Abstract
In vitro single-vesicle fusion assays are important tools to analyze the details of SNARE-mediated fusion processes. In this study, we employed planar pore-spanning membranes (PSMs) prepared on porous silicon substrates with large pore diameters of 5 μm, allowing us to compare the process of vesicle docking and fusion on the supported parts of the PSMs (s-PSMs) with that on the freestanding membrane parts (f-PSM) under the exact same experimental conditions. The PSMs harbor the t-SNARE ΔN49-complex to investigate the dynamics and fusogenicity of single large unilamellar vesicles doped with the v-SNARE synaptobrevin 2 by means of spinning-disc confocal microscopy with a time resolution of 10 ms. Our results demonstrate that vesicles docked to the s-PSM were fully immobile, whereas those docked to the f-PSM were mobile with a mean diffusion coefficient of 0.42 μm2/s. Despite the different dynamics of the vesicles on the two membrane types, similar fusion kinetics were observed, giving rise to a common fusion mechanism. Further investigations of individual lipid mixing events on the s-PSMs revealed semi-stable post-fusion structures.
Introduction
SNARE-mediated fusion of synaptic vesicles in the nerve terminal of eukaryotic cells is a fundamental step during signal transduction. To understand this process on a molecular level, several in vitro fusion assays have been developed over the past decades. In particular, single-vesicle fusion assays have evolved as an important tool to explore the mechanism of SNARE-mediated membrane fusion (1, 2, 3). A major advantage of single-vesicle fusion assays over bulk assays is that fusion intermediates can be readily distinguished and kinetic aspects such as docking lifetimes can be quantified (4, 5, 6, 7). Starting from a membrane geometry that resembles the planar presynaptic target membrane on one side and the vesicular membrane on the other side, virtually all described setups are based on supported lipid bilayers combined with fluorescence microscopy techniques such as total internal reflection fluorescence (TIRF) microscopy (6, 8, 9). Although these membranes have advantages, such as long-term stability, they suffer from membrane-support interactions that considerably influence lipid and protein mobility, which can lead to large fractions of immobile proteins and docked vesicles, as well as SNAP 25-independent fusion events (1, 3). To overcome some of these drawbacks occurring in membranes directly deposited on glass surfaces, polymer-cushioned membranes were developed to decouple the bilayer from the support. In this membrane system, fusion was SNAP 25 dependent and very fast, but docked vesicles were also immobile (1, 10, 11). These findings are in contrast to recent studies revealing that synaptic vesicles that are primed to the pre-synaptic membrane exhibit lateral mobility (12), which implies that the in vitro model systems introduce additional parameters that affect mobility. To provide a membrane system with freestanding bilayer areas, we recently presented a single-vesicle fusion assay based on planar pore-spanning membranes (PSMs) to investigate neuronal SNARE-mediated fusion (13). In contrast to supported lipid bilayers, PSMs feature freestanding membranes spanning pores of a highly ordered micro array in SiO2/Si3N4, which are surrounded by supported membranes (14).
In this study, PSMs were prepared by spreading GUVs (diameter >20 μm) with reconstituted t-SNAREs on a porous support functionalized with a hydrophilic self-assembled monolayer on the rims. The resulting membrane patches are composed of supported membrane parts on the rims (s-PSMs) and freestanding membranes spanning the pores (f-PSMs). Vesicles doped with synaptobrevin 2 (syb 2) were added, and docking and fusion was monitored by spinning-disc confocal microscopy with 10 ms time resolution. This system enables us to compare the behavior of docked vesicles on the supported part of the membrane with their behavior on the freestanding part under the exact same experimental conditions. In particular, the dynamics of SNARE-mediated docked vesicles on the supported and freestanding membrane parts was analyzed in detail to shed some light on the question of how the mobility of docked vesicles depends on the underlying planar membrane, and how this might influence the fusion process.
Materials and Methods
Materials
Porous SiO2/Si3N4 substrates with pore diameters of 5 μm and a surface porosity (area occupied by the pores) of 36% were purchased from Aquamarijn (Zutphen, the Netherlands). The pores were 800 nm deep and open on both sides, i.e., they are sieve like structures. All lipids were purchased from Avanti Polar Lipids (Alabaster, AL), including 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-l-serine (POPS), and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE). TR 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine triethylammonium salt (TexasRed-DPPE), cholesterol, and 6-mercapto-1-hexanol were purchased from Sigma Aldrich (Taufkirchen, Germany). Atto488 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (Atto488-DPPE) was purchased from Atto-Tec (Siegen, Germany). The OregonGreen syx 1A-transmembrane domain (aa 257–288, M267A, C271A, I279A; sequence: OregonGreen488-X-YQSKARRKKIAIIIACVILGIIAAS-TIGGIFG-OH) was synthesized using Fmoc solid-phase peptide synthesis as described previously (15).
Protein expression and isolation
Hexahistidine-tagged SNAREs, originating from Rattus norvegicus, including full-length synaptobrevin 2 (aa 1–116), a soluble synaptobrevin 2 fragment (aa 49–96) for the formation of the ΔN49-complex, a soluble synaptobrevin 2 fragment (aa 1–96) to competitively block the fusion process, syntaxin 1A (aa 183–288) and SNAP 25a (aa 1–206 with all cysteines replaced by serine) were heterologously expressed in Escherichia coli BL21(DE3) carrying a pET28a expression vector. Purification was performed by Ni-NTA agarose affinity chromatography with subsequent ion-exchange chromatography on MonoQ or MonoS columns (Äkta purifying system, GE Healthcare, Little Chalfont, United Kingdom). Hexahistidine tags were cleaved by thrombin overnight before ion-exchange chromatography. Full-length syb 2 and syntaxin 1A were handled in the presence of 16 mM CHAPS. The t-SNARE ΔN49 complex was assembled from the proteins syntaxin 1A, SNAP 25a, and the soluble syb 2 fragment (aa 49–96) overnight at 4°C and was further purified by ion-exchange chromatography on a MonoQ column in the presence of 16 mM CHAPS.
