Abstract
Ketamine, a noncompetitive antagonist of N-methyl-D-aspartate-type glutamate receptors, is a pediatric anesthetic that has been shown to be neurotoxic in rodents and nonhuman primates when administered during the brain growth spurt. Recently, the zebrafish has become an attractive model for toxicity assays, in part because the predictive capability of the zebrafish model, with respect to chemical effects, compares well with that from mammalian models. In the transgenic (hb9:GFP) embryos used in this study, green fluorescent protein (GFP) is expressed in the motor neurons, facilitating the visualization and analysis of motor neuron development in vivo. In order to determine whether ketamine induces motor neuron toxicity in zebrafish, embryos of these transgenic fish were treated with different concentrations of ketamine (0.5 and 2.0 mM). For ketamine exposures lasting up to 20 h, larvae showed no gross morphological abnormalities. Analysis of GFP-expressing motor neurons in the live embryos, however, revealed that 2.0 mM ketamine adversely affected motor neuron axon length and decreased cranial and motor neuron populations. Quantitative reverse transcriptase-polymerase chain reaction analysis demonstrated that ketamine down-regulated the motor neuron-inducing zinc finger transcription factor Gli2b and the proneural gene NeuroD even at 0.5 mM concentration, while up-regulating the expression of the proneural gene Neurogenin1 (Ngn1). Expression of the neurogenic gene, Notch1a, was suppressed, indicating that neuronal precursor generation from uncommitted cells was favored. These results suggest that ketamine is neurotoxic to motor neurons in zebrafish and possibly affects the differentiating/differentiatedneurons rather than neuronal progenitors. Published 2011. This article is a US Government work and is in the public domain in the USA.
Keywords: neurotoxicity, ketamine, motor neuron, transgenic zebrafish, gene expression
INTRODUCTION
Ketamine, a pediatric anesthestic, is thought to act primarily through blockade of N-methyl-D-aspartate (NMDA)-type glutamate receptors to provide analgesia/anesthesia to children for painful procedures (Kohrs and Durieux, 1998). Rodent research has shown that ketamine can induce apoptosis when administered in high doses and/or for prolonged periods during susceptible periods of development (Lahti et al., 2001; Larsen et al., 1998; Malhotra et al., 1997; Maxwell et al., 2006). Although it is clear that ketamine causes neuronal cell death in rodent models when given repeatedly during the brain growth spurt period (Ikonomidou et al., 1999; Wang et al., 2006), it has only recently been demonstrated that a similar phenomenon also occurs in primates (Haberny et al., 2002; Slikker et al., 2007; Wang et al., 2006). However, plasma ketamine levels associated with a light surgical plane of anesthesia in neonatal monkeys (postnatal days 5–6) were 3–5 times higher (Slikker et al., 2007) than those typically observed in humans (Clements and Nimmo, 1981; Grant et al., 1981).
Earlier rodent and monkey studies suggest that limiting doses and durations of exposure reduces the potential for neurodegeneration caused by ketamine and other NMDA receptor antagonists (Ikonomidou et al., 1999, 2001; Jevtovic-Todorovic et al., 2000; Olney et al., 2002, 2004; Pohl et al., 1999; Slikker et al., 2007). Although the exact mechanisms underlying the neurotoxicity induced by ketamine are not known, the window of vulnerability to the neuronal effects of anesthetics is restricted to the period of rapid synaptogenesis, also known as the brain growth spurt (Slikker et al., 2007).
In addition to its small size, prolific reproductive capacity and easy maintenance, zebrafish maintain the typical complexity of vertebrate systems and accumulating evidence advocates its use in several areas of research with the prospect of extrapolating findings to other vertebrates and humans (Briggs, 2002; Parng et al., 2002; Powers, 1989; Vascotto et al., 1997). Since its early use, emphasis has been given to characterizing molecules within the zebrafish nervous system, a difficult task to undertake in mammals (Key and Devine, 2003). The zebrafish has also been used as a model for other areas of research such as aging (Gerhard, 2007; Gerhard and Cheng, 2002), neurological diseases (Bretaud et al., 2004), drug addiction (Ninkovic and Bally-Cuif, 2006), and other behavioral studies (Fetcho and Liu, 1998; Miklosi and Andrew, 2006; Salas et al., 2006).
NMDA receptor channels are heteromeric complexes consisting of essential NR1 subunits and one or more regulatory NR2 subunits (NR2A/B/C/D; Kutsuwada et al., 1992; Monyer et al., 1992). Regulation of NMDA receptor channel activity is critical for normal neural development (Cull-Candy et al., 2001), learning and memory (Abel and Lattal, 2001), and the pathological processes associated with stroke (Meldrum, 1990). There are 10 NMDA receptor subunits found in zebrafish (Cox et al., 2005). These subunits fall into five subtypes, each containing two paralogous genes. There are two NMDAR1 genes (NR1.1 and NR1.2), and eight NMDAR2 genes, designated NR2A.1 and NR2A.2, NR2B.1 and NR2B.2, NR2C.1 and NR2C.2, and NR2D.1 and NR2D.2. The predicted sequences of the NR1 paralogs display 90% identity with the human protein. The NR2 subunits show less identity, differing most at the N- and C-termini. Both the NR1 genes are expressed embryonically, although in a nonidentical manner. NR1.1 is found in brain, retina and spinal cord at 24 h postfertilization (hpf). NR1.2 is expressed in the brain at 48 hpf but not in the spinal cord. NR2 developmental gene expression varies: both paralogs of the NR2A are expressed at 48 hpf in the retina; only one paralog of the NR2B is expressed at low levels in the heart at 48 hpf; no NR2C paralogs are expressed embryonically; and NR2D.1 is expressed in the forebrain, retina, and spinal cord at 24 hpf, whereas NR2D.2 is only found in the retina (Cox et al., 2005). Studies based on immunohistochemistry with available antibodies have shown that NR2A subunits are expressed in primary motor neurons and axons in zebrafish larvae (Todd et al., 2004). Expression of other subunits in both primary and secondary motor neurons remains to be determined.
In adult zebrafish, 0.8% ketamine is an effective anesthetic dose and 0.2% is a subthreshold dose. At the subthreshold (subanesthetic) dose, zebrafish show a variety of abnormal behaviors, such as altered gill movement, stress responses and circling behavior, qualitatively analogous to those observed in humans and rodents treated with various drugs (Zakhary et al., 2011). Zebrafish larvae treated with 0.1–3.0 mM ketamine for 20 min show altered sensorimotor gating (Burgess and Granato, 2007). In our study on 28 hpf embryos, we used ketamine at 0.5 (0. 014%) and 2.0 mM (0.055%) concentrations for 20 h in order to study specifically its effects on motor neuron development.
In order to quantitate the effect of ketamine on motor neurons in vivo, hb9:green fluorescent protein (hb9:GFP) transgenic zebra-fish embryos were used, in which the promoter of the transcription factor hb9 that is found in developing motor neurons of both mammals (William et al., 2003) and zebrafish (Cheesman et al., 2004; Park et al., 2004), drives GFP expression in motor neurons (Flanagan-Steet et al., 2005).
