Abstract
A convenient and general zinc‐catalyzed borylation of aryl diazonium salts and aryltriazenes has been developed. With bis‐ (pinacolato)diboron as the borylation reagent, aryldiazonium tetrafluoroborate salts and aryltriazenes were transformed into the corresponding arylboronates in moderate to excellent yields under mild conditions. As a convenient and practical methodology, no additional ligands, base, or any other additives are required here.
Keywords: arylboronates, aryldiazonium salts, aryltriazenes, borylation, zinc catalysis
Arylboronates are very important intermediates in transition‐metal‐catalyzed carbon−carbon and carbon−heteroatom bond construction,1 and have also found widespread applications in medicinal chemistry.2 Owing to the low toxicity and high stability of arylboronates, increasing attention has been focused on their preparation. Classical synthetic methods for arylboronates mainly rely on the reaction of aryl Grignard or aryllithium reagents with trialkyl borates.3 However; these methods usually suffer from several drawbacks, such as narrow substrate scope and rigorous anhydrous conditions. As a result, a series of strategies based on the transition‐metal‐catalyzed borylation reaction have been established and have emerged as a general and efficient approach for the synthesis of arylboronates over the past decades. In particular, noble‐metal palladium,4 iridium,5 rhodium,6 and nickel7 catalysts have shown a predominant performance in these borylation reactions. Additionally, copper,8 iron,9 and cobalt10 salts have also been applied in borylation reactions, and metal‐free systems based on aryldiazonium salts (preformed or in situ generation) have been developed as well.11 However, strong bases and expensive ligands are demanded and a decreased reaction efficiency has been observed with these aforementioned catalysts. Thus, the development of efficient and environmentally friendly catalytic systems for arylboronate synthesis is still being pursued.
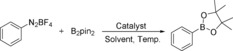
Zinc salt is a promising alternative to commonly used expensive metal catalysts, owing to advantages such as low cost, low toxicity, abundance, and because it is environmental benign.12 Compared with noble‐metal catalysts, zinc catalysts are rarely studied in borylation reactions. In 2008, Nozaki and co‐workers developed a stoichiometric Zn‐mediated C−B bond formation reaction through a zinc boryl complex.13 Knochel's group developed procedures for the preparation of stereochemically pure organozinc species via boron zinc transmetalation reactions.14 Uchiyama and co‐workers developed an interesting and general borylzincate‐complex‐mediated procedure for the borylation of aryl halides and borylzincation of benzynes/terminal alkynes.15 A zinc catalyst has also been applied in the dehydrogenative coupling of terminal alkynes with 1,8‐naphthalenediaminatoborane.16 Alkynylboranes were produced in good yields. Notably, in 2014, Marder and co‐workers reported an efficient Zn‐catalyzed borylation of alkyl halides; good yields of alkyl boronates can be prepared with ZnCl2 and IMes [1,3‐bis(2,4,6‐trimethylphenyl)imidazol‐2‐ylidene] as the catalytic system in the presence of KOtBu at room temperature.17 Soon, they succeeded in extending their procedure to aryl halides by using KOMe as the base.18 Herein, we wish to report our new results on the Zn‐catalyzed borylation of aryl diazonium salts and aryltriazenes. Various arylboronates can be isolated in good yields under mild conditions and no ligand or additive is required.
Initially, we selected phenyldiazonium tetrafluoroborate as the model substrate to react with B2pin2 in CH3OH at 40 °C, and various zinc salts were tested (Table 1, entries 1–7). To our delight, a 23 % yield of phenylboronate was obtained with Zn(OTf)2 as the catalyst (Table 1, entry 1). Zn(ClO4)2 provided the best results among all of the tested zinc salts (Table 1, entry 6). Other catalysts, including AgOTf, Mg(OTf)2, and Cu(OTf)2, were also investigated; the desired product can be obtained, but with lower efficiency (Table 1, entries 8–10). In the case of reaction temperature variation, the yields decreased in both cases (Table 1, entries 11–12). The yield of phenylboronate can be dramatically improved to 82 % when 1.2 equivalents of B2pin2 are added (Table 1, entry 13). Notably, 43 % of the desired product can still be obtained in the absence of catalyst. Then, several solvents were tested. To our surprise, the yield of 4,4,5,5‐tetramethyl‐2‐phenyl‐1,3,2‐dioxaborolane dropped to 5–11 % when using DMF or DMSO as the reaction medium (Table 1, entries 14,15). A moderate yield can be obtained in EtOH, whereas no product can be detected in toluene, MeCN, or THF (Table 1, entries 16–20). These results imply that an alcoholic solvent might react with the substrate during the reaction.