Protein reconstitution into vesicles
Proteins were reconstituted into vesicles by co-micellization in the presence of n-octyl-β-d-glycoside (n-OG) and subsequent detergent removal by size-exclusion chromatography, as described previously (16). Briefly, lipids (DOPC/POPS/POPE/cholesterol 5:1:2:2 (mol/mol), 0.5 mg total) dissolved in chloroform were mixed and the solvent was removed by a stream of nitrogen and further dried in vacuum for 30 min to remove remaining solvent. The lipid films were solubilized with 50 μL reconstitution buffer (20 mM HEPES, 100 mM KCl, and 1 mM EDTA, pH 7.4) and n-OG. Proteins were added to a total concentration of 75 mM n-OG and a protein to lipid ratio of 1:500. The mixed micelle solution was incubated for 30 min at room temperature and detergent was removed via size-exclusion chromatography (illustra NAP-25 G25 column, GE Healthcare) in reconstitution buffer. A subsequent second size-exclusion step was performed in ultrapure water to remove remaining detergent and salt. The proteoliposome suspension was then dried either on indium tin oxide slides in a desiccator over a saturated sodium chloride solution (relative humidity 80%) for the formation of GUVs by electroformation or in a glass cylinder for the preparation of large unilamellar vesicles (LUVs) by swelling and extrusion. Electroformation was carried out by applying a sinusoidal wave for 3 h (1.6 Vpeak-peak, 10 Hz) to the indium tin oxide slides filled with 200 mM sucrose solution. LUVs were produced by swelling the dried proteo-lipid film in reconstitution buffer for 15 min and then mixed for 1 min. This procedure was repeated three times. Afterwards, the suspended lipid film was extruded 31 times with a miniextruder (LiposoFast-Basic, Avestin, Ottawa, Ontario, Canada) through a polycarbonate membrane with a nominal pore diameter of 400 nm. Vesicle sizes were determined to be 240 ± 100 nm by dynamic light scattering. Successful reconstitution of full-length syb 2 into the LUVs was confirmed by density-gradient centrifugation and sodium dodecyl sulfate polyacrylamide gel electrophoresis (data not shown). Successful reconstitution of the t-SNARE ΔN49-complex into PSMs with the described protocol was verified indirectly by reconstituting the OregonGreen-labeled syntaxin 1A transmembrane domain. The fusogenicity of the LUVs and GUVs was analyzed by bulk lipid mixing experiments as shown previously (13).
PSMs
Porous SiO2/Si3N4 substrates with pore diameters of 5 μm and a surface porosity of 36% were rinsed with ethanol and dried under a stream of nitrogen. The surface was sputter coated (10 s, 40 mA, 0.08 mbar; Cressington sputter coater 108 auto, Elektronen-Optik-Service, Dortmund, Germany) with a thin titanium layer and subsequently coated with a 30-nm-thick gold layer deposited by thermal evaporation (Bal-Tec Med 020, Balzers, Liechtenstein). The substrates were immersed in a 1 mM n-propanolic 6-mercapto-1-hexanol solution overnight at room temperature. Functionalized substrates were rinsed with ethanol, dried under a stream of nitrogen, and placed in the buffer-filled measuring chamber. GUV solution (5–10 μL) was pipetted onto the substrates and incubated for 10–20 min, resulting in PSMs. GUVs that did not spread were carefully rinsed from the surface with a pipette. The formation of PSMs was monitored by fluorescence microscopy.
Fluorescence microscopy
An upright spinning-disc confocal microscope setup (spinning disc: Yokogawa CSU-X, Rota Yokogawa KG, Wehr, Germany; stand: Olympus custom-made, Olympus Deutschland, Hamburg, Germany; camera: iXON 897Ultra, Andor Technology, Belfast, United Kingdom) equipped with a water immersion objective (LUMFLN 60XW 60× NA 1.1, Olympus, Hamburg, Germany) was used for the experiments. In the single-color experiments, TexasRed-DPPE was excited at λ = 561 nm and selected with a 590 LP ET long-pass emission filter (AHF Analysentechnik, Tübingen, Germany). In the dual-color experiments, TexasRed-DPPE was excited at λ = 561 nm and selected with a 650/60 emission filter (AHF Analysentechnik), and Atto488-DPPE was excited at λ = 488 nm and selected with a 525/50 emission filter (AHF Analysentechnik). Each channel was then aligned onto one half of the 512 × 512 pixel2 detector using an optosplit (Acal BFi Germany, Dietzenbach, Germany). The pixel size was 222 × 222 nm2.
Data analysis
Docking and fusion of vesicles with PSMs was recorded over a period of 155 s with 10 ms time resolution in single-color mode and 20 ms time resolution in dual-color mode. The identification of docking and fusion of vesicles on PSMs was evaluated manually. Time-resolved fluorescence intensity data were analyzed by placing a 4 × 4 pixel2 region of interest (ROI) on the center of each individual vesicle. Vesicles that docked to the f-PSM were analyzed by using a dynamic ROI that follows the center of the tracked vesicle. Docking times of the vesicles were evaluated as detailed in the Supporting Material (Fig. S1). Mobile vesicles were tracked with the software TrackNTrace by fitting the intensity profile of the vesicle with the point spread function (17). This allows detection of displacements with subpixel resolution over several hundred frames to evaluate the mean-square displacement (MSD) over time. For the calculation of the diffusion coefficient, a linear function was fit to the first 15–20 data points (150–200 ms).
