Abstract
Cell migration is a major process that drives metastatic progression of cancers, the major cause of cancer death. Existing chemotherapeutic drugs have limited efficacy to prevent and/or treat metastasis, emphasizing the need for new treatments. We focus on triple negative breast cancer (TNBC), the subtype of breast cancer with worst prognosis and no standard chemotherapy protocols. Here we demonstrate that a group of natural compounds, known as phytochemicals, effectively block migration of metastatic TNBC cells. Using a novel cell micropatterning technology, we generate consistent migration niches in standard 96-well plates where each well contains a cell-excluded gap within a uniform monolayer of cells. Over time, cells migrate into and occupy the gap. Treating TNBC cells with non-toxic concentrations of phytochemicals significantly blocks motility of cells. Using a molecular analysis approach, we show that anti-migratory property of phytochemicals is partly due to their inhibitory effects on phosphorylation of ERK1/2. This study provides a framework for future studies to understand molecular targets of phytochemicals and evaluate their effectiveness in inhibiting metastasis in animal models of cancer.
1. Introduction
Triple negative breast cancer (TNBC), defined by lack of estrogen and progesterone receptors and amplification of human epidermal growth factor receptor 2 (ER−, PR−, HER2−), is the most aggressive subtype of breast cancer.1,2 TNBC does not respond to the most effective treatments for breast cancer: targeted endocrine therapies such as tamoxifen or aromatase inhibitors and HER2-directed therapy such as trastuzumab for ER+, PR+ and Her2+ disease, respectively.3 According to the National Cancer Institute, TNBC constitutes about 15% of breast cancers yet claims disproportionally more lives compared to other subtypes, especially among younger women and African–Americans.4–6 Higher rates of proliferation, recurrence, and distant metastasis primarily account for poor prognosis of TNBC.7 Given the lack of targeted therapies, cytotoxic chemotherapy remains the primary treatment option.8 Unfortunately, standard chemotherapy compounds are rarely effective and TNBC tumors often lead to incurable metastatic disease.7,9,10
Migration of cancer cells is a key process leading to local invasion and metastasis.11–13 Cancer cells migrate within the primary tumor stroma to access circulation and lymphatic system and within secondary sites in distant organs to form metastases. Histopathological examinations of tumor specimens show collective and individual migration of cancer cells, further highlighting the importance of cell migration in metastasis.12,14 Therefore, blocking cell migration can potentially inhibit or reduce metastatic progression of TNBC. Benefits of blocking cell migration were demonstrated recently using a novel organic inhibitor of an actin-bundling protein to block cellular motility and inhibit metastasis in a mouse model of breast cancer.15 However, standard chemotherapeutics mainly induce programmed cell death in rapidly dividing cancer cells through mechanisms including inhibition of cell division and interruption of DNA/RNA synthesis, and do not necessarily block cell motility. Therefore, tumor cells surviving or resisting chemotherapy can undergo migration and invasion to develop metastases.
The goal of this work is to examine inhibitory effects of a collection of natural compounds, phytochemicals, against migration of metastatic TNBC cells. Our rationale is based on several studies that show certain phytochemicals downregulate various malignant phenotypes of different cancer cells in vitro and prevent chemically-induced tumor formation and metastasis in animal models.16–21 Dietary phytochemicals such as curcumin, resveratrol, capsaicin, fisetin, epigallocatechin gallate (EGCG), 6-gingerol, indole-3-carbinol, and quercetin have shown a broad range of effects including apoptotic, anti-proliferative, anti-migratory, anti-invasive, and anti-angiogenic against various cancer cells.20,22–24 Therefore, phytochemicals may offer a new source of experimental drugs. The low rate of approval of new anti-cancer drugs emphasizes this need.25 Despite general consensus about anti-cancer properties of phytochemicals, there is a lack of systematic investigations to identify inhibitory effects of phytochemicals on migration of metastatic cancer cells including TNBC cells.
To address this need, we utilized our high throughput cell migration assay technology and screened efficacy of ten phyto-chemicals against migration of two aggressive TNBC cell lines. Our cell migration technology is based on the use of a polymeric aqueous two-phase system with polyethylene glycol (PEG) and dextran (DEX) as phase-forming polymers.26 A drop of the aqueous DEX phase is printed on the surface of each well of a 96-well plate and allowed to dehydrate to a disk. Cells are mixed with the aqueous PEG phase and added to each well. The DEX phase disk rehydrates back to a drop that remains immiscible from the aqueous PEG phase and prevents cells from adhering to its underlying surface. This results in a cell-excluded gap within a monolayer of adhered cells. The gap serves as a cell migration niche. We have previously evaluated this robotic technology for high throughput compound screening against cell migration and shown that it consistently returns a Z′ factor of greater than 0.7, demonstrating its quality and robust performance.27 Using this migration technology, our screening identified several natural compounds that effectively blocked motility of cells. Subsequent molecular analysis showed that phytochemicals interfered with dysregulated kinase signaling and scavenged excessive intracellular free radicals in TNBC cells. Overall, phytochemicals may offer a new source of anti-cancer drugs with the potential to block metastatic disease by inhibiting cancer cell migration. Our robotic high throughput technology will allow screening libraries of phytochemicals to identify effective compounds for subsequent validation studies in animal models.
2. Materials and methods
2.1 Preparation of aqueous two-phase system
Our cell migration assay utilized a polymeric aqueous two-phase system (ATPS) with 10.0% (w/v) polyethylene glycol (PEG), Mw 35 K, (Sigma-Aldrich) and 12.8% (w/v) dextran (DEX), Mw 500 K, (Pharmacosmos), as phase-forming polymers.26,28–30 Both poly-meric solutions were prepared with phenol red-free complete growth medium one day prior to experiments. To facilitate dissolution of polymers, solutions containing polymers were vortexed and incubated in a water bath at 37 °C for one hour. The solutions were stored at 4 °C. While the DEX phase solution was used at this concentration, the aqueous PEG phase was diluted to 5.0% (w/v) after mixing with cells, as explained below.