For this study, several genes involved in neuronal development were selected for analysis. Gli2, the zinc finger transcription factors, play a role in several cellular lineages – the ventral neural precursors, cranial motor neurons, interneurons and dorsal sensory neurons (Ke et al., 2005, 2008). Notch1a is a member of the Notch trans-membrane proteins that play a central role in the signaling events critical for nervous system development by lateral inhibition (reviewed in Lewis, 1996). In zebrafish, Notch-mediated lateral inhibition maintains a pool of neuronal precursors for later differentiation (Appel et al., 1999) and transient inhibition of Notch signaling using the gamma-secretase inhibitor DAPT {N-[N-(3,5-difluorophenacetyl)-1-alanyl]-S-phenylglycine t-butyl ester} during early neurogenesis produces excess primary motor neurons and KA′ (Kolmer–Agduhr′) interneurons in zebrafish (Shin et al., 2007). Neurogenin 1 (Ngn1), the bHLH (basic helix–loop–helix) transcription factor, is expressed in the precursors of motor neurons, interneurons and sensory neurons of zebrafish (Blader et al., 1997; Korzh et al., 1998). NeuroD, another bHLH transcription factor, and a downstream target of Ngn1, is expressed mostly in postmitotic neurons (Korzh et al., 1998; Mueller and Wullimann, 2002) and is required for terminal differentiation rather than for neuronal precursor commitment. Notch mutant mice up-regulate expression of proneuronal transcription factors (Ngn1 and NeuroD), indicative of premature and excess neuronal development (de la Pompa et al., 1997; Ishibashi et al., 1995) while expression of constitutively active forms of Notch block neuronal development and seemingly maintain neural cells in a precursor state (Gaiano et al., 2000). These observations reinforced the notion that Notch determines neuronal cell fate through lateral inhibition (reviewed in Gaiano and Fishell, 2002).
The homeobox transcription factor hb9 is expressed selectively in postmitotic motor neurons in developing vertebrates and serves as a marker for the motor neuron phenotype (Arber et al., 1999; Tanabe et al., 1998). Genetic studies in mice have shown its role in the consolidation and maintenance of motor neuron identity (Arber et al., 1999; Thaler et al., 1999). In the hb9:GFP fish, hb9 promoter-driven GFP expression can be monitored in the zebrafish embryos and larvae in vivo. In this work, taking advantage of the established transgenic zebrafish line (hb9-GFP), in which, owing to motor neuron-specific expression of GFP, only motor neuron development in the embryos can be monitored in vivo, we show that ketamine adversely affects motor neuron development and motor axons as assessed in vivo using a transgenic zebrafish line and the resulting phenotype correlates well with the changes in expression of relevant genes.
MATERIALS AND METHODS
Animals
Adult hb9-GFP transgenic zebrafish (Danio rerio, AB strain) were obtained from the Zebrafish International Resource Center at the University of Oregon (Eugene, OR, USA). The fish were kept in fish tanks (Aquatic Habitats) at the NCTR/FDA zebrafish facility (IACUC-approved protocol no. E0738701) containing buffered water (pH 7.2) at 28.5 °C, and were fed daily live brine shrimp and Zeigler dried flake food (Zeigler, Gardners, PA, USA). The day–night cycle was maintained at 14:10 h, and spawning and fertilization were stimulated by the onset of light. Fertilized zebrafish embryos were collected from the bottom of the tank. The eggs were placed in Petri dishes and washed thoroughly with buffered egg water [reverse osmosis water containing 60 mg sea salt (Crystal Sea®, Aquatic Eco-systems Inc., Apopka, FL, USA) per liter of water (pH 7.5)] and then allowed to develop in an incubator seat at 28.5 °C.
Treatment of Zebrafish Embryos with Ketamine
For each experiment, three sets of 28 hpf dechorionated (manual dechorionation using a pair of watchmakers’ forceps) embryos were used. Each set included 10 embryos placed in individual wells of six-well plates (n = 30/each experimental group), each well containing 5 ml egg water. Ketamine (ketamine hydrochloride from Sigma, St Louis, MO, USA; catalog no. K2753) was dissolved as a stock of 100 mg ml−1 in buffered egg water. The solution was made fresh and treatment (static exposure) at various doses (0.5 and 2.0 mM) continued for 2 or 20 h. Ketamine stock solution was added to the embryos in 5 ml of egg water to a final concentration of either 0.5 or 2.0 mM ketamine. After 2 h of ketamine treatment, the embryos were examined microscopically (epifluorescence using the FITC filter). Since there was no difference in GFP fluorescence levels in the treated and control embryos, eventually, 20 h static exposure was chosen as there were no changes in the intensities of GFP expression after 2 h treatments, although locomotory behavior changes in zebrafish larvae have been observed after minutes of ketamine treatment (Burgess and Granato, 2007). The rationale behind this longer exposure was to make any subtle effects of ketamine on the phenotype microscopically detectable. An untreated control group of 10 embryos/set (n = 30) was examined in parallel. Embryos were incubated at 28.5 °C.
Live Embryo Morphological Assessment
Morphological changes were examined by visually monitoring (using a Nikon SMZ1000 binocular microscope) the following endpoints: body length, contour and curvature, head morphology and yolk morphology (color of yolk sac and shape of yolk extension). Ten embryos from each experimental group were examined for these parameters.
Live Embryo Imaging
Post-treatment with ketamine, images of the hb9-GFP Tg embryos were acquired using an Olympus SZX 16 binocular microscope and DP72 camera. Higher magnification images were acquired using a Nikon Eclipse 80i microscope and Nikon DXM1200C digital camera. After 2 h of ketamine treatment, when the embryos were 30 hpf, GFP expression in the motor neurons was monitored under the microscope for changes in GFP expression. In embryos treated with ketamine for 20 h (static exposure), GFP-expressing spinal motor neurons in the trunk region (three hemisegments following the distal end of the yolk extension) were counted per specific hemisegments following a procedure used earlier (Kanungo et al., 2009). The values from 10 embryos each per experimental group were averaged to obtain the number of neurons/hemisegment. Relative motor axon (GFP-positive) lengths were measured using a micrometer.
RNA Extraction and cDNA Synthesis
Total RNA (from pooled 30 embryos/treatment group) was extracted from whole embryos (48 hpf) using the Trizol reagent (Invitrogen, Carlsbad, CA, USA). An aliquot of each RNA sample was used to spectrophotometrically (using a NanoDrop ND-1000; NanoDrop Technology, Wilmington, DE, USA) to determine RNA quality (A260/A280 >2.0) and concentration. First-strand cDNA was synthesized from total RNA (1 μg; 20 μl final reaction volume) with oligo(dT) priming using SuperScript II reverse transcriptase (Invitrogen) according to the manufacturer’s instructions.
Primers
The following primers were used for the quantitative polymerase chain reaction (qPCR) assays: Gli2a forward 5′- AAAAACAGGGCGGGACTACT-3′ and reverse 5′-ATGCTGGGTTGGAGGTACAG-3′; Gli2b forward 5′-TTTGCTGGAGCCAGAAAGTT-3′ andreverse5′-TTCGCTGAAGACGTTTCCTT-3′; Notch1a forward 5′-TTCTGGCATTCACTGTGAGC-3′ and reverse 5′-TCTCTCTGTCCTGGCAGGTT-3′; Ngn1 forward 5′-AAG-CAGGGCAAGTCAAGAGA-3′ and reverse 5′-ACGTCGGTTTGCAAGTATCC-3′; NeuroD forward 5′- CAGCAAGTGCTTCCTTTTCC-3′ and reverse 5′-TAAGGGGTCCGTCAAATGAG-3′; GAPDH forward 5′- GATACACGGAGCACCAGGTT-3′ and reverse 5′-GCCATCAGGTCACATACACG-3′.