Results
To investigate docking and fusion of vesicles with planar membranes dependent on the support of the bilayer, we reconstituted the minimal neuronal SNARE fusion machinery, composed of the v-SNARE syb 2 and a stabilized t-SNARE complex (ΔN49-complex) that consists of the SNAREs syntaxin 1A, SNAP 25a, and a soluble fragment of syb 2 (aa 49–96). Full-length syb 2 was reconstituted into LUVs (protein/lipid 1:500) with diameters of 240 ± 100 nm (Fig. S2), and the ΔN49-complex was reconstituted into planar PSMs (protein/lipid 1:500), as described previously (13). Vesicles and PSMs were both composed of DOPC/POPE/POPS/cholesterol (5:2:1:2, mol/mol), similar to the lipid composition of synaptic vesicles (18). The ΔN49-complex was chosen as it has been shown to avoid the formation of a syntaxin 1A/SNAP 25a 2:1 dead-end complex, which significantly reduces fusion kinetics (16). In the ΔN49-complex, the soluble syb 2 fragment can be displaced by full-length syb 2 forming the highly conserved four-helix SNARE bundle (16).
Docking of syb-2-containing vesicles to ΔN49-complex-doped PSMs
Docking of syb-2-doped vesicles to PSMs containing the ΔN49-complex was observed by spinning-disc confocal microscopy. The vesicles were fluorescently labeled with 1 mol% TexasRed-DPPE and the PSM with 1 mol% Atto488-DPPE. Before each fusion experiment, an image of the PSM was recorded to verify an intact continuous membrane and to obtain a mask for the positions of the freestanding (f-PSMs) and supported membrane areas (s-PSMs). This mask allowed a distinct assignment of the docking and fusion sites on the PSM for each vesicle in the analysis of the time series. Image acquisition was started by exciting and recording the TexasRed (TR) fluorescence, and syb-2-containing vesicles were added atop the individual PSM. The LUVs freely diffused in the volume above the PSMs until they randomly docked to the planar membrane. Specificity of the SNARE-induced docking was analyzed by blocking the ΔN49-complex in the PSM before addition of the syb-2-doped vesicles using a soluble syb 2 fragment (aa 1–96) lacking the transmembrane domain that irreversibly binds to the ΔN49-complex, preventing vesicle docking and fusion (16). If the ΔN49-complex was blocked with the syb 2 fragment, no docking of syb 2 vesicles to the PSMs was observed. Moreover, without SNAP 25a, no docking and fusion was observed, as reported in our previous study (13).
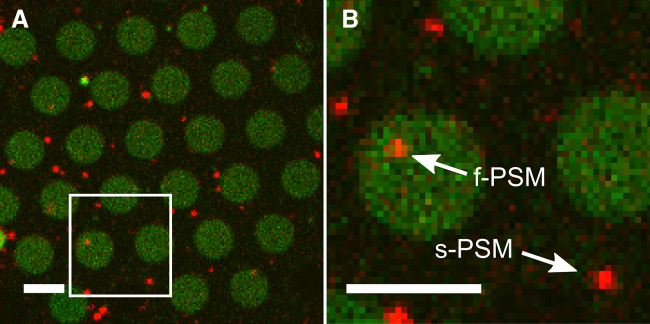
Fig. 1 A shows an overlay of a fluorescence micrograph of a PSM (Atto488-DPPE; green channel) with vesicles that docked seconds after their injection (TR-DPPE; red channel). The red fluorescence of docked vesicles is clearly visible on the f-PSMs and on the s-PSMs, whereas the green fluorescence of the PSM can only be observed on the f-PSMs. This is a result of the height-dependent fluorescence quenching at distances <15 nm from the underlying gold surface (19, 20, 21). Evidence for the formation of a continuous lipid bilayer on top of the substrate was obtained by fluorescence recovery after photobleaching (FRAP) experiments (Fig. S3).
Figure 1.
(A) Fluorescence micrograph of an Atto488-DPPE-labeled PSM composed of DOPC/POPE/POPS/cholesterol (5:2:1:2), containing the t-SNARE ΔN49-complex, to which TR-DPPE-labeled vesicles composed of the same lipid composition doped with full-length syb 2 were docked. (B) Magnification of the marked area (white box) of the fluorescence micrograph shown in (A), highlighting that vesicles dock to supported PSMs (s-PSMs) and freestanding PSMs (f-PSMs). Scale bars, 5 μm. To see this figure in color, go online.
Each individual docked vesicle on the s-PSM as well as on the f-PSM can be assigned (Fig. 1 B (magnification of the boxed area in Fig. 1 A)). Analysis of the docking sites of 664 docked vesicles, observed in seven different preparations on 17 PSM patches, revealed that only 16% of the vesicles initially docked to the f-PSM, whereas 84% docked to the s-PSM. Considering that the area percentage occupied by the f-PSM is 36%, the docking probability on the f-PSM is lower by a factor of ∼2 than on the s-PSM. We do not assume largely different protein concentrations in the f-PSM and the s-PSM, which could be an explanation for the different docking probabilities, as FRAP experiments revealed that an OregonGreen-labeled syntaxin 1A transmembrane domain (OG-syx 1A TMD) can diffuse between the s-PSM and the f-PSM (Fig. S3). It is, however, conceivable that immobile proteins in the s-PSMs slightly increase the docking probability, as discussed below.
Mobility of docked vesicles
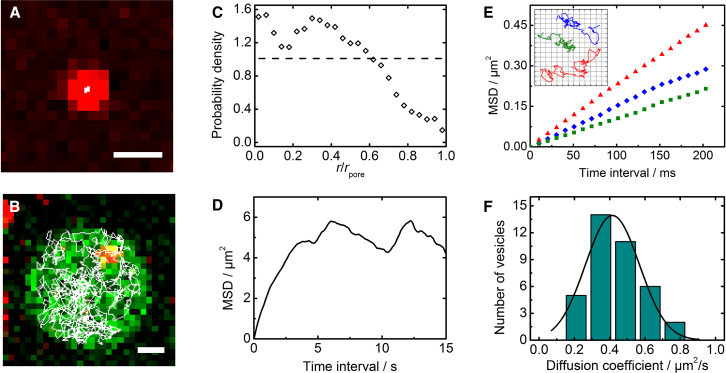
By video microscopy, we recorded the mobility of the docked vesicles. The vast majority of the vesicles docked to s-PSMs appeared to be fully immobile (>99%), whereas all observed vesicles docked to f-PSMs were mobile inside the f-PSMs (Movies S1 and S2). We analyzed the mobility of s-PSM and f-PSM docked vesicles by single-particle tracking with subpixel resolution (17). From the individual positions, trajectories were derived by visualizing the random walk of the vesicles. Fig. 2 depicts typical trajectories of a docked vesicle on an s-PSM (Fig. 2 A) and on an f-PSM (Fig. 2 B) over a time period of several seconds, with the underlying fluorescence micrographs displaying the first frame of the trajectory, i.e., the vesicle’s starting position. The trajectory of the s-PSM docked vesicle appears as a white dot as it is located only within an area much smaller than a single pixel. All analyzed s-PSM docked vesicles exhibit identical trajectories, indicating that they are immobile. This observation is consistent with findings of other groups who used solid supported or polymer-cushioned membranes (1, 3, 8, 9, 22). In none of these systems have mobile SNARE-mediated docked vesicles been reported.
