Conspectus
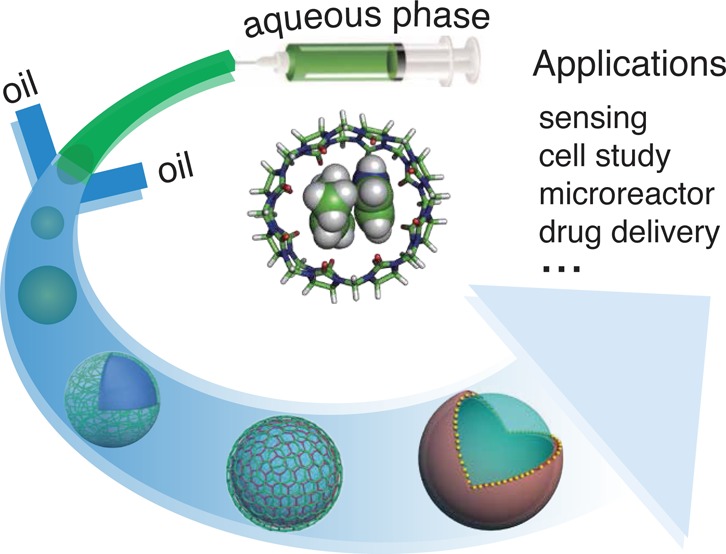
Microencapsulation is a fundamental concept behind a wide range of daily applications ranging from paints, adhesives, and pesticides to targeted drug delivery, transport of vaccines, and self-healing concretes. The beauty of microfluidics to generate microcapsules arises from the capability of fabricating monodisperse and micrometer-scale droplets, which can lead to microcapsules/particles with fine-tuned control over size, shape, and hierarchical structure, as well as high reproducibility, efficient material usage, and high-throughput manipulation. The introduction of supramolecular chemistry, such as host–guest interactions, endows the resultant microcapsules with stimuli-responsiveness and self-adjusting capabilities, and facilitates hierarchical microstructures with tunable stability and porosity, leading to the maturity of current microencapsulation industry.
Supramolecular architectures and materials have attracted immense attention over the past decade, as they open the possibility to obtain a large variety of aesthetically pleasing structures, with myriad applications in biomedicine, energy, sensing, catalysis, and biomimicry, on account of the inherent reversible and adaptive nature of supramolecular interactions. As a subset of supramolecular interactions, host–guest molecular recognition involves the formation of inclusion complexes between two or more moieties, with specific three-dimensional structures and spatial arrangements, in a highly controllable and cooperative manner. Such highly selective, strong yet dynamic interactions could be exploited as an alternative methodology for programmable and controllable engineering of supramolecular architectures and materials, exploiting reversible interactions between complementary components. Through the engineering of molecular structures, assemblies can be readily functionalized based on host–guest interactions, with desirable physicochemical characteristics.
In this Account, we summarize the current state of development in the field of monodisperse supramolecular microcapsules, fabricated through the integration of traditional microfluidic techniques and interfacial host–guest chemistry, specifically cucurbit[n]uril (CB[n])-mediated host–guest interactions. Three different strategies, colloidal particle-driven assembly, interfacial condensation-driven assembly and electrostatic interaction-driven assembly, are classified and discussed in detail, presenting the methodology involved in each microcapsule formation process. We highlight the state-of-the-art in design and control over structural complexity with desirable functionality, as well as promising applications, such as cargo delivery stemming from the assembled microcapsules. On account of its dynamic nature, the CB[n]-mediated host–guest complexation has demonstrated efficient response toward various external stimuli such as UV light, pH change, redox chemistry, and competitive guests. Herein, we also demonstrate different microcapsule modalities, which are engineered with CB[n] host–guest chemistry and also can be disrupted with the aid of external stimuli, for triggered release of payloads. In addition to the overview of recent achievements and current limitations of these microcapsules, we finally summarize several perspectives on tunable cargo loading and triggered release, directions, and challenges for this technology, as well as possible strategies for further improvement, which will lead to substainitial progress of host–guest chemistry in supramolecular architectures and materials.
1. Introduction
The synthesis and self-assembly of polymer building blocks for the construction of functional supramolecular micro/nano-structures are a major part of the emerging field of Supramolecular Polymer Chemistry.1,2 The introduction of noncovalent supramolecular interactions such as hydrogen bonding, host–guest complexation, or electrostatic interactions offers great opportunities to impart novel features and functions to these polymer systems.3 As an example of such self-assembled architectures, microcapsules have promising applications ranging from drug delivery, cosmetics, and encapsulation, to microreactors and tissue engineering, on account of their low effective density, high specific surface area, and remarkable encapsulation capability.4−6 Currently, microcapsules are commonly fabricated in the presence of soft or solid templates (e.g., droplets, polymer colloids, silica, and calcium carbonate particles). The encapsulation methods based on bulk emulsion with liquid droplets as templates are unable to control the shape, size, as well as the structure of a microcapsule. On the other hand, solid templates, with desirable morphologies, can be used to generate microcapsules by physical adsorption, sol–gel chemistry, or layer-by-layer (LbL) techniques, followed by template removal via calcination or chemical etching.5 Despite the uniform size achieved, these approaches typically involve multistep processes, requiring careful examination of the experimental conditions in each step. Moreover, the presence of a template might significantly limit the loading capacity of the resulting microcapsules based on these postloading strategies.7 Thus, in order to expand the applicability of such microcapsules, development of facile and effective fabrication and encapsulation pathways are needed.
Since some of the earliest microcapsules produced from microfluidics were reported by Weitz and co-workers,9 intensive studies have enabled the rapid development of microdroplet techniques for the fabrication of monodisperse capsules on the microscale.4,5,10 In these cases, encapsulation is generally achieved by the formation of single or double microdroplet emulsions, containing synthetic polymers, biopolymers, lipids or amphiphiles, followed by shell solidification as a result of solvent evaporation, polymerization or dewetting. Microfluidic techniques have been demonstrated to overcome challenges inherent to sacrificial template strategies, such as multiple synthesis steps and limited encapsulation efficiency.11 Periodic shear of the stream can be induced by flow-focusing with an immiscible sheath fluid, giving rise to uniformed microdroplets composed of precursor solutions. Microcapsule skins are generated upon subsequent solidification at the microdroplet interface.12 The reliance on covalent cross-linking to give a stable microcapsule structure could, in some circumstances, impede on-demand cargo release via structural destruction.13,14 Introduction of noncovalent interactions, such as supramolecular host–guest complexation and hydrophobic interactions, can impart flexibility to control both assembly and disassembly of the microcapsule motifs.15 On account of the dynamic binding and feasibility for building highly organized mesostructures, microcapsules that are engineered with supramolecular interactions are adaptive, dynamic, and self-repairing.16 Since adaptive dynamics are crucial in biological systems as well for artificial biomimetic systems,17,18 such features enable the microcapsules to be exploited in numerous biomedical applications, including drug delivery, biomedical diagnostics, living-cell-integrated assays, construction of artificial organelles, and regenerative biomedicines.19
2. Cucurbit[n]uril-Mediated Host–Guest Chemistry in Microdroplets
A supramolecular host–guest inclusion complex, formed via molecular recognition, involves noncovalent interactions between a receptor (host molecule) and a guest molecule.20,21 The host molecules usually possess a hydrophobic or hydrophilic cavity, which can accommodate guest molecules, such as organic compounds, metal ions, nanoparticles, and biomacromolecules.16 Cucurbit[n]urils (CB[n], n = 5–8, 10) are a class of aqueous soluble macrocyclic hosts with a rigid hydrophobic cavity and two identical carbonyl-fringed portals.16,22,23 As stimuli-responsive properties can be inherited from both the building units and host–guest recognition motifs, this self-assembly strategy enriches the corresponding supramolecular integrities with a broad range of responsiveness and features.17 Similar to other macrocyclic analogues, such as cyclodextrins20 and pillar[n]arenes,21 the utility of CB[n] host–guest conjugation in various supramolecular architectures and functional materials has been widely investigated. However, as for the microcapsule motifs, very limited work has been reported in the past few years. Thus far, through the combination of CB[n] host–guest interactions and microfluidic techiniques, microcapsules have been formed by colloidal particle-driven assembly, interfacial condensation-driven assembly or electrostatic interaction-driven assembly, as discussed in the following sections.