2.2 Cell culture
We used two tumorigenic, mesenchymal-like TNBC cells, MDA-MB-231 and MDA-MB-157, originally derived from pleural fluid.31 Both cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% glutamine, and 1% antibiotic. One passage prior to migration experiments, phenol red-free DMEM was used to culture cells. Cells were cultured in T75 flasks in a humidified incubator at 37 °C with 5% CO2 until they formed a monolayer about 90% confluent. Then, cells were dislodged using 4 ml of a Hank’sbased enzyme-free cell dissociation buffer (Life Technologies) for 30 min in an incubator, collected with 4 ml of complete growth medium, and centrifuged down at 1000 rpm for 5 min. After removing supernatant and re-suspending the cell pellet in 1 ml of phenol red-free DMEM, cells were counted with a hemocytometer.
2.3 Aqueous two-phase mediated patterning of migration niche
We used an ATPS-mediated cell-exclusion patterning to perform cell migration experiments.26,32 Aqueous DEX phase drops of 1 μl volume were simultaneously printed in 96-well plates (Corning) using a robotic liquid handler (SRT Bravo, Agilent), one drop in the center of each well. The plate, covered with its lid, was kept inside a sterile biological safety cabinet for 24 h to allow the drops to dry. This protocol consistently gives dehydrated DEX phase drops of 2.00 ± 0.1 mm diameter.27 Cell suspensions adjusted to a density of 500 × 103 ml−1 MDA-MB-157 cells and 350 × 103 ml−1 MDA-MB-231 cells were thoroughly mixed with an equal volume of the 10.0% (w/v) aqueous PEG phase. Each well was loaded with 100 μl of the resulting suspension. This addition rehydrated the DEX phase drop. The interfacial tension between the two immiscible aqueous phases prevented the cells from entering into the drop such that cells could only adhere to the well surface around the drop.26,33 This resulted in a well-defined cell-excluded gap within a monolayer in each well. After 12 hours of incubation, the ATPS was replaced with growth medium only (control) or medium containing a test compound. The area of the cell-excluded gap within well plates averaged to 3.33 ± 0.09 mm2.27
2.4 Preparation of test compounds
We evaluated the potential of ten phytochemicals (Table 1 and Table S1, ESI†) against migration of TNBC cells. We note that methyl amooranin is a semi-synthetic compound. Except for methyl amooranin and black currant extract that were synthesized in-house,34,35 remaining phytochemicals were commercially available (Table S1, ESI†). Stock solution for each phytochemical was prepared on the day of the experiment by dissolving the powder in a designated solvent (Table 1). Each mixture was vortexed to facilitate dissolution of the compound and stored at 4 °C until use. Stock solutions were kept at −20 °C for long-term storage. Working concentrations were diluted from respective stock solutions in complete growth medium prepared with phenol red-free DMEM. All solutions were protected from light.
Table 1.
Phytochemicals, solvents and solubility values, and maximum concentration from each compound used for migration experiments
| Phytochemical | Solvent/solubility (mg ml−1) | Maximum concentration (μM) |
|---|---|---|
| Fisetin | Ethanol/5 | 200 |
| Quercetin | DMSO/30 | 200 |
| Methyl amooranin | DMSO/10 | 100 |
| Indole-3-carbinol | DMSO/30 | 200 |
| Resveratrol | DMSO/2.24 | 100 |
| Curcumin | DMSO/10 | 15 |
| Capsaicin | Ethanol/30 | 100 |
| Black currant extract | Water/2 | 2 (mg ml−1) |
| Epigallocatechin gallate (EGCG) | Water/20 | 30 |
| 6-Gingerol | Water/5 | 200 |
2.5 Cell viability
An AlamarBlue assay (Life Technologies) was used to determine viability of TNBC cells treated with phytochemicals. The Alamar Blue reagent contains an active ingredient, resazurin, which is reduced by metabolically active cells to a fluorescent form, resorufin. The level of fluorescent signal directly correlates with cell viability. TNBC cells were seeded in a 96-well plate and maintained for 48 h in the presence of phytochemicals in the concentration range of 0–1 mM. Then, 10% of the total well volume of the AlamarBlue reagent was added to compound-treated and non-treated (control) cells. After 6 h of incubation at 37 °C, fluorescent measurements were done using a plate reader (Synergy H1M, Biotek Instruments). Only those concentrations from each phytochemical that resulted in a viability of greater than 90% were used for subsequent migration experiments.
2.6 Cell migration
TNBC cells were micropatterned in 96-well plates as a monolayer containing a cell-excluded gap. Cells were allowed to migrate into the gaps for 48 h in the presence of different concentrations of each compound. In all experiments, a control condition was set in the presence of culture medium only and without any phytochemical (no treatment). Each condition had 32 replicates. Then, cells were stained with 2 μM Calcein AM (Life Technologies) for 30 min. Each well containing fluorescing cells was imaged using an inverted fluorescent microscope (AxioObserver, Zeiss) equipped with an AxioCam MRm camera (Zeiss). The initial gap area (A1) and final gap area after 48 h of incubation (A2) were computed using a script developed in Matlab (MathWorks). Cell migration was quantified as gap closure = 1 − A2/A1. Resulting data were plotted versus concentrations of each test compound. Inhibition of migration at each concentration of a phytochemical was determined using 1 − gap closure.
2.7 Immunocytochemistry
TNBC cells were seeded at a density of 1.5 × 104 cells per well of a black-wall 96-well plate and incubated overnight. Cells were treated with 200 μM fisetin, 100 μM resveratrol, and 10 μM of a specific inhibitor of ERK phosphorylation, PD98059 (Life Technologies). Cells were incubated for 6 h, fixed, and stained with primary antibodies for pERK and tERK (Cell Signaling Technology). Protein expression was detected using a fluorescent secondary antibody (Jackson ImmunoResearch). Images of cells were captured from each well maintaining imaging conditions identical for all treatments. Each condition had eight replicates. The fluorescent intensity from each image was measured in ImageJ and an average value was determined for each condition.