Figure 2.
Fluorescence micrographs of a TR-doped syb-2-containing vesicle docked to (A) a ΔN49-complex-doped s-PSM and (B) a ΔN49-complex-doped f-PSM together with the corresponding trajectories derived by single-particle tracking (white line) over a time period of several seconds. Scale bars, 1 μm. (C) Probability density of the position of the docked vesicle in the f-PSM. (D) MSD versus time-interval plot derived from the trajectory of the f-PSM docked vesicle shown in (B) over a larger time period of 15 s, showing confined diffusion inside the f-PSM. (E) MSD-versus-time-interval plots of the first 20 time steps of three exemplary vesicles docked to f-PSMs, showing that the vesicles freely diffuse on a short timescale. Diffusion coefficients, D, of 0.55 (red), 0.36 (blue), and 0.27 μm2/s (green) were obtained from the slope (4D). The corresponding trajectories are depicted in the inset of (E) for a time lapse of 1 s. The grid lines are 200 nm long. (F) Distribution of the diffusion coefficients obtained for 38 vesicles docked to f-PSMs. A Gaussian distribution was fit to the histogram with a maximum of 0.42 μm2/s (σ = 0.15 μm2/s). To see this figure in color, go online.
Of note, we found that all vesicles docked to the f-PSM were highly mobile. The trajectories covered large areas of the f-PSM, but never exceeded the pore rim (Fig. 2 B). During the docking time period, most of the vesicles (89%) that docked on the f-PSM became immobilized on the boundary between s-PSM and f-PSM and did not regain their mobility. This means that initially counted f-PSM docked vesicles turn into s-PSM fusing vesicles. This phenomenon leads to the very low fusion probability eventually observed on the f-PSM, as discussed in more detail below.
To investigate whether the vesicles diffuse within the entire f-PSM with the same probability, we determined the radial distribution of 38 vesicles docked to the f-PSM. For this purpose, the f-PSMs were segmented into annuli with a width of 100 nm. The distance from each tracking point to the pore center was then determined and assigned to the corresponding annulus, and the counts per annuli were corrected for the different annulus sizes (Fig. 2 C). The probability density demonstrates that the vesicle is capable of exploring the entire f-PSM; however, the probability of finding a vesicle on the edge of the f-PSM (r = 2.5 μm) is significantly reduced. This finding implies an energy penalty for the vesicle to be located at the rim of the f-PSM, which might be a result of the curved membrane structure in the rim area, as shown previously (23).
The diffusion velocity of the vesicle is, however, independent of the position of the docked vesicle. To quantify the diffusion constant of a docked vesicle, we tracked the vesicle and computed the MSD (Fig. 2 D). The MSD approaches a constant value on large timescales, demonstrating the confinement of the f-PSM area (20 μm2). On short timescales (several 100 ms), the diffusion of the vesicle is unhindered, being linear with a slope of 4D (Fig. 2 E). This observation is reasonable, as the f-PSM area is significantly larger than the average displacement per time interval and the size of the vesicle. We determined the diffusion coefficients of 38 f-PSM docked vesicles with a mean diffusion coefficient of 0.42 ± 0.15 μm2/s (mean ± SD) (Fig. 2 F). Compared to the diffusion coefficient of an OG-syx 1A TMD in the f-PSM of 3.4 ± 0.2 μm2/s (mean ± SD) (13), the diffusion constant of the docked vesicles is reduced by a factor of 8.
Fusion of docked vesicles
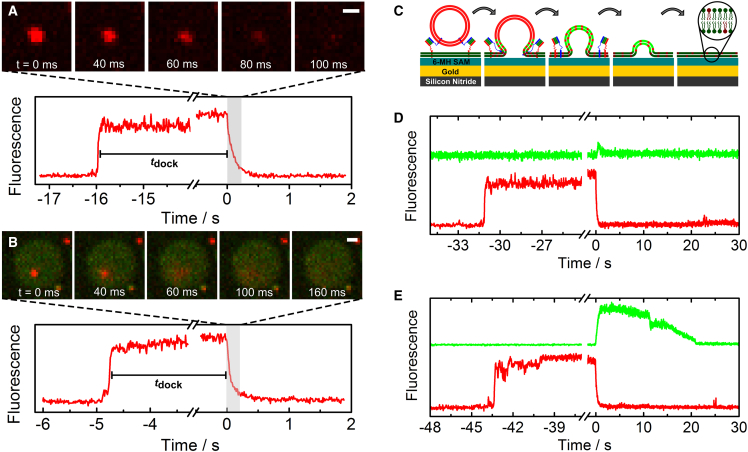
After vesicle docking, part of the vesicles proceeded to fusion. We counted 664 vesicles docked to the PSMs. Of these vesicles, 47% proceeded to fusion and the rest remained docked until the end of the time series. In total, 312 fusion events were observed on s-PSMs and only 11 fusion events were monitored on the f-PSM. This low number of fusion events on f-PSMs is a result of the two-times-reduced probability that the vesicles will dock to the f-PSMs (i.e., 16%, with 36% being the expectation if the docking site was independent of s-PSM and f-PSM) and the observation that most of the f-PSM docked vesicles are immobilized on the pore rim before fusion, thus counting as an s-PSM fusion event. Fusion of vesicles with the s-PSM was analyzed by reading out the TR intensity in a fixed ROI (4 × 4 pixel2) on the center of each docked vesicle. Due to their mobility, f-PSM docked vesicles were tracked during the analysis. Fluorescence micrographs of a vesicle fusing with the s-PSM and one fusing with the f-PSM, together with the corresponding time-resolved TR intensity traces of the center ROI, are shown in Fig. 3, A and B, respectively. Docking of the vesicle in the ROI leads to an increase in TR intensity to a constant level. Upon onset of fusion, the TR intensity decreases to the TR intensity before docking, as TR radially distributes into the planar membrane. This radial TR distribution around the fusing vesicle can be observed on the f-PSM by applying a donut-shaped ROI around the vesicle (Fig. S4), but this will not work for fusion events on the s-PSM due to the gold-induced fluorescence quenching. We found that the majority of the fusing vesicles (87%) showed full TR release to the predocking intensity within a single step, as shown in Fig. 3, A and B. These events are interpreted as full fusion events, as no stable fusion intermediates are observable with the given time resolution of 10 ms. Only a small fraction of the fusing vesicles (13%) showed intermediate TR intensity levels, from which 64% proceeded to full fusion in a second step (Fig. S5). These intermediates cannot be unambiguously assigned to hemifusion states, as they could also arise from transient fusion products or inhomogeneities of the vesicle suspension (Supporting Material; Fig. S5) (24, 25). Further experiments are currently in progress to elucidate this aspect in more detail.
