Abstract
Rationale
Peroxiredoxin 2 (Prdx2), a thiol-specific peroxidase, has been reported to regulate proinflammatory responses, vascular remodeling, and global oxidative stress.
Objective
Although Prdx2 has been proposed to retard atherosclerosis development, no direct evidence and mechanisms have been reported.
Methods and Results
We show that Prdx2 is highly expressed in endothelial and immune cells in atherosclerotic lesions and blocked the increase of endogenous H2O2 by atherogenic stimulation. Deficiency of Prdx2 in apolipoprotein E–deficient (ApoE−/−) mice accelerated plaque formation with enhanced activation of p65, c-Jun, JNKs, and p38 mitogen-activated protein kinase; and these proatherogenic effects of Prdx2 deficiency were rescued by administration of the antioxidant ebselen. In bone marrow transplantation experiments, we found that Prdx2 has a major role in inhibiting atherogenic responses in both vascular and immune cells. Prdx2 deficiency resulted in increased expression of vascular adhesion molecule-1, intercellular adhesion molecule-1, and monocyte chemotactic protein-1, which led to increased immune cell adhesion and infiltration into the aortic intima. Compared with deficiency of glutathione peroxidase 1 or catalase, Prdx2 deficiency showed a severe predisposition to develop atherosclerosis.
Conclusions
Prdx2 is a specific peroxidase that inhibits atherogenic responses in vascular and inflammatory cells, and specific activation of Prdx2 may be an effective means of antiatherogenic therapy.
Keywords: peroxiredoxin 2, atherosclerosis, inflammation, VCAM-1, ICAM-1
Oxidative stress by reactive oxygen species (ROS), including superoxide anions and H2O2, has been implicated in contributing to the initiation and progression of atherosclerosis.1 ROS are produced by many endogenous and exogenous contributors, such as hypercholesterolemia, hyperglycemia, hypertension, and shear stress in vascular and inflammatory cells.2 ROS mediate various signaling pathways that upregulate a number of atherogenic processes, such as monocyte infiltration, platelet activation, smooth muscle cell migration, and cell adhesion.2 Therefore, increased anti-oxidant defense against accumulation of oxidative stress has been proposed to retard the development of atherosclerosis.
Peroxiredoxins (Prdxs) are thiol-specific antioxidant proteins found in mammals, yeast, and bacteria and are classified largely on the basis of having either 1 (1-Cys) or 2 (2-Cys) conserved cysteine residues.3 Among the 6 isoforms of Prdxs, Prdx1 and Prdx2 are the most abundant, constituting a total of 0.2% to 1% of soluble protein in cultured mammalian cells,3 and exhibit higher affinity toward low concentrations of H2O2.4 Prdx1 is induced by exposing macrophages to oxidized LDL5 and by laminar shear stress in endothelial cells.6 In addition, Prdx1 deficiency enhanced the regulated secretion pathway in endothelial cells by promotion of excessive release of several proinflammatory components of Weibel-Palade bodies, such as P-selectin and von Willebrand factor.7 However, Prdx1 deficiency did not affect transcriptional regulation of receptors such as intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule-1 (VCAM-1). Prdx2 removes transiently produced H2O2 in response to activation of various cell surface receptors.8 Prdx2 regulates platelet-derived growth factor (PDGF) signaling, including enhanced activation of the PDGF receptor and phospholipase Cγ1, and vascular remodeling, including PDGF-dependent neointimal thickening of vascular smooth muscle cells.9 Prdx2 also inhibits general immune cell responsiveness through scavenging low levels of ROS,10 modulates lipopolysaccharide-induced proinflammatory responses, and protects against endotoxin-induced lethal shock.11 Consistent with this notion, the roles of Prdxl and Prdx2 are also apparent in phenotypes of the corresponding knockout mice, including hemolytic anemia and cellular senescence,12,13 and differences in tissue distribution.14 A recent report showed that Prdx2 is more susceptible than Prdx1 to hyperoxidation in cells subjected to sustained global oxidative stress,15 which suggests that Prdx2 deficiency may lead to accelerated atherosclerosis due to failure to eliminate ROS. However, Prdx2 has not yet been reported to be connected to atherosclerosis.
Previous studies showed that dietary antioxidants or antioxidant enzymes can protect against atherosclerosis by reducing oxidative stress.16–18 However, many antioxidant drugs or agents have shown poor outcomes. Therefore, we focused on identifying the specific antioxidant enzyme responsible for modulating H2O2 levels as part of in vivo atherogenic signaling pathways. In the present study, we found that Prdx2 is a specific peroxidase critical for proinflammatory and atherogenic responses in the vasculature.
Methods
Experimental Animals
Animal study protocols were approved by the Animal Care Committee of Ewha Womans University. To generate Prdx2−/− ApoE−/− animals, Prdx2-deficient mice were crossed with apolipoprotein E–deficient (ApoE−/−) mice. Both strains were C57BL/6 congenic lines backcrossed more than 10 times with C57BL/6J mice. The atherogenic cholate-containing diet contained 0.15% cholesterol, 20% fat, and 0.05% sodium cholate (all wt/wt; Research Diets Inc, New Brunswick, NJ; C12348). Mice were euthanized and hearts and aortas perfused with phosphate-buffered saline (PBS) through the left ventricle. Hearts were embedded in OCT (Sakura, Tokyo, Japan) and frozen on dry ice. Aortas were dissected from the proximal ascending aorta to the bifurcation of the iliac artery, and adventitial fat was removed. For en face analysis, aortas were split longitudinally, pinned onto flat black silicone plates, and fixed in 10% (vol/vol) formaldehyde in PBS overnight. Fixed aortas were stained with oil red O for 4 hours, washed with PBS briefly, and digitally photographed at a fixed magnification. Total aortic areas and lesion areas were calculated with AxioVision (Carl Zeiss, Jena, Germany). For analysis of aortic sinus plaque lesions and aortic arch lesions, cryosectioning was performed. Each section was stained with oil red O overnight, and images were digitized. Plasma lipid levels were measured with an automatic blood chemical analyzer (Hitachi, Tokyo, Japan).
Statistical Analysis
Results were analyzed with the Wilcoxon rank sum test for comparison of 2 groups or the Kruskal-Wallis test followed by Wilcoxon rank sum test for multiple comparisons. For matched experiments, the results were analyzed with the Wilcoxon signed rank test.
Supplemental Methodology
An expanded Methods section is available in the Online Data Supplement at http://circres.ahajournals.org. For detailed methods related to analysis of atherosclerosis, bone marrow transplantation, infusion protocol for ebselen, cell culture and aortic organ culture, in vitro and ex vivo adhesion assay, in vitro transmigration assay, in vitro transmigration rate measure, en face confocal imaging, amplex red assay, quantitative real-time polymerase chain reaction polymerase chain reaction, reagents, and immunostaining, see the online-only Data Supplement.
Results
Prdx2 Is Highly Expressed in Endothelial and Immune Cells in Atherosclerosis-Prone Areas and Regulates Endogenous H2O2 Production
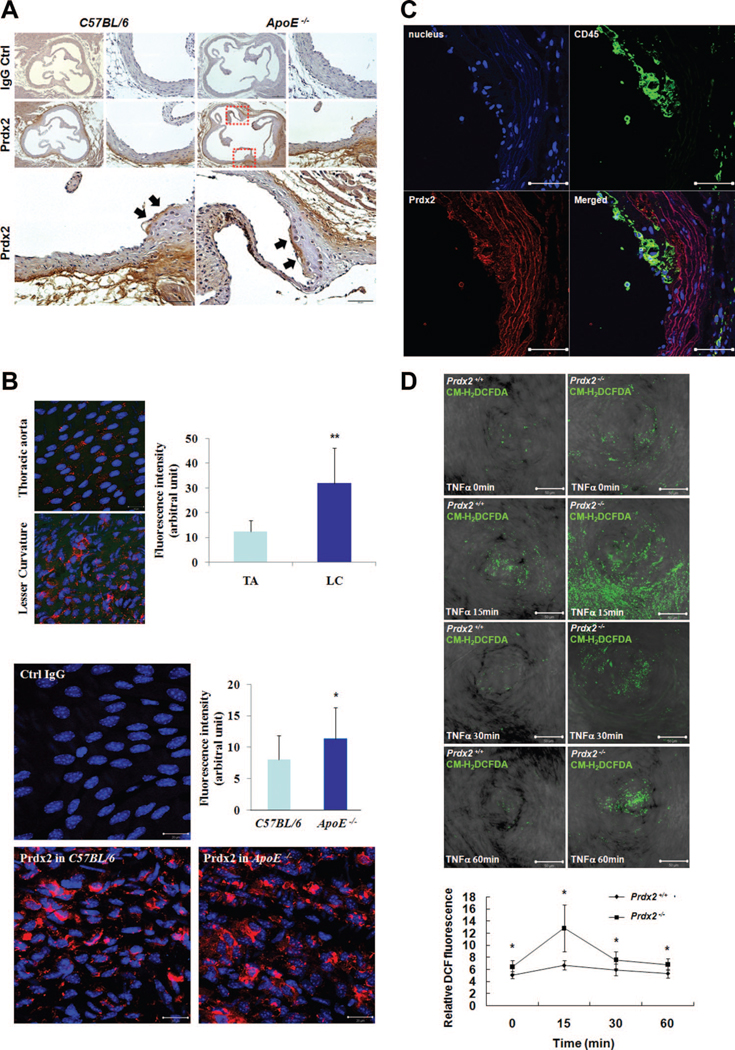
To study the expression pattern of Prdx2 in the vasculature, we stained Prdx2 in the aorta from 8-week-old C57BL/6 and ApoE−/− mice (Figures 1A and 1B). Prdx2 was not only expressed in the adventitia region of both mice but was also detected in the endothelial layer of ApoE−/− mice predisposed to atherosclerosis (Figure 1A). To focus on specific Prdx2 expression in the endothelial layer in the atherosclerosis-prone condition, we stained for Prdx2 using en face aorta preparation. Prdx2 expression in the lesser curvature (LC) was higher than that in the thoracic aorta from C57BL/6 mice (Figure 1B, upper panels), and Prdx2 expression was upregulated in the LC of atherosclerosis-prone ApoE−/− mice compared with normal C57BL/6 mice (Figure 1B, lower panels). In addition, CD45+ cells that infiltrated into the plaque formed in the aortic intima of 60-week-old ApoE−/− mice highly expressed Prdx2 (Figure 1C). To identify the effect of endogenous H2O2 production associated with Prdx2 deficiency in the vasculature, the aorta segments from 8-week-old Prdx2+/+ or Prdx2−/− mice were incubated with or without tumor necrosis factor-α (TNF-α). As expected, Prdx2 deficiency upregulated the endogenous H2O2 level in both basal and inflammation conditions (Figure 1D).