Table 1.
Zn‐catalyzed borylation: screening of reaction conditions.[a]
| Entry | Catalyst | Solvent | Temp. [°C] | Yield[b] [%] |
|---|---|---|---|---|
| 1 | Zn(OTf)2 | CH3OH | 40 | 23 |
| 2 | ZnO | CH3OH | 40 | 24 |
| 3 | Zn(acac)2 | CH3OH | 40 | 33 |
| 4 | Zn(OAc)2 | CH3OH | 40 | 33 |
| 5 | ZnCl2 | CH3OH | 40 | 31 |
| 6 | Zn(ClO4)2 | CH3OH | 40 | 37 |
| 7 | Zn(NO3)2 | CH3OH | 40 | 34 |
| 8 | AgOTf | CH3OH | 40 | 33 |
| 9 | Mg(OTf)2 | CH3OH | 40 | 28 |
| 10 | Cu(OTf)2 | CH3OH | 40 | 33 |
| 11 | Zn(ClO4)2 | CH3OH | 30 | 28 |
| 12 | Zn(ClO4)2 | CH3OH | 50 | 24 |
| 13[c] | Zn(ClO4)2 | CH3OH | 40 | 82 |
| 14[c] | Zn(ClO4)2 | DMF | 40 | 11 |
| 15[c] | Zn(ClO4)2 | DMSO | 40 | 5 |
| 16[c] | Zn(ClO4)2 | C2H5OH | 40 | 55 |
| 17[c] | Zn(ClO4)2 | H2O | 40 | 30 |
| 18[c] | Zn(ClO4)2 | Toluene | 40 | 0 |
| 19[c] | Zn(ClO4)2 | CH3CN | 40 | 0 |
| 20[c] | Zn(ClO4)2 | THF | 40 | 0 |
[a] Reaction conditions: PhN2BF4 (1.0 mmol), B2pin2 (0.5 mmol), catalyst (5 mol %), solvent (4 mL), 12 h. [b] Yields were determined by GC using dodecane as an internal standard. [c] B2pin2 (1.2 mmol).
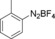
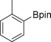
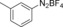
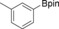
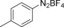
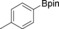
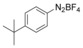
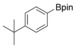
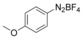
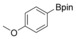
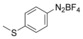
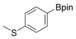
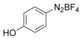
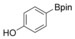
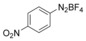
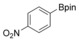
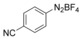
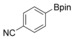
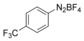
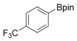
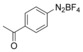
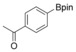
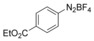
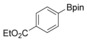
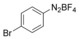
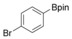
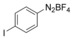
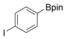
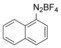
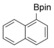
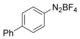
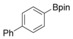
With the best reaction conditions in hand, we subsequently started testing the substrates generality. A variety of aryldiazonium tetrafluoroborate salts were tested and, in general, good to excellent yields of the target products can be isolated under standard reaction conditions. Substrates with electron‐donating groups, such as methyl, tert‐butyl, methoxy, methylthio, and hydroxyl groups, resulted in the desired products in very good yields (Table 2, entries 2–8). Those alkyl groups located at the para‐position worked better than ortho‐ and meta‐substitution (Table 2, entries 2 and 3 vs. 4). Electron‐withdrawing groups, including nitro, nitrile, trifluoromethyl, ketone, and ester groups, can give the corresponding products in moderate to excellent yields as well (Table 2, entries 9–13). Aryldiazonium tetrafluoroborate salts with halogen substitutions can be tolerated well and provide the desired arylboronate products in moderate‐to‐high yields (Table 2, entries 14–17). Notably, double substitution on the phenyl ring resulted in a lower yield compared with mono‐substitution (Table 2, entries 18–19). Additionally, naphthyl and biphenyl groups were also studied; 61 and 90 % yields of the desired boronates were obtained, respectively, without further optimizations (Table 2, entries 20 and 21).