2.8 Detection of reactive oxygen species (ROS)
Endogenous reactive oxygen species (ROS) levels in TNBC cells with and without treatment with fisetin were measured using an ROS kit (Abcam).36 This kit contains a fluorescent reagent, 2′,7′-dichlorofluorescein diacetate (DCFDA), which is deacety-lated to a non-fluorescent compound and then oxidized by hydroxyl, peroxyl and other ROS into 2′,7′-dischlorofluorescin (DCF). DCF is excited at 495 nm and detected at 529 nm. TNBC cells were seeded at a density of 2.5 × 104 per each well of a black wall 96-well plate (Corning). Cells were incubated overnight, washed with a 1× buffer included in the kit, and then stained with 25 μM DCFDA diluted in the 1× buffer, for 45 minutes at 37 °C. Next, cells were washed once with the 1× buffer and then 100 μl of fisetin at 200 μM was added to wells. Fresh medium of similar volume without the phytochemical was added to control wells. Cells were imaged at 2 and 4 h of incubation. The fluorescent signal was measured and normalized against control (no treatment). In parallel, cells were treated with 50 μM of tert-butyl hydrogen peroxide provided in the kit to stimulate production of intracellular free radicals.
2.9 Statistical analysis
Statistical analysis was performed in Excel using a two-tailed distribution and heteroscedastic student’s t-test. For immuno-cytochemistry studies, p-values were found using a pERK/tERK value from each treatment (fisetin, resveratrol, and inhibitor) compared to the corresponding value from the control condition (no treatment). For cell viability studies, p-values were obtained using viability of cells treated with a phytochemical and the control condition. Statistical significance was defined as p-values less than a 5% significance level.
3. Results and discussion
3.1 Evaluation of toxicity of phytochemicals to TNBC cells
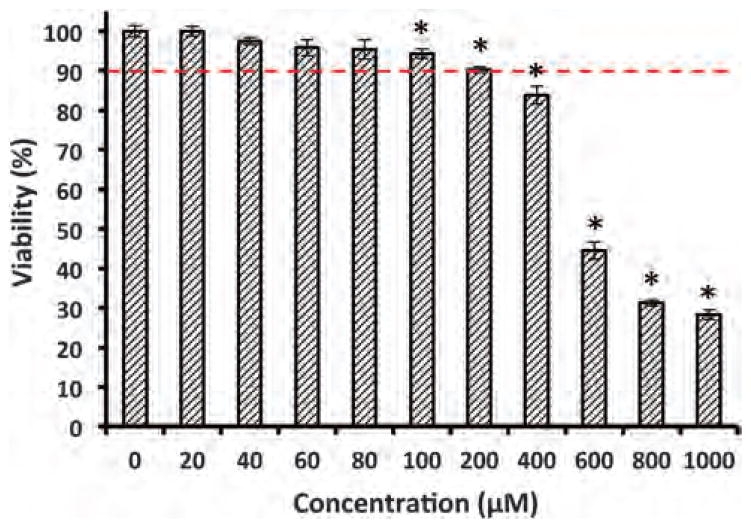
Prior to migration experiments, dose-dependent cell viability tests were performed with each compound to determine a range of working concentrations that maintain viability of over 90%. Avoiding toxicity to cells was crucial to selectively examine anti-migratory effects of phytochemicals. The duration of these tests and migration experiments was identical, i.e., 48 h. A typical result is shown in Fig. 1 for MDA-MB-231 cells treated with fisetin over a concentration range of 0–1 mM. Cells retained a high viability up to 200 μM treatment. Increasing fisetin concentration reduced cellular viability below 90% with a significant shift between 400–600 μM. Based on this experiment, a concentration range of 0–200 μM was selected to investigate anti-migratory effects of fisetin on MDA-MB-231 cells. These experiments were performed for the entire collection of phytochemicals. We ensured the lack of toxicity from the solvent by evaluating viability of cells treated with the solvent of each compound at its highest concentration. In addition, cells were visually examined to ensure a normal morphology. Table 1 lists the phytochemicals and shows the highest working concentration of each compound used for cell migration experiments.
Fig. 1.
Viability of MDA-MB-231 cells treated with fisetin for 48 h is measured using an AlamarBlue assay. The dashed line represents the cutoff concentration for cell viability of 90%. Asterisks denote statistically significant difference between viability of cells treated with fisetin and the control condition (0 μM) (p < 0.05).
