expression could be down-regulated in cultured HMECs by exposure to reconstituted extracellular matrix material, while exposure to transforming growth factor type beta increased its relative abundance. The protein encoded by NB-1 may have Ca2+ binding properties and perform functions similar to those of authentic calmodulin. Its possible roles in differentiation and/or suppression of tumorigenicity in epithelial tissues remain to be examined.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Blum J. L., Zeigler M. E., Wicha M. S. Regulation of rat mammary gene expression by extracellular matrix components. Exp Cell Res. 1987 Dec;173(2):322–340. doi: 10.1016/0014-4827(87)90274-6. [DOI] [PubMed] [Google Scholar]
- Chafouleas J. G., Lagacé L., Bolton W. E., Boyd A. E., 3rd, Means A. R. Changes in calmodulin and its mRNA accompany reentry of quiescent (G0) cells into the cell cycle. Cell. 1984 Jan;36(1):73–81. doi: 10.1016/0092-8674(84)90075-8. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Clark R., Stampfer M. R., Milley R., O'Rourke E., Walen K. H., Kriegler M., Kopplin J., McCormick F. Transformation of human mammary epithelial cells by oncogenic retroviruses. Cancer Res. 1988 Aug 15;48(16):4689–4694. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fischer R., Koller M., Flura M., Mathews S., Strehler-Page M. A., Krebs J., Penniston J. T., Carafoli E., Strehler E. E. Multiple divergent mRNAs code for a single human calmodulin. J Biol Chem. 1988 Nov 15;263(32):17055–17062. [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelstein V. I., Tchypysheva T. A., Ermilova V. D., Litvinova L. V., Troyanovsky S. M., Bannikov G. A. Monoclonal antibody mapping of keratins 8 and 17 and of vimentin in normal human mammary gland, benign tumors, dysplasias and breast cancer. Int J Cancer. 1988 Aug 15;42(2):147–153. doi: 10.1002/ijc.2910420202. [DOI] [PubMed] [Google Scholar]
- Hennings H., Michael D., Cheng C., Steinert P., Holbrook K., Yuspa S. H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980 Jan;19(1):245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Hassell J. R., Star V. L., Cannon F. B., Laurie G. W., Martin G. R. Basement membrane complexes with biological activity. Biochemistry. 1986 Jan 28;25(2):312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- Koller M., Strehler E. E. Characterization of an intronless human calmodulin-like pseudogene. FEBS Lett. 1988 Oct 24;239(1):121–128. doi: 10.1016/0014-5793(88)80558-1. [DOI] [PubMed] [Google Scholar]
- Li M. L., Aggeler J., Farson D. A., Hatier C., Hassell J., Bissell M. J. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc Natl Acad Sci U S A. 1987 Jan;84(1):136–140. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima H., Kishi K., Sokabe H. Multiple calmodulin mRNA species are derived from two distinct genes. Mol Cell Biol. 1987 May;7(5):1873–1880. doi: 10.1128/mcb.7.5.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putkey J. A., Carroll S. L., Means A. R. The nontranscribed chicken calmodulin pseudogene cross-hybridizes with mRNA from the slow-muscle troponin C gene. Mol Cell Biol. 1987 Apr;7(4):1549–1553. doi: 10.1128/mcb.7.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen C. D., Means A. R. Calmodulin is involved in regulation of cell proliferation. EMBO J. 1987 Dec 20;6(13):3961–3968. doi: 10.1002/j.1460-2075.1987.tb02738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudland P. S., Hughes C. M. Immunocytochemical identification of cell types in human mammary gland: variations in cellular markers are dependent on glandular topography and differentiation. J Histochem Cytochem. 1989 Jul;37(7):1087–1100. doi: 10.1177/37.7.2471725. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SenGupta B., Friedberg F., Detera-Wadleigh S. D. Molecular analysis of human and rat calmodulin complementary DNA clones. Evidence for additional active genes in these species. J Biol Chem. 1987 Dec 5;262(34):16663–16670. [PubMed] [Google Scholar]
- Soule H. D., McGrath C. M. A simplified method for passage and long-term growth of human mammary epithelial cells. In Vitro Cell Dev Biol. 1986 Jan;22(1):6–12. doi: 10.1007/BF02623435. [DOI] [PubMed] [Google Scholar]
- Stampfer M. R., Bartley J. C. Induction of transformation and continuous cell lines from normal human mammary epithelial cells after exposure to benzo[a]pyrene. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2394–2398. doi: 10.1073/pnas.82.8.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Stampfer M., Bartek J., Lewis A., Boshell M., Lane E. B., Leigh I. M. Keratin expression in human mammary epithelial cells cultured from normal and malignant tissue: relation to in vivo phenotypes and influence of medium. J Cell Sci. 1989 Nov;94(Pt 3):403–413. doi: 10.1242/jcs.94.3.403. [DOI] [PubMed] [Google Scholar]
- Trask D. K., Band V., Zajchowski D. A., Yaswen P., Suh T., Sager R. Keratins as markers that distinguish normal and tumor-derived mammary epithelial cells. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2319–2323. doi: 10.1073/pnas.87.6.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walen K. H., Stampfer M. R. Chromosome analyses of human mammary epithelial cells at stages of chemical-induced transformation progression to immortality. Cancer Genet Cytogenet. 1989 Feb;37(2):249–261. doi: 10.1016/0165-4608(89)90056-3. [DOI] [PubMed] [Google Scholar]
- Wawrzynczak E. J., Perham R. N. Isolation and nucleotide sequence of a cDNA encoding human calmodulin. Biochem Int. 1984 Aug;9(2):177–185. [PubMed] [Google Scholar]