Abstract
Ketamine, a dissociative anesthetic, is a noncompetitive antagonist of N-methyl-D-aspartate-type glutamate receptors. In rodents and non-human primates as well as in zebrafish embryos, ketamine has been shown to be neurotoxic. In cyclic female rats, ketamine has been shown to decrease serum estradiol-17β(E2) levels. E2 plays critical roles in neurodevelopment and neuroprotection. Cytochrome p450 (CYP) aromatase catalyzes E2 synthesis from androgens. Although ketamine down-regulates a number of CYP enzymes in rodents, its effect on the CYP aromatase (CYP19) is not known. Zebrafish have been used as a model system for examining mechanisms underlying drug effects. Here, using wild-type (WT) zebrafish (Danio rerio) embryos, we demonstrate that ketamine significantly reduced E2 levels compared with the control. However, the testosterone level was elevated in ketamine-treated embryos. These results are concordant with data from mammalian studies. Ketamine also attenuated the expression of the ovary form of CYP aromatase (cyp19a1a) at the transcriptional level but not the brain form of aromatase, cyp19a1b. Exogenous E2 potently induced the expression of cyp19a1b and vtg 1, both validated biomarkers of estrogenicity and endocrine disruption, but not cyp19a1a expression. Attenuation of activated ERK/MAPK levels, reportedly responsible for reduced human cyp19 transcription, was also observed in ketamine-treated embryos. These results suggest that reduced E2 levels in ketamine-treated embryos may have resulted from the suppression of cyp19a1a transcription. Published 2013. This article is a U.S. Government work and is in the public domain in the USA.
Keywords: ketamine, zebrafish, estradiol-17β, CYP aromatase, gene expression, testosterone
Introduction
Ketamine [2-(2-chlorophenyl)-2-(methylamino)-cyclohexanone], a pediatric anesthetic, is thought to act primarily through blockade of N-methyl-D-aspartate (NMDA)-type glutamate receptors to provide analgesia/anesthesia to children for painful procedures (Kohrs and Durieux, 1998). Several previous reports have illustrated that ketamine can induce neuronal apoptosis when administered in high doses and/or for prolonged durations during susceptible periods of development in rodents (Lahti et al., 2001; Larsen et al., 1998; Malhotra et al., 1997; Maxwell et al., 2006) and primates (Haberny et al., 2002; Slikker et al., 2007; Wang et al., 2006) and these effects can manifest as later disruptions in cognitive function (Paule et al., 2011). We have previously reported that ketamine induces motor neuron toxicity in zebrafish embryos (Kanungo et al., 2013).
Estrogens are known to play key roles in a wide range of physiological functions, including immune responses, the central nervous system and normal somatic cell growth (Filby and Tyler 2005; Gustafsson 2003). Among the anesthetics, ketamine, but not pentobarbital, has been shown to reduce serum concentrations of estradiol-17β (E2) in adult cyclic female rats (Lee et al., 2000) although any direct action of anesthetics on the ovary is not known. Ketamine does not affect ovulation and serum levels of FSH (follicle-stimulating hormone) and LH (luteinizing hormone) in cyclic rats (Clarke and Doughton, 1983). E2 synthesis from androgens in gonadal and extragonadal tissues is carried out by cytochrome p450 (CYP) aromatase encoded by the gene cyp19 (Meinhardt and Mullis, 2002). CYPs are a diverse superfamily of the heme-containing monooxygenase enzymes (Nebert and Dalton 2006; Nebert et al., 1991) involved in the metabolism and bioactivation of both endogenous and exogenous chemicals (Nebert et al., 1991; Zhang and Yang, 2009).
Zebrafish have a total of 94 CYP genes, distributed among 18 gene families found also in mammals (Goldstone et al., 2010). While expressions of CYPs are transcriptionally regulated by a variety of xenobiotics (Gotoh, 2012), it has been shown that ketamine suppresses CYP3A4 gene expression in mammals (Chang et al., 2009; Chen and Chen, 2010) that could result in endoplasmic reticulum stress, heat shock and apoptotic response (Acharya et al., 2009). However, ketamine’s effect on CYP19 aromatase is not known.
With its small size, prolific reproductive capacity and easy maintenance, zebrafish maintain the typical complexity of vertebrate systems and accumulating evidence advocates its use in several areas of research with the prospect of extrapolating findings to other vertebrates and humans (Briggs 2002; Parng et al., 2002; Powers 1989; Vascotto et al., 1997). Exposure to environmental estrogens in oviparous organisms, such as zebrafish, induces a molecular response that is mediated by the estrogen receptor (ER) where binding leads to induction of more ER, increased production of estrogen and subsequent synthesis of the egg yolk precursor protein vitellogenin (vtg) in the liver (Jobling et al., 2006). Induction of vtg is a widely accepted biomarker of exposure to estrogenic compounds (Sumpter and Jobling, 1995). Cyp19a1b is the brain form of aromatase in zebrafish (Chiang et al., 2001; Tchoudakova et al., 2001) and is highly responsive to estrogens (Menuet et al., 2005). Both the ER and aromatases expressed early in development in zebrafish and xenobiotic response elements have been shown to exist in the promoter region of cyp19a1b, thus, providing another mechanistic link between these systems (Kazeto et al., 2004). Data obtained from studies using a transgenic cyp19a1b-GFP zebrafish line indicate that cyp19a1b expression increases between 24 and 48 h post fertilization (hpf) whereas the expression is blocked in embryos exposed to anti-estrogens (Mouriec et al., 2009). As the aromatase is highly expressed in radial glial cells that are progenitors for generating neurons throughout the zebrafish life cycle (Adolf et al., 2006; Pellegrini et al., 2005), estrogens are now strongly implicated in the process of embryonic, adult or reparative neurogenesis (Barha et al., 2009; De Nicola et al., 2009; Galea 2008; Garcia-Segura et al., 2001; Martinez-Cerdeno et al., 2006).
Previously, we have shown that ketamine induces developmental neurotoxicity in zebrafish embryos (Kanungo et al., 2013). The goal of the present study was to evaluate whether in these embryos, ketamine modulated steroid hormone levels and expression of aromatase genes, cyp19a1a and cyp19a1b.
Materials and Methods
Materials
Ketamine (ketamine HCL) and E2 were purchased from Phoenix (St. Joseph, MO, USA) and Sigma (St. Louis, MO, USA), respectively. All high-performance liquid chromatography (HPLC) reagents, unless otherwise indicated, were obtained from Sigma (St. Louis, MO, USA).
Animals
Adult wild-type (WT) zebrafish (Danio rerio, AB strain) were obtained from the Zebrafish International Resource Center at the University of Oregon (Eugene, OR, USA). The fish were kept in fish tanks (Aquatic Habitats) at the NCTR/FDA zebrafish facility containing buffered water (pH 7.5) at 28 °C, and were fed daily live brine shrimp and Zeigler dried flake food (Zeiglers, Gardeners, PA, USA). Each 3-l tank housed 8 adult males or 8 females. Handling and maintenance of zebrafish were in compliance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the NCTR/FDA IACUC. The day–night cycle was maintained at 14:10 h, and spawning and fertilization were stimulated by the onset of light at 08.30 hours. For in-system breeding, crosses of males and females were set up the previous day with partitions that were taken off the following morning at the time of light onset at 08.30 hours. Fertilized zebrafish embryos were collected from the bottom of the tank as soon as they were laid. The eggs/embryos were placed in Petri dishes and washed thoroughly with buffered egg water [reverse osmosis water containing 60 mg of sea salt (Crystal Sea®, Aquatic Eco-systems, Inc., Apopka, FL, USA) per liter of water (pH 7.5)] and then allowed to develop in an incubator at 28.5 °C for further experiments.