3.2 Inhibitory effects of phytochemicals on migration of TNBC cells
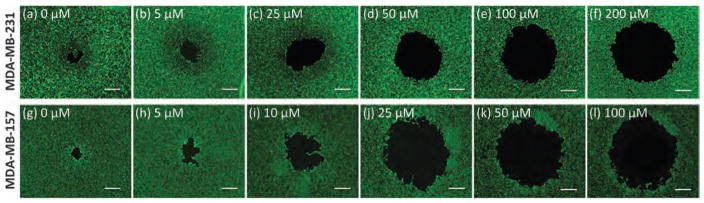
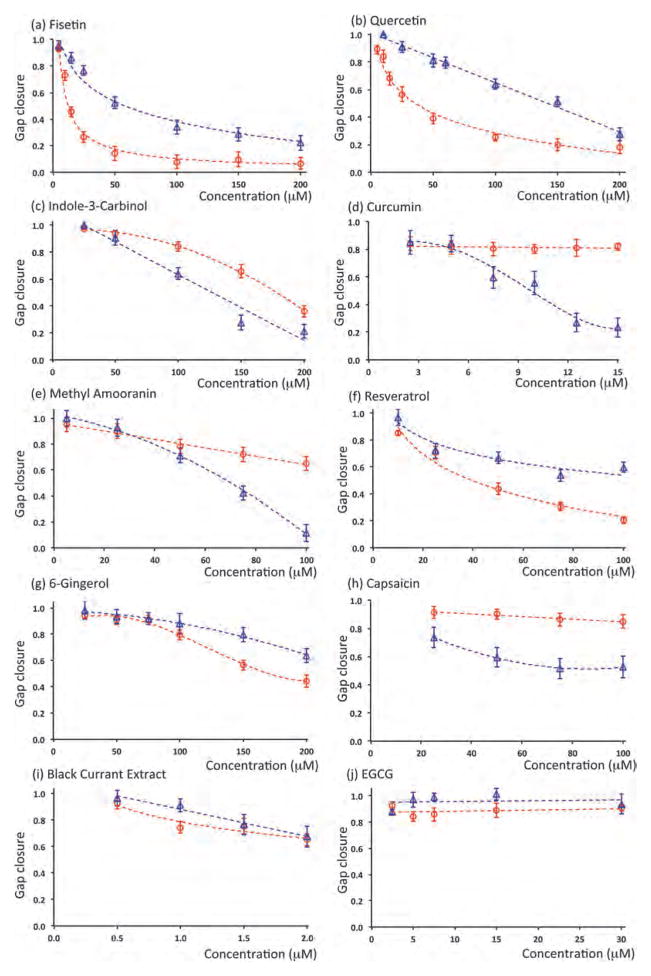
Anti-migratory effect of phytochemicals was evaluated against two TNBC cell lines. Each compound was used in a wide range of non-toxic concentrations determined from the viability tests above. Fig. 2 shows a typical result with fisetin treatment of MDA-MB-231 and MDA-MB-157 cells. Increasing the concentration of fisetin dose-dependently inhibits migration of both cell lines. Using pre-migration and post-migration images of cells, the gap closure was calculated at every concentration for each phytochemical.26 Fig. 3 summarizes the dose–response of both MDA-MB-231 and MDA-MB-157 cells to treatment with all ten phytochemicals. A single data point in each panel of this figure represents 32 individual migration experiments (replicates). Taken all these experiments together, data in this figure represent over 12000 individual migration experiments, demonstrating the power of this high throughput robotic technology for cell migration studies.
Fig. 2.
Post-migration images of MDA-MB-231 cells (a–f) and MDA-MB-157 cells (g–l) treated with fisetin shows that increasing the concentration of the compound blocks cell migration into the gap dose-dependently. Scale bar 500 μm.
Fig. 3.
Dose–response of MDA-MB-157 (circles, red) and MDA-MB-231 (triangles, blue) each treated with ten phytochemicals at non-toxic concentrations. All data are normalized against control (no treatment).
This comprehensive screening identified compounds that were highly effective against motility of both cells. Fisetin, quercetin, and indole-3-carbinol dose-dependently blocked migration of both TNBC cells. Several compounds including curcumin, methyl amooranin, and resveratrol were highly effective against one cell line and had moderate or no effect against the second TNBC cell line. Remaining phytochemicals, i.e., 6-gingerol, capsacin, black currant extract, and EGCG were only moderately effective or ineffective. To quantitatively evaluate the results and compare the effectiveness of compounds, we defined a metric to determine the concentration of each phytochemical that resulted in inhibition of migration of each TNBC cell by 50% of control condition (no treatment). This metric allows comparing the effectiveness of all ten phytochemicals against each TNBC cell line. For each compound–cell pair, this concentration was identified by curve fitting to data in Fig. 3 (dashed lines). The results are shown in Table 2. Only those phytochemicals that reduced migration of at least one TNBC cell by 50% of its control were included. Three compounds that did not meet this criterion were thus eliminated.
Table 2.
Compounds that inhibit migration of at least one TNBC cell line by 50% of control (no treatment) are listed. The 50% inhibitory concentration values are shown in micromolar units. These values are obtained by curve fitting to data of Fig. 3
| MDA-MB-157 | MDA-MB-231 | |
|---|---|---|
| Fisetin | 12.3 | 57.7 |
| Quercetin | 35.9 | 142.3 |
| Indole-3-carbinol | 167.9 | 126.8 |
| Curcumin | — | 9.2 |
| Methyl amooranin | — | 66.4 |
| Resveratrol | 39.3 | — |
| 6-Gingerol | 158.1 | — |
Among effective compounds, fisetin was the most promising as it significantly blocked motility of both TNBC cells (Fig. 3a). Fisetin was particularly potent against MDA-MB-157 cells with a 50% inhibitory concentration of 12.3 μM and a maximum migration inhibition of 93 ± 3%. With MDA-MB-231 cells, the required concentration of fisetin for 50% inhibition was 57.7 μM and a maximum inhibition of 78 ± 5% was reached. Both quercetin (Fig. 3b) and indole-3-carbinol (Fig. 3c) satisfied the metric for 50% inhibition of migration with each TNBC cell line, but at substantially higher concentrations compared to fisetin. Maximum inhibition of migration for each TNBC cell line treated with these two compounds was also lower than that obtained with fisetin treatment.
Four of the phytochemicals met the 50% inhibition criterion only with one TNBC cell line. Curcumin (Fig. 3d) and methyl amooranin (Fig. 3e) were effective against MDA-MB-231 cells. Considering concentrations of compounds, curcumin ranked higher since it only required 9.2 μM to block cell motility by 50% of control and enabled a maximum inhibition of 77 ± 7% at 15.0 μM. Methyl amooranin resulted in a greater maximum inhibition of 89 ± 6% but at a substantially higher concentration of 100.0 μM. Both compounds had only a marginal inhibitory effect on migration of MDA-MB-157 cells.