Figure 1. Prdx2 regulates endogenous H2O2 production and is highly expressed in endothelial cells in atherosclerosis-prone areas and immune cells.
A and B, Expression pattern of Prdx2 in the vasculature from 8-week-old C57BL/6 and ApoE−/− mice. A, Representative immunostaining of aortic sinus sections. Scale bars, 200 μm or 50 μm. Lower panels are higher magnification images of dashed boxes. Prdx2 is expressed in the adventitia region of both mice and the endothelial layer of ApoE−/− mice. Scale bars,50 μm. B, Representative en face immunofluorescence staining of the lesser curvature (LC) of aortic arch and thoracic aorta (TA) from C57BL/6 mice (n=3) for Prdx2; quantitative graph in upper panel. Representative en face immunofluorescence staining of the LC from C57BL/6 mice (n=5) and ApoE−/− mice (n=5); quantitative graph in lower panel. *P<0.05, **P<0.01 compared with counterpart. Scale bars, 20 μm. C, Representative immunostaining for CD45 (green) and Prdx2 (red) of atherosclerotic plaques in 60-week-old ApoE−/− mice. Prdx2 is expressed in CD45+ cells on plaques. Scale bars,50 μm. D, CM-H2DCFDA fluorescence image of TNF-α–stimulated aortic tissue. Four isolated pieces of aorta tissue from 8-week-old Prdx2+/+ (n=6) and Prdx2−/− (n=6) mice were incubated with or without TNF-α (10 ng/mL). Quantitative data in the graph represent relative DCF fluorescence intensity. In the endothelial layer of the aortas, intracellular H2O2 production was higher in Prdx2−/− mice than in Prdx2+/+ mice. *P<0.05 compared with counterpart. Scale bars,50μm.
Prdx2 Deficiency Accelerates Atherosclerotic Plaque Formation
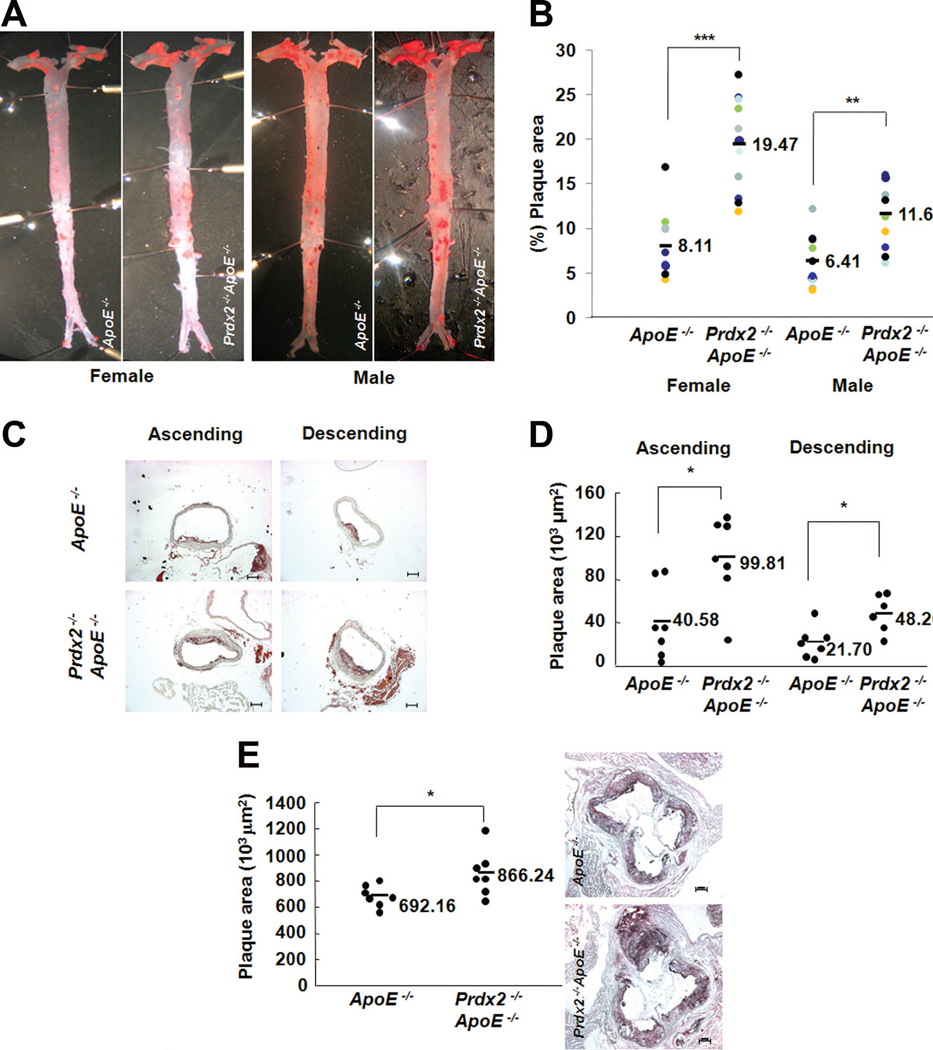
To test the effect of Prdx2 deficiency on the development of atherosclerosis, we generated double-knockout mice lacking ApoE and Prdx2 (Online Figure I) and compared plaque formation in Prdx2−/− ApoE−/− and ApoE−/− control litter-mates at 2 different stages. The plaque area in the aortic arch was significantly higher in 20-week-old female Prdx2−/− ApoE−/− mice (16.4%) than in their ApoE−/− counterparts (10.4%, P<0.01). The same was true of male Prdx2−/− ApoE−/− mice (9.63% versus 6.41% in ApoE−/− males, P<0.01; Online Figure II). After a 10-week atherogenic cholate-containing diet, plaque formation was significantly higher in female Prdx2−/− ApoE−/− mice (19.5%) than in female ApoE−/− mice (8.1%, P<0.001; Figures 2A and 2B, left panels). In addition, plaques in the ascending and descending regions of the aortic arch (Figures 2C and 2D) and aortic sinus (Figure 2E) were also significantly larger in Prdx2−/− ApoE−/− mice than in ApoE−/− mice, which expressed Prdx2 abundantly (Online Figure III). Prdx2 deficiency also exacerbated atherosclerosis in male mice, as demonstrated by the significantly larger plaques in Prdx2−/− ApoE−/− mice than in ApoE−/− mice after 10 weeks on the atherogenic cholate-containing diet (Figures 2A and 2B, right panels). However, compared with ApoE−/− mice, Prdx2−/− Apoe−/− mice did not have altered body weight (data not shown), total cholesterol, LDL cholesterol, HDL cholesterol, or triglyceride levels when fed either the normal chow diet or atherogenic cholate-containing diet (Online Table I).
Figure 2. Prdx2 deficiency accelerates atherosclerotic plaque formation in ApoE−/− mice.
A and B, ApoE−/− and Prdx2−/− ApoE−/− mice were fed an atherogenic cholate-containing diet for 10 weeks. Representative en face images (A) showing oil red O-stained plaque areas of aortas and (B) percentage of plaque areas (mean±SD; n=10 to 12). **P<0.01 and ***P<0.001. C, Frozen sections of ascending and descending aortas of ApoE−/− and Prdx2−/− ApoE−/− female mice fed an atherogenic cholate-containing diet for 10 weeks were stained with oil red O; D, plaque areas were quantified. Quantitative data (mean±SD; n=6 to 7). *P<0.05. Scale bars, 200 μm. E, Atherosclerotic plaques in the aortic sinuses of ApoE−/− and Prdx2−/− ApoE−/− female mice. Quantitative data (mean±SD; n=7). *P<0.05. Scale bars, 200 μm.