Treatment of Zebrafish Embryos with Ketamine and E2
Embryos were developmentally staged according to methods described previously (Kimmel et al., 1995). For ketamine exposure, ketamine (2.0 mM) was added directly to the embryo medium. E2 was used at a 1-nM concentration. Exposure was for 24 h in 6-well cell culture plates (20 embryos per well).
Determination of Ketamine Levels in Zebrafish Embryos Using Reverse Phase HPLC
The embryos were collected in microcentrifuge tubes (1.5 ml), centrifuged in a microcentrifuge (1700 × g) and the aqueous layer was discarded and replaced with fresh deionized water (0.5 ml) three additional times. After the final centrifugation, the embryo samples were alkalinized with 0.35 ml of 0.2 mol l−1 borate buffer and sonicated at 30% intensity for 15 s and then extracted with dichloromethane: ethyl acetate (80:20 v/v) by mixing (75 × g, 10 min). The samples were centrifuged (1500 g, 3 min) and the organic layer was transferred to a borate test, and the samples were extracted again using dichloromethane: ethyl acetate (80:20 v/v). The combined organic layer was back-extracted with perchloric acid (2 N), and the organic layer was discarded. The acidic aqueous layer was evaporated to dryness at 45 °C. The dried residue was reconstituted in 0.1 ml of mobile phase and 50 μl was injected into the column, and the amount of ketamine was determined by an HPLC method described below.
The amount of ketamine was determined by a rapid HPLC method on a Waters 2695 separations module coupled to a Waters 2996 PDA (Milford, MA, USA). Briefly, the isocratic HPLC separation of ketamine was achieved on a SB-C18 Zorbax column (150 × 4.6 mm, 5 μm) (Agilent, Santa Clara, CA, USA) with a simple mobile phase consisting of acetonitrile: 0.03 mol l−1 phosphate buffer (23:77% v/v) adjusted to pH 7.2 at a flow rate of 1.5 ml min−1. The effluents were monitored at 210 nm and quantified using the area under the peak from standard solutions dissolved in deionized water (0.75–200 μmol l−1). The accumulated amount of ketamine per embryo was empirically derived by determining the fraction of the dose absorbed in the embryo from triplicate samples. The data are expressed as means (amount per embryo) ± SD.
Determination of E2 Levels in Zebrafish Embryos by Reverse Phase HPLC
The embryos were collected in microcentrifuge tubes (1.5 ml), centrifuged in a microcentrifuge (2000 rpm) and the aqueous layer was discarded and replaced with fresh deionized water (0.5 ml) three additional times. After the final centrifugation, the embryo samples were alkalinized with 0.35 ml of perchloric acid (0.2 N) and sonicated at 30% intensity for 15 s and then centrifuged (14000 g, 20 min) and the aqueous layer was transferred to a borate test tube and evaporated to dryness at 45 °C. The dried residue was reconstituted in 0.1 ml of mobile phase and 50 μl was injected into the column, and the amount of E2 was determined by an HPLC method described below.
The amount of E2 was determined using a rapid HPLC method on a Waters 2695 separations module coupled to a Waters 2996 PDA (Milford, MA, USA). Briefly, the isocratic HPLC separation of E2 was achieved on a SB-C18 Zorbax column (150 × 4.6 mm, 5 um) (Agilent) with a simple mobile phase consisting of acetonitrile: water (35:65% v/v) at a flow rate of 1.5 ml min−1. The effluents were monitored at 215 nm and quantified using the area under the peak from standard solutions dissolved in mobile phase (0.15 to 50 μmol l−1). The data are expressed as mean (amount/embryo) ± SD from triplicate samples.
RNA Extraction and cDNA Synthesis
Total RNA (from 50 pooled embryos/treatment group) was extracted from whole embryos using the Trizol reagent (Invitrogen, Carlsbad, CA, USA). An aliquot of each RNA sample was used to spectrophotometrically (using a NanoDrop ND-1000; NanoDrop Technology, Wilmington, DE, USA) determine RNA quality (A260/A280 > 2.0) and concentration. First-strand cDNA was synthesized from total RNA (1 μg; 20 μl final reaction volume) with oligo(dT) priming using SuperScript II reverse transcriptase (Invitrogen) according to the manufacturer’s instructions.
Primers
Zebrafish gene-specific primers (Table 1) were used for the quantitative real-time polymerase chain reaction (RT-qPCR) assays to quantify GAPDH, cyp19a1a (GenBank entry no. AF226620), cyp19a1b (GenBank entry no. AF226619) and vtg1 (GenBank entry no. AF406784.1).
Table 1.
List of primers used in quantitative real-time polymerase chain reaction (RT-qPCR) assays
| Gene | Forward primer | Reverse primer |
|---|---|---|
| cyp19a1a | 5′-TCTGCTTCAGAAGATTCATAAATACTTT-3′ | 5′-CCTGCAACTCCTGAGCATCTC-3′ |
| cyp19a1b | 5′-AAAGAGTTACTAATAAAGATCCACCGGTAT-3′ | 5′-TCCACAAGCTTTCCCATTTCA-3′ |
| vtg 1 | 5′-CTGCGTGAAGTTGTCATGCT-3′ | 5′-GACCAGCATTGCCCATAACT-3′ |
| GAPDH | 5′-GATACACGGAGCACCAGGTT-3′ | 5′- GCCATCAGGTCACATACACG-3′ |
RT-qPCR
Real-time qPCR was performed using a CFX96 C1000 (Bio-Rad, Hercules, CA, USA) detection system with SYBR green fluorescent label (Bio-Rad). Samples (25 μl final vol) contained the following: 1× SYBR green master mix (Bio-Rad), 5 pmol of each primer and 0.25 μl of the reverse transcriptase (RT) reaction mixture. Samples were run in triplicate in optically clear 96-well plates. Cycling parameters were as follows: 50 C × 2 min, 95 °C 10 min, then 40 cycles of 95 °C × 15 s, 60 °C × 1 min. A melting temperature-determining dissociation step was performed at 95 C × 15 s, 60 °C × 15 s and 95 °C × 15 s at the end of the amplification phase. The 2 −ΔΔCt method was used to determine the relative gene expression (Livak and Schmittgen, 2001). The GAPDH gene was the internal control for all qPCR experiments. Data from each group (n = 3) were averaged and shown as normalized gene expression ±SD. One-way ANOVA and Holm–Sidak pair-wise multiple comparison post-hocs (Sigma Stat 3.1 for analysis) were used to determine statistical significance with P < 0.05.
Sample Preparation for ELISA to Determine Testosterone and E2 Levels in Embryos
The embryos (125 per sample in triplicate) at 48 hpf were treated either with or without ketamine (2 mM) for 24 h. Post-treatment, embryos were collected in microcentrifuge tubes (1.5 ml). The embryos were suspended in deionized water (0.5 ml), sonicated (30% intensity for 30 s) and then centrifuged (14000 g, 20 min). The aqueous layer was transferred to a glass vial and dichloromethane (2 ml) was added followed by thorough mixing. The mixture was incubated on a shaker for 2 h. After incubation, the samples were centrifuged (1000 g, 30 s.), and the bottom organic layers was transferred to a clean test tube and evaporated to dryness at 45 °C overnight. The dried residue was reconstituted in 0.4 ml of EIA buffer supplied with the ELISA kits, and the amount of E2 or testosterone was determined by ELISA methods as described in the manufacturer’s protocol (Cayman Chemical Company, Ann Arbor, MI, USA). The data are presented as the amount of compound of interest per mg protein as determined by the Pierce BCA protein assay.