2.1. Colloidal Particles Driven Assembly of Microcapsules
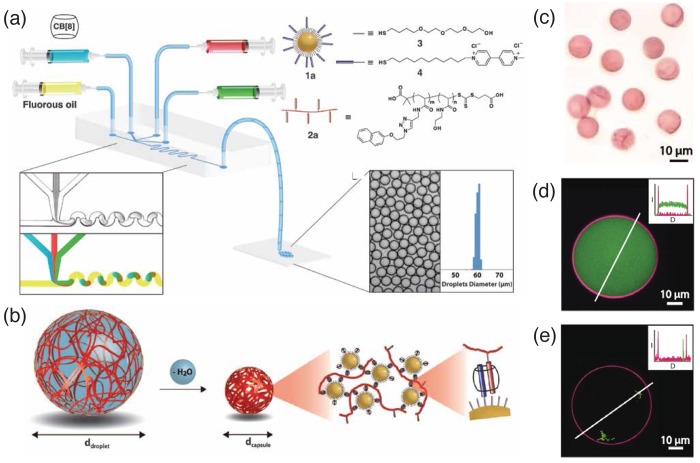
In a Pickering emulsion, colloidal particles tend to localize at the interface between immiscible liquids, such as the water/oil interface, in order to minimize the interfacial energy of immiscible phases. Despite this phenomena being discovered more than a century ago, the effect has only been exploited in the past decade for the fabrication of stable microcapsules from nano- or micro- particles.25 By integrating Pickering emulsions and CB[8] supramolecular host–guest chemistry, Zhang et al.8 reported a new class of supramolecular microcapsules fabricated in a single step using a microdroplet-based microfluidic platform. Microdroplets were first generated in a microfluidic device using a simple T-junction geometry (Figure 2a). The oil carrier phase was directed perpendicular to the aqueous dispersion phase, consisting of three inlets for aqueous solutions of CB[8], gold nanoparticles functionalized with methylviologen (MV2+) moieties (Figure 1a), and a naphthol-functionalzed copolymer (Figure 2a). The gold nanoparticles directed the supramolecular self-assembly to the water/oil interface, pulling with them the complementary polymers for complexation, leading to the formation of a microcapsule shell (Figure 2b). Individual microcapsules were readily isolated after evaporation of the water droplet, and shown to retain a spherical geometry upon subsequent rehydration (Figure 2c). During microcapsule formation, cargo loading (fluorescein isothiocyanate-dextran, FITC-dextran, or Esherichia coli cells) could be readily accessed in the same step with a high yield (Figure 2d and e).
Figure 2.
Schematic representation of (a) the microdroplet generation process using a microfluidic device; and (b) the microcapsule formation process from the initial droplet stage to the dehydrated stable capsules, where gold nanoparticles serve to direct the supramolecular self-assembly at the water/oil interface. (c) Brightfield micrographs of the isolated microcapsules. Laser scanning confocal microscope images of the microcapsules containing (d) FITC-dextran; and (e) Esherichia coli cells, respectively. Insets in (d) and (e) display the corresponding fluorescence intensity profiles (I = intensity; D = distance). Adapted with permission from ref (8). Copyright 2012 AAAS.
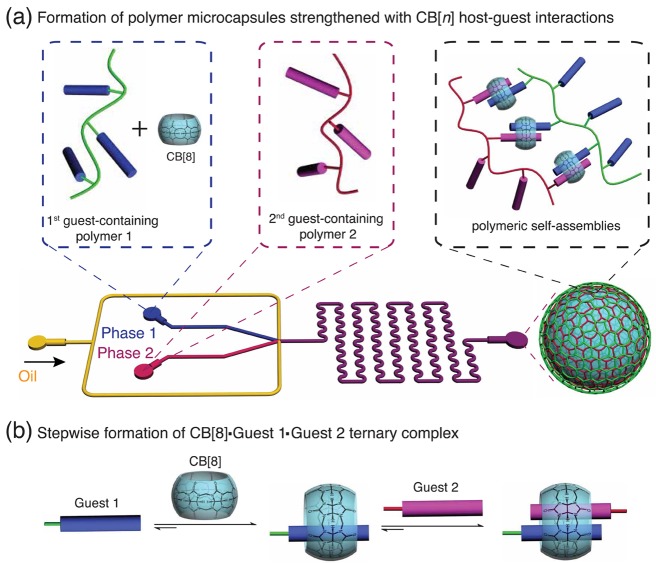
Figure 1.
(a) Schematic illustration of supramolecular polymer microcapsules assembled at the interface of microfluidic droplets. By using a microfluidic flow-focusing device, an aqueous phase carrying CB[8] and first guest-containing polymer 1 intersects with another phase consisted of second guest-containing polymer 2, at a flow-focusing microchannel junctions to form a periodic flow of oil-in-water microdroplets. (b) Stepwise formation of a supramolecular heteroternary complexation of CB[8] and guest 1 (electron deficient, such as methylviologen) and then guest 2 (electron rich, such as naphthol, azobenzene, benzyl, phenylalanine, etc.).
