Abstract
For patients with hematologic malignancies undergoing allogeneic hematopoietic cell transplantation, umbilical cord blood transplantation (UCBT) has become an acceptable alternative donor source in the absence of a matched sibling or unrelated donor. However, there have been few published series dedicated solely to describing the outcomes of adult patients with myelodysplastic syndrome (MDS) who have undergone UCBT.
From 2004 to 2013, 176 adult MDS patients underwent UCBT as reported to the Center for International Blood and Marrow Transplant Research. Median age at the time of transplant was 56 years (range 18–73 years), with 10% having very low, 23% low, 19% intermediate, 19% high, and 13% very high-risk Revised International Prognostic Scoring System (IPSS-R) scores, respectively. The 100-day probability of Grade 2–4 acute graft-versus-host disease (GVHD) was 38%, and the 3-year probability of chronic GVHD was 28%. The probability of relapse and transplant-related mortality (TRM) at 3 years was 32% and 40%, respectively, leading to a 3-year, disease-free survival (DFS) of 28%, and overall survival (OS) of 31%. In multivariate analysis increasing IPSS-R score at time of HCT was associated with inferior TRM (P=.0056), DFS (P=.018), and OS (P=.0082), but not with GVHD or relapse. Pre-transplant comorbidities were associated with TRM (P=.001), DFS (P=.02), and OS (P=.001). Reduced conditioning intensity was associated with increased risk of relapse (RR 3.95; 95% CI 1.78–8.75, P<.001), and although a higher proportion of myeloablative UCBTs were done for those with high-risk disease, the effect of conditioning regimen intensity was the same regardless of IPSS-R score.
For those who lack a matched sibling or unrelated donor, UCBT can result in long-term, disease-free survival for some patients. However, the success of UCBT in this population is hampered by a high rate of TRM.
Keywords: umbilical cord transplantation, blood and marrow transplantation, myelodysplastic syndrome
Introduction
Widespread application of innovative sequencing technologies is rapidly unraveling the biologic underpinnings driving the pathogenesis of the myelodysplastic syndromes (MDS). Nonetheless, allogeneic hematopoietic cell transplantation (HCT) remains the only therapeutic modality that has demonstrated curative potential, with patients surviving in unmaintained remission for more than two decades after HCT [1]. However, only one-in-four patients will have a sibling donor that is matched for human leukocyte antigens (HLA), which is likely to decline as the average family size in the United States declines [2]. Moreover, older MDS patients are likely to have older siblings, increasing the chance of potential donors being found to be unsuitable due to comorbid conditions. With the development of robust donor registries, an HLA-matched donor can be identified for approximately 75% of Caucasian recipients, with successful matching being much more limited for other ethnic groups [3, 4]. Therefore, alternative donor sources have been actively explored. Umbilical cord blood (UCB) is an alternative hematopoietic cell source with two distinct advantages. One being a relative tolerance of HLA disparity, and the other, as a cryopreserved stem cell source, a rapid availability with flexible timing of transplant [5, 6]. A major drawback of UCB as a donor source is the limited number of cells leading to a delayed time to engraftment and immune reconstitution. This has been overcome, in part, by the use of two cord blood units, or ex vivo expansion of a cord blood unit prior to infusion [7, 8]. Over the past decade, improved cord blood unit selection, conditioning, and supportive care have all lead to improved outcomes in adults after UCB transplant (UCBT). Contemporary retrospective analyses suggest that disease-free survival after UCBT for hematologic malignancies is comparable to that of matched-related or unrelated donors [9–11].
Many of the large studies evaluating UCBT have included patients with MDS, however their post-transplant outcomes can only be described through subgroup analyses. There have been few published series solely describing the outcomes of adult patients who have undergone UCBT for MDS [12–14]. In the absence of substantial data, patients that could benefit from UCBT may not be offered the treatment option when they otherwise would be considered for matched related or unrelated donors. Likewise, clinical trials in HCT also may exclude cord blood as a donor source, such as Blood and Marrow Clinical Trials Network (BMT-CTN) 1102 [15]. Treatment decisions and clinical trial design can be aided by providing a description of UCBT for MDS that incorporates a large number of patients from multiple centers. Therefore, we conducted a descriptive analysis of patients who have undergone UCBT for MDS as reported to the Center for International Blood and Marrow Transplantation Research (CIBMTR). We also sought to validate the ability of MDS disease-risk models to predict post-HCT outcomes.
Patients and Methods
Data Sources
The CIBMTR is a combined research program of the Medical College of Wisconsin and the National Marrow Donor Program, which consists of a voluntary network of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous transplants to a centralized statistical center. Observational studies conducted by CIBMTR are performed in compliance with all applicable Federal regulations pertaining to the protection of human research participants. Protected health information issued in the performance of such research is collected and maintained in CIBMTR’s capacity as a Public Health Authority under the Health Insurance Portability and Accountability Act Privacy Rule. Additional details regarding the data source are described elsewhere [16].
Patients
Adult patients (age ≥18 years) with MDS who underwent their first transplant between 2004 and 2013 were included (N=2709). Patients with HLA-matched sibling, matched or mismatched unrelated donor (URD), haploidentical or syngeneic donor, and those missing donor data, were excluded from this analysis (N=2518). An additional 15 patients were excluded due to missing 100-day follow-up data.
Cytogenetics were classified based on those identified by the MDS Comprehensive Cytogenetic Scoring System [17]. Monosomal karyotype was defined as persons that have monosomy of two or more chromosomes or one single autosomal monosomy in the presence of other structural abnormalities [18]. Overall disease risk at the time of transplant was stratified by the Revised International Prognostic Scoring System (IPSS-R) [19].
Study Endpoints
Primary endpoints included transplant-related mortality (TRM), relapse, overall (OS), and disease-free survival (DFS). TRM was defined as death from any cause in the first 28 days post-transplantation irrespective of relapse status. Death beyond day +28 was considered to be transplant-related if the disease state was remission. DFS was defined as time to relapse or death from any cause. OS was defined as time from transplantation to death from any cause. Patients were censored at last follow-up.