Real-time PCR (qPCR)
Real-time PCR was performed using a CFX96 C1000 (Bio-Rad, Hercules, CA, USA) detection system with SYBR green fluorescent label (Bio-Rad). Samples (25 ml final volume) contained the following: 1 × SYBR green master mix (Bio-Rad), 5 pmol of each primer, and 0.25 μl of the reverse transcriptase (RT) reaction mixture. Samples were run in triplicate in optically clear 96-well plates. Cycling parameters were as follows: 50 °C × 2 min, 95 °C 10 min, then 40 cycles of 95 °C × 15 s, 60 °C × 1 min. A melting temperature-determining dissociation step was performed at 95 C × 15 s, 60 C × 15 s, and 95 C × 15 s at the end of the amplification phase. The 2−ΔΔCt method was used to determine the relative gene expression (Livak and Schmittgen, 2001). The GAPDH gene was the internal control for all qPCR experiments. Data from pools of embryos (n = 30, from triplicate wells containing 10 per well) were averaged and shown as normalized gene expression ± SEM. One-way ANOVA (‘exposure’ or ‘ketamine dose’ as factor) and Holm–Sidak pair-wise multiple comparison post-hocs (Sigma Stat 3.1 for analysis) were used to determine statistical significance with P < 0.05.
RESULTS
Motor Neuron-specific GFP (Reporter) Expression in Transgenic Zebrafish Exposed to Ketamine
Based on reported observations that ketamine is neurotoxic in developing rodents and monkeys, the goal of this study was to test whether similar effects manifest in zebrafish, an emerging alternate vertebrate animal model for drug screening and drug safety assessments. In order to assess the effect of ketamine on the developing zebrafish nervous system in vivo, we employed a transgenic zebrafish line (hb9:GFP) that has motor neurons specifically identified by the expression of GFP. Based on prior doses of ketamine used with zebrafish larvae for behavioral studies (Burgess and Granato, 2007), we chose two different doses, 0.5 and 2.0 mM, that were directly added to the water. Embryos (28 hpf) exposed for 2 h to ketamine showed no effect on motor neurons when monitored in vivo (data not shown). A prolonged exposure to ketamine for 20 h, however, resulted in a reduction in the GFP expression intensity in the motor neuron population at the 2.0 mM dose, but the defects were not so obvious at the lower 0.5 mM dose (Fig. 1). Although no obvious morphological (body contour and curvature, head morphology, yolk sac color and contour of yolk extension) abnormalities were noted after ketamine treatment, scanning for GFP expression in live embryos revealed that, when compared with the control untreated embryos (Fig. 1A), 0.5 mM ketamine-treated embryos did not show any differences (Fig. 1B) while the 2.0 mM ketamine-treated embryos showed reduced GFP expression in the brain and spinal cord (Fig. 1 C).
Figure 1.
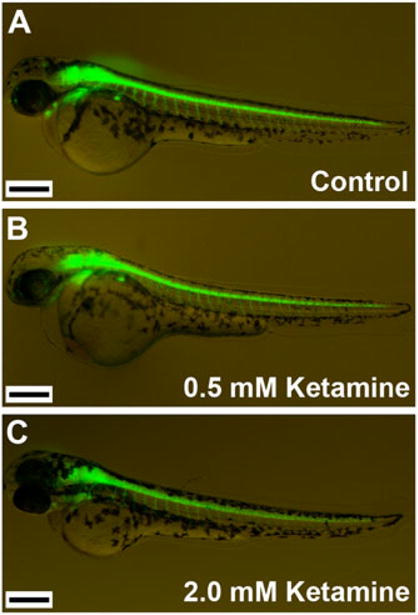
Effect of ketamine on zebrafish embryos. Ketamine does not have a drastic effect on zebrafish morphology but adversely affects the central nervous system as indicated by differences in relative GFP fluorescence. In the hb9:GFP transgenic fish motor neurons express hb9 promoter-driven GFP. Embryos at 28 hpf were treated with ketamine. After 20 h of treatment (48 hpf actual age), images of the live embryos were acquired for assessment of GFP fluorescence. In this experiment, MS-222 was not used (a routine procedure) to immobilize the untreated control embryos for photography in order to avoid any interference with the effect produced by ketamine. The experiment was repeated three times with n = 30 (replicates of 10 each) for each group in each experiment. Lateral views of the embryos are shown with dorsal side up. Embryos in different experimental groups are (A) control, (B) 0.5 mM ketamine-treated, and (C) 2.0 mM ketamine-treated. Scale bar = 280 μM.
Ketamine’s Adverse Effects on the Motor Neurons and Motor Axon Development in Transgenic Zebrafish
In the brain, exact quantification of GFP-positive neurons either in the control or in the adversely affected 2.0 mM ketamine-treated embryos was not possible The qualitative changes indicated a reduced GFP expression intensity in the ketamine-treated brain compared with the control (Fig. 2A, B). A similarly reduced GFP expression in the spinal cord motor neuron population was noticeable in the 2.0 mM ketamine-treated compared with the control (Fig. 2C, D). In these hb9:GFP transgenic embryos, the motor axons, projecting from the spinal motor neurons, also express GFP making it possible to visualize them in vivo. On comparison between control and 2.0 mM ketamine-treated embryos, a significant reduction in axon length (20%) was obvious in the latter group (Fig. 2E).
Figure 2.
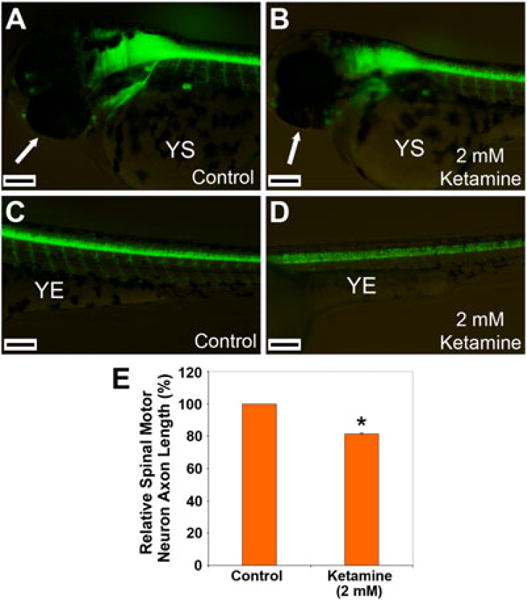
Adverse effects of ketamine on cranial and spinal motor neurons. Hb9:GFP transgenic fish embryos (28 hpf) were treated with 2.0 mM ketamine. After 20 h of treatment (48 hpf actual age), images of the live embryos (lateral views with dorsal side up and anterior side to the left) were acquired for assessment of GFP fluorescence. GFP expressing motor neurons are shown in control (A) and ketamine-treated (B) brains, and control (C) and ketamine-treated (D) spinal cords. The arrows indicate the eyes, YS indicates yolk sac, and YE indicates yolk extension. Since the brain has a high density of GFP-positive motor neurons, the difference in motor neuron numbers could not be quantitatively determined. However, overall GFP fluorescence in ketamine-treated embryos was reduced compared with the untreated controls. Spinal motor neurons, however, could be visualized individually. Scale bar = 120 μM. Axon lengths from specific areas in the spinal cord region were measured using a micrometer in the microscope and the mean difference (%) between the control and ketamine-treated embryos from three different replications is presented (E). The value for the control does not have an error bar (SEM) because the spinal motor axon lengths were normalized relative to the lengths of the control. Student’s t-test was performed to determine statistical significance between observed differences and significance (*) was set at P < 0.05 (D).
Significant Reduction in Spinal Motor Neurons in Transgenic Zebrafish Exposed to Ketamine
Although it was not possible to visualize or count the cranial motor neurons, using fluorescence microscopy, quantification of GFP-positive spinal motor neurons in specific hemisegments in these embryos was accomplished. By visually counting the neurons in the three hemisegments distal to the yolk extension and obtaining the mean value, the average neuron numbers per hemisegment were calculated. Compared with the control, in the 0.5 mM ketamine-treated embryos, there was no difference in the GFP-positive motor neuron numbers (Fig. 3A, B). However, in the 2.0 mM ketamine-treated embryos, there was a significant reduction (30%) in the number of spinal motor neurons compared with the untreated embryos (Fig. 3C, D).