Figure 3.
Fluorescence micrograph sequences of a docked vesicle fusing with (A) an s-PSM and (B) an f-PSM, together with the corresponding time-resolved TR intensity traces analyzed in a 4 × 4 pixel2 ROI on the center of each vesicle. Scale bars, 1 μm. (C) Cartoon of a vesicle fusing with the s-PSM, indicating the diffusion of TR and Atto488 and the observable fluorescence signal as a function of the distance to the gold layer. Time-resolved intensity traces of TR and Atto488 recorded simultaneously in dual-color mode showing (D) a fast-collapsing vesicle and (E) a much-slower-collapsing vesicle. The small dip in the Atto488 trace (t = 11 s) in (E) is a result of a microscope vibration. To see this figure in color, go online.
Using dual-color fluorescence readout, we were further able to investigate what happens to the vesicle ghost, i.e., the vesicle that has started to fuse with the planar membrane. Fig. 3 C shows a schematic drawing of the scenario of a fusing vesicle on the gold-covered pore rims. The vesicle ghost can immediately collapse into the s-PSM after fusion, as observed by Kiessling et al. (26) for small vesicles of d = 50 nm, or it can remain in an Ω-shaped structure for a certain time period (27). To illuminate this process, 60 fusion events were analyzed by simultaneously reading out the fluorescence intensity of Atto488 and TR in a 4 × 4 pixel2 ROI placed around the center of each vesicle. Upon onset of fusion, TR diffuses out of the vesicle ghost and is quenched, whereas Atto488 diffuses into the vesicle ghost and is dequenched (Fig. 3 C). If the vesicle ghost collapses very fast into the s-PSM, only a short Atto488 peak is observed after TR decay (Fig. 3 D).
If, however the vesicle ghost remains in a three-dimensional Ω-shaped structure, a significant increase in the Atto488 signal is observed after full TR decay (Fig. 3 E) as a result of the diffusion of the Atto488 molecules into the three-dimensional vesicle ghost separating the Atto488 fluorophores from the quenching gold surface. We found that 47% of the fusion events showed rapid collapse (<1 s) of the vesicle ghost into the s-PSM, whereas the residual 53% of the vesicles collapsed much more slowly or even remained in an Ω-shaped structure until the end of the time series.
Kinetics of single fusion events
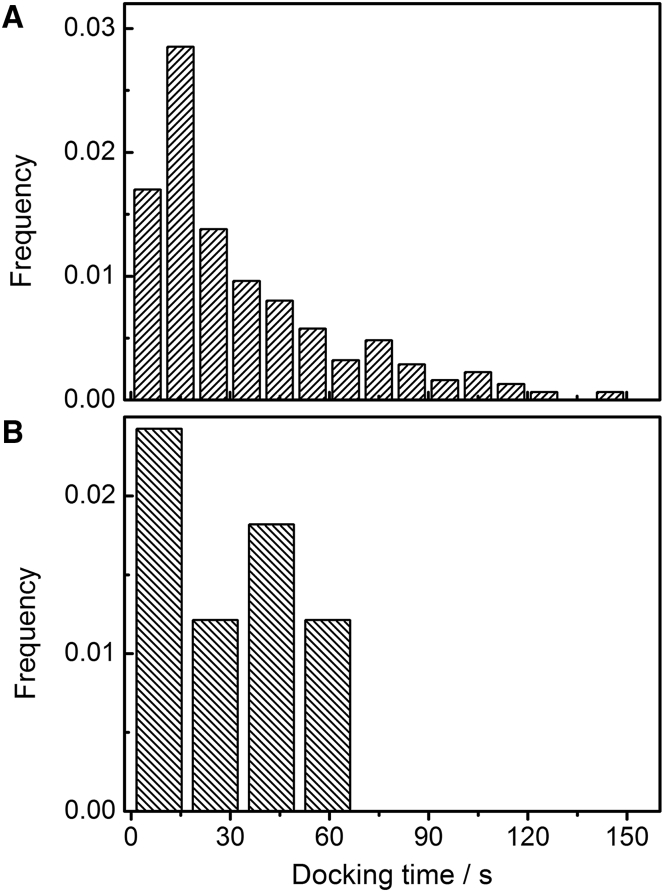
We further analyzed the docking time, which is defined as the time between docking of a vesicle to the PSM and the onset of fusion, as a function of whether the vesicle fused with the s-PSM or the f-PSM (Fig. 4, A and B). Fig. 4 A shows the histogram of the docking times of all fusion events recorded on s-PSMs. The docking times are broadly distributed between 5 and 150 s, with a main peak at 15 s and a tail toward longer times. The docking times of the 11 events we observed on the f-PSM range from 4 to 60 s (Fig. 4 B) and are thus in the same range as those observed on the s-PSMs, even though the statistics is low, which is a result of the low probability of observing a freely diffusing vesicle fusing with the f-PSM (3.3%), as discussed above. However, from the results, we can still safely conclude that the support of the s-PSM does not significantly alter the docking time of the vesicles. Even though only the vesicles docked to f-PSMs are mobile, their docking lifetimes are very similar to those docked on the s-PSMs.