Secondary endpoints included hematopoietic recovery, as well as the incidence of acute and chronic graft-versus-host disease (GVHD). Neutrophil and platelet engraftment were defined as the time from transplantation to a neutrophil count (ANC) >0.5 × 109/L (first of 3 consecutive days), and time to platelets ≥20 × 109/L (first of 3 consecutive days and no platelet transfusions 7 days prior), respectively. GVHD, both acute and chronic, were graded per consensus criteria [20, 21]. Conditioning regimen intensity was determined according to the CIBMTR Reduced-Intensity Conditioning (RIC) Regimen Workshop [22].
Statistical Analysis
Descriptive tables of patient, disease, and transplant-related variables for patients receiving UCBT for MDS were generated. Univariate probabilities of OS and DFS were calculated using the Kaplan-Meier estimator; the log-rank test was used for univariate comparisons. Probabilities of graft failure, acute and chronic GVHD, TRM, and relapse were calculated using cumulative incidence functions to accommodate competing risks.
Assessment of potential risk factors for post-HCT outcome was evaluated in multivariate analyses using the Cox proportional hazards model that included age and Karnofsky performance status at time of transplantation, comorbidity score (HCT-CI), recipient CMV status, MDS risk score, primary versus secondary MDS, single versus double cord blood units transplanted, nucleated cell dose, year of transplantation, conditioning regimen intensity, use of serotherapy (anti-thymocyte globulin or alemtuzumab) in the condition regimen, and type of GVHD prophylaxis (tacrolimus-based versus cyclosporine-based versus other) as covariates. Two models were built: 1) with IPSS-R as the main effect; and 2) with the proposed CIBMTR MDS Transplantation Risk Score [23] as the main effect. The latter model was developed and validated among MDS patients (training n = 1,151; validation n = 577) who underwent allogeneic HCT from either an identical sibling donor or a well-matched unrelated donor. The two risk scores were compared using concordance probability [24]. To account for center effect, the marginal Cox model was performed [25]. Backward elimination procedure, with a p-value <.05, was used to select significant covariates. Interactions between the main effect and significant covariates were examined.
Results
Patient, Disease, and Transplantation Characteristics
Patient and disease characteristics are presented in Table 1. We identified 176 adult MDS patients, including 21 with CMML, who underwent UCBT, at 59 centers, between 2004 and 2013. The median number of transplants per center was 2 (range 1–26). With 34% being over the age of 60 years, the median age at the time of transplantation was 56 years (range 18–73). Most patients had Karnofsky Performance Scores (KPS) of 90–100%, and 32%, 27%, 34% of patients had HCT-CI scores of 0, 1–2, or ≥3, respectively.
Table 1.
Characteristics of patients received allogeneic umbilical cord blood transplantation for MDS between 2004 and 2013
| Variable | N (%) |
|---|---|
| Number of patients | 176 |
| Patient-related | |
| Age, median (range) | 56 (18–73) |
| Gender | |
| Male | 99 (56) |
| Female | 77 (44) |
| Karnofsky score | |
| 90–100% | 127 (72) |
| < 90% | 47 (27) |
| Missing | 2 (1) |
| Comorbitidy score (HCT-CI) | |
| 0 | 57 (32) |
| 1–2 | 48 (27) |
| ≥3 | 60 (34) |
| Not available before 2007 | 11 (6) |
| Recipient CMV status | |
| Negative | 58 (33) |
| Positive | 117 (66) |
| Not tested | 1 (<1) |
| Disease-related | |
| Secondary MDS | |
| No | 146 (83) |
| Yes | 25 (14) |
| Missing | 5 (3) |
| Pre-transplantation cytoreductive therapy | |
| Hypomethylating agent only | 87 (49) |
| Intensive chemotherapy only | 19 (11) |
| Hypomethylating agent & intensive chemotherapy | 24 (14) |
| None | 40 (23) |
| Missing | 6 (3) |
| Bone marrow myeloblasts prior to transplantation | |
| < 5% | 127 (73) |
| 5–10% | 23 (13) |
| > 10% | 13 (7) |
| Missing | 13 (7) |
| Blast in blood prior to transplant | |
| ≤3% | 121 (69) |
| > 3% | 12 (7) |
| Missing | 43 (24) |
| Platelet count prior to transplant | |
| ≤ 50 × 109/L | 68 (39) |
| > 50 × 109/L | 108 (61) |
| Cytogenetic risk prior to conditioning | |
| Good | 61 (35) |
| Intermediate | 34 (19) |
| Poor | 34 (19) |
| Very poor | 3 (2) |
| Monosomal Karyotype | 30 (17) |
| Not tested | 2 (1) |
| Missing/unable to classify | 12 (7) |
| IPSS-R prior to transplant | |
| Very low | 18 (10) |
| Low | 41 (23) |
| Intermediate | 33 (19) |
| High | 34 (19) |
| Very high | 22 (13) |
| Missing | 28 (16) |
| CIBMTR MDS transplantation risk score [23] | |
| Low | 20 (11) |
| Intermediate | 78 (44) |
| High | 42 (24) |
| Very high | 1 (<1) |
| Missing | 35 (20) |
| Time between diagnosis and transplant | |
| 0–3 months | 54 (31) |
| 3–6 months | 56 (32) |
| ≥ 6 months | 66 (38) |
| Transplant-related | |
| Number of cord blood units | |
| Single cord | 36 (20) |
| Double cord | 140 (80) |
| Cord blood HLA matching | |
| 3/6 | 4 (2) |
| 4/6 | 102 (58) |
| 5/6 | 53 (30) |
| 6/6 | 4 (2) |
| Missing | 13 (7) |
| CD34+ cell dose, median (range), × 105/kg | 2 (<1–73) |
| CD34+ cell doses | |
| 0–2 × 105/kg | 83 (47) |
| 2–4 × 105/kg | 51 (29) |
| 4–8 × 105/kg | 16 (9) |
| > 8 × 105/kg | 14 (8) |
| Missing | 12 (7) |
| Nucleated cell doses, median (range), × 107/kg | 4 (<1–29) |
| Nucleated cell doses | |
| 0–2 × 107/kg | 13 (7) |
| 2–4 × 107/kg | 74 (42) |
| 4–8 × 107/kg | 75 (43) |
| > 8 × 107/kg | 5 (3) |
| Missing | 9 (5) |
| Donor-recipient sex match (1st cord blood unit) | |
| Male-Male | 38 (22) |
| Male-Female | 33 (19) |
| Female-Male | 41 (23) |
| Female-Female | 33 (19) |
| Missing | 31 (18) |
| Donor-recipient sex match (2nd cord blood unit) | |
| Male-Male | 20 (11) |
| Male-Female | 19 (11) |
| Female-Male | 24 (14) |
| Female-Female | 15 (9) |
| NA | 36 (20) |
| Missing | 62 (35) |
| Year of transplantation | |
| 2004–2007 | 24 (14) |
| 2008–2009 | 62 (35) |
| 2010–2011 | 46 (26) |
| 2012–2013 | 44 (25) |
| Conditioning regimen | |
| Myeloablative | 61 (35) |
| RIC/NMA | 115 (65) |
| Serotherapy used in conditioning | |
| ATG alone | 76 (43) |
| alemtuzumab alone | 1 (<1) |
| No ATG or alemtuzumab | 99 (56) |
| GVHD prophylaxis | |
| Tacrolimus-based | 81 (46) |
| Cyclosporine-based | 83 (47) |
| Other(s) | 8 (5) |
| Missing | 4 (2) |
| Median follow-up of survivors (range), months | 37 (3–78) |
List of abbreviations: Hematopoietic cell transplantation comorbidity index (HCT-CI), cytomegalovirus (CMV), human leukocyte antigen (HLA), reduced intensity conditioning (RIC), non-myeloablative (NMA), graft-versus-host disease (GVHD)
The median time from diagnosis to UCBT was 9 months (range 1–147). Cytogenetic data and IPSS-R scores at the time of transplant were available for 92% and 84% of patients, respectively. A majority of patients (77%) received some form of cytoreductive therapy, predominantly hypomethylating agents, prior to transplantation, and 72% had 5% blasts or less on their pre-HCT bone marrow biopsy.