Prdx2 Deficiency Activates Redox-Dependent Signaling
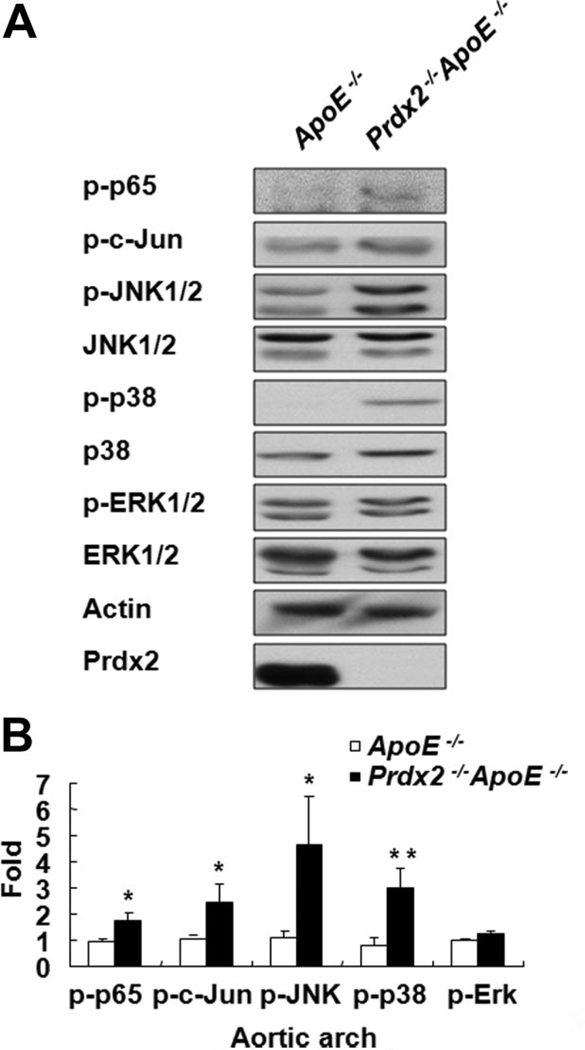
To find mechanisms of accelerated plaque formation induced by Prdx2 deficiency, we tested redox-dependent signaling involving nuclear factor-кB (NF-кB), activator protein-1 (AP-1), and mitogen-activated protein kinases (MAPKs). In association with increased atherosclerotic lesions, 20-week-old Prdx2−/− ApoE−/− mice displayed enhanced activation of redox-dependent signaling molecules such as p65, c-Jun, c-Jun N-terminal kinases (JNKs), and p38 MAPK, but not extracellular signal-related kinases (ERKs; Figure 3A and B), compared with the control mice, as shown in Online Figure II.
Figure 3. Prdx2 deficiency enhances activation of redox-dependent signaling molecules.
A, Western blot analyses of p65, c-Jun, JNK1/2, p38 MAPK, and ERK1/2 in extracts from the aortic arch (pooling at least 3 samples) of 20-week-old ApoE−/− or Prdx2−/−ApoE−/− mice. β-Actin, JNK1/2, p38 MAPK, and ERK1/2 levels were used as loading controls. The blot is representative of 3 independent experiments. B, Quantitative data (mean±SD; n=3). *P<0.05; **P<0.01.
To examine whether the reduction of elevated endogenous H2O2 levels associated with Prdx2 deficiency could ameliorate atherosclerotic progression, we administered ebselen19 to Prdx2−/− ApoE−/− mice using osmotic pumps. After infusion, the 2 control groups (dimethyl sulfoxide [DMSO]-treated ApoE−/− and Prdx2−/− ApoE−/− mice) and 2 antioxidant-treated groups (ebselen-treated ApoE−/− and Prdx2−/− ApoE−/− mice) were examined and compared after being fed an atherogenic cholate-containing diet. Consistent with Figure 2, the plaque area of the aorta in DMSO-treated Prdx2−/− ApoE−/− mice was greater than that in DMSO-treated ApoE−/− mice. The plaque in ApoE−/− mice and Prdx2−/− ApoE−/− mice was attenuated by treatment with ebselen compared with DMSO-treated ApoE−/− and Prdx2−/− ApoE−/− mice, respectively. Atherosclerotic plaques in ebselen-treated Prdx2−/− ApoE−/− mice were reduced to a level similar to that in DMSO-treated ApoE−/− mice (Online Figure IV).
Exacerbation of Atherosclerosis by Prdx2 Deficiency Is Mediated by Both Vascular and Hematopoietic Cells
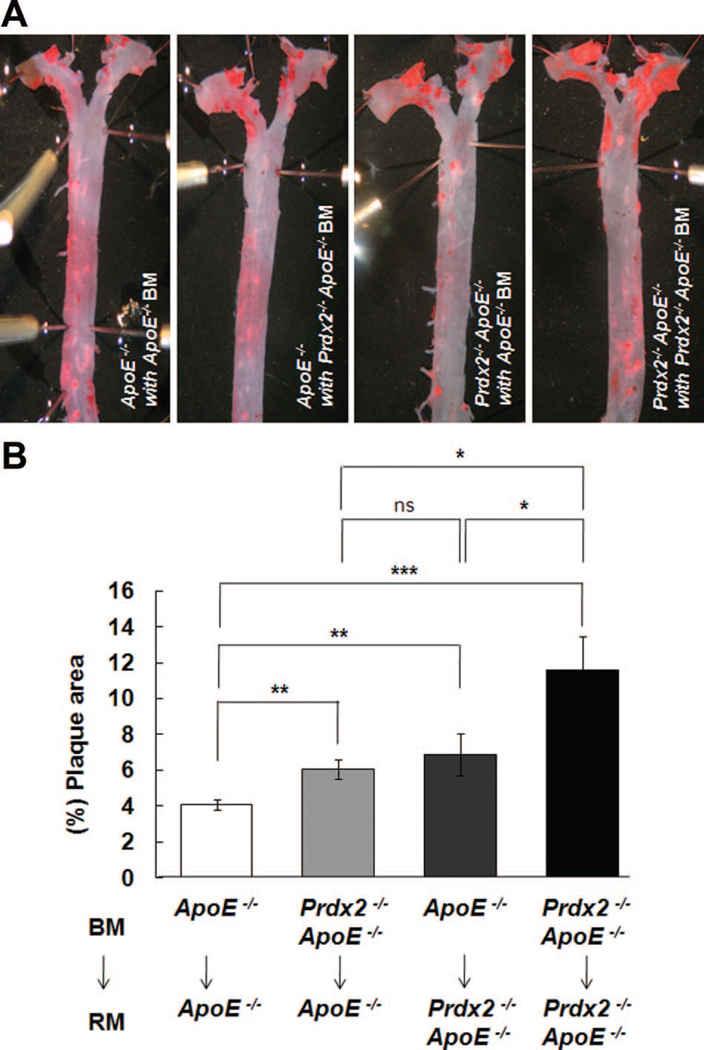
To determine whether the increased plaque formation in Prdx2-deficient ApoE−/− mice was due to Prdx2 deficiency in either arterial walls or hematopoietic cells, we conducted bone marrow transplantation experiments. Bone marrow from ApoE−/− or Prdx2−/− ApoE−/− mice was transplanted into 4-week-old lethally irradiated ApoE−/− or Prdx2−/− ApoE−/− mice, respectively. After undergoing a 4-week recovery, the mice were fed an atherogenic cholate-containing diet for 10 additional weeks. Successful chimerism of bone marrow cells was confirmed by polymerase chain reaction in blood cells (Online Figure V, A). Comparing recipient mice of identical genotypes, we confirmed that Prdx2-deficient hematopoietic cells accelerated plaque formation compared with Prdx2 wild-type cells. In addition, Prdx2−/− ApoE−/− recipient mice showed significantly more plaque formation than ApoE−/− recipient mice when transplanted with identical bone marrow cells (Figures 4A and 4B). To examine the accumulation of immune cells in the plaque of bone marrow transplanted mice, we stained CD45+ cell in aortic sinus of each group of mice. The proportion of the CD45+ area in the plaque was increased by Prdx2-null bone marrow transplantation in both ApoE−/− and Prdx2−/− ApoE−/− recipient mice (Online Figure V, B; P<0.01, respectively).
Figure 4. The proatherogenic effects of Prdx2 deficiency are mediated by both vasculature and hematopoietic cells.
A, Representative images for oil red O staining of aortas from ApoE−/− or Prdx2−/−ApoE−/− bone marrow (BM)–transplanted ApoE−/− and Prdx2−/−ApoE−/− recipient mice (RM) fed an atherogenic cholate-containing diet for 10 weeks. B, Quantitative data (mean±SEM; n=5 to 8). *P<0.05, **P<0.01, ***P<0.001.