Table 2.
Zn‐catalyzed borylation of aryldiazonium tetrafluoroborates.[a]
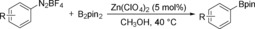
| Entry | Substrate | Product | Yield[b] [%] |
|---|---|---|---|
| 1 |
|
|
80 |
| 2 |
|
|
52 |
| 3 |
|
|
65 |
| 4 |
|
|
83 |
| 5 |
|
|
78 |
| 6 |
|
|
73 |
| 7 |
|
|
87 |
| 8 |
|
|
80 |
| 9 |
|
|
94 |
| 10 |
|
|
85 |
| 11 |
|
|
67 |
| 12 |
|
|
82 |
| 13 |
|
|
58 |
| 14 |
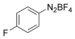
|
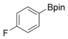
|
84 |
| 15 |
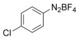
|
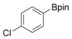
|
87 |
| 16 |
|
|
70 |
| 17 |
|
|
61 |
| 18 |
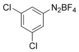
|
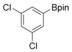
|
46 |
| 19 |
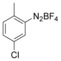
|
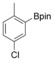
|
51 |
| 20 |
|
|
61 |
| 21 |
|
|
90 |
[a] Reaction conditions: ArN2BF4 (1.0 mmol), B2pin2 (1.2 mmol), Zn(ClO4)2 (5 mol %), CH3OH (4 mL), 8–15 h, 40 °C. [b] Isolated yields.
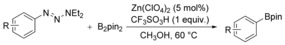
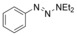
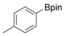
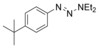
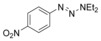
Considering that aryltriazenes have been applied as stable diazonium salt precursors, and can readily be transformed into the corresponding diazonium salt in the presence of acid, we tested aryltriazenes as substrates under our conditions. Under the same catalytic system and with the addition of 1 equivalent of CF3SO3H, good yields of the desired products can be obtained (Table 3). Both electron‐donating and electron‐withdrawing substituents are well tolerated.
Table 3.
Zn‐catalyzed borylation of aryltriazenes.[a]
| Entry | Substrate | Product | Yield[b] [%] |
|---|---|---|---|
| 1 |
|
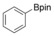
|
88 |
| 2 |
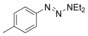
|
|
91 |
| 3 |
|
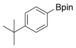
|
71 |
| 4 |
|
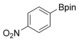
|
76 |
| 5 |
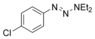
|
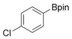
|
83 |
| 6 |
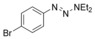
|
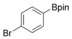
|
84 |
| 7 |
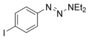
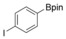
|
|
72 |
[a] Reaction conditions: aryltriazene (1.0 mmol), B2pin2 (1.2 mmol), Zn(ClO4)2 (5 mol %), CF3SO3H (1 equiv), CH3OH (4 mL), 10–15 h, 60 °C. [b] Isolated yields.
To gain more information about the mechanism of the reaction, a series of control experiments were set up under different conditions. When 1 equivalent of TEMPO was added to the reaction mixture, the borylation process was suppressed and the yield dropped from 82 to 47 % [Scheme 1, Eq. (1)]. Whereas, when 4 equivalents of TEMPO were applied, no desired product could be observed and 76 % yield of the TEMPO‐trapped compound was isolated [Scheme 1, Eq. (2)]. These results suggest that this borylation procedure is a radical‐involved pathway. As visible light is known to activate the aryldiazonium salt to give the corresponding aryl radical, we performed the reaction in the dark and protected the reaction tube with aluminum paper; 71 % yield of the target product can still be produced [Scheme 1, Eq. (3)].
Scheme 1.