Myeloablative conditioning regimens were given to 61 (35%), and 77 (30% of myeloablative, 51% of RIC/non-myeloablative) patients received either anti-thymocyte globulin or alemtuzumab as part of their conditioning. Double cord blood units were used in 80% of transplants, with median total nucleated cell dose (TNC) of 4 × 107/kg (range <1–29 × 107/kg). As expected, conditioning regimen intensity was associated with age at the time of transplantation (P=.001), but was not associated with receiving a single or double cord blood unit (P=.58).
Considering the unit with the higher number of human leukocyte antigen (HLA) incompatibilities with the recipient, 60% of recipients had 2 or more mismatches. GVHD prophylaxis regimens were primarily based on a combination calcineurin inhibitor (tacrolimus or cyclosporine) with mycophenolate mofetil (80%). The median follow-up of survivors was 37 months (range 3–78 months). The completeness of follow up was 98%, 93%, and 89% at 1, 2, and 3 years, respectively [26].
Hematopoietic Recovery
The cumulative incidence of neutrophil recovery at 28 and 100 days after UCBT was 92% (95% CI 88–95%) and 97% (95% CI 95–99%), respectively. The corresponding values for platelet recovery at 28 and 100 days was 66% (95% CI 59–72%) and 86% (95% CI 81–90%), respectively.
GVHD, Relapse, Treatment-Related Mortality, and Survival Outcomes
The cumulative incidence acute GVHD at day 100 was 38% (95% CI 30–45%) and 14% (95% CI 9–20%), for Grades 2–4 and 3–4, respectively (Table 2). The probability of chronic GVHD at 1 year was 26% (95% CI 19–33%), and 28% (95% CI 21–36%) at 3 years.
Table 2.
Univariate analysis results for patients undergoing umbilical cord blood transplantation for MDS between 2004 and 2013
| Outcome | Probability of event (95% CI) |
|---|---|
| Grade 2–4 acute GVHD | |
| 100-day | 38 (30–45)% |
| Grade 3–4 acute GVHD | |
| 100-day | 14 (9–20)% |
| Chronic GVHD | |
| 1-year | 26 (19–33)% |
| 3-year | 28 (21–36)% |
| Relapse | |
| 1-year | 27 (21–34)% |
| 3-year | 32 (25–40)% |
| Transplant-related mortality | |
| 1-year | 34 (27–42)% |
| 3-year | 40 (33–48)% |
| Disease-free survival | |
| 1-year | 38 (31–46)% |
| 3-year | 28 (21–35)% |
| Overall survival | |
| 1-year | 47 (40–55)% |
| 3-year | 31 (24–39)% |
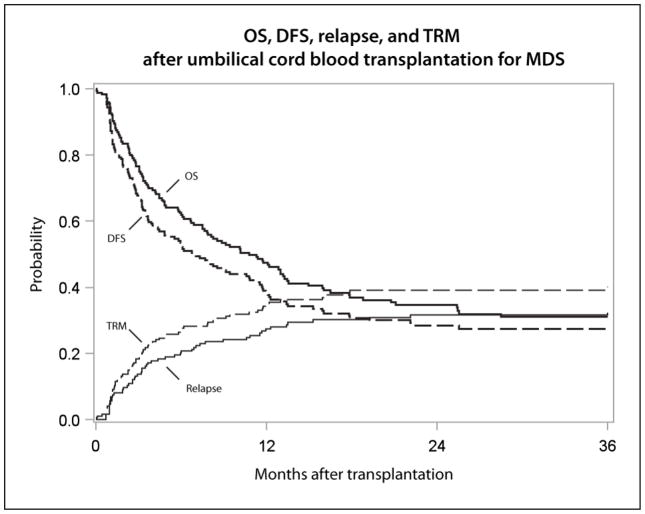
The probability of relapse at 3 years was 32% (95% CI 25–40%), with the latest relapse occurring at 22 months after UCBT (Table 2, Figure 1).
Figure 1.
Overall survival, disease free survival, relapse, and transplant-related mortality after umbilical cord blood transplantation for MDS
The 3-year probabilities of TRM, DFS, and OS were 40% (95% CI 33–48%), 28% (95% CI 21–35%), and 31% (95% CI 24–39%), respectively (Table 2, Figure 1). The most common cause of death was persistence or relapse of MDS (45%), followed by infection (16%) and organ failure (13%). Graft failure accounted for only 3% of deaths (Table 3)
Table 3.