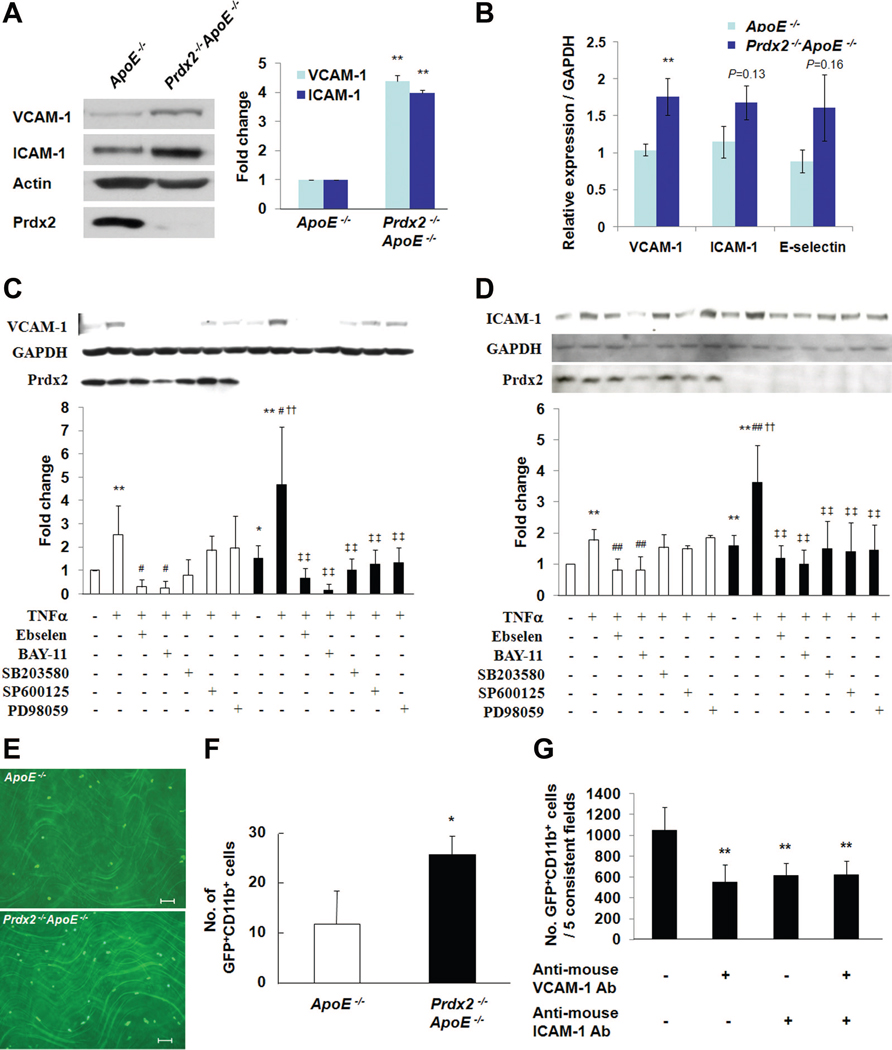
Adhesion of Immune Cells on Endothelial Cells Is Enhanced by Prdx2 Deficiency via Increased VCAM-1 and ICAM-1 Expression
Leukocytes attach to endothelial cells that express selective adhesion molecules on their surfaces.20 In particular, ICAM-1 and VCAM-1 preferentially bind to monocytes and T lymphocytes.21 The aortic arch of 20-week-old Prdx2−/− ApoE−/− mice showed higher expression of VCAM-1 and ICAM-1 than ApoE−/− control littermates (Figures 5A and 5B). Compared with control mice, VCAM-1 expression was elevated in the LC of the aorta, but not in the thoracic aorta, of Prdx2-deficient mice fed an atherogenic cholate-containing diet for 2 weeks (Online Figure VI, upper panels). ICAM-1 expression was higher in both the LC and thoracic aorta of Prdx2-deficient mice than in control mice (Online Figure VI, lower panels). As shown in Figures 5C and 5D, TNF-α–induced expression of VCAM-1 (P<0.05) and ICAM-1 (P<0.01) was significantly higher in Prdx2−/− aortas than in Prdx2−/− aortas. Importantly, incubation of aortic segments with the antioxidant ebselen22 for 30 minutes before TNF-α stimulation effectively reduced H2O2 levels (unpublished data) and aortic VCAM-1 and ICAM-1 expression in aortas of both Prdx2−/− and Prdx2−/− mice (Figures 5C and 5D, third and tenth lanes), which suggests a role for H2O2 in aortic expression of adhesion molecules. Next, we studied signaling pathways that regulate TNF-α–dependent adhesion molecule expression.22 MAPK inhibitors including the p38 MAPK inhibitor SB203580, JNK inhibitor SP600125, and ERK inhibitor PD98059 only marginally reduced the TNF-α–induced expression of VCAM-1 and ICAM-1 in Prdx2+/+ aortas, in contrast to the significant reduction on treatment with the NF-кB inhibitor BAY-11. However, all the inhibitors significantly reduced adhesion molecule expression in Prdx2−/− aortas (Figures 5C and 5D), which suggests their involvement. These results showed that both MAPK activation and NF-кB signaling are important in TNF-α–dependent adhesion molecule expression in Prdx2−/− aortas.
Figure 5. Adhesion of monocytes is enhanced by Prdx2 deficiency via increasing expression of VCAM-1 and ICAM-1.
A, VCAM-1 and ICAM-1 expression in aortic arches from Prdx2−/− ApoE−/− mice was higher than in ApoE−/− mice. The blot is representative of 3 experiments. **P<0.01 compared with control group. B, mRNA levels of VCAM-1, ICAM-1, and E-selectin were increased in the aortic arch of 20-week-old Prdx2−/− ApoE−/− mice compared with ApoE−/− mice (n=3), as determined by quantitative reverse transcription—polymerase chain reaction. **P<0.01 compared with control group. C and D, VCAM-1 (C) and ICAM-1 (D) expression in extracts from the aortas of Prdx2+/+ and Prdx2−/− mice. Aortas were treated with or without TNF-α (10 ng/mL) either with or without inhibitors for 12 hours ex vivo. VCAM-1 and ICAM-1 expression in extracts from the aorta of Prdx2−/− mice were increased compared with Prdx2+/+ mice by TNF-α stimulation, and inhibitors reduced adhesion molecule expression to basal levels in Prdx2−/− aortas. Quantitative data in C and D are mean±SD (n=3 to 10) of fold changes. *P<0.05 and **P<0.01 versus Prdx2+/+ control, #P<0.05 and ##P<0.01 versus Prdx2+/+ treated with TNF-α,††P<0.01 versus Prdx2−/− ctrl, and ‡‡P<0.01 versus Prdx2−/− treated with TNF-α. E, Representative ex vivo binding assay of GFP+CD11b+ cells to aortas isolated from ApoE−/− or Prdx2−/− ApoE−/− mice (n=4). Scale bars, 50 μm. F, Number of bound cells in panel E were significantly increased in Prdx2−/− ApoE−/− mice. *P<0.05 compared with aortas of ApoE−/− mice. G, The number of bound GFP+CD11b+ cells on Prdx2 −/− MAECs treated with TNF-α for 12 hours with blockade of VCAM-1 and/or ICAM-1. **P<0.01 compared with number of GFP+CD11b+ cells in MAECs without anti-VCAM-1 and anti-ICAM-1 Ab.
To compare the interaction between monocytes and aortic walls in ApoE−/− or Prdx2−/− ApoE−/− mice, we performed ex vivo adhesion assays with aortas isolated from ApoE−/− and Prdx2−/− ApoE−/− mice.23 Consistent with the results of the expression of VCAM-1 and ICAM-1, the number of CD11b+GFP+monocytes bound to Prdx2−/− ApoE−/− aortas was significantly higher than that bound to ApoE−/− aortas (Figures 5E and 5F). In isolated mouse aortic endothelial cells (MAECs) from Prdx2+/+ or Prdx2−/− mice,23 Prdx2 deficiency increased the expression of adhesion molecules (unpublished data) and binding of CD11b+GFP+ monocytic cells (Online Figure VII). The blockade of VCAM-1 and ICAM-1 with antibodies reduced monocytic cell binding (Figure 5G).