Treatment with resveratrol (Fig. 3f) and 6-gingerol (Fig. 3g) reduced migration of MDA-MB-157 cells more than 50% of control. Resveratrol was more promising because of a significantly lower 50% inhibitory concentration of 39.3 μM. In addition, it resulted in a maximum inhibition of 79 ± 4% at 100.0 μM, whereas at this concentration, 6-gingerol blocked migration of MDA-MB-157 cells only by 20 ± 5%. Both compounds had a moderate effect against motility of MDA-MB-231 cells. Finally, capsaicin (Fig. 3h), black currant extract (Fig. 3i), and EGCG (Fig. 3j) did not satisfy the 50% inhibition metric with either of the two TNBC cells.
This screening study demonstrated the potential of phyto-chemicals to block migration of metastatic TNBC cells. In particular, compounds such as fisetin and quercetin that were effective against both TNBC cell lines may be broadly useful against migration of metastatic TNBC cells. Our technology provides a unique screening tool to examine this question by validating anti-migratory effects of the compounds against a panel of TNBC cells.
Although both TNBC cells studied here were classified as a mesenchymal-like subtype,31 several phytochemicals only blocked migration of one of the TNBC cells. This is most likely due to differences in molecular drivers of cell migration in these cells. Furthermore, MDA-MB-157 was originally derived from an African–American patient, whereas MDA-MB-231 was from a Caucasian patient. Recent studies demonstrated significant differences in mutation of genes and activity of migration-modulating cellular pathways such as phosphatidyl inositol-3 kinase (PI-3K) and MAP Kinase in these cohorts.31,37,38 Our screening approach enables addressing such potential ethnic-specific differences in cellular responses to phytochemicals by systematic screening of migration of cells from different ethnic groups.
3.3 Inhibition of cell migration by short exposure to phytochemicals
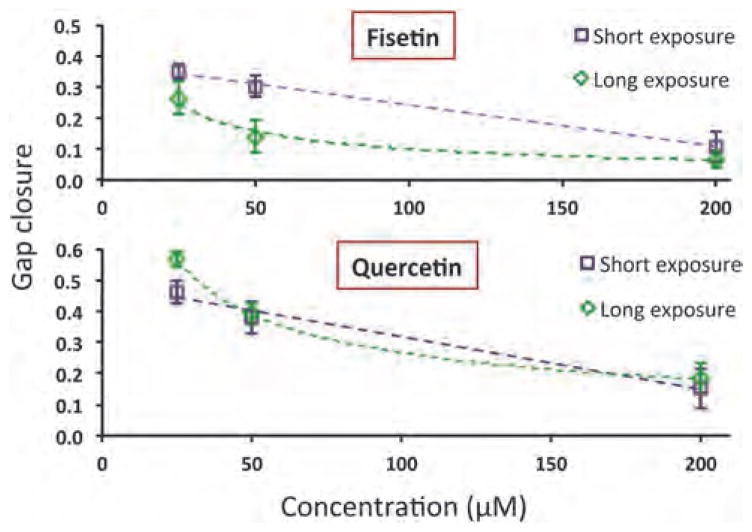
To investigate whether continuous, long-term exposure of phytochemicals (such as for Fig. 3) is required to block cell migration, we treated MDA-MB-157 cells with fisetin and quer-cetin at concentrations of 25, 50, and 200 μM for 12 h. Then, phytochemical solutions were washed out and replaced with culture media. Migration experiments continued for 48 h. Next, cells were fluorescently stained and imaged for analysis of migration. Fig. 4 shows the results from short exposure of cells to both phytochemicals and compares it with those from long exposure experiments. Only a 12 h exposure to fisetin and quercetin reduced cell migration dose-dependently at levels similar to those from long exposure experiments. Therefore, anti-migratory effects of phytochemicals last even after removal of compounds, at least with these two compounds and within the timeframe of our experiments.
Fig. 4.
Effect of a short exposure (12 h) of fisetin and quercetin on migration of MDA-MB-157 cells (squares). Gap closure results from exposing cells to compounds for 48 h (diamonds) are included for comparison.
3.4 Phytochemicals downregulate phosphorylation of extracellular signal-regulated kinases 1 and 2 (ERK1/2)
ERK 1 and 2 are protein kinases in the Ras/Raf/MEK/ERK (MAPK) signal transduction pathway and regulate a variety of processes including cell migration.39,40 Binding of mitogens and growth factors to cell surface receptors such as EGFR and IGFR activates this pathway to phosphorylate ERK1/2 ( pERK). pERK catalyzes phosphorylation of various nuclear substrates including regulatory molecules and transcription factors that lead to increased cell motility and invasiveness.41–43 Recent molecular analyses show increased activity of MAPK pathway in TNBC cells.37,44,45 In human breast cancer cells, the activity of ERK1/2 was necessary for re-assembly of stress fibers, which are involved in the extension of cell protrusions and the translocation of the cell body, disassembly of adhesions at the cell front, and rear retraction of cells during migration.46 Inhibition of ERK phosphorylation by PD98059, a specific inhibitor of the upstream kinase MEK, blocked cell migration in a concentration-dependent manner.39,47