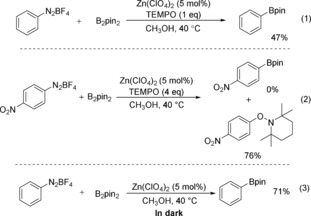
Control experiments.
With these results in hand, the most possible reaction pathway is proposed (Scheme 2). Firstly, aryldiazonium tetrafluoroborate reacts with MeOH to give 1‐methoxy‐2‐aryldiazene as the intermediate. Aryldiazene is not stable; it immediately decomposes to aryl radical with the assistance of the of zinc catalyst, which can then be trapped by TEMPO. Finally, the aryl radical reacts with bis(pinacolato)diboron to give the final arylboronates.
Scheme 2.
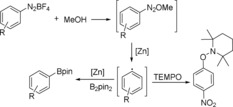
Proposed reaction pathway.
In summary, a general and convenient zinc‐catalyzed borylation of aryldiazonium tetrafluoroborate salts with B2pin2 as the boron source has been developed. With environmental benign and abundant zinc salt as the catalyst, moderate‐to‐excellent yields of the desired arylboronates can be isolated. A wide range of functional groups can be tolerated under these mild conditions.
Experimental Section
Aryldiazonium salts (1.0 mmol), bis(pinacolato)diboron (1.2 mmol), and Zn(ClO4)2 (5 mol %) were added to a 15 mL Schlenk tube under a nitrogen atmosphere. Then, CH3OH (4 mL) was added through a syringe. The mixture was stirred for 8–15 h at 40 °C. After the reaction was complete, the reaction was stopped. After cooling to room temperature, the reaction mixture was filtered, concentrated, and purified by using column chromatography (petroleum ether/ethyl acetate 50:1) to give the pure product.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
The authors thank the financial support of the NSFC (21472174, 21602201, 21602204) and Zhejiang Natural Science Fund for Distinguished Young Scholars (LR16B020002). X.‐F.W. appreciates the general support from Professor Matthias Beller in LIKAT.
X. Qi, L.-B. Jiang, C. Zhou, J.-B. Peng, X.-F. Wu, ChemistryOpen 2017, 6, 345.
References
- 1.
- 1a. Jana R., Pathak T. P., Sigman M. S., Chem. Rev. 2011, 111, 1417–1492; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1b. Rudolph A., Lautens M., Angew. Chem. Int. Ed. 2009, 48, 2656–2670; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2009, 121, 2694–2708; [Google Scholar]
- 1c. Imao D., Glasspoole B. W., Laberge V. S., Crudden C. M., J. Am. Chem. Soc. 2009, 131, 5024–5025; [DOI] [PubMed] [Google Scholar]
- 1d. Frisch A. C., Beller M., Angew. Chem. Int. Ed. 2005, 44, 674–688; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2005, 117, 680–695; [Google Scholar]
- 1e. Miyaura N., Suzuki A., Chem. Rev. 1995, 95, 2457–2483; [Google Scholar]
- 1f. Yamaguchi J., Yamaguchi A. D., Itami K., Angew. Chem. Int. Ed. 2012, 51, 8960–9009; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 9092–9142; [Google Scholar]
- 1g. Xu L., Zhang S., Li P., Chem. Soc. Rev. 2015, 44, 8848–8858. [DOI] [PubMed] [Google Scholar]
- 2.
- 2a. Beenen M. A., An C., Ellman J. A., J. Am. Chem. Soc. 2008, 130, 6910–6911; [DOI] [PubMed] [Google Scholar]
- 2b. Milo L. J., J. H. Lai, Jr. , Wu W., Liu Y., Maw H., Li Y., Jin Z., Shu Y., Poplawski S. E., Wu Y., Sanford D. G., Sudmeier J. L., Bachovchin W. W., J. Med. Chem. 2011, 54, 4365–4377; [DOI] [PubMed] [Google Scholar]
- 2c. Einsele H., Recent Results Cancer Res. 2010, 184, 173–187. [DOI] [PubMed] [Google Scholar]
- 3.