Figure 3.
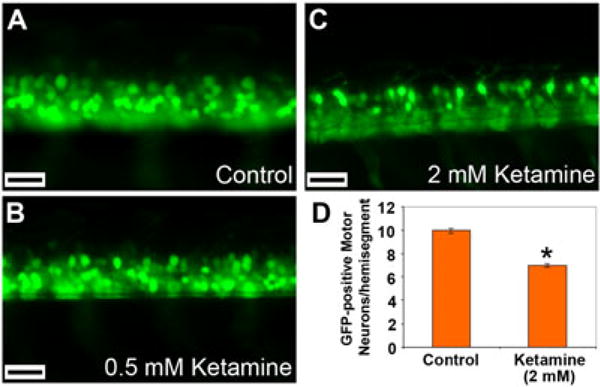
Effect of ketamine on spinal motor neuron numbers. Embryos at 28 hpf were treated with 0.5 and 2.0 mM ketamine for 20 h (static exposure). Higher magnification of the trunk region showing GFP positive neurons in control (A), 0.5 mM ketamine treated- (B) and 2.0 mM ketamine-treated (C) embryos (48 hpf actual age). GFP-positive motor neurons in specific hemi-segments were counted, and Student’s t-test was performed to determine statistical significance between observed differences with significance (*) set at P < 0.05 (D). In each of three replications, spinal motor neurons were counted in 10 embryos each from the control and ketamine-treated groups. Scale bar = 30 μM.
Changes in the Expressions of Specific Genes Involved in Motor Neuron Development upon Ketamine Exposure
The above adverse effects of ketamine indicated that either there was a reduction in the differentiation of the motor neurons or the differentiated neurons simply did not survive, thus contributing toward a reduction in number. In order to further address these possibilities, we examined the expression of a number of specific genes that are known to regulate motor neuron induction and differentiation as well as some that are expressed in differentiated neurons. Of the neurogenic genes, we choose Notch1a (Appel and Eisen, 1998; Chitnis and Kintner, 1996; Chitnis, 1995; Dornseifer et al., 1997; Haddon et al., 1998) and of the proneural genes, Neurogenin1 (Ngn1) and NeuroD (Blader et al., 1997; Kim et al., 1997; Lee, 1997). Of the genes that have specific motor neuron inductive ability essential for neuronal differentiation from undifferentiated cells, a candidate gene is Gli2b (Karlstrom et al., 2003; Ke et al., 2005, 2008). We chose to analyze both Gli2b and Gli2a since Gli2a has been shown to be involved in the development of ventral neurons in the developing mouse brain (Blaess et al., 2006).
Quantitative RT-PCR assays to determine mRNA expression levels (Fig. 4) demonstrated that, compared with the control embryos, 0.5 mM ketamine significantly down-regulated Gli2b expression (0.33-fold, P < 0.0001), even though no adverse effects on the number or GFP-fluorescence of motor neurons were apparent. Gli2a expression was unchanged while the expression of the neurogenic gene, Notch1a, was slightly down-regulated (0.8-fold), indicating that ketamine at this dose positively affected the signaling pathway responsible for driving cells toward neuronal commitment. In support of this supposition, the pro-neural gene Ngn1 was up-regulated (1.5-fold, P < 0.005). Since Ngn1 is a downstream target of Notch inhibition, the results are consistent in showing that the neuronal induction pathway associated with ketamine treatment not only remained intact, but was also somehow favored. Surprisingly, however, NeuroD, a direct downstream target of Ngn1, was significantly down-regulated (0.7-fold, P < 0.03) in the 0.5 mM ketamine-treated embryos. It is possible that, as a gene required for terminal neuronal differentiation in differentiated neurons, NeuroD expression began to lessen as a secondary effect while the expression of its inducer, Ngn1, remained favored, when differentiating/differentiated neurons were entering, subtly, an apoptotic or disintegrative pathway. This subtle insult, however, was not yet translated to reduced GFP expression, as the motor neurons still existed. If this were the case and if such down-regulation occurred at the threshold level of an insult at which neurons could remain alive, one would expect any further down-regulation of NeuroD to be deleterious to the differentiated motor neurons. Anticipating such an outcome, gene expression changes were analyzed in embryos exposed to 2.0 mM ketamine, a 4-fold higher dose. Gli2b expression, although significantly less than in controls (0.5-fold, P 0.0004), was higher than in embryos treated with 0.5 mM, suggesting that, perhaps since Gli2b is also expressed in undifferentiated neurons and their precursors, there could be enhancement of the development of these cells. Gli2a expression remained unaltered as in the embryos treated with the 0.5 mM dose, but Notch1a expression was further down-regulated (0.75-fold, P < 0.005) from levels seen in embryos treated with the 0.5 mM dose. Accordingly, Ngn1 expression remained up-regulated (1.24-fold, P < 0.02). These results further support the hypothesis that ketamine favored the expression of the pro-neural gene, Ngn1, by antagonizing Notch signaling. It is important to note that ketamine, at both doses tested, had negative effects on the expression of the neurogenic gene, NeuroD, and the motor neuron inductive gene, Gli2b. At 2.0 mM, NeuroD was down-regulated (0.45-fold, P < 0.006). With respect to the NeuroD expression pattern, phenotypic comparisons between the 0.5 and 2.0 mM exposure groups suggested that, at the higher dose, the down-regulation of NeuroD expression could have reached a threshold level so as to induce neuronal degeneration. The expression pattern of these selected neuron-specific and motor-neuron specific genes that accompanies ketamine treatment compares well with the observed phenotype where motor neurons are adversely affected. At the same time, these results also demonstrate that ketamine does not seem to have any deleterious effects on neuronal commitment, an event that occurs early in neurogenesis, since Notch1a was inhibited (a process that promotes neuronal commitment of progenitor cells) and, as would be expected of its downstream effector, Ngn1 expression was induced. Together, the phenotypic analyses and the specific gene expression studies suggest that initiation of ketamine’s deleterious effects on the motor neurons probably begins at the lower 0.5 mM dose, becoming more obvious at the higher 2.0 mM dose, a scenario that probably depends upon the real exposure (internal dose) of the embryos to ketamine.
Figure 4.
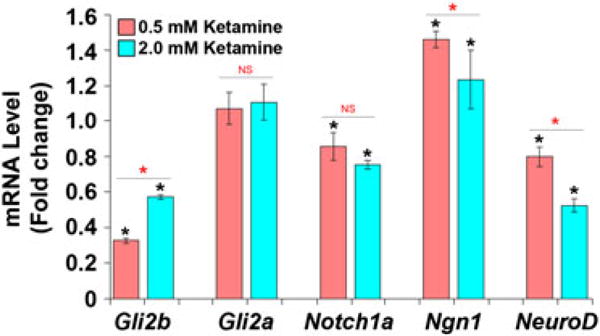
Effect of ketamine on expression of specific proneural and neurogenic genes. Ketamine induces changes in gene expression in zebrafish embryos. Expression of the proneural gene Notch1a, and the neurogenic genes Ngn1, NeuroD, Gli2a and Gli2b at the mRNA level was analyzed. Embryos at 28 hpf were treated with 0.5 and 2.0 mM ketamine. After 20 h of treatment (48 hpf actual age), total RNA was isolated from control (untreated) and the two ketamine-treated groups. Following first-strand cDNA synthesis from the RNA, qPCR was performed. The 2−ΔΔCt method was used to determine the relative gene expression. The GAPDH gene was the internal control for all qPCR experiments. The mean Ct values for GAPDH expression were 21.47 ± 0.11 (control), 21.28 ± 0.07 (0.5 mM ketamine) and 21.02 ± 0.073 (2 mM ketamine). Data from biological replicates were averaged and are shown as normalized gene expression ± SEM. For all pairwise multiple comparison procedures the Holm–Sidak method was used for data analysis with overall significance level set at P < 0.05. Lower-level ANOVAs were performed to determine further differences (between different ketamine doses).