This work represents a facile protocol for direct construction of microcapsules, combining the advantages of Pickering emulsion and supramolecular host–guest chemistry. The ability to form a colloidal microcapsule is dictated by the ability of colloidal particles to self-assemble at the interface. Such simultaneous encapsulation of both the colloidal components and molecular cargo or living cells during microcapsule generation ensured both high reproducibility and loading efficiency. Further exploitation based on this Pickering emulsion strategy includes employing MV2+-functionalized polystyrene colloids, in order to avoid reliance on metallic nanoparticles while increasing the mechanical strength and stability of the microcapsules, leading to prolonged cargo retention.26,27
2.2. Interfacial Condensation-Driven Assembly of Microcapsules
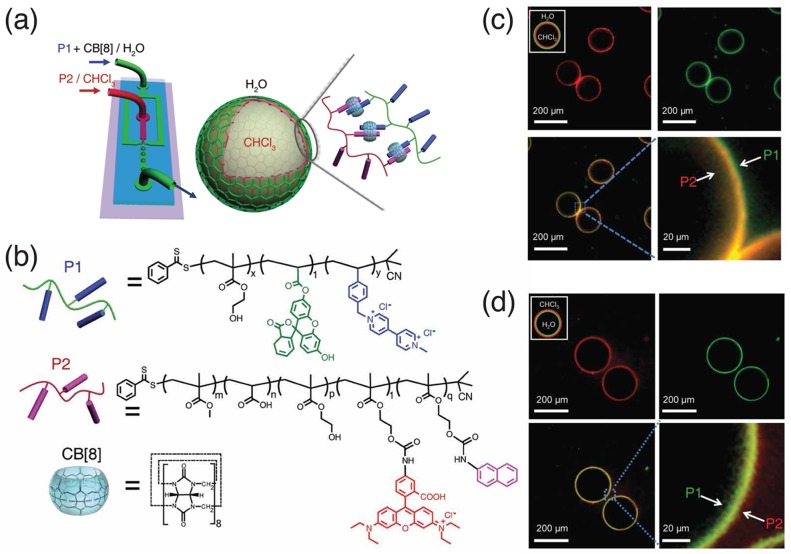
Inspired by interfacial reaction, microcapsules have been fabricated by condensation across the interface of the microfluidic droplets, involving interfacial polymerization and interfacial molecular cross-links.28,29 Zheng and Yu et al.24 reported the formation of supramolecular microcapsules via the self-assembly of two polymers bearing different guest moieties at the interface of aqueous droplets in chloroform, exploiting such an interfacial condensation strategy within microdroplets (Figure 3). In this case, guest-containing polymers in two incompatible phases were assembled and disassembled at the microdroplet interface, mediated by noncovalent interactions (Figure 3a). Specifically, MV2+ (first guest)-containing hydrophilic poly(HEMA-co-StMV) (P1) in water and naphthol (second guest)-containing hydrophobic poly(MMA-co-AA-co-HEMA-co-HEMANP) (P2) in chloroform were brought together at the water/chloroform interface of a microfluidic droplet, leading to the formation of a supramolecular polymeric skin, and subsequently robust polymer microcapsules (Figure 3c and d). Both water-soluble and oil-soluble polymers can be used for the skins of the microcapsules, using either oil-in-water or water-in-oil microdroplets as templates. As a result, this allows for sophisticated control over the hierarchical structures, properties and functions of the as-formed microcapsules, such as segregated domains, skin thickness, permeability, and an expanded tool set for triggered release.
Figure 3.
(a) Schematic representation of the microdroplet generation process using a microfluidic flow-focusing device and (b) chemical structures of the CB[8] host molecule, methylviologen (first guest)-containing polymer P1, and naphthol (second guest)-containing polymer P2; (c) fluorescence micrographs of the monodisperse chloroform-in-water microdroplets, demonstrating the interfacial assembly of P2 (rhodamine tagged, red) within the droplet and P1 (fluorescein tagged, green) present in the external media. (d) Fluorescence micrographs of the inverted water-in-chloroform microdroplets, illustrating reversal of P1 and P2 at the droplet interface. Adapted with permission from ref (24). Copyright 2014 Nature Publishing Group.
2.3. Electrostatic Interaction-Driven Assembly of Microcapsules
A powerful alternative approach for the assembly of microcapsules relies on electrostatic interactions to direct the accumulation of the structural components at the microdroplet interface.31−33 For electrostatic self-assembly, charged components are selectively partitioned at the microdroplet interface by a complementary charged surfactant. The shell thickness and integrity of the microcapsules produced in a single step can be sufficient to offer robustness and improved stability as cargo carriers.
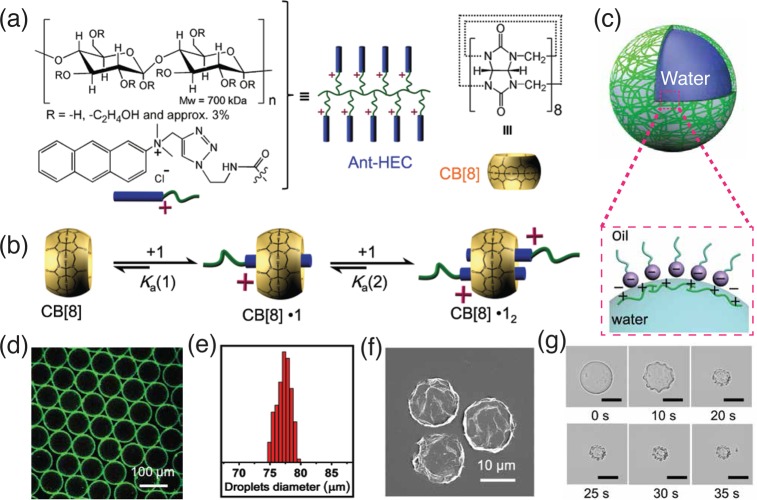
Side-group functionalization of hydroxyethyl cellulose (HEC) with anthracene moieties enables dynamic cross-linking through a 2:1 homoternary complexation with CB[8] molecules (Figure 4).30 The electrostatic attractions at the droplet interface between positively charged anthracene-functionalized HEC and carboxylate head groups on the surfactants, lead to accumulation of polymer components at the interface (Figure 4d). The 2:1 host–guest complexation of anthracene with CB[8] complexation ultimately results in the formation of a supramolecular hydrogel skins (Figure 4g). Even though the biocompatibility of such anthracene-functionalized HEC-based microcapsules has not been reported, nevertheless, some investigation into the cytotoxicity of CB[n]-based assemblies has been carried out. In the CB[n]-based colloidal assemblies, cytotoxicity of the MV2+-containing colloids was substantially inhibited, upon formation of heteroternary complexes containing MV2+, naphthol and CB[8].35 Similar findings were also reported by Rotello and co-workers.36 Thus, we might speculate that an anthracene-functionalized HEC system might also exhibit high biocompatibility, which can be exploited in further studies.
Figure 4.
(a) Anthracene-functionalized hydroxyethyl cellulose and CB[8]; and (b) schematic representation of the formation of homoternary complexes by accommodating two anthracene moieties in the cavity of CB[8]. (c) Schematic representation of the microcapsules generation using a charged surfactant within the carrier phase of oil, while CB[8] and anthracene functionalized HEC are dispersed in the water phase. (d) Laser-scanning confocal fluorescence image of microdroplets, confirming that Ant-HEC is localized at the microdroplet interface; (e) normalized distribution of microdroplet diameters, exhibiting a narrow size distribution (coefficient of variation 1.5%). (f) Scanning electron microscopy (SEM) image of dried microcapsules. (g) Brightfield micrographs of the microcapsule drying process, resulting in a collapsed structure (scale bars: 20 μm). Adapted with permission from ref (30). Copyright 2015 The Royal Society of Chemistry.