Prdx2 Deficiency Increases the Accumulation of Immune Cells in Atherosclerotic Lesions
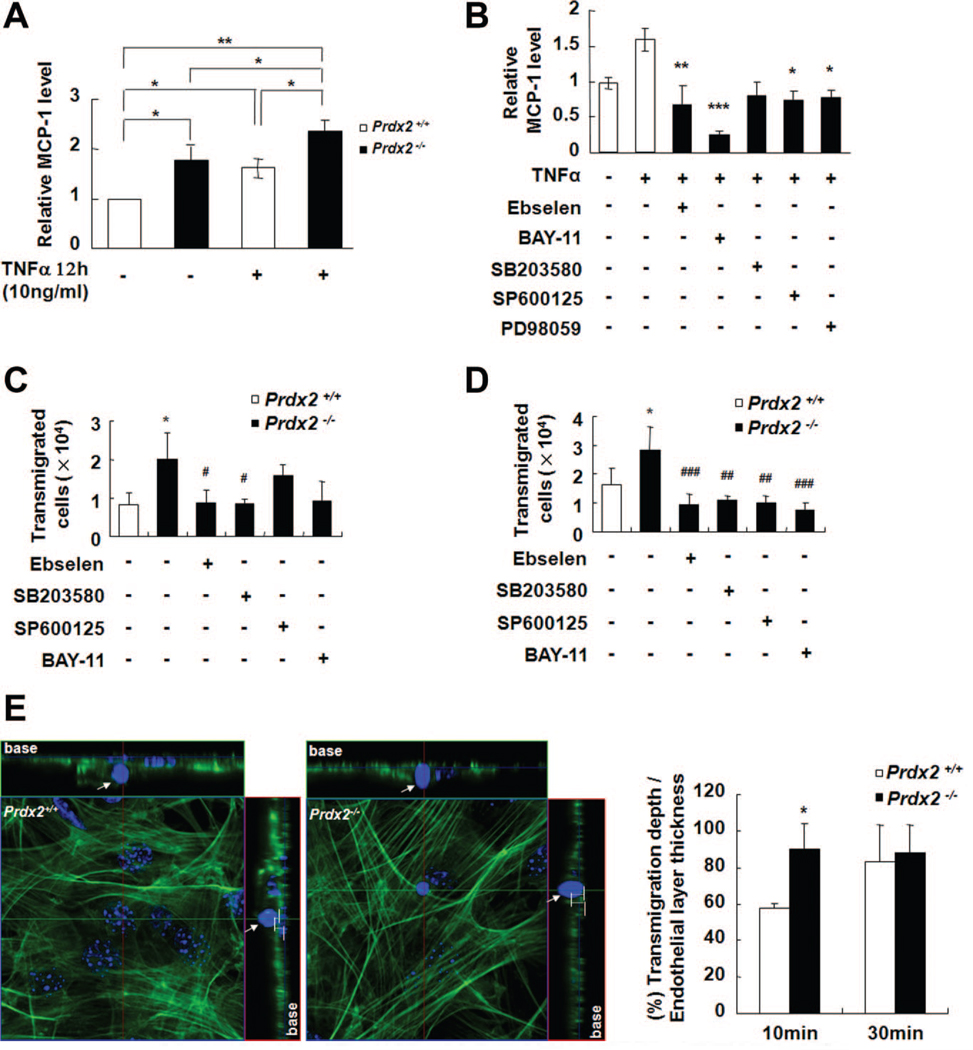
To assess leukocyte transmigration, we examined the effect of Prdx2 deficiency on the expression of monocyte chemotactic protein-1 (MCP-1). MCP-1 was significantly higher in the culture medium of TNF-α–treated aortas from Prdx2−/− mice than in Prdx2+/+ controls (Figure 6A, P<0.05). The enhanced production of MCP-1 in Prdx2−/− aortas was blocked by ebselen, BAY-11, and MAPK inhibitors (Figure 6B). To test the effect of Prdx2 deficiency on leukocyte transmigration, we performed an in vitro transmigration assay. After MAECs on transwells were stimulated with TNF-α for 6 hours, CD11b+ monocytes were added and incubated for 4 or 18 hours. In Prdx2-deficient MAECs, transmigrated monocytes were increased at both time points (Figures 6C and 6D). Pretreatment with the antioxidant ebselen, SB203580, SP600125, or BAY-11 before TNF-α stimulation on MAECs showed that ebselen and SB203580 effectively suppressed the transmigration of CD11b+ monocytes at both time points, whereas SP600125 and BAY-11 only inhibited the transmigration of CD11b+ monocytes at 18 hours. To investigate whether Prdx2 deletion affects the transmigration rate, we obtained confocal microscopic images and measured transmigration rates by Z stack analysis. TNF-α–stimulated MAECs from Prdx2+/+ or Prdx2−/− mice were incubated with CD11b+ monocytes for 10 or 30 minutes, and we confirmed that Prdx2 depletion in MAECs accelerated the transmigration rate of cells after 10 minutes (Figure 6E).
Figure 6. Prdx2 deficiency increases MCP-1 production and leukocyte transmigration.
A, TNF-α–induced MCP-1 secretion in isolated aortas from Prdx2−/− mice is increased compared with Prdx2+/+ mice. Isolated aortas were cultured with or without TNF-α (10 ng/mL) for 12 hours and media were analyzed by ELISA (mean±SEM; n=5to7). *P<0.05, **P<0.01 compared with control group. B, TNF-α–induced MCP-1 secretion by isolated aortas of Prdx2−/− mice. Isolated aortas were cultured with or without TNF-α (10 ng/mL) for 12 hours with or without inhibitors. *P<0.05, **P<0.01, ***P<0.001 relative to Prdx2−/− mice treated with TNF-α. C and D, In vitro transmigration assay. MAECs from Prdx2+/+ and Prdx2−/− mice in transwells were activated by TNF-α (10 ng/mL) for 6 hours. Inhibitors were added to MAECs for 1 hour before TNF-α treatment. After 4 hours (C)or18 hours (D), CD11b+ monocytes that transmigrated into the low chamber were counted. *P<0.05 compared with Prdx2+/+ controls. #P<0.05, ##P<0.01, ###P<0.001 compared with Prdx2−/− MAECs without inhibitors. E, Representative confocal images for analyzing in vitro transmigration rate after 10 minutes and quantitative graph. Arrow indicates the CD11b+ monocyte. Transmigration rate was estimated as percentage of transmigration depth of leukocyte over endothelial layer thickness and was accelerated in Prdx2-deficient MAECs after 10 minutes.*P<0.05 compared with counterpart.
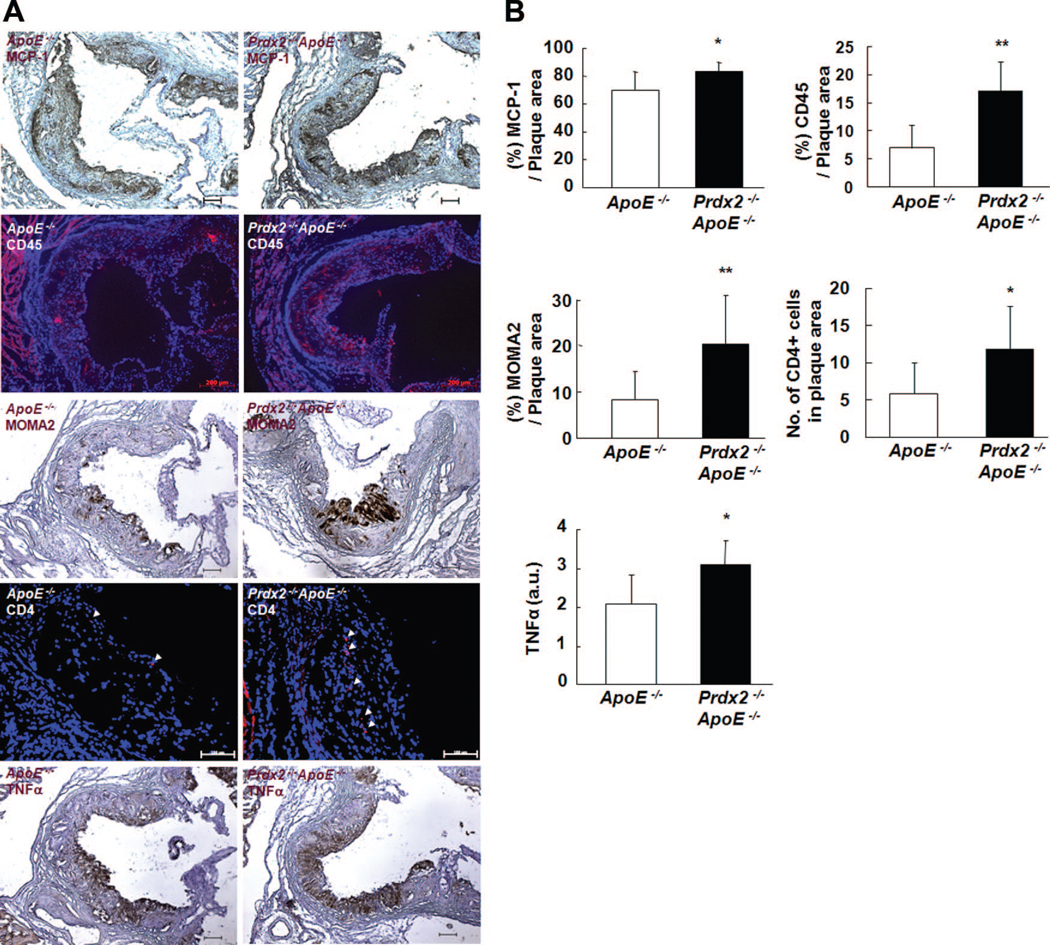
Consistent with the in vitro transmigration studies, plasma levels of MCP-1 were significantly higher in Prdx2−/− ApoE−/− mice (36.0±1.5 pg/mL) than in ApoE−/− mice (32.6±0.9 pg/mL, P<0.05), and focal expression of MCP-1 in the plaque was also increased in Prdx2−/− ApoE−/− mice. After the increased MCP-1 levels, CD45+ cells, macrophages, and CD4+ cells were more abundant in the plaques of Prdx2−/− ApoE−/− mice than in ApoE−/− mice (Figures 7A and 7B). In addition, TNF-α focal expression in the plaque was more pronounced in Prdx2−/− ApoE−/− mice than in ApoE−/− controls (Figure 7A, fifth row, and Figure 7B).
Figure 7. Prdx2 deficiency increases accumulation of inflammatory cells in plaques.
A, Representative immunostaining for MCP-1, CD45, MOMA2, CD4, and TNF-α in aortic sinus plaques from ApoE−/− and Prdx2−/− ApoE−/− mice fed an atherogenic cholate-containing diet for 10 weeks. Arrows indicate CD4+ cells. Nuclei were stained with hematoxylin or DAPI. Scale bars, 100 μm. B, Quantitative data (mean±SD) in the graph represent the positively stained area percentage of plaque area and show an increased stained area in Prdx2−/− ApoE−/− mice compared with ApoE−/− mice. *P<0.05, **P<0.01 relative to ApoE−/− mice.