- 3a. Zweifel G., Brown H. C., Org. React. 1963, 13, 1–54; [Google Scholar]
- 3b. Brown H. C., Cole T. E., Organometallics 1983, 2, 1316–1319; [Google Scholar]
- 3c. Brown H. C., Srebnik M., Cole T. E., Organometallics 1986, 5, 2300–2303. [Google Scholar]
- 4.
- 4a. Chow W. K., So C. M., Lau C. P., Kwong F. Y., Chem. Eur. J. 2011, 17, 6913–6917; [DOI] [PubMed] [Google Scholar]
- 4b. Molander G. A., Trice S. L. J., Dreher S. D., J. Am. Chem. Soc. 2010, 132, 17701–17703; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4c. Tang W., Keshipeddy S., Zhang Y., Wei X., Savoie J., Patel N. D., Yee N. K., Senanayake C. H., Org. Lett. 2011, 13, 1366–1369; [DOI] [PubMed] [Google Scholar]
- 4d. Kawamorita S., Ohmiya H., Iwai T., Sawamura M., Angew. Chem. Int. Ed. 2011, 50, 8363–8366; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 8513–8516; [Google Scholar]
- 4e. Molander G. A., Trice S. L. J., Kennedy S. M., Org. Lett. 2012, 14, 4814–4817; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4f. Molander G. A., Trice S. L. J., Kennedy S. M., J. Org. Chem. 2012, 77, 8678–8688; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4g. Chow W. K., Yuen O. Y., So C. M., Wong W. T., Kwong F. Y., J. Org. Chem. 2012, 77, 3543–3548; [DOI] [PubMed] [Google Scholar]
- 4h. Molander G. A., Trice S. L. J., Kennedy S. M., Dreher S. D., Tudge M. T., J. Am. Chem. Soc. 2012, 134, 11667–11673; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4i. Guerrand H. D. S., Marciasini L. D., Jousseaume M., Vaultier M., Pucheault M., Chem. Eur. J. 2014, 20, 5573–5579; [DOI] [PubMed] [Google Scholar]
- 4j. Pandarus V., Marion O., Gingras G., Béland F., Ciriminna R., Pagliaro M., ChemCatChem 2014, 6, 1340–1348; [Google Scholar]
- 4k. Xu L., Li P., Chem. Commun. 2015, 51, 5656–5659; [DOI] [PubMed] [Google Scholar]
- 4l. Smith K. B., Logan K. M., You W., Brown M. K., Chem. Eur. J. 2014, 20, 12032–12036; [DOI] [PubMed] [Google Scholar]
- 4m. Bhanuchandra M., Baralle A., Otsuka S., Nogi K., Yorimitsu H., Org. Lett. 2016, 18, 2966–2969; [DOI] [PubMed] [Google Scholar]
- 4n. Dzhevakov P. B., Topchiy M. A., Zharkove D. A., Morozov O. S., Asachenko A. F., Nechaev M. S., Adv. Synth. Catal. 2016, 358, 977–983; [Google Scholar]
- 4o. Ma Y., Song C., Jiang W., Xue G., Cannon J. F., Wang X., Andrus M. B., Org. Lett. 2003, 5, 4635–4638; [DOI] [PubMed] [Google Scholar]
- 4p. Willis D. M., Strongin R. M., Tetrahedron Lett. 2000, 41, 8683–8686. [Google Scholar]
- 5.