DISCUSSION
Ketamine is a dissociative anesthetic introduced in the 1960s that produces anesthesia, analgesia, suppression of fear and anxiety, and amnesia. Most of ketamine’s effects are mediated by antagonism of NMDA receptors (Anis et al., 1983). Activation of NMDARs is essential for long-term potentiation and spatial learning and memory (Malenka and Bear, 2004), and NMDAR blockade results in impaired synaptic plasticity manifested in adverse effects on learning and memory (Sakimura et al., 1995; Shimizu et al., 2000). Conversely, enhanced NMDAR function improves memory (Tang et al., 2001). It has been shown that higher doses of ketamine can induce apoptosis in rodents (Ikonomidou et al., 1999; Lahti et al., 2001; Larsen et al., 1998; Malhotra et al., 1997; Maxwell et al., 2006; Wang et al., 2006) and primates (Haberny et al., 2002; Slikker et al., 2007; Wang et al., 2006) during the brain growth spurt (Slikker et al., 2007).
Results from the present study on the effects of ketamine on zebrafish embryos provide evidence that, after 20 h of exposure, 2.0 mM ketamine significantly reduces both cranial and spinal motor neuron populations. These data are concordant with reported data from mammalian studies (neurotoxicity induced by ketamine in rodents and monkeys). It has been shown that, in postnatal day 7 rat pups, repeated doses of 20 mg kg−1 ketamine resulted in a blood level of 14 μg ml−1 concentration and induced neuroaopotosis in the dorsolateral thalamus (Scallet et al., 2004). The blood level of 14 μg ml−1 is approximately 7-fold greater than anesthetic blood levels in humans (Malinovsky et al., 1996; Mueller and Hunt, 1998). The doses we used, 0.5 (1.37 mg ml−1 water) and 2.0 mM (5.48 mg ml−1 water) are in the range of the effective doses that induce behavioral changes in zebrafish larvae (Burgess and Granato, 2007). In the present study, the focus was specifically on motor neurons as we took advantage of the transgenic line that makes motor neurons visible in vivo. Ketamine induced motor neuron toxicity in vivo as assessed using hb9-GFP transgenic embryos. There was also a significant reduction in spinal motor axon length, indicating that either ketamine had a direct effect on the axonogenesis or the exposed neurons were producing stunted axons.
In order to gain more insight into the occurrence of such phenotypes, the expression of selected target genes that are associated with motor neuron development and axonogenesis was analyzed. Gene expression profiling in the developing rat brain exposed to ketamine has revealed the up-regulation of several NMDA receptor subunits (Shi et al., 2010). In zebrafish, ketamine treatment began at 28 hpf, when only NR1.1 and NR 2D.1 are found in brain and spinal cord. NR1.2 is expressed in the brain at 48 hpf but not in the spinal cord (Cox et al., 2005), a time point when ketamine exposure was terminated. In this initial study, these time points were chosen since the goal was to monitor ketamine’s effects in vivo and, beyond 48 hpf, excess pigmentation hinders visualization of GFP signals. Although development of zebrafish pigmentation can be inhibited by early treatment of embryos with phenylthiourea to facilitate visualization of tissues and organs and, therefore, GFP signals, phenylthiourea is a known toxic compound and was not used in these experiments. Our immediate goal was to focus on a set of specific genes, Gli2a, Gli2b, Notch1a, Ngn1 and NeuroD that are essential for motor neuron development and axonogenesis.
During neurodevelopment in vertebrates, the formation of oligodendrocytes and motor neurons depends on hedgehog (Hh), a family of secretory proteins, and Glis, the zinc finger proteins that act as mediators of Hh signaling (Ruiz i Altaba, 1999). Mice lacking sonic hedgehog (Shh) function display severe neural patterning defects, including a lack of most ventral neuronal cell types in the spinal cord (Chiang et al., 1996). It has also been suggested that Shh signaling is required to promote the timely appearance of motor neuron progenitors in the mouse developing spinal cord (Oh et al., 2009). In mouse embryos lacking Gli genes, the ventral telencephalon is highly disorganized with intermingling of different neuronal cell types (Yu et al., 2009). Gli2−/−; Gli3−/−double mutant mice have an enlarged ventral neuroepithelium resulting from enhanced cell proliferation (Bai et al., 2004). There are two Gli2 genes, Gli2a and Gli2b, in zebrafish (Ke et al., 2005) that play a role in the development of several cellular lineages, such as the ventral neural precursors, cranial motor neurons, interneurons and dorsal sensory neurons (Ke et al., 2008). Gli2a is preferentially expressed early on in the lateral mesoderm. Gli2b is also expressed early on, predominantly in the neural plate (Karlstrom et al., 2003), and its absence causes defects in the neural tube, including the reduction of mitotic neural precursors (Karlstrom et al., 2003; Ke et al., 2005). In ketamine-treated zebrafish embryos, Gli2a expression remained unaltered while that of Gli2b was down-regulated. Since Gli2b but not Gli2a is directly involved in motor neuron development, ketamine-induced motor neuron loss could be the result of such a down-regulation. In our studies, stunted axonogenesis in ketamine-treated embryos is consistent with the report that loss of Gli2b function causes abnormal axonogenesis (Ke et al., 2008). A possible mechanism of how Gli2b down-regulation could influence motor neuron toxicity is schematically presented in Fig. 5.
Figure 5.
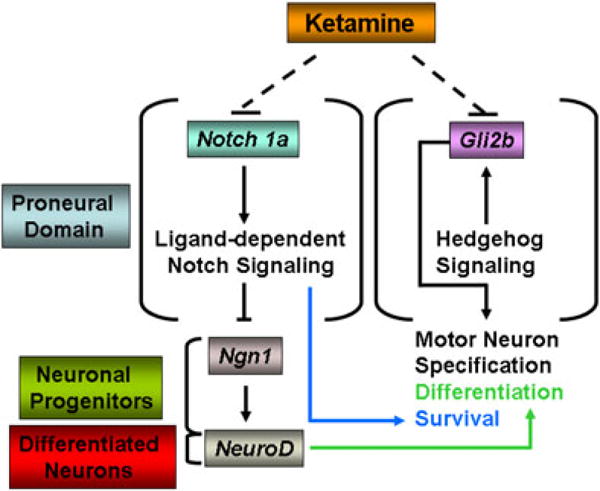
Schematic representation of pathways potentially affected by ketamine. Ketamine has toxic effects on motor neurons in the zebrafish embryos. Down-regulation of the transmembrane receptor gene, Notch1a, could negatively affect ligand-dependent Notch signaling and inhibition of Notch signaling in the proneural domain is known to up-regulate the neurogenic gene, Ngn1, in the neuronal progenitors. Ngn1 is a direct inducer of NeuroD that is critical for neuronal differentiation and NeuroD is expressed in differentiated neurons. At the same time, in differentiated neurons, sustained inhibition of Notch signaling can adversely affect neuron survival. Ketamine-induced down-regulation of Gli2b, a mediator of Hedgehog signaling, can disrupt Hedgehog signaling, thereby adversely affecting motor neuron specification,differentiation and survival. Down-regulation of NeuroD expression in spite of an up-regulation of its inducer, Ngn1, could be the consequence of fewer surviving differentiated neurons.