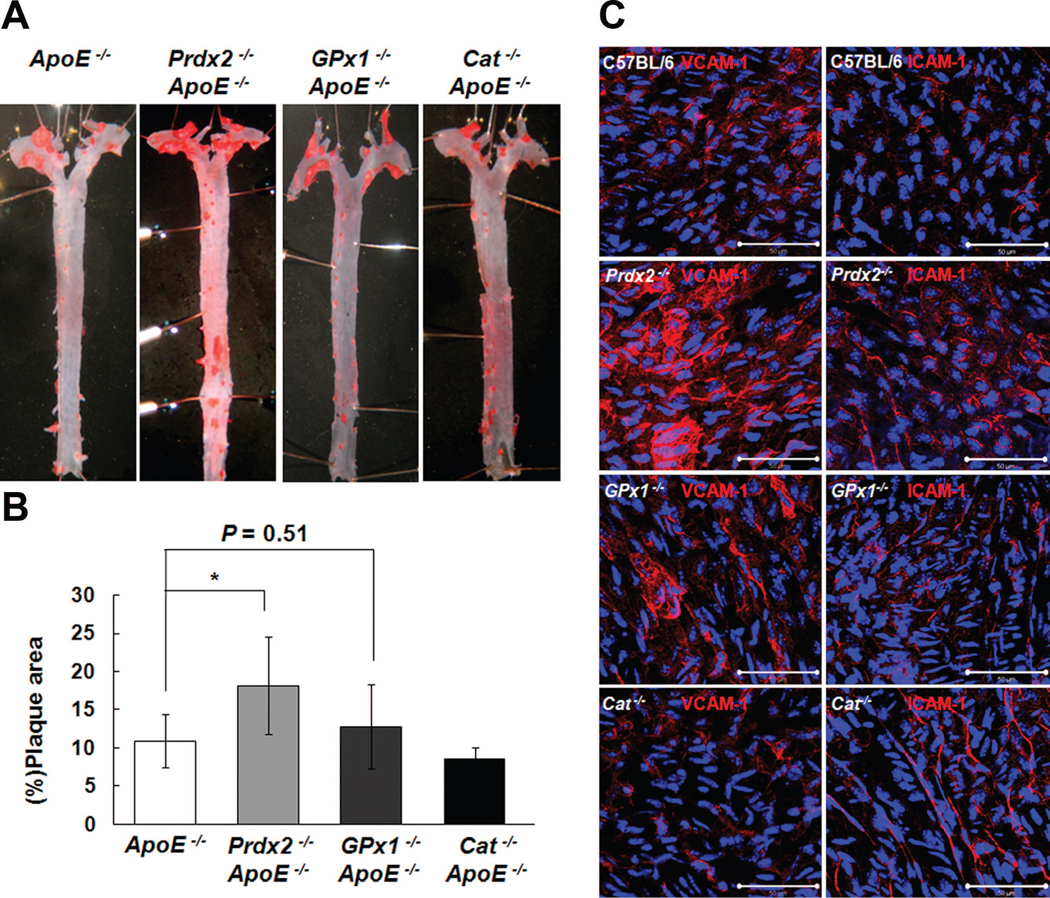
Prdx2 Deficiency Shows More Severe Atherosclerosis Development Than Deficiency of Glutathione Peroxidase 1 or Catalase
To compare the effect of Prdx2 to other major peroxidases such as glutathione peroxidase 1 (GPx1) and catalase on the development of atherosclerosis, we generated double knockout mice lacking ApoE and GPx1 or catalase (Online Figure I). The distributions of Prdx2, GPx1, and catalase in tissues from C57BL/6 mice were confirmed in Online Figure VIII, then we identified that Prdx2 and GPx1 were highly expressed compared with catalase in the aortas. Next, male mice of each group were fed an atherogenic cholate-containing diet for 10 weeks to examine plaque formation at an early stage of atherosclerosis. In spite of Gpx1 deficiency showing increased tendency to develop atherosclerosis, plaque formation in GPx1−/− ApoE−/− was less than that in Prdx2−/− ApoE−/− mice (Figures 8A and 8B). Our results from GPx1−/− ApoE−/− mice are consistent with previous reports that GPx1−/− ApoE−/− mice developed more atherosclerosis on the Western diet than control ApoE−/− mice.17 En face immunofluorescence staining of the corresponding aortic lesion areas was performed. Prdx2-deficient mice showed highly elevated VCAM-1 or ICAM-1 expression in the LC of the aorta compared with GPx1-deficient mice, whereas the expression level in catalase-deficient mice was similar to control mice (Figure 8C).
Figure 8. Prdx2 deficiency shows severe predisposition to develop atherosclerosis than deficiency of GPx1 or catalase.
A and B, ApoE−/−, Prdx2−/− ApoE−/−, GPx1−/− ApoE−/−, and Cat−/− ApoE−/− male mice were fed an atherogenic cholate-containing diet for 10 weeks. A, Representative en face images showing oil red O-stained plaque areas of aortas. B, Percentage of plaque areas (mean±SD; n = 7 to 11). *P<0.05. C, Prdx2 deficiency increases VCAM-1 and ICAM-1 expression compared with deficiency of GPx1 or catalase in the arterial wall. En face immunofluorescence staining of VCAM-1 and ICAM-1 expression in the LC region of aortas from C57BL/6, Prdx2−/−, GPx1−/−, and Cat−/− mice (n=3) fed an atherogenic cholate-containing diet for 2 weeks.
Discussion
Prdx2 expression is more pronounced in endothelial and immune cells on atherosclerotic-prone regions and plaques. Endothelial and immune cells activated by hyperlipidemia are major contributors to the development of atherosclerosis. We therefore investigated which cells have major roles in accelerating plaque formation in Prdx2-deficient mice. Transplantation of bone marrow into ApoE−/− or Prdx2−/− ApoE−/− mice showed that Prdx2 deficiency in either vascular tissue or blood cells was sufficient to mediate the observed exacerbation of plaque formation. In addition, Prdx2 deficiency in both vascular and blood cells synergistically increased atherosclerotic plaque formation. We also confirmed that accumulation of CD45+ cells was significantly increased in the plaques of ApoE−/− and Prdx2−/− ApoE−/− recipient mice transplanted with Prdx2−/− ApoE−/− bone marrow than in those transplanted with ApoE−/− bone marrow. Moreover, the size of the necrotic core in the lesions of Prdx2−/− ApoE−/− recipient mice increased significantly compared with ApoE−/− recipient mice (Online Figure V, B), which probably leads to the increasing CD45 antigen degradation responsible for the reduction in CD45 staining in Prdx2−/− ApoE−/− recipient mice compared with ApoE−/− recipient mice.
Atherosclerosis preferentially develops in branched or curved arterial regions exposed to turbulent flow, including oscillatory shear stress.24 Turbulent flow induces inflammation via increasing ROS production from NADPH oxidases and accelerates atherosclerosis, including upregulated expression of VCAM-1 and ICAM-1. We showed that Prdx2 is more abundant in the aortic LC exposed to turbulent flow than in the thoracic aorta exposed to undisturbed laminar shear stress, and the expression of VCAM-1 and ICAM-1 was enhanced in the LC of Prdx2-deficient mice. These results suggest that upregulated Prdx2 in the LC has a protective role in inhibiting the expression of VCAM-1 and ICAM-1. Mowbray et al6 reported that exposure to chronic laminar shear stress upregulated Prdx1 and Prdx5 levels in bovine aortic endothelial cells and suggested that Prdx1 is a novel mechanosensitive antioxidant. On the other hand, our studies showed that Prdx2 levels in the LC were regulated by turbulent flow in mice.
Increased expression of adhesion molecules by activated endothelial cells is a key feature of atherosclerosis.25 VCAM-1 and ICAM-1 are expressed by endothelial cells in regions predisposed to atherosclerotic lesion formation.21 We showed that VCAM-1 and ICAM-1 expression is also highly dependent on vessel structure. In endothelial cells, VCAM-1 and ICAM-1 expression is stimulated by ERKs, JNKs, p38MAPK, AP-1, and NF-кB.26 Consistent with these results, we found that adhesion molecule expression was stimulated by accumulation of H2O2 and accompanied by MAPK and NF-кB activation in isolated aortic tissue stimulated with TNF-α. We also showed that MAPKs, NF-кB, and c-Jun were activated by endogenous H2O2 in the Prdx2-deficient aorta. In particular, the active, phosphorylated forms of JNK and p38 MAPK were significantly elevated in Prdx2-deficient mice, which suggests that JNKs and p38 MAPK are the major mediators of in vivo atherogenic signaling initiated by H2O2.
Leukocyte transmigration has been shown to be regulated by signaling pathways initiated by clustering of ICAM-1 and VCAM-1.27 ROS participate in these pathways, activating Src and p38 MAPK and inactivating protein tyrosine phosphatases.27 In Prdx2-deficient endothelial cells, leukocyte transmigration was increased through the upregulation of H2O2 and activation of p38 MAPK. To block intracellular H2O2 for leukocyte transmigration, Prdx2 null MAECs were treated with ebselen. The present data show that ebselen effectively suppressed the transmigration of CD11b+ monocytes. Others have shown that activation of p38 MAPK was required for ICAM-1–induced cytoskeletal remodeling, such as the generation of actin stress fibers.27 Here, increasing transmigration in Prdx2-deficient MAECs was inhibited by a p38 MAPK inhibitor, and JNKs and NF-кB inhibitors also reduced leukocyte transmigration at 18 hours. This may be the effect of abrogation on VE-cadherin phosphorylation and junctional separation by the JNK inhibitor28 and reduced expression of VCAM-1 and ICAM-1 by the NF-кB inhibitor. Taken together, these data suggest that upregulated H2O2 stimulates enhanced leukocyte transmigration in Prdx2-deficient endothelial cells via activation of p38 MAPK, JNKs, and NF-кB.