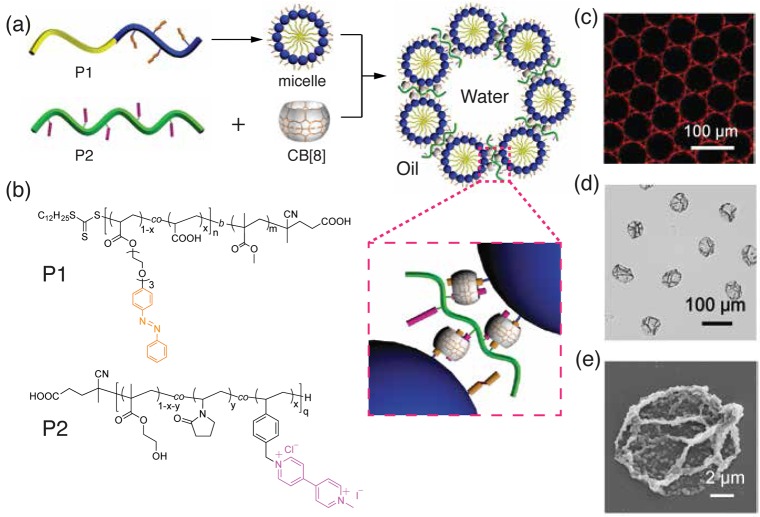
Yu et al.34 further developed a hierarchical capsule assembly process, in which self-assembled micelles from amphiphilic block copolymers were immobilized at the interface of microfluidic droplets via electrostatic and CB[8] host–guest interactions, leading to multicompartmentalized microcapsules (Figure 5). The first step of supramolecular assembly involved micellization of an amphiphilic poly(methyl methacrylate)-block-poly(acrylic acid) copolymer containing pendant azobenzene moieties in the hydrophilic block (P1, Figure 5a). The presence of azobenzene moieties in the corona of the micelles facilitated complexation via CB[8] to MV2+ moieties pendant from poly(N-vinylpyrrolidone)-co-poly(hydroxyethyl methacrylate)-co-poly(MV2+-styrene) (P2), resulting in the formation of a dynamically cross-linked polymer skin. The microdroplets exhibited a low polydispersity and the presence of red fluorescence within the shell of microcapsules confirmed the exclusive localization of Nile red-loaded micelles at the water/oil interface (Figure 5c). Evaporation of water from the capsules resulted in the collapse and distortion of the flexible polymer shell (Figure 5d). SEM images showed the skin of the microcapsule consisting of a composite network structure of the micelles embedded within the polymer matrix (Figure 5e). This architecture draws parallels between biomimetic systems for artificial organelles and multicompartmental microreactors with partitioned channels.
Figure 5.
(a) Schematic representation of hierarchical assembly of supramolecular microcapsules from amphiphilic blocks copolymers; and (b) chemical structures of polymer P1 and polymer P2. (c) Fluorescence micrographs of microdroplets, illustrating the assembly of Nile red-loaded P1 micelles at the droplet interface. (d) Brightfield micrograph and (e) SEM image of dried microcapsules. Adapted with permission from ref (34). Copyright 2016 American Chemical Society.
Parker et al.33 exploited electrostatic interactions between charged supramolecular polymer complexes in microdroplets and charged surfactants around the microdroplets to selectively assemble capsule-forming components at the interface, dynamically cross-linked via CB[8] host–guest interactions. The supramolecular microcapsules are formed by the electrostatic interactions with a complementarily charged surfactant at the water/oil interface, which results from initial accumulation of the charged building blocks as a thin and diffusive layer at the interface. Once the microcapsule formation is subjected to water evaporation, the interfacial polymer layer dynamically flows and reorganizes to decrease the microdroplet surface area, however, it remains confined to the water/oil interface on account of the supramolecular CB[8] cross-linking. At a critical point this layer undergoes a phase change, resulting in a supramolecular polymer film that buckles and collapses upon further evaporation. As an extension of this electrostatic approach, the location of orthogonally charged copolymers can also be independently manipulated within a microdroplet, allowing for selective assembly at the interface. The controllable assembly offers the advantage to explore the formation of capsules-in-capsules and opens the prospect of carrying diverse cargoes for sequential release. Moreover, the electrostatically directed assembly of small molecules to the interface of an aqueous microdroplet has also been recently demonstrated by Groombridge et al., whereby accumulation and subsequent cross-linking via CB[8] led to the formation of an interfacial supramolecular gel.37
To better understand the formation of CB[8]-mediated supramolecular skins surrounding liquid droplets, Salmon et al.38 proposed a formation mechanism that can account for a phase transition upon interfacial compression. The compression-induced phase change causes the onset of buckling at the microdroplet interface. Upon water evaporation in microdroplets, the interfacial film increases in both thickness and density, until it reaches a critical density and the “buckling transition” happens. This phase change is a gelation process, which relies on a sufficient cross-link density, thus, the microcapsule properties can be controlled by tuning the compressive phase changes.
3. Cargo Encapsulation and Triggered Release
Development of microcapsules for cargo delivery and release is of utmost importance for applications in the pharmaceutical, agriculture, food and the cosmetic industry.4−6,39,40 While a number of reports have demonstrated that the microfluidic strategy offers a high-throughput method for microcapsule fabrication, the microdroplet-based supramolecular self-assembly approach we introduced provides a platform that guarantees an extremely high encapsulation efficiency under mild conditions and in a single step.4,5,41 For microcapsule systems with permanent cross-linking, release of the payload can only be achieved through a diffusion mechanism, swelling and/or degradation of the shell.42 Fortunately, the introduction of supramolecular cross-linking motifs not only strengthens the shell stability, but also bestows the polymer shell with extra benefits including dynamic adaptability, and thus tunable release kinetics.
3.1. Controlled Release of Cargoes
In comparison to conventional disruptive conditions (pressure or shear stress) or molecular diffusion, supramolecular CB[n] host–guest interactions make possible a number of elegant routes to enable triggered release of cargoes. In addition to manipulation of the initial degree of cross-linking, partial or complete dissociation of CB[n] host–guest interaction can be activated by the addition of a reducing agent,8 competitive guest,43 or by remote photoirradiation,24 subsequently leading to swelling of the microcapsule shell and increased pore sizes, enabling release of the cargoes.