Cause of death after umbilical cord blood transplantation
| IPSS-R Risk Group | Total (%) | |||
|---|---|---|---|---|
| Cause of death | Very low/Low (%) | Intermediate (%) | High/Very high (%) | |
| Primary disease/Relapse | 17 (47) | 12 (63) | 13 (34) | 42 (45) |
| Infection | 7 (19) | 3 (16) | 5 (13) | 15 (16) |
| Organ failure | 4 (11) | 0 (0) | 8 (21) | 12 (13) |
| GVHD | 3 (8) | 1 (5) | 4 (11) | 8 (9) |
| Idiopathic pneumonitis/ARDS | 2 (6) | 1 (5) | 4 (11) | 7 (8) |
| Other cause | 3 (8) | 1 (5) | 2 (5) | 6 (6) |
| Graft failure | 0 (0) | 1 (5) | 2 (5) | 3 (3) |
Impact of IPSS-R at the time of UCBT
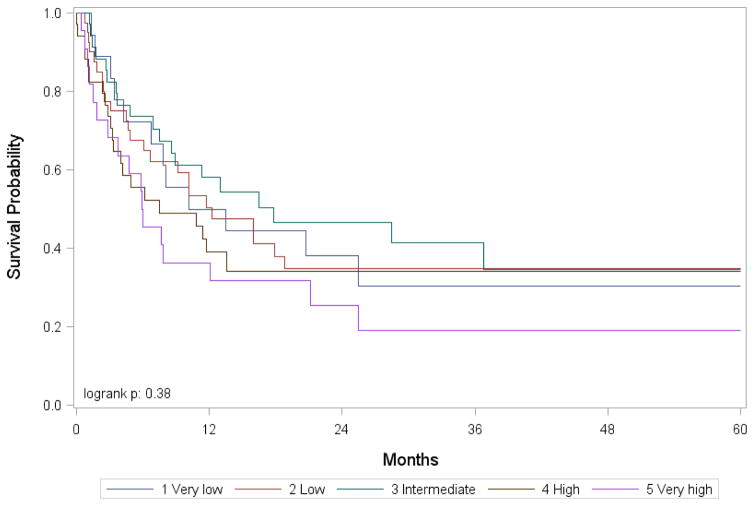
In multivariate analysis using marginal Cox model to adjust for center effect, IPSS-R at the time of HCT was associated with TRM (P=.006), DFS (P=.02), and OS (P=.008, Table 4, Figure 2). IPSS-R was not associated with the incidence of acute (P=.52) or chronic GVHD (P=.66), as well as disease relapse (P=.86). RIC/non-meyloablative (NMA) conditioning regimens were associated with an increased risk of relapse relative to myeloablative regimens (HR 3.95, 95% CI 1.78–8.75; P=.0007, Supplemental Figure 2), and a higher proportion of myeloablative HCTs were done for those with high-risk disease by IPSS-R. However, the interaction between conditioning intensity and IPSS-R for relapse (P=.17) and TRM (P=. 0.33) were not significant. In this model, HCT-CI was also associated with TRM (P=.001), DFS (P=.02), and OS (P=.001, Table 4, Supplemental Figure 1).
Table 4.
Multivariate analysis for adult MDS patients who received umbilical cord blood transplantation between 2004 and 2013
| Relapse | ||||||
|---|---|---|---|---|---|---|
| 95% CI | 95% CI | Overall | ||||
| IPSS-R | N | RR | Lower Limit | Upper Limit | p-value | p-value |
| Very low/Low | 64 | 1 | 0.8611 | |||
| Intermediate | 34 | 1.184 | 0.577 | 2.429 | 0.646 | |
| High/Very high | 54 | 1.143 | 0.576 | 2.268 | 0.7017 | |
| Missing | 28 | 1.391 | 0.65 | 2.977 | 0.3955 | |
| Conditioning regimen | ||||||
| Myeloablative | 62 | 1 | 0.0007 | |||
| RIC/NMA | 118 | 3.947 | 1.781 | 8.746 | 0.0007 | |
| TRM | ||||||
| 95% CI | 95% CI | Overall | ||||
| IPSS-R | N | RR | Lower Limit | Upper Limit | p-value | p-value |
| Very low/Low | 64 | 1 | 0.0056 | |||
| Intermediate | 34 | 0.682 | 0.344 | 1.352 | 0.2726 | |
| High/Very high | 54 | 1.755 | 1.12 | 2.751 | 0.0141 | |
| Missing | 28 | 2.046 | 0.926 | 4.52 | 0.0766 | |
| HCT-CI | ||||||
| 0 | 58 | 1 | 0.0013 | |||
| 1–2 | 50 | 1.178 | 0.68 | 2.04 | 0.5585 | |
| ≥3 | 61 | 2.395 | 1.393 | 4.118 | 0.0016 | |
| Missing | 11 | 0.743 | 0.358 | 1.539 | 0.4234 | |
| DFS | ||||||
| 95% CI | 95% CI | Overall | ||||
| IPSS-R | N | RR | Lower Limit | Upper Limit | p-value | p-value |
| Very low/Low | 64 | 1 | 0.0177 | |||
| Intermediate | 34 | 0.832 | 0.504 | 1.374 | 0.4728 | |
| High/Very high | 54 | 1.393 | 1.017 | 1.906 | 0.0387 | |
| Missing | 28 | 1.632 | 0.946 | 2.814 | 0.0782 | |
| HCT-CI | ||||||
| 0 | 58 | 1 | 0.0241 | |||
| 1–2 | 50 | 1.086 | 0.632 | 1.867 | 0.7646 | |
| ≥3 | 61 | 1.908 | 1.164 | 3.126 | 0.0104 | |
| Missing | 11 | 1.229 | 0.715 | 2.113 | 0.4551 | |
| OS | ||||||
| 95% CI | 95% CI | Overall | ||||
| IPSS-R | N | RR | Lower Limit | Upper Limit | p-value | p-value |
| Very low/Low | 65 | 1 | 0.0082 | |||
| Intermediate | 35 | 0.876 | 0.51 | 1.504 | 0.6303 | |
| High/Very high | 55 | 1.548 | 1.119 | 2.142 | 0.0084 | |
| Missing | 28 | 1.724 | 0.911 | 3.262 | 0.0943 | |
| HCT-CI | ||||||
| 0 | 60 | 1 | 0.0014 | |||
| 1–2 | 50 | 1.108 | 0.618 | 1.988 | 0.7297 | |
| ≥3 | 61 | 2.221 | 1.314 | 3.755 | 0.0029 | |
| Missing | 12 | 1.478 | 0.868 | 2.517 | 0.1506 |
Figure 2.