Inhibition of Notch signaling results in the formation of excess primary neurons whereas forced expression of constitutively active Notch blocks neuronal differentiation (Atkinson and Roy, 1995; Chitnis, 1995; Coffman et al., 1993). During neuronal differentiation, pro-neural genes, such as Ngn1, a direct downstream target of Notch inhibition, induce downstream bHLH genes, such as NeuroD, to elicit the transition from proliferative neural precursor cells to postmitotic neurons (Blader et al., 1997; Kim et al., 1997; Lee, 1997). Our results demonstrated that ketamine induced a down-regulation of Notch1a with an up-regulation of Ngn1 expression, an occurrence that favors neuronal precursor commitment. What could then be deleterious to the motor neurons may be the down-regulation of NeuroD, which is the only gene that showed a dose-dependent response to ketamine, thus explaining a scenario where only the higher dose resulted in a significant loss of motor neurons. NeuroD is a direct downstream target of Ngn1 that is expressed in differentiated neurons and is essential for terminal differentiation of neurons (Korzh et al., 1998; Mueller and Wullimann, 2002). In our studies, while Ngn1 was up-regulated, surprisingly, NeuroD was down-regulated. One possible explanation of this observation is that, since ketamine induced Notch1a down-regulation and Notch signaling is necessary for neuron survival (Breunig et al., 2007; Mason et al., 2006; Oishi et al., 2004), a sustained Notch inhibition in differentiated neurons may have triggered motor neuron degeneration, although the same Notch inhibition in the proneural domain is required for the generation of neuronal progenitors (Fig. 5). Since NeuroD is expressed in differentiated neurons but not in their progenitors, its low expression may be a consequence of fewer living neurons post ketamine treatment.
Taken together, our in vivo data suggest that ketamine is toxic to motor neuron development in zebrafish. Phenotype-specific selected gene expression studies indicate that ketamine could potentially favor neuronal commitment but negatively affect neuronal differentiation and survival.
Disclaimer
This document has been reviewed in accordance with United States Food and Drug Administration (FDA) policy and approved for publication. Approval does not signify that the contents necessarily reflect the position or opinions of the FDA, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the FDA.
Acknowledgments
This work was supported by the National Center for Toxicological Research/US Food and Drug Administration. We thank Melanie Dumas for her help in zebrafish breeding.
References
- Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;11(2):180–187. doi: 10.1016/s0959-4388(00)00194-x. [DOI] [PubMed] [Google Scholar]
- Anis NA, Berry SC, Burton NR, Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol. 1983;79(2):565–575. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel B, Eisen JS. Regulation of neuronal specification in the zebrafish spinal cord by Delta function. Development. 1998;125(3):371–380. doi: 10.1242/dev.125.3.371. [DOI] [PubMed] [Google Scholar]
- Appel B, Fritz A, Westerfield M, Grunwald DJ, Eisen JS, Riley BB. Delta-mediated specification of midline cell fates in zebrafish embryos. Curr Biol. 1999;9(5):247–256. doi: 10.1016/s0960-9822(99)80113-4. [DOI] [PubMed] [Google Scholar]
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23(4):659–674. doi: 10.1016/s0896-6273(01)80026-x. [DOI] [PubMed] [Google Scholar]
- Atkinson A, Roy D. In vivo DNA adduct formation by bisphenol A. Environ Mol Mutagen. 1995;26(1):60–66. doi: 10.1002/em.2850260109. [DOI] [PubMed] [Google Scholar]
- Bai CB, Stephen D, Joyner AL. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev Cell. 2004;6(1):103–115. doi: 10.1016/s1534-5807(03)00394-0. [DOI] [PubMed] [Google Scholar]
- Blader P, Fischer N, Gradwohl G, Guillemot F, Strahle U. The activity of neurogenin1 is controlled by local cues in the zebrafish embryo. Development. 1997;124(22):4557–4569. doi: 10.1242/dev.124.22.4557. [DOI] [PubMed] [Google Scholar]
- Blaess S, Corrales JD, Joyner AL. Sonic hedgehog regulates Gli activator and repressor functions with spatial and temporal precision in the mid/hindbrain region. Development. 2006;133(9):1799–1809. doi: 10.1242/dev.02339. [DOI] [PubMed] [Google Scholar]
- Bretaud S, Lee S, Guo S. Sensitivity of zebrafish to environmental toxins implicated in Parkinson’s disease. Neurotoxicol Teratol. 2004;26(6):857–864. doi: 10.1016/j.ntt.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Breunig JJ, Silbereis J, Vaccarino FM, Sestan N, Rakic P. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc Natl Acad Sci USA. 2007;104(51):20558–20563. doi: 10.1073/pnas.0710156104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs JP. The zebrafish: a new model organism for integrative physiology. Am J Physiol Regul Integr Comp Physiol. 2002;282(1):R3–9. doi: 10.1152/ajpregu.00589.2001. [DOI] [PubMed] [Google Scholar]
- Burgess HA, Granato M. Sensorimotor gating in larval zebrafish. J Neurosci. 2007;27(18):4984–4994. doi: 10.1523/JNEUROSCI.0615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheesman SE, Layden MJ, Von Ohlen T, Doe CQ, Eisen JS. Zebrafish and fly Nkx6 proteins have similar CNS expression patterns and regulate motoneuron formation. Development. 2004;131(21):5221–5232. doi: 10.1242/dev.01397. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383(6599):407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Chitnis AB. The role of Notch in lateral inhibition and cell fate specification. Mol Cell Neurosci. 1995;6(4):311–321. doi: 10.1006/mcne.1995.1024. [DOI] [PubMed] [Google Scholar]
- Chitnis A, Kintner C. Sensitivity of proneural genes to lateral inhibition affects the pattern of primary neurons in Xenopus embryos. Development. 1996;122(7):2295–301. doi: 10.1242/dev.122.7.2295. [DOI] [PubMed] [Google Scholar]
- Clements JA, Nimmo WS. Pharmacokinetics and analgesic effect of ketamine in man. Br J Anaesth. 1981;53(1):27–30. doi: 10.1093/bja/53.1.27. [DOI] [PubMed] [Google Scholar]
- Coffman CR, Skoglund P, Harris WA, Kintner CR. Expression of an extracellular deletion of Xotch diverts cell fate in Xenopus embryos. Cell. 1993;73(4):659–71. doi: 10.1016/0092-8674(93)90247-n. [DOI] [PubMed] [Google Scholar]
- Cox JA, Kucenas S, Voigt MM. Molecular characterization and embryonic expression of the family of N-methyl-D-aspartate receptor subunit genes in the zebrafish. Dev Dyn. 2005;234(3):756–766. doi: 10.1002/dvdy.20532. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11(3):327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- de la Pompa JL, Wakeham A, Correia KM, Samper E, Brown S, Aguilera RJ, Nakano T, Honjo T, Mak TW, Rossant J, Conlon RA. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development. 1997;124(6):1139–1148. doi: 10.1242/dev.124.6.1139. [DOI] [PubMed] [Google Scholar]