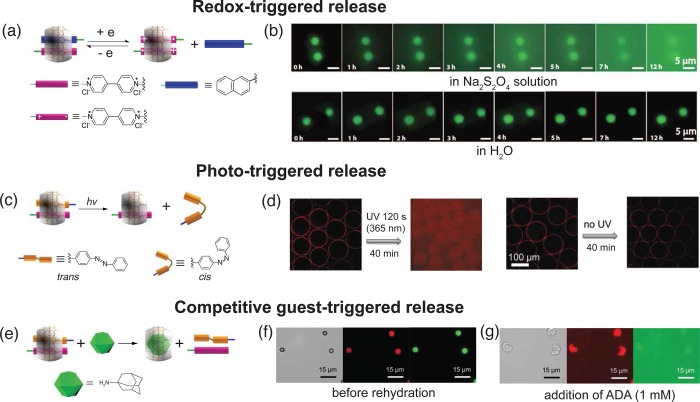
In a microcapsule system composed of CB[8]-mediated host–guest cross-linking between MV2+ and azobenzene moieties,24 the addition of a reducing agent, sodium dithionite, can induce the reduction of MV2+ to MV+•, causing the dissociation of heteroternary complexes (Figure 6a), activating the release of FITC-dextran cargo (Figure 6b). Alternatively, the use of azobenzene moieties imparts the microcapsules with photoresponsiveness.44 Photoisomerization of azobenzene units prompts the dissociation of existing heterotenary complexes (Figure 6c), leading to a remote light-triggered release in the supramolecular assembly (Figure 6d). The simple addition of a competitive guest with a higher affinity for CB[8] molecules is another straightforward route to trigger cargo release. 1-Adamantylamine, a commonly used competitive guest (Ka of 1013 M–1),16 was employed to competitively disassemble the heterotenary complexes (Figure 6e) and trigger cargo release (Figure 6f and g).
Figure 6.
(a) Schematic representation of preferential formation of the ternary complexation of two MV+· with CB[8] over the CB[8]·MV2+·naphthol complexes in the presence of a reducing agent. (b) Fluorescence micrographs of the disintegration process of the microcapsule wall material in Na2S2O4 solution or H2O over 12 h. (c) Ultraviolet-light-driven photoisomerization of azobenzene moiety can be used to disassemble the heteroternary supramolecular complexes between MV2+, azobenzene, and CB[8], resulting in the release of the entrapped cargo from the microcapsules. (d) Fluorescence micrographs of the release of the molecular cargo from microcapsules with and without initial ultraviolet irradiation. (e) Schematic disassembly of the ternary complexes of MV, azobenzene, and CB[8], in the presence of competitive guest, 1-adamantylamine. Optical and fluorescent micrographs of dual cargo-loaded (Nile Red and 500 kDa FITC-dextran) microcapsules (f) before and (g) after hydration for 3 min in 1-adamantylamine solution (1 mM). Adapted with permission from ref (24). Copyright 2014 Nature Publishing Group.
3.2. Improved Cargo Loading Stability
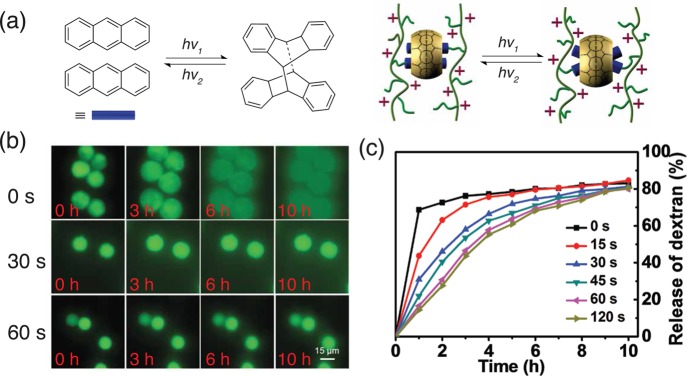
An ideal cargo delivery system would be expected to encapsulate a large amount of payload, without premature release during storage and transportation. Subsequently, triggered release of the cargo should be readily achieved in a controlled manner upon application of a stimulus.40 Incorporation of covalent cross-linking, derived from CB[n] host–guest complexation, can increase encapsulation stability. It has been reported that the photoinduced [4 + 4] dimerization of anthracene derivatives facilitated by CB[8] host molecules at 350 nm was 10 times faster than that of free anthracene molecules in solution, ascribed to the parallel alignment of two anthracene moieties within the CB[8] cavity (Figure 7a).45 In the case of microcapsules comprising of anthracene-functionalized HEC polymers, a photoinduced dimerization of anthracene moieties can convert noncovalent cross-links to covalent cross-links.30 An improved encapsulation stability in the microcapsules was detected post UV-irradiation, demonstrated by a retarded release of FITC-dextran (250 kDa) compared to the control in the absence of any UV irradiation (Figure 7b). Extending the UV irradiation further slows the release kinetics because of a higher degree of dimerization/cross-linking up to 120 s irradiation, when complete covalent cross-linking was achieved (Figure 7c). This approach demonstrates the potential of post-treatment modification to enhance supramolecular microcapsules to a specific release profile.
Figure 7.
(a) Schematic representation of the [4 + 4]-photodimerization of anthracene derivatives in the CB[8] cavity. (b) Fluorescent micrographs of the release of FITC-dextran from microcapsules as a function of UV irradiation time in water over 12 h. (c) FITC-dextran release profiles from the microcapsules as a function of the UV irradiation time. Adapted with permission from ref (30). Copyright 2015 The Royal Society of Chemistry.
3.3. Dual Cargo Loading and Orthogonal Delivery
Inspired by precisely organized complex structures in Nature, chemists and in particular supramolecular chemists, have attempted to mimic biological structures and produce artificially compartmentalized assemblies, following a simple prokaryotic model or even eukaryotic level of sophistication.46,47 The introduction of a specific molecular recognition motif in the complementary microcapsule wall, mimicking a biofunctional membrane, might allow for selective permeation among segregated domains.
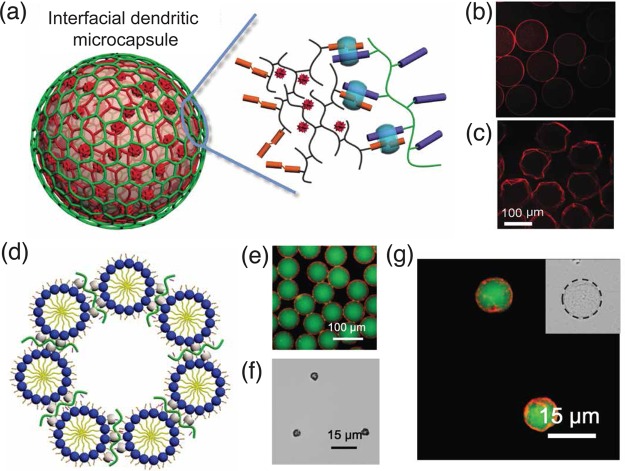
Double-, triple-, and multiple-emulsion microfluidic droplets have been developed, such as microcapsule-in-microcapsule architectures, for selective encapsulation of different cargoes.48,49 The encapsulation process of such nested microdroplets requires complicated microfluidic devices, which involve multistage sequential emulsion generation and precise control over the wettability of each droplet.50 In contrast, a dendritic copolymer is able to entrap a hydrophilic small molecule, such as Congo red, on account of its unique three-dimensional nanoscale structure (Figure 8a). By using dendritic polymers as the construction blocks for the microcapsules, Zheng and Yu et al. reported the exclusive localization of Congo red within the microcapsule shell, in the hydrated (Figure 8b) or dehydrated states (Figure 8c).24 In the micelle-based microcapsules. segregated domains are generated within the microcapsule core and micellar shell. This multicompartmental hierarchy enabled simultaneous encapsulation of both nonwater-soluble (Nile red, in the micelle) and water-soluble (FITC-dextran, green, in the capsule core) cargoes (Figure 8d–g).34 This system benefits from dynamic host–guest interactions, which are able to synergistically deliver cargoes with triggered release behaviors through photocontrolled porosity.