Overall survival after umbilical cord blood transplantation for MDS by pre-transplantation IPSS-R score
Validation of CIBMTR MDS Transplantation Risk Score
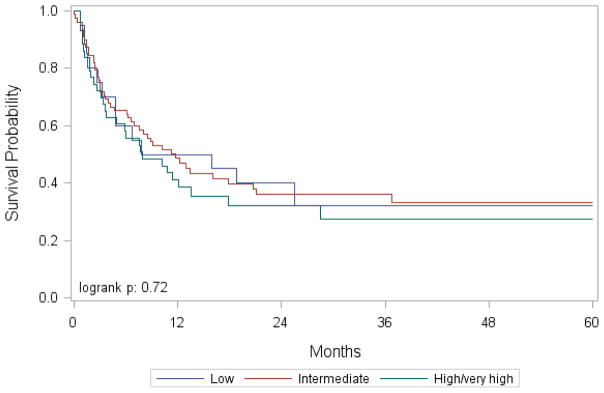
In multivariate analysis using marginal Cox model to adjust for center effect, the CIBMTR MDS risk score was not associated with any of the outcomes (Table 5, Figure 3). As with the previous model, where IPSS-R was the main effect, RIC/NMA conditioning regimens, relative to myeloablative regimens, were associated with relapse (P=.0008). Also, HCT-CI was associated with TRM (P=.02), DFS (P=.03), and OS (P=.001) as seen when IPSS-R is the main effect.
Table 5.
Multivariate analysis results for patients undergoing umbilical cord blood transplantation for MDS between 2004 and 2013, with the CIBMTR MDS Transplantation Score as the main effect
| Relapse | ||||||
|---|---|---|---|---|---|---|
| 95% CI | 95% CI | Overall | ||||
| CIBMTR MDS score | N | RR | Lower Limit | Upper Limit | p-value | p-value |
| Low | 19 | 1 | 0.3072 | |||
| Intermediate | 83 | 0.749 | 0.281 | 1.997 | 0.5631 | |
| High/Very high | 44 | 0.869 | 0.304 | 2.486 | 0.7932 | |
| Missing | 34 | 1.378 | 0.499 | 3.805 | 0.5366 | |
| Conditioning regimen | ||||||
| Myeloablative | 62 | 1 | 0.0008 | |||
| RIC/NMA | 118 | 3.963 | 1.775 | 8.85 | 0.0008 | |
| TRM | ||||||
| 95% CI | 95% CI | Overall | ||||
| CIBMTR MDS score [23] | N | RR | Lower Limit | Upper Limit | p-value | p-value |
| Low | 19 | 1 | 0.7711 | |||
| Intermediate | 83 | 0.752 | 0.32 | 1.768 | 0.5131 | |
| High/Very high | 44 | 1.044 | 0.463 | 2.352 | 0.9177 | |
| Missing | 34 | 0.802 | 0.318 | 2.02 | 0.6397 | |
| HCT-CI | ||||||
| 0 | 58 | 1 | 0.0225 | |||
| 1–2 | 50 | 1.085 | 0.611 | 1.928 | 0.781 | |
| ≥3 | 61 | 2.132 | 1.249 | 3.638 | 0.0055 | |
| Missing | 11 | 0.963 | 0.418 | 2.22 | 0.9301 | |
| DFS | ||||||
| 95% CI | 95% CI | Overall | ||||
| CIBMTR MDS score | N | RR | Lower Limit | Upper Limit | p-value | p-value |
| Low | 19 | 1 | 0.3668 | |||
| Intermediate | 83 | 0.845 | 0.401 | 1.782 | 0.6591 | |
| High/Very high | 44 | 1.058 | 0.535 | 2.092 | 0.8706 | |
| Missing | 34 | 1.212 | 0.595 | 2.468 | 0.5968 | |
| HCT-CI | ||||||
| 0 | 58 | 1 | 0.0308 | |||
| 1–2 | 50 | 1.093 | 0.64 | 1.865 | 0.7454 | |
| ≥3 | 61 | 1.877 | 1.115 | 3.161 | 0.0178 | |
| Missing | 11 | 1.336 | 0.809 | 2.206 | 0.2575 | |
| OS | ||||||
| 95% CI | 95% CI | Overall | ||||
| CIBMTR MDS score | N | RR | Lower Limit | Upper Limit | p-value | p-value |
| Low | 20 | 1 | 0.6573 | |||
| Intermediate | 85 | 0.81 | 0.377 | 1.738 | 0.5881 | |
| High/Very high | 44 | 1.062 | 0.507 | 2.227 | 0.8726 | |
| Missing | 34 | 0.939 | 0.467 | 1.886 | 0.8589 | |
| HCT-CI | ||||||
| 0 | 60 | 1 | 0.0016 | |||
| 1–2 | 50 | 1.064 | 0.591 | 1.915 | 0.8364 | |
| ≥3 | 61 | 2.084 | 1.228 | 3.537 | 0.0065 | |
| Missing | 12 | 1.738 | 1.056 | 2.86 | 0.0295 |
Figure 3.
Overall survival after umbilical cord blood transplantation for MDS by CIBMTR MDS Transplantation Risk Score
Discussion
The extensive adoption of disease-modifying drugs, such as the hypomethylating agents and lenalidomide, along with better supportive care, have contributed to better outcomes for patients with MDS over time. In the same fashion, outcomes for patients undergoing allogenic hematopoietic cell transplantation have improved [27], which can also contribute a positive effect, and potential of cure, for patients with MDS. However, in a disease common to an older population, suitable sibling donors may not be available. In the absence of matched unrelated donors, alternative donor sources are often considered. In the current study, we described the outcomes of 176 patients who underwent UBCT for MDS as reported to the CIBMTR. We found that the 3-year probabilities of chronic GVHD, relapse, TRM, DFS, and OS were 28%, 32%, 40%, 28%, and 31%, respectively.