Electrophoresis and Western Blot Analysis
Embryos were prepared for immunoblotting (Western blot) through sonication (one embryo per sample) in Eppendorf tubes in loading buffer [0.125 M Tris–HCl, pH 6.8; 4% sodium dodecyl sulfate (SDS); 20% glycerol; 0.2 M dithiothreitol (DTT); 0.02% bromophenol blue in distilled water) along with an additional 0.1 mM DTT and 1 mM phenylmethylsulfonyl fluoride (protease inhibitor)]. Protein samples (one embryo equivalent/lane) were run in 4–20% gradient SDS-polyacrylamide gels purchased from Bio-Rad (Hercules, CA, USA) at 30–32 mA for protein separation. Proteins were then transferred onto a nitrocellulose membrane in transfer buffer (20% methanol, 25 mM Tris, 192 mM glycine, 0.1% SDS in distilled water). Transfer membranes were blocked with 2% bovine serum albumin (BSA) in Tris-borate saline with 0.5% Tween 20 (blocking buffer) and probed with phospho-MAPK specific antimouse IgG diluted in blocking buffer (1:2500; Sigma-Aldrich, St. Louis, MO, USA). After development for chemiluminescent signals, the same membrane was stripped from the bound antibodies using the stripping buffer (Thermo Scientific, Rockford, IL, USA) and reprobed with the total ERK1 anti-rabbit IgG (1:500; Santa Cruz Biotech., Santa Cruz, CA, USA). Advanced ECL Western Blotting Substrate (GE Healthcare, Piscataway, NJ, USA) was used to detect HRP-conjugate bound secondary antibodies. For quantification, densitometry of the immunoblot signals was performed using ImageJ (National Institutes of Health, Bethesda, MD).
Results
Time Course of Changes in Ketamine Accumulation in Zebrafish Embryos
Based on the reports that ketamine decreases plasma levels of E2 in rodents, and our previous studies showing ketamine as an inducer of neurotoxicity in zebrafish embryos (Kanungo et al., 2013), we tested whether similar effects of ketamine in reducing E2 also manifest in zebrafish embryos, a suitable vertebrate animal model for drug screening and drug safety assessments. To this end, WT zebrafish embryos at 48 hpf were treated with 2 mM ketamine for up to 24 h (static exposure), the dose and duration selected on the basis of our previous report on ketamine-induced neurotoxicity (Kanungo et al., 2013). Internal ketamine concentrations in these embryos were determined by reverse-phase HPLC and were well within the limits of quantification at 2, 4 and 20 h of static exposures. At a ketamine dose 2 mM, the amount of ketamine absorbed by the embryos was determined to be ~7.27 μM per embryo (Fig. 1). Differences in the amount of ketamine absorbed by the embryos after 2, 4 and 20 h were minimal and insignificant, indicating that the internal concentrations of ketamine reached steady-state conditions in the embryos within 2 h of exposure.
Figure 1.
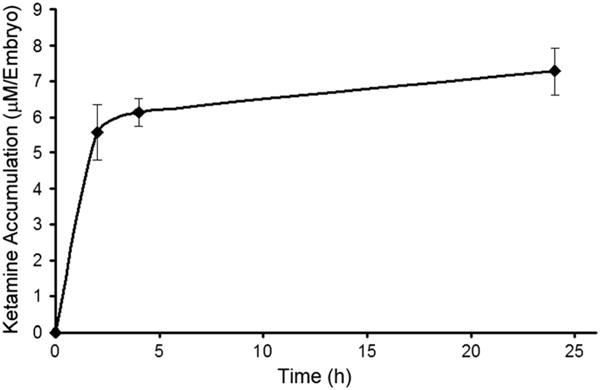
Ketamine accumulation (internal ketamine concentrations) in zebrafish embryos after static exposure to 2 mM ketamine. Embryos at 48 h post fertilization (hpf) were exposed for 2, 4 and 24 h. Post exposure, embryos were washed extensively with quick changes of embryo water three times. Reverse-phase high-performance liquid chromatography (HPLC) was employed to measure ketamine concentrations in the embryos (n = 15 per group). Data from three separate estimations were averaged and are presented as the mean ± SD.
Reduced E2 and Elevated Testosterone Levels in Ketamine-Treated Embryos
Ketamine-treated embryos were processed to determine the internal levels of E2 using reverse-phase HPLC and the observed amounts fell well within the limits of quantification. The results showed a significant reduction in E2 levels (approximately four-fold) in ketamine-treated embryos compared with untreated controls (Fig. 2). The four-fold decrease in E2 suggested that ketamine may have interfered with E2 synthesis that is mediated by cyp19 aromatase, otherwise known as estrogen synthase. It is important to note that without sex chromosomes, zebrafish early life stages up to 21 days post-fertilization (dpf) develop as females and sex differentiation begins at 21 dpf.
Figure 2.
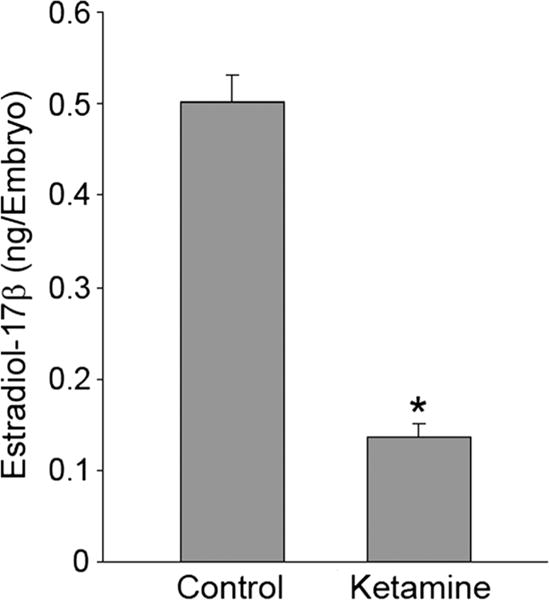
Effect of ketamine on E2 levels in zebrafish embryos. Embryos at 48 h post fertilization (hpf) were exposed to 2 mM ketamine for 24 h (static exposure). Post exposure, embryos were washed extensively with quick changes of embryo water three times. Reverse-phase high-performance liquid chromatography (HPLC) was employed to measure estradiol-17β (E2) levels in the embryos (n = 50 per group). Data from three separate experiments were averaged and are presented as mean ± SD. Student’s t test was used to determine significance P < 0.05 (*).
Further analysis using ELISA demonstrated a significant reduction in E2 levels but elevated testosterone levels in ketamine-treated embryos compared with the controls (Fig. 3).
Figure 3.