Figure 8.
(a) Schematic representation of an interfacial dendritic microcapsule prepared using a dendritic copolymer as one of the building blocks, and the preferential localization of hydrophobic oil-soluble Congo Red (red luminance) within a dendritic microcapsule shell before (b) and after (c) dehydration. (d) Schematic illustration of a supramolecular microcapsules assembled from micelles of an amphiphilic blocks copolymer; (e) fluorescence micrographs of monodisperse microcapsules containing both water-soluble FITC-dextran cargo (500 kDa, green) in the core and the hydrophobic Nile Red dye (red) in the microcapsule shell; (f) brightfield micrographs of dehydrated microcapsules; and (g) fluorescent micrograph of microcapsules after rehydration, confirming that partitioning of cargo was retained during after the dehydration/rehydration processes. Adapted with permission from ref (24), Copyright 2014 Nature Publishing Group; and ref (34), Copyright 2016 American Chemical Society.
4. Challenges, Concluding Remarks, and Future Directions
This Account briefly summarizes recent advancements in monodisperse microcapsules and microcapsule-based materials, fabricated by the integrating of microfluidics and CB[n] supramolecular host–guest chemistry. These microcapsules are attractive for fundamental studies of supramolecular polymer shell mechanics, leading to the engineering of sophisticated hierarchical structures and materials and their applications such as cargo delivery. Additionally, dynamic host–guest interactions bestow microcapsules with intrinsic reversibility and adaptability, thus dynamic assembly/disassembly activated by diverse external stimuli and stimuli-triggered release of payloads.
One limitation of this microfluidic technique is that it produces capsules that are tens to hundreds of micrometers in diameter. Therefore, a big challenge remains in using standard microfluidic devices for certain applications, which require exceptionally small dimensions down to the nanoscale. Capillaries with small orifices and manipulation of high pressure under extremely high throughputs have been demonstrated to reduce the size of the capsules to nanoscale.51 Nevertheless, routine preparation of nanoscale capsules requires future investigation, device design, and process optimization. An important direction for future work will be expanding the range of stimuli, which can be incorporated in microcapsule formulations, featuring quantitative functions and responses that can be activated in these dynamic assemblies in a well-controlled manner. A future challenge is to develop a monitoring strategy, which not only controls the microcapsule integrity, but also tracks in situ cargoes delivery. Further in vitro and in vivo studies will be indispensable before CB[n]-based microcapsules are used for any biomedical applications, as most current studies focus on the methodology of microcapsule construction and techniques to trigger the structural destruction with internal or external stimuli, thus release of the cargoes. Addressing these issues will benefit the design and fabrication of next-generation supramolecular hierarchical structures and functional materials.
Acknowledgments
J.L. is financially supported by the Marie Curie FP7 SASSYPOL ITN (607602) programme. C.S.Y.T. thanks the Ministry of Education of Malaysia and Universiti Teknologi MARA for their financial support. Z.Y. and C.A. were in receipt of funding from BBSRC sLoLa award reference BB/L002957/1, EPSRC and Institutional Sponsorship 2012-University of Cambridge EP/K503496/1 and the Translational Grant EP/H046593/1. O.A.S thanks the EPRSC (EP/F0355351 and EP/G060649/1) and the ERC (ASPiRe, 240629) for their funding.
Biographies
Ji Liu obtained his Ph.D. in 2013 under the cosupervision of Prof. Christine Jerome at the University of Liege and Prof. Etienne Duguet at the University of Bordeaux. He joined the group of Prof. Oren Scherman at the University of Cambridge as a postdoctoral research associate in 2014 and then as a Marie Curie research fellow since 2015. His current research is mainly focused on cucurbit[n]uril-based supramolecular host–guest interactions for polymeric self-assembly, supramolecular transient networks, and dynamic interfacial adhesion.
Yang Lan completed his Ph.D. at the University of Cambridge in 2014 under the supervision of Prof. Oren Scherman. He continued as a joint postdoctoral research associate with Prof. Oren Scherman and Dr. Erika Eiser in the Cavendish Laboratory. His current research is centred on colloidal self-assemblies with the assistance of cucurbit[n]uril host–guest chemistry.
Ziyi Yu received his Ph.D. at the Nanjing University of Technology in 2012 under the supervision of Prof. Su Chen, and then joined Prof. Chris Abell’s group at the University of Cambridge as a postdoctoral research associate. His research interest is on developing droplet-based microfluidics in cucurbit[n]uril-based supramolecular assemblies, colloidal structures, and single-cell analysis.
Cindy S. Y. Tan obtained her Ph.D. at the University of Cambridge in 2016, under the supervision of Prof. Oren Scherman. Her research focuses on supramolecular polymer networks based on cucurbit[n]uril host–guest interactions. She is currently a Senior Lecturer at Universiti Teknologi MARA in Malaysia.
Richard M. Parker graduated with a Ph.D. in chemistry from the University of Southampton in 2011. He is currently a postdoctoral research associate at the University of Cambridge, where he utilizes self-assembly within microfluidic droplets to prepare novel material architectures, with interests ranging from supramolecular microcapsules and gels to bioinspired photonics.
Chris Abell is the Pro-Vice-Chancellor for Research and the Professor of Biological Chemistry at the University of Cambridge. He was elected into the Academy of Medical Sciences in 2012 and a Fellow of the Royal Society in 2016. His research focus is on using fragment-based methods in drug discovery and chemical biology. In 1999, he cofounded Astex, which grew to become the world-leading company in fragment-based drug discovery. He also has research interests in developing microdroplets as a novel experimental platform, which led to the cofounding of Sphere Fluidics (2010) and Aqdot (2013).
Oren A. Scherman is the Director of the Melville Laboratory for Polymer Synthesis in the Department of Chemistry at the University of Cambridge and Professor of Supramolecular and Polymer Chemistry. His current research projects include the application of macrocyclic host–guest chemistry using cucurbit[n]urils in the development of novel supramolecular hydrogels and microcapsules, drug-delivery systems based on dynamic hydrogels, the conservation and restoration of important historical artefacts through the exploitation of supramolecular polymer chemistry and sensing and catalysis using self-assembled nanophotonic systems.
The authors declare no competing financial interest.