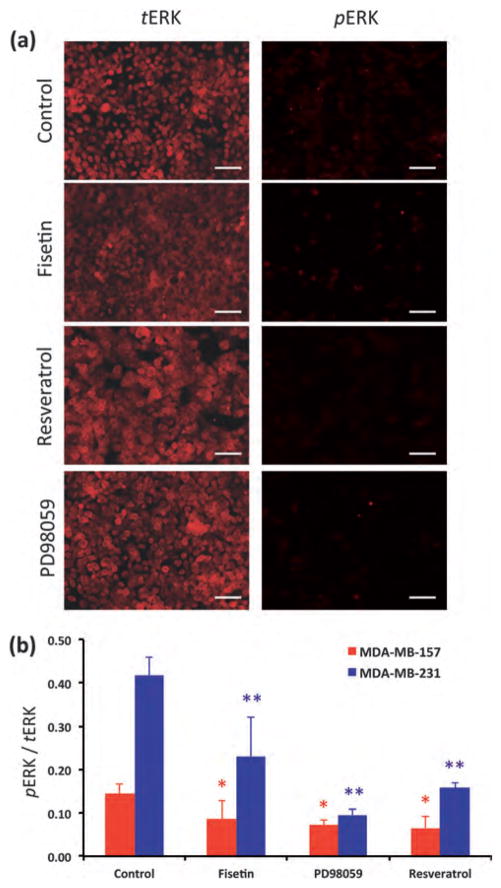
We examined whether blocking of TNBC cell migration by phytochemicals was due to inhibition of phosphorylation of ERK. Both TNBC cells were treated with fisetin, resveratrol, or PD98059. Levels of pERK and tERK were determined from images of immunostained cells and normalized with respect to number of cells in each image known from nuclear staining of cells. The ratio of pERK/tERK was found for each condition (Fig. 5). MDA-MB-231 cells showed substantially higher levels of ERK activity compared to MDA-MB-157 cells. Treating MDA-MB-231 cells with fisetin and resveratrol inhibited ERK1/2 phosphorylation by about 45% and 62% of the control, respectively. With MDA-MB-157 cells, fisetin treatment reduced pERK levels by 40%, whereas resveratrol inhibited ERK1/2 phosphorylation by 55%. These results demonstrate that phytochemicals downregulate ERK1/2 activity and attenuate pERK-induced signaling in TNBC cells. With both cells, resveratrol has a stronger inhibitory effect against ERK1/2 phosphorylation. However, considering that fisetin blocked migration of both cells to a greater extent compared to resveratrol (Fig. 3), it is conclusive that signaling molecules other than ERK1/2 also regulate motility of TNBC cells. We confirmed this conclusion by conducting a migration experiment with MDA-MB-231 cells in the presence of PD98059. Treatment of cells with 10 μM PD98059 reduced cell migration by 29 ± 9% of control, whereas at this concentration, PD98059 reduced pERK/tERK levels by ~77% of control. A detailed molecular analysis is required to gain a complete understanding of the role of inhibitory effects of phytochemicals on signaling molecules, particularly in MAPK and PI-3K pathways, modulating cell migration.
Fig. 5.
(a) Inhibition of ERK1/2 phosphorylation in MDA-MB-157 cells by treatment with fisetin, resveratrol, and PD98050 is compared to control, non-treated cells. (b) Quantitative comparison of pERK/tERK levels in MDA-MB-157 and MDA-MB-231 cells under different treatment conditions. With both cell lines, the pERK/tERK level in all treatments is statistically different from control. * and ** denote statistically significant difference between treatments and control (p < 0.05) for MDA-MB-157 and MDA-MB-231 cells, respectively. Scale bar 100 μm.
3.5 Phytochemicals scavenging of reactive oxygen species (ROS)
Cancer cells, including TNBC, have high levels of intracellular free radicals such as reactive oxygen species (ROS) beyond the capacity of endogenous antioxidative defense mechanisms to detoxify them.48,49 ROS cause DNA damage and increased activity of MAPK and PI-3K signaling pathways that lead to activation of transcription factors driving cell motility.50,51 Deregulated kinase signaling, which is prevalent in TNBC, also leads to excessive production of intracellular ROS.52 This vicious cycle involving sustained oxidative stresses and upregulated kinase signaling significantly aggravates the metastatic phenotypes of TNBC cells.
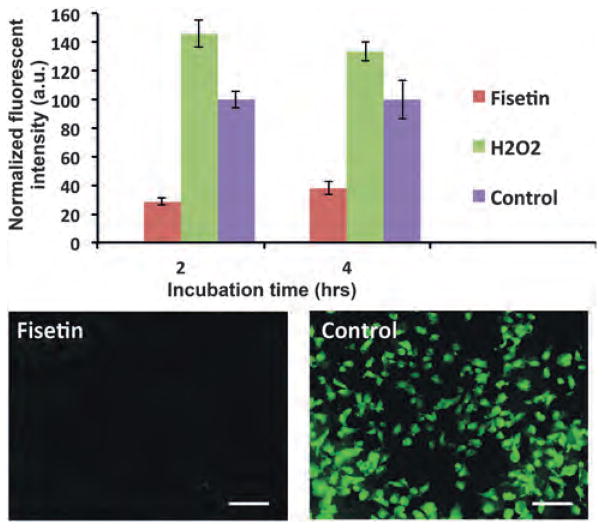
Phytochemicals are natural antioxidants with the ability to scavenge free radicals.22 In a proof-of-concept study, we treated MDA-MB-231 cells with fisetin and measured the reduction of ROS compared to two control conditions, no treatment and H2O2 treatment (Fig. 6). Fisetin eliminated intracellular ROS by up to 77% of control only after 2 h of treatment. On the other hand, H2O2 stimulation of cells increased the production of ROS by 46% from the base level. The effects were maintained at 4 h of detection time point, albeit with slight changes. Taken together with the downregulation of ERK1/2 phosphorylation, these data suggest that phytochemicals disrupt the elevated kinase signaling – ROS production cycle in TNBC cells.
Fig. 6.
Fisetin treatment of MDA-MB-231 cells significantly reduces endogenous ROS levels from the base levels in the control group and from H2O2-treated cells. Scale bar 100 μm.