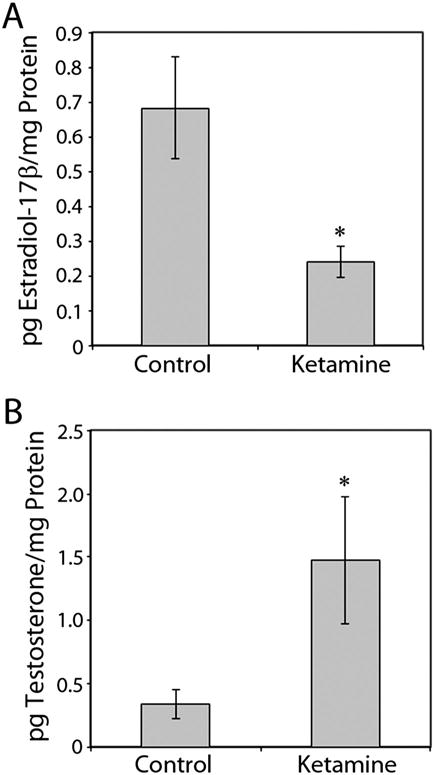
Determination of the effects of ketamine on E2 (estradiol-17β) and testosterone levels in zebrafish embryos. Embryos (n = 125 per sample) at 48 h post fertilization (hpf) were treated either with or without ketamine (2 mM) for 24 h. Estimations of E2 (A) and testosterone (B) levels were performed by ELISA as described in the Materials and Methods. Data from three separate experiments were averaged and are presented as the mean ± SD. Student’s t test was used to determine significance P < 0.05 (*).
Effects of E2 on the Expression of Estrogen-Responsive and Non-Responsive Genes
In order to test whether ketamine also altered the expression of cyp19 aromatases, we monitored the expression of both cyp19a1a and cyp19a1b aromatase genes along with their responsiveness to exogenous E2. First, as well-established controls, after RT-qPCR analyzes of cDNA derived from RNA isolated from 48 hpf embryos treated with E2 for 24 h (actual age of the embryo being 72 hpf during analysis), expression of both the aromatases and vtg 1, the gene encoding the estrogen-responsive, liver-synthesized protein vitellogenin, were quantified. The brain form of aromatase, cyp19a1b, and vtg 1 were significantly induced by 1 nM E2, the lowest dose that reportedly evokes a response by estrogen-inducible genes. These genes serve as biomarkers of estrogenicity or endocrine disruption. However, E2 did not alter cyp19a1a expression (Fig. 4A) as it is known to be recalcitrant to E2 induction (Cheshenko et al., 2007; McElroy et al., 2012).
Figure 4.
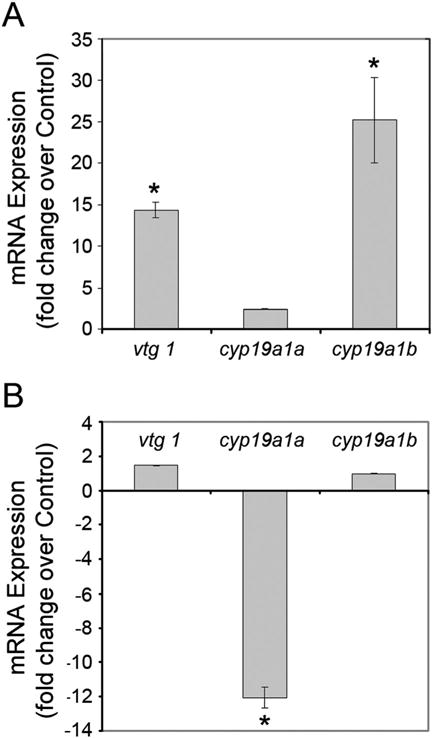
Effects of E2 (A) and ketamine (B) on the expression of vtg 1, cyp19a1a and cyp19a1b genes in zebrafish embryos. Embryos at 48 h post fertilization (hpf) were treated with either 1 nM estradiol-17β (E2) or 2 mM ketamine for 24 h (static exposure). Total RNA was isolated from the embryos. After first strand cDNA synthesis from the RNA, quantitative real-time polymerase chain reaction (RT-qPCR) was performed. The 2-ΔΔCT method was used to determine relative gene expression. The GAPDH gene was the internal control for all RT-qPCR experiments. Data were averaged and shown as normalized (fold change over control) gene expression ± SD. One-way ANOVA and Holm Sidak pair-wise multiple comparison post-hocs (Sigma Stat 3.1 for analysis) were used to determine statistical significance with P < 0.05 (*).
Ketamine’s Effect on the Expression of CYP19 Aromatase Genes
The next step was to ascertain whether ketamine has any effect on the aromatase and vtg 1 gene expression, embryos at 48 hpf were treated with 2 mM ketamine for 24 h (actual age at the time of analysis being 72 hpf). RT-qPCR analyzes showed that compared with the control, there was no significant difference in the expression of vtg 1 or cyp19a1b transcripts (Fig. 4B). However, there was a significant reduction (~ 12-fold) in cyp19a1a expression. These results demonstrated that ketamine does not possess estrogenic activity. While the expression of brain aromatase, cyp19a1b, remained unaltered, suppression of the expression of ovary aromatase, cyp19a1a, by ketamine suggests that the significant reduction in E2 levels in the embryos could be the consequence of reduced expression of the latter, although both the aromatases are capable of mediating E2 synthesis from androgens.
Involvement of ERK/MAPK signaling in inducing cyp19 transcription in a human cell line (MCF-7) have been reported (Catalano et al., 2003; Ye et al., 2009) and our previous study has shown that ketamine attenuates activated (phosphorylated) ERK/MAPK (p-ERK) levels in 28 hpf zebrafish embryos treated for 20 h with 2 mM ketamine (Kanungo et al., 2012). Here, we analyzed the ERK/MAPK levels in the 48 hpf embryos treated for 24 h with 2 mM ketamine and the results showed a significant reduction in p-ERK levels although E2 did not induce any significant change (Fig. 5). These results suggested that similar to human MCF-7 cells, ERK/MAPK signaling may be involved in cyp19a1a transcription.
Figure 5.
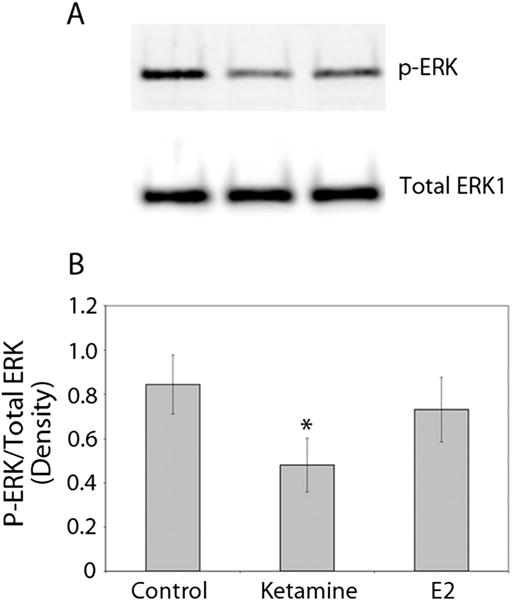
Effect of ketamine and estradiol-17β (E2) on activated ERK (phosphorylated ERK or p-ERK) levels in zebrafish embryos. Ketamine (2 mM) or E2 (1 nM) treatment of 48 h post fertilization (hpf) embryos was for 24 h (static exposure). (A) Western blot analysis conducted on whole-embryo lysates (one embryo/lane) shows activated ERK (p-ERK) and total ERK levels (on the same blot used after stripping). (B) Densitometric analysis (presented as the ratio of the densities of p-ERK to total ERK) of the Western blots. Data from three separate experiments were averaged and shown as the mean ±SD. Student’s t test was used to compare the densitometric values of the P-ERK/Total ERK of the control and the respective treated groups. *Significance was set at P < 0.05.