References
- Brunsveld L.; Folmer B.; Meijer E.; Sijbesma R. Supramolecular polymers. Chem. Rev. 2001, 101, 4071–4098. 10.1021/cr990125q. [DOI] [PubMed] [Google Scholar]
- Tsivgoulis G. M.; Lehn J. M. Photonic molecular devices: reversibly photoswitchable fluorophores for nondestructive readout for optical memory. Angew. Chem., Int. Ed. Engl. 1995, 34, 1119–1122. 10.1002/anie.199511191. [DOI] [Google Scholar]
- Aida T.; Meijer E.; Stupp S. Functional supramolecular polymers. Science 2012, 335, 813–817. 10.1126/science.1205962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncanson W. J.; Lin T.; Abate A. R.; Seiffert S.; Shah R. K.; Weitz D. A. Microfluidic synthesis of advanced microparticles for encapsulation and controlled release. Lab Chip 2012, 12, 2135–2145. 10.1039/c2lc21164e. [DOI] [PubMed] [Google Scholar]
- Datta S. S.; Abbaspourrad A.; Amstad E.; Fan J.; Kim S.-H.; Romanowsky M.; Shum H. C.; Sun B.; Utada A. S.; Windbergs M.; Zhou S.; Weitz D. A. 25th anniversary article: double emulsion templated solid microcapsules: mechanics and controlled release. Adv. Mater. 2014, 26, 2205–2218. 10.1002/adma.201305119. [DOI] [PubMed] [Google Scholar]
- van Dongen S. F.; de Hoog H.-P. M.; Peters R. J.; Nallani M.; Nolte R. J.; van Hest J. C. Biohybrid polymer capsules. Chem. Rev. 2009, 109, 6212–6274. 10.1021/cr900072y. [DOI] [PubMed] [Google Scholar]
- Sun T.; Morgan H. Single-cell microfluidic impedance cytometry: a review. Microfluid. Nanofluid. 2010, 8, 423–443. 10.1007/s10404-010-0580-9. [DOI] [Google Scholar]
- Zhang J.; Coulston R. J.; Jones S. T.; Geng J.; Scherman O. A.; Abell C. One-step fabrication of supramolecular microcapsules from microfluidic droplets. Science 2012, 335, 690–694. 10.1126/science.1215416. [DOI] [PubMed] [Google Scholar]
- Kim J.-W.; Utada A. S.; Fernández-Nieves A.; Hu Z.; Weitz D. A. Fabrication of monodisperse gel shells and functional microgels in microfluidic devices. Angew. Chem. 2007, 119, 1851–1854. 10.1002/ange.200604206. [DOI] [PubMed] [Google Scholar]
- Schloss A. C.; Liu W.; Williams D. M.; Kaufman G.; Hendrickson H. P.; Rudshteyn B.; Fu L.; Wang H.; Batista V. S.; Osuji C.; Yan E. C.; Regan L. Fabrication of modularly functionalizable microcapsules using protein-based technologies. ACS Biomater. Sci. Eng. 2016, 2, 1856–1861. 10.1021/acsbiomaterials.6b00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.-T.; Wang J.; Han J.-J. Fabrication of advanced particles and particle-based materials assisted by droplet-based microfluidics. Small 2011, 7, 1728–1754. 10.1002/smll.201001913. [DOI] [PubMed] [Google Scholar]
- Wang W.; Zhang M.-J.; Chu L.-Y. Functional polymeric microparticles engineered from controllable microfluidic emulsions. Acc. Chem. Res. 2014, 47, 373–384. 10.1021/ar4001263. [DOI] [PubMed] [Google Scholar]
- Johnston A. P.; Cortez C.; Angelatos A. S.; Caruso F. Layer-by-layer engineered capsules and their applications. Curr. Opin. Colloid Interface Sci. 2006, 11, 203–209. 10.1016/j.cocis.2006.05.001. [DOI] [Google Scholar]
- Wang Y.; Angelatos A. S.; Caruso F. Template synthesis of nanostructured materials via layer-by-layer assembly. Chem. Mater. 2008, 20, 848–858. 10.1021/cm7024813. [DOI] [Google Scholar]
- Kong F.; Zhang X.; Zhang H.; Qu X.; Chen D.; Servos M.; Mäkilä E.; Salonen J.; Santos H. A.; Hai M.; Weitz D. Inhibition of multidrug resistance of cancer cells by co-delivery of DNA nanostructures and drugs using porous silicon nanoparticles@ giant liposomes. Adv. Funct. Mater. 2015, 25, 3330–3340. 10.1002/adfm.201500594. [DOI] [Google Scholar]
- Barrow S. J.; Kasera S.; Rowland M. J.; del Barrio J.; Scherman O. A. Cucurbituril-based molecular recognition. Chem. Rev. 2015, 115, 12320–12406. 10.1021/acs.chemrev.5b00341. [DOI] [PubMed] [Google Scholar]
- Boekhoven J.; Stupp S. I. 25th anniversary article: supramolecular materials for regenerative medicine. Adv. Mater. 2014, 26, 1642–1659. 10.1002/adma.201304606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X.; Wang F.; Zheng B.; Huang F. Stimuli-responsive supramolecular polymeric materials. Chem. Soc. Rev. 2012, 41, 6042–6065. 10.1039/c2cs35091b. [DOI] [PubMed] [Google Scholar]
- Whitesides G. M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- Harada A.; Takashima Y.; Nakahata M. Supramolecular polymeric materials via cyclodextrin-guest interactions. Acc. Chem. Res. 2014, 47, 2128–2140. 10.1021/ar500109h. [DOI] [PubMed] [Google Scholar]
- Yu G.; Jie K.; Huang F. Supramolecular amphiphiles based on host-guest molecular recognition motifs. Chem. Rev. 2015, 115, 7240–7303. 10.1021/cr5005315. [DOI] [PubMed] [Google Scholar]
- Isaacs L. Stimuli responsive systems constructed using cucurbit[n]uril-type molecular containers. Acc. Chem. Res. 2014, 47, 2052–2062. 10.1021/ar500075g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf K. I.; Nau W. M. Cucurbiturils: from synthesis to high-affinity binding and catalysis. Chem. Soc. Rev. 2015, 44, 394–418. 10.1039/C4CS00273C. [DOI] [PubMed] [Google Scholar]
- Zheng Y.; Yu Z.; Parker R. M.; Wu Y.; Abell C.; Scherman O. A. Interfacial assembly of dendritic microcapsules with host-guest chemistry. Nat. Commun. 2014, 5, 5772. 10.1038/ncomms6772. [DOI] [PubMed] [Google Scholar]
- Park J. I.; Saffari A.; Kumar S.; Günther A.; Kumacheva E. Microfluidic synthesis of polymer and inorganic particulate materials. Annu. Rev. Mater. Res. 2010, 40, 415–443. 10.1146/annurev-matsci-070909-104514. [DOI] [Google Scholar]
- Stephenson G.; Parker R. M.; Lan Y.; Yu Z.; Scherman O. A.; Abell C. Supramolecular colloidosomes: fabrication, characterisation and triggered release of cargo. Chem. Commun. 2014, 50, 7048–7051. 10.1039/c4cc01479k. [DOI] [PubMed] [Google Scholar]
- Yu Z.; Lan Y.; Parker R. M.; Zhang W.; Deng X.; Scherman O. A.; Abell C. Dual-responsive supramolecular colloidal microcapsules from cucurbit[8]uril molecular recognition in microfluidic droplets. Polym. Chem. 2016, 7, 5996–6002. 10.1039/C6PY01171C. [DOI] [Google Scholar]