Overall, this systematic high throughput screening demonstrated the potential of phytochemicals to inhibit or reduce the migration of metastatic TNBC cells. Importantly, anti-migratory effects of these compounds were achieved at non-toxic concentrations. This investigation provided a framework for future studies in several directions. Our collection of ten phytochemicals included compounds such as fisetin and quercetin that potently blocked migration of both MDA-MB-231 and MDA-MB-157 cells, whereas compounds such as methyl amooranin and curcumin were more effective against one cell line. Screening of these compounds against a panel of TNBC cell lines will determine how broadly specific phytochemicals inhibit motility of TNBC cells. Considering that these two cell lines were originally derived from patients of different ethnicities (MDA-MB-231 cells from a Caucasian patient and MDA-MB-157 cells from an African–American patient), selecting TNBC cells from different ethnic groups for this screening will elucidate whether certain phytochemicals may have ethnic-specific effects on blocking motility of cancer cells. Our molecular analysis using both TNBC cells treated with fisetin and resveratrol showed that both compounds have inhibitory effects against phosphorylation of ERK1/2, which is a major driver of cell migration. Nevertheless, fisetin that blocked cell migration more effectively than resveratrol was less potent against ERK1/2 phosphorylation, indicating involvement of other signaling molecules. A comprehensive protein expression analysis in migration-modulating pathways such as PI-3K and MAPK is necessary to identify molecular targets of phytochemicals. In addition to these in vitro studies, establishing that phytochemicals have preventive effects against cancer metastasis may lead to a new source of chemotherapy agents. This is supported by several recent studies that show specific phytochemicals prevent chemically-induced tumor formation and metastasis in animal models.16–21
4. Conclusion
We utilized our high throughput cell migration technology and conducted a comprehensive screening of anti-migratory effects of ten phytochemicals against triple negative breast cancer cells. This high throughput screening identified compounds that effectively blocked migration of cells at non-toxic concentrations and in a dose-dependent manner. Anti-migratory effects of phytochemicals were maintained even with a short treatment and after removing them from cultures. Protein expression analysis showed that phyto-chemicals significantly inhibit phosphorylation of ERK1/2 that is a driver of migration of metastatic cancer cells. In addition, these natural antioxidant compounds effectively scavenged intracellular reactive oxygen species found at excessively high levels in cancer cells. Given the key role of cell migration in cancer metastasis, upregulated kinase signaling, and presence of excess free radicals in cancer cells, the use of phytochemicals as chemotherapeutics may prove effective in downregulating malignant cell phenotypes and blocking metastasis.
Insight, innovation, integration.
Migration of cells is a major driver of cancer metastasis. Inhibition of motility of cancer cells may help block metastasis. We demonstrate that certain natural compounds, phytochemicals, provide strong anti-migratory effects against highly metastatic triple negative breast cancer cells. We achieve this using a robotic, high throughput cell migration technology that enables systematic compound screening. We show that migration inhibition by phytochemicals is in part due to interference with kinase signaling and scavenging of intracellular free radicals. This study provides preliminary evidence that the use of phytochemicals as chemotherapeutics may offer major benefits.
Acknowledgments
This work is supported by grants from NIH (R21CA182333, Tavana), NSF (CBET-0954360, Yun), and The University of Akron Faculty Research Committee (Tavana).
Footnotes
Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ib00121h
References
- 1.Hudis CA, Gianni L. Oncologist. 2011;16(suppl 1):1–11. doi: 10.1634/theoncologist.2011-S1-01. [DOI] [PubMed] [Google Scholar]
- 2.Gluz O, Liedtke C, Gottschalk N, Pusztai L, Nitz U, Harbeck N. Ann Oncol. 2009;20:1913–1927. doi: 10.1093/annonc/mdp492. [DOI] [PubMed] [Google Scholar]
- 3.Arnedos M, Bihan C, Delaloge S, Andre F. Ther Adv Med Oncol. 2012;4:195–210. doi: 10.1177/1758834012444711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McBride R, Hershman D, Tsai WY, Jacobson JS, Grann V, Neugut AI. Cancer. 2007;110:1201–1208. doi: 10.1002/cncr.22884. [DOI] [PubMed] [Google Scholar]
- 5.Servick K. Science. 2014;343:1452–1453. doi: 10.1126/science.343.6178.1452. [DOI] [PubMed] [Google Scholar]
- 6.Stead LA, Lash TL, Sobieraj JE, Chi DD, Westrup JL, Charlot M, Blanchard RA, Lee JC, King TC, Rosenberg CL. Breast Cancer Res. 2009;11:R18. doi: 10.1186/bcr2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pogoda K, Niwinska A, Murawska M, Pienkowski T. Med Oncol. 2013;30:388. doi: 10.1007/s12032-012-0388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masuda H, Baggerly KA, Wang Y, Zhang Y, Gonzalez-Angulo AM, Meric-Bernstam F, Valero V, Lehmann BD, Pietenpol JA, Hortobagyi GN, Symmans WF, Ueno NT. Clin Cancer Res. 2013;19:5533–5540. doi: 10.1158/1078-0432.CCR-13-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, Cristofanilli M, Hortobagyi GN, Pusztai L. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 10.Oakman C, Moretti E, Galardi F, Biagioni C, Santarpia L, Biganzoli L, Di Leo A. Breast. 2011;20(suppl 3):S135–141. doi: 10.1016/S0960-9776(11)70311-3. [DOI] [PubMed] [Google Scholar]