Figure 4.
Single-vesicle fusion kinetics. Histogram of docking times of syb-2-containing vesicles fusing with ΔN49-complex containing (A) s-PSMs (n = 312) and (B) f-PSMs (n = 11).
Discussion
We established a SNARE-mediated single-vesicle fusion assay based on planar PSMs that consist of supported and freestanding lipid bilayers covering pores with a diameter of 5 μm. This system enabled us to compare SNARE-mediated vesicle docking and fusion on supported and freestanding membranes under the same conditions in one experiment to gather information about the influence of the support on the docking and fusion behavior of individual vesicles. Our results clearly demonstrate that docking and fusion of syb-2-doped vesicles with t-SNARE (ΔN49-complex) containing PSMs is SNARE specific and depends on the presence of syntaxin 1A and SNAP 25a in the target membrane, in accordance with results obtained with polymer-cushioned membranes (2, 8). In contrast to these observations, fusion on SiO2-supported membranes was frequently reported to be independent of SNAP 25 (1, 3). We assume that the PEG layer (2, 8) and the self-assembled monolayer in our system efficiently decouple the target-membrane from the support, which significantly reduces the lateral tension as well as defects in the membrane, thus preventing nonspecific fusion.
The syb-2-doped vesicles dock onto both s-PSMs and f-PSMs, but the probability of vesicles docking to the f-PSM was two times lower than expected from the available f-PSM area (36%), which might be a result of an immobile protein fraction in the s-PSMs, as discussed below (3). Vesicles that docked to the s-PSMs were immobile over the entire observation time or until fusion occurred, whereas a single syntaxin 1A transmembrane domain and the lipids were proven to be mobile in the membrane. Immobility of SNARE-mediated docked vesicles was reported in various single vesicle fusion assays using planar supported membranes (1, 2, 3, 6, 8, 9), even though different membrane preparation techniques were employed, leading to different immobile fractions. While bilayers produced by direct spreading of t-SNARE-containing vesicles onto SiO2 show very large fractions of immobile proteins (93–97%) (1), membranes prepared by spreading t-SNARE-doped vesicles on a preformed lipid monolayer deposited on a PEG cushion exhibited immobile protein fractions of only 17–23% (8). Despite these large deviations in the immobile fraction, in all cases, the docked vesicles were fixed to their positions, suggesting that only a minor fraction of immobile t-SNAREs is sufficient to fully immobilize the docked vesicle. Even though we cannot directly analyze the amount of immobile t-SNAREs in our system owing to the gold-induced fluorescence quenching on the pore rims, it is well conceivable that a fraction of the ΔN49-complex is indeed immobile in the s-PSMs, resulting in immobile docked vesicles on that membrane part.
In contrast to the vesicles that docked to the s-PSM, those that docked to the f-PSM were mobile, with an average diffusion coefficient of 0.42 μm2/s suggesting the lack of immobile ΔN49-complexes in this membrane part. To our knowledge, this is the first report of mobile SNARE-mediated docked vesicles in an artificial membrane system that was observed in a time-resolved manner. This result implies that the formation of a trans-SNARE complex itself is not sufficient to immobilize the docked vesicle on the membrane. The result further demonstrates that SNARE-mediated vesicle docking per se is independent of whether mobile or immobile t-SNARE complexes are present in the membrane. Along the same line, the docking times also do not depend on the mobility of the docked vesicles (see Fig. 4).
Vesicles, docked to the f-PSM, diffuse within the entire f-PSM area but remain entrapped within the f-PSM, similar to caged diffusion of synaptic vesicles observed in synaptic boutons (12). FRAP experiments using a fluorescently labeled syntaxin 1A transmembrane domain and the lipid Atto488-DPPE revealed that these single molecules can diffuse between s-PSM and f-PSM with diffusion coefficients of 1–3 μm2/s (Fig. S3). This implies that there is a barrier for the docked vesicles, but not for the individual membrane components. We have shown in previous studies that the PSM is bent at the boundary between s-PSM and f-PSM (23). This curvature might introduce a barrier for the docked vesicle, also leading to a reduced probability density of the vesicle in these areas. Although this barrier seems to reduce the probability of finding a vesicle near this boundary, it does not prevent most of the vesicles (89%) from becoming immobilized at the border between s-PSM and f-PSM after some time. Once the docked vesicle approaches the boundary to the s-PSM, it can interact with immobilized ΔN49-complexes, thus immobilizing the vesicle. Hence, an f-PSM docked vesicle turns into an s-PSM fusing vesicle. This behavior and the fact that the docking probability in the f-PSM is by a factor of 2 lower ultimately leads to the very low number of fusion events occurring on the f-PSM.
Compared to the diffusion coefficient of the single syntaxin 1A transmembrane domain, anchoring the ΔN49-complex in the planar membrane, which was determined by FCS to be D = 3.4 μm2/s (13), the diffusion coefficient of the vesicles docked to the f-PSM are lower by a factor of 8. We propose two reasons to explain our observation. 1) It is likely that more than one SNARE complex is involved in docking the vesicle to the target membrane, as shown by others (8, 28, 29, 30, 31). 2) We assume close contact of the vesicle membrane and the planar membrane, with a partly displaced intermembrane water layer, induced by the formed SNARE complex, which would lead to an increased frictional coupling between the membranes.