Discussion
Anesthetics have complex neuroendocrine effects and ketamine is known to block spontaneous GnRH (gonadotropin-releasing horomone) release and to decrease peripheral LH levels in adult rats (Cohen et al., 1983; Emanuele et al., 1987; Matzen et al., 1987; Sherwood et al., 1980). However, in male Sprague–Dawley rats, ketamine treatment increased testosterone concentrations, but decreased LHRH (luteinizing hormone-releasing hormone) after 1 of administration and continued to significantly decrease after 24 h (Gould, 2008). In the present study, an effort to investigate whether ketamine influenced E2 concentration and CYP aromatase expression in zebrafish embryos was based on the reports that ketamine regulates a number of CYPs in mammals (Chen and Chen, 2010) and alters the serum concentrations of several hormones, such as progesterone, testosterone and E2 in cyclic rats (Lee et al., 2000). Estrogens are fundamental in the growth and development of the ovary in females (Richards et al., 1976), and spermatogenesis in males (Mahato et al., 2001; O’Donnell et al., 2001). In addition, estrogens are known to play key roles in the central nervous system, and normal somatic cell growth (Filby and Tyler, 2005; Gustafsson, 2003). It has been suggested that differences in estrogen levels in zebrafish embryos and larvae occur from an early developmental stage (Lee et al., 2012). Our previous studies illustrated that 2 mM ketamine had an adverse effect on the central nervous system (Kanungo et al., 2013). The present study shows the ketamine concentration to be ~ 7.27 μM per embryo after exposure to 2 mM ketamine for 24 h. The data are comparable with 10 μM ketamine inducing neuronal damage in primary neurons from rat forebrain in culture (Liu et al., 2012). Additionally, our current data demonstrate that 48 hpf embryos with such low internal ketamine concentrations had significantly lower levels of E2 compared with the control, an effect concordant with that in mammals in which a single dose of ketamine reduces E2 levels (Lee et al., 2000).
Ketamine-treated embryos showed an increase in testosterone level, which is in agreement with data reported in male boars (Estienne and Barb 2002), male collared peccary (Tayassu tajacu) (Hellgren et al., 1985) and female Norway rats (Gould, 2008). In contrast, ketamine has been shown to cause a reduction in plasma testosterone levels in men (Oyama et al., 1977), domestic tom cats (Johnstone and Bancroft, 1988) and cyclic female Sprague–Dawley rats (Lee et al., 2000). In humans, although there have been some reports of decreased testosterone levels after general anesthesia, possibly owing to surgical trauma, more often an increase in the testosterone level occurred with ketamine anesthesia 1 h after surgery (Cartensen et al., 1973). A lack of any significant change in testosterone levels in ketamine-treated male rhesus monkeys (Puri et al., 1981; Zaidi et al., 1982) and male cynomolgus (Malaivijitnond et al., 1998) has also been reported. These differences may be attributed to variations in the dose and duration of ketamine treatment as well as sex and age of the subjects.
CYP19 aromatase is a crucial steroidogenic enzyme that catalyzes the final, rate-limiting step in the conversion of androgens into estrogens (Simpson et al., 1994). In mammals, with the exception of pigs, there is a single CYP19 gene which is expressed in a variety of tissues (Simpson et al., 2002). However, owing to genome duplication during evolution, teleosts including zebrafish contain a pair of aromatase genes: cyp19a1a and cyp19a1b (Cheshenko et al., 2008). Changes in estradiol levels during sexual differentiation are directly correlated with the changes in aromatase transcript expression in the gonads of Japanese flounders (Kitano et al., 1999). It has been reported that in zebrafish larvae exposed to E2 between 17 and 20 dpf, a time just prior to the onset of sex differentiation, there was no significant effect on cyp19a1a expression (Callard et al., 2001). In contrast, short-term exposure of zebrafish larvae to E2 strongly up-regulated cyp19a1b expression (Cheshenko et al., 2007; McElroy et al., 2012) supporting similar reports on the response of zebrafish embryos and larvae exposed to E2 (Kishida and Callard, 2001). Therefore, cyp19a1b has proven to be a promising marker of estrogenic compounds in zebrafish early life stages (Menuet et al., 2005). In contrast to the dramatic alteration of cyp19a1b expression, E2 has no effect on cyp19a1a expression in zebrafish larvae (Kazeto et al., 2004; Trant et al., 2001). Consistent with these reports, our data show 1 nM E2 significantly inducing cyp19a1b but not cyp19a1a expression in 48-hpf embryos.
Decades ago, in rodents, ketamine pretreatment was first reported to result in a two-fold increase in metabolism in vitro in hepatic microsomes suggesting a self-induction of ketamine metabolism (Marietta et al., 1976) and implicating ketamine as an inducer of CYP enzymes. However, later studies in rodents showed that ketamine decreased CYP (CYP1A, CYP2A, CYP2B, CYP2C, CYP2D1 and CYP3A) activities (Loch et al., 1995; Lupp et al., 2003; Meneguz et al., 1999). In human hepatoma HepG2 cells, ketamine suppresses CYP3A4 mRNA synthesis (Chang et al., 2009). Moreover, ketamine-induced mitochondrial dysfunction and inhibition of calcium mobilization has been linked to suppression of CYP3A4 (Chang et al., 2009). However, the exact molecular mechanism of ketamine-induced suppression of CYP gene expressions remains obscure. In our previous report, we have shown that ketamine-induced ERK/MAPK down regulation and an attenuated cardiac rate (Kanungo et al., 2012). Suppression of cyp19 aromatase gene transcription in a human breast cancer cell line (MCF-7) has been shown to occur when ERK/MAPK activation is prevented (Catalano et al., 2003; Ye et al., 2009). Although our results are consistent in showing that ketamine caused attenuation of activated ERK/MAPK (p-ERK), whether ketamine’s suppressive effect on cyp19a1a expression and concomitant reduction of E2 in zebrafish embryos are direct consequences of the modulated ERK/MAPK signaling pathway remain to be elucidated.
Aromatase knockout (ArKO) female mice are infertile as their reproductive organs do not develop properly (Simpson, 2004). These mice show impaired functionality in the amygdala and hypothalamus (Pierman et al., 2008) and increased apoptosis in the frontal cortex (Hill et al., 2008). Various studies on ArKO mice have implicated estrogen in neurodevelopment (Balthazart et al., 1991; Sasahara et al., 2007). Whether ketamine-induced down-regulation of the ovary form of aromatase (cyp19a1a) adversely affects the nervous system in zebrafish and is linked to ketamine-induced neurotoxicity (Kanungo et al., 2013) need to be examined.
In conclusion, our data show that ketamine reduces E2 levels while elevating testosterone levels in zebrafish embryos. The single static exposure, however, did not alter estrogen-responsive gene (vtg 1 and cyp19a1b) transcription. Down-regulation of the ovary form of aromatase cyp19a1a further suggested that the reduced E2 levels in ketamine-treated embryos could result from the inhibition of its synthesis from androgens. Finally, our data showing ketamine’s effects on E2 and testosterone levels in zebrafish embryos are concordant with data from mammals. These findings further emphasize the importance of cross-species comparisons to delineate molecular mode of action of drugs.
Acknowledgments
This work was supported by the National Center for Toxicological Research (NCTR)/U.S. Food and Drug Administration (FDA). We thank Melanie Dumas for zebrafish breeding.
Footnotes
Disclaimer
This document has been reviewed in accordance with United States Food and Drug Administration (FDA) policy and approved for publication. Approval does not signify that the contents necessarily reflect the position or opinions of the FDA nor does mention of trade names or commercial products constitute endorsement or recommendation for use. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the FDA.