- Dornseifer P, Takke C, Campos-Ortega JA. Overexpression of a zebrafish homologue of the Drosophila neurogenic gene Delta perturbs differentiation of primary neurons and somite development. Mech Dev. 1997;63(2):159–171. doi: 10.1016/s0925-4773(97)00037-3. [DOI] [PubMed] [Google Scholar]
- Fetcho JR, Liu KS. Zebrafish as a model system for studying neuronal circuits and behavior. Ann NY Acad Sci. 1998;860:333–345. doi: 10.1111/j.1749-6632.1998.tb09060.x. [DOI] [PubMed] [Google Scholar]
- Flanagan-Steet H, Fox MA, Meyer D, Sanes JR. Neuromuscular synapses can form in vivo by incorporation of initially aneural postsynaptic specializations. Development. 2005;132(20):4471–4481. doi: 10.1242/dev.02044. [DOI] [PubMed] [Google Scholar]
- Gaiano N, Fishell G. The role of notch in promoting glial and neural stem cell fates. Annu Rev Neurosci. 2002;25:471–490. doi: 10.1146/annurev.neuro.25.030702.130823. [DOI] [PubMed] [Google Scholar]
- Gaiano N, Nye JS, Fishell G. Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron. 2000;26(2):395–404. doi: 10.1016/s0896-6273(00)81172-1. [DOI] [PubMed] [Google Scholar]
- Gerhard GS. Small laboratory fish as models for aging research. Ageing Res Rev. 2007;6(1):64–72. doi: 10.1016/j.arr.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Gerhard GS, Cheng KC. A call to fins! Zebrafish as a gerontological model. Aging Cell. 2002;1(2):104–111. doi: 10.1046/j.1474-9728.2002.00012.x. [DOI] [PubMed] [Google Scholar]
- Grant IS, Nimmo WS, Clements JA. Pharmacokinetics and analgesic effects of i.m. and oral ketamine. Br J Anaesth. 1981;53(8):805–810. doi: 10.1093/bja/53.8.805. [DOI] [PubMed] [Google Scholar]
- Haberny KA, Paule MG, Scallet AC, Sistare FD, Lester DS, Hanig JP, Slikker W., Jr Ontogeny of the N-methyl-D-aspartate (NMDA) receptor system and susceptibility to neurotoxicity. Toxicol Sci. 2002;68(1):9–17. doi: 10.1093/toxsci/68.1.9. [DOI] [PubMed] [Google Scholar]
- Haddon C, Smithers L, Schneider-Maunoury S, Coche T, Henrique D, Lewis J. Multiple delta genes and lateral inhibition in zebrafish primary neurogenesis. Development. 1998;125(3):359–370. doi: 10.1242/dev.125.3.359. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283(5398):70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Koch C, Genz K, Hoerster F, Felderhoff-Mueser U, Tenkova T, Dikranian K, Olney JW. Neurotransmitters and apoptosis in the developing brain. Biochem Pharmacol. 2001;62(4):401–405. doi: 10.1016/s0006-2952(01)00696-7. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Ang SL, Shiota K, Nakanishi S, Kageyama R, Guillemot F. Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix–loop–helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev. 1995;9(24):3136–3148. doi: 10.1101/gad.9.24.3136. [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Benshoff N, Olney JW. Ketamine potentiates cerebrocortical damage induced by the common anaesthetic agent nitrous oxide in adult rats. Br J Pharmacol. 2000;130(7):1692–1698. doi: 10.1038/sj.bjp.0703479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanungo J, Zheng YL, Amin ND, Kaur S, Ramchandran R, Pant HC. Specific inhibition of cyclin-dependent kinase 5 activity induces motor neuron development in vivo. Biochem Biophys Res Commun. 2009;386(1):263–267. doi: 10.1016/j.bbrc.2009.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlstrom RO, Tyurina OV, Kawakami A, Nishioka N, Talbot WS, Sasaki H, Schier AF. Genetic analysis of zebrafish gli1 and gli2 reveals divergent requirements for gli genes in vertebrate development. Development. 2003;130(8):1549–1564. doi: 10.1242/dev.00364. [DOI] [PubMed] [Google Scholar]
- Ke Z, Emelyanov A, Lim SE, Korzh V, Gong Z. Expression of a novel zebrafish zinc finger gene, gli2b, is affected in Hedgehog and Notch signaling related mutants during embryonic development. Dev Dyn. 2005;232(2):479–486. doi: 10.1002/dvdy.20242. [DOI] [PubMed] [Google Scholar]
- Ke Z, Kondrichin I, Gong Z, Korzh V. Combined activity of the two Gli2 genes of zebrafish play a major role in Hedgehog signaling during zebrafish neurodevelopment. Mol Cell Neurosci. 2008;37(2):388–401. doi: 10.1016/j.mcn.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Key B, Devine CA. Zebrafish as an experimental model: strategies for developmental and molecular neurobiology studies. Meth Cell Sci. 2003;25(1–2):1–6. doi: 10.1023/B:MICS.0000006849.98007.03. [DOI] [PubMed] [Google Scholar]
- Kim P, Helms AW, Johnson JE, Zimmerman K. XATH-1, a vertebrate homolog of Drosophila atonal, induces a neuronal differentiation within ectodermal progenitors. Dev Biol. 1997;187(1):1–12. doi: 10.1006/dbio.1997.8572. [DOI] [PubMed] [Google Scholar]
- Kohrs R, Durieux ME. Ketamine: teaching an old drug new tricks. Anesth Analg. 1998;87(5):1186–1193. doi: 10.1097/00000539-199811000-00039. [DOI] [PubMed] [Google Scholar]
- Korzh V, Sleptsova I, Liao J, He J, Gong Z. Expression of zebrafish bHLH genes ngn1 and nrd defines distinct stages of neural differentiation. Dev Dyn. 1998;213(1):92–104. doi: 10.1002/(SICI)1097-0177(199809)213:1<92::AID-AJA9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M, Mishina M. Molecular diversity of the NMDA receptor channel. Nature. 1992;358(6381):36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25(4):455–467. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- Larsen B, Hoff G, Wilhelm W, Buchinger H, Wanner GA, Bauer M. Effect of intravenous anesthetics on spontaneous and endotoxin-stimulated cytokine response in cultured human whole blood. Anesthesiology. 1998;89(5):1218–1227. doi: 10.1097/00000542-199811000-00023. [DOI] [PubMed] [Google Scholar]
- Lee JE. Basic helix–loop–helix genes in neural development. Curr Opin Neurobiol. 1997;7(1):13–20. doi: 10.1016/s0959-4388(97)80115-8. [DOI] [PubMed] [Google Scholar]
- Lewis J. Neurogenic genes and vertebrate neurogenesis. Curr Opin Neurobiol. 1996;6(1):3–10. doi: 10.1016/s0959-4388(96)80002-x. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, Breier A. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17(3):141–150. doi: 10.1016/S0893-133X(97)00036-5. [DOI] [PubMed] [Google Scholar]
- Malinovsky JM, Servin F, Cozian A, Lepage JY, Pinaud M. Ketamine and norketamine plasma concentrations after i.v., nasal and rectal administration in children. Br J Anaesth. 1996;77(2):203–207. doi: 10.1093/bja/77.2.203. [DOI] [PubMed] [Google Scholar]
- Mason HA, Rakowiecki SM, Gridley T, Fishell G. Loss of notch activity in the developing central nervous system leads to increased cell death. Dev Neurosci. 2006;28(1–2):49–57. doi: 10.1159/000090752. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Ehrlichman RS, Liang Y, Trief D, Kanes SJ, Karp J, Siegel SJ. Ketamine produces lasting disruptions in encoding of sensory stimuli. J Pharmacol Exp Ther. 2006;316(1):315–324. doi: 10.1124/jpet.105.091199. [DOI] [PubMed] [Google Scholar]
- Meldrum B. Protection against ischaemic neuronal damage by drugs acting on excitatory neurotransmission. Cerebrovasc Brain Metab Rev. 1990;2(1):27–57. [PubMed] [Google Scholar]