Even though a freestanding membrane mimics the natural situation more closely than a supported membrane, the supported membrane part of the PSM system provides some advantages as a result of the fluorescence quenching on the gold-coated pore rims. In the dual-color single-vesicle fusion experiments, we were able to observe vesicle ghosts that did not fully collapse into the target membrane after the onset of fusion but rather slowly flattened or even retained part of their Ω-shape. This behavior has also been described for secretory granules that only rarely collapsed into the presynaptic membranes after exocytosis (27, 32). The authors resolved an enlargement or a shrinkage of the vesicle, which they explained by a randomly closing fusion pore that stabilizes the partly merged vesicle, as also observed in other in vitro systems (33, 34).
Conclusions
Using PSMs, we were able to simultaneously analyze SNARE-induced docking and fusion on supported and freestanding lipid bilayers with 10 ms time resolution. Vesicles docked to the s-PSM were immobile, as observed in other in vitro single-vesicle fusion assays, probably due to an immobile t-SNARE fraction. In contrast, vesicles docked to the f-PSM were highly mobile before fusion, with a mean diffusion coefficient of 0.42 μm2/s indicating the presence of mobile t-SNAREs in the f-PSMs and implying that the formation of a trans-SNARE complex itself is not sufficient to immobilize the docked vesicle on the membrane. Despite the observed difference in vesicle mobility, the vesicle docking times on both membranes were similar, indicating that vesicle docking and fusion are independent of the presence of immobile t-SNAREs in the membrane. However, the diffusion constant of f-PSM docked vesicles is significantly lower than that of a single t-SNARE. This gives rise to the assumption that a tight contact between the vesicle and the planar membrane is formed as a result of fully zippered SNARE complexes at the contact zone, which slows down the lateral movement of the vesicle. The observation of partially stable postfusion structures and possible fusion intermediates suggests the formation of transient fusion pores. However, further investigations using dye-filled vesicles in combination with pore cavities instead of open pore arrays will be required to elucidate fusion pore formation in more detail in the near future.
Author Contributions
J.W.K. performed the experiments and analyzed the data. M.J. synthesized and labeled the syx 1A-TMD. J.W.K., U.D., and C.S. wrote the manuscript.
Acknowledgments
The authors thank the Deutsche Forschungsgemeinschaft for financial support (SFB 803, projects A01 and B04), S. Stein and J. Thiart for the tracking software TrackNTrace, R. Jahn for providing the SNARE constructs, and I. Mey and B. Geil for fruitful discussions.
Editor: Heiko Heerklotz.
Footnotes
Supporting Materials and Methods, six figures, and two movies are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(17)30448-4.
Supporting Citations
References (35, 36, 37, 38) appear in the Supporting Material.
Supporting Material
References
- 1.Bowen M.E., Weninger K., Chu S. Single molecule observation of liposome-bilayer fusion thermally induced by soluble N-ethyl maleimide sensitive-factor attachment protein receptors (SNAREs) Biophys. J. 2004;87:3569–3584. doi: 10.1529/biophysj.104.048637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karatekin E., Di Giovanni J., Rothman J.E. A fast, single-vesicle fusion assay mimics physiological SNARE requirements. Proc. Natl. Acad. Sci. USA. 2010;107:3517–3521. doi: 10.1073/pnas.0914723107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu T., Tucker W.C., Weisshaar J.C. SNARE-driven, 25-millisecond vesicle fusion in vitro. Biophys. J. 2005;89:2458–2472. doi: 10.1529/biophysj.105.062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diao J., Su Z., Ha T. A single-vesicle content mixing assay for SNARE-mediated membrane fusion. Nat. Commun. 2010;1:54. doi: 10.1038/ncomms1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreutzberger A.J., Kiessling V., Tamm L.K. High cholesterol obviates a prolonged hemifusion intermediate in fast SNARE-mediated membrane fusion. Biophys. J. 2015;109:319–329. doi: 10.1016/j.bpj.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang T., Smith E.A., Weisshaar J.C. Lipid mixing and content release in single-vesicle, SNARE-driven fusion assay with 1-5 ms resolution. Biophys. J. 2009;96:4122–4131. doi: 10.1016/j.bpj.2009.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon T.-Y., Okumus B., Ha T. Multiple intermediates in SNARE-induced membrane fusion. Proc. Natl. Acad. Sci. USA. 2006;103:19731–19736. doi: 10.1073/pnas.0606032103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domanska M.K., Kiessling V., Tamm L.K. Single vesicle millisecond fusion kinetics reveals number of SNARE complexes optimal for fast SNARE-mediated membrane fusion. J. Biol. Chem. 2009;284:32158–32166. doi: 10.1074/jbc.M109.047381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fix M., Melia T.J., Simon S.M. Imaging single membrane fusion events mediated by SNARE proteins. Proc. Natl. Acad. Sci. USA. 2004;101:7311–7316. doi: 10.1073/pnas.0401779101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka M., Sackmann E. Polymer-supported membranes as models of the cell surface. Nature. 2005;437:656–663. doi: 10.1038/nature04164. [DOI] [PubMed] [Google Scholar]
- 11.Wagner M.L., Tamm L.K. Reconstituted syntaxin1a/SNAP25 interacts with negatively charged lipids as measured by lateral diffusion in planar supported bilayers. Biophys. J. 2001;81:266–275. doi: 10.1016/S0006-3495(01)75697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordan R., Lemke E.A., Klingauf J. Visualization of synaptic vesicle movement in intact synaptic boutons using fluorescence fluctuation spectroscopy. Biophys. J. 2005;89:2091–2102. doi: 10.1529/biophysj.105.061663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwenen L.L.G., Hubrich R., Steinem C. Resolving single membrane fusion events on planar pore-spanning membranes. Sci. Rep. 2015;5:12006. doi: 10.1038/srep12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mey I., Steinem C., Janshoff A. Biomimetic functionalization of porous substrates: towards model systems for cellular membranes. J. Mater. Chem. 2012;22:19348–19356. [Google Scholar]