References
- Acharya P, Engel JC, Correia MA. Hepatic CYP3A suppression by high concentrations of proteasomal inhibitors: a consequence of endoplasmic reticulum (ER) stress induction, activation of RNA-dependent protein kinase-like ER-bound eukaryotic initiation factor 2alpha (eIF2alpha)-kinase (PERK) and general control nonderepressible-2 eIF2alpha kinase (GCN2), and global translational shutoff. Mol Pharmacol. 2009;76:503–515. doi: 10.1124/mol.109.056002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Adolf B, Chapouton P, Lam CS, Topp S, Tannhauser B, Strahle U, Gotz M, Bally-Cuif L. Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev Biol. 2006;295:278–293. doi: 10.1016/j.ydbio.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Foidart A, Surlemont C, Harada N. Neuroanatomical specificity in the co-localization of aromatase and estrogen receptors. J Neurobiol. 1991;22:143–157. doi: 10.1002/neu.480220205. [DOI] [PubMed] [Google Scholar]
- Barha CK, Lieblich SE, Galea LA. Different forms of oestrogen rapidly upregulate cell proliferation in the dentate gyrus of adult female rats. J Neuroendocrinol. 2009;21:155–166. doi: 10.1111/j.1365-2826.2008.01809.x. [DOI] [PubMed] [Google Scholar]
- Briggs JP. The zebrafish: a new model organism for integrative physiology. Am J Physiol Regul Integr Comp Physiol. 2002;282:R3–9. doi: 10.1152/ajpregu.00589.2001. [DOI] [PubMed] [Google Scholar]
- Callard GV, Tchoudakova AV, Kishida M, Wood E. Differential tissue distribution, developmental programming, estrogen regulation and promoter characteristics of cyp19 genes in teleost fish. J Steroid Biochem Mol Biol. 2001;79:305–314. doi: 10.1016/s0960-0760(01)00147-9. [DOI] [PubMed] [Google Scholar]
- Catalano S, Marsico S, Giordano C, Mauro L, Rizza P, Panno ML, Ando S. Leptin enhances, via AP-1, expression of aromatase in the MCF-7 cell line. J Biol Chem. 2003;278:28668–28676. doi: 10.1074/jbc.M301695200. [DOI] [PubMed] [Google Scholar]
- Chang HC, Chen TL, Chen RM. Cytoskeleton interruption in human hepatoma HepG2 cells induced by ketamine occurs possibly through suppression of calcium mobilization and mitochondrial function. Drug Metab Dispos. 2009;37:24–31. doi: 10.1124/dmd.108.023325. [DOI] [PubMed] [Google Scholar]
- Chen JT, Chen RM. Mechanisms of ketamine-involved regulation of cytochrome P450 gene expression. Expert Opin Drug Metab Toxicol. 2010;6:273–281. doi: 10.1517/17425250903505108. [DOI] [PubMed] [Google Scholar]
- Cheshenko K, Pakdel F, Segner H, Kah O, Eggen RI. Interference of endocrine disrupting chemicals with aromatase CYP19 expression or activity, and consequences for reproduction of teleost fish. Gen Comp Endocrinol. 2008;155:31–62. doi: 10.1016/j.ygcen.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Cheshenko K, Brion F, Le Page Y, Hinfray N, Pakdel F, Kah O, Segner H, Eggen RI. Expression of zebra fish aromatase cyp19a and cyp19b genes in response to the ligands of estrogen receptor and aryl hydrocarbon receptor. Toxicol Sci. 2007;96:255–267. doi: 10.1093/toxsci/kfm003. [DOI] [PubMed] [Google Scholar]
- Chiang EF, Yan YL, Guiguen Y, Postlethwait J, Chung B. Two Cyp19 (P450 aromatase) genes on duplicated zebrafish chromosomes are expressed in ovary or brain. Mol Biol Evol. 2001;18:542–550. doi: 10.1093/oxfordjournals.molbev.a003833. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Doughton BW. Effect of various anaesthetics on resting plasma concentrations of luteinizing hormone, follicle-stimulating hormone and prolactin in ovariectomized ewes. J Endocrinol. 1983;98:79–89. doi: 10.1677/joe.0.0980079. [DOI] [PubMed] [Google Scholar]
- Cohen H, Guillaumot P, Sabbagh I, Bertrand J. A new hypoprolactinemic rat strain. Prolactin, luteinizing hormone, follicle-stimulating hormone, testosterone and corticosterone levels in males and effects of two anesthetics. Biol Reprod. 1983;28:122–127. doi: 10.1095/biolreprod28.1.122. [DOI] [PubMed] [Google Scholar]
- De Nicola AF, Pietranera L, Beauquis J, Ferrini MG, Saravia FE. Steroid protection in aging and age-associated diseases. Exp Gerontol. 2009;44:34–40. doi: 10.1016/j.exger.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Emanuele MA, Tentler J, Kirsteins L, Reda D, Emanuele NV, Lawrence AM. Anaesthesia with alphaxalone plus alphadolone acetate decreases serum concentrations of LH in castrated rats. J Endocrinol. 1987;115:221–223. doi: 10.1677/joe.0.1150221. [DOI] [PubMed] [Google Scholar]
- Estienne MJ, Barb CR. Modulation of growth hormone, luteinizing hormone, and testosterone secretion by excitatory amino acids in boars. Reprod Biol. 2002;2:13–24. [PubMed] [Google Scholar]
- Filby AL, Tyler CR. Molecular characterization of estrogen receptors 1, 2a, and 2b and their tissue and ontogenic expression profiles in fathead minnow (Pimephales promelas) Biol Reprod. 2005;73:648–662. doi: 10.1095/biolreprod.105.039701. [DOI] [PubMed] [Google Scholar]
- Galea LA. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev. 2008;57:332–341. doi: 10.1016/j.brainresrev.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Goldstone JV, McArthur AG, Kubota A, Zanette J, Parente T, Jonsson ME, Nelson DR, Stegeman JJ. Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish. BMC Genomics. 2010;11:643. doi: 10.1186/1471-2164-11-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh O. Evolution of cytochrome p450 genes from the viewpoint of genome informatics. Biol Pharm Bull. 2012;35:812–817. doi: 10.1248/bpb.35.812. [DOI] [PubMed] [Google Scholar]
- Gould EM. The effect of ketamine/xylazine and carbon dioxide on plasma luteinizing hormone releasing hormone and testosterone concentrations in the male Norway rat. Lab Anim. 2008;42:483–488. doi: 10.1258/la.2007.007090. [DOI] [PubMed] [Google Scholar]
- Gustafsson JA. What pharmacologists can learn from recent advances in estrogen signalling. Trends Pharmacol Sci. 2003;24:479–485. doi: 10.1016/S0165-6147(03)00229-3. [DOI] [PubMed] [Google Scholar]
- Haberny KA, Paule MG, Scallet AC, Sistare FD, Lester DS, Hanig JP, Slikker W., Jr Ontogeny of the N-methyl-D-aspartate (NMDA) receptor system and susceptibility to neurotoxicity. Toxicol Sci. 2002;68:9–17. doi: 10.1093/toxsci/68.1.9. [DOI] [PubMed] [Google Scholar]
- Hellgren EC, Lochmiller RL, Amoss MS, Jr, Grant WE. Endocrine and metabolic responses of the collared peccary (Tayassu tajacu) to immobilization with ketamine hydrochloride. J Wildl Dis. 1985;21:417–425. doi: 10.7589/0090-3558-21.4.417. [DOI] [PubMed] [Google Scholar]