In the current study, the median age was 56 years, which indicates an older cohort as compared to other published studies. For example, in the report by Sato and collogues, the median age at the time of transplantation was 42 years [12]. Other key differences in baseline characteristics between this study and ours, make a direct comparison of the results difficult. For example, all of the patients in the current study had MDS, excluding those who progressed to AML, whereas in the study by Sato et al, 79% of patients had transformed to AML.
Although a formal statistical analysis was not performed, we found that post-HCT survival in this cohort was substantially lower than what has been described in a contemporary cohort that included matched related and unrelated donors also conducted by the CIBMTR [23]. There is a stark difference between the 3-year DFS of 28% (95% CI 21–35%) in this study, compared to matched unrelated donors in the contemporary study (41%, 95% CI 38–44% for the training cohort; 44%, 95% CI 40–48% for the validation cohort). This difference is a product of a relative increase in the incidence of TRM, and to a lesser extent, relapse.
The European Group for Blood and Marrow Transplantation (EBMT) reviewed a group of 129 patients with MDS that underwent UCBT, finding similar results with 2-year probabilities of chronic GVHD, relapse, TRM, DFS, and OS of 23%, 30%, 42%, 28%, and 30%, respectively [14]. One key difference between the two studies, is that a majority (71%) of the patients in the EMBT analysis had progressed to acute myeloid leukemia prior to transplantation, with less than half (48%) of them in remission prior to transplantation. In the current study, only 28% has a blast count over 5% at the time of transplantation. In the EBMT study, the investigators also went on to compare the outcomes of MDS patients who underwent UCBT with 502 contemporary patients who had matched related or unrelated donors using peripheral blood (PB) as a hematopoietic progenitor cell source. As compared to UCBT, those who underwent PB transplantation had similar rates of GVHD and relapse, but a better 2-year TRM (31% vs. 42%, P=.03), DFS (44% vs. 28%, P<.0001), and OS (49% vs. 30%, P<.0001).
Another report from Japan Society for Hematopoietic Cell Transplantation Data Registry, described the outcomes of 431 patients that underwent UCBT and compared to a contemporary cohort of 1093 patients that underwent unrelated donor transplant [13]. They found that the estimated 5-year OS was inferior for UCBT as compared to unrelated donors (32% vs. 46%, P<.0001). UCBT and unrelated donor transplant had similar rates of TRM (3-year cumulative incidence of TRM was 34% vs. 36%), however UCBT had a higher incidence of relapse (20% vs. 10%, P<.001).
In contrast to other reports, the current study is unique in that it validated predictive models for post-HCT outcomes. Interestingly, IPSS-R, a model that specifically quantifies disease risk, calculated at the time of HCT did not predict for post-HCT relapse, but did for TRM. While it is clear that disease burden before transplantation is a predictor of relapse [28, 29], the optimal pre-HCT therapy has yet to be defined [30, 31]. Approximately three quarters of the patients received some form of pre-HCT cytoreductive therapy. There was no association between pre-HCT therapy and post-HCT outcome. Although, relapse was the most common cause of death for patients with lower and intermediate-risk disease, mortality from transplant-related complications was increased in those with higher-risk disease (Table 3). Those with higher IPSS-R may have received pre-HCT therapies of greater duration and intensity, and cumulative toxicity may explain the association between disease-risk and TRM. However, the number or types of pre-HCT therapy did not vary with the pre-transplant IPSS-R risk-groups. Another limitation of this analysis is the fact that the pre-HCT IPSS-R score was missing in 16% of patients.
The CIBMTR MDS Transplantation Risk Score, a model specifically developed to predict post-HCT outcomes in patients with MDS [23], was not found to be predictive in this analysis. While the donor sources were different, the current study’s cohort had a similar median age, performance status, pre-transplant blast count, and cytogenetic risk to the cohort used to build the MDS transplant risk model. However, in the current study, a higher proportion of patients received pre-transplant cytoreductive therapy, and there were noteworthy differences in the conditioning regimens that patients received. In the current study, more patients received either antithymocyte globulin or alemtuzumab as part of conditioning, and a larger proportion of patients in the MDS transplant risk model derivation cohort underwent meyloablative conditioning. It is important to note that the CIBMTR MDS Transplantation Risk Score was missing for 35 (20%) patients, therefore limiting the statistical power to determine the utility of the model in this population.
The median time from diagnosis to UCBT in the current study is comparable to previous reports [12, 14]. In patients with limited donor options, increased effort is put in to the search process and evaluation of alternative donor sources. This can add time to the pre-transplant period resulting in a lead-time bias. This bias could positively influence post-HCT outcomes, as patients with more aggressive disease may not have enough time to secure a donor and undergo HCT, therefore are not included in the subsequent analysis. Conversely, lead-time bias could be deleterious to aggregate outcomes as a result of transplanting patients later in their disease course. Nonetheless, time from diagnosis to HCT for MDS is not associated with outcome in the matched-related or matched-unrelated donor setting [23], as well as the UCBT setting [14].
While the existing data suggest that outcomes with matched related and unrelated transplantation are superior, UCBT does offer long-term disease-free survival for some patients. With relapse being the primary contributor to mortality after transplantation, strategies to reduce relapse are needed to improve outcomes irrespective of donor source [32]. This is particularly important for UCBT, as the option of graft manipulation with donor cellular infusion is not available. When comparing donor source, the rates of relapse are similar between different donor sources, where TRM from delayed immunologic recovery stands out as a heightened barrier to success specific to UCBT. Therefore, several manipulation and expansion strategies, with the aim of increasing the cell dose and modifying the composition of cord blood units, are being developed [33, 34]. With increased focus on health care costs and delivering value-based care, the cost of cord blood unit acquisition compared to obtaining a graft from other donor sources presents another potential barrier to wide-spread adoption of UCBT.