- Quevedo E.; Steinbacher J.; McQuade D. T. Interfacial polymerization within a simplified microfluidic device: capturing capsules. J. Am. Chem. Soc. 2005, 127, 10498–10499. 10.1021/ja0529945. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Tumarkin E.; Peerani R.; Nie Z.; Sullan R. M. A.; Walker G. C.; Kumacheva E. Microfluidic production of biopolymer microcapsules with controlled morphology. J. Am. Chem. Soc. 2006, 128, 12205–12210. 10.1021/ja0635682. [DOI] [PubMed] [Google Scholar]
- Yu Z.; Zhang J.; Coulston R. J.; Parker R. M.; Biedermann F.; Liu X.; Scherman O. A.; Abell C. Supramolecular hydrogel microcapsules via cucurbit[8]uril host–guest interactions with triggered and UV-controlled molecular permeability. Chem. Sci. 2015, 6, 4929–4933. 10.1039/C5SC01440A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman G.; Boltyanskiy R.; Nejati S.; Thiam A. R.; Loewenberg M.; Dufresne E. R.; Osuji C. O. Single-step microfluidic fabrication of soft monodisperse polyelectrolyte microcapsules by interfacial complexation. Lab Chip 2014, 14, 3494–3497. 10.1039/C4LC00482E. [DOI] [PubMed] [Google Scholar]
- Kim M.; Yeo S. J.; Highley C. B.; Burdick J. A.; Yoo P. J.; Doh J.; Lee D. One-step generation of multifunctional polyelectrolyte microcapsules via nanoscale interfacial complexation in emulsion (NICE). ACS Nano 2015, 9, 8269–8278. 10.1021/acsnano.5b02702. [DOI] [PubMed] [Google Scholar]
- Parker R. M.; Zhang J.; Zheng Y.; Coulston R. J.; Smith C. A.; Salmon A. R.; Yu Z.; Scherman O. A.; Abell C. Electrostatically directed self-assembly of ultrathin supramolecular polymer microcapsules. Adv. Funct. Mater. 2015, 25, 4091–4100. 10.1002/adfm.201501079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z.; Zheng Y.; Parker R. M.; Lan Y.; Wu Y.; Coulston R. J.; Zhang J.; Scherman O. A.; Abell C. Microfluidic droplet-facilitated hierarchical assembly for dual cargo loading and synergistic delivery. ACS Appl. Mater. Interfaces 2016, 8, 8811–8820. 10.1021/acsami.6b00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y.; Loh X. J.; Geng J.; Walsh Z.; Scherman O. A. A supramolecular route towards core-shell polymeric microspheres in water via cucurbit[8]uril complexation. Chem. Commun. 2012, 48, 8757–8759. 10.1039/c2cc34016j. [DOI] [PubMed] [Google Scholar]
- Kim C.; Agasti S. S.; Zhu Z.; Isaacs L.; Rotello V. M. Recognition-mediated activation of therapeutic gold nanoparticles inside living cells. Nat. Chem. 2010, 2, 962–966. 10.1038/nchem.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groombridge A. S.; Palma A.; Parker R. M.; Abell C.; Scherman O. A. Aqueous interfacial gels assembled from small molecule supramolecular polymers. Chem. Sci. 2017, 10.1039/C6SC04103E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon A. R.; Parker R. M.; Groombridge A. S.; Maestro A.; Coulston R. J.; Hegemann J.; Kierfeld J.; Scherman O. A.; Abell C. Microcapsule buckling triggered by compression-induced interfacial phase change. Langmuir 2016, 32, 10987–10994. 10.1021/acs.langmuir.6b03011. [DOI] [PubMed] [Google Scholar]
- Das M.; Zhang H.; Kumacheva E. Microgels: old materials with new applications. Annu. Rev. Mater. Res. 2006, 36, 117–142. 10.1146/annurev.matsci.36.011205.123513. [DOI] [Google Scholar]
- Liu J.; Detrembleur C.; Mornet S.; Jérôme C.; Duguet E. Design of hybrid nanovehicles for remotely triggered drug release: an overview. J. Mater. Chem. B 2015, 3, 6117–6147. 10.1039/C5TB00664C. [DOI] [PubMed] [Google Scholar]
- Zhao C.-X. Multiphase flow microfluidics for the production of single or multiple emulsions for drug delivery. Adv. Drug Delivery Rev. 2013, 65, 1420–1446. 10.1016/j.addr.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Liu J.; Debuigne A.; Detrembleur C.; Jérôme C. Poly (N-vinylcaprolactam): a thermoresponsive macromolecule with promising future in biomedical field. Adv. Healthcare Mater. 2014, 3, 1941–1968. 10.1002/adhm.201400371. [DOI] [PubMed] [Google Scholar]
- Xu X.; Appel E. A.; Liu X.; Parker R. M.; Scherman O. A.; Abell C. Formation of cucurbit[8]uril-based supramolecular hydrogel beads using droplet-based microfluidics. Biomacromolecules 2015, 16, 2743–2749. 10.1021/acs.biomac.5b01048. [DOI] [PubMed] [Google Scholar]
- Tan C. S. Y.; del Barrio J.; Liu J.; Scherman O. A. Supramolecular polymer networks based on cucurbit[8]uril host-guest interactions as aqueous photo-rheological fluids. Polym. Chem. 2015, 6, 7652–7657. 10.1039/C5PY01115A. [DOI] [Google Scholar]
- Biedermann F.; Ross I.; Scherman O. A. Host-guest accelerated photodimerisation of anthracene-labeled macromolecules in water. Polym. Chem. 2014, 5, 5375–5382. 10.1039/C4PY00627E. [DOI] [Google Scholar]
- Marguet M.; Bonduelle C.; Lecommandoux S. Multicompartmentalized polymeric systems: towards biomimetic cellular structure and function. Chem. Soc. Rev. 2013, 42, 512–529. 10.1039/C2CS35312A. [DOI] [PubMed] [Google Scholar]
- Schoonen L.; van Hest J. Compartmentalization approaches in soft matter science: from nanoreactor development to organelle mimics. Adv. Mater. 2016, 28, 1109–1128. 10.1002/adma.201502389. [DOI] [PubMed] [Google Scholar]
- Shum H. C.; Zhao Y. J.; Kim S.-H.; Weitz D. A. Multicompartment polymersomes from double emulsions. Angew. Chem. 2011, 123, 1686–1689. 10.1002/ange.201006023. [DOI] [PubMed] [Google Scholar]
- Delcea M.; Yashchenok A.; Videnova K.; Kreft O.; Möhwald H.; Skirtach A. G. Multicompartmental micro- and nanocapsules: hierarchy and applications in biosciences. Macromol. Biosci. 2010, 10, 465–474. 10.1002/mabi.200900359. [DOI] [PubMed] [Google Scholar]
- Paleos C. M.; Pantos A. Molecular recognition and organizational and polyvalent effects in vesicles induce the formation of artificial multicompartment cells as model systems of eukaryotes. Acc. Chem. Res. 2014, 47, 1475–1482. 10.1021/ar4002679. [DOI] [PubMed] [Google Scholar]
- Valencia P. M.; Farokhzad O. C.; Karnik R.; Langer R. Microfluidic technologies for accelerating the clinical translation of nanoparticles. Nat. Nanotechnol. 2012, 7, 623–629. 10.1038/nnano.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]