- Hill RA, Simpson ER, Boon WC. Evidence for the existence of an estrogen-responsive sexually dimorphic group of cells in the medial preoptic area of the 129SvEv mouse strain. Int J Impot Res. 2008;20:315–323. doi: 10.1038/ijir.2008.2. [DOI] [PubMed] [Google Scholar]
- Jobling S, Williams R, Johnson A, Taylor A, Gross-Sorokin M, Nolan M, Tyler CR, van Aerle R, Santos E, Brighty G. Predicted exposures to steroid estrogens in U.K. rivers correlate with widespread sexual disruption in wild fish populations. Environ Health Perspect. 2006;114(Suppl 1):32–39. doi: 10.1289/ehp.8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone IP, Bancroft BJ. The effects of different anesthetics on blood steroid concentrations in domestic tom-cats. Aust Vet J. 1988;65:382–385. doi: 10.1111/j.1751-0813.1988.tb14278.x. [DOI] [PubMed] [Google Scholar]
- Kanungo J, Cuevas E, Ali SF, Paule MG. l-Carnitine rescues ketamine-induced attenuated heart rate and MAPK (ERK) activity in zebrafish embryos. Reprod Toxicol. 2012;33:205–212. doi: 10.1016/j.reprotox.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanungo J, Cuevas E, Ali SF, Paule MG. Ketamine induces motor neuron toxicity and alters neurogenic and proneural gene expression in zebrafish. J Appl Toxicol. 2013;33:410–417. doi: 10.1002/jat.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazeto Y, Place AR, Trant JM. Effects of endocrine disrupting chemicals on the expression of CYP19 genes in zebrafish (Danio rerio) juveniles. Aquat Toxicol. 2004;69:25–34. doi: 10.1016/j.aquatox.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kishida M, Callard GV. Distinct cytochrome P450 aromatase isoforms in zebrafish (Danio rerio) brain and ovary are differentially programmed and estrogen regulated during early development. Endocrinology. 2001;142:740–750. doi: 10.1210/endo.142.2.7928. [DOI] [PubMed] [Google Scholar]
- Kitano T, Takamune K, Kobayashi T, Nagahama Y, Abe SI. Suppression of P450 aromatase gene expression in sex-reversed males produced by rearing genetically female larvae at a high water temperature during a period of sex differentiation in the Japanese flounder (Paralichthys olivaceus) J Mol Endocrinol. 1999;23:167–176. doi: 10.1677/jme.0.0230167. [DOI] [PubMed] [Google Scholar]
- Kohrs R, Durieux ME. Ketamine: teaching an old drug new tricks. Anesth Analg. 1998;87:1186–1193. doi: 10.1097/00000539-199811000-00039. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25:455–467. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- Larsen B, Hoff G, Wilhelm W, Buchinger H, Wanner GA, Bauer M. Effect of intravenous anesthetics on spontaneous and endotoxin-stimulated cytokine response in cultured human whole blood. Anesthesiology. 1998;89:1218–1227. doi: 10.1097/00000542-199811000-00023. [DOI] [PubMed] [Google Scholar]
- Lee CJ, Do BR, Kim JK, Yoon YD. Pentobarbital and ketamine suppress serum concentrations of sex hormones in the female rat. J Anesth. 2000;14:187–190. doi: 10.1007/s005400070003. [DOI] [PubMed] [Google Scholar]
- Lee O, Takesono A, Tada M, Tyler CR, Kudoh T. Biosensor zebrafish provide new insights into potential health effects of environmental estrogens. Environ Health Perspect. 2012;120:990–996. doi: 10.1289/ehp.1104433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Patterson TA, Sadovova N, Zhang X, Liu S, Zou X, Hanig JP, Paule MG, Slikker W, Jr, Wang C. Ketamine-induced neuronal damage and altered N-methyl-D-aspartate (NMDA) receptor function in rat primary forebrain culture. Toxicol Sci. 2012;131:548–557. doi: 10.1093/toxsci/kfs296. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loch JM, Potter J, Bachmann KA. The influence of anesthetic agents on rat hepatic cytochromes P450 in vivo. Pharmacology. 1995;50:146–153. doi: 10.1159/000139276. [DOI] [PubMed] [Google Scholar]
- Lupp A, Kerst S, Karge E. Evaluation of possible pro- or antioxidative properties and of the interaction capacity with the microsomal cytochrome P450 system of different NMDA-receptor ligands and of taurine in vitro. Exp Toxicol Pathol. 2003;54:441–448. doi: 10.1078/0940-2993-00280. [DOI] [PubMed] [Google Scholar]
- Mahato D, Goulding EH, Korach KS, Eddy EM. Estrogen receptor-alpha is required by the supporting somatic cells for spermatogenesis. Mol Cell Endocrinol. 2001;178:57–63. doi: 10.1016/s0303-7207(01)00410-5. [DOI] [PubMed] [Google Scholar]
- Malaivijitnond S, Takenaka O, Sankai T, Yoshida T, Cho F, Yoshikawa Y. Effects of single and multiple injections of ketamine hydrochloride on serum hormone concentrations in male cynomolgus monkeys. Lab Anim Sci. 1998;48:270–274. [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, Breier A. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17:141–150. doi: 10.1016/S0893-133X(97)00036-5. [DOI] [PubMed] [Google Scholar]
- Marietta MP, White PF, Pudwill CR, Way WL, Trevor AJ. Biodisposition of ketamine in the rat: self-induction of metabolism. J Pharmacol Exp Ther. 1976;196:536–544. [PubMed] [Google Scholar]
- Martinez-Cerdeno V, Noctor SC, Kriegstein AR. Estradiol stimulates progenitor cell division in the ventricular and subventricular zones of the embryonic neocortex. Eur J Neurosci. 2006;24:3475–3488. doi: 10.1111/j.1460-9568.2006.05239.x. [DOI] [PubMed] [Google Scholar]
- Matzen S, Knigge U, Warberg J. Effect of anaesthetics on PRL and LH secretion in rats. Importance of pre-anaesthetic adaptation and involvement of monoaminergic neurons. Acta Endocrinol (Copenh) 1987;115:528–536. doi: 10.1530/acta.0.1150528. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Ehrlichman RS, Liang Y, Trief D, Kanes SJ, Karp J, Siegel SJ. Ketamine produces lasting disruptions in encoding of sensory stimuli. J Pharmacol Exp Ther. 2006;316:315–324. doi: 10.1124/jpet.105.091199. [DOI] [PubMed] [Google Scholar]
- McElroy A, Clark C, Duffy T, Cheng B, Gondek J, Fast M, Cooper K, White L. Interactions between hypoxia and sewage-derived contaminants on gene expression in fish embryos. Aquat Toxicol. 2012;108:60–69. doi: 10.1016/j.aquatox.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Meinhardt U, Mullis PE. The essential role of the aromatase/p450arom. Semin Reprod Med. 2002;20:277–284. doi: 10.1055/s-2002-35374. [DOI] [PubMed] [Google Scholar]
- Meneguz A, Fortuna S, Lorenzini P, Volpe MT. Influence of urethane and ketamine on rat hepatic cytochrome P450 in vivo. Exp Toxicol Pathol. 1999;51(4–5):392–396. doi: 10.1016/S0940-2993(99)80027-X. [DOI] [PubMed] [Google Scholar]