As UCBT continues its development, it will do so in parallel with other alternative donor sources including mismatched unrelated and haploidentical donor sources. Use of single mismatch donors significantly increases the available donor pool [4]. However, use of these donors leads to rates of GVHD and TRM that are much higher than expected for fully-matched donor HCT. Multiple strategies have been sought to identify “permissible mismatches” associated with improved outcomes of a single-allele mismatched unrelated donor HCT [35]. Haploidentical transplantation, facilitated by the administration of cyclophosphamide after cellular infusion, has the advantages of following a logistical pattern similar to matched sibling transplantation, and a time to engraftment on par with matched related and unrelated donor transplants [36]. However, data on long-term outcomes is lacking, and like UCBT, the published reports include MDS as a disease subset, not as a primary focus. In order to answer these questions, there is clear need for a prospective study randomizing patients with MDS between haploidentical transplantation and UCBT similar to the ongoing BMT-CTN 1101 study (ClinicalTrials.gov Identifier: NCT01597778). By focusing on the outcomes when different transplantation strategies are applied to individual patient populations, treatment decisions and clinical trial design can better informed. These are moving targets, and as both traditional and alternative donor transplants are refined, they will need continuous evaluation.
Supplementary Material
Supplemental Figure 1. Overall survival after umbilical cord blood transplantation for MDS by HCT-CI Score adjusted for IPSS-R risk
Supplemental Figure 2. Cumulative incidence of relapse after umbilical cord blood transplantation for MDS by conditioning regimen adjusted for IPSS-R risk
Highlights.
Use of umbilical cord blood transplant (UCBT) for MDS is not well-described
Relapse and overall survival at 3 years was 32 and 31%, respectively
Transplant-related mortality (TRM) at 3 years was 40%
Disease risk, comorbidities, and conditioning intensity predict outcomes
UCBT can offer long-term success for some, but is hampered by a high rate of TRM
Acknowledgments
CIBMTR Support List
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement 5U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from Alexion; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Be the Match Foundation; *Bristol Myers Squibb Oncology; *Celgene Corporation; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Genentech, Inc.; Genzyme Corporation; *Gilead Sciences, Inc.; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; *Jazz Pharmaceuticals, Inc.; Jeff Gordon Children’s Foundation; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Merck & Co, Inc.; Mesoblast; *Millennium: The Takeda Oncology Co.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Otsuka America Pharmaceutical, Inc.; Otsuka Pharmaceutical Co, Ltd. – Japan; Oxford Immunotec; Perkin Elmer, Inc.; Pharmacyclics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; *Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Telomere Diagnostics, Inc.; TerumoBCT; Therakos, Inc.; University of Minnesota; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Footnotes
Corporate Members
-
Designed the study, analyzed data, and wrote the manuscript:Aaron GerdsMatt KalaycioKwang Ahn WooZhen-Huan HuRonald SobecksWael Saber
- Designed the study and wrote the manuscript: Writing committee members (listed from author list)
- No conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gerds AT, Deeg HJ. Transplantation for myelodysplastic syndrome in the era of hypomethylating agents. Current opinion in hematology. 2012;19:71–5. doi: 10.1097/MOH.0b013e32834ff562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United States Census Bureau. [Accessed September 20, 2016];Families and Living Arrangements - Households. 2016 http://www.census.gov/hhes/families/data/households.html.
- 3.Appelbaum FR. Pursuing the goal of a donor for everyone in need. N Engl J Med. 2012;367:1555–6. doi: 10.1056/NEJMe1209982. [DOI] [PubMed] [Google Scholar]
- 4.Gragert L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371:339–48. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker JN, Byam CE, Kernan NA, Lee SS, Hawke RM, Doshi KA, et al. Availability of cord blood extends allogeneic hematopoietic stem cell transplant access to racial and ethnic minorities. Biol Blood Marrow Transplant. 2010;16:1541–8. doi: 10.1016/j.bbmt.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker JN, Krepski TP, DeFor TE, Davies SM, Wagner JE, Weisdorf DJ. Searching for unrelated donor hematopoietic stem cells: availability and speed of umbilical cord blood versus bone marrow. Biol Blood Marrow Transplant. 2002;8:257–60. doi: 10.1053/bbmt.2002.v8.pm12064362. [DOI] [PubMed] [Google Scholar]
- 7.Dahlberg A, Delaney C, Bernstein ID. Ex vivo expansion of human hematopoietic stem and progenitor cells. Blood. 2011;117:6083–90. doi: 10.1182/blood-2011-01-283606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scaradavou A, Brunstein CG, Eapen M, Le-Rademacher J, Barker JN, Chao N, et al. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood. 2013;121:752–8. doi: 10.1182/blood-2012-08-449108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunstein CG, Eapen M, Ahn KW, Appelbaum FR, Ballen KK, Champlin RE, et al. Reduced-intensity conditioning transplantation in acute leukemia: the effect of source of unrelated donor stem cells on outcomes. Blood. 2012;119:5591–8. doi: 10.1182/blood-2011-12-400630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunstein CG, Gutman JA, Weisdorf DJ, Woolfrey AE, Defor TE, Gooley TA, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116:4693–9. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YB, Aldridge J, Kim HT, Ballen KK, Cutler C, Kao G, et al. Reduced-intensity conditioning stem cell transplantation: comparison of double umbilical cord blood and unrelated donor grafts. Biol Blood Marrow Transplant. 2012;18:805–12. doi: 10.1016/j.bbmt.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Sato A, Ooi J, Takahashi S, Tsukada N, Kato S, Kawakita T, et al. Unrelated cord blood transplantation after myeloablative conditioning in adults with advanced myelodysplastic syndromes. Bone Marrow Transplant. 2011;46:257–61. doi: 10.1038/bmt.2010.91. [DOI] [PubMed] [Google Scholar]
- 13.Ishiyama K, Aoki J, Aoki K, Itonaga H, Ishikawa T, Miyazaki Y, et al. Outcomes Of Umbilical Cord Blood Transplantation For Patients With Myelodysplastic Syndrome: A Nationwide Survey. Haematologica. 2013;98:253–4. (abstract S600) [Google Scholar]