- 5a. Mkhalid I. A. I., Barnard J. H., Marder T. B., Murphy J. M., Hartwig J. F., Chem. Rev. 2010, 110, 890–931; [DOI] [PubMed] [Google Scholar]
- 5b. Ishiyama T., Miyaura N., J. Organomet. Chem. 2003, 680, 3–11; [Google Scholar]
- 5c. Hartwig J. F., Chem. Soc. Rev. 2011, 40, 1992–2002; [DOI] [PubMed] [Google Scholar]
- 5d. Hartwig J. F., Acc. Chem. Res. 2012, 45, 864–873; [DOI] [PubMed] [Google Scholar]
- 5e. Ishiyama T., Isou H., Kikuchi T., Miyaura N., Chem. Commun. 2010, 46, 159–161; [DOI] [PubMed] [Google Scholar]
- 5f. Kawamorita S., Miyazaki T., Ohmiya H., Iwai T., Sawamura M., J. Am. Chem. Soc. 2011, 133, 19310–19313; [DOI] [PubMed] [Google Scholar]
- 5g. Preshlock S. M., Plattner D. L., Maligres P. E., Krska S. W., R. E. Maleczka, Jr. , M. R. Smith III , Angew. Chem. Int. Ed. 2013, 52, 12915–12919; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 13153–13157; [Google Scholar]
- 5h. Konishi S., Kawamorita S., Iwai T., Steel P. G., Marder T. B., Sawamura M., Chem. Asian J. 2014, 9, 434–438; [DOI] [PubMed] [Google Scholar]
- 5i. Ros A., Fernández R., Lassaletta J. M., Chem. Soc. Rev. 2014, 43, 3229–3243; [DOI] [PubMed] [Google Scholar]
- 5j. B. A. Vanchura II , Preshlock S. M., Roosen P. C., Kallepalli V. A., Staples R. J., R. E. Maleczka Jr , Singleton D. A., M. R. Smith III , Chem. Commun. 2010, 46, 7724–7726; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5k. Tajuddin H., Harrisson P., Bitterlich B., Collings J. C., Sim N., Batsanov A. S., Cheung M. S., Kawamorita S., Maxwell A. C., Shukla L., Morris J., Lin Z., Marder T. B., Steel P. G., Chem. Sci. 2012, 3, 3505–3515; [Google Scholar]
- 5l. Ishiyama T., Takagi J., Ishida K., Miyaura N., Anastasi N. R., Hartwig J. F., J. Am. Chem. Soc. 2002, 124, 390–391; [DOI] [PubMed] [Google Scholar]
- 5m. Cho J.-Y., Tse M. K., Holmes D., R. E. Maleczka, Jr. , M. R. Smith III , Science 2002, 295, 305–308; [DOI] [PubMed] [Google Scholar]
- 5n. Mkhalid I. A. I., Coventry D. N., Albesa-Jove D., Batsanov A. S., Howard J. A. K., Perutz R. N., Marder T. B., Angew. Chem. Int. Ed. 2006, 45, 489–491; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2006, 118, 503–505; [Google Scholar]
- 5o. Tajuddin H., Shukla L., Maxwell A. C., Marder T. B., Steel P. G., Org. Lett. 2010, 12, 5700–5703; [DOI] [PubMed] [Google Scholar]
- 5p. Preshlock S. M., Ghaffari B., Maligres P. E., Krska S. W., R. E. Maleczka, Jr. , M. R. Smith III , J. Am. Chem. Soc. 2013, 135, 7572–7582; [DOI] [PubMed] [Google Scholar]
- 5q. Larsen M. A., Hartwig J. F., J. Am. Chem. Soc. 2014, 136, 4287–4299. [DOI] [PubMed] [Google Scholar]
- 6.
- 6a. Chen H., Schlecht S., Semple T. C., Hartwig J. F., Science 2000, 287, 1995–1997; [DOI] [PubMed] [Google Scholar]
- 6b. Cho J.-Y., Iverson C. N., M. R. Smith III , J. Am. Chem. Soc. 2000, 122, 12868–12869; [Google Scholar]
- 6c. Shimada S., Batsanov A. S., Howard J. A. K., Marder T. B., Angew. Chem. Int. Ed. 2001, 40, 2168–2171; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2001, 113, 2226–2229. [Google Scholar]
- 7.