- Menuet A, Pellegrini E, Brion F, Gueguen MM, Anglade I, Pakdel F, Kah O. Expression and estrogen-dependent regulation of the zebrafish brain aromatase gene. J Comp Neurol. 2005;485:304–320. doi: 10.1002/cne.20497. [DOI] [PubMed] [Google Scholar]
- Mouriec K, Gueguen MM, Manuel C, Percevault F, Thieulant ML, Pakdel F, Kah O. Androgens upregulate cyp19a1b (aromatase B) gene expression in the brain of zebrafish (Danio rerio) through estrogen receptors. Biol Reprod. 2009;80:889–896. doi: 10.1095/biolreprod.108.073643. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Nelson DR, Coon MJ, Estabrook RW, Feyereisen R, Fujii-Kuriyama Y, Gonzalez FJ, Guengerich FP, Gunsalus IC, Johnson EF, et al. The P450 superfamily: update on new sequences, gene mapping, and recommended nomenclature. DNA Cell Biol. 1991;10:1–14. doi: 10.1089/dna.1991.10.1. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer. 2006;6:947–960. doi: 10.1038/nrc2015. [DOI] [PubMed] [Google Scholar]
- O’Donnell L, Robertson KM, Jones ME, Simpson ER. Estrogen and spermatogenesis Endocr Rev. 2001;22:289–318. doi: 10.1210/edrv.22.3.0431. [DOI] [PubMed] [Google Scholar]
- Oyama T, Toyota M, Shinozaki Y, Kudo T. Effects of morphine and ketamine anaesthesia and surgery on plasma concentrations of luteinizing hormone, testosterone and cortisol in man. Br J Anaesth. 1977;49:983–990. doi: 10.1093/bja/49.10.983. [DOI] [PubMed] [Google Scholar]
- Parng C, Seng WL, Semino C, McGrath P. Zebrafish: a preclinical model for drug screening. Assay Drug Dev Technol. 2002;1(1 Pt 1):41–48. doi: 10.1089/154065802761001293. [DOI] [PubMed] [Google Scholar]
- Paule MG, Li M, Allen RR, Liu F, Zou X, Hotchkiss C, Hanig JP, Patterson TA, Slikker W, Jr, Wang C. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol. 2011;33:220–230. doi: 10.1016/j.ntt.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini E, Menuet A, Lethimonier C, Adrio F, Gueguen MM, Tascon C, Anglade I, Pakdel F, Kah O. Relationships between aromatase and estrogen receptors in the brain of teleost fish. Gen Comp Endocrinol. 2005;142:60–66. doi: 10.1016/j.ygcen.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Pierman S, Sica M, Allieri F, Viglietti-Panzica C, Panzica GC, Bakker J. Activational effects of estradiol and dihydrotestosterone on social recognition and the arginine-vasopressin immunoreactive system in male mice lacking a functional aromatase gene. Horm Behav. 2008;54:98–106. doi: 10.1016/j.yhbeh.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers DA. Fish as model systems. Science. 1989;246:352–358. doi: 10.1126/science.2678474. [DOI] [PubMed] [Google Scholar]
- Puri CP, Puri V, Anand Kumar TC. Serum levels of testosterone, cortisol, prolactin and bioactive luteinizing hormone in adult male rhesus monkeys following cage-restraint or anaesthetizing with ketamine hydrochloride. Acta Endocrinol (Copenh) 1981;97:118–124. doi: 10.1530/acta.0.0970118. [DOI] [PubMed] [Google Scholar]
- Richards JS, Ireland JJ, Rao MC, Bernath GA, Midgley AR, Jr, Reichert LE., Jr Ovarian follicular development in the rat: hormone receptor regulation by estradiol, follicle stimulating hormone and luteinizing hormone. Endocrinology. 1976;99:1562–1570. doi: 10.1210/endo-99-6-1562. [DOI] [PubMed] [Google Scholar]
- Sasahara K, Shikimi H, Haraguchi S, Sakamoto H, Honda S, Harada N, Tsutsui K. Mode of action and functional significance of estrogen-inducing dendritic growth, spinogenesis, and synaptogenesis in the developing Purkinje cell. J Neurosci. 2007;27:7408–7417. doi: 10.1523/JNEUROSCI.0710-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood NM, Chiappa SA, Sarkar DK, Fink G. Gonadotropin-releasing hormone (GnRH) in pituitary stalk blood from proestrous rats: effects of anesthetics and relationship between stored and released GnRH and luteinizing hormone. Endocrinology. 1980;107:1410–1417. doi: 10.1210/endo-107-5-1410. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994;15:342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Clyne C, Rubin G, Boon WC, Robertson K, Britt K, Speed C, Jones M. Aromatase–a brief overview. Annu Rev Physiol. 2002;64:93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- Simpson ER. Models of aromatase insufficiency. Semin Reprod Med. 2004;22:25–30. doi: 10.1055/s-2004-823024. [DOI] [PubMed] [Google Scholar]
- Slikker W, Jr, Paule MG, Wright LK, Patterson TA, Wang C. Systems biology approaches for toxicology. J Appl Toxicol. 2007;27:201–217. doi: 10.1002/jat.1207. [DOI] [PubMed] [Google Scholar]
- Sumpter JP, Jobling S. Vitellogenesis as a biomarker for estrogenic contamination of the aquatic environment. Environ Health Perspect. 1995;103(Suppl 7):173–178. doi: 10.1289/ehp.95103s7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchoudakova A, Kishida M, Wood E, Callard GV. Promoter characteristics of two cyp19 genes differentially expressed in the brain and ovary of teleost fish. J Steroid Biochem Mol Biol. 2001;78:427–439. doi: 10.1016/s0960-0760(01)00120-0. [DOI] [PubMed] [Google Scholar]
- Trant JM, Gavasso S, Ackers J, Chung BC, Place AR. Developmental expression of cytochrome P450 aromatase genes (CYP19a and CYP19b) in zebrafish fry (Danio rerio) J Exp Zool. 2001;290:475–483. doi: 10.1002/jez.1090. [DOI] [PubMed] [Google Scholar]
- Vascotto SG, Beckham Y, Kelly GM. The zebrafish’s swim to fame as an experimental model in biology. Biochem Cell Biol. 1997;75:479–785. [PubMed] [Google Scholar]
- Wang C, Sadovova N, Hotchkiss C, Fu X, Scallet AC, Patterson TA, Hanig J, Paule MG, Slikker W., Jr Blockade of N-methyl-D-aspartate receptors by ketamine produces loss of postnatal day 3 monkey frontal cortical neurons in culture. Toxicol Sci. 2006;91:192–201. doi: 10.1093/toxsci/kfj144. [DOI] [PubMed] [Google Scholar]
- Ye L, Gho WM, Chan FL, Chen S, Leung LK. Dietary administration of the licorice flavonoid isoliquiritigenin deters the growth of MCF-7 cells overexpressing aromatase. Int J Cancer. 2009;124:1028–1036. doi: 10.1002/ijc.24046. [DOI] [PubMed] [Google Scholar]
- Zaidi P, Wickings EJ, Nieschlag E. The effects of ketamine HCl and barbiturate anaesthesia on the metabolic clearance and production rates of testosterone in the male rhesus monkey, Macaca mulatta. J Steroid Biochem. 1982;16:463–466. doi: 10.1016/0022-4731(82)90061-9. [DOI] [PubMed] [Google Scholar]
- Zhang YY, Yang L. Interactions between human cytochrome P450 enzymes and steroids: physiological and pharmacological implications. Expert Opin Drug Metab Toxicol. 2009;5:621–629. doi: 10.1517/17425250902967648. [DOI] [PubMed] [Google Scholar]