- 11.Chambers AF, Groom AC, MacDonald IC. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 12.Friedl P, Gilmour D. Nat Rev Mol Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 13.Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I, Morony S, Rubin E, Sarao R, Hojilla CV, Komnenovic V, Kong YY, Schreiber M, Dixon SJ, Sims SM, Khokha R, Wada T, Penninger JM. Nature. 2006;440:692–696. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- 14.Friedl P, Hegerfeldt Y, Tusch M. Int J Dev Biol. 2004;48:441–449. doi: 10.1387/ijdb.041821pf. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Yang S, Jakoncic J, Zhang JJ, Huang XY. Nature. 2010;464:1062–1066. doi: 10.1038/nature08978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aggarwal BB, Shishodia S. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Weng CJ, Yen GC. Cancer Treat Rev. 2012;38:76–87. doi: 10.1016/j.ctrv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Bishayee A, Mbimba T, Thoppil RJ, Haznagy-Radnai E, Sipos P, Darvesh AS, Folkesson HG, Hohmann J. J Nutr Biochem. 2011;22:1035–1046. doi: 10.1016/j.jnutbio.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Mandal A, Bhatia D, Bishayee A. Mol Cell Biochem. 2013;384:239–250. doi: 10.1007/s11010-013-1803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bishayee A, Thoppil RJ, Waghray A, Kruse JA, Novotny NA, Darvesh AS. Curr Cancer Drug Targets. 2012;12:1191–1232. [PubMed] [Google Scholar]
- 21.Parikh NR, Mandal A, Bhatia D, Siveen KS, Sethi G, Bishayee A. Phytochem Rev. 2014;13:793–810. doi: 10.1007/s11101-014-9337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta SC, Kim JH, Prasad S, Aggarwal BB. Cancer Metastasis Rev. 2010;29:405–434. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siveen KS, Sikka S, Surana R, Dai X, Zhang J, Kumar AP, Tan BK, Sethi G, Bishayee A. Biochim Biophys Acta. 2014;1845:136–154. doi: 10.1016/j.bbcan.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Gullett NP, Ruhul Amin ARM, Bayraktar S, Pezzutod JM, Shin DM, Khuri FR, Aggarwal BB, Surh Y-J, Kucuk O. Semin Oncol. 2010;37:258–281. doi: 10.1053/j.seminoncol.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Ward DJ, Martino OI, Simpson S, Stevens AJ. BMJ Open. 2013;3:e002088. doi: 10.1136/bmjopen-2012-002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tavana H, Kaylan K, Bersano-Begey T, Luker KE, Luker GD, Takayama S. Adv Funct Mater. 2011;21:2920–2926. doi: 10.1002/adfm.201002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemmo S, Nasrollahi S, Tavana H. Biotechnol J. 2014;9:426–434. doi: 10.1002/biot.201300227. [DOI] [PubMed] [Google Scholar]
- 28.Tavana H, Mosadegh B, Takayama S. Adv Mater. 2010;22:2628–2631. doi: 10.1002/adma.200904271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tavana H, Jovic A, Mosadegh B, Lee QY, Liu X, Luker KE, Luker GD, Weiss SJ, Takayama S. Nat Mater. 2009;8:736–741. doi: 10.1038/nmat2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemmo Ham S, Atefi A, Fyffe D, Tavana H. J Visualized Exp. 2015;98:e52754. doi: 10.3791/52754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frampton JP, White JB, Abraham AT, Takayama S. J Visualized Exp. 2013;73:e50304. doi: 10.3791/50304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atefi E, Mann JA, Jr, Tavana H. Langmuir. 2014;30:9691–9699. doi: 10.1021/la500930x. [DOI] [PubMed] [Google Scholar]
- 34.Bishayee A, Haznagy-Radnai E, Mbimba T, Sipos P, Morazzoni P, Darvesh AS, Bhatia D, Hohmann J. Nat Prod Commun. 2010;5:1613–1618. [PubMed] [Google Scholar]
- 35.Bishayee A, Mandal A, Thoppil RJ, Darvesh AS, Bhatia D. Int J Cancer. 2013;133:1054–1063. doi: 10.1002/ijc.28108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu S, Hu X, Yu Z, Peng Y, Zhu J, Liu X, Li M, Han S, Zhu C. PLoS One. 2015;10:e0123519. doi: 10.1371/journal.pone.0123519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.N. Cancer Genome Atlas. Nature. 2012;490:61–70. [Google Scholar]
- 38.Lindner R, Sullivan C, Offor O, Lezon-Geyda K, Halligan K, Fischbach N, Shah M, Bossuyt V, Schulz V, Tuck DP, Harris LN. PLoS One. 2013;8:e71915. doi: 10.1371/journal.pone.0071915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krueger JS, Keshamouni VG, Atanaskova N, Reddy KB. Oncogene. 2001;20:4209–4218. doi: 10.1038/sj.onc.1204541. [DOI] [PubMed] [Google Scholar]
- 40.Roskoski R., Jr Pharmacol Res. 2012;66:105–143. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Hartmann G, Weidner KM, Schwarz H, Birchmeier W. J Biol Chem. 1994;269:21936–21939. [PubMed] [Google Scholar]
- 42.Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Block ER, Tolino MA, Lozano JS, Lathrop KL, Sullenberger RS, Mazie AR, Klarlund JK. Mol Biol Cell. 2010;21:2172–2181. doi: 10.1091/mbc.E09-12-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Notte A, Ninane N, Arnould T, Michiels C. Cell Death Dis. 2013;4:e638. doi: 10.1038/cddis.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Umemura S, Yoshida S, Ohta Y, Naito K, Osamura RY, Tokuda Y. Cancer Sci. 2007;98:1889–1892. doi: 10.1111/j.1349-7006.2007.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teranishi S, Kimura K, Nishida T. Invest Ophthalmol Visual Sci. 2009;50:5646–5652. doi: 10.1167/iovs.08-2534. [DOI] [PubMed] [Google Scholar]
- 47.Yang Y, Cheon S, Jung MK, Song SB, Kim D, Kim HJ, Park H, Bang SI, Cho D. Biochem Biophys Res Commun. 2015;459:379–386. doi: 10.1016/j.bbrc.2015.02.108. [DOI] [PubMed] [Google Scholar]
- 48.Karihtala P, Kauppila S, Soini Y, Arja Jukkola V. BMC Cancer. 2011;11:262. doi: 10.1186/1471-2407-11-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Visconti R, Grieco D. Curr Opin Drug Discovery Dev. 2009;12:240–245. [PubMed] [Google Scholar]
- 50.Liou GY, Storz P. Free Radical Res. 2010;44:479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, Pae HO. J Signal Transduction. 2011;2011:792639. doi: 10.1155/2011/792639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torres M, Forman HJ. Biofactors. 2003;17:287–296. doi: 10.1002/biof.5520170128. [DOI] [PubMed] [Google Scholar]