An atherogenic diet that includes cholesterol or cholate components can induce hepatic inflammation, which causes increased complication of atherosclerotic plaque.29,30 In particular, mice fed an atherogenic diet containing 0.5% sodium cholate increased accumulation of collagen and extracellular matrix components in liver, which induce fibrosis, and expression of genes related to the response to chronic inflammation.30 Although we used an atherogenic diet that contained low-dose sodium cholate (0.05%), we could not ignore the effects of cholate on an atherogenic cholate-containing diet in ApoE−/− mice undergoing hyperlipidemia and inflammatory responses themselves. Therefore, we tested the antiatherogenic effects of Prdx2 in ApoE−/− mice fed a normal chow diet and confirmed that deficiency of Prdx2 in ApoE−/− mice revealed significantly increased plaque formation compared with control mice (Online Figure II).
An increased antioxidant defense against accumulation of oxidative stress has been proposed to retard the development of atherosclerosis. Ectopic overexpression of catalase, known to remove H2O2, has protective effects in cardiovascular disease, such as inhibition of oxidized LDL-induced proliferation of vascular smooth muscle cells31 and retardation of atherosclerosis in ApoE−/− mice.18 Interestingly, atherosclerosis in ApoE−/− mice overexpressing Cu/Zn-superoxide dismutase was comparable to that in ApoE−/− control mice. However, catalase deficiency in ApoE−/− mice has not been reported to date. In the present study, we found that catalase expression is lower than GPx1 or Prdx2 expression in the vasculature, and catalase deficiency did not affect atherosclerosis development in ApoE−/− mice. The major isoform of GPx, GPx1, is located in the cytosol and mitochondria. In patients with coronary artery disease, the low activity of red blood cell GPx1 is associated with the risk of cardiovascular events.32 GPx1 deficiency accelerates the progression of atherosclerosis in ApoE−/− mice.33 These results showed that GPx1−/− ApoE−/− mice developed significantly more atherosclerosis after 24 weeks, but not at 6 or 12 weeks, on the Western diet than control ApoE−/− mice. Haan et al reported that GPx1 deficiency does not increase atherosclerosis in C57BL/J6 mice fed a high-fat diet34 or in nondiabetic ApoE−/− mice.35 The present results showed that deficiency of Gpx1 in ApoE−/− mice accelerated atherosclerosis slightly. Like Prdx2, Prdx6 is located in the cytosol and is expressed in the endothelium and macrophages and regulates oxidative stress and H2O2 as a second messenger. Paigen et al reported that Prdx6 deficiency in a B6;129 background showed significantly larger aortic root lesions than in wild-type controls, but there was no difference in other genetic backgrounds, which suggests that Prdx6 does not play a major antiatherogenic role in mice. Furthermore, Prdx6 overexpression in ApoE−/− background did not affect atherosclerosis.36 However, we still think that the effect of Prdx6 deficiency on atherosclerosis should be conclusively shown in ApoE-deficient mice. Peroxiredoxin 2 function in human atherogenesis has yet to be investigated. In ruptured abdominal aortic aneurysms, the expression of PRDX2 was increased compared with unruptured controls.37 Although the pathophysiological processes are somewhat different between atherogenesis and aneurysm, the 2 diseases share many common factors, including several inflammatory mediators and oxidative stress. Therefore, research on polymorphisms of the PRDX2 gene and activity of PRDX2 in human atherosclerosis patients may help to identify a novel biomarker of cardiovascular events.
In summary, Prdx2 was highly expressed in endothelial and immune cells in atherosclerotic lesions and blocked upregulation of endogenous H2O2 by proinflammatory cytokines. Deficiency of Prdx2 in ApoE−/− mice accelerated plaque formation after enhanced activation of p65, c-Jun, JNKs, and p38 MAPK. These proatherogenic effects of Prdx2 deficiency were rescued in part by administration of the antioxidant ebselen and mediated not only by vascular cells but also by bone marrow–derived inflammatory cells. Prdx2 deficiency increased the expression of VCAM-1, ICAM-1, and MCP-1. Immune cell infiltration into the plaque and TNF-α production by inflammatory cells were also increased by Prdx2 deficiency. Compared with glutathione peroxidase 1 and catalase, Prdx2 deficiency revealed a severe predisposition to develop atherosclerosis. In conclusion, Prdx2 is a specific peroxidase that inhibits atherogenic responses in vasculature and immune cells, and specific activation of Prdx2 may be an effective means of antiinflammatory and antiatherogenic therapy.
Supplementary Material
Novelty and Significance.
What Is Known?
Reactive oxygen species (ROS) has been implicated in the initiation and progression of atherosclerosis.
Peroxiredoxin 2 (Prdx2), which is an antioxidant enzyme, regulates proinflammatory responses, vascular remodeling, and global oxidative stress; however, little is known about its roles in pathogenesis of atherosclerosis.
What New Information Does This Article Contribute?
Expression of Prdx2 in endothelial cell is increased in atherosclerosis-prone area and hyperlipidemic mice.
Prdx2 is a specific antioxidant enzyme that inhibits endogenous ROS level and thereby attenuates the activation of the redox-dependent signaling molecules including p65, c-Jun, c-Jun N-terminal kinases (JNKs), and p38 mitogen-activated protein kinase (MAPK) on atherogenic stimulation.
Deficiency of Prdx2 in apolipoprotein E—deficient (ApoE−/−) mice accelerates atherosclerosis by increasing the infiltration of immune cells into plaques after increased expression of vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1).
Although several animal studies show that antioxidants or antioxidant enzymes can protect against atherosclerosis, clinical trials with antioxidant interventions have led to poor therapeutic outcomes Therefore, we focused on identifying the specific antioxidant enzyme responsible for regulating H2O2 levels as part of in vivo atherogenic signaling pathways. We show that Prxd2 is highly expressed in vascular endothelial cells in atherosclerosis-prone areas and in immune cells in plaques. Deficiency of Prdx2 in the atherosclerosis-prone ApoE−/− mice accelerated atherogenesis. We found that increased activation of redox-dependent signaling molecules enhanced the expression of VCAM-1 and ICAM-1 and increased infiltration of immune cells into plaques. These atherogenic effects by Prdx2 deficiency were rescued by the administering the antioxidant ebselen. The antiatherogenic effects of Prdx2 were found to be important for both vascular and immune cell function, as shown by bone marrow transplantation experiments. Finally, Prdx2 deficiency led to more severe atherosclerosis than the deficiency of glutathione peroxidase 1 or catalase in ApoE−/− mice. Our study demonstrates for the first time that Prdx2 is a critical regulator in the pathogenesis of atherosclerosis. Therefore, specific activation of Prdx2 could be an effective approach for developing antiatherogenic therapy.
Acknowledgments
The authors are grateful to Dr Sun Hee Yim for critical comments and Dr Eun-Kyung Suh for reviewing the English.
Sources of Funding
This work was supported by the Research Program for the National Research Laboratory (R0A-2007-000-20016-0), a Korean Ministry of Health and Welfare grant (A000385 and A090264), a National Core Research Center grant (R15-2006-020), and the National Research Foundation (20100019542) from the Ministry of Education, Science & Technology, South Korea.
Non-standard Abbreviations and Acronyms
- ApoE−/−
apolipoprotein E deficient
- Cys
cysteine residue
- GPx
glutathione peroxidase
- LC
lesser curvature
- MAEC
mouse aortic endothelial cell
- Prdx
peroxiredoxin
Footnotes
Disclosures
None.