- 7a. Rosen B. M., Huang C., Percec V., Org. Lett. 2008, 10, 2597–2600; [DOI] [PubMed] [Google Scholar]
- 7b. Wilson D. A., Wilson C. J., Rosen B. M., Percec V., Org. Lett. 2008, 10, 4879–4882; [DOI] [PubMed] [Google Scholar]
- 7c. Rosen B. M., Wilson D. A., Wilson C. J., Peterca M., Won B. C., Huang C., Lipski L. R., Zeng X., Ungar G., Heiney P. A., Percec V., J. Am. Chem. Soc. 2009, 131, 17500–17521; [DOI] [PubMed] [Google Scholar]
- 7d. Wilson V., Wilson C. J., Moldoveanu C., Resmerita A. M., Corcoran P., Hoang L. M., Rosen B. M., Percec V., J. Am. Chem. Soc. 2010, 132, 1800–1801; [DOI] [PubMed] [Google Scholar]
- 7e. Moldoveanu C., Wilson D. A., Wilson C. J., Corcoran P., Rosen B. M., Percec V., Org. Lett. 2009, 11, 4974–4977; [DOI] [PubMed] [Google Scholar]
- 7f. Moldoveanu C., Wilson D. A., Wilson C. J., Leowanawat P., Resmerita A.-M., Liu C., Rosen B. M., Percec V., J. Org. Chem. 2010, 75, 5438–5452; [DOI] [PubMed] [Google Scholar]
- 7g. Yamamoto T., Morita T., Takagi J., Yamakawa T., Org. Lett. 2011, 13, 5766–5769; [DOI] [PubMed] [Google Scholar]
- 7h. Molander G. A., Cavalcanti L. N., García-García C., J. Org. Chem. 2013, 78, 6427–6439; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7i. Huang K., Yu D.-G., Zheng S.-F., Wu Z.-H., Shi Z.-J., Chem. Eur. J. 2011, 17, 786–791; [DOI] [PubMed] [Google Scholar]
- 7j. Liu X.-W., Echavarren J., Zarate C., Martin R., J. Am. Chem. Soc. 2015, 137, 12470–12473; [DOI] [PubMed] [Google Scholar]
- 7k. Hu J., Sun H., Cai W., Pu X., Zhang Y., Shi Z., J. Org. Chem. 2016, 81, 14–24. [DOI] [PubMed] [Google Scholar]
- 8.
- 8a. Zhu W., Ma D., Org. Lett. 2006, 8, 261–263; [DOI] [PubMed] [Google Scholar]
- 8b. Kleeberg C., Dang L., Lin Z., Marder T. B., Angew. Chem. Int. Ed. 2009, 48, 5350–5354; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2009, 121, 5454–5458; [Google Scholar]
- 8c. Grigg R. D., Van Hoveln R., Schomaker J. M., J. Am. Chem. Soc. 2012, 134, 16131–16134; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8d. Labre F., Gimbert Y., Bannwarth P., Olivero S., Duñach E., Chavant P. Y., Org. Lett. 2014, 16, 2366–2369; [DOI] [PubMed] [Google Scholar]
- 8e. Ando S., Matsunaga H., Ishizuka T., J. Org. Chem. 2015, 80, 9671–9681; [DOI] [PubMed] [Google Scholar]
- 8f. Niwa T., Ochiai H., Watanabe Y., Hosoya T., J. Am. Chem. Soc. 2015, 137, 14313–14318; [DOI] [PubMed] [Google Scholar]
- 8g. Zhang J., Wang X., Yu H., Ye J., Synlett 2012, 23, 1394–1396. [Google Scholar]
- 9.
- 9a. Marciasini L. D., Richy N., Vaultier M., Pucheault M., Adv. Synth. Catal. 2013, 355, 1083–1088; [Google Scholar]
- 9b. Bedford R. B., Brenner P. B., Carter E., Clifton J., Cogswell P. M., Gower N. J., Haddow M. F., Harvey J. N., Kehl J. A., Murphy D. M., Neeve E. C., Neidig M., Nunn J., Snyder B. E. R., Taylor J., Organometallics 2014, 33, 5767–5780. [Google Scholar]
- 10.