- Miklosi A, Andrew RJ. The zebrafish as a model for behavioral studies. Zebrafish. 2006;3(2):227–234. doi: 10.1089/zeb.2006.3.227. [DOI] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256(5060):1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Mueller RA, Hunt R. Antagonism of ketamine-induced anesthesia by an inhibitor of nitric oxide synthesis: a pharmacokinetic explanation. Pharmacol Biochem Behav. 1998;60(1):15–22. doi: 10.1016/s0091-3057(97)00450-4. [DOI] [PubMed] [Google Scholar]
- Mueller T, Wullimann MF. Expression domains of neuroD (nrd) in the early postembryonic zebrafish brain. Brain Res Bull. 2002;57(3–4):377–379. doi: 10.1016/s0361-9230(01)00694-3. [DOI] [PubMed] [Google Scholar]
- Ninkovic J, Bally-Cuif L. The zebrafish as a model system for assessing the reinforcing properties of drugs of abuse. Methods. 2006;39(3):262–274. doi: 10.1016/j.ymeth.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Oh S, Huang X, Liu J, Litingtung Y, Chiang C. Shh and Gli3 activities are required for timely generation of motor neuron progenitors. Dev Biol. 2009;331(2):261–269. doi: 10.1016/j.ydbio.2009.05.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Kamakura S, Isazawa Y, Yoshimatsu T, Kuida K, Nakafuku M, Masuyama N, Gotoh Y. Notch promotes survival of neural precursor cells via mechanisms distinct from those regulating neurogenesis. Dev Biol. 2004;276(1):172–184. doi: 10.1016/j.ydbio.2004.08.039. [DOI] [PubMed] [Google Scholar]
- Olney JW, Wozniak DF, Jevtovic-Todorovic V, Farber NB, Bittigau P, Ikonomidou C. Drug-induced apoptotic neurodegeneration in the developing brain. Brain Pathol. 2002;12(4):488–498. doi: 10.1111/j.1750-3639.2002.tb00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Young C, Wozniak DF, Ikonomidou C, Jevtovic-Todorovic V. Anesthesia-induced developmental neuroapoptosis. Does it happen in humans? Anesthesiology. 2004;101(2):273–275. doi: 10.1097/00000542-200408000-00004. [DOI] [PubMed] [Google Scholar]
- Park HC, Shin J, Appel B. Spatial and temporal regulation of ventral spinal cord precursor specification by Hedgehog signaling. Development. 2004;131(23):5959–5969. doi: 10.1242/dev.01456. [DOI] [PubMed] [Google Scholar]
- Parng C, Seng WL, Semino C, McGrath P. Zebrafish: a preclinical model for drug screening. Assay Drug Dev Technol. 2002;1(1 Pt 1):41–48. doi: 10.1089/154065802761001293. [DOI] [PubMed] [Google Scholar]
- Pohl D, Bittigau P, Ishimaru MJ, Stadthaus D, Hubner C, Olney JW, Turski L, Ikonomidou C. N-Methyl-D-aspartate antagonists and apoptotic cell death triggered by head trauma in developing rat brain. Proc Natl Acad Sci USA. 1999;96(5):2508–2513. doi: 10.1073/pnas.96.5.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers DA. Fish as model systems. Science. 1989;246(4928):352–358. doi: 10.1126/science.2678474. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A. Gli proteins encode context-dependent positive and negative functions: implications for development and disease. Development. 1999;126(14):3205–3216. doi: 10.1242/dev.126.14.3205. [DOI] [PubMed] [Google Scholar]
- Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, Mishina M. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature. 1995;373(6510):151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- Salas C, Broglio C, Duran E, Gomez A, Ocana FM, Jimenez-Moya F, Rodriguez F. Neuropsychology of learning and memory in teleost fish. Zebrafish. 2006;3(2):157–171. doi: 10.1089/zeb.2006.3.157. [DOI] [PubMed] [Google Scholar]
- Scallet AC, Schmued LC, Slikker W, Jr, Grunberg N, Faustino PJ, Davis H, Lester D, Pine PS, Sistare F, Hanig JP. Developmental neurotoxicity of ketamine: morphometric confirmation, exposure parameters, and multiple fluorescent labeling of apoptotic neurons. Toxicol Sci. 2004;81(2):364–370. doi: 10.1093/toxsci/kfh224. [DOI] [PubMed] [Google Scholar]
- Shi Q, Guo L, Patterson TA, Dial S, Li Q, Sadovova N, Zhang X, Hanig JP, Paule MG, Slikker W, Jr, Wang C. Gene expression profiling in the developing rat brain exposed to ketamine. Neuroscience. 2010;166(3):852–863. doi: 10.1016/j.neuroscience.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Tang YP, Rampon C, Tsien JZ. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science. 2000;290(5494):1170–1174. doi: 10.1126/science.290.5494.1170. [DOI] [PubMed] [Google Scholar]
- Shin J, Poling J, Park HC, Appel B. Notch signaling regulates neural precursor allocation and binary neuronal fate decisions in zebrafish. Development. 2007;134(10):1911–1920. doi: 10.1242/dev.001602. [DOI] [PubMed] [Google Scholar]
- Slikker W, Jr, Zou X, Hotchkiss CE, Divine RL, Sadovova N, Twaddle NC, Doerge DR, Scallet AC, Patterson TA, Hanig JP, Paule MG, Wang C. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci. 2007;98(1):145–158. doi: 10.1093/toxsci/kfm084. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, William C, Jessell TM. Specification of motor neuron identity by the MNR2 homeodomain protein. Cell. 1998;95(1):67–80. doi: 10.1016/s0092-8674(00)81783-3. [DOI] [PubMed] [Google Scholar]
- Tang YP, Wang H, Feng R, Kyin M, Tsien JZ. Differential effects of enrichment on learning and memory function in NR2B transgenic mice. Neuropharmacology. 2001;41(6):779–790. doi: 10.1016/s0028-3908(01)00122-8. [DOI] [PubMed] [Google Scholar]
- Thaler J, Harrison K, Sharma K, Lettieri K, Kehrl J, Pfaff SL. Active suppression of interneuron programs within developing motor neurons revealed by analysis of homeodomain factor HB9. Neuron. 1999;23(4):675–687. doi: 10.1016/s0896-6273(01)80027-1. [DOI] [PubMed] [Google Scholar]
- Todd KJ, Slatter CA, Ali DW. Activation of ionotropic glutamate receptors on peripheral axons of primary motoneurons mediates transmitter release at the zebrafish NMJ. J Neurophysiol. 2004;91(2):828–840. doi: 10.1152/jn.00599.2003. [DOI] [PubMed] [Google Scholar]
- Vascotto SG, Beckham Y, Kelly GM. The zebrafish’s swim to fame as an experimental model in biology. Biochem Cell Biol. 1997;75(5):479–485. [PubMed] [Google Scholar]
- Wang C, Sadovova N, Hotchkiss C, Fu X, Scallet AC, Patterson TA, Hanig J, Paule MG, Slikker W., Jr Blockade of N-methyl-D-aspartate receptors by ketamine produces loss of postnatal day 3 monkey frontal cortical neurons in culture. Toxicol Sci. 2006;91(1):192–201. doi: 10.1093/toxsci/kfj144. [DOI] [PubMed] [Google Scholar]
- William CM, Tanabe Y, Jessell TM. Regulation of motor neuron subtype identity by repressor activity of Mnx class homeodomain proteins. Development. 2003;130(8):1523–1536. doi: 10.1242/dev.00358. [DOI] [PubMed] [Google Scholar]
- Yu W, Wang Y, McDonnell K, Stephen D, Bai CB. Patterning of ventral telencephalon requires positive function of Gli transcription factors. Dev Biol. 2009;334(1):264–275. doi: 10.1016/j.ydbio.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Zakhary SM, Ayubcha D, Ansari F, Kamran K, Karim M, Leheste JR, Horowitz JM, Torres G. A behavioral and molecular analysis of ketamine in zebrafish. Synapse. 2011;65(2):160–162. doi: 10.1002/syn.20830. [DOI] [PMC free article] [PubMed] [Google Scholar]