References
- 1.Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury, part II: animal and human studies. Circulation. 2003;108:2034–2040. doi: 10.1161/01.CIR.0000093661.90582.c4. [DOI] [PubMed] [Google Scholar]
- 2.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 3.Chae HZ, Kim HJ, Kang SW, Rhee SG. Characterization of three isoforms of mammalian peroxiredoxin that reduce peroxides in the presence of thioredoxin. Diabetes Res Clin Pract. 1999;45:101–112. doi: 10.1016/s0168-8227(99)00037-6. [DOI] [PubMed] [Google Scholar]
- 4.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 5.Conway JP, Kinter M. Dual role of peroxiredoxin I in macrophage-derived foam cells. J Biol Chem. 2006;281:27991–28001. doi: 10.1074/jbc.M605026200. [DOI] [PubMed] [Google Scholar]
- 6.Mowbray AL, Kang DH, Rhee SG, Kang SW, Jo H. Laminar shear stress up-regulates peroxiredoxins (PRX) in endothelial cells: PRX 1 as a mechanosensitive antioxidant. J Biol Chem. 2008;283:1622–1627. doi: 10.1074/jbc.M707985200. [DOI] [PubMed] [Google Scholar]
- 7.Kisucka J, Chauhan AK, Patten IS, Yesilaltay A, Neumann C, Van Etten RA, Krieger M, Wagner DD. Peroxiredoxin1 prevents excessive endothelial activation and early atherosclerosis. Circ Res. 2008;103:598–605. doi: 10.1161/CIRCRESAHA.108.174870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhee SG. Cell signaling: H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 9.Choi MH, Lee IK, Kim GW, Kim BU, Han YH, Yu DY, Park HS, Kim KY, Lee JS, Choi C, Bae YS, Lee BI, Rhee SG, Kang SW. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005;435:347–353. doi: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]
- 10.Moon EY, Noh YW, Han YH, Kim SU, Kim JM, Yu DY, Lim JS. T lymphocytes and dendritic cells are activated by the deletion of peroxiredoxin II (Prx II) gene. Immunol Lett. 2006;102:184–190. doi: 10.1016/j.imlet.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Yang CS, Lee DS, Song CH, An SJ, Li S, Kim JM, Kim CS, Yoo DG, Jeon BH, Yang HY, Lee TH, Lee ZW, El-Benna J, Yu DY, Jo EK. Roles of peroxiredoxin II in the regulation of proinflammatory responses to LPS and protection against endotoxin-induced lethal shock. J Exp Med. 2007;204:583–594. doi: 10.1084/jem.20061849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee TH, Kim SU, Yu SL, Kim SH, Park DS, Moon HB, Dho SH, Kwon KS, Kwon HJ, Han YH, Jeong S, Kang SW, Shin HS, Lee KK, Rhee SG, Yu DY. Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood. 2003;101:5033–5038. doi: 10.1182/blood-2002-08-2548. [DOI] [PubMed] [Google Scholar]
- 13.Han YH, Kim HS, Kim JM, Kim SK, Yu DY, Moon EY. Inhibitory role of peroxiredoxin II (Prx II) on cellular senescence. FEBS Lett. 2005;579:4897–4902. doi: 10.1016/j.febslet.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 14.Godoy JR, Funke M, Ackermann W, Haunhorst P, Oesteritz S, Capani F, Elsasser HP, Lillig CH. Redox atlas of the mouse immunohistochemical detection of glutaredoxin-, peroxiredoxin-, and thioredoxin-family proteins in various tissues of the laboratory mouse. Biochim Biophys Acta. 2010;1810:2–92. doi: 10.1016/j.bbagen.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG. Inactivation of peroxiredoxin I by phosphorylation allows localized H(2)O(2) accumulation for cell signaling. Cell. 2010;140:517–528. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 16.De Rosa S, Cirillo P, Paglia A, Sasso L, Di Palma V, Chiariello M. Reactive oxygen species and antioxidants in the pathophysiology of cardiovascular disease: does the actual knowledge justify a clinical approach? Curr Vasc Pharmacol. 2010;8:259–275. doi: 10.2174/157016110790887009. [DOI] [PubMed] [Google Scholar]
- 17.Torzewski M, Ochsenhirt V, Kleschyov AL, Oelze M, Daiber A, Li H, Rossmann H, Tsimikas S, Reifenberg K, Cheng F, Lehr HA, Blankenberg S, Forstermann U, Munzel T, Lackner KJ. Deficiency of glutathione peroxidase-1 accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:850–857. doi: 10.1161/01.ATV.0000258809.47285.07. [DOI] [PubMed] [Google Scholar]
- 18.Yang H, Roberts LJ, Shi MJ, Zhou LC, Ballard BR, Richardson A, Guo ZM. Retardation of atherosclerosis by overexpression of catalase or both Cu/Zn-superoxide dismutase and catalase in mice lacking apolipoprotein E. Circ Res. 2004;95:1075–1081. doi: 10.1161/01.RES.0000149564.49410.0d. [DOI] [PubMed] [Google Scholar]
- 19.Weber DS, Rocic P, Mellis AM, Laude K, Lyle AN, Harrison DG, Griendling KK. Angiotensin II-induced hypertrophy is potentiated in mice overexpressing p22phox in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2005;288:H37–H42. doi: 10.1152/ajpheart.00638.2004. [DOI] [PubMed] [Google Scholar]
- 20.Huo Y, Ley K. Adhesion molecules and atherogenesis. Acta Physiol Scand. 2001;173:35–43. doi: 10.1046/j.1365-201X.2001.00882.x. [DOI] [PubMed] [Google Scholar]
- 21.Iiyama K, Hajra L, Iiyama M, Li H, DiChiara M, Medoff BD, Cybulsky MI. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res. 1999;85:199–207. doi: 10.1161/01.res.85.2.199. [DOI] [PubMed] [Google Scholar]
- 22.Yoshizumi M, Fujita Y, Izawa Y, Suzaki Y, Kyaw M, Ali N, Tsuchiya K, Kagami S, Yano S, Sone S, Tamaki T. Ebselen inhibits tumor necrosis factor-alpha-induced c-Jun N-terminal kinase activation and adhesion molecule expression in endothelial cells. Exp Cell Res. 2004;292:1–10. doi: 10.1016/j.yexcr.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Jeon HJ, Choi JH, Jung IH, Park JG, Lee MR, Lee MN, Kim B, Yoo JY, Jeong SJ, Kim DY, Park JE, Park HY, Kwack K, Choi BK, Kwon BS, Oh GT. CD137 (4–1BB) deficiency reduces atherosclerosis in hyperlipidemic mice. Circulation. 2010;121:1124–1133. doi: 10.1161/CIRCULATIONAHA.109.882704. [DOI] [PubMed] [Google Scholar]
- 24.Jo H, Song H, Mowbray A. Role of NADPH oxidases in disturbed flow-and BMP4- induced inflammation and atherosclerosis. Antioxid Redox Signal. 2006;8:1609–1619. doi: 10.1089/ars.2006.8.1609. [DOI] [PubMed] [Google Scholar]
- 25.Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2292–2301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 26.Berk BC, Abe JI, Min W, Surapisitchat J, Yan C. Endothelial atheroprotective and anti-inflammatory mechanisms. Ann N Y Acad Sci. 2001;947:93–109. doi: 10.1111/j.1749-6632.2001.tb03932.x. [DOI] [PubMed] [Google Scholar]
- 27.Wittchen ES. Endothelial signaling in paracellular and transcellular leukocyte transmigration. Front Biosci. 2009;14:2522–2545. doi: 10.2741/3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nwariaku FE, Liu Z, Zhu X, Nahari D, Ingle C, Wu RF, Gu Y, Sarosi G, Terada LS. NADPH oxidase mediates vascular endothelial cadherin phos-phorylation and endothelial dysfunction. Blood. 2004;104:3214–3220. doi: 10.1182/blood-2004-05-1868. [DOI] [PubMed] [Google Scholar]
- 29.Getz GS, Reardon CA. Diet and murine atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:242–249. doi: 10.1161/01.ATV.0000201071.49029.17. [DOI] [PubMed] [Google Scholar]
- 30.Vergnes L, Phan J, Strauss M, Tafuri S, Reue K. Cholesterol and cholate components of an atherogenic diet induce distinct stages of hepatic inflammatory gene expression. J Biol Chem. 2003;278:42774–42784. doi: 10.1074/jbc.M306022200. [DOI] [PubMed] [Google Scholar]
- 31.Lin SJ, Shyue SK, Shih MC, Chu TH, Chen YH, Ku HH, Chen JW, Tam KB, Chen YL. Superoxide dismutase and catalase inhibit oxidized low-density lipoprotein-induced human aortic smooth muscle cell proliferation: role of cell-cycle regulation, mitogen-activated protein kinases, and transcription factors. Atherosclerosis. 2007;190:124–134. doi: 10.1016/j.atherosclerosis.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 32.Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, Smieja M, Cambien F, Meyer J, Lackner KJ. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med. 2003;349:1605–1613. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]
- 33.Espinola-Klein C, Rupprecht HJ, Bickel C, Schnabel R, Genth-Zotz S, Torzewski M, Lackner K, Munzel T, Blankenberg S. Glutathione peroxidase-1 activity, atherosclerotic burden, and cardiovascular prognosis. Am J Cardiol. 2007;99:808–812. doi: 10.1016/j.amjcard.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 34.de Haan JB, Witting PK, Stefanovic N, Pete J, Daskalakis M, Kola I, Stocker R, Smolich JJ. Lack of the antioxidant glutathione peroxidase-1 does not increase atherosclerosis in C57BL/J6 mice fed a high-fat diet. J Lipid Res. 2006;47:1157–1167. doi: 10.1194/jlr.M500377-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Lewis P, Stefanovic N, Pete J, Calkin AC, Giunti S, Thallas-Bonke V, Jandeleit-Dahm KA, Allen TJ, Kola I, Cooper ME, de Haan JB. Lack of the antioxidant enzyme glutathione peroxidase-1 accelerates atherosclerosis in diabetic apolipoprotein E-deficient mice. Circulation. 2007;115:2178–2187. doi: 10.1161/CIRCULATIONAHA.106.664250. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Phelan SA, Petros C, Taylor EF, Ledinski G, Jurgens G, Forsman-Semb K, Paigen B. Peroxiredoxin 6 deficiency and atherosclerosis susceptibility in mice: significance of genetic background for assessing atherosclerosis. Atherosclerosis. 2004;177:61–70. doi: 10.1016/j.atherosclerosis.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Urbonavicius S, Lindholt JS, Vorum H, Urbonaviciene G, Henneberg EW, Honore B. Proteomic identification of differentially expressed proteins in aortic wall of patients with ruptured and nonruptured abdominal aortic aneurysms. J Vasc Surg. 2009;49:455–463. doi: 10.1016/j.jvs.2008.08.097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.