- 10a. Adams C. J., Baber R. A., Batsanov A. S., Bramham G., Charmant J. P. H., Haddow M. F., Howard J. A. K., Lam W. H., Lin Z., Marder T. B., Norman N. C., Orpen A. G., Dalton Trans. 2006, 1370–1373; [DOI] [PubMed] [Google Scholar]
- 10b. Frank R., Howell J., Campos J., Tirfoin R., Phillips N., Zahn S., Mingos D. M. P., Aldridge S., Angew. Chem. Int. Ed. 2015, 54, 9586–9590; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 9722–9726; [Google Scholar]
- 10c. Zhang L., Peng D., Leng X., Huang Z., Angew. Chem. Int. Ed. 2013, 52, 3676–3680; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 3764–3768; [Google Scholar]
- 10d. Zhang L., Zuo Z., Wan X., Huang Z., J. Am. Chem. Soc. 2014, 136, 15501–15504; [DOI] [PubMed] [Google Scholar]
- 10e. Zhang L., Zuo Z., Leng X., Huang Z., Angew. Chem. Int. Ed. 2014, 53, 2696–2700; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 2734–2738; [Google Scholar]
- 10f. Zhang L., Huang Z., J. Am. Chem. Soc. 2015, 137, 15600–15603; [DOI] [PubMed] [Google Scholar]
- 10g. Jia X., Huang Z., Nat. Chem. 2016, 8, 157–161; [DOI] [PubMed] [Google Scholar]
- 10h. Yao W., H, Fang , Peng S., Wen H., Zhang L., Hu A., Organometallics 2016, 35, 1559–1564. [Google Scholar]
- 11.
- 11a. Mo F., Dong G., Zhang Y., Wang J., Org. Biomol. Chem. 2013, 11, 1582–1593; [DOI] [PubMed] [Google Scholar]
- 11b. Zhu C., Yamane M., Org. Lett. 2012, 14, 4560–4563; [DOI] [PubMed] [Google Scholar]
- 11c. Yu J., Zhang L., Yan G., Adv. Synth. Catal. 2012, 354, 2625–2628; [Google Scholar]
- 11d. Mo F., Jiang Y., Qiu D., Zhang Y., Wang J., Angew. Chem. Int. Ed. 2010, 49, 1846–1849; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2010, 122, 1890–1893; [Google Scholar]
- 11e. Qiu D., Jin L., Zheng Z., Meng H., Mo F., Wang X., Zhang Y., Wang J., J. Org. Chem. 2013, 78, 1923–1933; [DOI] [PubMed] [Google Scholar]
- 11f. Erb W., Hellal A., Albini M., Rouden J., Blanchet J., Chem. Eur. J. 2014, 20, 6608–6612. [DOI] [PubMed] [Google Scholar]
- 12.
- 12a. Wu X. F., Chem. Asian J. 2012, 7, 2502–2509; [DOI] [PubMed] [Google Scholar]
- 12b. Wu X. F., Neumann H., Adv. Synth. Catal. 2012, 354, 3141–3160; [Google Scholar]
- 12c. Wu X. F., Chem. Rec. 2015, 15, 949–963. [DOI] [PubMed] [Google Scholar]
- 13. Kajiwara T., Terabayashi T., Yamashita M., Nozaki K., Angew. Chem. Int. Ed. 2008, 47, 6606–6610; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2008, 120, 6708–6712. [Google Scholar]
- 14.
- 14a. Hupe E., Calaza M. I., Knochel P., J. Organomet. Chem. 2003, 680, 136–142; [Google Scholar]
- 14b. Hupe E., Calaza M. I., Knochel P., Chem. Eur. J. 2003, 9, 2789–2796. [DOI] [PubMed] [Google Scholar]
- 15. Nagashima Y., Takita R., Yoshida K., Hirano K., Uchiyama M., J. Am. Chem. Soc. 2013, 135, 18730–18733. [DOI] [PubMed] [Google Scholar]
- 16. Tsuchimoto T., Utsugi H., Sugiura T., Horio S., Adv. Synth. Catal. 2015, 357, 77–82. [Google Scholar]
- 17. Bose S. K., Fucke K., Liu L., Steel P. G., Marder T. B., Angew. Chem. Int. Ed. 2014, 53, 1799–1803; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 1829–1834. [Google Scholar]
- 18.
- 18a. Bose S. K., Marder T. B., Org. Lett. 2014, 16, 4562–4565; [DOI] [PubMed] [Google Scholar]
- 18b. Bose S. K., Deißenberger A., Eichhorn A., Steel P. G., Lin Z., Marder T. B., Angew. Chem. Int. Ed. 2015, 54, 11843–11847; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 12009–12014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