- 14.Robin M, Ruggeri A, Labopin M, Niederwieser D, Tabrizi R, Sanz G, et al. Comparison of unrelated cord blood and peripheral blood stem cell transplantation in adults with myelodysplastic syndrome after reduced-intensity conditioning regimen: a collaborative study from Eurocord (Cord blood Committee of Cellular Therapy & Immunobiology Working Party of EBMT) and Chronic Malignancies Working Party. Biol Blood Marrow Transplant. 2015;21:489–95. doi: 10.1016/j.bbmt.2014.11.675. [DOI] [PubMed] [Google Scholar]
- 15.Saber W, Le Rademacher J, Sekeres M, Logan B, Lewis M, Mendizabal A, et al. Multicenter biologic assignment trial comparing reduced-intensity allogeneic hematopoietic cell transplant to hypomethylating therapy or best supportive care in patients aged 50 to 75 with intermediate-2 and high-risk myelodysplastic syndrome: Blood and Marrow Transplant Clinical Trials Network #1102 study rationale, design, and methods. Biol Blood Marrow Transplant. 2014;20:1566–72. doi: 10.1016/j.bbmt.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horowitz M. The role of registries in facilitating clinical research in BMT: examples from the Center for International Blood and Marrow Transplant Research. Bone Marrow Transplant. 2008;42(Suppl 1):S1–S2. doi: 10.1038/bmt.2008.101. [DOI] [PubMed] [Google Scholar]
- 17.Schanz J, Tuchler H, Sole F, Mallo M, Luno E, Cervera J, et al. New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J Clin Oncol. 2012;30:820–9. doi: 10.1200/JCO.2011.35.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breems DA, Van Putten WL, Lowenberg B. The impact of abn(17p) and monosomy -5/del(5q) on the prognostic value of the monosomal karyotype in acute myeloid leukemia. Blood. 2013;121:3056–7. doi: 10.1182/blood-2013-01-475012. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Sole F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–65. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rowlings PA, Przepiorka D, Klein JP, Gale RP, Passweg JR, Henslee-Downey PJ, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–64. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 21.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–17. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 22.Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:367–9. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaffer BC, Ahn KW, Hu ZH, Nishihori T, Malone AK, Valcarcel D, et al. Scoring System Prognostic of Outcome in Patients Undergoing Allogeneic Hematopoietic Cell Transplantation for Myelodysplastic Syndrome. J Clin Oncol. 2016 doi: 10.1200/JCO.2015.65.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gönen M, Heller G. Concordance probability and discriminatory power in proportional hazards regression. Biometrika. 2005;92:965–70. [Google Scholar]
- 25.Lee EW, Wei LJ, Amato D. Cox-Type Regression Analysis for Large Numbers of Small Groups of Correlated Failure Time Observations. In: Klein JP, Goel PK, editors. Survival Analysis: State of the Art. Dordrecht, the Netherlands: Kluwer Academic Publishers; 1992. pp. 237–47. [Google Scholar]
- 26.Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet. 2002;359:1309–10. doi: 10.1016/s0140-6736(02)08272-7. [DOI] [PubMed] [Google Scholar]
- 27.Hahn T, McCarthy PL, Jr, Hassebroek A, Bredeson C, Gajewski JL, Hale GA, et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J Clin Oncol. 2013;31:2437–49. doi: 10.1200/JCO.2012.46.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warlick ED, Cioc A, Defor T, Dolan M, Weisdorf D. Allogeneic stem cell transplantation for adults with myelodysplastic syndromes: importance of pretransplant disease burden. Biol Blood Marrow Transplant. 2009;15:30–8. doi: 10.1016/j.bbmt.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Cutler CS, Lee SJ, Greenberg P, Deeg HJ, Pérez WS, Anasetti C, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104:579–85. doi: 10.1182/blood-2004-01-0338. [DOI] [PubMed] [Google Scholar]
- 30.Gerds AT, Gooley TA, Estey EH, Appelbaum FR, Deeg HJ, Scott BL. Pretransplantation therapy with azacitidine vs induction chemotherapy and posttransplantation outcome in patients with MDS. Biol Blood Marrow Transplant. 2012;18:1211–8. doi: 10.1016/j.bbmt.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Damaj G, Duhamel A, Robin M, Beguin Y, Michallet M, Mohty M, et al. Impact of Azacitidine Before Allogeneic Stem-Cell Transplantation for Myelodysplastic Syndromes: A Study by the Societe Francaise de Greffe de Moelle et de Therapie-Cellulaire and the Groupe-Francophone des Myelodysplasies. J Clin Oncol. 2012 doi: 10.1200/JCO.2012.44.3499. [DOI] [PubMed] [Google Scholar]
- 32.Wayne AS, Giralt S, Kroger N, Bishop MR. Proceedings from the National Cancer Institute’s Second International Workshop on the Biology, Prevention, and Treatment of Relapse after Hematopoietic Stem Cell Transplantation: introduction. Biol Blood Marrow Transplant. 2013;19:1534–6. doi: 10.1016/j.bbmt.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horwitz ME. Ex Vivo Expansion or Manipulation of Stem Cells to Improve Outcome of Umbilical Cord Blood Transplantation. Curr Hematol Malig Rep. 2016;11:12–8. doi: 10.1007/s11899-015-0297-7. [DOI] [PubMed] [Google Scholar]
- 34.Horwitz M, Montesinos P, Kurtzberg J, Valcarcel D, Jagasia MH, Cilloni D, et al. NiCord single unit expanded umbilical cord blood transplantation: Results of phase I/II trials. Journal of Clinical Oncology. 2016;34 abstr 7004. [Google Scholar]
- 35.Lazaryan A, Wang T, Spellman SR, Wang HL, Pidala J, Nishihori T, et al. Human leukocyte antigen supertype matching after myeloablative hematopoietic cell transplantation with 7/8 matched unrelated donor allografts: A report from the Center for International Blood and Marrow Transplant Research. Haematologica. 2016 doi: 10.3324/haematol.2016.143271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Stasi A, Milton DR, Poon LM, Hamdi A, Rondon G, Chen J, et al. Similar transplantation outcomes for acute myeloid leukemia and myelodysplastic syndrome patients with haploidentical versus 10/10 human leukocyte antigen-matched unrelated and related donors. Biol Blood Marrow Transplant. 2014;20:1975–81. doi: 10.1016/j.bbmt.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Overall survival after umbilical cord blood transplantation for MDS by HCT-CI Score adjusted for IPSS-R risk
Supplemental Figure 2. Cumulative incidence of relapse after umbilical cord blood transplantation for MDS by conditioning regimen adjusted for IPSS-R risk