- 15.van den Bogaart G., Meyenberg K., Jahn R. Membrane protein sequestering by ionic protein-lipid interactions. Nature. 2011;479:552–555. doi: 10.1038/nature10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pobbati A.V., Stein A., Fasshauer D. N- to C-terminal SNARE complex assembly promotes rapid membrane fusion. Science. 2006;313:673–676. doi: 10.1126/science.1129486. [DOI] [PubMed] [Google Scholar]
- 17.Stein S.C., Thiart J. TrackNTrace: a simple and extendable open-source framework for developing single-molecule localization and tracking algorithms. Sci. Rep. 2016;6:37947. doi: 10.1038/srep37947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breckenridge W.C., Morgan I.G., Vincendon G. Adult rat brain synaptic vesicles. II. Lipid composition. Biochim. Biophys. Acta. 1973;320:681–686. doi: 10.1016/0304-4165(73)90148-7. [DOI] [PubMed] [Google Scholar]
- 19.Kocun M., Lazzara T.D., Janshoff A. Preparation of solvent-free, pore-spanning lipid bilayers: modeling the low tension of plasma membranes. Langmuir. 2011;27:7672–7680. doi: 10.1021/la2003172. [DOI] [PubMed] [Google Scholar]
- 20.Reineck P., Gómez D., Bach U. Distance and wavelength dependent quenching of molecular fluorescence by Au@SiO2 core-shell nanoparticles. ACS Nano. 2013;7:6636–6648. doi: 10.1021/nn401775e. [DOI] [PubMed] [Google Scholar]
- 21.Chi Y.S., Byon H.R., Choi I.S. Polymeric rulers: distance-dependent emission behaviors of fluorophores on flat gold surfaces and bioassay platforms using plasmonic fluorescence enhancement. Adv. Funct. Mater. 2008;18:3395–3402. [Google Scholar]
- 22.Kiessling V., Ahmed S., Tamm L.K. Rapid fusion of synaptic vesicles with reconstituted target SNARE membranes. Biophys. J. 2013;104:1950–1958. doi: 10.1016/j.bpj.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Böcker M., Muschter S., Schäffer T.E. Imaging and patterning of pore-suspending membranes with scanning ion conductance microscopy. Langmuir. 2009;25:3022–3028. doi: 10.1021/la8034227. [DOI] [PubMed] [Google Scholar]
- 24.Parmar M.M., Edwards K., Madden T.D. Incorporation of bacterial membrane proteins into liposomes: factors influencing protein reconstitution. Biochim. Biophys. Acta. 1999;1421:77–90. doi: 10.1016/s0005-2736(99)00118-2. [DOI] [PubMed] [Google Scholar]
- 25.Mayer L., Hope M., Cullis P. Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim. Biophys. Acta. 1986;858:161–168. doi: 10.1016/0005-2736(86)90302-0. [DOI] [PubMed] [Google Scholar]
- 26.Kiessling V., Domanska M.K., Tamm L.K. Single SNARE-mediated vesicle fusion observed in vitro by polarized TIRFM. Biophys. J. 2010;99:4047–4055. doi: 10.1016/j.bpj.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiang H.-C., Shin W., Wu L.-G. Post-fusion structural changes and their roles in exocytosis and endocytosis of dense-core vesicles. Nat. Commun. 2014;5:3356. doi: 10.1038/ncomms4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez J.M., Stein A., Jahn R. Membrane fusion intermediates via directional and full assembly of the SNARE complex. Science. 2012;336:1581–1584. doi: 10.1126/science.1221976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernandez J.M., Kreutzberger A.J.B., Jahn R. Variable cooperativity in SNARE-mediated membrane fusion. Proc. Natl. Acad. Sci. USA. 2014;111:12037–12042. doi: 10.1073/pnas.1407435111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohrmann R., de Wit H., Sørensen J.B. Fast vesicle fusion in living cells requires at least three SNARE complexes. Science. 2010;330:502–505. doi: 10.1126/science.1193134. [DOI] [PubMed] [Google Scholar]
- 31.Montecucco C., Schiavo G., Pantano S. SNARE complexes and neuroexocytosis: how many, how close? Trends Biochem. Sci. 2005;30:367–372. doi: 10.1016/j.tibs.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Anantharam A., Onoa B., Axelrod D. Localized topological changes of the plasma membrane upon exocytosis visualized by polarized TIRFM. J. Cell Biol. 2010;188:415–428. doi: 10.1083/jcb.200908010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rawle R.J., van Lengerich B., Boxer S.G. Vesicle fusion observed by content transfer across a tethered lipid bilayer. Biophys. J. 2011;101:L37–L39. doi: 10.1016/j.bpj.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stratton B.S., Warner J.M., O’Shaughnessy B. Cholesterol increases the openness of SNARE-mediated flickering fusion pores. Biophys. J. 2016;110:1538–1550. doi: 10.1016/j.bpj.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu X., Zhang F., Shin Y.K. Membrane fusion induced by neuronal SNAREs transits through hemifusion. J. Biol. Chem. 2005;280:30538–30541. doi: 10.1074/jbc.M506862200. [DOI] [PubMed] [Google Scholar]
- 36.Liu T., Wang T., Weisshaar J.C. Productive hemifusion intermediates in fast vesicle fusion driven by neuronal SNAREs. Biophys. J. 2008;94:1303–1314. doi: 10.1529/biophysj.107.107896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giraudo C.G., Hu C., Rothman J.E. SNAREs can promote complete fusion and hemifusion as alternative outcomes. J. Cell Biol. 2005;170:249–260. doi: 10.1083/jcb.200501093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elhamdani A., Azizi F., Artalejo C.R. Double patch clamp reveals that transient fusion (kiss-and-run) is a major mechanism of secretion in calf adrenal chromaffin cells: high calcium shifts the mechanism from kiss-and-run to complete fusion. J. Neurosci. 2006;26:3030–3036. doi: 10.1523/JNEUROSCI.5275-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.